Abstract
Background
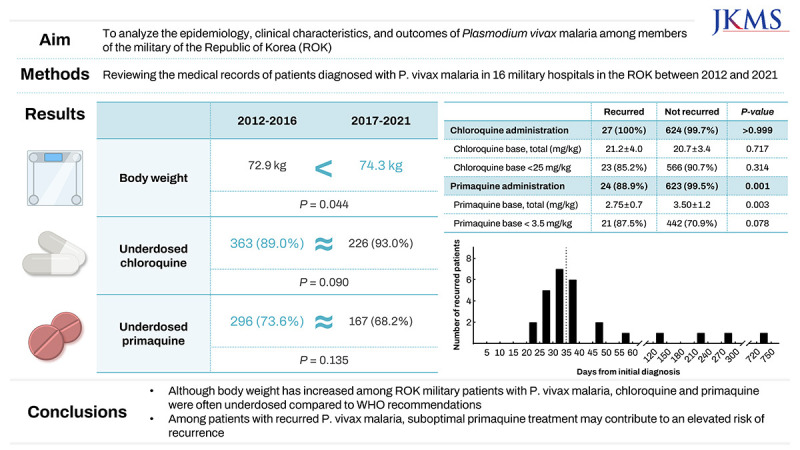
We aimed to analyze the epidemiology, clinical characteristics, and outcomes of malaria caused by Plasmodium vivax among military members of the Republic of Korea (ROK).
Methods
We reviewed the medical records of patients diagnosed with P. vivax malaria in 16 military hospitals in the ROK between 2012–2021, excluding other types of malaria, as well as imported cases and those treated in civilian hospitals.
Results
In total, 653 patients were treated for P. vivax malaria. Their mean age was 22.0 ± 3.8 years, and their mean body weight was 73.4 ± 10.8 kg. Hospitalization occurred in 92.0% (n = 601) of the cases, with 4.4% (n = 29) recurring. The mean administered dose was 20.7 ± 3.4 mg/kg for the chloroquine (CQ) base and 3.5 ± 1.2 mg/kg for the primaquine (PQ) base. Between 2012–2016 and 2017–2021, the mean patient body weight increased (72.9 ± 11.1 vs. 74.3 ± 10.3 kg, P = 0.044). Correspondingly, the total administered doses of CQ (1,476.0 ± 144.0 vs. 1,515.1 ± 155.1 mg, P = 0.010) and PQ (242.6 ± 79.7 vs. 265.7 ± 92.3 mg, P < 0.001) were increased. However, there was no difference in the weight-based dosage of CQ (20.7 ± 3.6 vs. 20.7 ± 3.2 mg/kg, P = 0.580) or PQ (3.33 ± 1.1 vs. 3.64 ± 1.3 mg/kg, P = 0.256), nor in the percentage of patients who received sub-recommended doses. Among the 27 patients who experienced recurrence and had available initial treatment data, the proportion of those prescribed PQ (24 [88.9%] vs. 623 [99.5%], P = 0.001) and the mean PQ dose (2.75 ± 0.7 vs. 3.50 ± 1.2 mg/kg, P = 0.003) were significantly lower in the recurrence group.
Conclusion
Over time, as the body weight of patients with P. vivax malaria in the ROK military has increased, the administered dosages of CQ and PQ have correspondingly risen. However, these dosages often remain suboptimal when compared to the body weight-based recommendations by the World Health Organization. Of particular concern is the continued administration of antimalarial drugs at suboptimal doses, which may contribute to an elevated risk of recurrence. Further education may therefore be beneficial to ensuring appropriate dosing for more effective malaria treatment.
Keywords: Malaria, Plasmodium vivax, Military Personnel, Epidemiology, Body Weight
Graphical Abstract
INTRODUCTION
Malaria is a global disease that affects > 240 million people annually, resulting in up to 600,000 deaths every year.1 The World Health Organization (WHO) regularly updates its guidelines for effective malaria management, emphasizing the appropriate use of medications, including optimal dosing based on patient weight.1 Ensuring optimal dosing is crucial because inappropriate dosing is associated with treatment failure and relapse.2,3,4 This issue is particularly relevant for the forms of malaria caused by Plasmodium vivax and Plasmodium ovale, which have high relapse rates—owing to the dormant hypnozoite liver stages of these parasites’ life cycles. As a result, determining the correct dosage of anti-malaria medication based on each patient’s weight is critical for effective treatment.
