Abstract
Auditory fear conditioning with tone bursts followed by electric leg stimulation activates neurons not only in the auditory and somatosensory systems but also in many other regions of the brain and elicits shifts in the best frequencies (BFs) of collicular and cortical neurons, i.e., reorganization of the frequency (co-chleotopic) maps in the inferior colliculus and auditory cortex (AC). What are the neural elements minimally necessary for evoking long-term cortical BF shifts? We found that: (i) both electric stimulation and acetylcholine applied to the AC evoke the long-term cortical BF shift as does the conditioning; (ii) both electric stimulation of the AC and acetylcholine applied to the inferior colliculus increase the short-term collicular BF shift evoked by the cortical electric stimulation but do not change it into long-term; and (iii) as this short-term collicular BF shift is blocked by atropine, the development of the long-term cortical BF shift becomes slow and small. Therefore, the most essential neural elements for evoking the long-term cortical BF shift are the AC, corticofugal feedback and the cholinergic nucleus. Our current data support the Gao–Suga model, which hypothesizes that the small short-term cortical BF shifts are evoked by tonal stimuli without the association of conditioned and unconditioned stimuli in the multisensory thalamic nuclei and that these BF shifts are augmented and changed into the large long-term BF shifts by cholinergic neurons.
Keywords: bat, best frequency, conditioning, corticofungal modulation, frequency tuning
Large long-term cortical best frequency (BF) shifts can be evoked by either auditory fear conditioning (1–5) or tone bursts paired with electric stimulation of the cholinergic basal forebrain (6–9). Tone bursts used as a conditioned stimulus (CS) activate neurons in the ascending and descending auditory systems, association cortex, multisensory subcortical nuclei, amygdala, and modulatory systems such as the cholinergic basal forebrain, etc. Electric leg (or foot) stimulation used as an unconditioned stimulus (US) activates neurons in the ascending and descending somatosensory systems and in other brain regions, as do the tone bursts. Electric stimulation of the cholinergic basal forebrain activates neurons not only in the cholinergic basal forebrain but also in the cerebral cortex, and amygdala, etc. (10). What are the neural elements minimally necessary to evoke the large long-term cortical BF shift?
There have been five major neurophysiological findings directly related to the cortical and collicular BF shifts.
The direction of the cortical and collicular BF shifts evoked by focal electric stimulation of the auditory cortex (AC) changes when an antagonist, bicuculline (11, 12), or an agonist, muscimol (12), of GABAA receptors is applied to the AC. This finding indicates that the neural circuit within the AC plays an important role in evoking the BF shifts.
Inactivation of the somatosensory cortex by muscimol does not affect cortical auditory responses but selectively abolishes the development of the conditioning-dependent long-term cortical and short-term collicular BF shifts (3). Electric stimulation of the somatosensory cortex after, but not before, tone-burst stimulation or electric stimulation of the AC augments the cortical and collicular BF shifts. This augmentation does not occur if the basal forebrain is lesioned (9). These findings indicate that the somatosensory cortex, through the cholinergic basal forebrain, plays an essential role in the development of the conditioning-dependent BF shifts.
Muscimol applied to the AC blocks the development of the conditioning-dependent short-term collicular BF shift (2). An antagonist of muscarinic acetylcholine (ACh) receptors, atropine applied to the AC blocks the development of the conditioning-dependent long-term cortical BF shift but not the collicular BF shift. Atropine applied to the inferior colliculus (IC) abolishes the development of the conditioning-dependent collicular BF shift, reduces the cortical BF shift, and makes it short-term (4). These findings and our current findings indicate that the corticofugal system evokes the conditioning-dependent short-term collicular BF shift, that this collicular BF shift contributes to the development of the large long-term cortical BF shift, and that muscarinic ACh receptors play an essential role in evoking the BF shifts.
Electric stimulation of the cholinergic basal forebrain augments the development of the collicular and cortical BF shifts evoked by the focal electric stimulation of the AC (9) or by a long train of tone-burst stimuli (6–9). ACh applied to the AC does not evoke BF shifts without acoustic stimuli or electric stimulation of the AC. However, when paired, ACh augments the development of the short-term cortical BF shift evoked by electric stimulation of the AC and changes it into the long-term BF shift (our present paper). These findings indicate that ACh released in the AC by the cholinergic basal forebrain augments the development of the cortical and collicular BF shifts evoked by both the AC and the corticofugal system and makes the cortical BF shift long-term. These findings also indicate that the BF shifts can be evoked without the signals associating CS and US from the multisensory thalamic nuclei to the AC.
