Abstract
It has long been hypothesized that morphological and numerical alterations in dendritic spines underlie long-term structural encoding of experiences. Here we investigate the efficacy of aversive experience in the form of acute immobilization stress (AIS) and chronic immobilization stress (CIS) in modulating spine density in the basolateral amygdala (BLA) of male rats. We find that CIS elicits a robust increase in spine density across primary and secondary branches of BLA spiny neurons. We observed this CIS-induced spinogenesis in the BLA 1 d after the termination of CIS. In contrast, AIS fails to affect spine density or dendritic arborization when measured 1 d later. Strikingly, the same AIS causes a gradual increase in spine density 10 d later but without any effect on dendritic arbors. Thus, by modulating the duration of immobilization stress, it is possible to induce the formation of new spines without remodeling dendrites. However, unlike CIS-induced spine formation, the gradual increase in spine density 10 d after a single exposure to AIS is localized on primary dendrites. Finally, this delayed induction of BLA spinogenesis is paralleled by a gradual development of anxiety-like behavior on the elevated plus-maze 10 d after AIS. These findings demonstrate that stressful experiences can lead to the formation of new dendritic spines in the BLA, which is believed to be a locus of storage for fear memories. Our results also suggest that stress may facilitate symptoms of chronic anxiety disorders like post-traumatic stress disorder by enhancing synaptic connectivity in the BLA.
Keywords: anxiety, dendritic remodeling, immobilization, synapse, rats
The search for cellular substrates underlying experience-related plasticity has focused on dendritic spines ever since Ramón y Cajal (1, 2) proposed that the storage of long-term memory involves strengthening of synaptic connections (or even a building of new connections) among central neurons. More recently, the focus has shifted toward understanding the physiological and molecular basis of synaptic plasticity mechanisms, such as long-term potentiation (LTP), and their relationship to spine plasticity and ultimately behavioral memory (3, 4). A majority of these studies have examined the hippocampus. Although the hippocampus is required for the acquisition and temporary storage of declarative memory, studies with human subjects and animal models suggest that more permanent morphological correlates of long-term memory storage are unlikely to reside in the hippocampus (5–7). In this context, the amygdala, for which the neural circuit underlying emotional memory formation is well characterized (8), provides a significant advantage. The basolateral amygdala (BLA) is believed to be a site of storage for memories of fearful or stressful experiences (9–12). Furthermore, recent studies indicate that the synthesis of new proteins in the BLA is involved in the long-term consolidation of emotional memories (13). Thus, the BLA presents an attractive locus to investigate structural encoding of aversive experiences.
Recent reports using rodent models of fear and stress are beginning to identify putative cellular and molecular determinants of structural plasticity in the amygdala (14). For example, there is evidence that the Rho GTPase pathway (15) and BDNF (brain-derived neurotrophic factor) signaling (16), both important regulators of neuronal structure, are involved in fear memory formation and consolidation in the lateral amygdala. Tissue plasminogen activator, which plays a key role in spine plasticity during visual cortical development (17), is also a critical component in the sequence of molecular events linking repeated restraint stress-induced neuronal remodeling in the amygdala with the development of anxiety-like behavior (18). These recent findings, taken together with earlier reports on the role of NMDA receptors in the BLA in fear (19, 20) and anxiety (21), all provide plausible mechanisms that can lead to spine formation as a result of aversive experience. However, there is no direct morphological evidence for experience-induced spine formation in the BLA. In the present study we hypothesize that because animal models of chronic stress potentiate both fear (22, 23) and anxiety (24), they are likely to serve as a useful tool for amplifying molecular mechanisms underlying amygdalar spinogenesis, thereby eliciting robust and detectable changes in spines in the BLA. Hence, here we investigate whether aversive experience, in the form of chronic immobilization stress, leads to numerical alterations in dendritic spines in the rat BLA.
Materials and Methods
Experimental Animals. Male Wistar rats were used for chronic immobilization stress (CIS) and acute immobilization stress (AIS) protocols. At the beginning of the experiments, CIS animals were between 3 and 3.5 mo of age, whereas the age of AIS and chronic unpredictable stress (CUS) animals at the onset of experiments was 2.5–3 mo. All animals (National Centre for Biological Sciences, Bangalore, India) were housed in groups of three with access to food and water ad libitum, unless specified otherwise in stress protocols. Control animals, which were littermates of the stress-treated animals, were housed in separate cages. Animals were maintained in a temperature-controlled room, with a light/dark cycle of 12 h (lights on at 7:00 a.m.). All procedures related to animal maintenance and experimentation were approved by the Institutional Animal Ethics Committee (National Centre for Biological Sciences).
Stress Protocols. Rats, randomly assigned to experimental groups, were subjected to CIS, AIS, or CUS. CIS consisted of complete immobilization (2 h/d, 10 a.m.–noon) in rodent immobilization bags without access to either food or water, for 10 consecutive d (25, 26). AIS consisted of a single immobilization session of 2 h, after which either 1 d (AIS-1) or 10 d (AIS-10) were allowed to lapse before behavioral or morphological analysis was conducted. CUS, as described earlier, involved exposing rats to several types of stressors, which varied from day to day, for a period of 10 d (26, 27). Control animals were not subjected to any type of stress.
Elevated Plus-Maze. The elevated plus-maze, consisting of two opposite open arms (60 × 15 cm) and two enclosed arms (60 × 15 cm, surrounded by a 15-cm-high opaque wall), was elevated 75 cm from the ground. The animals were tested on the maze 24 h after the termination of the stress paradigm. Individual trials lasted for 5 min each and were videotaped for subsequent off-line analysis. At the beginning of each trial, animals were placed at the center of the maze, facing an enclosed arm. All trials were conducted between 10 a.m. and 2 p.m., and the maze was cleaned with 5% (vol/vol) ethanol solution after each trial.