In the Republic of Korea (ROK), P. vivax malaria is an endemic disease. Although no indigenous cases of P. vivax malaria were reported for approximately 10 years from the early 1980s, it re-emerged in 1993 within military units near the Demilitarized Zone (DMZ).5,6 Currently, the areas in the ROK with malaria epidemics are primarily located near the DMZ bordering the Democratic People’s Republic of Korea (DPRK), placing ROK soldiers serving in these areas at higher risk of infection. As a result, approximately one-third of endemic malaria cases in the ROK occur among active or discharged military personnel.5,7
Since the re-emergence of malaria, the number of newly reported malaria cases has rapidly increased, reaching up to 4,000 per year in the late 1990s. In 1997, the ROK’s Ministry of National Defense (MND) introduced chemoprophylaxis during the mosquito season, consisting of a combination of hydroxychloroquine and primaquine (PQ) for military personnel deployed to high-risk areas.8 Conventionally, a 15-week course of weekly hydroxychloroquine (400 mg per week) followed by a 14-day course of daily PQ (15 mg per day) between July and October had been used as the mass chemoprophylaxis regimen. Recently, the MND revised the mass chemoprophylaxis guidelines to improve compliance. Starting in 2024, a 15-week course of weekly hydroxychloroquine (400 mg per week) beginning in June, accompanied by at least one compliance and safety monitoring session, has been implemented, along with a 7-day (30 mg per day) or 14-day (15 mg per day) course of daily PQ in March. In addition, the ROK army has implemented an annual education program on non-pharmacological prevention for high-risk military units.8 The program includes cleaning the environment (e.g., removal of empty cans to control mosquito larvae), using insect screens and mosquito nets, and applying repellents. Regular application of pesticides targeting adult mosquitoes with space sprays (≥ 2 times/week) and residual sprays (≥ 1 time/month) is an important component of vector control. With the introduction of chemoprophylaxis in combination with vector control and education programs, the incidence of malaria gradually decreased in the 2000s. However, it remains endemic, with > 100 newly diagnosed malaria cases annually.7 Furthermore, patients with recurred or relapsed malaria cases continue to be reported, and the possibility of treatment failure has increased—thus warranting further analysis to ensure that proper treatments are being administered.9,10 To address this, we studied the epidemiology and clinical characteristics of P. vivax malaria among military personnel serving in the ROK.
METHODS
Study design and population
This retrospective study was conducted using the Defense Medical Information System (DEMIS) database of the Korean Armed Forces Medical Command. The study population consisted of military personnel and military-civilian employees who received outpatient or inpatient treatment at 16 military medical institutions in the ROK and were coded with International Classification of Diseases, Tenth Revision (ICD-10) codes for malaria (B50.0–B54.1-01) between 2012–2021. The inclusion criteria were: patients diagnosed with P. vivax malaria confirmed by a peripheral blood smear (PBS) or malaria polymerase chain reaction (PCR) test, those who received outpatient or inpatient treatment at military medical institutions, and those for whom complete medical records were accessible. The exclusion criteria were: imported cases of P. vivax, Plasmodium falciparum, P. ovale, and Plasmodium malariae malaria contracted during deployment or travel outside of the ROK, and patients who were treated in civilian hospitals (for whom medical records were inaccessible).
Variables
The demographic variables used in this study included age, sex, body weight, branch of service, and service location. The clinical variables included the date of symptom onset and diagnosis, tests used to make the definitive diagnosis, occurrence of relapse, and whether chemoprophylaxis was administered. The time from symptom onset to diagnosis was also calculated. The total dose of hydroxychloroquine administered during the treatment period was converted into an equivalent chloroquine (CQ) base dose per body weight. The daily dose and duration of PQ administration were recorded and converted to the total dose per body weight. Follow-up PBS results were recorded, noting whether the PBS was positive 72 hours after the administration of the initial CQ base dose.
