Abstract
Clostridioides difficile (C. difficile) was a gram-positive anaerobic bacterium in the gut, exhibiting clinical manifestations ranging from mild diarrhoea to fatal pseudomembranous colitis. C. difficile infection (CDI) remains a serious public health problem and accounted for an estimated 360,075 cases in the United States in 2021. It has attracted the utmost attention of the world health organization (WHO). Since publication of a review of the diagnosis of CDI in adults, new clinical diagnostic assays have become available and clinical practice guidelines were updated. This paper presents a comprehensive review of contemporary laboratory diagnostic approaches for CDI in adult patients, with a focus on the utilisation and potential advancements of five sophisticated methodologies, CRISPR in conjunction with nucleic acid amplification tests (NAATs), gene sequencing technology, ultra-high performance liquid chromatography-mass spectrometry, Raman spectroscopy, and real-time cell analysis (RTCA). It can provide new perspectives and ideas for the early diagnosis of CDI in clinical settings.
Keywords: Clostridioides difficile infection, adults, diagnostic assays, CRISPR, nucleic acid amplification tests, gene sequencing technology
1. Introduction
C. difficile was a spore-producing anaerobic bacterium that has become a major pathogen causing hospital-acquired infections (HAIs) and community-acquired infections (CAIs) worldwide, especially in North America ( Figure 1 ) (Feuerstadt et al., 2023; Okafor et al., 2023). Once antibiotic treatment disrupted the normal intestinal flora, C. difficile colonised the colon and induced diarrhoea and pseudomembranous colitis (McDonald and Lee, 2015; Theriot and Young, 2015). The global burden of CDI was exacerbated by the emergence of highly virulent and antibiotic-resistant strains (Åkerlund et al., 2008; Kuijper et al., 2006; Moore et al., 2013). A report from the Centers for Disease Control and Prevention (CDC) 2021 emphasised the persistent risk of CDI infection to patient health (National Center for Emerging and Zoonotic Infectious Diseases (U.S.). Division of Healthcare Quality Promotion. 2023). Women and Caucasians were at higher risk of developing CDI infection, which increased with age (Guh et al., 2020). To accommodate evolving clinical requirements and epidemiological traits, Conventional diagnostic assays, including toxigenic culture (TC) and cytotoxicity detection (CCTA) (Planche and Wilcox, 2011), were time-intensive and inefficient in rapidly differentiating between toxigenic and non-toxigenic strains. It could not meet the current requirements of high volume, high speed and high accuracy. With the innovation of molecular technology, quantitative polymerase chain reaction (qPCR) and loop-mediated isothermal amplification (LAMP) technologies were used by NAATs to rapidly amplify and detect target nucleic acids (El-Daly, 2024). As a result, CDI could be diagnosed earlier, with greater sensitivity, specificity, and accuracy, which allowed effective treatment strategies to be implemented more quickly and efficiently. Meanwhile, C. difficile mainly produces three exotoxins, including TcdA, TcdB and CDT, of which TcdB is the key pathogenic factor (Alam and Madan, 2024; Luo et al., 2022). Based on sequence variations, TcdB had been classified into several subtypes, mainly including TcdB1, TcdB2, TcdB3, and TcdB4. There were significant differences in receptor recognition and pathological effects among different TcdB subtypes, with TcdB1 being able to utilize Chondroitin Sulfate Proteoglycan 4 (CSPG4) and Frizzled (FZD), TcdB2 relying predominantly on CSPG4, TcdB3 favouring FZD, and TcdB4 not relying on these known receptors (Pan et al., 2021). This difference in receptor preference directly affected the severity of infection and pathological changes. Mouse models showed that TcdB1 caused the most severe pathological effects, whereas TcdB4 caused the least, further validating the significant difference in disease progression between the two subtypes. Thus, detection of different toxin subtypes of CDI not only helped to identify the infection, but also provided important information about the severity of infection and disease progression, which was crucial for clinicians to choose the appropriate therapeutic regimen. And complex molecular technologies including next-generation sequencing (NGS) and nanopore sequencing (NS). Not only could it better understand the epidemiology and molecular mechanisms of CDI (Goodwin et al., 2016), but we might also effectively differentiate between toxin types. In settings with limited resources, CRISPR-Cas was known for its fast and sensitive detection (Yang, 2022; Jiang et al., 2023a). Ultra-high performance liquid chromatograph-mass spectrometry (UPLC-MS) and Raman spectroscopy (RS) have also been introduced into CDI diagnosis (Mullish et al., 2022; Malyshev et al., 2023; Koya et al., 2018). Infection-specific biomarkers could be rapidly identified by metabolic profiling and molecular vibration analysis. In transitioning from conventional diagnostic assays to advanced diagnostic assays, these technological advancements aimed to improve CDI diagnosis accuracy and clinical response times. Each new approach has built on the advantages of previous approaches, it made up for some limitations. This paper can offer valuable perspectives for clinical practitioners, researchers, and public health policymakers to refine strategies for diagnosing and managing CDI. It is designed to offer valuable perspectives for clinical practitioners, researchers, and public health policymakers to refine strategies for diagnosing and managing CDI.
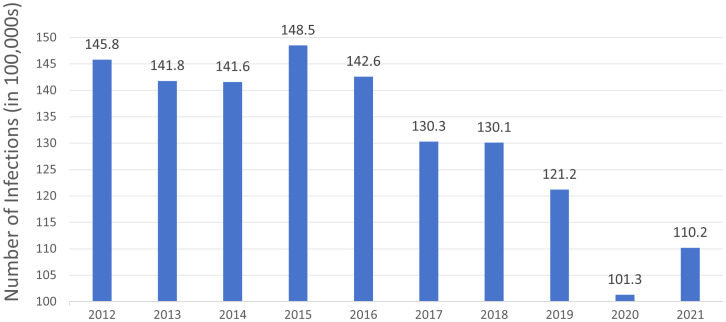
Figure 1.
The number of Clostridioides difficile infection cases in North America from 2012 to 2021 over a decade. The data is derived from the Centers for Disease Control and Prevention (CDC) Annual Reports for the Emerging Infections Program for Clostridioides difficile Infection from 2012 to 2021.
2. Traditional laboratory diagnostic assays for C. difficile
2.1. Microbiological culture
2.1.1. Clostridioides difficile anaerobic culture
C. difficile was a specialised anaerobic bacterium, with strict requirements for culture conditions, it was not easy to grow under conventional anaerobic culture conditions. When anaerobically cultured in cycloserin-cefoxitin-fructose agar (CCFA) medium for 24-48 h, it could form white or yellowish colonies with irregular edges and typical horse faeces odour, and yellow-green fluorescence might be seen under ultraviolet irradiation (Zhou Yong et al., 2024). After the culture of CCFA, it could be identified according to the morphology of the colonies, fluorescence characteristics, odour and the results of Gram staining, and the majority of the tests could be completed in 24 hours, and the sensitivities of its detection at 24 hours and 48 hours could be up to 91.9%, 97.3%, and the sensitivity of its detection at 24 hours and 48 hours was up to 97.3% (Yang et al., 2014). Therefore, C. difficile isolation and culture, as a traditional assay, which could provide a more comprehensive understanding of its epidemiological characteristics and provide a basis for clinical treatment due to its high sensitivity of 94%-100% and high specificity of 99% (Napolitano and Edmiston, 2017), and the availability of the corresponding strains for molecular typing and drug-resistance analysis. It was mostly used as a gold standard for comparison of new detection assays, evaluation of new treatment assays and monitoring of clinical drug resistance. However, its shortcomings should not be ignored. Separation and culture took a long time, and it could not clearly distinguish between virulent and non-virulent strains, thus providing no basis for rapid clinical diagnosis.
2.1.2. Cytotoxicity assays
C. difficile is divided into toxin-producing strains and non-toxin-producing strains, and its identification relies on CCTA (Antonara and Leber, 2016). Fresh patient faeces were diluted, centrifuged, and incubated with Vero or HepG2 cells with or without antitoxin A/B-neutralising antibodies for 24-48 hours. TcdA and TcdB were determined by detecting the cytopathic effect (CPE). Confirmation of C. difficile in the sample as a toxin-producing strain if CPE occurs. CCTA was considered the gold standard of traditional laboratory reference assays for confirming the diagnosis of CDI (Planche and Wilcox, 2011). However, the sensitivity of TC was generally considered to be between 75% and 85%, which was a highly sensitive and specific test. While it could identify CDI presence in samples, its time-consuming and labour-intensive nature increased the risk of contamination during complex sampling, culturing, and experimental procedures (Burnham and Carroll, 2013). In addition, CCTA was not suitable for immediate diagnosis in the laboratory due to instrumentation and laboratory space requirements.
2.1.3. Toxigenic culture
The TC test consists of a two-part C. difficile culture and a toxin-producing assay.TC is performed only when the culture result is positive, and is usually initially confirmed by adding a drop of treated faecal filtrate to a monolayer of human foreskin fibroblasts and to other monolayers of human fibroblasts on a microtitre plate, and observing for the presence of CPE to initially confirm toxigenic C. difficile (Strachan et al., 2013). Studies have shown that the sensitivity of toxin-producing tests usually ranges from 75% to 90%, while the specificity ranges from 80% to 100%. TC has high sensitivity and specificity, but because TC only detects the in vitro toxin-producing ability of C. difficile, it cannot determine toxin production by C. difficile in vivo, and it cannot differentiate whether the toxin-producing C. difficile is causing a true infection in vivo. Similar to CCTA, TC has two major disadvantages of being time-consuming and labour-intensive, and is not conducive to immediate diagnosis; therefore, TC is mainly one of the gold standards of traditional laboratory reference assays for confirming CDI (Planche and Wilcox, 2011). It is generally not applicable to clinical laboratory diagnosis.
