Abstract
We examined the role of CD4+CD25+ regulatory T cells in the development of 3-methylcholanthrene (MCA)-induced tumors. Immunization of wild-type BALB/c mice with a series of SEREX (serological identification of antigens by recombinant expression cloning)-defined broadly expressed self-antigens results in the development of highly active CD4+CD25+ regulatory T cells. Accelerated tumor development was observed in mice immunized with self-antigens and was abolished by antibody-mediated depletion of CD4+ T cells or CD25+ T cells. A similar acceleration of tumorigenesis was also observed in mice adoptively transferred 2 or 4 weeks after MCA injection with CD4+CD25+ T cells derived from mice immunized with DnaJ-like 2, one of these self-antigens. Experiments with Jα281-/- mice lacking invariant natural killer (iNK) T cells indicated that iNK T cells, known for their protective role in the development of MCA-induced tumors, were suppressed in immunized hosts. NK cells, also known to play a protective role in MCA induced-tumorigenesis, were also suppressed in mice immunized with serologically defined self-antigens in a CD4+CD25+ T cell-dependent manner. We propose that CD4+CD25+ regulatory T cells generated by immunization with these self-antigens enhance susceptibility to MCA induced-tumorigenesis by down-regulating iNK T and NK reactivity, and suggest that these observations provide direct evidence for the existence of cancer immunosurveillance in this system of chemical carcinogenesis.
Keywords: immunosurveillance, self-antigen immunization
We have recently reported that immunization with serologically defined self-antigens leads to heightened susceptibility to challenge with syngeneic tumor lines observed as enhancement of pulmonary metastasis (1). These broadly expressed self-antigens have been identified by serological identification of antigens by recombinant expression cloning (SEREX), a serological expression cloning method that has been widely used to identify immunogenic molecules in murine and human tumors (2-4). Using sera of animals bearing 3-methylcholanthrene (MCA)-induced sarcoma lines of BALB/c origin, four of the most frequently detected gene products were selected and used as immunogenic self-antigens after verifying the lack of gene mutations based on the registered sequences in GenBank (4).
Although these self-antigens were identified by IgG type antibodies and thus considered to be recognized by CD4+ helper T cells, immunization with these SEREX-defined self-antigens surprisingly enhanced pulmonary metastasis via elicitation of self-antigen-specific regulatory CD4+CD25+ T cells with strong suppressive activity on CD4+ T and CD8+ T cell activity (1, 4, 5). In mice immunized with one of the SEREX-defined self-antigens, DnaJ-like 2, the number of α-galactosylceramide (GalCer)/CD1d tetramer+CD3+ T cells [representing invariant natural killer T (iNKT) cells] was reduced in the pulmonary compartment, whereas no evident change in the number of other T cell subsets was observed. Experiments with Jα281-/- mice lacking most iNKT cells indicate that iNKT cells are primarily responsible for metastasis suppression and that their activity is inhibited by immunization with DnaJ-like 2 (1). Taken together, we have speculated that SEREX identifies a pool of self-antigens that maintains and regulates immunological homeostasis via CD4+CD25+ regulatory T cells.
In this study, we show that immunization with these SEREX-defined self-antigens, but not SEREX-unrelated self-antigens or heterologous antigens, accelerates tumor development induced by MCA in wild-type BALB/c mice. Acceleration of tumor development in animals immunized with SEREX-defined self-antigens is mediated by CD4+CD25+ T cells. Similar to our findings in the pulmonary metastasis model, iNKT cells are critical in protecting against MCA-induced tumor development and can be down-regulated by CD4+CD25+ regulatory T cells responding to SEREX-defined self-antigens. In addition, NK cell activity is also greatly reduced by immunization with SEREX-defined self-antigens in a CD4+CD25+ T cell-dependent manner.
Materials and Methods
Mice. Female BALB/c mice and BALB/cnu/nu mice were obtained from CLEA Japan (Osaka, Japan) and used at 7-10 weeks of age. Jα281-/- mice were established by specific deletion of the Jα281 gene segment as described (6), backcrossed 10 times to BALB/c background, and used at 7-9 weeks of age. Mice were maintained under specific pathogen-free condition at the Animal Center of Mie University School of Medicine (Mie, Japan). The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Mie University School of Medicine.
