Abstract
Although methamphetamine (METH) and other addictive substance use disorders are a major social problem worldwide, appropriate pharmacotherapies have not yet been discovered. Subtype‐nonselective opioid receptor antagonists, such as naltrexone (NTX), have been reported to suppress METH addiction, but unclear are the opioid receptor subtypes that are involved in this beneficial effect. To clarify the role of μ‐opioid receptors (MOPs), we examined effects of the novel nonpeptidic MOP‐selective antagonist UD‐030 on the acquisition and expression of METH‐induced conditioned place preference (CPP) using behavioral tests in C57BL/6J mice. UD‐030 was found to inhibit both the acquisition and expression of METH‐induced CPP in a dose‐dependent manner, with effects comparable to those observed with NTX. These findings suggest that UD‐030 has the potential to mitigate METH‐related reward mechanisms and may serve as a promising candidate for MOP‐selective pharmacotherapy targeting METH addiction.
Keywords: addiction, CPP, methamphetamine, naltrexone, opioid antagonist
Although methamphetamine (METH) and other addictive substance use disorders are a major social problem worldwide, appropriate pharmacotherapies have not yet been discovered. The novel nonpeptidic μ‐opioid receptor (MOP)‐selective antagonist UD‐030 dose‐dependently inhibited the acquisition and expression of METH‐induced conditioned place preference (CPP) in mice. These results indicate that UD‐030 may effectively inhibit rewarding effects of METH and suggest that a novel MOP‐selective antagonist may be a candidate for the development of pharmacotherapies for METH addiction.
1. INTRODUCTION
Drug addiction is a complex disorder that is characterized by behaviors that lead to continued drug seeking despite harmful consequences. Methamphetamine (METH) is a highly addictive psychostimulant that increases dopamine release in the brain's reward pathways, leading to euphoria, greater energy, and higher alertness. 1 , 2 These effects contribute to reinforcing the properties of METH and increase the risk of addiction. Despite adverse health consequences of METH use, effective pharmacotherapies for METH addiction remain limited. 3 The development of novel treatments for METH addiction is an urgent unmet medical need.
It is well established that the endogenous opioid system plays a significant role in mediating the rewarding properties of METH. Previous studies have demonstrated that opioid receptor antagonists, including naltrexone (NTX), can attenuate the reinforcement associated with METH use in rodent models. 4 , 5 Naltrexone is a Food and Drug Administration‐approved opioid receptor antagonist that has been used clinically to treat alcohol and opioid addiction. 6 , 7 Opioids exert their pharmacological effects by acting on μ‐, κ‐, and δ‐opioid receptors. Naltrexone is a nonselective opioid receptor antagonist that has low selectivity for the μ‐opioid receptor (MOP). Little is known about the utility of MOP antagonism in the inhibitory effects of NTX on METH reward. Although some moderately potent ligands are available, few optimal nonpeptide receptor antagonists have been developed that selectively target the MOP. We recently reported that the novel nonpeptide ligand UD‐030 is a highly selective antagonist of the MOP relative to the other subtypes. 8 Peptides are generally not orally administered because digestive enzymes quickly degrade them in the gastrointestinal tract. For this reason, we hypothesized that UD‐030 could be a useful candidate therapeutic agent that could be administered orally. UD‐030 was rapidly absorbed after a single oral dose and remained in plasma for at least 8 h in pharmacokinetic analysis. 8 Oral UD‐030 administration dose‐dependently suppressed the acquisition and expression of rewarding effects of morphine. 8 These findings suggest that UD‐030 may have potential as a seed compound in the development of treatments for opioid use disorder. However, its effects on other addictive substances, particularly METH, are still unknown. Thus, the present study conducted conditioned place preference (CPP) tests to evaluate the inhibitory effect of UD‐030 on METH preference compared with NTX.
2. METHODS
Male C57BL/6J mice (Japan CLEA) were used for the behavioral analyses at 7 weeks of age. The mice were housed 4–6 per cage in an environment at 23°C ± 1°C and 50% ± 5% humidity with free access to food and water under a 12 h/12 h light/dark cycle.