Conditioning-dependent changes in impulse discharges occur in the cholinergic basal forebrain before the AC (13). The latency of a conditioning-dependent change in discharge is 10–20 ms (14–16) in the lateral amygdala. The latency is longer in the AC than in the lateral amygdala: >20 ms (17), 30–50 ms (15), and 500–1,500 ms (18). The development of long-latency conditioning-dependent discharges in the AC is abolished by a lesion of the amygdala (18). The discharge rate change in the AC is presumably evoked by the cholinergic basal forebrain through the amygdala and may be related to the augmentation of the cortical BF shift which has beenevoked by the neural net in the AC and corticofugal system. (It should be noted that BF shifts in the lateral amygdala have not yet been studied.) It also is found that inactivation of the amygdala prevents the development of conditioning-dependent plastic changes in the medial division of the medial geniculate body (MGBm) (19, 20).
Because of the findings obtained by 1998, Gao and Suga (2) proposed the neural net model to explain the tone-specific cortical and collicular plasticity (BF shifts), not the conditioned behavioral responses, elicited by auditory fear conditioning (CS-US). Their model has been further supported by the findings obtained after 1998. The Gao–Suga model (2) states that small and short-term cortical and collicular BF shifts specific to tone bursts (CS) are evoked by the AC and corticofugal system activated by CS alone, and that this cortical BF shift is augmented and changed into long-term by ACh released into the AC by the cholinergic basal forebrain, which is activated by the auditory and somatosensory cortices through the association cortex and the amygdala. The collicular BF shift also is increased by the augmented cortical BF shift through the corticofugal system and contributes to the development of the large long-term cortical BF shift.
If this model is correct, the minimally necessary neural elements for evoking the large long-term cortical BF shift would be the AC, corticofugal feedback, and neurons releasing ACh into the AC. However, it has not yet been demonstrated that the short-term cortical BF shift evoked by focal electric stimulation of the AC is changed into the long-term cortical BF shift by ACh applied to the AC. Our present paper reports that both focal electric stimulation and ACh applied to the AC do evoke the large long-term cortical BF shift as does auditory fear conditioning. Therefore, our finding supports the Gao–Suga model and contributes to the further understanding of the neural circuit involved in the long-term plastic changes in the AC elicited by auditory experiences, including auditory fear conditioning.
Methods
General. Surgery, acoustic and electric stimulation, recording of neural activity, and data acquisition and processing were the same as those described in ref. 9. Drug applications to the AC or IC were the same as those described in ref. 4. The animal studies committee of Washington University approved the protocol for this research.
Nine adult big brown bats (Eptesicus fuscus) were used. Under neuroleptanalgesia (Innovar 4.08 mg/kg of body weight), a 1.5-cm-long metal post was glued on the dorsal surface of the bat's skull. A local anesthetic (lidocaine HCl) and antibiotic ointment (Furacin) were applied to the surgical wound. Three days after the surgery, the awake animal was placed in a body mold, which was hung with an elastic band at the center of a 31°C soundproof room. The metal post glued on the skull was attached to a metal rod with set screws to immobilize the animal's head. The head was adjusted to face directly at the loudspeaker located 74 cm away. A few holes (50–100 μm in diameter) were made in the skull covering the AC and IC. A pair of tungsten-wire electrodes (≈7-μm tip diameter, 20–35 μm apart, one proximal to the other) was inserted to a 500- to 700-μm depth in the AC through one of the holes. The responses (action potentials) of neurons to tone bursts were recorded, and the BF to excite the neurons was measured. Then, this electrode pair was used to electrically stimulate the neurons. A single tungsten-wire electrode (≈7-μm tip diameter) also was inserted into the AC or the central nucleus of the IC to record the responses of a single neuron to tone bursts and to examine the effect of electric stimulation on the responses. In addition to the cortical electric stimulation, ACh was applied to the stimulation site in the AC with or without an atropine application to the IC. The effects of these drugs were then examined on the BF shifts of the cortical and collicular neurons evoked by the cortical electric stimulation.
Acoustic Stimulation. Acoustic stimuli were 20-ms-long tone bursts with a 0.5-ms rise-decay time. They were delivered at a rate of 5/s with a leaf tweeter. The frequency and amplitude of the tone bursts were either varied manually or computer-controlled. When computer-controlled, the frequency of a tone burst in the frequency scan was randomly varied with a stimulus-control and recording software (Tucker-Davis Technologies, Alachua, FL). The amplitude was calibrated with a microphone (model no. 4135, Brüel & Kjær Instruments, Marlborough, MA), and the sound pressure level was expressed in decibels.
The frequency-tuning curve of a single cortical or collicular neuron was first manually measured. Then, the amplitude of a tone burst was fixed at 10 decibels above the minimum threshold of the neuron, and a computer-controlled frequency scan was delivered. The frequency scan consisted of 21 200-ms-long time blocks. In the first 20 blocks, the frequency was changed in 0.3- or 0.5-kHz steps, and in the 21st (last) block, no stimulus was presented to count background discharges. An identical frequency scan was repeated 50 times with a 200-ms-long time interval.
