Abstract
Objective
To investigate the association between thyroid dysfunction or thyroid autoimmunity (TAI) and diminished ovarian reserve (DOR).
Methods
A total of 2,867 women undergoing their first in-vitro fertilization (IVF) cycle at Shenzhen Zhongshan Obstetrics & Gynecology Hospital between January 1, 2013 and June 30, 2021, were enrolled in this study. The participants had documented thyroid and ovarian reserve metrics. They were categorized into three groups based on their thyroid function: normal thyroid function (N = 2,540), subclinical/overt hypothyroidism (SCH/OH) (N = 290), and subclinical/overt hyperthyroidism (N = 37). Anti-Mullerian hormone (AMH) and antral follicle count (AFC) were assessed and collected. Women with AMH <1.2 ng/mL and AFC < 5 were diagnosed with DOR. Basic characteristics and ovarian reserve-related parameters were compared among the three groups. The association between thyroid function and ovarian reserve function was further analyzed using logistical regression analyses. In addition, the euthyroid population was stratified using a thyroid-stimulating hormone (TSH) threshold of 2.5 µIU/mL, and the ovarian reserve-related parameters were compared among women with low-normal TSH (TSH < 2.5 µIU/mL), high-normal TSH (2.5 µIU/mL ≤ TSH ≤ 4.2 µIU/mL) and SCH/OH.
Results
Women with SCH/OH had lower AMH levels (2.79 ng/mL vs. 3.41 ng/mL, P < 0.001) and a significantly higher prevalence of AMH level < 1.2ng/mL (17.2% vs. 12.1%, P = 0.015) compared to those with normal thyroid function. The prevalence of DOR was also higher among women with SCH/OH (10.0% vs. 6.5%, P = 0.036). There were no significant differences in ovarian reserve between women with normal thyroid function and those with subclinical/overt hyperthyroidism. Logistic regression analyses showed that the odds ratio (OR) of women with SCH/OH suffering from DOR was 1.666 (95% CI: 1.079-2.572) compared to those with normal thyroid function, after adjusting for TAI status and basic clinical characteristics. When the euthyroid group was stratified according to TSH levels, women with SCH/OH showed significantly lower AMH levels compared to women with low-normal TSH (2.79 ng/mL vs. 3.44 ng/mL, P < 0.001) and a significantly higher prevalence of DOR (10.0% vs. 6.0%, P = 0.010). Logistic regression analyses showed that the women with SCH/OH had an increased prevalence of DOR (OR: 1.819, 95% CI: 1.158-2.858) compared to those with low-normal TSH, after adjusting for TAI status and basic clinical characteristics. However, the OR for DOR among women with high-normal TSH was not significantly elevated compared to those with low-normal TSH (OR: 1.310, 95% CI: 0.936-1.832).
Conclusion
SCH/OH may be associated with DOR, irrespective of TAI status.
Keywords: infertility, hypothyroidism, thyroid stimulating hormone, thyroid autoimmunity, ovarian reserve, anti-Müllerian hormone
1. Introduction
Diminished ovarian reserve (DOR) is a reproductive condition characterized by a depletion of the ovarian follicle pool, resulting in a decreased number of eggs and/or a decline in the quality of the remaining eggs (1). DOR may reduce fertility in women attempting spontaneous conception or those undergoing assisted reproductive technologies (ART) such as in vitro fertilization (IVF) (2–4). Research into the etiology of this condition is crucial for improving diagnostic and therapeutic strategies for women suffering from DOR. Although the exact cause is multifactorial, studies have identified several contributing factors, such as genetic predisposition, environmental influences, and specific medical conditions (5–8). Recent studies have found that endocrine abnormalities, such as thyroid dysfunction, may be associated with DOR (9–11).
The thyroid is the largest endocrine gland in the human body and thyroid hormones, such as thyroid-stimulating hormone (TSH), free triiodothyronine (FT3) and free thyroid hormone (FT4), play a significant role in the growth and maturation of various organs (12–15). Thyroid dysfunction is a common endocrine disorder that significantly affects the fertility of women of reproductive age (16). Hyperthyroidism, characterized by elevated FT4 levels and decreased TSH levels, often manifests with menstrual irregularities such as amenorrhea, hypomenorrhea, and oligomenorrhea (17, 18). Overt hypothyroidism (OH) and subclinical hypothyroidism (SCH), identified by elevated levels of TSH, have been associated with miscarriage and preterm birth (19). Interestingly, thyroid hormone and TSH receptors have been observed on granulosa cells and oocytes in the ovary, where they act either directly or synergistically with follicle-stimulating hormone (FSH) to influence follicular growth and development (20). This finding has drawn attention to the relationship between thyroid dysfunction and DOR. However, the conclusions on this relationship remain controversial (21–24). For instance, Michalakis et al. found that elevated preconception levels of TSH were associated with DOR (21). However, Kucukler et al. found that ovarian reserve function was not different among women with OH, SCH, and normal thyroid function (23).
Previous studies also demonstrated that women who were diagnosed with thyroid autoimmunity (TAI) are more likely to develop thyroid dysfunction, such as OH or SCH (25, 26). Some studies have indicated that TAI is also a risk factor for impaired ovarian reserve function (27, 28). The complex link between TAI and thyroid dysfunction makes it difficult to distinguish their independent effect on ovarian reserve function, and most previous studies have ignored the interaction between these two factors (23, 29), limiting the clarity of their respective roles in ovarian reserve function.
In light of these considerations, this study aimed to explore the relationship between thyroid dysfunction and DOR in infertile women. Additionally, the potential modifying effect of TAI on this relationship was evaluated.
2. Materials and methods
2.1. Ethical approval
The study was approved by the Ethics Committee of Shenzhen Zhongshan Obstetrics & Gynecology Hospital (formerly Shenzhen Zhongshan Urology Hospital) (approval number: 2023SZM-LW006). A waiver of informed consent was granted for this study.
2.2. Subjects
A cross-sectional study was conducted on infertile women who underwent ART at Shenzhen Zhongshan Obstetrics & Gynecology Hospital (formerly Shenzhen Zhongshan Urology Hospital) between January 1, 2013 and June 30, 2021.
The inclusion criteria were as follows: (1) women aged 20-40 years old; (2) women undergoing their first IVF/ICSI treatment at our hospital; (3) women with integrated electronic medical record data; and (4) women who met the criteria for normal thyroid function, OH, SCH, overt hyperthyroidism or subclinical hyperthyroidism.