Statistical analysis
A descriptive analysis was conducted to identify the general characteristics of the study population. The 10-year study period was divided into two halves to analyze the changes in variables over time. χ2, Fisher’s exact, and Mann-Whitney U tests were performed to compare the patient characteristics between two periods. Among patients who revisited the military medical institutions for malaria recurrence, treatment data from the initial diagnosis were compared to those of patients who did not revisit for malaria (considered as non-recurrent cases). χ2, Fisher’s exact, and Mann-Whitney U tests were performed to compare the treatment variables. All statistical analyses were performed using IBM SPSS Statistics version 29.0.2.0 (IBM Corp., Armonk, NY, USA), with statistical significance set at P < 0.05.
Artificial intelligence statement
Generative artificial intelligence was used only to correct the grammar and spelling in the additional materials section.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of the Armed Forces Medical Command of the ROK (IRB No. AFMC-202206-HR-032-01). The requirement for informed consent was waived, owing to the retrospective nature of the study.
RESULTS
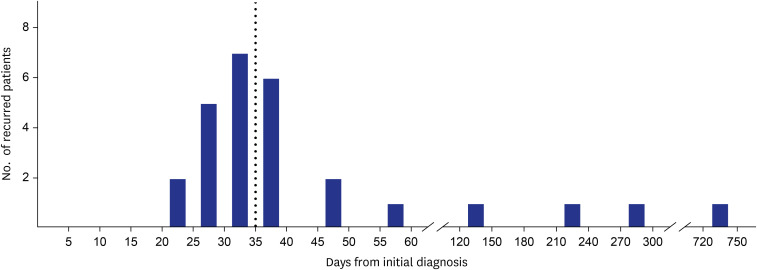
In total, 653 patients were treated for P. vivax malaria (Table 1). The vast majority of patients were male, with only one reported female patient. The mean age of the patients was 22.0 ± 3.8 years, and the mean body weight was 73.4 ± 10.8 kg. The distribution of patients by military branch was: army, 609 (93.3%); marine corps, 32 (4.9%); navy, 6 (0.9%); air force, 3 (0.5%); and other, 3 (0.5%). PBSs were the major diagnostic method used to make definitive diagnoses (n = 641, 98.2%), followed by malaria PCR (n = 4, 0.6%). The median time from the onset of initial symptoms to diagnosis was 5 days (interquartile range [IQR], 3–7 days). Most of the patients (n = 601, 92.0%) were hospitalized. Among the patients diagnosed with malaria, 121 (18.5%) reported receiving antimalarial chemoprophylaxis prior to their diagnosis. However, detailed information regarding chemoprophylaxis compliance was unavailable. Hydroxychloroquine was the initial drug of choice for most of the patients (n = 651, 99.7%), and subsequent anti-relapse treatment with PQ was administered to 647 (99.1%). Prior to PQ administration, only 155 of the patients (23.7%) were tested for glucose-6-phosphate dehydrogenase (G6PD) deficiency. However, no patients developed hemolytic anemia attributed to G6PD deficiency. The mean dose of CQ base administered during the treatment course was 1,490.6 ± 149.3 mg, and the weight-based dose was 20.7 ± 3.4 mg/kg. The mean total administered PQ base was 251.4 ± 85.4 mg, with a weight-based dose of 3.5 ± 1.2 mg/kg over 14.0 ± 1.0 days. Among 653 reported patients with P. vivax malaria, 29 (4.4%) experienced recurrence. The median time from initial diagnosis to recurrence was 35 days (IQR, 30–43) among 27 patients, excluding 2 patients for whom the date of initial diagnosis was unavailable. Among the 27 patients who experienced recurrence, 23 recurred within 1–2 months of the initial diagnosis and 4 recurred after 4 months (Fig. 1). Within 35 days of the initial diagnosis, 14 patients (51.9%) experienced recurrence, while 13 patients (48.1%) experienced recurrence after more than 35 days. The majority of repeated hospitalizations with P. vivax malaria (n = 26, 89.7%) were diagnosed during the high-risk season (May to October), as were the patients initially diagnosed with P. vivax malaria (n = 567, 90.9%) (P = 0.743).