CPP experiments were performed as outlined in prior studies. 8 On day 1 (habituation) and day 2 (pretest), mice were permitted to explore both compartments freely for 15 min. During these exploratory sessions, the time spent in each compartment and locomotor activity were recorded using an infrared sensor system (Neuroscience, Osaka, Japan). The conditioning phase took place over four consecutive days (days 3–6), with a single session per day. On day 3, mice received an intraperitoneal (i.p.) injection of either METH (2.0 mg/kg) or saline and were subsequently confined to one of the two compartments (black or white) for 60 min. On day 4, the treatment was reversed: mice were injected with either saline or METH and confined to the alternate compartment for another 60‐min session. The same conditioning protocol was repeated on days 5 and 6, with each mouse receiving alternating treatments. On day 7, during the postconditioning phase, mice were allowed free access to both compartments for 15 min, and the time spent in each compartment was recorded. The conditioned place preference (CPP) score was calculated as the difference in time spent in the drug‐paired compartment between day 7 and day 2. METH hydrochloride (Sumitomo Pharma, Osaka, Japan) was dissolved and diluted in saline and administered in a volume of 10 mL/kg body weight. UD‐030 (the chemical structure is as we have previously reported 8 ) and NTX hydrochloride (Tocris Bioscience, Bristol, UK) were dissolved and diluted in sterilized 0.5 w/v% Methyl Cellulose 400 Solution (FUJIFILM Wako Pure Chemical Co., Osaka, Japan) and administered orally (p.o.) in a volume of 10 mL/kg body weight. All experiments were conducted with a dose of METH (2 mg/kg) that is sufficient to elicit CPP.
Statistical analysis was performed using two‐way repeated‐measures ANOVA, followed by Sidak's multiple‐comparison test for post hoc analysis. Additionally, one‐way ANOVA with Dunnett's post hoc test was used where appropriate. Data were processed using GraphPad Prism, and statistical significance was set at p < 0.05.
3. RESULTS
To assess the inhibitory effect of UD‐030 on the acquisition of METH‐induced re‐warding effects, we conducted the CPP test. The mice were pretreated with UD‐030 (0, 3, and 10 mg/kg, oral [p.o.]) or NTX (10 mg/kg, p.o.) 60 min before each METH treatment during the conditioning phase. The two‐way repeated‐measures analysis of variance (ANOVA) revealed significant changes in the time spent in the drug‐paired compartment when comparing the preconditioning and postconditioning phases (pre vs. post: F 1,32 = 5.81, p = 0.022; interaction: F 3,32 = 5.85, p = 0.003; Figure 1A). In control mice pretreated with vehicle, METH administration resulted in a significant increase in time spent in the drug‐paired compartment (p < 0.001, Sidak's multiple‐comparison post hoc test). However, no notable differences were observed between the preconditioning and postconditioning phases for the groups pretreated with UD‐030 or NTX. Analysis of CPP scores using ANOVA indicated significant differences among the pretreated drugs before METH treatment (F 3,32 = 5.85, p = 0.003; Figure 1B). Post hoc testing revealed a significant reduction in METH‐induced CPP scores in mice pretreated with 10 mg/kg UD‐030 or 10 mg/kg NTX compared to the vehicle control group (p = 0.001 and p = 0.010, respectively; Dunnett's multiple‐comparison post hoc test).
FIGURE 1.
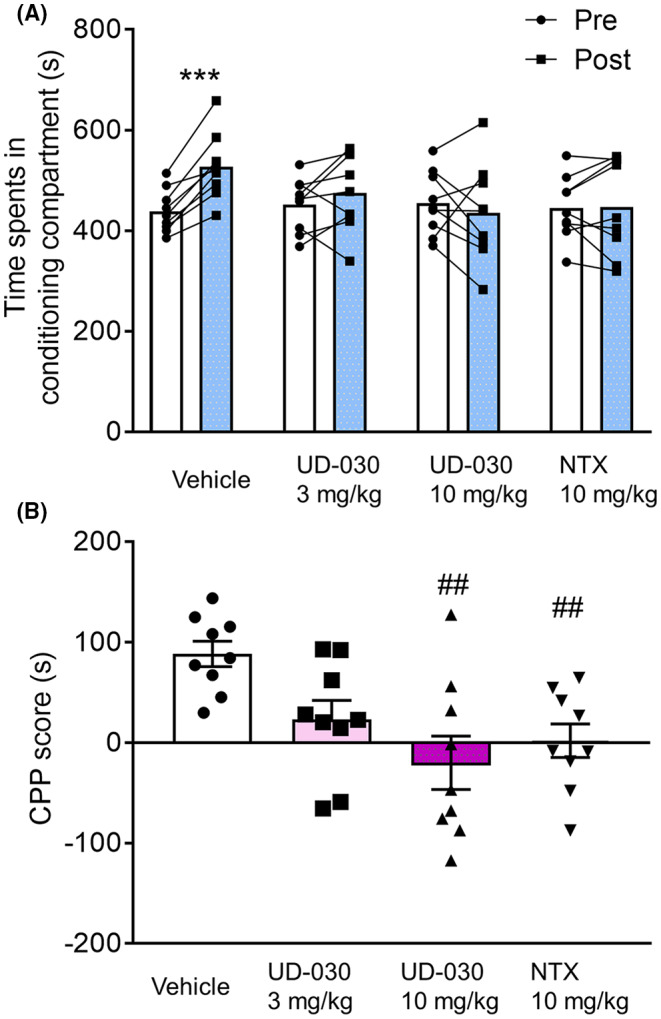
UD‐030 inhibits the acquisition of METH‐induced conditioned place preference (CPP). (A) Time spent in the METH‐paired compartment (2 mg/kg, i.p.) during the preconditioning (pre, white bars) and postconditioning (post, gray bars) phases. Mice (n = 9 per group) were pretreated with UD‐030 (0, 3, and 10 mg/kg, p.o.) or NTX (10 mg/kg, p.o.) 60 min before each METH treatment (2 mg/kg, i.p.) in the conditioning phase. Lines between data points indicate individual values for each mouse. Bars show the mean. ***p < 0.001, difference between pre‐ and postconditioning phases for each treatment. (B) CPP scores for each treatment in mice. The bars and error lines represent the mean ± SEM. ## p < 0.01, compared with vehicle‐pretreated (control) mice.