2.2. Immunologic tests
2.2.1. Glutamate dehydrogenase
The antigenic protein GDH is widespread and highly expressed on the surface of all C. difficile strains. In patient samples, GDH is detected using the GDH assay to make a diagnosis. The GDH assay is a brief, cost-effective test with high sensitivity and a strong negative predictive value (NPV) (Shetty et al., 2011). There is a 90-100% sensitivity, a negative predictive value of more than 99%, and a negative GDH result can be considered negative. CDI can be excluded if the test is negative for GDH, while positive tests for toxins or toxin genes are required to confirm the test, and are often used in conjunction with enzyme immunoassay (EIA) (Zangiabadian et al., 2023), GeneXpert real-time PCR (RT-PCR) (Kameli et al., 2024), and so on. However, because both toxin-producing strains and non-toxin-producing strains produce GDH, and other strains can also produce GDH, its specificity is low, so it cannot be applied solely to laboratory diagnostic tests. As a result, it is often used as a preliminary screening test for CDI.
2.2.2. Enzyme immunoassay
Using antibodies coupled to enzymes, the EIA detects specific biomarkers by binding antigens and converting the substrate into an observable product. Traditionally, GDH, the exotoxins TcdA and TcdB have been used to achieve laboratory diagnosis. The GDH EIA demonstrated a sensitivity of 82% (95% CI: 79-84) and a specificity of 91% (95% CI: 90-92), while the Tox A/B EIA showed a sensitivity of 75% (95% CI: 70-79) and a specificity of 95% (95% CI: 94-96) (Zangiabadian et al., 2023). EIA includes ELISA and immunochromatographic assay. Ramakrishnan et al. used the Duoset ELISA kit (R&D Systems) to assess neutrophil elastase and lipid transporter protein-2 levels in plasma samples (Ramakrishnan et al., 2024). Neutrophil elastase and lipid transporter-2 levels in patient plasma were determined at a 1:120 and 1:100 ratio, respectively. By comparing ELISA results from patients with CDI and healthy controls, the researchers found that plasma levels of neutrophil elastase and lipid transfer protein-2 were significantly elevated in patients with acute CDI. As faecal levels of calreticulin (FC) and lactoferrin (FL) may be associated with the severity and risk of recurrence of CDI (Gallo et al., 2018; Kim et al., 2017). Fernández et al. applied EIA in order to help predict recurrence and severity of CDI (Ágreda Fernández, 2024). They extracted faecal samples from patients using a CALEX® bottle cap extraction device, and on the one hand, FC levels were measured by an FDA-approved commercial ELISA kit (Bühlmann fCAL® ELISA) (Hamer et al., 2022). On the other hand, stool FL levels were measured by a commercial EIA kit (DRG EIA6038) and extracted using the EIA-5674 sample preparation system. The FC and FL prediction study results for CDI were then compared between different threshold values to determine diagnostic accuracy. The Youden Index was used to determine the optimal cut-off values to maximise the overall validity of the CDI diagnostic test (Youden, 1950). Among its advantages, EIA has the advantage of being simple and highly specific for distinguishing between toxin-producing and non-toxin-producing C. difficile strains. The disadvantage is that time-consuming, the sensitivity fluctuates greatly and false-negative results are easy to occur. Its positive predictive value (PPV) is low, resulting in omission of diagnosis for patients with mild disease, and strains cannot be obtained for further research, such as genotyping. The existing studies suggest it cannot be used separately as a diagnosis of CDI.
2.3. Nucleic acid amplification tests
2.3.1. Quantitative polymerase chain reaction
qPCR is a molecular diagnostic technique that allows for the continuous monitoring of DNA amplification, with data acquisition occurring in sync with each cycle of the amplification process. This assay is crucial for correlating the initial copy number of the target nucleic acid with the rate of fluorescence increase, where a higher copy number is indicative of an earlier and more pronounced rise in fluorescence. The sensitivity of qPCR is paramount, as it enables the detection of even minute quantities of nucleic acid, which is particularly relevant for the detection of CDI. The most commonly used product assays for the qPCR process are SYBR Green and hydrolysed probes (Busi, 2020). Adrianne C Maestri et al. compared two qPCR diagnostic assays targeting the CDI tcdB gene (Maestri, 2022). Firstly, primers were designed using Primer3 software and C. difficile 630 (Costa et al., 2016; Quesada-Gómez et al., 2015), and the specificity of primers was verified by MegaX software and Primer-blast (Kumar et al., 2018). Then real-time PCR designs and controls were amplified and detected using ABI 7500, and PCR reaction conditions were established, including annealing temperature and amplification cycle. Sensitivity, reproducibility, specificity, and accuracy were analysed. Finally, DNA was extracted from C. difficile ATCC BAA1870 bacterial suspension and tested for sensitivity and specificity at different concentrations. These two qPCR CDI assays were compared with the conventional GDH plus TC CDI assay. 97.9% sensitivity and 99.1% specificity were achieved with the SYBR Green assay, while 87.5% sensitivity and 100.0% specificity were achieved with the hydrolysis probe assay (Maestri, 2022). Both had diagnostic accuracies of 99.0% and 98.5%, respectively, and these findings underscore the ability of qPCR to reliably detect CDI in clinical samples. Xiao-xi Jia et al. designed a single-tube multiplex qPCR assay for the direct detection of toxin-producing C. difficile in faecal samples (Jia et al., 2023), which facilitates the diagnostic efficiency of CDI. The assay can also differentiate between multiple toxin genes and subtypes, such as tcdA, tcdB, and cdtB (Kubota et al., 2014; Luna et al., 2011). These genes are key for the detection of CDI. Additionally, the assay includes the internal control gene chip. It is built to work with various qPCR platforms, ensuring flexibility. The assay also integrates with the point-of-care-testing (POCT) system. This integration significantly boosts the efficiency of detection. Furthermore, it improves the sensitivity of the assay. The assay exhibits a limit of detection (Lod) from 101 to 103 gene copies per microliter, with coefficients of variation below 3% for both inter-batch and intra-batch analyses, signifying excellent reproducibility. In faecal samples, the limit of detection was found to range between 5×102 to 5×105 colony-forming units per gram (CFU/g), underscoring the robustness of the assay for CDI diagnosis. The qPCR assay exhibited a high concordance rate of 98.4% with conventional PCR and ELISA (Costa et al., 2016), demonstrating equivalent sensitivity in identifying both toxin-producing and non-toxin-producing strains of C. difficile. This technique offers the advantages of high specificity and rapid detection, allowing for quantitative analysis compared to traditional PCR. However, it requires the use of specific fluorescently labelled probes and specialised qPCR equipment, which can be cost-prohibitive. Additionally, the assay necessitates sophisticated experimental manipulation and data analysis, mandating the expertise of trained professionals for both operation and result interpretation.
2.3.2. Loop-mediated isothermal amplification
LAMP is a nucleic acid amplification technique used to detect and quantify DNA molecules. The most important feature is that it is performed at a constant temperature, usually 60-65°C. There is no need to rely on temperature cycling equipment such as PCR, simplifying the experimental conditions. LAMP uses four specific primers to recognise six target regions, including two outward primers, two inward primers, and two loop primers. The aim is to recognise the six regions of target DNA within a short period of time (30 ~ 60 min). Using rapid amplification of nucleic acids, different parts of the target DNA are combined to produce many product DNA molecules. The colour change or turbidity is visible without the use of subsequent detection assays such as gel electrophoresis, thus enabling rapid detection of the target gene. LAMP, an assay that detected the presence of binary toxin genes, was used by Lan Yu et al. to diagnose CDI (Yu et al., 2017). For the binary toxin encoding genes of C. difficile, the cdtA and cdtB gene sequences were analysed using Primer Explorer Version 4 software, and eight primers for cdtA and six primers for cdtB were designed (Lin et al., 2022). The LAMP reaction was then performed using the Loopamp® DNA amplification kit from Eiken Chemical Co. Ltd. The optimal reaction conditions were determined to be 60 min at 60°C based on the turbidity change data at 650 nm from a real-time turbidity monitor. The reaction system was supplemented with Loopamp® Fluorescence Detection Reagent from Eiken Chemical Co. Ltd. This reagent contains a metal indicator compatible with the reaction system. Visual judgement was made by colour change (from orange to green) (Girão, 2021). Subsequently, a specificity test was carried out to test the specificity of the LAMP reaction using 25 different bacterial strains (including C. difficile), which showed that the LAMP reaction only reacted to C. difficile and was negative for all other bacteria. The sensitivity test, which compared the LAMP assay and traditional PCR for diagnosing CDI, found that the detection limit of LAMP is 24.8 pg/µL, which is 10 times lower than that of traditional PCR (Yu et al., 2017). Lin M. et al. developed a LAMP assay for the tetM gene in C. difficile strains cultured from faeces, which efficiently amplified the target DNA within 60 min at a constant temperature of 62°C, with a Lod of 36.1 pg/μL, representing a 100-fold increase in sensitivity over traditional PCR (Lin et al., 2022). Hiber Gene’s LAMP assay uses a novel LAMP targeting the tcdA and tcdB genes to diagnose CDI. When compared to the GDH toxin A/B test (C. diff Quik Chek®), Hiber Gene’s LAMP demonstrated 100% sensitivity and 95.8% specificity. When compared to the multiplexed NAATs FilmArray™ GI panel® (BioFire), the sensitivity was 81.2%, the specificity was 100%, and the concordance was 96.38% (Gandelman et al., 2011). Therefore, LAMP technology offers advantages such as high specificity and selectivity, high sensitivity, fast reaction speed, and a simple operational procedure, with results that can be visualised and observed in a short time (Norén et al., 2014).