Table 1. Demographic characteristics of patients diagnosed with Plasmodium vivax malaria in Republic of Korea military hospitals between 2012–2021.
| Characteristics | Values (N = 653) | ||
|---|---|---|---|
| Age, yr | 22.0 ± 3.8 | ||
| Sex | |||
| Male | 652 (99.8) | ||
| Female | 1 (0.2) | ||
| Body weight, kg | 73.4 ± 10.8 | ||
| Branch of service | |||
| Army | 609 (93.3) | ||
| Navy | 6 (0.9) | ||
| Air force | 3 (0.5) | ||
| Marine | 32 (4.9) | ||
| Othersa | 3 (0.5) | ||
| Diagnostic method | |||
| Peripheral blood smear | 641 (98.2) | ||
| Malaria PCR | 4 (0.6) | ||
| Unknown | 8 (1.2) | ||
| Time to diagnosis, days | 5 (3–7) | ||
| Medical care | |||
| Inpatient | 601 (92.0) | ||
| Outpatient | 52 (8.0) | ||
| Antimalaria chemoprophylaxis | |||
| Yes | 121 (18.5) | ||
| No | 78 (11.9) | ||
| Unknown | 454 (69.5) | ||
| G6PD test before PQ | 155 (23.7) | ||
| Antimalarial treatment | |||
| CQ administration | 651 (99.7) | ||
| CQ base, total, mg | 1,490.6 ± 149.3 | ||
| CQ base, total, mg/kg | 20.7 ± 3.4 | ||
| PQ administration | 647 (99.1) | ||
| PQ base, total, mg | 251.4 ± 85.4 | ||
| PQ base, total, mg/kg | 3.5 ± 1.2 | ||
| PQ duration, days | 14.0 ± 1.0 | ||
| Recurrence | 29 (4.4) | ||
Values are presented as numbers (%), mean ± standard deviation, or median (interquartile range).
PCR = polymerase chain reaction, G6PD = glucose-6-phosphate dehydrogenase, PQ = primaquine, CQ = chloroquine.
aDesignated unit under the Ministry of National Defense.
Fig. 1. Time interval distribution between the initial and recurrent diagnosis of Plasmodium vivax malaria. Of the 29 patients with recurrence, the time interval for 27 patients was available. Among these, 23 (85.2%) experienced a recurrence within 2 months. Fourteen (51.9%) experienced a recurrence within 35 days (dotted line). One patient experienced recurrence after nearly 2 years (possibly due to re-infection).
When comparing the periods 2012–2016 and 2017–2021 (Table 2), there was an observed increase in the mean body weight of the patients (72.9 ± 11.1 vs. 74.3 ± 10.3 kg, P = 0.044). The median time to diagnosis decreased from 5 days (IQR, 3–7) to 4 (IQR, 3–7), but this change was not statistically significant. Total CQ base administered dosage was increased over time (1,476.0 ± 144.0 vs. 1,515.1 ± 155.1 mg, P = 0.010) despite no significant differences in the total weight-based CQ base dosage (20.7 ± 3.6 vs. 20.7 ± 3.2 mg/kg, P = 0.580) or in the proportion of patients in the underdosing group (< 25 mg/kg; 363 [89.0%] vs. 226 [93.0%], P = 0.090) between the two periods. Similarly, there was an increasing trend in the total PQ base dosage administered (242.6 ± 79.7 vs. 265.7 ± 92.3 mg, P < 0.001), despite no significant differences in total weight-based PQ base dosage administered (3.38 ± 1.1 vs. 3.64 ± 1.3 mg/kg, P = 0.256) or the proportion of patients in the underdosing group (< 3.5 mg/kg; 296 [73.6%] vs. 167 [68.2%], P = 0.135] between the two periods. The persistence of parasitemia 72 hours after the initial CQ dose also did not differ significantly between the two periods (73/116 [62.9%] vs. 49/80 [61.3%], P = 0.811). The number of patients with relapsed malaria was not significantly different between the two periods (22 [5.4%] vs. 7 [2.9%], P = 0.128).