The exclusion criteria for women in this study were as follows: a history of medication use, including thyroid hormone, anti-thyroid medication, and thyroid surgery (N = 24); a diagnosis of polycystic ovarian syndrome, endometriosis, or ovarian tumor (N = 689); a history of chemotherapy, radiotherapy, or ovarian surgery (N = 222); and X chromosome abnormalities (N = 18).
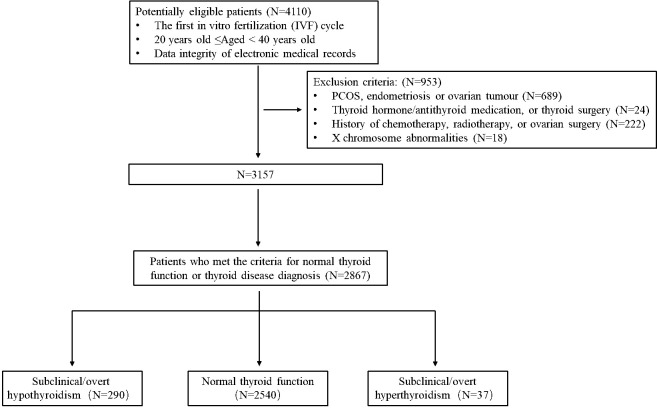
To ensure the accuracy of the study, we thoroughly examined each electronic record to confirm that all participants met the specified inclusion and exclusion criteria. Finally, 2867 women were enrolled in the analysis ( Figure 1 ). Based on their TSH and FT4 levels, the participants were categorized into three groups: those with normal thyroid function (N = 2,540), SCH/OH (N = 290), and those with subclinical/overt hyperthyroidism (N = 37).
Figure 1.
Diagram of the study and distribution of the patients investigated.
2.3. Variable measurement and collection procedures
2.3.1. Demographic characteristics
Height and weight were measured for all patients. In addition, detailed medical background information was collected, including age, duration of infertility, and type of infertility.
2.3.2. Sex hormones
Endocrine hormones, including FSH, luteinizing hormone (LH), and estradiol (E2), were measured using a chemiluminescence immunoassay (Roche Diagnostics, Germany) conducted on the 2nd to 5th day of the menstrual cycle. These measurements were taken before ovarian stimulation, following standard protocols. In our laboratory, the reference ranges for basal hormone levels were as follows: FSH, 3.5 to 12.5 IU/L, LH, 2.4 to 12.6 IU/L, and E2, 0 to 160 pg/mL, as provided by the manufacturer. The sensitivity threshold for measuring FSH and LH was set at 0.1 IU/L.
2.3.3. Thyroid function examination
Thyroid function parameters, including TSH, FT4, and FT3, were measured using the electrochemical luminescence method on a Cobas E601 analyzer (Roche Diagnostics GmbH, Germany). Thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) were assessed using a commercial chemiluminescence immunoassay (YHLO Biotech, China). The minimum detectable levels for TPOAb and TgAb were 0.1 IU/mL, with an intra-assay coefficient of variation below 10%. The reference ranges for thyroid function tests were as follows, TSH: 0.27-4.20 μIU/mL, FT3: 2.0-4.4 pg/mL, and FT4: 0.93-1.70 ng/mL. The sensitivity threshold for measuring TSH was 0.005 μIU/mL. A TPOAb titer above 34 IU/mL or a TgAb titer above 115 IU/mL was considered positive, and positivity for either or both antibodies was used to define TAI. Thyroid dysfunction was categorized as follows: OH, TSH > 4.2 mIU/L with FT4 < 0.93 ng/mL; SCH, TSH > 4.2 mIU/L with FT4 levels within the normal range; overt hyperthyroidism, TSH < 0.1 mIU/L with FT4 > 1.7 ng/mL or FT3 > 4.4 pg/mL; and subclinical hyperthyroidism, TSH < 0.1 mIU/L with FT4 and FT3 levels within the normal range.
2.3.4. Ovarian reserve function parameters
Serum anti-Mullerian hormone (AMH) levels were measured using a commercial chemiluminescence immunoassay (YHLO Biotech, China). In statistical analysis, AMH values below the detection limit (0.01ng/mL) were recorded as 0.01 ng/mL. Antral follicle count (AFC) referred to the total number of early sinusoidal follicles (2-10 mm independent diameter) observed via transvaginal ultrasound on both ovaries.
2.4. Outcomes
This study analyzed the prevalence of women with DOR, defined as an AMH level < 1.2 ng/mL and AFC level < 5. In addition, the prevalence of women with an AMH level < 1.2 ng/mL regardless of AFC level, and those with an AFC level < 5 regardless of AMH level, was examined.
2.5. Statistical analyses
All statistical analyses and graphical representations were performed using SPSS version 26.0 and R version 4.2.1. The normality of continuous variables was assessed by combining the Kolmogorov-Smirnov test and quantile-quantile plot. For continuous variables with non-normal distribution, data were presented as medians (with interquartile ranges) and compared using non-parametric tests. The Kruskal-Wallis rank-sum test was used to compare continuous variables across three groups, with pairwise comparisons performed using Dunn’s with Bonferroni correction. Qualitative variables were presented as absolute values and percentages (%). Differences in outcomes between qualitative variables were analyzed using the chi-square test, while pairwise comparisons among multiple groups were conducted using the chi-square partitioning method. Fisher’s exact probability test was used when the expected frequency was less than 5.
To assess the impact of thyroid dysfunction and TAI on ovarian reserve, binary and multinomial logistic regression models were employed to calculate odds ratios (OR) and 95% confidence intervals (CI). The logistic regression models were adjusted for potential confounders, including TAI, female age, BMI, infertility duration and infertility type. A P-value of 0.05 was considered statistically significant.