3. Advanced laboratory diagnostic assays for Clostridioides difficile
3.1. Next-generation sequencing
As a DNA sequencing assay, NS is also known as high-throughput sequencing (HTS). It is based on the principles of PCR and Gene Chip, which enables sequencing while synthesising. NGS technology determines the DNA sequence by capturing special markers (usually fluorescent molecules) on bases added during DNA replication. This technology is characterised by its high throughput and short read lengths. Major technology platforms include the Roche 454 FLX, Illumina MiSeq, and Illumina HiSeq, etc. NGS is particularly suited for amplicon sequencing, such as 16S, 18S rRNA genes and variable regions of internal transcribed spacers (ITS). For sequencing genomic and macrogenomic DNA, Metagenomic Shotgun Sequencing (MSS) is required (Falony et al., 2019; Vasilescu et al., 2022), which involves breaking DNA into small fragments (e.g., 30bp) for sequencing (Quince et al., 2017). In a study to identify diarrhoea-associated pathogens in faecal specimens. Two sequencing technologies using NGS platforms were compared. Sequencing of the 16S rRNA gene started with total nucleic acids from C. difficile extracted from faecal samples for prior sample processing, followed by PCR amplification of the V3 to V5 regions of the 16S rRNA gene using specific primers (357F and 926R). The amplicons were then purified as well as mixed equimolarly. Finally, pyrophosphate sequencing was performed on a Roche 454 Titanium platform. Low-quality reads (average mass <35), short reads (<200 bp), and reads containing sequences from the chimeric 16S rRNA gene were removed, while high-quality sequences were classified from phylum to genus level by the Ribosomal Database Project Naive Bayesian Classifier. Of the 22 C. difficile-positive samples, 20 (90.9%) were detected by 16S rRNA gene sequencing (Woo et al., 2005; Zhou et al., 2016). MSS first performed sample processing to convert total C. difficile nucleic acids to DNA and construct single-indexed sequencing libraries. The DNA is then interrupted using a Covaris instrument and subjected to end repair, A-tail addition, junction ligation and PCR amplification. Finally, MSS was performed on the Illumina HiSeq platform to generate 100 bp paired-end reads. Reads were mass trimmed, host contamination removed and low complexity regions masked. Approximately 5000 reference genomes were aligned using RTG mapping to identify bacterial and fungal species. MSS was detected in 86.3% of C. difficile-positive samples detected (Zhou et al., 2016). Both assays have their advantages and limitations. 16S rRNA gene sequencing is less costly but may have PCR-related bias and limited classification accuracy. MSS, on the other hand, is more costly and has a huge amount of data, requiring large computational resources for data processing and storage. These two assays can complement each other to improve the diagnostic accuracy of CDI. Whole Genome Sequencing (WGS) is one of the most widespread applications of NGS technology (Goodwin et al., 2016). It specifically refers to the process of sequencing the entire genome, focusing on obtaining sequence information about the entire genome. WGS can obtain complete genomic information about a C. difficile strain infecting a patient by sequencing the entire genome of the strain. This information encompasses the strain genome sequence, the proteins encoded by the genes, and data regarding other related genes (Capobianchi et al., 2013; Miller et al., 2013). Study by Helena et al., the WGS technique was able to provide more detailed and precise information about the strain compared to traditional PCR-ribotyping (Seth-Smith et al., 2021). Single nucleotide polymorphisms (SNPs) can be detected by WGS, which is particularly useful in tracing the source of infection and the chain of transmission between cases (Kuenzli et al., 2020). In contrast, PCR-ribotyping, although commonly used and rapid, has limited resolution and does not provide the same detailed genetic information. Meanwhile, WGS analysis enables the tracking of genetic variation in C. difficile and the identification of affinities between different strains, which can lead to inferences about the source of infection and the path of transmission (Goyal et al., 2020). A Canadian study utilised whole genome sequencing to identify genetic and epidemiological correlations between CDI cases and previously infected or colonised individuals, highlighting the role of colonised patients in transmission. Laboratory diagnosis of C. difficile strains was conducted using WGS (Hargreaves et al., 2016). By comparing genomic sequence differences between C. difficile strains, CDI can be identified and targeted therapeutic measures can be implemented more accurately.
3.2. Nanopore sequencing
NS is also called ab initio sequencing technology, i.e., single-molecule real-time DNA sequencing. NS is represented by PacBio’s single molecule real time sequencing (SMRT) and Oxford nanopore technologies (ONT). Their greatest advantage over the previous two generations is single-molecule sequencing, which does not require PCR amplification. Conventional short-read-length sequencing techniques tend to lead to fragmentation of genome sequences, especially when dealing with bacteria with complex genome structures and repetitive sequences. In contrast, SMRT sequencing can generate high-quality complete genome sequences, which is particularly important for pathogens such as C. difficile. Through SMRT, the researchers identified genomic differences and rearrangements between different isolates of C. difficile (Hargreaves et al., 2016). These differences include insertions and deletions of mobile genetic elements (MGEs), such as the differential presence of the Tn6164 and Tn6293 transposons in different strains. These MGEs not only affect the structure of the genome but may also transfer genes between strains through horizontal gene transfer mechanisms. In addition, SMRT was able to detect the presence of methylation modifications. An enzyme called C. difficile adenine methyltransferase a (CamA) has also been found to play an important role in C. difficile reproduction (Oliveira, 2019), especially during spore formation. When the DNA methylation corresponding to CamA was lost, C. difficile spore formation was reduced by about half. This suggests that detecting the DNA methylation status of C. difficile could serve as a potential indicator for diagnosing CDI, which is crucial for understanding the regulation of bacterial gene expression and adaptation to different environments. In contrast to 16S rRNA gene sequencing, which is limited in revealing the structure of microbial communities due to the taxonomic resolution is restricted to the genus or family level. PacBio’s SMRT, which is capable of generating very long read lengths, avoids primer bias and therefore has a significant advantage in terms of resolution and accuracy. Sadowsky et al. demonstrated that microbial communities can be analysed more efficiently with SMRT, making the identification of C. difficile more accurate (Sadowsky et al., 2017). The role of nanopore electrical signal sequencing in CDI is to analyse in detail the species and strains of the donor gut microbiota and how they colonize and affect the recipient in a high-resolution manner. This technique enables rapid sequencing of bacterial ribosomal RNA gene clusters, including the 16S-ITS-23S region, to accurately identify strains of C. difficile. In this way, researchers such as Benítez-Páez can track the transfer process of specific bacteria such as C. difficile (Benítez-Páez et al., 2022).
3.3. Clustered regularly interspaced short palindromic repeats/CRISPR-associated
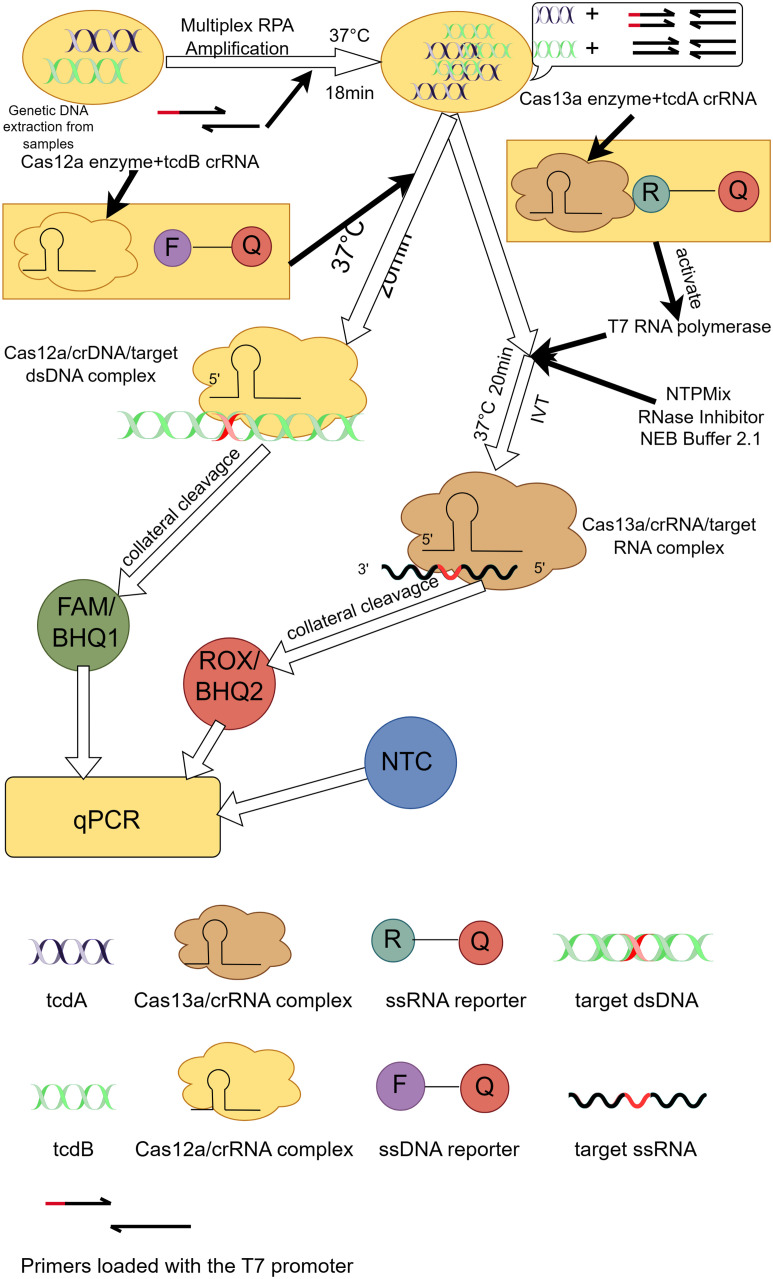
The evolution of CRISPR-Cas technology in diagnostics demonstrates its diverse applications as a revolutionary molecular biology tool (Li et al., 2023a). Initially, the CRISPR-Cas system was discovered as a natural immune system for bacteria and archaea and attracted extensive interest from the scientific community. Subsequently, researchers began to explore its potential in gene editing and molecular biology. Important advances in the use of CRISPR-Cas technology for diagnostic applications date back to 2015, when Zetsche et al. first described Cas12a (formerly Cpf1), a single RNA-guided nucleic acid endonuclease capable of cleaving double-stranded DNA in cis (Zetsche et al., 2015). Subsequently, Chen et al. found that Cas12a also demonstrated the ability to cut single-stranded DNA in trans, which laid the foundation for the development of novel nucleic acid detection platforms (Chen et al., 2018). Building on this discovery, platforms such as DNA nucleic acid endonuclease targeting CRISPR trans reporter genes (DETECTR) and the 1-hour low-cost versatile and efficient system (HOLMES) have been developed, combining Cas12a with recombinase polymerase amplification (RPA) for rapid detection of various pathogens (Chen et al., 2018; Li et al., 2018). On the other hand, breakthroughs in specific nucleic acid detection were triggered by the discovery of Cas13a (earlier known as C2c2), which exhibited trans-cleavage activity against RNA, leading to the development of the specific highly sensitive enzyme reporter gene unlocking (SHERLOCK) platform (Gootenberg et al., 2017). The platform utilized the non-specific cleavage activity of Cas13a on single-stranded RNA (ssRNA) in combination with fluorescent or other indicators to enable rapid detection of a wide