Antibodies and Reagents. Anti-CD25 (PC61, rat IgG1) was purified from ascitic fluid on a DEAE Toyopearl 650S column (Tosoh, Tokyo). The mAb specific for CD4 (GK1.5, rat IgG2b) was used in the form of ascites. Anti-CD4 mAb (25-μl ascites) or anti-CD25 mAb (250 μg) were injected mice 2 weeks after MCA inoculation. Mice treated with anti-CD4 mAb and anti-CD25 mAb were also treated with the respective mAb every 4 weeks that resulted in the depletion of respective cell population during entire period of tumor induction experiment as described (7, 8). MCA was purchased from Sigma. α-GalCer was kindly provided by Pharmaceutical Research Laboratories (Kirin Brewery, Gunma, Japan).
Induction of Tumors by MCA. Groups of 15-36 mice were inoculated s.c. in the right hind flank with 0.2 ml of peanut oil (Sigma) containing 50 μg of MCA and monitored weekly for the development of tumors. Tumors >5 mm in diameter and demonstrating progressive growth over 3 weeks were counted as primary tumors.
Plasmids. SEREX-defined molecules and control SEREX-unrelated molecules were cloned into pBK-CMV and purified by using EndoFree Plasmid Mega kit (Qiagen, Hilden, Germany) (1, 4, 5).
Immunization by Gene Gun. Gold particles coated with plasmid DNA (1 μg per injection) were prepared and delivered into shaved skin of the abdominal wall of BALB/c mice by a Helios Gene Gun System (Bio-Rad, Hercules, CA) at a helium discharge pressure of 350-400 psi, as described (1, 4, 5).
Transfer of CD4+CD25+ or CD4+CD25- T Cells. CD4+CD25+ and CD4+CD25- T cells were prepared as described (1, 5) and were confirmed to contain >96% and >93% of respective cell population. Purified CD4+CD25+ T cells or CD4+CD25- T cells were inoculated into BALB/c mice through the lateral tail vein.
Reconstitution of Jα281-/- Mice with α-GalCer/CD1d Tetramer+ Cells. Splenocytes from BALB/c mice were enriched for α-GalCer/CD1d tetramer+ cells by using anti-phycoerythrin microbeads (Miltenyi Biotec) as described (1).
51Cr-Release Assays. Thy1+ cells were depleted from splenic populations by using anti-Thy1 microbeads (Miltenyi Biotec). The resultant Thy1- cells were further enriched for DX5+ cells by positive selection on a MACS column after reacting with anti-DX5 microbeads (Miltenyi Biotec) and used as NK cells. Cytotoxic assays were performed as described with some modifications (9). Briefly, target cell lines, YAC-1 or P1.HTR labeled with 51Cr were added to varying numbers of effector cells in U-bottom plates. After 18-h incubation, supernatants were collected and the percent-specific lysis was calculated by using the formula [(cpm released from the test sample - spontaneous release)/(cpm released by detergent - spontaneous release) × 100%].
Statistical Analysis. Statistical significance was evaluated by a Mann-Whitney U test using spss 12.01 for Windows (SPSS, Chicago).