Tissue Preparation. After completion of stress protocols, animals were killed under deep anesthesia. The brain was removed quickly, and blocks of tissue containing the amygdala were dissected and processed for rapid Golgi staining as described (26, 28). Coronal sections (120 μm thick) were prepared as described (26). Slides were coded before quantitative analysis, and the code was broken only after the analysis was completed.
Analysis of Dendritic Arborization. To be selected for analysis, Golgi-impregnated neurons (Fig. 1A) had to satisfy the following criteria that have been applied in similar morphometric studies (26, 29–31): (i) presence of untruncated dendrites, (ii) consistent and dark impregnation along the entire extent of all dendrites, and (iii) relative isolation from neighboring impregnated neurons to avoid interfering with analysis. Both spiny pyramidal-like and stellate neurons from the BLA were selected for analysis on the basis of morphological criteria described in the literature (26, 32, 33). As described (26), our analysis of BLA neurons was restricted to those located between bregma -2.0 mm and -3.2 mm. To analyze effects of AIS on dendritic length and the number of branch points, we carried out morphometry in six neurons per animal in each experimental group (control, AIS-1, and AIS-10). To this end, 3D reconstructions of the selected neurons were accomplished by using the NeuroLucida image analysis system (MicroBrightField, Wiliston, VT) attached to an Olympus BX61 microscope (40×, 0.75 numerical aperture).
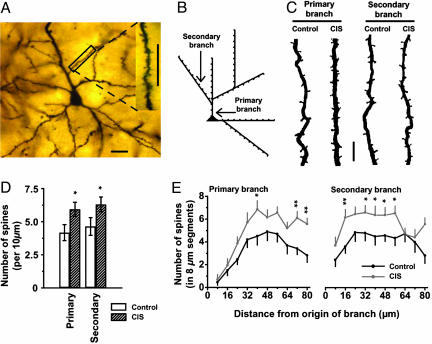
Fig. 1.
Effects of CIS on spiny BLA neurons. (A) Low-power photomicrograph of a Golgi stain-impregnated pyramidal neuron in the BLA. (Scale bar, 20 μm.) (Inset) High-power image of spines on a secondary dendrite from the same neuron. (Scale bar, 20 μm.) (B) Schematic drawing classifying types of apical dendrites selected for spine density analysis. In our analysis, a dendritic branch emanating directly from the cell soma (▴) was defined as a primary branch, whereas a dendrite originating from a primary branch was defined as a secondary branch. Spines were counted, starting from the origin of a branch, in 10 consecutive segments of 8 μm each (small tick marks). (C) Photomicrographs of representative segments of primary (Left) and secondary (Right) branches from control and CIS neurons, demonstrating an increase in the number of spines. (Scale bar, 5 μm.) (D) Mean (±SEM) values for spine-density (calculated as the average number of spines per 10 μm) of primary (Left) and secondary (Right) branches of spiny BLA neurons from CIS and control groups. *, P < 0.05, compared with control, Student's t test; control, n = 20 neurons; CIS, n = 20 neurons. (E) Segmental analysis of the mean (±SEM) number of spines in each successive 8-μm segment along primary (Left) and secondary (Right) branches as a function of the distance of that segment from the origin of the branch. *, P < 0.05; **, P < 0.01, compared with control, Student's t test.
Analysis of Dendritic Spine Density. By using the same NeuroLucida system (100×, 1.3 numerical aperture, Olympus BX61), all protrusions, irrespective of their morphological characteristics, were counted as spines if they were in direct continuity with the dendritic shaft (Fig. 1 A Inset and C). For the purpose of this study, dendrites directly originating from cell soma were classified as primary dendrites, and those originating from primary dendrites were classified as secondary dendrites (Fig. 1B). Moreover, we always selected the first branch that emerged from the primary branch (Fig. 1B) and designated it as the secondary branch to be analyzed. Starting from the origin of the branch, and continuing away from the cell soma, spines were counted along an 80-μm stretch of the dendrite. This total length of 80 μm was further subjected to a detailed segmental analysis, which consisted of counting the number of spines in successive steps of 8 μm each, for a total of 10 steps. The values for number of spines from each 8-μm segment, at a given distance from the origin of the branch, were then averaged across all neurons in a particular experimental group. For spine density analysis in chronic stress experiments, five neurons per animal were used in each experimental group (control, CIS, and CUS). Finally, it may be noted that our analysis, like all those involving Golgi staining, is likely to lead to a systematic underestimation of spine density because it is not possible to visualize spines pointing directly toward the surface or extending beneath the dendrite (34–36). In the present study, however, no attempt was made to correct for these hidden spines, because of previously reported validation (37) of the use of visible spine counts for comparison between different experimental conditions.
Statistical Analysis. Values are reported as mean ± SEM [along with coefficient of variance (CV)], and percentage changes are calculated with respect to corresponding control values. In all cases, n refers to the number of neurons used for morphometry, and N refers to the number of rats used. Statistical significance for the effects of CIS and CUS on density of spines was calculated by using Student's t test. Effects of AIS-1 and AIS-10 on spine density were analyzed by ANOVA with stress group as between-subject factor and with the number of neurons as the n. Significant effects were further analyzed by least significant difference posthoc test. Additional analysis was done by using a more stringent nonparametric randomized Mann–Whitney test with number of animals as the N. In cases where the two analyses concurred, the more conservative estimate (i.e., the higher value) from the P values obtained from both the ANOVA and randomized Mann–Whitney tests are quoted. In cases where the two P values differed considerably, both values are quoted (for N = number of animals and n = number of neurons). Effects of AIS-1 and AIS-10 on anxiety-like behavior were analyzed by using the nonparametric Kruskal–Wallis H test. Parameters exhibiting significant effects in Kruskal–Wallis H test were further analyzed pairwise by using the nonparametric Mann–Whitney U test.