range of pathogens, including SARS-CoV-2 (Li et al., 2023b, He et al., 2023). With further technological evolution, the introduction of orthogonal CRISPR strategies further enhanced the possibility of multiplexed detection, and Gootenberg et al. introduced the SHERLOCK platform (Gootenberg et al., 2018), which, for the first time, utilised an orthogonal CRISPR system to achieve simultaneous detection of multiple targets in a single reaction. This advancement not only expanded the range of detection, but also improved the sensitivity and specificity of the assay. Tian et al. further extended this concept by developing a portable fluorescent detection device for rapid detection of pathogens such as SARS-CoV-2 in a single tube, which greatly facilitated the application and popularization of immediate diagnostic technologies (Tian et al., 2022). Tong Jiang et al. took it a step further by investigating the development of a new diagnostic platform for laboratory diagnosis of CDI called orthogonal CRISPR system combined with multiplex recombinase polymerase amplification (OC-MAB) ( Figure 2 ) (Jiang et al., 2023b), which combines two CRISPR systems (Cas12a and Cas13a) and multiplex recombinase polymerase amplification (RPA) technology designed to simultaneously recognize the toxin genes of C. difficile tcdA and tcdB. C. difficile DNA was first extracted from the samples. specific primers were then designed to target the C. difficile toxin genes, tcdA and tcdB, and the target DNA sequences were isothermally amplified using RPA technology (Kaminski et al., 2021). T7 RNA polymerase was then used to transcribe tcdA into single-stranded RNA (ssRNA), while tcdB was left intact as double-stranded DNA (dsDNA). Next, the Cas12a and Cas13a proteins were specifically recognised by DNA and RNA, respectively, and their non-specific cleavage activity was activated (Chen et al., 2018; Myhrvold et al., 2018). This leads to cleavage of the labelled DNA and RNA probes, releasing a fluorescent signal. On the one hand, the fluorescence signal after cleavage can be detected using the dual-channel fluorescence detection system of the qPCR instrument. And the presence or absence and intensity of the fluorescent signal can be used to determine whether the C. difficile toxin gene is present in the sample. On the other hand, the cutting product of the CRISPR-Cas system can be combined with immunochromatographic test strips labelled with antibodies for visual detection. A colour change is displayed on the test strip, allowing a visual determination of the presence or absence of the C. difficile toxin gene in the sample. The OC-MAB platform not only excels in a laboratory setting but also has the potential to be used in resource-poor areas or where rapid detection is required. It has an extremely low detection limit of 102 to 101 copies/mL with excellent sensitivity and specificity (Jiang et al., 2023b). The OC-MAB platform demonstrates comparable or superior performance to traditional qPCR in detecting C. difficile.
Figure 2.
Principle of the OC-MAB platform. DNA samples of C. difficile were isothermally amplified in multiplex RPA, tcdA, tcdB were subjected to CRISPR dual-system cleavage, and the cleavage signals and NTC negative controls were compared with the results by fluorescence. This figure was drawn by Figdraw.
3.4. Ultra-performance liquid chromatography-mass spectrometry
The coupling of high-performance liquid chromatography (HPLC) and mass spectrometry (MS) has significantly improved the separation capability and sensitivity of sample analysis. It enables rapid and accurate detection and identification of components in complex mixtures, providing a wealth of data and quantitative analysis capabilities for research. UPLC-MS has proven invaluable for diagnosing CDI by providing a comprehensive analysis of the metabolome in faecal samples (Zhou et al., 2018). UHPLC-MS was chromatographically separated using a Waters ACQUITY UPLC system and a BEH C18 analytical column (Rathod et al., 2019). Separation efficiency and selectivity were improved by mobile phase A (water/formic acid) and mobile phase B (acetonitrile/formic acid) (Cao et al., 2011; Huang et al., 2013). A linear gradient elution procedure was used for faecal sample analysis. Mass spectrometry analysis was then performed in positive ion electrospray mode. Peak intensities in the raw UHPLC-MS data were detected, integrated, and normalised using MarkerLynx Applications Manager software (MarkerLynx). Pattern recognition analysis was performed using principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) (Mbunge et al., 2022; Yahaya et al., 2016). Linear discriminant analysis (LDA) based on sequential feature selection was performed using Matlab software to diagnose the cause of diarrhoea. Finally, the Human Metabolome Database and PubChem Compound Database were searched and mass spectra and retention times of potential biomarkers were compared with authentic standards for identification. The C. difficile-positive, C. difficile-negative, and healthy control groups showed significant clustering differences in the PCA score plots. In the PLS-DA model, the C. difficile-positive group showed a clearer separation from the C. difficile-negative group and the healthy controls. A total of eight classes (13 types) of markers were identified, including fatty amides, sphingomyelins, bile acids, amino acids, carnitine, lecithin (LPC), and esters. An LDA model based on capsiamide, dihydrosphingosine, and glycochenodeoxycholic acid was developed for the identification of CDI in diarrhoea (Dawkins et al., 2022). The model showed leave-one-out cross-validated CDI diagnosis (LOOCV) accuracy, sensitivity, and specificity of the training set of 90.00%, 93.33%, and 86.67% respectively. While the predicted accuracy, sensitivity, and specificity of CDI diagnosis for the external validation set were 78.57%, 83.33%, and 75.00% respectively (Mbunge et al., 2022). The area under the receiver operating characteristic curve (AUC) was 0.9 and 0.7917 for the training and validation sets, respectively. C. difficile diarrhoea has a unique faecal metabolomic profile compared to other hospital-onset diarrhoea. The UPLC-MS metabolomics model is expected to be a useful tool for diagnosing C. difficile diarrhoea (Weingarden et al., 2014). The disadvantages of UHPLC-MS technology include high cost, complexity of operation and data processing, low throughput, and limitations for specific analytes, which limit its widespread use.
3.5. Raman spectroscopy
Raman spectroscopy is an analytical spectroscopy technique that utilizes the Raman effect to study the molecular vibration and rotation states of matter (Koya et al., 2019). In Raman spectroscopy, a sample is excited by monochromatic light (usually a laser) and scatters. When the laser beam interacts with the molecules in the sample, every 106 to 108 photons undergo elastic scattering (Rayleigh scattering, where there is no change in the energy of the scattered versus incident photons), while about 1 photon undergoes inelastic scattering (Raman scattering, which involves a change in the energy of the scattered versus incident photons) (Hassanain et al., 2021). Hassanain et al. developed a novel laboratory diagnostic tool based on surface-enhanced Raman scattering (SERS)-based lateral flow assay (LFA). This technique uses gold nanoparticles (AuNPs) as an enhancement substrate for SERS signals. Specific antibodies and Raman reporter molecules (e.g. malachite green isothiocyanate, MGITC) were bound to the surface of AuNPs by physical adsorption to form SERS nanotags. These nanotags specifically recognise and bind to target biomarkers (SlpA and TcdB) (Hassanain et al., 2021). The capture antibodies were then sprayed at specific locations on the nitrocellulose membrane to form test and control lines. The test line is used to capture specific biomarkers while the control line is used to confirm the functionality of the test strip. The SERS nanolabels are mixed with the sample such that the antibodies on the nanolabels form immune complexes with the biomarkers in the sample (Wang et al., 2024). Two separate test lines, corresponding to SlpA and TcdB, were designed on a lateral flow test strip. The formed immune complex solution was added dropwise to the lateral flow test strip, and capillary action was used to cause the solution to migrate along the test strip. When the immune complex reaches the test line, it binds to the pre-immobilised capture antibody and forms a ‘sandwich’ structure, resulting in a change in the colour of the test line, thus enabling the visualisation of the biomarker. A handheld Raman spectrometer can be used to scan the test line and further collect Raman signals generated by the SERS nanotag (Joung et al., 2022). The concentration of biomarkers can be quantified by analysing the intensity changes of specific Raman peaks. Compared to traditional assays such as ELISA, SERS-based LFA not only offers higher sensitivity (minimum observed concentration of 0.01 pg/μL) and specificity, but also completes the assay in a shorter period of time (only 20 minutes) using dual detection and flow measurement test strips. The platform can be used in the field with a handheld Raman spectrometer suitable for use at the point-of-care (POC), facilitating rapid detection in the field and reducing sample handling and analysis time, enabling faster diagnosis and management of CDI cases (Hassanain et al., 2021). However, RS still requires some technical knowledge and equipment support.