Results
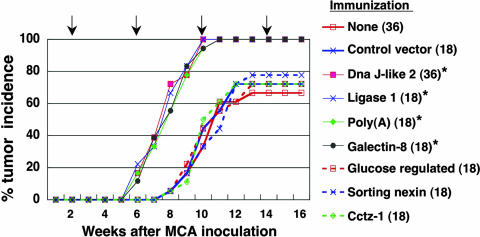
Tumor Development Is Accelerated in Animals Immunized with SEREX-Defined Self-Antigens. s.c. injection of 50 μg of MCA mixed with peanut oil resulted in tumor development in 70-80% of control BALB/c mice in 12-14 weeks. To examine the effect of immunization with SEREX-defined self-antigens on MCA-induced tumorigenesis, mice injected with MCA received subsequent immunization with plasmids encoding SEREX-defined self-antigens or control plasmids. Immunization commenced 2 weeks after MCA injection and was repeated every 4 weeks. Tumor development was regularly checked in the 18-36 animals in each immunization group. As shown in Fig. 1, tumors developed much sooner and in higher frequency in mice immunized with plasmids encoding any one of the four SEREX-defined self-antigens: Mus heat shock protein, DnaJ-like 2 (GenBank accession no. AF055664), Mus DNA ligase 1 (Ligase 1) (GenBank accession no. U19604), Mus galectin-8 (Galectin-8) (GenBank accession no. AF218069), and Mus poly(A)-binding protein, cytoplasmic 1 [Poly(A)] (GenBank accession no. X65553), compared with control mice. In contrast, no acceleration of tumor development was observed in mice immunized with following control plasmids (i.e., plasmids encoding murine molecules not detected in repeated SEREX analyses and selected randomly from SEREX screening library): Mus sorting nexin 1 (Sorting nexin) (GenBank accession no. AB019214), Mus glucose regulated protein (Glucose regulated) (GenBank accession no. D78645), and Mus Cctz-1 gene for chaperon containing TCP-1-ζ-1 subunit (Cctz-1) (GenBank accession no. AB022159), or heterologous plasmids: Homo sapiens HMBA-inducible (GenBank accession no. XM_008348), human retinoic acid-responsive protein (GenBank accession no. U50383), and OVA (Fig. 1 and data not shown).
Fig. 1.
Immunization with SEREX-defined self-antigens accelerates development of MCA-induced tumors. BALB/c mice were inoculated s.c. with 50 μg of MCA in peanut oil. Immunization with plasmids encoding the indicated antigens using a gene gun commenced 2 weeks after MCA inoculation and was repeated every 4 weeks (indicated by the arrows). Mice were monitored weekly for tumor development. The number of mice in each group is shown in parentheses. Statistical significance of immunization was determined by the Mann-Whitney U test. % tumor frequency of each group at 10 weeks was compared with BALB/c mice receiving 50 μg of MCA alone (*, P < 0.001). These experiments were repeated four times with similar results.
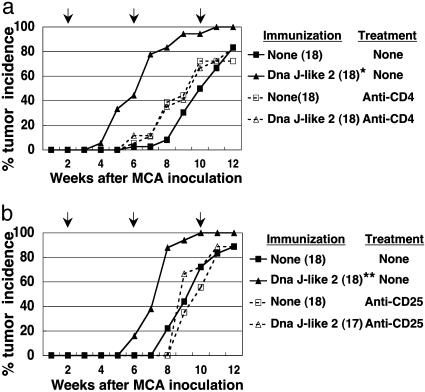
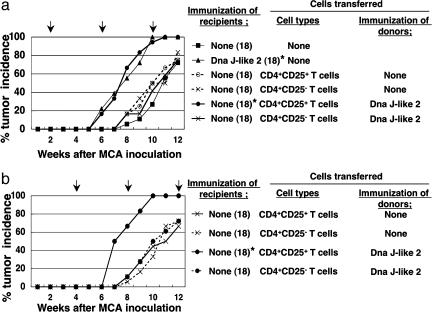
Acceleration of Tumor Development Depends on CD4+CD25+ T Cells. Pretreatment of mice with anti-CD4 mAb (GK1.5) or anti-CD25 mAb (PC61) abolished the accelerated tumor development induced by the SEREX-defined self-antigen DnaJ-like 2 (Fig. 2 a and b). To analyze the role of CD4+CD25+ T cells in tumor development, we investigated whether CD4+CD25+ T cells derived from DnaJ-like 2 immunized mice could transfer the accelerated tumor development to recipients. As shown in Fig. 3, CD4+CD25+ T cells, but not CD4+CD25- T cells, derived from animals immunized with DnaJ-like 2 clearly mediated the accelerated tumor development in recipients. Accelerated tumor development was observed when T cells were transferred either 2 or 4 weeks after MCA injection, whereas no such acceleration was observed in recipients of CD4+CD25+ T cells derived from naive mice (Fig. 3 a and b).