Electric Stimulation. Electric stimulation delivered to the AC was a 6.2-ms-long train of four monophasic electric pulses (100 nA, 0.2-ms duration, 2.0-ms interval). The train of electric stimuli was delivered at a rate of 10/s for 30 min to the AC. Such stimulation was estimated to influence neurons within a 60-μm radius around the electrode tip and to cause a shift of their tuning curves (21). The bat showed no behavioral response at all to such weak electric stimulation.
Applications of ACh or Atropine. The drugs applied to the AC and IC were, respectively, 2.0–4.0 μl of 1 M AChCl (dissolved in distilled water) and 0.5 μl of 0.4 M atropine sulfate (dissolved in 0.9% saline), both from Sigma. A glass micropipette (≈10-μm tip diameter) filled with the ACh solution was placed at the site where the BF of electrically stimulated cortical neurons was measured, whereas a glass micropipette filled with the atropine solution was placed on the dorsal surface of the IC. The drugs were applied with a Picospritzer II (General Valve, Fairfield, NJ). The Picospritzer delivered a 20-kPa pressure for a 30- to 60-min-long ACh application (0.9–1.1 μl of ACh solution per 15 min) and a 55-kPa pressure for a 1.0-s-long atropine application (0.5 μl of atropine solution per s).
Data Acquisition. Action potentials of a single cortical or collicular neuron tuned to a particular frequency were selected with a time-amplitude window-discriminator software (Tucker-Davis Technologies). The action potential (i.e., template) stored and displayed on the monitor screen was compared with action potentials during data acquisition. The responses of the neuron to a frequency scan delivered 50 times were recorded before and after electric stimulation with or without ACh and/or atropine and were displayed as an array of poststimulus time (PST) histograms. The data were stored in the computer hard drive and used for off-line analysis. In a 1-day experiment, only one neuron was studied for the effect of and recovery from electric stimulation and/or ACh and/or atropine applications.
Off-Line Data Processing. The magnitude of auditory responses of a neuron was expressed by a number of impulses per 50 identical stimuli and was plotted as the function of frequency to show the frequency-response curve of that neuron. A BF was determined as the frequency to which the neuron showed the largest response. Because an identical frequency scan was delivered 50 times, there were 50 samples of BFs that could be used to compute a mean and a standard deviation of the BFs and to perform statistical analysis: a two-tailed, paired t test to determine whether the difference in response magnitude between a BF and adjacent frequencies and between the BFs obtained before and after the electric stimulation and/or drug application was significantly different for P < 0.05.
Both BF shifts and the recovery of BF shifts were significant in all neurons studied (paired t test; P < 0.05). Therefore, these BF shifts were highly significant (P < 0.0025). In other words, the BF shift evoked by cortical electric stimulation with or without ACh or atropine was highly significant because it shifted back (i.e., recovered) to the BF in the control condition.
Results
The data were obtained from 71 cortical and 38 collicular neurons. The number of neurons studied for a given ACh or atropine application is shown by a number at the end of each curve in Figs. 3, 4, 5.
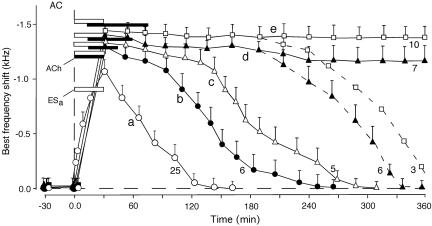
Fig. 3.
The time courses of the BF shifts of cortical neurons evoked by 30-min-long focal cortical electric stimulation (ESa) without (curve a) or with (curves b–e) a cortical ACh application. The duration of an ACh application was 30 min (b and c), 45 min (d), or 60 min (e) and is expressed by the filled horizontal bars. The duration of ESa is expressed by the open horizontal bar. The cortical BF shift recovered 4–5 h after the onset of ESa for the 30-min-long ACh application (b and c). However, the cortical BF shift showed no sign of recovery at 3 h after ESa for an ACh application >30 min. In 7 of 13 neurons studied with a 45-min-long ACh application (d) and in 10 of 13 neurons studied with a 60-min-long ACh application (e), the BF shift showed no sign of recovery even at 360 min after ESa. The number at the end of each curve indicates the number of single neurons studied to obtain the curve. Each data point and a vertical bar indicate a mean and standard error.
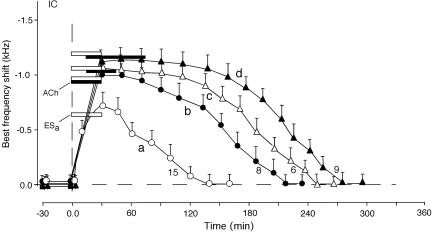
Fig. 4.
The time courses of the BF shifts of collicular neurons evoked by a focal cortical electric stimulation (ESa) without (curve a) or with (curves b–d) a cortical ACh application. The duration of an ACh application was either 30 min (b and c) or 60 min (d). Unlike the BF shifts of cortical neurons, the BF shifts of all collicular neurons recovered within 5 h.