3. Results
3.1. Prevalence of DOR was increased in infertile women with SCH/OH compared to women with normal thyroid function
To investigate the differences in ovarian reserve function among women with different thyroid conditions, we compared ovarian reserve parameters among infertile women with normal thyroid function, SCH/OH, and subclinical/overt hyperthyroidism ( Table 1 ). The concentration of TPOAb (9.33 IU/mL vs. 32.90 IU/mL vs. 6.35 IU/mL, P < 0.001) and TgAb (14.69 IU/mL vs. 108.3 IU/mL vs. 12.55 IU/mL, P < 0.001) was significantly increased in the women with SCH/OH and subclinical/overt hyperthyroidism compared to those with normal thyroid function. Similarly, the prevalence of TPOAb (27.2% vs. 48.6% vs. 10.3%, P < 0.001) and TgAb (28.3% vs. 48.6% vs. 12.9%, P < 0.001) was notably elevated among women with SCH/OH and subclinical/overt hyperthyroidism compared to women with normal thyroid function.
Table 1.
Baseline characteristics and ovarian reserve parameters of participants with different thyroid conditions.
| Variables | Normal thyroid function |
SCH/OH | Subclinical/overt hyperthyroidism |
P |
|---|---|---|---|---|
| N | 2540 | 290 | 37 | |
| Age (years) | 32.00 [29.00, 35.00] | 32.00 [29.00, 35.00] | 31.00 [29.00, 34.00] | 0.139 |
| BMI (kg/m2) | 20.81 [19.31, 22.66] | 21.36 [20.03, 24.03] | 21.08 [19.47, 23.24] | <0.001a |
| Duration of infertility (years) | 3.00 [2.00, 5.00] | 3.00 [2.00, 5.00] | 3.00 [2.00, 5.00] | 0.657 |
| Infertility type, n (%) | ||||
| Primary infertility | 1178 (46.4) | 156 (53.8) | 24 (64.9) | 0.006a |
| Secondary infertility | 1362 (53.6) | 134 (46.2) | 13 (35.1) | |
| TSH (µIU/mL) | 1.95 [1.42, 2.68] | 5.08 [4.54, 6.34] | 0.02 [0.00, 0.03] | <0.001a,b,c |
| FT4 (ng/dL) | 1.25 [1.14, 1.35] | 1.20 [1.10, 1.30] | 1.77 [1.44, 2.11] | <0.001a,b,c |
| FT3 (pg/mL) | 2.94 [2.73, 3.17] | 2.99 [2.75, 3.20] | 4.14 [3.21, 5.06] | <0.001b, c |
| TPOAb (IU/mL) | 6.35 [1.68, 12.27] | 9.33 [3.42, 42.90] | 32.90 [14.13, 143.10] | <0.001a,b,c |
| TgAb (IU/mL) | 12.55 [5.23, 23.27] | 14.69 [7.93, 187.00] | 108.30 [15.30, 696.80] | <0.001a,b,c |
| Positive TPOAb, n(%) | 261 (10.3) | 79 (27.2) | 18 (48.6) | <0.001a, b,c |
| Positive TgAb, n(%) | 327 (12.9) | 82 (28.3) | 18 (48.6) | <0.001a,b,c |
| Positive TAI, n(%) | 396 (15.6) | 95 (32.8) | 19 (51.4) | <0.001a,b,c |
| LH (IU/L) | 4.91 [3.76, 6.41] | 4.80 [3.59, 6.45] | 4.86 [3.90, 6.07] | 0.948 |
| E2 (pg/mL) | 34.90 [26.00, 47.50] | 32.60 [24.15, 45.65] | 41.10 [33.60, 58.80] | 0.004b,c |
| AMH (ng/mL) | 3.41 [1.94, 5.47] | 2.79 [1.46, 4.82] | 3.64 [1.54, 4.91] | 0.002a |
| AFC (n) | 10.00 [6.00, 14.00] | 9.00 [6.25, 14.00] | 9.00 [6.00, 14.00] | 0.849 |
| FSH (mIU/mL) | 6.78 [5.85, 8.01] | 7.03 [6.06, 8.09] | 6.72 [5.75, 7.69] | 0.237 |
| Prevalence of AMH< 1.2 ng/mL, n(%) | 308 (12.1) | 50 (17.2) | 5 (13.5) | 0.045a |
| Prevalence of AFC<5, n(%) | 329 (13.0) | 43 (14.8) | 3 (8.1) | 0.445 |
| Prevalence of DOR, n(%) | 166 (6.5) | 29 (10.0) | 1 (2.7) | 0.052 |
All data is presented as median [25th percentile, 75th percentile] or n (%).
Superscripts indicate significant differences between aNormal thyroid function and SCH/OH, b SCH/OH and subclinical/overt hyperthyroidism, and cNormal thyroid function and subclinical/overt hyperthyroidism.
Kruskal-Wallis rank-sum test is applied for comparisons among three groups of continuous variables, with pairwise comparisons via Dunn’s with Bonferroni correction. Categorical variables are compared using chi-squared test, with pairwise comparisons among multiple groups are conducted using the chi-square partitioning method.
SCH/OH, Subclinical/overt hypothyroidism; BMI, body–mass index; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TPOAb, thyroid peroxidase antibody; TgAb, thyroglobulin antibody; TAI, thyroid autoimmunity; LH, luteinizing hormone; E2, estradiol; AMH, anti-müllerian hormone; AFC, antral follicle count; FSH, follicle stimulating hormone; DOR, diminished ovarian reserve.
An elevated E2 level was observed predominantly in the subclinical/overt hyperthyroidism group (41.10 pg/mL vs. 34.90 pg/mL vs. 32.60 pg/mL, P = 0.004) compared to women with normal thyroid function and those with SCH/OH. Notably, AMH levels were significantly lower in women with SCH/OH compared to those with normal thyroid function (2.79 ng/mL vs. 3.41 ng/mL, P < 0.001). In addition, the prevalence of women with AMH <1.2 ng/mL was significantly higher in the SCH/OH group compared to the normal thyroid function group (17.2% vs. 12.1%, P = 0.015). Moreover, there was a noticeable trend toward a higher prevalence of DOR in women with SCH/OH compared to those with normal thyroid function (10.0% vs. 6.5%, P = 0.036).
3.2. SCH/OH may be associated with DOR in infertile women
Univariate logistic regression analysis showed a significant association between SCH/OH and DOR (OR: 1.589, 95% CI: 1.05-2.406; Table 2 ), while overt/subclinical hyperthyroidism was not related to the occurrence of DOR (OR: 0.397, 95% CI: 0.054-2.916). Interestingly, TAI positivity did not significantly increase the prevalence of DOR (OR: 1.005, 95% CI: 0.688-1.468).
Table 2.