Fig. 2.
CD4+/CD25+ T cells are responsible for acceleration of tumor development induced by immunization with SEREX-defined self-antigen. BALB/c mice were injected i.p. with anti-CD4 mAb (25-μl ascites) (a) or anti-CD25 mAb (250 μg) (b) 2 weeks after MCA inoculation. The mice were subsequently treated with the respective mAb every 4 weeks. Mice were inoculated s.c. with 50 μg of MCA mixed with peanut oil. Immunization with plasmids encoding DnaJ-like 2 using a gene gun commenced 2 weeks after MCA inoculation and was repeated every 4 weeks. Mice were monitored weekly for tumor development. The number of mice in each group is shown in parentheses. The arrows indicate days of mAb treatment and immunization. % tumor frequency of each group at 10 weeks was compared with BALB/c mice receiving 50 μg of MCA alone by using Mann-Whitney U test (*, P = 0.003; **, P = 0.018). These experiments were repeated three times with similar results.
Fig. 3.
Accelerated development of MCA-induced tumors is adoptively transferred by CD4+CD25+ T cells derived from mice immunized with SEREX-defined self-antigen. Purified 1 × 105 CD4+CD25+ T cells or CD4+CD25- T cells derived from BALB/c mice (naive or DnaJ-like 2 immunized) were transferred into naive BALB/c mice 2 (a) or 4 (b) weeks after MCA inoculation and repeated every 4 weeks. Mice were monitored weekly for tumor development. The number of mice in each group is shown in parentheses. The arrows indicate days of cell transfer. % tumor frequency of each group at 10 weeks was compared with BALB/c mice receiving 50 μg of MCA alone by using Mann-Whitney U test (*, P < 0.001). These experiments were repeated three times (a) or twice (b) with similar results.
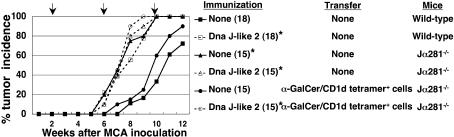
α-GalCer/CD1d Tetramer+ Cells Are Involved in the Acceleration of Tumor Development. An essential role of iNKT cells in the inhibition of tumor development by MCA has been reported (10). Therefore, we investigated the role of iNKT cells in the acceleration of tumor development induced by immunization with SEREX-defined self-antigens and the possible suppression of iNKT cells by CD4+CD25+ T cells. Jα281-/- mice lacking iNKT cells showed acceleration of MCA-induced tumor development compared with wild-type mice. Acceleration was significantly inhibited in mice reconstituted with α-GalCer/CD1d tetramer+ cells from wild-type mice. However, Jα281-/- mice reconstituted with α-GalCer/CD1d tetramer+ cells and subsequently immunized with DnaJ-like 2 showed acceleration of tumor development as observed in DnaJ-like 2 immunized wild-type mice (Fig. 4).
Fig. 4.
Accelerated MCA-induced tumor development is caused by down-regulation of NKT cells. Jα281-/- mice were reconstituted with 2 × 105 α-GalCer/CD1d tetramer+ cells derived from naive wild-type BALB/c mice. MCA was inoculated 2 weeks before reconstitution. Immunization with plasmids encoding DnaJ-like 2 was performed at the time of reconstitution and subsequently repeated every 4 weeks. Mice, either reconstituted with α-GalCer/CD1d tetramer+ cells or not, and also immunized with DnaJ-like 2 or not, were monitored weekly for tumor development. The number of mice in each group is shown in parentheses. The arrows indicate days of cell transfer and immunization. % tumor frequency of each group at 10 weeks was compared with BALB/c mice receiving 50 μg of MCA alone by using Mann-Whitney U test (*, P < 0.001). These experiments were repeated three times with similar results.
