Abstract
Asthma, is a common, significant and diverse condition marked by persistent airway inflammation, with a major impact on human health worldwide. The predisposing factors for asthma are complex and widespread. The beneficial effects of omega-3 (n-3) polyunsaturated fatty acids (PUFAs) in asthma have increasingly attracted attention recently. In asthma therapy, n-3 PUFAs may reduce asthma risk by controlling on levels of inflammatory cytokines and regulating recruitment of inflammatory cells in asthma. The specialized pro-resolving mediators (SPMs) derived from n-3 PUFAs, including the E- and D-series resolvins, protectins, and maresins, were discovered in inflammatory exudates and their biosynthesis by lipoxygenase mediated pathways elucidated., SPMs alleviated T-helper (Th)1/Th17 and type 2 cytokine immune imbalance, and regulated macrophage polarization and recruitment of inflammatory cells in asthma via specific receptors such as formyl peptide receptor 2 (ALX/FPR2) and G protein-coupled receptor 32. In conclusion, the further study of n-3 PUFAs and their derived SPMs may lead to novel anti-inflammatory asthma treatments.
Keywords: asthma, inflammation, n-3 fatty acid, maresin, protectin, resolvin
1. Introduction
Asthma, a common, non-communicable condition, with substantial morbidity, impacted 262 million individuals worldwide and resulted in 455,000 deaths, according to recent analyses (1, 2). The diversity and universality of pathogenic factors account for its widespread prevalence. Genetic risk factors, including family history and gender; lifestyle factors such as diet, exercise, stress, obesity and environmental factors, particularly inhalant allergens (dust mites, pollen), air pollution, smoke and occupational exposures (3), all affect the prevalence and mortality of asthma globally (4). The pathogenesis of asthma is extremely complex, involving multiple inflammatory mechanisms including Type 2 inflammation, T-helper (Th)1/Th17 immune imbalance, increased inflammatory cytokines, inflammatory cell recruitment and ultimately pathologic changes in the airways (5).
Omega-3 (n-3) polyunsaturated fatty acids (PUFAs) comprise a group of polyunsaturated fats, represented by Docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), essential nutrients, found in many foods. Published research suggests that n-3 PUFAs exhibit immunologic activity, affecting a variety of physiologic and pathologic processes, including cognitive function (6), vascular and myocardial function (7), inflammation (8), atopic disease (9), and cardiovascular diseases (10). In recent years, n-3 PUFAs-derived lipid mediators called specialized pro-resolving mediators (SPMs) were discovered and found to be biosynthesized by lipoxygenase mediated pathways, with the reports on their pro-resolving effects and anti-inflammatory activity. SPMs were able to regulate various inflammatory mechanisms in asthma and were the potential active mediators of the anti-asthma effects of n-3 PUFAs (11). This review aims to assess the established benefits of n-3 PUFAs in asthma, focusing on the n-3 PUFA-derived specialized pro-resolving lipid mediators and their anti-inflammatory properties.
2. Asthma phenotyping
Asthma is a diverse condition characterized by fluctuating respiratory symptoms, particularly wheeze, cough and breathlessness, which vary in intensity and frequency over time, associated with reversible expiratory airflow limitation, which may persist and become irreversible (12). Among the primary pathologic traits of asthma are airway hyper-responsiveness (AHR), airway remodeling, disrupted mucosal immunity, and persistent airway inflammation (13, 14).
Asthma has been classified into different phenotypes (15): according to age (childhood (16), adolescent (17), adult (18), and elderly asthma (19)); severity (severe and non-severe asthma (20)); inducing factors (allergic, non-allergic and occupational asthma (12), obesity asthma (21), etc.); biomarkers (eosinophilic, neutrophilic asthma, etc. (22)). Cluster analysis studies have defined the main phenotypes of asthma including early-onset allergic asthma, early-onset allergic moderate-to-severe remodeled asthma, late-onset nonallergic eosinophilic asthma, and late-onset nonallergic noneosinophilic asthma etc. (23). Endotypes, subtypes of disease defined functionally and pathologically by a molecular mechanism or by treatment, more succinctly classify asthma as type 2 (T2) and non-T2 types (24).