Table 2. Demographic and clinical data of patients diagnosed with Plasmodium vivax malaria in Republic of Korea military hospitals, by study period.
| Variables | Years | P value | |||
|---|---|---|---|---|---|
| 2012–2016 (n = 408) | 2017–2021 (n = 245) | ||||
| Age, yr | 21.8 ± 3.4 | 22.2 ± 4.4 | 0.712 | ||
| Sex | |||||
| Male | 407 (99.8) | 245 (100.0) | > 0.999 | ||
| Female | 1 (0.2) | - | - | ||
| Body weight, kg | 72.9 ± 11.1 | 74.3 ± 10.3 | 0.044 | ||
| Time to diagnosis, days | 5 (3–7) | 4 (3–7) | 0.231 | ||
| PBS follow-up | 121 (29.7) | 89 (36.3) | 0.077 | ||
| Antimalarial treatment | |||||
| CQ administration | 408 (100.0) | 243 (99.2) | 0.140 | ||
| CQ base, total, mg | 1,476.0 ± 144.0 | 1,515.1 ± 155.1 | 0.010 | ||
| CQ base, total, mg/kg | 20.7 ± 3.6 | 20.7 ± 3.2 | 0.580 | ||
| CQ base < 25 mg/kg | 363 (89.0) | 226 (93.0) | 0.090 | ||
| PQ administration | 402 (98.5) | 245 (100) | 0.089 | ||
| PQ base, total, mg | 242.6 ± 79.7 | 265.7 ± 92.3 | < 0.001 | ||
| PQ base, total, mg/kg | 3.38 ± 1.1 | 3.64 ± 1.3 | 0.256 | ||
| PQ base < 3.5 mg/kg | 296 (73.6) | 167 (68.2) | 0.135 | ||
| Parasitemia at 72 hr after 1st CQ dose | 73/116 (62.9) | 49/80 (61.3) | 0.811 | ||
| Recurrence | 22 (5.4) | 7 (2.9) | 0.128 | ||
Values are presented as numbers (%), mean ± standard deviation, or median (interquartile range).
PBS = peripheral blood smear, CQ = chloroquine, PQ = primaquine.
Among the 29 patients who experienced recurrence, initial treatment data were available for 27 patients (Table 3). Compared to those who did not revisit for recurrence (including patients treated for either the initial or recurrent diagnosis), the proportion of patients prescribed PQ was significantly lower among those who experienced recurrence (24 [88.9%] vs. 623 [99.5%], P = 0.001). Among patients prescribed PQ, the total weight-based PQ dose was significantly lower in the recurrent group compared to the non-recurrent group (2.75 ± 0.7 vs. 3.50 ± 1.2 mg/kg, P = 0.003). The proportion of PQ-underdosed patients was higher in the recurrent group, although the difference was not statistically significant (21 [87.5%] vs. 442 [70.9%], P = 0.078). There was no statistically significant difference between the two groups regarding CQ administration, dosage, and the proportion of CQ-underdosed cases.
Table 3. Association between recurrence of Plasmodium vivax malaria and antimalarial treatment in Republic of Korea military hospitals, 2012–2021.
| Variables | Recurred after treatment (n = 27a) | Not recurred after treatment (n = 626) | P value | |
|---|---|---|---|---|
| CQ administration | 27 (100.0) | 624 (99.7) | > 0.999 | |
| CQ base, total, mg | 1,500.0 ± 83.2 | 1,490.2 ± 151.6 | 0.750 | |
| CQ base, total, mg/kg | 21.2 ± 4.0 | 20.7 ± 3.4 | 0.717 | |
| CQ base < 25 mg/kg | 23 (85.2) | 566 (90.7) | 0.314 | |
| PQ administration | 24 (88.9) | 623 (99.5) | 0.001 | |
| PQ base, total, mg | 199.4 ± 36.6 | 253.4 ± 86.1 | 0.001 | |
| PQ base, total, mg/kg | 2.75 ± 0.7 | 3.50 ± 1.2 | 0.003 | |
| PQ base < 3.5 mg/kg | 21 (87.5) | 442 (70.9) | 0.078 | |
Values are presented as numbers (%), mean ± standard deviation.