3.6. Real-time cell analysis
Real-time Cell Analysis (RTCA) was a marker-free, non-invasive assay for monitoring cell status. Cell growth and response to toxins were assessed by detecting changes in electrical impedance on cell culture plates (Solly et al., 2004). RTCA quantifies TcdB toxin concentration by changes in cell index (CI) associated with changes in electrical impedance, thereby reflecting changes in the number, morphology, and degree of adhesion of toxin cells. In 2010, Ryder et al. monitored cytotoxic effects using the hypersensitive nature of HS27 cells to TcdB toxin in the RTCA platform. By using the platform it was learned that the Lod was 0.2 ng/mL, the specificity was 99.6%, and the sensitivity was 87.5%. Based on these results, the RTCA system might be used to determine clinical CDI severity and to monitor therapeutic efficacy using toxin concentration measurements (Ryder et al., 2010). Subsequently, Huang et al. combined the immunomagnetic bead separation and enrichment technique with an improved RTCA system (RTCA version 2) in 2014. Not only was the sensitivity increased to 96.2%, but also the Lod was reduced to 0.12 ng/mL. The test results of those samples could be obtained within 24 hours (Huang et al., 2014). By 2024, Shen et al. assessed the sensitivity to TcdB using four cell lines and selected the HS27 cell line as the target cell line for testing TcdB. Compared with RTCA, neither ELISA nor PCR had high resolution. RTCA could effectively distinguish CDI from C. difficile colonisation (CDC) through quantifying functional and toxical TcdB (Shen et al., 2024). Therefore, RTCA could be used as an effective complementary diagnostic tool for ELISA and PCR. However, this technology faced challenges such as operational complexity, high cost, and the possibility of inhibitors in the sample that affected the accuracy of the determination.
4. Discussion
Laboratory diagnosis of CDI is a multistep process involving a variety of traditional and advanced techniques. Traditional assays, such as TC and CCTA, are time-consuming and do not allow rapid differentiation between virulence-producing and non-virulence-producing strains, despite their high specificity (Luo et al., 2022). In contrast, anaerobic incubation in CCFA medium can detect C. difficile within 24 to 48 hours, with sensitivities of up to 91.9% at 24 hours and 97.3% at 48 hours, respectively (J. J. Yang et al., 2014). However, this assay cannot provide a basis for rapid clinical diagnosis, limiting its application in immediate diagnosis. Immunological tests, although simple and time-consuming, have low specificity and are usually used as a primary screening tool. The sensitivity of the GDH test is 90% to 100%, with a negative predictive value of more than 99%, and advances in GDH-EIA allow for rapid real-time diagnosis of CDI (101). NAATs, especially qPCR and LAMP, have significantly improved the sensitivity and specificity of CDI. The development of qPCR technology demonstrates high sensitivity and specificity; the SYBR Green assay has a sensitivity of 97.9% and specificity of 99.1%, and the hydrolysed probe assay has a sensitivity of 87.5% and specificity of 100.0% and can be combined with automated molecular assays to achieve faster and more sensitive diagnostics, such as the GeneXpert system (Makristathis et al., 2017). LAMP technology has shown great potential for immediate detection with its fast, simple process and visualisation of results, and is up to 10 times more sensitive than traditional PCR. Advanced diagnostic assays, with NGS observing significant concordance across typing assays, offer advantages that have transformed epidemiologic studies, enabling high-resolution genomic analysis of C. difficile (Maestri et al., 2023). By contrast, NS Technology uses ONT sequencing to detect CDI of sequence type (ST)2 as part of its recent prospective genomic surveillance system using multilocus sequence type (MLST) ( Table 1 ), which provides in-depth genomic information relating to C. difficile epidemiology and molecular mechanisms of infection (Abad-Fau et al., 2023). RTCA demonstrated significant advantages in this study. It is able to quantify functional and virulent C. difficile TcdB in clinical samples by monitoring changes in CI in real time. Compared to traditional assays such as ELISA and PCR, RTCA can distinguish CDI from CDC more accurately (98). Because it directly measures the biological activity of the toxin and not just the presence of toxin genes or proteins. This assay is not only rapid but also highly sensitive and specific, providing an effective clinical tool for better diagnosis and management of CDI. CRISPR-Cas technology, with its rapidity and sensitivity, offers a new strategy for the diagnosis of CDI, especially in resource-constrained settings (Bloomfield et al., 2024). Technologies such as UPLC-MS and RS provide a new dimension to the diagnosis of CDI by analysing the metabolome and molecular vibrations of samples. These technologies enable rapid identification of infection-specific biomarkers that contribute to early diagnosis and treatment, further improving diagnostic accuracy and clinical responsiveness ( Table 2 ). In summary, the development of novel diagnostic platforms offers new possibilities for rapid and accurate diagnosis of CDI. Future research should focus on improving the accuracy, accessibility, and cost-effectiveness of detection technologies to better serve clinical needs.
Table 1.
Comparison Table of NGS and NS.
| Technology Type | Sequencing Principle | Read Length Features | Throughput Characteristics | Sequencing Accuracy | Sample Enrichment | Cost | Advantages | Limitations | CDI Research Applications | References |
|---|---|---|---|---|---|---|---|---|---|---|
| NGS | Sequencing by synthesis with reversible termination | Short read lengths, suitable for amplicon sequencing | High throughput, suitable for large-scale sample analysis | Lower error rates, requires reference sequences | Requires PCR amplification | Higher than PCR, lower than NS | High throughput, low cost, precise short read lengths | Limitations of short read lengths, requires reference sequences | Pathogen identification, 16S rRNA gene sequencing | (Goodwin, et al., 2016) |
| NS | Single-molecule real-time sequencing, no PCR required | Long read lengths, suitable for complex genomes | Medium to low throughput, suitable for long sequence analysis | Higher error rates, correctable by coverage | Direct sequencing, no PCR required | Particularly high | Long read lengths, no PCR required, complex genome resolution | High error rates, high costs | Genomic variation tracking, source of infection transmission chain analysis | (Hargreaves et al., 2016; Koya et al., 2019) |
Table 2.
Comparison table of traditional and advanced laboratory diagnosis of Clostridioides difficile infection.
| Diagnostic Assay |
Detected Substances | Time Required | Cost | Specificity | Sensitivity | Accessibility | Challenge level | References |
|---|---|---|---|---|---|---|---|---|
| C. difficile anaerobic culture | Colony morphology and color | 1-3 days | Low | High | High | Medium accessibility, low cost of instruments, one of the gold standards | Medium challenge, time-consuming, medium personnel and operational requirements, unable to distinguish non-toxigenic strains, cannot POCT | (Zhou et al., 2024) |
| CCTA | Tcd B | 1-3 days | High | High | Low | Medium accessibility, low cost of instruments, can distinguish between toxigenic and non-toxigenic strains, one of the gold standards | Medium challenge, time-consuming, medium personnel and operational requirements, cannot POCT | (Antonara and Leber, 2016) |
| TC | Toxinogenic C. difficile | 3-5 days | High | High | Medium to high | Medium accessibility, low cost of instruments, one of the gold standards | Medium challenge, time-consuming, medium personnel and operational requirements, unable to distinguish non-toxigenic strains, cannot POCT | (Strachan et al., 2013) |
| GDH | GDH, TcdA and TcdB | Several hours | Low | Low | High | High accessibility, short time consumption, low cost, high NPV, high reliability, often used as an initial screening test for CDI | Low challenge, unable to distinguish between toxigenic and non-toxigenic strains | (Shetty et al., 2011) |
| EIA | Tcd A/TcdB | 3-5 hours | Low | High | Low | High accessibility, easy to operate, can distinguish between toxigenic and non-toxigenic strains | Low challenge, low cost, time-consuming, sensitivity fluctuates greatly, prone to false negatives. Low PPV | (Zangiabadian et al., 2023) |
| NAAT | Toxin Genes | 1-3 hours | High | High | High | High accessibility, short time, quantitative analysis possible, can implement POCT | Low challenge, high cost, medium personnel and operational requirements | (Lin et al., 2022; Maestri, 2022) |
| NGS | DNA sequences (tcdA, tcdB, etc.) | Several hours to one day | High | High | High | Low accessibility, high throughput, capable of metagenomic sequencing | High challenge, requires PCR amplification, high equipment cost | (Goodwin et al., 2016) |
| NS | DNA sequences (tcdA, tcdB, etc.), methyltransferases (CamA, etc.) | Several hours | High | High | Relatively high | Low accessibility, long read length, no PCR required, real-time sequencing | High challenge, higher error rate, relatively high equipment and time costs | (Hargreaves et al., 2016; Koya et al., 2019) |
| CRISPR-Cas | tcdA, tcdB | About 1 hour | Medium | High | High | High accessibility, short time consumption, high portable, suitable for POCT | Medium challenge, medium personnel and operational requirements | (Jiang et al., 2023b) |
| UPLC-MS | Metabolic markers (lipids, amino acids, etc.) | Several hours | High | High | Medium to high | Low accessibility, can diagnose unique fecal metabolomic features | High challenge, high cost, complex operation and data processing, low throughput, and limitations to specific analytes | (Zhou et al., 2018) |
| RS | Biomarkers (SlpA, TcdB, etc.) | Less than 30 minutes | Low to medium | Medium to high | High | High accessibility, short time consumption, portable, suitable for POCT | Medium challenge, medium personnel and operational requirements | (Hassanain et al., 2021) |
| RTCA | TcdB | Less than 24h | High | High | High | High accessibility, effective differentiation between infection and colonisation, quantitative detection of toxins | Medium challenge, operational complexity, high cost, subject to inhibitors | (Shen et al., 2024) |
5. Conclusion
In summary, in order to effectively diagnose and treat CDI, medical laboratories have now adopted a variety of detection techniques, which include microbiological culture, immunological detection, and molecular biology detection. Microbiological culture is the most basic assay, but it takes a long time to obtain the results. Immunological detection has high sensitivity but may be interfered with by other antibodies. Molecular biology testing can quickly and accurately detect the genetic material of C. difficile, but it is more demanding under experimental conditions. With the continuous progress of biotechnology, new laboratory diagnostic techniques for C. difficile have emerged, facing different needs for different technologies, if one needs to get the test results quickly, NAATs may be a better choice. Out of the same need, new diagnostic platforms for CDI, such as RTCA, have become available. can not only diagnose CDI but can further differentiate between specific toxin-type aspects. In cases where limited resources and expertise are available, RS may be a more practical option. CRISPR technologies (Kozovska et al., 2021). With the development of CRISPR-Cas technology, the combination of isothermal amplification and CRISPR-Cas technology can rapidly detect DNA/RNA, effectively promoting the development of CRISPR-Cas technology in POCT (Mao et al., 2023). On the other hand, these technologies are indispensable tools when it comes to gene editing and in-depth molecular mechanism studies (Li et al., 2023a). These technologies will provide more options for future clinical diagnostics. However, these advanced detection technologies are not foolproof, there are still many challenges to be faced in their practical application. For example, diagnostic criteria may differ between different laboratories, which will affect the accuracy and comparability of diagnostic results. These techniques are difficult to operate and require experienced laboratory personnel. In addition, the cost of these techniques is relatively high, which restricts their popularization and application in primary healthcare institutions (Li et al., 2023b). It is only through overcoming these challenges that we will be able to continue developing, improving, and providing more timely and effective diagnostic and treatment services for patients with CDI.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by Zhejiang Shuren University Basic Scientific Research Special Funds (2024XZ011) and 2024 National Student Innovation and Entrepreneurship Training Program Project (202411842049). These funders did not participate in the designing, performing, or reporting of the current study.