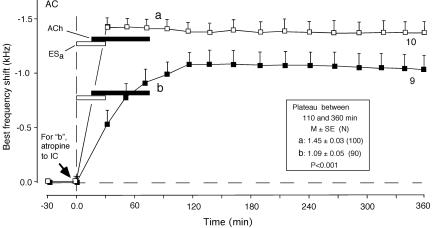
Fig. 5.
Effects of atropine applied to the IC on the development of cortical BF shifts evoked by focal cortical electric stimulation (ESa) plus a 60-min-long cortical ACh application. Shown are the BF shift evoked by ESa plus ACh without an atropine application (curve a) and after an atropine application to the IC (curve b). Atropine applied to the IC blocks the development of the short-term collicular BF shift evoked by ESa by means of the corticofugal projection (4). Therefore, curve b indicates that without the short-term collicular BF shift, the long-term cortical BF shift developed slowly to the plateau. (Inset) The data pooled between 110 and 360 min after ESa are significantly different in plateau value between curves a and b (P < 0.001).
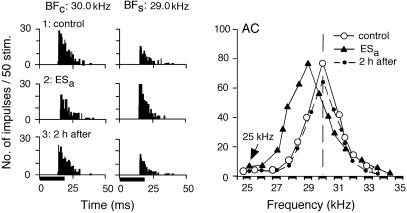
Focal electric stimulation of the AC (ESa) evokes BF shifts of cortical and collicular neurons (9, 22–25). ACh applied to the AC without conditioning or cortical electric stimulation evokes neither cortical nor collicular BF shifts, but it augments BF shifts evoked by conditioning (4) or cortical electric stimulation (12). In Fig. 1, the cortical neuron was tuned to 30.0 kHz (○). That is, the BF of the neuron in the control condition (BFc) was 30.0 kHz. When 25.0-kHz-tuned cortical neurons were electrically stimulated, the response at 30.0 kHz was inhibited, whereas the response at 29.0 kHz was facilitated. As the result of such frequency-dependent facilitation and inhibition, the frequency-tuning curve of the neuron shifted toward 25.0 kHz (▴). The BF in the shifted condition (BFs) was 29.0 kHz. The BF returned to 30.0 kHz ≈120 min after the onset of the electric stimulation (•). Fig. 1 Left and Center show the PST histograms displaying the responses at 30.0 and 29.0 kHz. The response was larger at 30.0 kHz than at 29.0 kHz in the control condition (1: control), whereas it was smaller at 30.0 kHz than at 29.0 kHz 30 min after the onset of the electric stimulation (2: ESa). These neural responses returned to those in the control condition 120 min after (3: 2 h after).
Fig. 1.
Changes in the responses and frequency-response curve of a single cortical neuron evoked by 30-min-long focal electric stimulation (ESa) of the AC without an ACh application to the AC. (Left) Each PST histogram displays the response of the cortical neuron to a sound delivered 50 times at the BF in the control (BFc) or shifted (BFs) condition. (1–3) PST histograms recorded before (control), immediately after ESa (i.e., 30 min after the onset of ESa), and 2 h after ESa. The horizontal bars at the bottom of the histograms indicate 20-ms-long acoustic stimuli: tone bursts. (Right) The frequency-response curves of the neuron measured before ESa (○), immediately after ESa (▴), and 2 h after ESa (•). The oblique arrow in the graph indicates the 25-kHz BF of the cortical neurons that received ESa.
For focal cortical electric stimulation, collicular neurons showed changes that were similar to those shown by cortical neurons, as reported in ref. 25. However, the time course of a BF shift evoked by cortical electric stimulation accompanied with ACh applied to the AC was quite different between cortical and collicular neurons. In the following, the BF shifts of a cortical and a collicular neuron are explained.
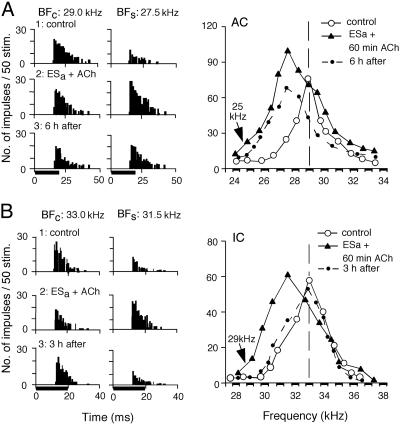
In Fig. 2A, the cortical neuron was tuned to 29.0 kHz (○). A 60-min-long ACh application plus a 30-min-long electric stimulation of 25.0-kHz-tuned cortical neurons augmented the auditory responses of the neuron and shifted its BF from 29.0 to 27.5 kHz (▴), as in Fig. 1. However, the shifted BF showed no sign of recovery even 6 h after the electric stimulation, although the response at the shifted BF decreased to that of the control BF (•). That is, the BF shift became long-term, as further documented later. [We operationally define short-term plasticity as changes that recover within 3.5 h of cortical electric stimulation or conditioning, whereas long-term plasticity refers to changes that show no sign of recovery 3.0 h after the electric stimulation or conditioning. Intermediate-term plasticity refers to changes between these two (9).]