3. T2 asthma
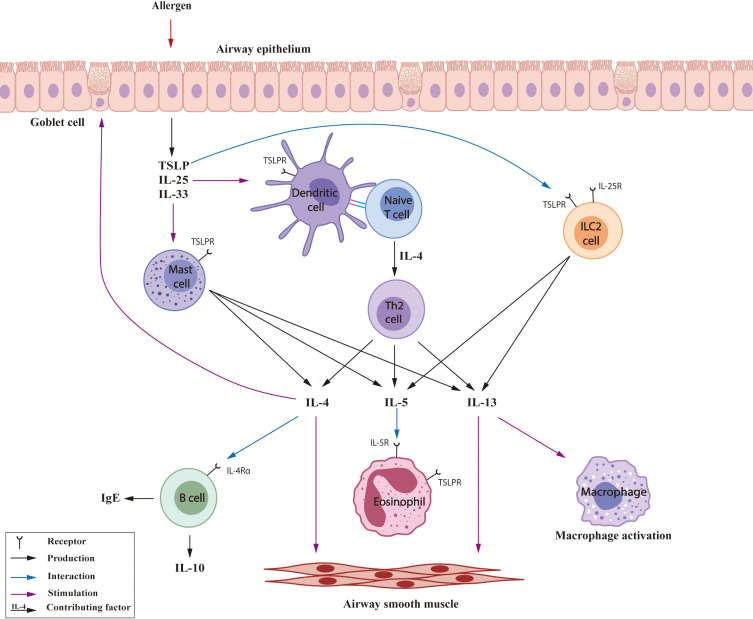
Type 2 immune processes represent a classic mechanism of allergy and an essential feature of asthma ( Figure 1 ). Type 2 inflammation plays a major role in eosinophilic and allergic asthma, and has been observed in 50% - 70% asthma patients (25). Inhaled allergens stimulate airway epithelial cells to release alarmins (26), which may interact with dendritic cells (DCs) and induce differentiation of naive T cells into Th2 cells (27). In addition, Th2 cells and type 2 innate lymphoid cells (ILC2s) produce a variety of type 2 cytokines, especially interleukins including interleukin (IL)-4, IL-5 and IL-13 (28, 29). IL-4 promotes the differentiation of Th2 cells, B cell switching and IgE production, goblet cell hyperplasia and mucus production, epithelial barrier disruption and tissue remodeling, airway smooth contraction and AHR (30, 31). Although the major effects of IL-13 are very similar to those of IL-4, some independent pathways of eosinophilia (32) and M2 macrophage polarization (33) have been reported for IL-13. IL-5 has a pivotal role in facilitating the maturation and recruitment of eosinophils (34); it is also released by mast cells and ILC2s, particularly after interaction with thymic stromal lymphopoietin (TSLP) (33, 35). Mixed granulocytic asthma, with elevation of sputum (and airway) neutrophils and eosinophils is a rarer phenotype, but it tends to feature Type 2 inflammation with the anticipated responses (22, 36).
Figure 1.
T2 inflammatory mechanisms in asthma. TSLP: thymic stromal lymphopoietin.
4. Non-T2 asthma
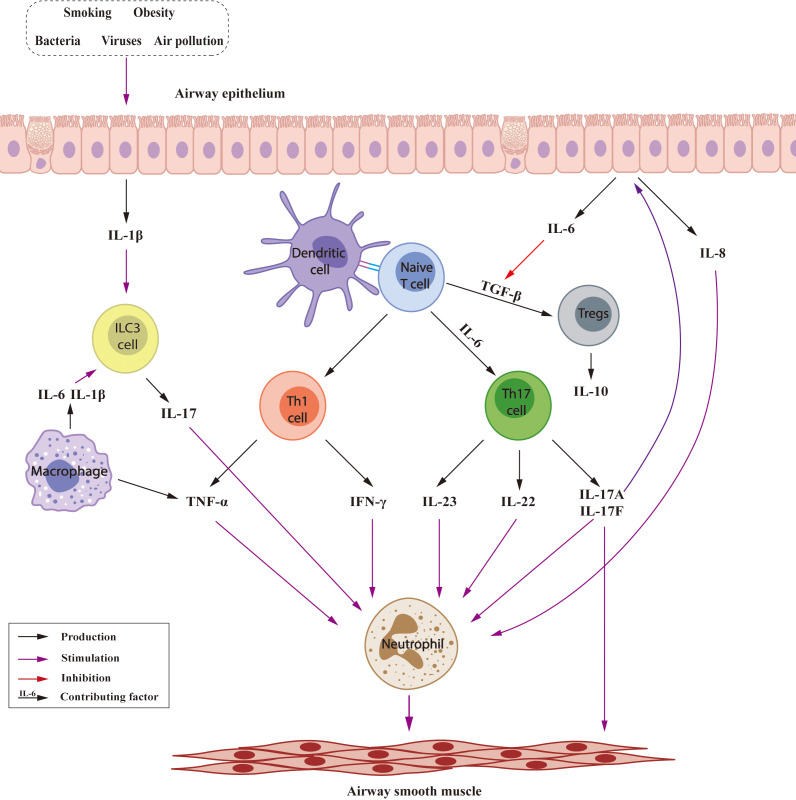
Non-T2 asthma is characterized by neutrophilic and paucigranulocytic inflammation, and may be triggered by factors including smoking, obesity, bacteria, viruses, and air pollution ( Figure 2 ) (37). In non-T2 asthma, naive T cells differentiate into Th1, Th17 cells. Th1 cells produce tumor necrosis factor-α (TNF-α) and interferon-gamma (IFN-γ) while Th17 cells produce a variety of cytokines (38, 39); together contributing to recruitment and activation of neutrophils leading to AHR and airway remodeling (40, 41). IL-17 stimulated airway epithelial cells release IL-6, which promotes differentiation of naive T cells into Th17 cells (42) and inhibition of transforming growth factor-β (TGF-β)-induced production of regulatory T cells (Tregs) (43). IL-8, is also produced by airway epithelial cells, increasing neutrophil numbers (44). In addition, innate lymphoid cells (ILC) 3 cells are another source of IL-17 (45), and macrophage-derived IL-6 and IL-1β could stimulate ILC3 to produce IL-17 (46, 47). Tregs, generated from naive T cells, suppress the Th2 response in asthma, inhibition TGF-β may exacerbate airway inflammation and remodeling by Treg downregulation (48). Tregs (49), B cells (50) and CD8+ T cells (51) produce IL-10, which decrease tissue mast cell and eosinophil counts and may prevent neutrophilic asthma.
Figure 2.
Inflammatory mechanisms involved in Non-T2 asthma.
Paucigranulocytic asthma may account for up to 40% of patients with asthma (52) and though it was usually well controlled on treatment, or intermittent in the Severe Asthma Research Program cohort (53), it has been relatively little studied. It has been suggested that the number of granulocytes may reflect depletion of eosinophils by steroid therapy. By contrast with the immune imbalance in neutrophilic asthma,paucigranulocytic asthma may be more strongly associated with neural regulation as suggested by high levels of nerve growth factor (NGF) (54) and sphingolipid synthesis inhibition (55) induced AHR, and bronchoconstrictor signaling (56) are also involved in the pathogenesis of paucigranulocytic asthma.
5. PUFAs in asthma
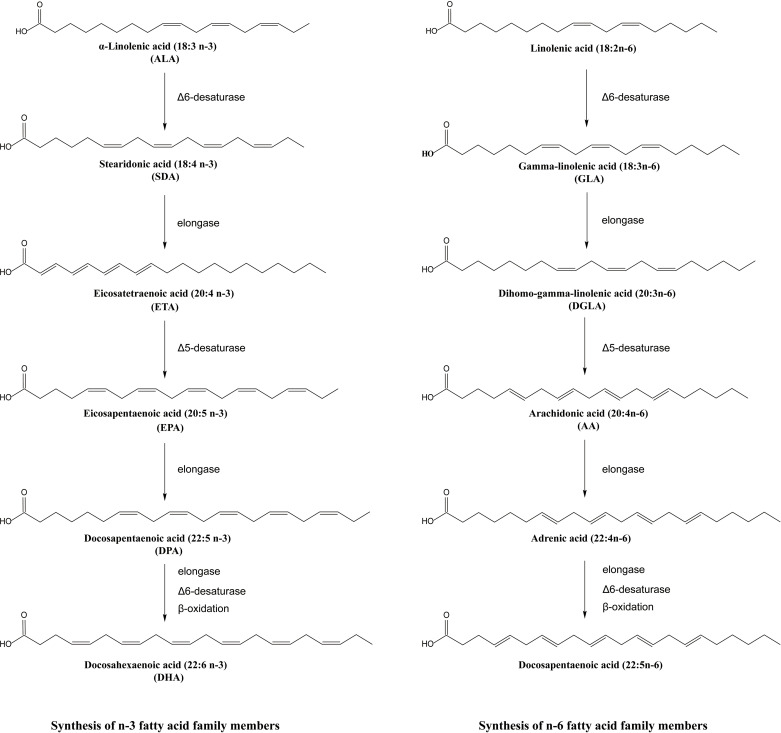
PUFAs are defined as fatty acids characterized by the presence of multiple double bonds, with a terminal methyl carbon at one end and the iconic hydroxyl group at the other (57). They are sometimes called essential fatty acids as they cannot be synthesized by humans and must be obtained through the diet. PUFAs are classified as omega-3 or n-3 PUFAs when their first double bond is situated between the third and fourth carbon atoms (58) and omega-6 PUFAs when the carbon-carbon double bond is at the n-6 position. A series of enzymatic reactions catalyzed the synthesis of n-3 PUFAs from the precursor alpha-linoleic acid (ALA), including EPA, DHA, and docosapentaenoic acid (DPA) and the biosynthesis of n-6 PUFAs including gamma-γ-linolenic acid (GLA), dihomo-gamma-linolenic acid (DGLA), and arachidonic acid (AA) (59, 60) as shown in Figure 3 . The Δ5 