Author contributions
D-AL: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. RH: Writing – review & editing. YQ: Writing – review & editing. YX: Writing – review & editing. KC: Writing – review & editing. JY: Writing – review & editing. XZ: Writing – review & editing, Writing – original draft, Project administration, Supervision. XL: Writing – review & editing, Writing – original draft, Project administration, Supervision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abad-Fau A., Sevilla E., Martín-Burriel I., Moreno B., Bolea R. (2023). Update on commonly used molecular typing methods for clostridioides difficile. Microorganisms 11, 1752. doi: 10.3390/microorganisms11071752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ágreda Fernández M., Origüen J., Rodriguez-Goncer I., San Juan R., López-Medrano F., Parra P., et al. (2024). Predictive value of fecal calprotectin and lactoferrin levels for negative outcomes in Clostridioides difficile infection. Eur. J. Clin. Microbiol. 43(2), 313–324. doi: 10.1007/s10096-023-04729-z [DOI] [PubMed] [Google Scholar]
- Åkerlund T., Persson I., Unemo M., Norén T., Svenungsson B., Wullt M., et al. (2008). Increased sporulation rate of epidemic clostridium difficile type 027/NAP1. J. Clin. Microbiol. 46, 1530–1533. doi: 10.1128/JCM.01964-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M. Z., Madan R. (2024). Clostridioides difficile toxins: host cell interactions and their role in disease pathogenesis. Toxins 16, 241. doi: 10.3390/toxins16060241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonara S., Leber A. L. (2016). Diagnosis of clostridium difficile infections in children. J. Clin. Microbiol. 54, 1425–1433. doi: 10.1128/JCM.03014-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-Páez A., Hartstra A. V., Nieuwdorp M., Sanz Y. (2022). Species- and strain-level assessment using rrn long-amplicons suggests donor’s influence on gut microbial transference via fecal transplants in metabolic syndrome subjects. Gut Microbes 14, 2078621. doi: 10.1080/19490976.2022.2078621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield M., Hutton S., Burton M., Tarring C., Velasco C., Clissold C., et al. (2024). Early identification of a ward-based outbreak of Clostridioides difficile using prospective multilocus sequence type-based Oxford Nanopore genomic surveillance. Infection Control Hosp. Epidemiol. 45(9), 1–7. doi: 10.1017/ice.2024.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham C. A., Carroll K. C. (2013). Diagnosis of clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin. Microbiol. Rev. 26, 604–630. doi: 10.1128/CMR.00016-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busi F. (2020). Fluorescent Oligonucleotide Probes for the Quantification of RNA by Real-Time qPCR. Methods Mol Biol. 2113, 263–280. doi: 10.1007/978-1-0716-0278-2_18 [DOI] [PubMed] [Google Scholar]
- Cao H., Huang H., Xu W., Chen D., Yu J., Li J., et al. (2011). Fecal metabolome profiling of liver cirrhosis and hepatocellular carcinoma patients by ultra performance liquid chromatography–mass spectrometry. Analytica Chimica Acta. 691(1–2), 68–75. doi: 10.1016/j.aca.2011.02.038 [DOI] [PubMed] [Google Scholar]
- Capobianchi M. R., Giombini E., Rozera G. (2013). Next-generation sequencing technology in clinical virology. Clin. Microbiol. Infection 19, 15–22. doi: 10.1111/1469-0691.12056 [DOI] [PubMed] [Google Scholar]
- Chen J. S., Ma E., Harrington L. B., Da Costa M., Tian X., Palefsky J. M., et al. (2018). CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439. doi: 10.1126/science.aar6245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C. L., López-Ureña D., De Oliveira Assis T., Ribeiro R. A., Silva R. O. S., Rupnik M., et al. (2016). A MLST Clade 2 Clostridium difficile strain with a variant TcdB induces severe inflammatory and oxidative response associated with mucosal disruption. Anaerobe. 40, 76–84. doi: 10.1016/j.anaerobe.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Dawkins J. J., Allegretti J. R., Gibson T. E., McClure E., Delaney M., Bry L., et al. (2022). Gut metabolites predict Clostridioides difficile recurrence. Microbiome 10, 87. doi: 10.1186/s40168-022-01284-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Daly M. M. (2024) Advances and challenges in SARS-coV-2 detection: A review of molecular and serological technologies. Diagnostics 14, 519. doi: 10.3390/diagnostics14050519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G., Vandeputte D., Caenepeel C., Vieira-Silva S., Daryoush T., Vermeire S., et al. (2019). The human microbiome in health and disease: Hype or hope. Acta Clinica Belgica 74, 53–64. doi: 10.1080/17843286.2019.1583782 [DOI] [PubMed] [Google Scholar]
- Feuerstadt P., Theriault N., Tillotson G. (2023). The burden of CDI in the United States: A multifactorial challenge. BMC Infect. Dis. 23, 132. doi: 10.1186/s12879-023-08096-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A., Vallone C., Sabatelli L., Ventura G., Covino M., Cammarota G., et al. (2018). Fecal calprotectin in management of Clostridium difficile infection: A longitudinal study. Scandinavian J. Gastroenterol. 53, 567–572. doi: 10.1080/00365521.2017.1392598 [DOI] [PubMed] [Google Scholar]
- Gandelman O., Jackson R., Kiddle G., Tisi L. (2011). Loop-mediated amplification accelerated by stem primers. Int. J. Mol. Sci. 12, 9108–9124. doi: 10.3390/ijms12129108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girão E. S. (2021). Evaluation of loop-mediated isothermal amplification assay for detection of Clostridioides difficile infection: A prospective diagnostic performance study. Anaerobe. 71, 102410. doi: 10.1016/j.anaerobe.2021.102410 [DOI] [PubMed] [Google Scholar]
- Goodwin S., McPherson J. D., McCombie W. R. (2016). Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 17, 333–351. doi: 10.1038/nrg.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. S., Abudayyeh O. O., Kellner M. J., Joung J., Collins J. J., Zhang F. (2018). Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360, 439–444. doi: 10.1126/science.aaq0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. S., Abudayyeh O. O., Lee J. W., Essletzbichler P., Dy A. J., Joung J., et al. (2017). Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442. doi: 10.1126/science.aam9321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M., Hauben L., Pouseele H., Jaillard M., De Bruyne K., Van Belkum A., et al. (2020). Retrospective definition of clostridioides difficile PCR ribotypes on the basis of whole genome polymorphisms: A proof of principle study. Diagnostics 10, 1078. doi: 10.3390/diagnostics10121078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh A. Y., Mu Y., Winston L. G., Johnston H., Olson D., Farley M. M., et al. (2020). Trends in U.S. Burden of clostridioides difficile infection and outcomes. New Engl. J. Med. 382, 1320–1330. doi: 10.1056/NEJMoa1910215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer H. M., Mulder A. H. L., De Boer N. K., Crouwel F., Van Rheenen P. F., Spekle M., et al. (2022). Impact of preanalytical factors on calprotectin concentration in stool: A multiassay comparison. J. Appl. Lab. Med. 7, 1401–1411. doi: 10.1093/jalm/jfac057 [DOI] [PubMed] [Google Scholar]
- Hargreaves K. R., Thanki A. M., Jose B. R., Oggioni M. R., Clokie M. R. J. (2016). Use of single molecule sequencing for comparative genomics of an environmental and a clinical isolate of Clostridium difficile ribotype 078. BMC Genomics 17, 1020. doi: 10.1186/s12864-016-3346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanain W. A., Spoors J., Johnson C. L., Faulds K., Keegan N., Graham D. (2021). Rapid ultra-sensitive diagnosis of clostridium difficile infection using a SERS-based lateral flow assay. Analyst 146, 4495–4505. doi: 10.1039/D1AN00726B [DOI] [PubMed] [Google Scholar]
- He J., Zhu S., Zhou J., Jiang W., Yin L., Su L., et al. (2023). Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay. Front Bioeng Biotechnol. 10, 1090281. doi: 10.3389/fbioe.2022.1090281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Zhang A. Y., Cao H. C., Lu H. F., Wang B. H., Xie Q., et al. (2013) Metabolomic analyses of faeces reveals malabsorption in cirrhotic patients. Digestive Liver Disease. 45(8), 677–682. doi: 10.1016/j.dld.2013.01.001 [DOI] [PubMed] [Google Scholar]