Effect of thyroid conditions for DOR.
| Variables | Model 1 OR(95% CI) | Model 2 OR(95% CI) | Model 3 OR(95% CI) | Model 4 OR(95% CI) |
|---|---|---|---|---|
| Thyroid conditions | ||||
| Normal thyroid function | Ref | Ref | Ref | |
| SCH/OH | 1.589(1.050,2.406)* | 1.598(1.050,2.432)* | 1.666(1.079,2.572)* | |
| Subclinical/overt hyperthyroidism | 0.397(0.054,2.916) | 0.402(0.055,2.965) | 0.463(0.062,3.459) | |
| Thyroid autoimmunity | ||||
| TAI(-) | Ref | Ref | Ref | |
| TAI(+) | 1.005(0.688,1.468) | 0.966(0.658,1.420) | 0.9(0.608,1.333) | |
| Basic clinical characteristics | ||||
| Female age (years) | 1.223(1.168,1.281)* | |||
| BMI (kg/m2) | 1.025(0.973,1.079) | |||
| Duration of infertility (years) | 1.015(0.968,1.065) | |||
| Infertility type | ||||
| Primary infertility | Ref | |||
| Secondary infertility | 0.64(0.468,0.875)* | |||
Model 1: Univariate logistic regression analysis of thyroid function. Model 2: Univariate logistic regression analysis of TAI. Model 3: Adjusted for thyroid autoimmunity. Model 4: Model 3, with additional adjustments for female age, BMI, infertility duration, infertility type.
*P value<0.05.
DOR, diminished ovarian reserve; SCH/OH, Subclinical/overt hypothyroidism; TAI, thyroid autoimmunity.
Multivariate logistic regression analyses indicated that SCH/OH remained significantly associated with DOR after adjusting for confounding factors, including TAI status, female age, BMI, duration of infertility, and type of infertility (OR: 1.666, 95% CI: 1.079-2.572).
To further analyze the impact of TAI status on the relationship between SCH/OH and DOR, interaction analyses were performed ( Table 3 ). Interaction analyses showed that SCH/OH alone was significantly associated with DOR (adjusted OR: 1.839; 95% CI: 1.116-3.031). In contrast, the presence of TAI alone was not significantly associated with DOR (adjusted OR: 0.996; 95% CI: 0.645-1.538). In addition, no significant interaction was found between TAI and thyroid conditions (adjusted OR: 1.174; 95% CI: 0.550-2.508).
Table 3.
Modification of association between different thyroid conditions and DOR by thyroid autoimmunity.
| Variables | Number of patients | Prevalence of DOR, n(%) | Adjusted OR (95% CI) |
|---|---|---|---|
| TAI(-) | |||
| Normal thyroid function | 2144 | 139(6.48) | Ref |
| SCH/OH | 195 | 21(10.77) | 1.871(1.133,3.089)* |
| TAI(+) | |||
| Normal thyroid function | 396 | 27(6.82) | Ref |
| SCH/OH | 95 | 8(8.42) | 1.126(0.472,2.687) |
| TAI&Thyroid condition | |||
| TAI(-)&Normal thyroid function | 2144 | 139(6.48) | Ref |
| TAI(-)&SCH/OH | 195 | 21(10.77) | 1.839(1.116,3.031) * |
| TAI(+)&Normal thyroid function | 396 | 27(6.82) | 0.996(0.645,1.538) |
| TAI(+)&SCH/OH | 95 | 8(8.42) | 1.247(0.581,2.676) |
| Interaction of TAI and Thyroid condition | 1.174(0.550,2.508) | ||
Adjusted OR: Adjusted for female age, BMI, infertility duration, infertility type.
*P value<0.05.
DOR, diminished ovarian reserve; SCH/OH, Subclinical/overt hypothyroidism; TAI, thyroid autoimmunity.
3.3. Prevalence of DOR was similar between women with high-normal TSH and low-normal TSH
Owing to the limited number of cases with overt or subclinical hyperthyroidism, we opted not to conduct further stratified analyses for these conditions. Instead, women with normal thyroid function were divided into two groups, low-normal TSH and high-normal TSH, based on a threshold of 2.5 µIU/mL. It was found that TPOAb (9.33 IU/mL vs. 7.31 IU/mL vs. 5.95 IU/mL, P < 0.001) and TgAb (14.69 IU/mL vs. 13.41 IU/mL vs. 12.16 IU/mL, P < 0.001) levels were markedly elevated in both the SCH/OH group and the high-normal TSH group compared to the low-normal TSH group ( Table 4 ). In alignment with this, the prevalence of positive TPOAb (27.2% vs. 12.6% vs. 9.3%, P < 0.001) TgAb (28.3% vs. 17.0% vs. 11.1%, P < 0.001) and TAI (32.8% vs. 19.5% vs. 13.9%, P < 0.001) was markedly elevated in the SCH/OH and high-normal TSH groups compared to the low-normal TSH group.
Table 4.
Clinical characteristics of participants with normal thyroid function and SCH/OH.