CQ = chloroquine, PQ = primaquine.
aAmong the 29 patients with recurrence, treatment data during the initial diagnosis was unavailable for 2 patients (treated in civilian hospitals).
DISCUSSION
We analyzed the epidemiological and clinical characteristics of ROK military personnel diagnosed with P. vivax malaria who were treated at 16 military hospitals. Despite the increase in patients’ average body weight over time, the issue of underdosing antimalarial medication compared to WHO guidelines persists, with no significant reduction, especially in the case of PQ.1 Indeed, a previous study in the ROK, which included civilian patients, suggested an association between underdosed CQ and treatment failure. From 2000 to 2016, patients prescribed < 25 mg/kg of CQ had significantly higher recurrence rates (4.7% vs. 1.4%), and the mean dose per body weight of PQ was also significantly lower in the CQ-underdosed group.10 In our study, the average weight of patients was over 70 kg, which is likely to result in inappropriate dosing of both CQ and PQ under fixed dosing regimens. Given that the prescribed weight-based PQ dosage was significantly lower in our recurred patient population, this underscores the importance of ensuring appropriate PQ dosing in malaria patients within the ROK military. Furthermore, a recent trial conducted in Brazil, as well as a recent meta-analysis, both demonstrated that a higher dose of PQ (7.0 mg/kg rather than 3.5 mg/kg) was more effective for preventing the relapse of P. vivax malaria.2,11 Although our data indicated a lower proportion of recurrence malaria cases (4.4%) among ROK military personnel compared to the 180-day recurrence reported in that meta-analysis (9.9% in the low-dose PQ group vs. 6.6% in the high-dose one), underdosing is known to be associated with treatment failure and relapse.2,3,4 Although the guidelines recommend weight-based dosing of antimalarials, practical application may be suboptimal due to the fixed-dose formulations of antimalarial tablets, which can be challenging to adjust precisely according to a patient’s weight. Consequently, this may result in dosing that falls slightly below the optimal therapeutic level. Such suboptimal dosing, particularly with PQ, could increase the risk of recurrence. To address this issue, enhancing education for military healthcare personnel and implementing monitoring of treatment practices may improve adherence to guidelines and subsequently optimize treatment outcomes in the ROK military.
In this study, only 23.7% of patients were tested for G6PD deficiency. The WHO guidelines recommend testing for G6PD deficiency before administering PQ due to the risk of hemolytic toxicity.1 This low testing rate is partly due to the low prevalence of G6PD deficiency in the ROK and the long turnaround time for test results in military hospitals. Recent studies with > 1,000 samples from malaria-endemic areas and a military training camp did not detect any cases of G6PD deficiency.12,13 In 2023, a study involving > 5,000 samples revealed that only 0.4% of Koreans had decreased G6PD activity, with G6PD deficiency defined by WHO guidelines confirmed in only 0.1% of cases.14 However, given the increasing number of children from multicultural families in the ROK, and the higher prevalence of G6PD deficiency among them—reported as 7.8% of male adolescents from multicultural families—compared to those of Korean ethnicity, it would be beneficial to consider more proactive G6PD deficiency testing in patients with endemic malaria who diagnosed in military medical facilities.14,15
In 2020, a study analyzing patients with malaria in the ROK from 2000 to 2016 showed an increasing trend in parasitemia clearance time (PCT).10 In our study, the proportion of patients with persistent parasitemia after 72 hours from initial treatment was 62.9% in 2012–2016 and 61.3% in 2017–2021, showing no significant difference between the periods. However, these figures are higher compared to the previous study which reported 30.5% (2000–2005), 36.3% (2006–2010), and 52.4% (2011–2016) of PCT > 72 hours.10 It might be because laboratories in ROK military hospitals counted gametocytes on the PBS slides, which should be reported but are not recommended to be routinely counted according to WHO’s malaria microscopy standard operating procedure.16 However, the increasing PCT may be an early indicator of the emergence of drug-resistant malaria and thus should be closely monitored to track potential drug resistance in the ROK, especially in the military under mass chemoprophylaxis.