- Huang B., Jin D., Zhang J., Sun J. Y., Wang X., Stiles J., et al. (2014). Real-time cellular analysis coupled with a specimen enrichment accurately detects and quantifies clostridium difficile toxins in stool. J. Clin. Microbiol. 52, 1105–1111. doi: 10.1128/JCM.02601-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Wang Y., Zhang W., Li W., Bai L., Lu J., et al. (2023). A rapid multiplex real-time PCR detection of toxigenic Clostridioides difficile directly from fecal samples. 3 Biotech. 13(2), 54. doi: 10.1007/s13205-022-03434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Hu X., Lin C., Xia Z., Yang W., Zhu Y., et al. (2023. a). Rapid visualization of Clostridioides difficile toxins A and B by multiplex RPA combined with CRISPR-Cas12a. Front. Microbiol. 14, 1119395. doi: 10.3389/fmicb.2023.1119395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T., Hu X., Shen J. (2023. b). Establishment of a novel detection platform for clostridioides difficile toxin genes based on orthogonal CRISPR. Microbiol. Spectr. 11, e01886–e01823. doi: 10.1128/spectrum.01886-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung Y., Kim K., Lee S., Chun B.-S., Lee S., Hwang J., et al. (2022). Rapid and accurate on-site immunodiagnostics of highly contagious severe acute respiratory syndrome coronavirus 2 using portable surface-enhanced raman scattering-lateral flow assay reader. ACS Sensors 7, 3470–3480. doi: 10.1021/acssensors.2c01808 [DOI] [PubMed] [Google Scholar]
- Kameli N., Basode V. K., Abdulhaq A., Alamoudi M. U. A., Zain K. A. M., Ghzwani A. H. (2024). Prevalence of toxigenic Clostridium difficile in hospitalized patients in the southwestern province of Saudi Arabia: Confirmation using the GeneXpert analysis. Libyan J. Med. 19, 2294571. doi: 10.1080/19932820.2023.2294571 [DOI] [PubMed] [Google Scholar]
- Kaminski M. M., Abudayyeh O. O., Gootenberg J. S., Zhang F., Collins J. J. (2021). CRISPR-based diagnostics. Nat. Biomed. Eng. 5, 643–656. doi: 10.1038/s41551-021-00760-7 [DOI] [PubMed] [Google Scholar]
- Kim J., Kim H., Oh H. J., Kim H. S., Hwang Y. J., Yong D., et al. (2017). Fecal calprotectin level reflects the severity of clostridium difficile infection. Ann. Lab. Med. 37, 53–57. doi: 10.3343/alm.2017.37.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya S. K., Brusatori M., Martin J. V., Yurgelevic S., Huang C., Liberati D. M., et al. (2018). 2018Rapid detection of clostridium difficile toxins in serum by raman spectroscopy. J. Surg. Res. 232, 195–201. doi: 10.1016/j.jss.2018.06.031 [DOI] [PubMed] [Google Scholar]
- Koya S. K., Yurgelevic S., Brusatori M., Huang C., Diebel L. N., Auner G. W. (2019). 2019Rapid detection of clostridium difficile toxins in stool by raman spectroscopy. J. Surg. Res. 244, 111–116. doi: 10.1016/j.jss.2019.06.039 [DOI] [PubMed] [Google Scholar]
- Kozovska Z., Rajcaniova S., Munteanu P., Dzacovska S., Demkova L. (2021). CRISPR: History and perspectives to the future. Biomedicine Pharmacotherapy 141, 111917. doi: 10.1016/j.biopha.2021.111917 [DOI] [PubMed] [Google Scholar]
- Kubota H., Sakai T., Gawad A., Makino H., Akiyama T., Ishikawa E., et al. (2014). Development of taqMan-based quantitative PCR for sensitive and selective detection of toxigenic clostridium difficile in human stools. PloS One 9, e111684. doi: 10.1371/journal.pone.0111684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzli A. B., Burri S., Casanova C., Sommerstein R., Buetti N., Seth-Smith H. M. B., et al. (2020). Successful management of a Clostridioides difficile ribotype 027 outbreak with a lean intervention bundle. J. Hosp. Infection 106, 240–245. doi: 10.1016/j.jhin.2020.07.034 [DOI] [PubMed] [Google Scholar]
- Kuijper E. J., Coignard B., Tüll P. (2006). Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infection 12, 2–18. doi: 10.1111/j.1469-0691.2006.01580.x [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.-Y., Cheng Q.-X., Wang J.-M., Li X.-Y., Zhang Z.-L., Gao S., et al. (2018). CRISPR-Cas12a-assisted nucleic acid detection. Cell Discovery 4, 20. doi: 10.1038/s41421-018-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhong J., Li H., Qiao Y., Mao X., Fan H., et al. (2023. a). Advances in the application of CRISPR-Cas technology in rapid detection of pathogen nucleic acid. Front. Mol. Biosci. 10, 1260883. doi: 10.3389/fmolb.2023.1260883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhu S., Zhang X., Ren Y., He J., Zhou J., et al. (2023. b). Advances in the application of recombinase-aided amplification combined with CRISPR-Cas technology in quick detection of pathogenic microbes. Front. Bioeng Biotechnol. 11, 1215466. doi: 10.3389/fbioe.2023.1215466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Li Z., Lin Q., Wang P., Liu W., Yuan J., et al. (2022). Development and clinical application of a rapid and visual loop-mediated isothermal amplification test for tetM gene in Clostridioides difficile strains cultured from feces. Int. J. Infect. Dis. 122, 676–684. doi: 10.1016/j.ijid.2022.07.032 [DOI] [PubMed] [Google Scholar]
- Luna R. A., Boyanton B. L., Mehta S., Courtney E. M., Webb C. R., Revell P. A., et al. (2011). Rapid stool-based diagnosis of clostridium difficile infection by real-time PCR in a children’s hospital. J. Clin. Microbiol. 49, 851–857. doi: 10.1128/JCM.01983-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Yang Q., Zhang X., Zhang Y., Wan L., Zhan X., et al. (2022). TFPI is a colonic crypt receptor for TcdB from hypervirulent clade 2 C. difficile. Cell 185, 980–994.e15. doi: 10.1016/j.cell.2022.02.010 [DOI] [PubMed] [Google Scholar]
- Maestri A. C., Raboni S. M., Cogo L. L., Rossi M. V., Nogueira K. S. (2022). Standardisation and validation of an in-house quantitative real-time polymerase chain reaction (qPCR) assay for the diagnosis of Clostridioides difficile infection. J. Microbiological Methods. 193, 106399. doi: 10.1016/j.mimet.2021.106399 [DOI] [PubMed] [Google Scholar]
- Maestri A. C., Nogueira K. S., Mialski R., Dos Santos E. M., Kraft L., Raboni S. M. (2023). Laboratory diagnosis of Clostridioides difficile infection in symptomatic patients: What can we do better? Braz. J. Microbiol. 54, 849–857. doi: 10.1007/s42770-023-00956-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makristathis A., Zeller I., Mitteregger D., Kundi M., Hirschl A. M. (2017). Comprehensive evaluation of chemiluminescent immunoassays for the laboratory diagnosis of Clostridium difficile infection. Eur. J. Clin. Microbiol. Infect. Dis. 36, 1253–1259. doi: 10.1007/s10096-017-2916-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyshev D., Jones I. A., McKracken M., Öberg R., Harper G. M., Joshi L. T., et al. (2023). Hypervirulent R20291 Clostridioides difficile spores show disinfection resilience to sodium hypochlorite despite structural changes. BMC Microbiol. 23, 59. doi: 10.1186/s12866-023-02787-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Xu M., Luo S., Yang Y., Zhong J., Zhou J., et al. (2023). Advancements in the synergy of isothermal amplification and CRISPR-cas technologies for pathogen detection. Front. Bioengineering Biotechnol. 11, 1273988. doi: 10.3389/fbioe.2023.1273988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbunge E., Chemhaka G., Batani J., Gurajena C., Dzinamarira T., Musuka G., et al. (2022). Predicting diarrhoea among children under five years using machine learning techniques. Artificial Intelligence Trends in Systems 502, 94–109. doi: 10.1007/978-3-031-09076-9_9 [DOI] [Google Scholar]
- Miller R. R., Montoya V., Gardy J. L., Patrick D. M., Tang P. (2013). Metagenomics for pathogen detection in public health. Genome Med. 5, 81. doi: 10.1186/gm485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P., Kyne L., Martin A., Solomon K. (2013). Germination efficiency of clinical Clostridium difficile spores and correlation with ribotype, disease severity and therapy failure. J. Med. Microbiol. 62, 1405–1413. doi: 10.1099/jmm.0.056614-0 [DOI] [PubMed] [Google Scholar]
- Mullish B. H., Martinez-Gili L., Chekmeneva E., Correia G. D. S., Lewis M. R., Horneffer-Van Der Sluis V., et al. (2022). Assessing the clinical value of faecal bile acid profiling to predict recurrence in primary Clostridioides difficile infection. Alimentary Pharmacol. Ther. 56, 1556–1569. doi: 10.1111/apt.17247 [DOI] [PubMed] [Google Scholar]
- Myhrvold C., Freije C. A., Gootenberg J. S., Abudayyeh O. O., Metsky H. C., Durbin A. F., et al. (2018). Field-deployable viral diagnostics using CRISPR-Cas13. Science 360, 444–448. doi: 10.1126/science.aas8836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano L. M., Edmiston C. E., Jr. (2017). Clostridium difficile disease: Diagnosis, pathogenesis, and treatment update. Surgery 162, 325–348. doi: 10.1016/j.surg.2017.01.018 [DOI] [PubMed] [Google Scholar]
- National Center for Emerging and Zoonotic Infectious Diseases (U.S.) Division of Healthcare Quality Promotion . (2023). Emerging Infections Program Healthcare-Associated Infections–Community Interface Report Clostridioides difficile Infection Surveillance, 2021, p.7. Available online at: https://stacks.cdc.gov/view/cdc/148753 [Google Scholar]