| Variables | TSH<2.5µIU/mL | 2.5µIU/mL≤TSH≤4.2µIU/mL | SCH/OH | P |
| N | 1771 | 769 | 290 | |
| Age (years) | 32.00 [29.00, 35.00] | 32.00 [29.00, 35.00] | 32.00 [29.00, 35.00] | 0.284 |
| BMI (kg/m2) | 20.76 [19.23, 22.58] | 20.96 [19.47, 22.86] | 21.36 [20.03, 24.03] | <0.001b,c |
| Duration of infertility (years) | 3.00 [2.00, 5.00] | 3.00 [2.00, 5.00] | 3.00 [2.00, 5.00] | 0.388 |
| Infertility type, n (%) | ||||
|---|---|---|---|---|
| Primary infertility | 815 (46.0) | 363 (47.2) | 156 (53.8) | 0.049c |
| Secondary infertility | 956 (54.0) | 406 (52.8) | 134 (46.2) | |
| TSH (µIU/mL) | 1.62 [1.23, 2.02] | 3.07 [2.76, 3.51] | 5.08 [4.54, 6.34] | <0.001a,b,c |
| FT4 (ng/dL) | 1.25 [1.15, 1.36] | 1.24 [1.13, 1.34] | 1.20 [1.10, 1.30] | <0.001b,c |
| FT3 (pg/mL) | 2.93 [2.71, 3.14] | 2.98 [2.76, 3.23] | 2.99 [2.75, 3.20] | <0.001a |
| TPOAb (IU/mL) | 5.95 [1.51, 11.72] | 7.31 [2.41, 13.89] | 9.33 [3.42, 42.90] | <0.001a,b,c |
| TgAb (IU/mL) | 12.16 [4.50, 22.18] | 13.41 [6.80, 34.15] | 14.69 [7.93, 187.00] | <0.001c |
| Positive TPOAb, n(%) | 164 (9.3) | 97 (12.6) | 79 (27.2) | <0.001a,b,c |
| Positive TgAb, n(%) | 196 (11.1) | 131 (17.0) | 82 (28.3) | <0.001a,b,c |
| Positive TAI, n(%) | 246 (13.9) | 150 (19.5) | 95 (32.8) | <0.001a,b,c |
| LH (IU/L) | 4.85 [3.69, 6.34] | 5.02 [3.90, 6.57] | 4.80 [3.59, 6.45] | 0.069 |
| E2 (pg/mL) | 35.00 [26.00, 47.30] | 34.00 [26.20, 48.00] | 32.60 [24.15, 45.65] | 0.087 |
| AMH (ng/mL) | 3.44 [1.96, 5.58] | 3.33 [1.91, 5.23] | 2.79 [1.46, 4.82] | <0.001c |
| AFC (n) | 10.00 [6.00, 14.00] | 10.00 [6.00, 14.00] | 9.00 [6.25, 14.00] | 0.852 |
| FSH (mIU/mL) | 6.72 [5.82, 7.98] | 6.93 [5.90, 8.14] | 7.03 [6.06, 8.09] | 0.062 |
| Prevalence of AMH< 1.2 ng/mL, n(%) | 204 (11.5) | 104 (13.5) | 50 (17.2) | 0.017c |
| Prevalence of AFC<5, n(%) | 214 (12.1) | 115 (15.0) | 43 (14.8) | 0.097 |
| Prevalence of DOR, n(%) | 106 (6.0) | 60 (7.8) | 29 (10.0) | 0.022c |
All data is presented as median [25th percentile, 75th percentile] or n (%).
Superscripts indicate significant differences between aTSH<2.5µIU/mL and 2.5µIU/mL≤TSH≤4.2µIU/mL, b2.5µIU/mL≤TSH≤4.2µIU/mL and SCH/OH, and cTSH<2.5µIU/mL and SCH/OH.
Kruskal-Wallis rank-sum test is applied for comparisons among three groups of continuous variables, with pairwise comparisons via Dunn’s with Bonferroni correction. Categorical variables are compared using chi-squared test, with pairwise comparisons among multiple groups are conducted using the chi-square partitioning method.
SCH/OH, Subclinical/overt hypothyroidism; BMI, body–mass index; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TPOAb, thyroid peroxidase antibody; TgAb, thyroglobulin antibody; TAI, thyroid autoimmunity; LH, luteinizing hormone; E2, estradiol; AMH, anti-müllerian hormone; AFC, antral follicle count; FSH, follicle stimulating hormone; DOR, diminished ovarian reserve.
AMH levels were significantly lower in the SCH/OH group compared with low-normal TSH group (2.79 ng/mL vs. 3.44 ng/mL, P < 0.001). Furthermore, the prevalence of women with AMH < 1.2 ng/mL (17.2% vs. 11.5%, P = 0.006) and those with DOR (10.0% vs. 6.0%, P = 0.01) was significantly higher in the SCH/OH group compared to the low-normal TSH group. However, no significant differences were observed in ovarian reserve-related parameters between the high-normal TSH group and the low-normal TSH group.
3.4. No association was found between high-normal TSH level and DOR
Univariate logistic regression indicated that SCH/OH was significantly associated with DOR (OR: 1.745, 95% CI: 1.134-2.686), while high-normal TSH level (OR: 1.329, 95% CI: 0.957-1.846) and TAI (OR: 1.045, 95% CI: 0.715-1.528) were not associated with DOR ( Table 5 ). Multivariate logistic regression analysis after adjusting for confounding factors revealed that SCH/OH still remained significantly associated with an increased prevalence of DOR (OR: 1.819, 95% CI: 1.158-2.858). However, the OR for DOR in women with high-normal TSH level, compared to those with low-normal TSH levels, was not significantly elevated (OR: 1.310, 95% CI: 0.936-1.832). Furthermore, to eliminate the impact of OH, we also analyzed the data of women with low-normal TSH, high-normal TSH, and SCH. The conclusions were consistent with the aforementioned results ( Supplementary Tables S1 , S2 ).
Table 5.
Effect of thyroid stimulating hormone levels on DOR.
| Variables | Model 1 OR(95% CI) | Model 2 OR(95% CI) | Model 3 OR(95% CI) | Model 4 OR(95% CI) |
|---|---|---|---|---|
| TSH levels (µIU/mL) | ||||
| TSH<2.5 | Ref | Ref | Ref | |
| 2.5≤TSH≤4.2 | 1.329(0.957,1.846) | 1.332(0.958,1.851) | 1.310(0.936,1.832) | |
| SCH/OH | 1.745(1.134,2.686)* | 1.757(1.135,2.72)* | 1.819(1.158,2.858)* | |
| Thyroid autoimmunity | ||||
| TAI(-) | Ref | Ref | Ref | |
| TAI(+) | 1.045(0.715,1.528) | 0.965(0.656,1.419) | 0.902(0.609,1.336) | |
| Basic clinical characteristics | ||||
| Female age (years) | 1.219(1.164,1.277)* | |||
| BMI (kg/m2) | 1.025(0.974,1.08) | |||
| Duration of infertility (years) | 1.016(0.969,1.066) | |||
| Infertility type | ||||
| Primary infertility | Ref | |||
| Secondary infertility | 0.649(0.475,0.888)* | |||
Model 1: Univariate logistic regression analysis of TSH levels. Model 2: Univariate logistic regression analysis of TAI. Model 3: Adjusted for thyroid autoimmunity. Model 4: Model 3, with additional adjustments for female age, BMI, infertility duration, infertility type.
*P value<0.05.
DOR, diminished ovarian reserve; SCH/OH, Subclinical/overt hypothyroidism; TAI, thyroid autoimmunity.