Fig. 2.
Changes in the responses and frequency-response curves of a cortical (A) and a collicular (B) neuron evoked by 30-min-long electric stimulation of cortical auditory neurons (ESa) plus a 60-min-long ACh application to the AC. In A, a cortical BF shift from 29.0 kHz (○) to 27.5 kHz (▴) showed no sign of recovery even 6 h after ESa (•). In B, a collicular BF shift from 33.0 kHz (○) to 31.5 kHz (▴) recovered within 3 h (•). Abbreviations are as in Fig. 1.
In Fig. 2B, the collicular neuron was tuned to 33.0 kHz (○). A 60-min-long ACh application plus cortical electric stimulation of 29.0-kHz-tuned cortical neurons evoked a BF shift from 33.0 kHz to 31.5 kHz (▴). Unlike cortical neurons, the shifted BF returned to the control BF 3 h after the onset of the electric stimulation (•). In Fig. 2, two columns of PST histograms display the changes in the responses at the BFs in the control and shifted conditions.
The time courses of cortical and collicular BF shifts were measured for different durations of cortical ACh applications accompanied by focal electric stimulation of the AC. Fig. 3, curve a shows the mean time course of the BF shifts of 25 cortical neurons evoked by electric stimulation alone. The BF shift was largest (mean ± standard error, 1.08 ± 0.06 kHz) at the end of the 30-min-long electric stimulation. The BF shift recovered ≈130 min after the onset of the electric stimulation.
For a 30-min-long ACh application plus the electric stimulation, the BF shift increased up to 1.20 ± 0.07 kHz, and its recovery time lengthened to 240 ± 12.5 min, n = 6 (Fig. 3 curve b). When the ACh application was delayed from the electric stimulation by 15 min, the BF shift at the peak did not significantly increase, but its recovery time became longer (P < 0.05; Fig. 3 curve c). Accordingly, a 45- or 60-min-long ACh application was delayed from the electric stimulation by 15 min. For the 45-min-long ACh application, the BF shift at the plateau was 1.38 ± 0.09 kHz, n = 13 (Fig. 3 curve d). Compared with that for the 30-min-long ACh application, this small increase in BF shift was insignificant. However, its recovery time significantly lengthened. All of the 13 neurons studied showed no sign of recovery 3.0 h after the electric stimulation. That is, they showed the long-term BF shift. Of the 13 neurons studied, 7 showed no sign of recovery of the BF shift even 360 min after the electric stimulation (Fig. 3 curve d, solid line). However, the BF shifts of the remaining 6 neurons recovered ≈340 min after the stimulation (Fig. 3 curve d, dashed portion). For the 60-min-long ACh application, all of the 13 neurons studied showed the long-term BF shift. The BF shift at the plateau was 1.43 ± 0.09 kHz on the average (Fig. 3 curve e). Of the 13 neurons, 10 showed no sign of recovery of the BF shift even 360 min after the electric stimulation (Fig. 3 curve e, solid line). However, the BF shifts of the remaining 3 neurons recovered ≈380 min after the stimulation (Fig. 3 curve e, dashed line).
The collicular BF shift evoked by cortical electric stimulation alone was similar in both amount and time course to the cortical BF shift evoked under the same condition (Fig. 4 curve a). The collicular BF shift evoked by electric stimulation plus a 30-min-long cortical ACh application to the AC also was very similar to the cortical BF shift evoked by these (Fig. 4 curves b and c). However, the collicular BF shift evoked by the electric stimulation plus a 60-min-long cortical ACh application was quite different from the cortical BF shift evoked under the same condition. That is, all of the BF shifts of nine collicular neurons studied recovered ≈270 min after the electric stimulation (Fig. 4 curve d). The collicular BF shift could not be changed from short-term to long-term.
Atropine applied to the IC before 30-min-long conditioning abolishes the development of the collicular BF shift elicited by the conditioning and reduces the development of the cortical BF shift, also changing it from long-term to short-term (4). When atropine was applied to the IC before focal cortical electric stimulation plus a 60-min-long cortical ACh application, the cortical BF shift slowly developed and plateaued at 1.1 ± 0.16 kHz (n = 9), which was significantly smaller than the plateau evoked by those neurons without the atropine application (P < 0.001, compare curves a and b in Fig. 5). These data indicate that the short-term collicular BF shift contributes to the faster and larger development of the cortical BF shift.