Results
CIS Causes an Extensive Increase in BLA Spine Density. We demonstrated (26) that CIS induces growth in dendritic arborization or hypertrophy of spiny neurons in the BLA. Therefore, in the present study we first examined the effects of CIS on spine density in BLA spiny neurons (Fig. 1 A). BLA neurons from CIS-treated animals exhibited significant (P < 0.05) increase in the number of spines per 10 μm (Fig. 1 B–D), as measured along an 80-μm segment of dendrite, for both primary (42% increase; control: 4.18 ± 0.60, CV = 0.64, n = 20 neurons, N = 4 animals, five neurons per animal; CIS: 5.94 ± 0.54, CV = 0.40, n = 20 neurons, N = 4 animals, five neurons per animal) and secondary branches (36% increase; control: 4.64 ± 0.66, CV = 0.63; CIS: 6.30 ± 0.56, CV = 0.40). These data on CIS-induced increase in spine density were gathered from a more detailed segmental analysis wherein the number of spines were counted in 10 consecutive steps of 8-μm segments, starting from the origin of the branch and radiating out from the soma for a total distance of 80 μm (Fig. 1 B and E). This detailed segmental analysis shows that, after an initial segment of ≈24 μm along the primary dendrite (Fig. 1E Left) where the number of spines is relatively low, there is a substantial increase (26–98%) in spine density in CIS neurons relative to their control counterparts. Moreover, this pattern of enhanced spine density carries over onto secondary branches as well (Fig. 1E Right). Thus, we conclude that exposure to CIS causes a significant increase in spine density spanning both primary and secondary branches of BLA neurons.
An earlier study demonstrated that, unlike CIS, another chronic stressor, CUS, was not capable of eliciting dendritic hypertrophy in BLA (26). Thus we examined whether the previously reported lack of CUS-induced effect on dendritic morphology is also accompanied by an absence of spine-related changes. Using the same framework described above for CIS, we observed no significant differences (P ≥ 0.3) in spine density along either primary (control: 3.96 ± 0.61, n = 20 neurons, N = 4 animals, five neurons per animal; CUS: 3.24 ± 0.33, n = 20 neurons, N = 4 animals, five neurons per animal) or secondary branches (control, 4.64 ± 0.68; CUS, 4.00 ± 0.31) after exposure to CUS.
AIS Elicits a Gradual and Spatially Restricted Increase in Spine Density. The results described thus far suggest a potential link between dendritic remodeling and spine density because whereas one stressor (CIS) elicits changes in both, the other (CUS) affects neither. But the nature of this putative link does not appear to be a simple inverse relationship: unlike hippocampal CA3 pyramidal neurons, where stress-induced changes in dendritic length and spine density appear to go in opposite directions (38, 39), both parameters increase in the BLA after CIS. This finding in turn raises the possibility that changes in spine density may not necessarily come about in response to modulation of dendritic length per se. Furthermore, the combined impact (more spines per unit length of dendrite multiplied by more dendrites) of CIS on the total number of spines in a typical BLA neuron would be considerable. Would it be possible to decouple dendritic remodeling from spinogenesis by eliciting more subtle changes in the BLA by titrating the intensity or duration of stress? To this end, we next explored the impact of a significantly milder form of stress by subjecting animals to a single 2-h episode of immobilization. Once again, we evaluated the effects of this AIS on BLA neuronal morphology (Fig. 2).
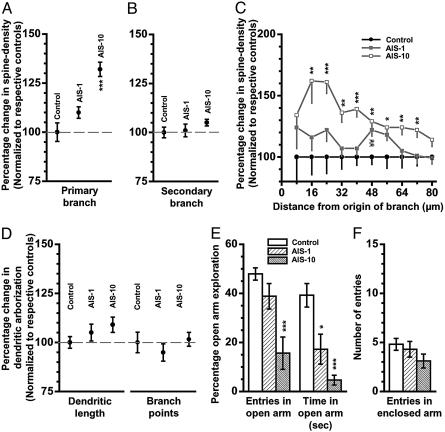
Fig. 2.
Effects of AIS on spine density, dendritic arborization, and anxiety-like behavior. For all graphs in A–D, summarizing changes in spine density or dendritic arbors, the ordinate depicts mean (±SEM) values for BLA spiny neurons from AIS-1 and AIS-10 animals, normalized to their respective control values (100%) for that parameter. (A) Percentage changes in spine density, relative to normalized control (100%), calculated from the total number of spines along an 80-μm segment of primary branch in spiny BLA neurons from AIS-1 and AIS-10 animals. Control, n = 18 neurons; AIS-1, n = 18 neurons; AIS-10, n = 30 neurons. One-way ANOVA; F(2, 63) = 19.2, P < 0.001 for differences between groups; least significant difference (LSD) posthoc test; ***, P < 0.001. (B) Percentage changes in spine density, relative to normalized control (100%), calculated from the total number of spines along an 80-μm segment of secondary branch in spiny BLA neurons from AIS-1 and AIS-10 animals. One-way ANOVA; F(2, 63) = 1.1, P > 0.3 for differences between groups. (C) Analysis of the percentage changes in spine density for each segment (mean ± SEM, normalized to respective control) for each successive 8-μm segment along primary branch as a function of the distance of that segment from the origin of the branch. Statistical significance was computed for each segment independently by using one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001 between control and AIS-10; LSD posthoc test. ¥¥, P < 0.01 between control and AIS-1. (D) Percentage changes, relative to normalized control (100%), in total dendritic length (Left) and number of branch points (Right) of BLA spiny neurons from control (n = 18 neurons), AIS-1 (n = 18 neurons), and AIS-10 (n = 30 neurons) groups. One-way ANOVA; total dendritic length, F(2, 63) = 2.7 and P > 0.2 for differences between groups; number of branch points, F(2, 63) = 0.6 and P > 0.5 for differences between groups. (E) Anxiety-like behavior on the elevated plus-maze for all three experimental groups (control, AIS, and AIS-10). Changes in open-arm exploration were measured as the percentage of open-arm entries (Left) and the percentage of time spent in open arm (Right). (F) No effect on locomotor activity as measured by entries in enclosed arm in the plus-maze. Control, N = 10 animals; AIS-1, N = 4 animals; AIS-10, N = 8 animals. Kruskal–Wallis test for differences between three independent samples; P < 0.01 for the percentage of open-arm entries, P < 0.001 for the percentage of open-arm time, and P > 0.2 for the number of entries in enclosed arms. Significant effects were further analyzed by Mann–Whitney test; *, P < 0.05; ***, P < 0.001; relative to control.