4. Discussion
Thyroid dysfunction is a common endocrine disorder in women of reproductive age (30). This study aimed to investigate the effect of different thyroid conditions on ovarian reserve in infertile women while considering the status of TAI. The findings revealed that women with SCH/OH had significantly lower AMH levels and an increased prevalence of AMH < 1.2 ng/mL compared to women with normal thyroid function. Logistic regression analysis indicated that SCH/OH might be associated with DOR, while TAI did not affect this result. Furthermore, stratification of TSH levels in women with normal thyroid function revealed that the prevalence of DOR in women with SCH/OH was significantly higher than that in women with TSH < 2.5 µIU/mL, with no significant difference observed in women with TSH levels between 2.5 µIU/mL and 4.2 µIU/mL. These findings suggest that SCH/OH may have an independent effect on ovarian reserve.
SCH/OH, characterized by TSH levels exceeding 4.2 µIU/mL, negatively impact women’s reproductive health (23, 31). Previous study have shown that women with SCH had significantly lower AMH concentrations and AFCs compared to euthyroid women (32). Consistent with these findings, our study found that SCH/OH was associated with reduced AMH levels and an increased prevalence of AMH <1.2 ng/mL. This reduction in AMH levels was significantly associated with DOR, underscoring the direct impact of hypothyroid states on the reproductive potential of women. Extensive researches have established a robust connection between thyroid dysfunction and DOR (21, 33, 34). Hiraoka et al. reported a significantly higher prevalence of DOR in patients with elevated TSH levels (TSH ≥4.5 µIU/mL) compared to those with normal TSH levels (46.2% vs 17.9%, P < 0.05) (35). Similarly, our study found reduced AMH levels and a higher prevalence of DOR in patients with SCH/OH compared to euthyroid women. DOR may be associated with SCH/OH (TSH > 4.2 µIU/mL). Conversely, there was no association between hyperthyroidism or subclinical hyperthyroidism and DOR. This suggests that thyroid function may influence ovarian reserve, with the impact may be more pronounced in hypothyroidism rather than in hyperthyroidism.
TAI is commonly observed in women of reproductive age and stands as the primary etiology of thyroid dysfunction (36). Thyroid autoantibodies may directly damage ovarian tissue, leading to impaired follicular development and premature ovarian insufficiency (POI) (37). Research suggested that the presence of thyroid autoantibodies in women has been linked to the deposition of autoantibodies in their ovarian tissue (38). However, previous studies have reported conflicting findings concerning the relationship between TAI and ovarian reserve in euthyroid women (39–42). A large cohort study involving 21,325 participants demonstrated that TAI significantly increased the risk of POI in women, aligning with the outcomes of a recent meta-analysis (41, 43). Conversely, Rao et al. suggested that while TPOAb positivity was not associated with ovarian reserve, SCH was associated with a lower AMH concentration (mean difference = -0.27 ng/mL [CI -0.43 to -0.12 ng/mL], P = 0.001), and lower AFC (mean difference = -0.7 [CI -1.3 to -0.2], P = 0.005) (32). In our study, no significant association was observed between TAI and DOR. These findings suggested that while autoimmunity processes may influence thyroid function, their direct impact on ovarian reserve may be less pronounced or may involve different mechanisms that require further investigation.
Several studies have demonstrated the correlation between TSH and ovarian reserve in infertile populations (22, 44, 45). Weghofer et al. conducted a retrospective study on 225 infertile women with TSH levels within the normal range (0.4-4.5 mIU/L) and found that TSH <3.0 μIU/mL was significantly correlated with higher AMH levels (34). Halici et al. classified the TSH range into four categories: ≤1 mIU/L, 1-2.5 mIU/L, 2.5-4 mIU/L, and >4 mIU/L, and discovered that the association between TSH and AMH was not linear, with the highest AMH levels observed at a TSH value of 2.88 mIU/L. Conversely, significantly lower AMH levels were reported in the group with TSH >4 mIU/L compared to the other three groups (46). Consistent with these findings, our study found that women with SCH/OH (TSH >4.2 µIU/mL) had significantly lower AMH levels and a significantly higher prevalence of DOR compared to those with TSH < 2.5 µIU/mL. Even after adjusting for confounding variables such as TAI, SCH/OH remained a significant association with DOR. These results suggest that abnormally elevated TSH level may impair ovarian reserve, while lower TSH levels exert a direct beneficial effect on follicular recruitment.
This study had some limitations that should be acknowledged. The retrospective cross-sectional nature of the study limited the ability to establish a causal relationship between thyroid function and ovarian reserve. While the study provided valuable insights into the potential association further research utilizing different study designs, such as prospective longitudinal studies, is necessary to definitively determine the nature of this relationship. Additionally, all patients were recruited from a single center in the study, which raises the possibility of selection bias affecting the characteristics and outcomes of the participants. Future should consider recruiting participants from multiple centers to enhance the generalizability of the findings and minimize the potential impact of selection bias on the results.
5. Conclusions
Our findings revealed that SCH/OH may be associated with a reduction in ovarian reserve among infertile women and may act as an independent factor affecting ovarian reserve. While the presence of TAI may potentially dilute or obscure the impact of SCH/OH on ovarian reserve, SCH/OH still may be associated with DOR. In summary, thyroid dysfunction may adversely affect a woman’s fertility, highlighting the importance of early recognition and management of thyroid-related issues to address possible barriers to conception.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by the National Natural Science Foundation of China (No. 82201859), the Guangdong Basic and Applied Basic Research Foundation (2022A1515110811).
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Shenzhen Zhongshan Obstetrics & Gynecology Hospital (formerly Shenzhen Zhongshan Urology Hospital). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Clinical data were exclusively extracted from the patients’ electronic medical record system for research purposes. The patients’ identities were anonymized throughout the research process to ensure the protection of personal privacy, and the study did not involve any issues related to personal privacy or commercial interests.