Subsequently, we evaluated the effect of UD‐030 on the expression of METH‐induced reward behaviors. Mice were administered with UD‐030 or NTX 60 min before the postconditioning phase. The two‐way repeated‐measures ANOVA indicated significant differences between the time spent in the pre‐ and postconditioning phases (pre vs. post: F 1,32 = 29.03, p < 0.0001; interaction: F 3,32 = 6.02, p = 0.002; Figure 2A). METH administration led to a significant increase in time spent in the METH‐paired compartment for both the vehicle control group and the 3 mg/kg UD‐030 group (p < 0.001 and p = 0.012, respectively; Sidak's multiple‐comparison post hoc test). In contrast, no significant changes were observed between the time spent in the pre‐ and postconditioning phases in the other groups. The one‐way ANOVA of CPP scores showed significant differences among the treated drugs before the preconditioning session (F 3,32 = 6.02, p = 0.002; Figure 2B). Post hoc Dunnett's multiple‐comparison tests indicated a significant reduction in METH‐induced CPP scores for both the 10 mg/kg UD‐030 group (p = 0.006) and the 10 mg/kg NTX group (p = 0.002) when compared to vehicle‐treated controls.
FIGURE 2.
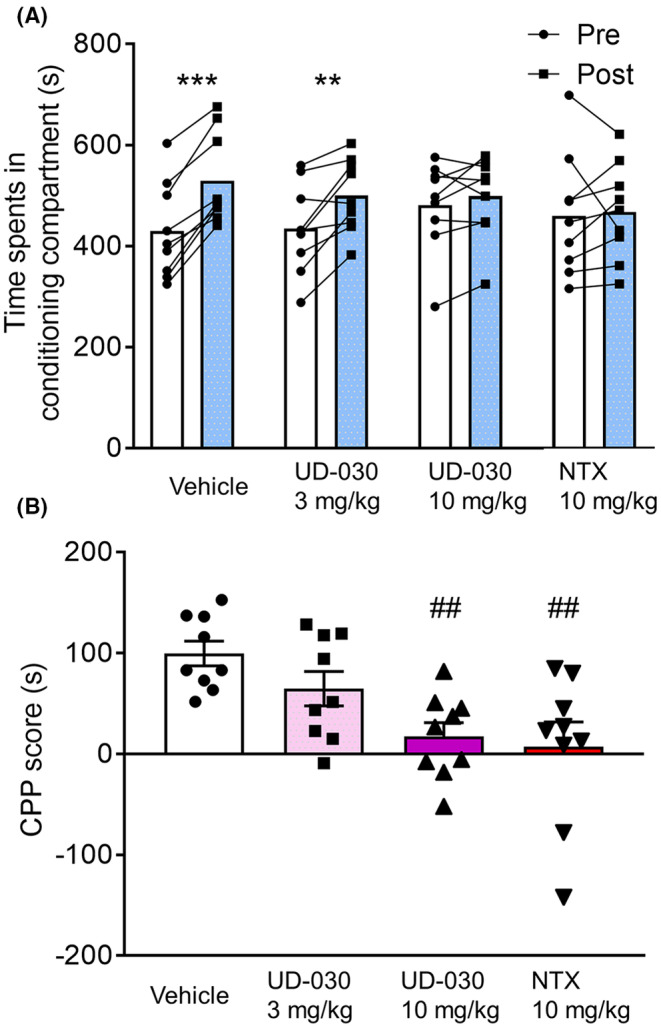
UD‐030 inhibits the expression of METH‐induced CPP. (A) Time spent in the METH‐paired compartment (2 mg/kg, i.p.) during the preconditioning (pre, white bars) and postconditioning (post, gray bars) phases. Mice (n = 9 per group) were pretreated with UD‐030 (0, 3, and 10 mg/kg, p.o.) or NTX (10 mg/kg, p.o.) 60 min before the postconditioning phase. Lines between data points indicate individual values for each mouse. Bars show the mean. ***p < 0.001, **p < 0.01, difference between pre‐ and postconditioning phases for each treatment. (B) CPP scores for each treatment. The bars and error lines represent the mean ± SEM. ## p < 0.01, compared with vehicle‐pretreated (control) mice.