desaturase and the Δ6 desaturase enzymes insert double bonds at the fifth and sixth carbon atoms, and the chain is shorted by β-oxidation (61). The shared desaturase and elongase enzymes lead to competition between n-3 and n-6 PUFAs, the n-6/n-3 ratio in organisms sometimes depends on the ingested ratio of substrates for n-6 and n-3 PUFAs (62).The importance of the n-6/n-3 ratio has been highlighted in cardiovascular disease (63), cancer (64), asthma (65) and other diseases. Because of the complicated combined actions of n-3 and n-6 PUFAs, beneficial effects of mixed fatty acids at an n-6/n-3 ratio of 5:1 were reported in asthma but at a ratio of 10:1, the effects became negative (66), suggesting meaningful roles for both n-3 and n-6 PUFAs in asthma.
Figure 3.
Synthesis of n-3 and n-6 fatty acid family members.
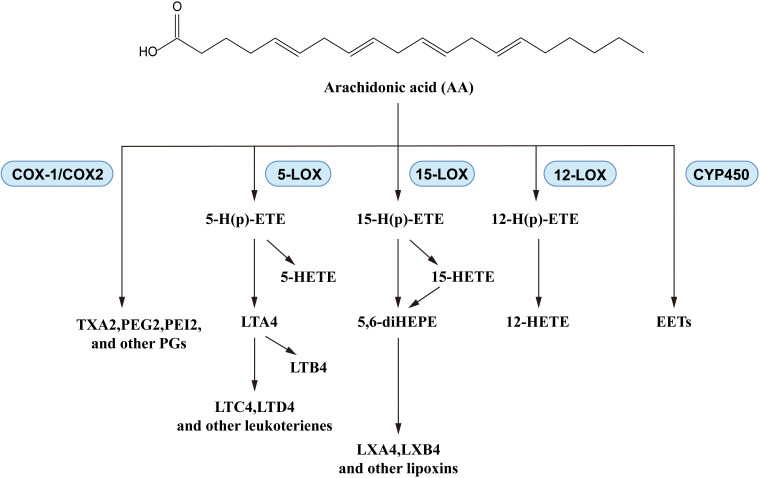
N-6 PUFAs, particularly AA, have demonstrated complex effects in asthma. In a large cross-sectional study, asthma risk was significantly negatively corelated with omega-6 fatty acid intake (67), as in the report from Lee-Sarwar et al. (9). However, asthma exacerbations influenced the levels of n-6 PUFAs in vivo: the plasma AA levels showed a positive correlation with childhood asthma attacks (68). Similar trends were also observed in lung cells of asthmatic mice (69), and in plasma levels of the AA-derived eicosanoids, prostaglandin E2 (PGE2) and thromboxane B2 (TXB2), in asthma patients (70). N-6 PUFAs generate mediators that play important roles in asthma development (71, 72), while AA produces leukotrienes, prostaglandins, and thromboxanes via a series of enzymatic reactions catalyzed by cyclooxygenase and lipoxygenase ( Figure 4 ). There are some reports about the pro-inflammatory activities of eicosanoids: leukotrienes increased vascular permeability and smooth-muscle contraction (73), prostaglandins induced allergen sensitization and Th2 immune response (74), and thromboxanes promoted bronchoconstriction and AHR (75). Taking into consideration the positive regulatory effect of n-6 PUFAs in asthma, further studies are needed to clarify the complex mechanisms of n-6 PUFAs effects in asthma. In fact, existing studies of PUFAs in asthma are more focused on the n-3 PUFAs: many clinical trials and animal experiments have elucidated their effects.