Author contributions
HZ: Conceptualization, Writing – review & editing. HQ: Investigation, Writing – original draft, Writing – review & editing. ZL: Data curation, Writing – original draft. WL: Supervision, Writing – review & editing. YW: Methodology, Writing – review & editing. CH: Conceptualization, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2024.1477665/full#supplementary-material
References
- 1. Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. (2018) 35:17–23. doi: 10.1007/s10815-017-1058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen D, Shen X, Wang L, Kuang Y. Cumulative live birth rates for low-prognosis women over 5 years or 9 frozen-thawed embryo transfer cycles. BMC Pregnancy Childbirth. (2022) 22:233. doi: 10.1186/s12884-022-04511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Busnelli A, Somigliana E, Cirillo F, Levi-Setti PE. Is diminished ovarian reserve a risk factor for miscarriage? Results of a systematic review and meta-analysis. Hum Reprod Update. (2021) 27:973–88. doi: 10.1093/humupd/dmab018 [DOI] [PubMed] [Google Scholar]
- 4. Yi Y, Fu J, Xie S, Zhang Q, Xu B, Wang Y, et al. Association between ovarian reserve and spontaneous miscarriage and their shared genetic architecture. Hum Reprod. (2023) 38:2247–58. doi: 10.1093/humrep/dead180 [DOI] [PubMed] [Google Scholar]
- 5. Luderer U, Lim J, Ortiz L, Nguyen JD, Shin JH, Allen BD, et al. Exposure to environmentally relevant concentrations of ambient fine particulate matter (PM(2.5)) depletes the ovarian follicle reserve and causes sex-dependent cardiovascular changes in apolipoprotein E null mice. Part Fiber Toxicol. (2022) 19:5. doi: 10.1186/s12989-021-00445-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olsen KW, Castillo-Fernandez J, Chan AC, la Cour Freiesleben N, Zedeler A, Bungum M, et al. Identification of a unique epigenetic profile in women with diminished ovarian reserve. Fertil Steril. (2021) 115:732–41. doi: 10.1016/j.fertnstert.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 7. Liu L, Cai B, Zhang X, Tan J, Huang J, Zhou C. Differential transcriptional profiles of human cumulus granulosa cells in patients with diminished ovarian reserve. Arch Gynecol Obstet. (2022) 305:1605–14. doi: 10.1007/s00404-022-06399-2 [DOI] [PubMed] [Google Scholar]
- 8. Jiao X, Meng T, Zhai Y, Zhao L, Luo W, Liu P, et al. Ovarian reserve markers in premature ovarian insufficiency: within different clinical stages and different etiologies. Front Endocrinol (Lausanne). (2021) 12:601752. doi: 10.3389/fendo.2021.601752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Serin AN, Birge O, Uysal A, Gorar S, Tekeli F. Hashimoto’s thyroiditis worsens ovaries in polycystic ovary syndrome patients compared to Anti-Mullerian hormone levels. BMC Endocr Disord. (2021) 21:44. doi: 10.1186/s12902-021-00706-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li F, Lu H, Huang Y, Wang X, Zhang Q, Li X, et al. A systematic review and meta-analysis of the association between Hashimoto’s thyroiditis and ovarian reserve. Int Immunopharmacol. (2022) 108:108670. doi: 10.1016/j.intimp.2022.108670 [DOI] [PubMed] [Google Scholar]
- 11. Yang X, Zhao Z, Fan Q, Li H, Zhao L, Liu C, et al. Cholesterol metabolism is decreased in patients with diminished ovarian reserve. Reprod BioMed. (2022) 44:185–92. doi: 10.1016/j.rbmo.2021.09.013 [DOI] [PubMed] [Google Scholar]
- 12. Kjaergaard AD, Marouli E, Papadopoulou A, Deloukas P, Kus A, Sterenborg R, et al. Thyroid function, sex hormones and sexual function: a Mendelian randomization study. Eur J Epidemiol. (2021) 36:335–44. doi: 10.1007/s10654-021-00721-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schiera G, Di Liegro CM, Di Liegro I. Involvement of thyroid hormones in brain development and cancer. Cancers (Basel). (2021) 13:2693. doi: 10.3390/cancers13112693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol. (2017) 14:39–55. doi: 10.1038/nrcardio.2016.174 [DOI] [PubMed] [Google Scholar]
- 15. Li M, Yang X, Li R, Wu B, Hao J, Qi Y, et al. Visceral fat area and subcutaneous fat area increase in hyperthyroidism patients after treatment-A single-group repeated-measures trial. Diabetes Metab Syndr Obes. (2024) 17:2165–76. doi: 10.2147/DMSO.S458486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akande AA, Isah IA, Aliyu IS, Adesiyun AG. Thyroid dysfunction in women of reproductive age: laboratory protocol for infertility evaluation. Ann Ib Postgrad Med. (2022) 20:53–7. [PMC free article] [PubMed] [Google Scholar]
- 17. Mintziori G, Kita M, Duntas L, Goulis DG. Consequences of hyperthyroidism in male and female fertility: pathophysiology and current management. J Endocrinol Invest. (2016) 39:849–53. doi: 10.1007/s40618-016-0452-6 [DOI] [PubMed] [Google Scholar]
- 18. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. (2016) 26:1343–421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 19. Maraka S, Ospina NM, O’Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, et al. Subclinical hypothyroidism in pregnancy: A systematic review and meta-analysis. Thyroid. (2016) 26:580–90. doi: 10.1089/thy.2015.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aghajanova L, Lindeberg M, Carlsson IB, Stavreus-Evers A, Zhang P, Scott JE, et al. Receptors for thyroid-stimulating hormone and thyroid hormones in human ovarian tissue. Reprod BioMed. (2009) 18:337–47. doi: 10.1016/s1472-6483(10)60091-0 [DOI] [PubMed] [Google Scholar]
- 21. Michalakis KG, Mesen TB, Brayboy LM, Yu B, Richter KS, Levy M, et al. Subclinical elevations of thyroid-stimulating hormone and assisted reproductive technology outcomes. Fertil Steril. (2011) 95:2634–7. doi: 10.1016/j.fertnstert.2011.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polyzos NP, Sakkas E, Vaiarelli A, Poppe K, Camus M, Tournaye H. Thyroid autoimmunity, hypothyroidism and ovarian reserve: a cross-sectional study of 5000 women based on age-specific AMH values. Hum Reprod. (2015) 30:1690–6. doi: 10.1093/humrep/dev089 [DOI] [PubMed] [Google Scholar]