In these experiments, we first measured BLA spine density 1 d after exposure to AIS (AIS-1; Fig. 2 A–C). We observed no significant difference in the total number of spines per 10 μm, counted in an 80-μm segment of primary (control: 5.70 ± 0.28, CV = 0.20, n = 18 neurons, N = 3 animals, six neurons per animal; AIS-1: 6.29 ± 0.16, CV = 0.11, n = 18 neurons, N = 3 animals, six neurons per animal; P > 0.07; Fig. 2 A) or secondary branch (control: 8.24 ± 0.23, CV = 0.12; AIS-1: 8.30 ± 0.26, CV = 0.13; P > 0.1; Fig. 2B). In light of the slightly greater trend toward spine density increase on primary vs. secondary dendrites (Fig. 2 A and B), we examined effects of AIS-1 on density of spines along primary dendrites in greater detail by using the same segmental analysis as described earlier (10 incremental steps of 8 μm each, Fig. 2C). This analysis demonstrates that except for one segment, most of the other segments exhibited a small but statistically insignificant increase in spine density (AIS-1, Fig. 2C, ▪). In the same set of BLA neurons, we also analyzed dendritic morphology (AIS-1, Fig. 2D). AIS failed to trigger any dendritic remodeling, because we did not observe significant changes in total dendritic length (control: 1,501 ± 45 μm, CV = 0.13, n = 18 neurons, N = 3 animals, six neurons per animal; AIS-1: 1,576 ± 65 μm, CV = 0.17, n = 18 neurons, N = 3 animals, six neurons per animal; P > 0.1; Fig. 2D Left) and number of branch points (control: 14.1 ± 0.7, CV = 0.21; AIS-1: 13.4 ± 0.6, CV = 0.25; P > 0.1; Fig. 2D Right).
In striking contrast to measurements made 24 h after AIS, a significant increase in spine density was detected in primary dendrites 10 d after the same AIS (control: 5.70 ± 0.28, CV = 0.20, n = 18 neurons, N = 3 animals, six neurons per animal; AIS-10: 7.51 ± 0.20, CV = 0.15, n = 30 neurons, N = 5 animals, six neurons per animal; P < 0.001, using n = number of neurons, and P < 0.05, using N = number of animals; 32% increase, Fig. 2A). Moreover, at this later time point, based on the detailed segmental analysis along the length of primary dendrites, a significant increase in spine numbers was detected in almost all of the segments (AIS-10, Fig. 2C, □; 22–62% increase, statistically significant between distances of 16 and 72 μm from the origin of the branch). Interestingly, this striking increase in spine density was confined to the primary dendrites and did not extend to the secondary dendrites (control: 8.24 ± 0.23, CV = 0.12; AIS-10: 8.61 ± 0.14, CV = 0.09; P > 0.1; Fig. 2B). Finally, this AIS-induced up-regulation of spine density was also unique in that it was not accompanied by dendritic hypertrophy. In other words, although AIS caused a gradual enhancement in spine density along primary dendrites that was detected 10 d later, it did not elicit an increase in dendritic length (control: 1,501 ± 45 μm, CV = 0.13, n = 18 neurons, N = 3 animals, six neurons per animal; AIS-10: 1637 ± 58 μm, CV = 0.19, n = 30 neurons, N = 5 animals, six neurons per animal; P > 0.07; Fig. 2D Left) and branch points (control: 14.1 ± 0.7, CV = 0.21; AIS-10: 14.3 ± 0.5, CV = 0.19; P > 0.1; Fig. 2D Right) of the same BLA neurons. Therefore, we conclude that, with the use of AIS, it is possible to elicit spinogenesis without changes in dendritic arborization in BLA neurons. Moreover, this increase in spine density is not as widespread as that seen after CIS and it is evident only after a 10-d delay after exposure to the AIS.
AIS also Induces a Gradual Facilitation of Anxiety-Like Behavior. The above results suggest that although a single 2-h episode of immobilization stress (AIS) triggers morphological changes that become evident as a relatively restricted increase in spine density 10 d later, repeating the same stressor for 10 consecutive d (CIS) causes a more widespread increase in the number of spines. The 10-d CIS paradigm has earlier been reported to also cause a significant facilitation in anxiety along with enhanced dendritic arborization (24, 26, 29). Can AIS, which leads to spinogenesis without dendritic hypertrophy, also affect anxiety-like behavior? To address this issue, we next examined the effects of AIS on anxiety-like behavior in the elevated plus-maze at the same time points when morphological changes were quantified, i.e., 1 d and 10 d after the AIS (Fig. 2E, AIS-1 and AIS-10). We observed no significant reduction in the percentage entries into open arms 1 d after AIS (control: 47.9 ± 2.5%, N = 10 animals; AIS-1: 38.8 ± 5.2%, N = 4 animals, P > 0.1; Fig. 2E Left) and a more significant decrease in the mean time spent in open arms (control: 118 ± 14.4 s; AIS-1: 51.5 ± 18.2 s; P < 0.05; Fig. 2E Right). When open exploration was measured 10 d after AIS (AIS-10, Fig. 2E), we observed a significant and greater degree of reduction in both percentage open-arm entries (67% decrease relative to control; control: 47.8 ± 2.5%, N = 10 animals; AIS-10: 15.6 ± 6.6%, N = 8 animals, P < 0.001; Fig. 2E Left) and the mean time spent in open arms (88% decrease relative to control; control: 118 ± 14.4 s; AIS-10: 14 ± 6.1 s; P < 0.001; Fig. 2E Right). Furthermore, the reduction in open-arm exploration could not be explained by changes in locomotor activity because there was no significant difference in the number of entries in enclosed arm between the groups (control, 4.8 ± 0.6; AIS-1, 4.3 ± 0.8; AIS-10, 3.1 ± 0.7; P = 0.2; Fig. 2F). Thus, AIS appears to trigger a gradual potentiation of anxiety-like behavior that is manifested 10 d later as a significant and robust increase in open-arm avoidance in the elevated plus-maze.