- Norén T., Unemo M., Magnusson C., Eiserman M., Matussek A. (2014). Evaluation of the rapid loop-mediated isothermal amplification assay Illumigene for diagnosis of Clostridium difficile in an outbreak situation. APMIS 122, 155–160. doi: 10.1111/apm.12121 [DOI] [PubMed] [Google Scholar]
- Okafor C. M., Clogher P., Olson D., Niccolai L., Hadler J. (2023). Trends in and risk factors for recurrent clostridioides difficile infection, new haven county, connecticut, US 2020. Emerging Infect. Dis. 29, 877–887. doi: 10.3201/eid2905.221294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira P. H., Ribis J. W., Garrett E. M., Trzilova D., Kim A., Sekulovic O., et al. (2020). Epigenomic characterization of Clostridioides difficile finds a conserved DNA methyltransferase that mediates sporulation and pathogenesis. Nat. Microbiol. 5(1), 166–180. doi: 10.1038/s41564-019-0613-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z., Zhang Y., Luo J., Li D., Zhou Y., He L., et al. (2021). Functional analyses of epidemic Clostridioides difficile toxin B variants reveal their divergence in utilizing receptors and inducing pathology. PloS Pathog. 17, e1009197. doi: 10.1371/journal.ppat.1009197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planche T., Wilcox M. (2011). Reference assays for Clostridium difficile infection: One or two gold standards? J. Clin. Pathol. 64, 1–5. doi: 10.1136/jcp.2010.080135 [DOI] [PubMed] [Google Scholar]
- Quesada-Gómez C., López-Ureña D., Acuña-Amador L., Villalobos-Zúñiga M., Du T., Freire R., et al. (2015). Emergence of an outbreak-associated clostridium difficile variant with increased virulence. J. Clin. Microbiol. 53, 1216–1226. doi: 10.1128/JCM.03058-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quince C., Walker A. W., Simpson J. T., Loman N. J., Segata N. (2017). Shotgun metagenomics, from sampling to analysis. Nature biotechnology. 35(9), 833–844. doi: 10.1038/nbt.3935 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G., Young M. K., Nayak U., Rigo I., Marrs A. S., Gilchrist C. A., et al. (2024). Systemic neutrophil degranulation and emergency granulopoiesis in patients with Clostridioides difficile infection. Anaerobe 87, 102840. doi: 10.1016/j.anaerobe.2024.102840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod R. H., Chaudhari S. R., Patil A. S., Shirkhedkar A. A. (2019). Ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS) in practice: Analysis of drugs and pharmaceutical formulations. Future J. Pharm. Sci. 5, 6. doi: 10.1186/s43094-019-0007-8 [DOI] [Google Scholar]
- Ryder A. B., Huang Y., Li H., Zheng M., Wang X., Stratton C. W., et al. (2010). Assessment of Clostridium difficile Infections by Quantitative Detection of tcdB Toxin by Use of a Real-Time Cell Analysis System. J. Clin. Microbiol. 48, 4129–4134. doi: 10.1128/JCM.01104-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Staley C., Heiner C., Hall R., Kelly C. R., Brandt L., et al. (2017). Analysis of gut microbiota – An ever changing landscape. Gut Microbes 8, 268–275. doi: 10.1080/19490976.2016.1277313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth-Smith H. M. B., Biggel M., Roloff T., Hinic V., Bodmer T., Risch M., et al. (2021). Transition from PCR-ribotyping to whole genome sequencing based typing of clostridioides difficile. Front. Cell. Infection Microbiol. 11, 681518. doi: 10.3389/fcimb.2021.681518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Lin S., You P., Chen Y., Luo Y., Song X., et al. (2024). Rapid discrimination between clinical Clostridioides difficile infection and colonization by quantitative detection of TcdB toxin using a real-time cell analysis system. Front. Microbiol. 15, 1348892. doi: 10.3389/fmicb.2024.1348892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty N., Wren M. W. D., Coen P. G. (2011). The role of glutamate dehydrogenase for the detection of Clostridium difficile in faecal samples: A meta-analysis. J. Hosp. Infection 77, 1–6. doi: 10.1016/j.jhin.2010.07.024 [DOI] [PubMed] [Google Scholar]
- Solly K., Wang X., Xu X., Strulovici B., Zheng W. (2004). Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay and drug development technologies. 2(4), 363–372. doi: 10.1089/adt.2004.2.363 [DOI] [PubMed] [Google Scholar]
- Strachan A. J., Evans N. E., Williams O. M., Spencer R. C., Greenwood R., Probert C. J. (2013). Comparison of a frozen human foreskin fibroblast cell assay to an enzyme immunoassay and toxigenic culture for the detection of toxigenic Clostridium difficile. Diagn. Microbiol. Infect. Dis. 75, 42–45. doi: 10.1016/j.diagmicrobio.2012.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriot C. M., Young V. B. (2015). Interactions between the gastrointestinal microbiome and clostridium difficile . Annu. Rev. Microbiol. 69, 445–461. doi: 10.1146/annurev-micro-091014-104115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Qiu Z., Jiang Y., Zhu D., Zhou X. (2022). Exploiting the orthogonal CRISPR-Cas12a/Cas13a trans-cleavage for dual-gene virus detection using a handheld device. Biosensors Bioelectronics 196, 113701. doi: 10.1016/j.bios.2021.113701 [DOI] [PubMed] [Google Scholar]
- Vasilescu I.-M., Chifiriuc M.-C., Pircalabioru G. G., Filip R., Bolocan A., Lazăr V., et al. (2022). Gut dysbiosis and clostridioides difficile infection in neonates and adults. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.651081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Srivastava S., Garg A., Xiao C., Hawks S., Pan J., et al. (2024). Digital surface-enhanced raman spectroscopy–lateral flow test dipstick: ultrasensitive, rapid virus quantification in environmental dust. Environ. Sci. Technol. 58, 4926–4936. doi: 10.1021/acs.est.3c10311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarden A. R., Chen C., Bobr A., Yao D., Lu Y., Nelson V. M., et al. (2014). Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection. Am J Physiol Gastrointest Liver Physiol. 306 (4), G310–G319. doi: 10.1152/ajpgi.00282.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P. C. Y., Lau S. K. P., Chan K., Fung A. M. Y., Tang B. S. F., Yuen K. (2005). Clostridium bacteraemia characterised by 16S ribosomal RNA gene sequencing. J. Clin. Pathol. 58, 301–307. doi: 10.1136/jcp.2004.022830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahaya S. S. S., Lim Y.-F., Ali H., Omar Z. (2016). Robust linear discriminant analysis. J. Mathematics Stat 12, 312–316. doi: 10.3844/jmssp.2016.312.316 [DOI] [Google Scholar]
- Yang Q. (2022). Protocol for genome-wide CRISPR knockout screens of bacterial cytotoxins in HeLa cells. STAR protocols. 3(3), 101595. doi: 10.1016/j.xpro.2022.101595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. J., Nam Y. S., Kim M. J., Cho S. Y., You E., Soh Y. S., et al. (2014). Evaluation of a chromogenic culture medium for the detection of clostridium difficile . Yonsei Med. J. 55, 994. doi: 10.3349/ymj.2014.55.4.994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youden W. J. (1950). Index for rating diagnostic tests. Cancer 3, 32–35. doi: [DOI] [PubMed] [Google Scholar]
- Yu L., Li H., Zhao X., Wang X., Wei X., Lin W., et al. (2017). Rapid visual detection of binary toxin producing Clostridium difficile by loop−mediated isothermal amplification. Exp. Ther. Med. 14(5), 4781–4788. doi: 10.3892/etm.2017.5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangiabadian M., Ghorbani A., Nojookambari N. Y., Ahmadbeigi Y., Hosseini S. S., Karimi-Yazdi M., et al. (2023). Accuracy of diagnostic assays for the detection of Clostridioides difficile: A systematic review and meta-analysis. J. Microbiological Methods 204, 106657. doi: 10.1016/j.mimet.2022.106657 [DOI] [PubMed] [Google Scholar]
- Zetsche B., Gootenberg J. S., Abudayyeh O. O., Slaymaker I. M., Makarova K. S., Essletzbichler P., et al. (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-cas system. Cell 163, 759–771. doi: 10.1016/j.cell.2015.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wylie K. M., El Feghaly R. E., Mihindukulasuriya K. A., Elward A., Haslam D. B., et al. (2016). Metagenomic approach for identification of the pathogens associated with diarrhea in stool specimens. J. Clin. Microbiol. 54(2), 368–375. doi: 10.1128/JCM.01965-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Zhou N., Shao L., Li J., Liu S., Meng X., et al. (2018). Diagnosis of Clostridium difficile infection using an UPLC–MS based metabolomics method. Metabolomics 14, 102. doi: 10.1007/s11306-018-1397-x [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wu Y., Zeng H., Chen C., Xie Q., He L. (2024). Analysis of Clostridioides difficile infection characteristics and risk factors in patients hospitalized fordiarrhea in 3 university hospitals in a mid-south city of China. Nan fang yi ke da xue xue bao. 44(5), 998–1003. doi: 10.12122/j.issn.1673-4254.2024.05.23 [DOI] [PMC free article] [PubMed] [Google Scholar]