Discussion
Duration of ACh Application. The time course of ACh release in the AC after 30-min-long conditioning, which evokes the large long-term cortical BF shift, has not yet been measured. Ji and Suga (5) found that atropine applied to the AC reduces the cortical BF shift elicited by the conditioning and that this atropine effect is large when it is applied within 120 min after the onset of the conditioning, small when applied at 160 min after the onset, and nonexistent when applied at 200 min after the onset. Therefore, they concluded that the role of ACh in the development of the cortical BF shift ends at ≈180 min after the conditioning. If their data are directly related to an increase in a cortical ACh level elicited by the conditioning, the 60-min-long ACh application to the AC, which evoked the long-term cortical BF shift as shown in our present experiment, would be much shorter than the duration of a conditioning-dependent ACh release by the cholinergic basal forebrain.
Time Courses of Cortical and Collicular BF Shifts. As shown in the present paper, electric stimulation accompanied with ACh applied to the AC evoked the long-term plastic change (BF shift) in the AC, as auditory fear conditioning does. This finding and others (3, 4, 11, 12) indicate that the short-term cortical BF shift is evoked by the neural circuit intrinsic to the AC and corticofugal feedback system and is augmented by the cholinergic basal forebrain, as hypothesized by Gao and Suga (2). However, there are several intriguing problems to be solved. One of them is related to the time course of the development of the collicular and cortical BF shifts. For 30-min-long electric stimulation of the AC, both the collicular and cortical BF shifts simultaneously reach the peak at the end of the stimulation (2, 9, 25). For 30-min-long auditory fear conditioning, the collicular BF shift also reaches the peak at the end of the conditioning, but the cortical BF shift reaches a plateau with a 50- to 150-min delay from the peak of the collicular one. The collicular BF shift is larger than the cortical one over the initial 50–90 min of the development of the BF shift (3, 4). Why does the cortical BF shift reach the plateau with such a significant delay from the collicular one? The cortical electric stimulation does not directly activate the ascending auditory system, but the conditioning acoustic stimulus does. Therefore, we speculate that the inhibitory neurons in the thalamic reticular nucleus slow down the development of the cortical BF shift. When NMDA is applied to the AC before the conditioning, the cortical BF shift reaches the plateau within 15 min after the onset of the conditioning (5). The cortical BF shift can rapidly develop to the plateau.
The time course of BF shifts elicited by the conditioning gives an impression that the collicular BF shift leads the cortical one, despite the fact that the collicular BF shift is evoked by the corticofugal system (2, 3). The latency of the development of the BF shifts has not yet been appropriately measured because our method to study BF shifts is unsuitable for such a measurement.
The conditioning-dependent discharge change develops in the MGBm before in the AC (26, 27). This finding appears to be incompatible with the Gao–Suga model and also with the finding that a lesion of the amygdala abolishes the change in the MGBm (19, 20). Further studies remain to be performed on the latencies of the conditioning-dependent discharge changes in the IC, MGBm, amygdala, and AC, as well as the latencies of BF shifts.
The Weinberger Model. It should be noted that the Gao–Suga model summarized in the Introduction is different from the Weinberger model (1). In the Weinberger model, CS–US association occurs in the multisensory thalamic nuclei: the MGBm and the posterior interlaminar nucleus. Then, the MGBm sends the “associated” signal to the AC and evokes a small short-term cortical BF shift. At the same time, the MGBm and posterior interlaminar nucleus send the associated signal to the amygdala. The amygdala then sends the associated signal to the cholinergic basal forebrain, which releases ACh in the AC. Then, the small short-term cortical BF shift is augmented and changed into long-term. However, neither electric stimulation nor lesion experiments have been performed to show that the MGBm–AC projection is essential to evoking the tone-specific cortical BF shift. Therefore, there have been no neurophysiological data conflicting with the Gao–Suga model.
Tone-Specific BF Shifts vs. Conditioned Behavioral Responses. The projection from the MGBm/posterior interlaminar nucleus to the amygdala (28, 29) and also the projections from the spinothalmic and spinoparabrachial tracts to the amygdala (30) play an essential role in eliciting conditioned behavioral responses (31–33). In the Gao–Suga model, the neural pathway for the cortical BF shift is mostly separated from that of conditioned behavioral responses. Several data reviewed below support this view.
Decorticated animals (34–36) and humans (37) retain or acquire conditioned behavioral responses. A large lesion of the AC does not prevent the acquisition of conditioned behavioral responses (28, 38, 39). These findings indicate that the cortical BF shift is not directly involved in evoking conditioned behavioral responses.
Tone bursts accompanied with electric stimulation of the cholinergic basal forebrain evoke the cortical BF shift (6–9) and induce tone-specific behavioral responses (40). These findings do not mean that the behavioral responses are induced by the activity of the cortical BF-shifted neurons, because decorticated animals show conditioned behavioral responses (34–36) and these behavioral responses are probably induced by the activation of the amygdala, which receives both auditory signals through the MGBm and neural signals from the electrically stimulated basal forebrain.