Discussion
In this study, we have investigated the efficacy of aversive experience in modulating the number of dendritic spines in the BLA. To this end, we modified the duration of immobilization stress (acute or chronic) and measured its effects, with variable delays (after 1 or 10 d), on structural plasticity and anxiety-like behavior in rats. We find that 2 h of immobilization stress per day repeated for 10 consecutive d (CIS), which has previously been reported to cause enhanced anxiety (24, 31) and dendritic growth in the BLA (26, 29), elicits a robust increase in spine density across both primary and secondary branches of BLA spiny neurons. Also, this facilitating effect is detected 1 d after the end of the 10-d CIS protocol. In contrast, a single 2-h episode of the same immobilization stress (AIS) fails to elicit any increase in spine density or dendritic arborization when measured 1 d later. Strikingly, the same AIS leads to a gradual up-regulation in spine density 10 d later but without any effect on dendritic arbors. In other words, an appropriately titrated stressor can eventually lead to spinogenesis without remodeling dendrites. However, unlike CIS-induced spine formation, this delayed increase in spine density 10 d after AIS is restricted to dendritic regions closer to the soma. Finally, this delayed BLA spinogenesis is accompanied by enhanced anxiety-like behavior 10 d after exposure to the AIS. Thus, 10 d after the end of AIS, we also observed a gradual build-up of significant open-arm avoidance in the elevated plus-maze.
Experience-Dependent Plasticity of Spines. Long-term encoding of experience-related neural activity is thought to involve changes in the structure and number of spine synapses (40). Improved imaging and morphometric techniques have demonstrated that dendritic spines are highly dynamic structures (41–43). During development and even in adulthood, the size and number of spines can be modified by learning, sensory experience, and steroid hormones, as well as many other factors (39, 42, 44–51). For example, there is evidence for greater numbers of synapses per neuron in visual cortex of rats reared in complex environments, and complex motor skill learning leads to an increase in synapse number within the cerebellar cortex (46, 52). Moreover, studies using in vitro techniques have demonstrated that formation of new dendritic protrusions and spines can be triggered by LTP, a synaptic plasticity mechanism thought to underlie learning and memory (4, 53, 54). Such LTP-induced spinogenesis could be mediated by de novo spine formation (48) or by splitting of preexisting spines (4, 51, 55, 56).
Accumulating data, derived mostly from studies in the hippocampus, suggest that NMDA receptors play a critical role in LTP and activity-dependent spinogenesis (4, 56, 57). The existence of LTP in the BLA, and its role in fear memory, is also well established (10, 58–60). Moreover, although NMDA-dependent LTP has been reported in the amygdala, the induction of a commonly studied form of amygdaloid LTP requires voltagegated calcium channels (11, 59–62). Thus, further experiments are necessary to determine the possible involvement of NMDA-receptor and voltage-gated calcium channel-dependent mechanisms in the stress-induced BLA spinogenesis described here.
Cellular and Molecular Mechanisms of Structural Plasticity in the Amygdala. Although the specific molecular and biochemical underpinnings of stress-induced morphological plasticity in the amygdala are yet to be characterized fully, several lines of recent evidence provide valuable insights into potential mechanisms. Lamprecht et al. (15) have demonstrated a role for the Rho-regulatory protein p190 RhoGAP in the lateral amygdala that may contribute to structural changes in synaptic connectivity required for long-term storage of fear memory. This model is particularly interesting because the Rho pathway is known to play a critical role in the developmental regulation of dendritic morphology as well as synaptic connectivity (63–66). Furthermore, there is growing evidence suggesting that the small GTPases of the Rho family might occupy a pivotal role in translating both intra- and extracellular signals into modifications of the actin cytoskeleton (41), which in turn could induce morphological changes of the kind observed after stress.
A second line of evidence from the amygdala goes beyond the traditional focus on intracellular signaling mechanisms and suggests that stress, through tissue plasminogen activator-mediated extracellular proteolysis, leads to neuronal remodeling and the development of anxiety-like behavior (18). This model is also interesting in light of reports suggesting a role for tissue plasminogen activator in spine dynamics (17) and facilitation of hippocampal LTP (67), and possibly potentiation of NMDA receptor signaling (68). Indeed, repeated restraint stress has been shown to amplify NMDA currents at the CA3 commissural-associational synapses in the hippocampus (69). These synaptic inputs impinge on the same CA3 apical dendritic region, which has been shown to undergo significant atrophy after repeated stress (26). As mentioned earlier, this CA3 dendritic atrophy is also accompanied by an increase in spine density (39). Taken together, these data from the hippocampus suggest that new spines formed in response to stress may contain synapses with enhanced NMDA conductance, which in turn could enhance calcium influx and affect relevant downstream targets. Therefore, it will be particularly interesting to study stress-induced changes in transmission and activity-dependent plasticity at glutamatergic synapses in the BLA.