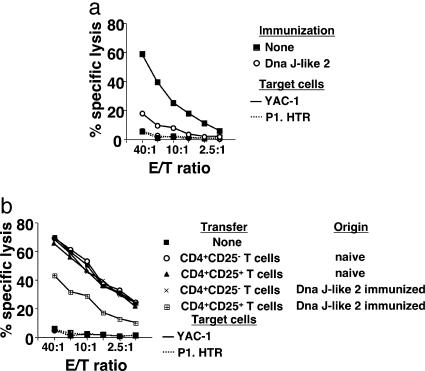
Cytolytic Activity of NK Cells Is Suppressed by CD4+CD25+ T Cells Derived from Mice Immunized with SEREX-Defined Self-Antigens. Collaboration of NK cells with iNKT cells in immunosurveillance has been reported (11). Therefore, we investigated whether immunization of mice with DnaJ-like 2 led to a change in NK cell activity. In mice immunized with DnaJ-like 2, no change in the number of NK cells defined as CD3-DX5+ cells was observed in the spleen and lungs compared with naive mice (data not shown). However, splenic NK cells from DnaJ-like 2 immunized mice (Fig. 5a) showed significantly reduced cytolytic activity against NK cell-sensitive target cells, YAC-1, compared with those derived from naive mice. We then asked whether the reduced cytolytic activity of NK cells was mediated by CD4+CD25+ T cells derived from DnaJ-like 2 immunized mice. For this purpose, we compared NK cell activity of BALB/cnu/nu mice adoptively transferred with CD4+CD25+ or CD4+CD25- T cells derived from either naive wild-type mice or DnaJ-like 2 immunized mice. As shown in Fig. 5b, NK cells obtained from BALB/cnu/nu mice adoptively transferred with CD4+CD25+ T cells derived from DnaJ-like 2 immunized mice showed significantly reduced cytolytic activities against YAC-1. Specifically, no reduction of NK cell activity was observed in hosts adoptively transferred with either CD4+CD25+ T cells derived from naive BALB/c mice or CD4+CD25- T cells derived from DnaJ-like 2 immunized mice, compared with lytic activities of NK cells of naive BALB/cnu/nu mice.
Fig. 5.
NK cell cytotoxicity is suppressed by CD4+CD25+ T cells derived from mice immunized with SEREX-defined self-antigen. Splenic NK cells were sorted from wild-type BALB/c mice immunized with DnaJ-like 2 or not (a) or BALB/cnu/nu reconstituted with CD4+CD25+ T cells or CD4+CD25- T cells derived from BALB/c mice (naive or DnaJ-like 2 immunized) (b). These cells were examined in 18-h 51Cr-release assays for direct lysis of labeled target cells, YAC-1 (bold line) and P1.HTR (dotted line). These experiments were repeated three times with similar results.
Discussion
Consistent with our recent findings in a tumor challenge model (1), immunization of wild-type BALB/c mice with each of the four SEREX-defined self-antigens resulted in accelerated MCA-induced tumor development. Neither the three nonimmunogenic molecules randomly selected from SEREX libraries nor the three heterologous antigens showed a similar acceleration of tumor development (Fig. 1). The similarity between results of the present study involving primary tumor induction and our past findings with transplanted tumors strongly supports the presence of common immunological mechanisms in these two systems of immunological tumor suppression (1). In both systems, accelerated tumor development induced by immunization with DnaJ-like 2, one of the SEREX-defined self-antigens, clearly depends on CD4+CD25+ T cells as shown in experiments involving antibody mediated depletion (Fig. 2) and T cell transfer (Fig. 3). Remarkably, accelerated primary tumor development was transferred to naive mice with as few as 1 × 105 CD4+CD25+ T cells obtained from DnaJ-like 2 immunized mice. Although antibody to DnaJ-like 2 was detected in mice immunized with DnaJ-like 2, none was found in mice receiving adoptive transfer of CD4+CD25+ T cells from such immunized mice (data not shown). This makes it highly unlikely that antibody-mediated tumor enhancement is the basis for the accelerated tumor development we observed.