Recurrence of P. vivax malaria within 1–2 months after initial treatment is referred to as recrudescence, caused by the failure to eliminate the intraerythrocytic stage of malaria due to insufficient treatment. Relapse refers to the reactivation of hypnozoites in the liver, resulting in recurrent infection.16 Of the 27 patients who experienced recurrence, 23 did so within 1–2 months and 4 after 4 months, indicating a bimodal distribution as the previous study.17 Re-infection can occur at any time in high-risk areas and during high-risk periods, making it difficult to clearly distinguish the cause of recurrence as either recrudescence, relapse, or re-infection except for 1 case (post-722 days). However, the above minimal inhibitory concentration level of CQ is maintained for approximately 30–35 days post-treatment,18 and in this study, 48.2% (n = 13) of cases experienced recurrence within this period. This suggests a high likelihood of recrudescence, potentially due to inappropriate dosing. Although there are no data on CQ blood concentration in this study, some cases exhibited a recurrence time as short as 22–29 days, raising suspicions of drug-resistant malaria. In addition, a study suggested that a host factor could be a risk factor for the recurrence of P. vivax malaria in South Korea.19 Among 139 samples from patients with P. vivax, including 48 samples designated as recurrent cases, reduced CYP2D6 activity phenotype and lower CYP2D6 activity were associated with recurrence. While our study did not perform molecular tests on recurred cases, making it difficult to determine the exact cause of recurrence, this limitation highlights the need for further research.
To the best of our knowledge, this study is the first to report on the treatment of military personnel patients with malaria in the ROK. Our findings may benefit the military forces of other countries as well—such as the U.S. Forces Korea. However, this study also had several key limitations worth noting. First, as this was a retrospective observational study, it was subject to inherent selection and information biases. Second, the study population underwent large-scale chemoprophylaxis. The ROK military administers chemoprophylaxis to military personnel stationed in high-risk areas between July and October annually. However, detailed information regarding the specific chemoprophylaxis regimens and compliance rates in our patient cohort was not available. Third, the study population mainly consisted of men serving under the conscription system. They are usually healthy, young males but are often continuously exposed to risks of malaria due to occupational reasons. It limits the generalizability of the findings.
Our study found that inappropriate dosing regimens of antimalarial drugs are currently being administered to ROK military personnel with P. vivax malaria. Such underdosed drugs may promote recurrence (or relapse) and the emergence of resistance due to insufficient treatment. It is therefore essential to educate healthcare providers in the country on the subject of proper dosing protocols and to monitor the prescriptions they write in order to ensure compliance. Further studies are warranted to evaluate changes in dosing and treatment outcomes before and after such interventions.
Footnotes
Funding: This research was supported by Hallym University Research Fund 2023 (HURF-2023-01).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Oh HS.
- Data curation: Hwang YH, Oh HS.
- Formal analysis: Hwang YH, Oh HS.
- Funding acquisition: Oh HS.
- Methodology: Oh HS.
- Project administration: Oh HS.
- Visualization: Hwang YH, Oh HS.
- Writing - original draft: Hwang YH, Oh HS.
- Writing - review & editing: Hwang YH, Yoon D, Go S, Yeom JS, Oh HS.