Functional Implications. Our results suggest that variations in the intensity of immobilization stress are capable of modulating the degree and nature of the BLA structural plasticity that follows. Because only one early and one late time points were examined in the present study, future investigations will be necessary to characterize the time course of cellular and behavioral changes triggered by stressors of varying intensity or duration. Our findings also raise the possibility that an acute episode of severe stress initiates plasticity mechanisms culminating in delayed and restricted spinogenesis, and this in itself may be sufficient to modulate anxiety-like behavior. But repeated exposure to the same stressor (CIS) pushes this intracellular machinery to scale up to a greater magnitude of spinogenesis along with enlargement of the dendritic tree. Furthermore, the fact that we observe higher spine density along dendrites that have previously been reported to undergo significant elongation (26, 31) implies that, on the average, CIS-treated BLA neurons end up with a considerably larger complement of spines as a whole. This increase, in turn, could lead to a significant strengthening in the overall synaptic connectivity of these BLA neurons. This would have a profound facilitating impact on the network output of the amygdala in terms of emotional behavior. Such a scenario is consistent with the large body of evidence showing that chronic stress enhances fear conditioning and anxiety-like behavior (21–23, 29, 70, 71).
We also observed that a brief but severe stressor (i.e., AIS) could trigger plasticity mechanisms, leading to a delayed increase in spine density that is paralleled by a gradual build-up of anxiety-like behavior. Although hippocampal volume loss has been the traditional focus of human studies on post-traumatic stress disorder (72, 73), our findings on the delayed manifestation of enhanced anxiety and BLA spinogenesis, triggered by a single temporally restricted episode of stress, may provide a new framework for studying cellular mechanisms of post-traumatic stress disorder in the amygdala. The delayed build-up of spines and anxiety after exposure to acute stress also highlights the unique temporal characteristics of stress-induced structural plasticity. Another recent report (31) using the same 10-d chronic immobilization stress used in the present study showed that amygdalar hypertrophy and anxiety endure for a number of weeks after termination of the stressor (31). Thus the amygdala appears to have special features, especially with respect its temporal manifestation and persistence, that fit well with the delayed and prolonged effects on fear and anxiety observed in human patients suffering from chronic anxiety disorders such as post-traumatic stress disorder (74).
The facilitating effects of stress on emotional behavior suggest that they may, at least in part, share common cellular and molecular substrates in the amygdala. In light of our findings on stress-induced increase in BLA spine density, it is interesting to note that one recent study reports an increase in the number of spinophilin-immunoreactive dendritic spines in the lateral amygdala of animals that underwent fear conditioning.¶ Importantly, the number of spinophilin puncta was significantly greater in animals that received paired presentations of conditioned and unconditioned stimuli, relative to those receiving random presentations of conditioned and unconditioned stimuli. As the study by Johnson et al. suggests, formation of new spines in the lateral amygdala, as a result of a precise pairing of conditioned and unconditioned stimuli, is likely to be more specific compared with cellular changes mediating a nonspecific enhancement in amygdaloid output, such as that embodied by a generalized increase in anxiety following chronic stress.¶ Another recent study found that BDNF mRNA is elevated transiently in the BLA 2 h after cued fear conditioning (16). This temporally restricted elevation of BDNF mRNA level and BDNF signaling in the BLA observed after the formation of a cue-specific fear is likely to elicit spatially localized modifications in spines. This result is in contrast to the more widespread CIS-induced structural remodeling in BLA, which is accompanied by cue-nonspecific and generalized fear, or anxiety. In this respect, the more subtle effects of AIS on localized increase in spine density and its delayed manifestation over time are intriguing and warrant more detailed examination of the potential interplay between fear-conditioning and temporally restricted forms of acute stress.∥
In summary, our data on the enhanced amygdalar synaptic connectivity caused by aversive experience suggest that, in addition to its regulatory influence on the stress response, the amygdala itself could be profoundly influenced by stress. Therefore, elucidation of molecular signaling mechanisms, and the physiological consequences, of stress-induced spinogenesis in the amygdala may well hold the key to a more comprehensive understanding of how severe stress leads to affective disorders.
Acknowledgments
We thank B. S. S. Rao for helpful advice. This work was supported by The Wellcome Trust.
Author contributions: R.M., B.S.M., A.V., and S.C. designed research; R.M., S.J., and A.V. performed research; R.M., S.J., A.V., and S.C. analyzed data; and R.M., A.V., and S.C. wrote the paper.
Abbreviations: AIS, acute immobilization stress; BLA, basolateral amygdala; CIS, chronic immobilization stress; CUS, chronic unpredictable stress; LTP, long-term potentiation; CV, coefficient of variance.
Footnotes
Johnson, L. R., Radley, J. J., Martino, J., Lamprecht, R., Hof, P. R., LeDoux, J. E. & Morrison, J. H. (2003) Abstract Viewer/Itinerary Planner (Soc. Neurosci., Washington, DC), Program no. 623.7.
Bush, D. E. A., McEwen, B. S. & LeDoux, J. E. (2004) Abstract Viewer/Itinerary Planner (Soc. Neurosci., Washington, DC), Program no. 208.4.
References
- 1.Ramón y Cajal, S. (1904) La Textura del Sistema Nerviosa del Hombre y los Vertebrados (Moya, Madrid).
- 2.Ramón y Cajal, S. (1893) Arch. Anat. Entwick., 319-428.