Although these SEREX-defined self-antigens were initially identified by screening cDNA libraries prepared from different MCA-induced sarcomas with sera from animals bearing cognate tumors, these antigens are ubiquitously expressed wild-type molecules that are in no sense tumor specific (4). We confirmed the expression of DnaJ-like 2 in emerging tumors in mice immunized with DnaJ-like 2 (data not shown). Through application of SEREX methodology to a wide variety of human and animal tumor systems, a number of antigens have been identified in tumor cells as immunogenic molecules in tumor-bearing host (2, 3). The basis for the immunogenicity of these widely expressed wild-type molecules is unknown, but may be related to higher expression levels and/or greater accessibility to the immune system due to spontaneously occurring tumor necrosis.
The present results, together with our recent findings (1), indicate that CD4+CD25+ regulatory T cells induced by SEREX-defined self-antigens suppress iNKT cells. Smyth and colleagues stressed the essential role of iNKT cells in immune protection against tumor development (10, 12). In their studies, Jα281-/- mice were found to be highly susceptible to MCA tumorigenesis (10, 12). In addition, they provided evidence for an important role of NK cells through interaction with iNKT cells in protection against MCA-induced sarcomas (10, 11). These findings of Smyth and colleagues in mice with a C57BL/6 background are essentially similar to our observations in BALB/c mice. More importantly, our reconstitution of resistance to tumor induction and tumor challenge (1) in Jα281-/- mice with α-GalCer/CD1d tetramer+ T cells provides additional direct evidence for the importance of iNKT cells in tumor immunity. The present results also indicate that NK cells are targets of CD4+CD25+ regulatory T cells induced by DnaJ-like 2. Because collaborative activity of iNKT cells and NK cells with distinct functions in immunosurveillance has been reported (10, 11), the significant suppression of both iNKT and NK cell activity by DnaJ-like 2 immunization may also explain the striking acceleration of tumor development in hosts immunized with SEREX-defined self-antigens.
The existence of cancer immunosurveillance has been a long disputed issue in tumor immunology (13-18). Recent analyses of genetically engineered animals have shed light on the immunosurveillance process during tumorigenesis induced by chemical carcinogens or transforming genes, or occurring spontaneously (18). In hosts lacking cellular components, such as αβ T cells (19, 20), γδ T cells (19-21), NKT cells (10, 12), NK cells (10, 11), both T and B cells (22), or immunoregulatory molecules such as IFN-γ (23-25), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (26, 27), or perforin (24, 28, 29), significant acceleration and higher frequency of tumor development have been observed, indicating that immune response functions as an effective tumor-suppressor system.
Although these findings strongly support the existence of an immunosurveillance system for cancer development, Qin et al. (30) proposed an alternative interpretation for the more rapid induction of chemically induced tumors in mice lacking IFN-γ receptor (IFN-γR). Confirming earlier studies, they found that MCA-induced tumor development was enhanced in IFN-γR-deficient mice. However, they argue that this is due to the lack of IFN-γ mediated encapsulation of MCA in IFN-γR-deficient mice, leading to diffusion of MCA and exposure of a larger number of cells to the transforming effect of MCA. Because encapsulated MCA can persist virtually life-long in mice without inducing tumors, Qin et al. concluded that the observed protective response induced by IFN-γ might not be directed against the transformed cells but against the carcinogen (30, 31). The finding that other immunological deficiencies not apparently involving IFN-γR, such as TRAIL (26, 27), and perforin (24, 28, 29), are also associated with increased frequency of MCA induced tumors, does not support this idea. In addition, our finding that CD4+CD25+ T cells derived from DnaJ-like 2 immunized mice accelerated tumor induction even when transferred as late as 4 weeks after MCA injection (allowing sufficient time for the cellular and other microenvironmental changes to occur at the site of MCA injection) is strong evidence in favor of immunoprotection against tumor development rather than simply sequestration of MCA by encapsulation.