4. DISCUSSION
This study demonstrates that UD‐030, a nonpeptidic and MOP‐selective antagonist, shows promising potential as a seed compound for the development of therapies targeting METH use disorder. Specifically, UD‐030 exhibited a dose‐dependent suppression of METH‐induced CPP when administered orally. The suppression of METH‐induced CPP by 10 mg/kg UD‐030 was comparable to that observed with 10 mg/kg NTX. Notably, both UD‐030 and NTX were effective in significantly attenuating both the acquisition and expression of METH‐induced CPP. Furthermore, MOP knockout mice were previously reported to be less sensitive to METH‐induced behavioral sensitization. 9 These findings suggest that among opioid receptor subtypes, MOP antagonism may be useful in suppressing METH addiction. It is important to note, however, that this study did not include a conditioning phase with UD‐030 alone, leaving open the possibility that any aversive effects induced solely by UD‐030 might have contributed to the inhibition of METH‐induced CPP acquisition. Despite this, UD‐030 effectively inhibited the expression of METH‐induced CPP following acquisition, indicating that its potential aversive effects might not solely oppose METH‐induced reward. Instead, UD‐030 may exert its effects by modulating endogenous opioid‐related neural circuits responsible for METH reward.
Activation of the mesolimbic dopaminergic pathway that projects from the ventral tegmental area to the nucleus accumbens (NAc) is well‐documented as a key neural mechanism underlying the rewarding properties of addictive substances. METH can bind to dopamine transporters in dopamine nerve terminals as a pseudo‐neurotransmitter, and the resulting increase in dopamine release in the NAc is one of the mechanisms that underlie its rewarding effects. The endogenous opioid system has been reported to tonically modulate this mesolimbic dopaminergic pathway. 10 μ‐Opioid receptors are Gi‐coupled receptors that are expressed on inhibitory nerve terminals that project to dopamine nerves. One possibility is that the inhibition of MOPs results in the modulation of dopaminergic neurons, thereby suppressing reward function. 10 , 11 However, it is also plausible that distinct brain regions and neural circuits involved in the reward system are regulated through MOP inhibition, and further research is needed to explore the mechanisms underlying the acquisition and expression of METH reward. Moreover, NTX has a well‐established role in the treatment of alcohol addiction. 12 Genetic polymorphisms of the MOP gene have been reported to be associated with addiction to not only opioids but also other addictive substances. 13 , 14 , 15 These observations support the notion that the endogenous opioid system, particularly MOP‐related pathways, could play a role in the common mechanisms of substance use disorders.
UD‐030 is rapidly absorbed after oral administration and remains in plasma for a relatively long time. 8 Since UD‐030 showed central pharmacological effects when administered orally, it is thought to cross the blood–brain barrier (BBB) and act in the brain. UD‐030 may serve as a seed compound for the development of treatments for substance use disorder. However, several limitations must be addressed before proceeding to clinical trials. For instance, it is not yet known how effectively UD‐030 crosses the BBB, the degree to which it enters and is maintained in the brain, or how its concentration behaves over time. Additionally, the occurrence and extent of adverse effects, as well as metabolism, should be confirmed in multiple animal species. In addition, drug relapse is often a problem in addicts, and the effectiveness of UD‐030 needs to be tested.
In summary, our findings highlight the involvement of MOPs in mediating METH's rewarding effects. UD‐030 was shown to inhibit both the acquisition and expression of METH‐induced CPP in a dose‐dependent manner, suggesting its potential efficacy in suppressing METH‐related reward behaviors. Taken together, these results indicate that UD‐030, as a highly selective MOP antagonist, shows promise as a candidate for the development of pharmacotherapeutic interventions aimed at treating METH addiction. Further preclinical and clinical studies are needed to validate its potential therapeutic use in humans.