The corticoamygdala and MGBm–amygdala projections converge into the same region of the lateral amygdala (41) and even on the same neuron (42). These two kinds of inputs must be different from each other in function. The corticoamygdala projection plays an important role in the conditioning-dependent augmentation of the tone-specific BF shift (3) and, presumably, in modulating the effect of the MGBm–amygdala projection on conditioned behavioral responses. On the other hand, the MGBm–amygdala projection plays an essential role in the development of behavioral responses to fear conditioning (32) and, presumably, in the development of sensitization. This working hypothesis remains to be tested. Tone-discrimination behavior trained with CS1–US vs. CS2 changes into fear responses to both CS1 and CS2 as the AC is lesioned (43). The corticoamygdala projection apparently has an influence on the function of the MGBm–amygdala projection. The functions of the corticoamygdala and MGBm–amygdala projections remain to be further explored by neurophysiologists.
Physiological Auditory Memory Trace vs. Physiological Associative Memory Trace. The cortical BF shift evoked by repetitive acoustic stimulation may be called a physiological auditory memory trace. It is still a physiological auditory memory trace even if it is augmented by electric stimulation of the cholinergic basal forebrain. Accordingly, it is still a physiological auditory memory trace even if it is augmented by the cholinergic basal forebrain activated by auditory fear conditioning. It is uncertain whether this cortical BF shift augmented by the conditioning is a physiological “associative” memory trace as described by Weinberger (1), because it has not yet been demonstrated that the BF-shifted neurons produced by the conditioning carry information of both auditory and somatosensory stimuli. If conditioned behavioral responses cannot be evoked without the activation of the cortical BF-shifted neurons, the cortical BF shift would be an associative memory trace. On the contrary, if conditioned behavioral responses can be evoked without the activation of the cortical BF-shifted neurons, the BF shift itself would not be an associative memory trace. As discussed above, the cortical BF shift is a neuronal change that can be separated from conditioned behavioral responses. Therefore, it is probably not a physiological associative memory trace.
Different from the data obtained from the decorticated animals (34–36), a lesion of the AC abolishes fear conditioning in the animal with the disrupted MGBm–amygdala projection. Therefore, it has been concluded that the AC is not necessary for but can mediate simple fear conditionings (38). The neuronal circuit in adult animals for auditory signal processing is plastic (44). When an animal with a disrupted MGBm–amygdala projection is trained for fear conditioning, its function in the normal animal might be taken over by the corticoamygdala projection. Therefore, the result of the above lesion experiment (38) is not necessarily contradictory to the data obtained from decorticated animals. It is an intriguing problem how the corticoamygdala and MGBm–amygdala projections interact with each other in the lateral amygdala.
Acknowledgments
This work was supported by National Institute on Deafness and Other Communicative Disorders Grant DC-00175.
Abbreviations: BF, best frequency; CS, conditioned stimulus; US, unconditioned stimulus; AC, auditory cortex; ACh, acetylcholine; IC, inferior colliculus; PST, poststimulus time; ESa, focal electric stimulation of the AC; MGBm, medial division of the medial geniculate body.
References
- 1.Weinberger, N. M. (1998) Neurobiol. Learn. Mem. 70, 226-251. [DOI] [PubMed] [Google Scholar]
- 2.Gao, E. & Suga, N. (1998) Proc. Natl. Acad. Sci. USA 95, 12663-12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao, E. & Suga, N. (2000) Proc. Natl. Acad. Sci. USA 97, 8081-8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji, W., Gao, E. & Suga, N. (2001) J. Neurophysiol. 86, 211-225. [DOI] [PubMed] [Google Scholar]
- 5.Ji, W. & Suga, N. (2003) J. Neurophysiol. 90, 1904-1909. [DOI] [PubMed] [Google Scholar]