References
- 1.World Health Organization (WHO) WHO Guidelines for Malaria. Geneva, Switzerland: WHO; 2023. [Google Scholar]
- 2.Commons RJ, Rajasekhar M, Edler P, Abreha T, Awab GR, Baird JK, et al. Effect of primaquine dose on the risk of recurrence in patients with uncomplicated Plasmodium vivax: a systematic review and individual patient data meta-analysis. Lancet Infect Dis. 2024;24(2):172–183. doi: 10.1016/S1473-3099(23)00430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commons RJ, Simpson JA, Thriemer K, Humphreys GS, Abreha T, Alemu SG, et al. The effect of chloroquine dose and primaquine on Plasmodium vivax recurrence: a WorldWide Antimalarial Resistance Network systematic review and individual patient pooled meta-analysis. Lancet Infect Dis. 2018;18(9):1025–1034. doi: 10.1016/S1473-3099(18)30348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg. 2006;75(3):402–415. [PubMed] [Google Scholar]
- 5.Chai JY. History and current status of malaria in Korea. Infect Chemother. 2020;52(3):441–452. doi: 10.3947/ic.2020.52.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feighner BH, Pak SI, Novakoski WL, Kelsey LL, Strickman D. Reemergence of Plasmodium vivax malaria in the republic of Korea. Emerg Infect Dis. 1998;4(2):295–297. doi: 10.3201/eid0402.980219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korea Disease Control and Prevention Agency (KDCA) Malaria Management Guideline. Cheongju, Korea: KDCA; 2024. [Google Scholar]
- 8.Im JH, Huh K, Yoon CG, Woo H, Lee JS, Chung MH, et al. Malaria control and chemoprophylaxis policy in the Republic of Korea Armed Forces for the previous 20 years (1997-2016) Malar J. 2018;17(1):295. doi: 10.1186/s12936-018-2449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwak YG, Lee HK, Kim M, Um TH, Cho CR. Clinical characteristics of vivax malaria and analysis of recurred patients. Infect Chemother. 2013;45(1):69–75. doi: 10.3947/ic.2013.45.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SY, Park YS, Park Y, Kwak YG, Song JE, Lee KS, et al. Increasing malaria parasite clearance time after chloroquine therapy, South Korea, 2000-2016. Emerg Infect Dis. 2020;26(8):1852–1855. doi: 10.3201/eid2608.190687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamma-Siqueira NN, Negreiros SC, Ballard SB, Farias S, Silva SP, Chenet SM, et al. Higher-dose primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2022;386(13):1244–1253. doi: 10.1056/NEJMoa2104226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goo YK, Ji SY, Shin HI, Moon JH, Cho SH, Lee WJ, et al. First evaluation of glucose-6-phosphate dehydrogenase (G6PD) deficiency in vivax malaria endemic regions in the Republic of Korea. PLoS One. 2014;9(5):e97390. doi: 10.1371/journal.pone.0097390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W, Lee SE, Lee MJ, Noh KT. Investigation of glucose-6-phosphate dehydrogenase (G6PD) deficiency prevalence in a Plasmodium vivax-endemic area in the Republic of Korea (ROK) Malar J. 2020;19(1):317. doi: 10.1186/s12936-020-03393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi R, Park W, Chun G, Lee SG, Lee EH. Utilization of glucose-6-phosphate dehydrogenase test and the prevalence of enzyme deficiency in Korea. J Clin Med. 2023;12(9):3179. doi: 10.3390/jcm12093179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahk YY, Ahn SK, Lee J, Im JH, Yeom JS, Park S, et al. A profile of glucose-6-phosphate dehydrogenase variants and deficiency of multicultural families in Korea. Korean J Parasitol. 2021;59(5):447–455. doi: 10.3347/kjp.2021.59.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus MB. Biological concepts in recurrent Plasmodium vivax malaria. Parasitology. 2018;145(13):1765–1771. doi: 10.1017/S003118201800032X. [DOI] [PubMed] [Google Scholar]
- 17.Ko DH, Kim SS, Choi BS, Seog W, Kim CH, Cho YK, et al. Studies on the vivax malaria readmitted in military hospital. Korean J Med. 2005;68(6):611–618. [Google Scholar]
- 18.Watson J, Chu CS, Tarning J, White NJ. Characterizing blood-stage antimalarial drug MIC values in vivo using reinfection patterns. Antimicrob Agents Chemother. 2018;62(7):e02476-17. doi: 10.1128/AAC.02476-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi S, Choi H, Park SY, Kwak YG, Song JE, Shin SY, et al. Association between CYP2D6 phenotype and recurrence of Plasmodium vivax infection in South Korean patients. Malar J. 2022;21(1):289. doi: 10.1186/s12936-022-04311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]