- 23. Kucukler FK, Gorkem U, Simsek Y, Kocabas R, Guler S. Evaluation of ovarian reserve in women with overt or subclinical hypothyroidism. Arch Med Sci. (2018) 14:521–6. doi: 10.5114/aoms.2016.58621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang Z, Xu Z, Liu J. Mendelian randomization study of thyroid function and anti-Mullerian hormone levels. Front Endocrinol (Lausanne). (2023) 14:1188284. doi: 10.3389/fendo.2023.1188284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: A review. JAMA. (2019) 322:153–60. doi: 10.1001/jama.2019.9052 [DOI] [PubMed] [Google Scholar]
- 26. Oppo A, Franceschi E, Atzeni F, Taberlet A, Mariotti S. Effects of hyperthyroidism, hypothyroidism, and thyroid autoimmunity on female sexual function. J Endocrinol Invest. (2011) 34:449–53. doi: 10.1007/BF03346712 [DOI] [PubMed] [Google Scholar]
- 27. Concepcion-Zavaleta MJ, Coronado-Arroyo JC, Quiroz-Aldave JE, Concepcion-Urteaga LA, Paz-Ibarra J. Thyroid dysfunction and female infertility. A comprehensive review. Diabetes Metab Syndr. (2023) 17:102876. doi: 10.1016/j.dsx.2023.102876 [DOI] [PubMed] [Google Scholar]
- 28. Dosiou C. Thyroid and fertility: recent advances. Thyroid. (2020) 30:479–86. doi: 10.1089/thy.2019.0382 [DOI] [PubMed] [Google Scholar]
- 29. Morales-Martinez FA, Sordia-Hernandez LH, Ruiz MM, Garcia-Luna S, Valdes-Martinez OH, Vidal-Gutierez O. Association between thyroid autoimmunity and ovarian reserve in women with hypothyroidism. Thyroid Res. (2021) 14:6. doi: 10.1186/s13044-021-00095-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. (2020) 111:1468–77. doi: 10.1111/cas.14363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu J, Guo M, Hu X, Weng X, Tian Y, Xu K, et al. Effects of thyroid dysfunction on reproductive hormones in female rats. Chin J Physiol. (2018) 61:152–62. doi: 10.4077/CJP.2018.BAG551 [DOI] [PubMed] [Google Scholar]
- 32. Rao M, Wang H, Zhao S, Liu J, Wen Y, Wu Z, et al. Subclinical hypothyroidism is associated with lower ovarian reserve in women aged 35 years or older. Thyroid. (2020) 30:95–105. doi: 10.1089/thy.2019.0031 [DOI] [PubMed] [Google Scholar]
- 33. Weghofer A, Himaya E, Kushnir VA, Barad DH, Gleicher N. The impact of thyroid function and thyroid autoimmunity on embryo quality in women with low functional ovarian reserve: a case-control study. Reprod Biol Endocrinol. (2015) 13:43. doi: 10.1186/s12958-015-0041-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weghofer A, Barad DH, Darmon S, Kushnir VA, Gleicher N. What affects functional ovarian reserve, thyroid function or thyroid autoimmunity? Reprod Biol Endocrinol. (2016) 14:26. doi: 10.1186/s12958-016-0162-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hiraoka T, Wada-Hiraike O, Hirota Y, Hirata T, Koga K, Osuga Y, et al. The impact of elevated thyroid stimulating hormone on female subfertility. Reprod Med Biol. (2016) 15:121–6. doi: 10.1007/s12522-015-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bucci I, Giuliani C, Di Dalmazi G, Formoso G, Napolitano G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front Endocrinol (Lausanne). (2022) 13:768363. doi: 10.3389/fendo.2022.768363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update. (2015) 21:378–87. doi: 10.1093/humupd/dmv004 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y, Wu Y, Tian M, Luo W, Zhang C, Liu Y, et al. Protein expression profile in IVF follicular fluid and pregnancy outcome analysis in euthyroid women with thyroid autoimmunity. ACS Omega. (2020) 5:11439–47. doi: 10.1021/acsomega.0c00463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ke H, Hu J, Zhao L, Ding L, Jiao X, Qin Y. Impact of thyroid autoimmunity on ovarian reserve, pregnancy outcomes, and offspring health in euthyroid women following in vitro fertilization/intracytoplasmic sperm injection. Thyroid. (2020) 30:588–97. doi: 10.1089/thy.2018.0657 [DOI] [PubMed] [Google Scholar]
- 40. Magri F, Schena L, Capelli V, Gaiti M, Zerbini F, Brambilla E, et al. Anti-Mullerian hormone as a predictor of ovarian reserve in ART protocols: the hidden role of thyroid autoimmunity. Reprod Biol Endocrinol. (2015) 13:106. doi: 10.1186/s12958-015-0103-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hsieh YT, Ho JYP. Thyroid autoimmunity is associated with higher risk of premature ovarian insufficiency-a nationwide Health Insurance Research Database study. Hum Reprod. (2021) 36:1621–9. doi: 10.1093/humrep/deab025 [DOI] [PubMed] [Google Scholar]
- 42. Unuane D, Velkeniers B, Bravenboer B, Drakopoulos P, Tournaye H, Parra J, et al. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod. (2017) 32:915–22. doi: 10.1093/humrep/dex033 [DOI] [PubMed] [Google Scholar]
- 43. Hasegawa Y, Kitahara Y, Osuka S, Tsukui Y, Kobayashi M, Iwase A. Effect of hypothyroidism and thyroid autoimmunity on the ovarian reserve: A systematic review and meta-analysis. Reprod Med Biol. (2022) 21:e12427. doi: 10.1002/rmb2.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bahri S, Tehrani FR, Amouzgar A, Rahmati M, Tohidi M, Vasheghani M, et al. Overtime trend of thyroid hormones and thyroid autoimmunity and ovarian reserve: a longitudinal population study with a 12-year follow up. BMC Endocr Disord. (2019) 19:47. doi: 10.1186/s12902-019-0370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuroda K, Uchida T, Nagai S, Ozaki R, Yamaguchi T, Sato Y, et al. Elevated serum thyroid-stimulating hormone is associated with decreased anti-Mullerian hormone in infertile women of reproductive age. J Assist Reprod Genet. (2015) 32:243–7. doi: 10.1007/s10815-014-0397-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halici M, Seker ME, Gebedek IY, Gokbak MN, Cetisli AF, Ciftci AB, et al. Thyroid hormones and ovarian reserve: a comprehensive study of women seeking infertility care. BMC Womens Health. (2023) 23:570. doi: 10.1186/s12905-023-02725-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.