- 3.Martin, S. J., Grimwood, P. D. & Morris, R. G. (2000) Annu. Rev. Neurosci. 23, 649-711. [DOI] [PubMed] [Google Scholar]
- 4.Yuste, R. & Bonhoeffer, T. (2001) Annu. Rev. Neurosci. 24, 1071-1089. [DOI] [PubMed] [Google Scholar]
- 5.Scoville, W. B. & Milner, B. (2000) J. Neuropsychiatry Clin. Neurosci. 12, 103-113. [DOI] [PubMed] [Google Scholar]
- 6.Rempel-Clower, N. L., Zola, S. M., Squire, L. R. & Amaral, D. G. (1996) J. Neurosci. 16, 5233-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zola-Morgan, S. M. & Squire, L. R. (1990) Science 250, 288-290. [DOI] [PubMed] [Google Scholar]
- 8.LeDoux, J. E. (2000) Annu. Rev. Neurosci. 23, 155-184. [DOI] [PubMed] [Google Scholar]
- 9.LeDoux, J. E. (1993) Behav. Brain Res. 58, 69-79. [DOI] [PubMed] [Google Scholar]
- 10.Rogan, M. T., Staubli, U. V. & LeDoux, J. E. (1997) Nature 390, 604-607. [DOI] [PubMed] [Google Scholar]
- 11.Blair, H. T., Schafe, G. E., Bauer, E. P., Rodrigues, S. M. & LeDoux, J. E. (2001) Learn. Mem. 8, 229-242. [DOI] [PubMed] [Google Scholar]
- 12.Schafe, G. E., Nader, K., Blair, H. T. & LeDoux, J. E. (2001) Trends Neurosci. 24, 540-546. [DOI] [PubMed] [Google Scholar]
- 13.Schafe, G. E. & LeDoux, J. E. (2000) J. Neurosci. 20, RC96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamprecht, R. & LeDoux, J. (2004) Nat. Rev. Neurosci. 5, 45-54. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht, R., Farb, C. R. & LeDoux, J. E. (2002) Neuron 36, 727-738. [DOI] [PubMed] [Google Scholar]
- 16.Rattiner, L. M., Davis, M., French, C. T. & Ressler, K. J. (2004) J. Neurosci. 24, 4796-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berardi, N., Pizzorusso, T. & Maffei, L. (2004) Neuron 44, 905-908. [DOI] [PubMed] [Google Scholar]
- 18.Pawlak, R., Magarinos, A. M., Melchor, J., McEwen, B. & Strickland, S. (2003) Nat. Neurosci. 6, 168-174. [DOI] [PubMed] [Google Scholar]
- 19.Maren, S., Aharonov, G., Stote, D. L. & Fanselow, M. S. (1996) Behav. Neurosci. 110, 1365-1374. [DOI] [PubMed] [Google Scholar]
- 20.Goosens, K. A. & Maren, S. (2004) Eur. J. Neurosci. 20, 537-548. [DOI] [PubMed] [Google Scholar]
- 21.Adamec, R. E., Burton, P., Shallow, T. & Budgell, J. (1999) Physiol. Behav. 65, 723-737. [DOI] [PubMed] [Google Scholar]
- 22.Conrad, C. D., LeDoux, J. E., Magarinos, A. M. & McEwen, B. S. (1999) Behav. Neurosci. 113, 902-913. [DOI] [PubMed] [Google Scholar]
- 23.Corodimas, K. P., LeDoux, J. E., Gold, P. W. & Schulkin, J. (1994) Ann. N.Y. Acad. Sci. 746, 392-393. [DOI] [PubMed] [Google Scholar]
- 24.Vyas, A. & Chattarji, S. (2004) Behav. Neurosci. 118, 1450-1454. [DOI] [PubMed] [Google Scholar]
- 25.Nibuya, M., Takahashi, M., Russell, D. S. & Duman, R. S. (1999) Neurosci. Lett. 267, 81-84. [DOI] [PubMed] [Google Scholar]
- 26.Vyas, A., Mitra, R., Shankaranarayana Rao, B. S. & Chattarji, S. (2002) J. Neurosci. 22, 6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortiz, J., Fitzgerald, L. W., Lane, S., Terwilliger, R. & Nestler, E. J. (1996) Neuropsychopharmacology 14, 443-452. [DOI] [PubMed] [Google Scholar]
- 28.Shankaranarayana Rao, B. S., Govindaiah, Laxmi, T. R., Meti, B. L. & Raju, T. R. (2001) Neuroscience 102, 319-327. [DOI] [PubMed] [Google Scholar]
- 29.Vyas, A., Mitra, R. & Chattarji, S. (2003) Ann. N.Y. Acad. Sci. 985, 554-555. [Google Scholar]
- 30.Vyas, A., Bernal, S. & Chattarji, S. (2003) Brain Res. 965, 290-294. [DOI] [PubMed] [Google Scholar]
- 31.Vyas, A., Pillai, A. G. & Chattarji, S. (2004) Neuroscience 128, 667-673. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, A. J. (1992) in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, ed. Aggleton, J. P. (Wiley–Liss, New York), pp. 67-96.