AUTHOR CONTRIBUTIONS
Conceptualization, S.I., N.I., K.A., M.K., S.U., and K.I.; methodology, S.I. and K.I.; software, S.I.; validation, S.I. and K.I.; formal analysis, S.I.; investigation, S.I.; resources, N.I., K.A., M.K., and S.U.; data curation, S.I.; writing – original draft preparation, S.I.; writing – review and editing, S.I., N.I., K.A., M.K., S.U., and K.I.; visualization, S.I.; supervision, S.I., N.I., and S.U.; project administration, S.I.; funding acquisition, S.I. and K.I. All authors read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This work was supported by research grants from Japan Society for the Promotion of Science (JSPS: 21K06814, 21H03028, 22H04922 [AdAMS], and 24K10049) and by a joint research fund from the UBE Corporation.
CONFLICT OF INTEREST STATEMENT
This research was conducted with a joint research fund from UBE Corporation. N.I., K.A., M.K., and S.U. have been involved as researchers in UBE Corporation. Dr. Ikeda is an Editorial Board member of Neuropsychopharmacology Reports and a co‐author of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
ETHICS STATEMENT
Approval of the Research Protocol by an Institutional Reviewer Board: All animal experiments were performed with approval from the Institutional Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science (protocol code 24–036 [2024/04/01]).
Informed Consent: N/A.
Registry and the Registration No. of the Study/Trial: N/A.
Animal Studies: All experimental procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Tokyo Metropolitan Institute of Medical Science.
Supporting information
Data S1.
ACKNOWLEDGMENTS
The authors are grateful to M. Arends for English editing. We also thank K. Taniko for evaluation.
Ide S, Iwase N, Arai K, Kojima M, Ushiyama S, Ikeda K. Inhibitory effects of the selective μ‐opioid receptor antagonist UD‐030 on methamphetamine‐induced conditioned place preference. Neuropsychopharmacol Rep. 2025;45:e12503. 10.1002/npr2.12503
DATA AVAILABILITY STATEMENT
The raw data of this study are presented as Supporting Information.
REFERENCES
- 1. Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31(5):301–313. [PMC free article] [PubMed] [Google Scholar]
- 2. Jayanthi S, Daiwile AP, Cadet JL. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp Neurol. 2021;344:113795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine‐type stimulant dependence: an update. Drug Alcohol Rev. 2013;32(5):449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiu CT, Ma T, Ho IK. Attenuation of methamphetamine‐induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull. 2005;67(1–2):100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang ZY, Guo LK, Han X, Song R, Dong GM, Ma CM, et al. Naltrexone attenuates methamphetamine‐induced behavioral sensitization and conditioned place preference in mice. Behav Brain Res. 2021;399:112971. [DOI] [PubMed] [Google Scholar]
- 6. Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2017. [DOI] [PubMed] [Google Scholar]
- 7. Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. J Subst Abuse Treat. 1997;14(6):529–534. [DOI] [PubMed] [Google Scholar]
- 8. Ide S, Iwase N, Arai K, Kojima M, Ushiyama S, Taniko K, et al. Inhibitory effects of a novel μ‐opioid receptor nonpeptide antagonist, UD‐030, on morphine‐induced conditioned place preference. Int J Mol Sci. 2023;24(4):3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen X, Purser C, Tien LT, Chiu CT, Paul IA, Baker R, et al. Mu‐opioid receptor knockout mice are insensitive to methamphetamine‐induced behavioral sensitization. J Neurosci Res. 2010;88(10):2294–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89(6):2046–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28(7):1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anton RF. Naltrexone for the management of alcohol dependence. N Engl J Med. 2008;359(7):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Al‐Eitan LN, Rababa'h DM, Alghamdi MA. Genetic susceptibility of opioid receptor genes polymorphism to drug addiction: a candidate‐gene association study. BMC Psychiatry. 2021;21(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, et al. Increased attributable risk related to a functional mu‐opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30(2):417–422. [DOI] [PubMed] [Google Scholar]
- 15. Ide S, Kobayashi H, Ujike H, Ozaki N, Sekine Y, Inada T, et al. Linkage disequilibrium and association with methamphetamine dependence/psychosis of mu‐opioid receptor gene polymorphisms. Pharmacogenomics J. 2006;6(3):179–188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The raw data of this study are presented as Supporting Information.