In vitro systems of MCA induced cellular transformation have shown that 3-8 weeks are required to yield transformed cells (32-34). If this were the case in vivo, e.g., first tumor cells appear at 3 weeks, it would then take 223 to 226 cell doublings to establish a detectable tumor mass (>5 mm in diameter) containing 107 to 108 cells. Given a doubling time of 12-24 h, initial tumors would theoretically be detected as soon as 5-7 weeks after injection of MCA. The actual observed time of tumor appearance in mice actively or adoptively immunized against DnaJ-like 2 is consistent with this theoretical estimate, and approximates what would be expected if no immunological restraint to tumor induction were exerted in vivo, whereas the longer induction time in nonimmunized mice is consistent with the actions of a protective immunosurveillance mechanism. In fact, the accelerated MCA tumor induction in mice receiving CD4+CD25+ T cells from donors immunized with DnaJ-like 2 can be taken as direct evidence for the existence of cancer immunosurveillance and provides a specific immunological maneuver to modulate the process.
Acknowledgments
We thank Drs. S. Gnjatic, E. Sato and H. Ikeda for helpful discussion and J. Suzuki for technical support. This work was supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author contributions: H.N., T.K., L.J.O., and H.S. designed research; H.N., I.T., T.T., K.S., and L.W. performed research; Y.I., H.W., T.N., and M.T. contributed new reagents/analytic tools; H.N., T.K., I.T., K.K., L.J.O., and H.S. analyzed data; and H.N., T.K., L.J.O., and H.S. wrote the paper.
Abbreviations: MCA, methylcholanthrene; SEREX, serological identification of antigens by recombinant expression cloning; iNKT cell, invariant natural killer T cell;α-GalCer,α-galactosylceramide.
References
- 1.Nishikawa, H., Kato, T., Tanida, K., Hiasa, A., Tawara, I., Ikeda, H., Ikarashi, Y., Wakasugi, H., Kronenberg, M., Nakayama, T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 10902-10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahin, U., Tureci, O., Schmitt, H., Cochlovius, B., Johannes, T., Schmits, R., Stenner, F., Luo, G., Schobert, I. & Pfreundschuh, M. (1995) Proc. Natl. Acad. Sci. USA 92, 11810-11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen, Y.-T., Scanlan, M. J., Obata, Y. & Old, L. J. (2000) Identification of Human tumor Antigens by Serological Expression Cloning (SEREX) (Lippincott, Williams & Wilkins, Philadelphia).
- 4.Nishikawa, H., Tanida, K., Ikeda, H., Sakakura, M., Miyahara, Y., Aota, T., Mukai, K., Watanabe, M., Kuribayashi, K., Old, L. J. & Shiku, H. (2001) Proc. Natl. Acad. Sci. USA 98, 14571-14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa, H., Kato, T., Tawara, I., Saito, K., Ikeda, H., Kuribayashi, K., Allen, P. M., Schreiber, R. D., Sakaguchi, S., Old, L. J. & Shiku, H. (2005) J. Exp. Med. 201, 681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui, J., Shin, T., Kawano, T., Sato, H., Kondo, E., Toura, I., Kaneko, Y., Koseki, H., Kanno, M. & Taniguchi, M. (1997) Science 278, 1623-1626. [DOI] [PubMed] [Google Scholar]
- 7.Udono, H., Levey, D. L. & Srivastava, P. K. (1994) Proc. Natl. Acad. Sci. USA 91, 3077-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onizuka, S., Tawara, I., Shimizu, J., Sakaguchi, S., Fujita, T. & Nakayama, E. (1999) Cancer Res. 59, 3128-3133. [PubMed] [Google Scholar]
- 9.Mukai, K., Yasutomi, Y., Watanabe, M., Kenjo, A., Aota, T., Wang, L., Nishikawa, H., Ishihara, M., Fujita, T., Kuribayashi, K. & Shiku, H. (2002) Gene Ther. 9, 879-888. [DOI] [PubMed] [Google Scholar]