- 33.McDonald, A. J. (1982) J. Comp. Neurol. 212, 293-312. [DOI] [PubMed] [Google Scholar]
- 34.Trommald, M., Jensen, V. & Andersen, P. (1995) J. Comp. Neurol. 353, 260-274. [DOI] [PubMed] [Google Scholar]
- 35.Feldman, M. L. & Peters, A. (1979) J. Comp. Neurol. 188, 527-542. [DOI] [PubMed] [Google Scholar]
- 36.Trommald, M. & Hulleberg, G. (1997) J. Comp. Neurol. 377, 15-28. [DOI] [PubMed] [Google Scholar]
- 37.Horner, C. H. & Arbuthnott, E. (1991) J. Anat. 177, 179-184. [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe, Y., Gould, E. & McEwen, B. S. (1992) Brain Res. 588, 341-345. [DOI] [PubMed] [Google Scholar]
- 39.Sunanda, Rao, M. S. & Raju, T. R. (1995) Brain Res. 694, 312-317. [DOI] [PubMed] [Google Scholar]
- 40.Bailey, C. H. & Kandel, E. R. (1993) Annu. Rev. Physiol. 55, 397-426. [DOI] [PubMed] [Google Scholar]
- 41.Bonhoeffer, T. & Yuste, R. (2002) Neuron 35, 1019-1027. [DOI] [PubMed] [Google Scholar]
- 42.Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G., Sanes, J. R., Welker, E. & Svoboda, K. (2002) Nature 420, 788-794. [DOI] [PubMed] [Google Scholar]
- 43.Fischer, M., Kaech, S., Knutti, D. & Matus, A. (1998) Neuron 20, 847-854. [DOI] [PubMed] [Google Scholar]
- 44.Woolley, C. S. & McEwen, B. S. (1994) J. Neurosci. 14, 7680-7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Federmeier, K. D., Kleim, J. A. & Greenough, W. T. (2002) Neurosci. Lett. 332, 180-184. [DOI] [PubMed] [Google Scholar]
- 46.Jones, T. A., Klintsova, A. Y., Kilman, V. L., Sirevaag, A. M. & Greenough, W. T. (1997) Neurobiol. Learn. Mem. 68, 13-20. [DOI] [PubMed] [Google Scholar]
- 47.Harris, K. M. (1999) Curr. Opin. Neurobiol. 9, 343-348. [DOI] [PubMed] [Google Scholar]
- 48.Harris, K. M., Fiala, J. C. & Ostroff, L. (2003) Philos. Trans. R. Soc. London B 358, 745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leuner, B., Falduto, J. & Shors, T. J. (2003) J. Neurosci. 23, 659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klintsova, A. Y. & Greenough, W. T. (1999) Curr. Opin. Neurobiol. 9, 203-208. [DOI] [PubMed] [Google Scholar]
- 51.Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. (1999) Nature 402, 421-425. [DOI] [PubMed] [Google Scholar]
- 52.Kleim, J. A., Swain, R. A., Armstrong, K. A., Napper, R. M., Jones, T. A. & Greenough, W. T. (1998) Neurobiol. Learn. Mem. 69, 274-289. [DOI] [PubMed] [Google Scholar]
- 53.Maletic-Savatic, M., Malinow, R. & Svoboda, K. (1999) Science 283, 1923-1927. [DOI] [PubMed] [Google Scholar]
- 54.Engert, F. & Bonhoeffer, T. (1999) Nature 399, 66-70. [DOI] [PubMed] [Google Scholar]
- 55.Toni, N., Buchs, P. A., Nikonenko, I., Povilaitite, P., Parisi, L. & Muller, D. (2001) J. Neurosci. 21, 6245-6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller, D., Toni, N. & Buchs, P. A. (2000) Hippocampus 10, 596-604. [DOI] [PubMed] [Google Scholar]
- 57.Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. (2000) Nat. Neurosci. 3, 887-894. [DOI] [PubMed] [Google Scholar]
- 58.McKernan, M. G. & Shinnick-Gallagher, P. (1997) Nature 390, 607-611. [DOI] [PubMed] [Google Scholar]
- 59.Chapman, P. F., Kairiss, E. W., Keenan, C. L. & Brown, T. H. (1990) Synapse 6, 271-278. [DOI] [PubMed] [Google Scholar]
- 60.Chapman, P. F. & Bellavance, L. L. (1992) Synapse 11, 310-318. [DOI] [PubMed] [Google Scholar]
- 61.Bauer, E. P., Schafe, G. E. & LeDoux, J. E. (2002) J. Neurosci. 22, 5239-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weisskopf, M. G., Bauer, E. P. & LeDoux, J. E. (1999) J. Neurosci. 19, 10512-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Threadgill, R., Bobb, K. & Ghosh, A. (1997) Neuron 19, 625-634. [DOI] [PubMed] [Google Scholar]
- 64.Brouns, M. R., Matheson, S. F., Hu, K. Q., Delalle, I., Caviness, V. S., Silver, J., Bronson, R. T. & Settleman, J. (2000) Development (Cambridge, U.K.) 127, 4891-4903. [DOI] [PubMed] [Google Scholar]
- 65.Brouns, M. R., Matheson, S. F. & Settleman, J. (2001) Nat. Cell Biol. 3, 361-367. [DOI] [PubMed] [Google Scholar]
- 66.Billuart, P., Winter, C. G., Maresh, A., Zhao, X. & Luo, L. (2001) Cell 107, 195-207. [DOI] [PubMed] [Google Scholar]
- 67.Madani, R., Hulo, S., Toni, N., Madani, H., Steimer, T., Muller, D. & Vassalli, J. D. (1999) EMBO J. 18, 3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicole, O., Docagne, F., Ali, C., Margaill, I., Carmeliet, P., MacKenzie, E. T., Vivien, D. & Buisson, A. (2001) Nat. Med. 7, 59-64. [DOI] [PubMed] [Google Scholar]
- 69.Kole, M. H., Swan, L. & Fuchs, E. (2002) Eur. J. Neurosci. 16, 807-816. [DOI] [PubMed] [Google Scholar]
- 70.LeDoux, J. E. (1994) Semin. Neurosci. 6, 231-237. [Google Scholar]
- 71.McGaugh, J. L. & Roozendaal, B. (2002) Curr. Opin. Neurobiol. 12, 205-210. [DOI] [PubMed] [Google Scholar]
- 72.Shin, L. M., Shin, P. S., Heckers, S., Krangel, T. S., Macklin, M. L., Orr, S. P., Lasko, N., Segal, E., Makris, N., Richert, K., et al. (2004) Hippocampus 14, 292-300. [DOI] [PubMed] [Google Scholar]
- 73.Bremner, J. D., Randall, P., Vermetten, E., Staib, L., Bronen, R. A., Mazure, C., Capelli, S., McCarthy, G., Innis, R. B. & Charney, D. S. (1997) Biol. Psychiatry 41, 23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yehuda, R. (2002) N. Engl. J. Med. 346, 108-114. [DOI] [PubMed] [Google Scholar]