- 6.Bakin, J. S. & Weinberger, N. M. (1996) Proc. Natl. Acad. Sci. USA 93, 11219-11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjordahl, T. S., Dimyan, M. A. & Weinberger, N. M. (1998) Behav. Neurosci. 112, 467-479. [DOI] [PubMed] [Google Scholar]
- 8.Kilgard, M. P. & Merzenich, M. M. (1998) Science 279, 1714-1718. [DOI] [PubMed] [Google Scholar]
- 9.Ma, X. & Suga, N. (2003) J. Neurophysiol. 89, 90-103. [DOI] [PubMed] [Google Scholar]
- 10.Wenk, G. L. (1997) Neurobiol. Learn. Mem. 67, 85-95. [DOI] [PubMed] [Google Scholar]
- 11.Xiao, Z. & Suga, N. (2002) Proc. Natl. Acad. Sci. USA 99, 15743-15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma, X, & Suga, N. (2004) J. Neurophysiol. 92, 3192-3199. [DOI] [PubMed] [Google Scholar]
- 13.Maho, C., Hars, B., Edeline, J.-M. & Hennevin, E. (1995) Psychobiology 23, 10-25. [Google Scholar]
- 14.Quirk, G. J., Repa, C. & LeDoux, J. E. (1995) Neuron 15, 1029-1039. [DOI] [PubMed] [Google Scholar]
- 15.Quirk, G. J., Armony, J. L. & LeDoux, J. E. (1997) Neuron 19, 613-624. [DOI] [PubMed] [Google Scholar]
- 16.Maren, S. (2000) Eur. J. Neurosci. 12, 4047-4054. [DOI] [PubMed] [Google Scholar]
- 17.Li, X. F., Armony, J. L. & LeDoux, J. E. (1996) Synapse 24, 115-124. [DOI] [PubMed] [Google Scholar]
- 18.Armony, J. L., Quirk, G. J. & LeDoux, J. E. (1998) J. Neurosci. 18, 2592-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poremba, A. & Gabriel, M. (2001) J. Neurosci. 21, 270-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maren, S., Yap, S. A. & Goosens, K. A. (2001) J. Neurosci. 21, RC135 (1-6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan, J. & Suga, N. (1996) Science 273, 1100-1103. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, Y., Suga, N. & Yan, J. (1997) Nature 387, 900-903. [DOI] [PubMed] [Google Scholar]
- 23.Yan, W. & Suga, N. (1998) Nat. Neurosci. 1, 54-58. [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury, S. A. & Suga, N. (2000) J. Neurophysiol. 83, 1856-1863. [DOI] [PubMed] [Google Scholar]
- 25.Ma, X. & Suga, N. (2001) J. Neurophysiol. 85, 1078-1087. [DOI] [PubMed] [Google Scholar]
- 26.Disterhoft, J. F. & Olds, J. (1972) J. Neurophysiol. 35, 665-679. [DOI] [PubMed] [Google Scholar]
- 27.Disterhoft, J. F & Stuart, D. K. (1976) J. Neurophysiol. 39, 266-281. [DOI] [PubMed] [Google Scholar]
- 28.LeDoux, J. E., Cicchetti, P., Xagoraris, A. & Romanski, L. M. (1990) J. Neurosci. 10, 1062-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner, B. H. & Herkenham, M. (1991) J. Comp. Neurol. 313, 295-325. [DOI] [PubMed] [Google Scholar]
- 30.Lanuza, E., Nader, K. & LeDoux, J. E. (2004) Neuroscience 125, 305-315. [DOI] [PubMed] [Google Scholar]
- 31.Iwata, J., LeDoux, J. E., Meeley, M. P., Arneric, S. & Reis, D. J. (1986) Brain Res. 383, 195-214. [DOI] [PubMed] [Google Scholar]
- 32.LeDoux, J. E., Sakaguchi, A. & Reis, D. J. (1984) J. Neurosci. 4, 683-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeDoux, J. E., Sakaguchi, A., Iwata, J. & Reis, D. J. (1986) Neuroscience 17, 615-627. [DOI] [PubMed] [Google Scholar]
- 34.DiCara, L. V., Braun, J. J. & Pappas, B. A. (1970) J. Comp. Physiol. Psychol. 73, 208-216. [DOI] [PubMed] [Google Scholar]
- 35.Norman, R. J., Villablanca, J. R., Brown, K. A, Schwafel, J. A, & Buchwald, J. S. (1974) Exp. Neurol. 44, 363-380. [DOI] [PubMed] [Google Scholar]
- 36.Mauk, M. D. & Thompson, R. F. (1987) Brain Res. 403, 89-95. [DOI] [PubMed] [Google Scholar]
- 37.Berntson, G. G., Tuber, D. S., Ronca, A. E. & Bachman, D. S. (1983) Exp. Neurol. 81, 77-88. [DOI] [PubMed] [Google Scholar]
- 38.Romanski, L. M. & LeDoux, J. E. (1993) Cereb. Cortex 3, 515-532. [DOI] [PubMed] [Google Scholar]
- 39.Armony, J. L., Servan-Schreiber, D., Romanski, L. M., Cohen, J. D. & LeDoux, J. E. (1997) Cereb. Cortex 7, 157-165. [DOI] [PubMed] [Google Scholar]
- 40.McLin, D. E., III, Miasnikov, A. A. & Weinberger, N. M. (2002) Proc. Natl. Acad. Sci. USA 99, 4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeDoux, J. E., Farb, C. R. & Romanski, L. M. (1991) Neurosci. Lett. 134, 139-144. [DOI] [PubMed] [Google Scholar]
- 42.Li, X. F., Stutzmann, G. E. & LeDoux, J. E. (1996) Learn. Mem. 3, 229-242. [DOI] [PubMed] [Google Scholar]
- 43.Jarrell, T. W., Gentile, C. G., Romanski, L. M., McCabe, P. M. & Schneiderman, N. (1987) Brain Res. 412, 285-294. [DOI] [PubMed] [Google Scholar]
- 44.Heffner, H. E.& Heffner, R. S. (1984) Science 226, 75-76. [DOI] [PubMed] [Google Scholar]