- 10.Smyth, M. J., Thia, K. Y., Street, S. E., Cretney, E., Trapani, J. A., Taniguchi, M., Kawano, T., Pelikan, S. B., Crowe, N. Y. & Godfrey, D. I. (2000) J. Exp. Med. 191, 661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smyth, M. J., Crowe, N. Y. & Godfrey, D. I. (2001) Int. Immunol. 13, 459-463. [DOI] [PubMed] [Google Scholar]
- 12.Crowe, N. Y., Smyth, M. J. & Godfrey, D. I. (2002) J. Exp. Med. 196, 119-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnet, F. M. (1970) Progr. Exp. Tumor Res. 13, 1-27. [DOI] [PubMed] [Google Scholar]
- 14.Prehn, R. T. (1971) J. Reticuloendothel. Soc. 10, 1-16. [PubMed] [Google Scholar]
- 15.Stutman, O. (1975) Adv. Cancer Res. 22, 261-422. [DOI] [PubMed] [Google Scholar]
- 16.Thomas, L. (1982) Yale J. Biol. Med. 55, 329-333. [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth, M. J., Godfrey, D. I. & Trapani, J. A. (2001) Nat. Immunol. 2, 293-299. [DOI] [PubMed] [Google Scholar]
- 18.Dunn, G. P., Old, L. J. & Schreiber, R. D. (2004) Annu. Rev. Immunol. 22, 329-360. [DOI] [PubMed] [Google Scholar]
- 19.Girardi, M., Oppenheim, D. E., Steele, C. R., Lewis, J. M., Glusac, E., Filler, R., Hobby, P., Sutton, B., Tigelaar, R. E. & Hayday, A. C. (2001) Science 294, 605-609. [DOI] [PubMed] [Google Scholar]
- 20.Gao, Y., Yang, W., Pan, M., Scully, E., Girardi, M., Augenlicht, L. H., Craft, J. & Yin, Z. (2003) J. Exp. Med. 198, 433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardi, M., Glusac, E., Filler, R. B., Roberts, S. J., Propperova, I., Lewis, J., Tigelaar, R. E. & Hayday, A. C. (2003) J. Exp. Med. 198, 747-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankaran, V., Ikeda, H., Bruce, A. T., White, J. M., Swanson, P. E., Old, L. J. & Schreiber, R. D. (2001) Nature 410, 1107-1111. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan, D. H., Shankaran, V., Dighe, A. S., Stockert, E., Aguet, M., Old, L. J. & Schreiber, R. D. (1998) Proc. Natl. Acad. Sci. USA 95, 7556-7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Street, S. E., Cretney, E. & Smyth, M. J. (2001) Blood 97, 192-197. [DOI] [PubMed] [Google Scholar]
- 25.Street, S. E., Trapani, J. A., MacGregor, D. & Smyth, M. J. (2002) J. Exp. Med. 196, 129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cretney, E., Takeda, K., Yagita, H., Glaccum, M., Peschon, J. J. & Smyth, M. J. (2002) J. Immunol. 168, 1356-1361. [DOI] [PubMed] [Google Scholar]
- 27.Takeda, K., Smyth, M. J., Cretney, E., Hayakawa, Y., Kayagaki, N., Yagita, H. & Okumura, K. (2002) J. Exp. Med. 195, 161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Broek, M. E., Kagi, D., Ossendorp, F., Toes, R., Vamvakas, S., Lutz, W. K., Melief, C. J., Zinkernagel, R. M. & Hengartner, H. (1996) J. Exp. Med. 184, 1781-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth, M. J., Thia, K. Y., Street, S. E., MacGregor, D., Godfrey, D. I. & Trapani, J. A. (2000) J. Exp. Med. 192, 755-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin, Z., Kim, H. J., Hemme, J. & Blankenstein, T. (2002) J. Exp. Med. 195, 1479-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blankenstein, T. & Qin, Z. (2003) Curr. Opin. Immunol. 15, 148-154. [DOI] [PubMed] [Google Scholar]
- 32.Laaksonen, M., Maki-Paakkanen, J. & Komulainen, H. (2001) Arch. Toxicol. 75, 613-617. [DOI] [PubMed] [Google Scholar]
- 33.Sakai, A. & Teshima, R. (2001) Cancer Lett. 168, 183-190. [DOI] [PubMed] [Google Scholar]
- 34.Wolfle, D. (2003) Toxicology 188, 139-147. [DOI] [PubMed] [Google Scholar]