Figure 4.
Biosynthesis of AA-derived lipid mediators.
6. Effects of n-3 PUFAs in asthma and lung inflammation
As critical nutrients in diets, the sources of n-3 PUFAs are multifarious. The main sources of EPA and DPA are fish and seafood, while ALA is found in leafy vegetables and nuts (57). DHA has played a beneficial role in cardiovascular disease, the brain and visual function and inflammation (76). EPA showed helpful influences on brain function, oxidative stress, inflammation, hyperlipidemia and neurodegenerative diseases (77). Fish and lean red meat are sources of DPA, and the effects of DPA such as anti-inflammatory actions, antiplatelet aggregation, and improvement of plasma lipid have been reported (78). Because of the high β-oxidation rate of ALA (79), the few sources and low conversion of SDA to DHA (80), these two kinds of n- 3 PUFAs are rarely used in clinical anti-inflammatory treatment. In this review, we mainly discuss EPA, DHA, and DPA that are easily obtained in the daily diet and frequently supplemented in asthma therapy. Positive outcomes associated with n-3 PUFAs have been documented in the context of preventive measures (81, 82) and disease control (83) of asthma. According to a related study (84), n-3 intake decreased asthma risk in a dose-dependent manner (< 59.0 mg/kg/day). Various types of n-3 PUFA supplements have been implemented; including the delivery of combinations of various PUFAs, fish oil and diets rich in PUFAs. This article will examine the impact of various forms of n-3 PUFAs, rather than n-6 PUFAs, on asthma prevalence, lung inflammation, asthma challenge testing, and clinical asthma, as reported in recent clinical trials [ Table 1 (85–88), Table 2 (89–97)] and in animal/cellular asthma models [ Table 3 (69, 98–103)].
Table 1.
Effects of n-3 PUFAs on asthma in clinical trials.
| Form of n-3 PUFAs |
Intervention | Participants | Outcomes | Reference |
|---|---|---|---|---|
| EPA and DHA | High dose (3.7 g EPA + 2.5 g DHA/d) x 21 days, low dose (1.8 g EPA + 1.3 g DHA/d) and placebo x 21 days | 8 male adults with asthma and HIB and 8 healthy male adult controls | Peak fall in FEV1 reduced by 34% and 30% (both p + 0.001): baseline fraction of exhaled NO was reduced by 24% and 31% (p = or < 0.02) | (85) |
| EPA and DHA | 180 mg EPA + 120 mg DHA/d x 3 months | 39 asthma patients (aged 4 - 14 y) | Two-point improvement in symptom score in 28 patients and in PEF and lower IL-17A and TNF-α levels (p < 0.05) | (86) |
| PUFA-enriched fat blend |
450 mg EPA + 180 mg DHA + 60 mg gamma linoleic acid + 60 mg SDA/d | 13 female and 10 male adults with asthma (aged 22 - 29 y) | eNO was significantly lower (p = 0.022) with lower levels of serum eosinophils (10.1 8 ± 0.1.84 vs. 5.79 ± 80.69%), eosinophilic cationic protein (20.5 8 ± 9.93 vs. -1.68 ± 4.36 ng/mL) and cysteinyl leukotriene release (2,889 ± 872 vs. 1,120 ± 173 ng/mL) (p < 0.05 each) in the n-3 PUFA group | (87) |
| EPA and DHA | (55% EPA+37% DHA) 2.4 g/day and placebo from 24 weeks’ gestation until 1week postpartum | Pregnant women and their offspring between birth and 3 to 5 years of age |
The cumulative risk of persistent wheeze or asthma were decreased from birth to 3 - 5 years (16.9% vs 23.7%, relative risk (RR) = 0.69, 95%CI 0.49 to 0.97, p = 0.035) and birth to 5 years (17.5% vs 24.6%, RR = 0.68, 95%CI 0.49 to 0.95, p = 0.024), but no difference in the risk of asthma exacerbations | (88) |
HIB, hyperpnoea-induced bronchoconstriction; FEV1, forced expiratory volume in 1 second; eNO, exhaled nitric oxide; y, years; PEF, peak expiratory flow; ELFE, Etude Longitudinale Francais depuis L’Enfance; LCPUFA, long-chain PUFA; RR, relative risk.
Table 2.
Effects of n-3 PUFA-enriched fish oil diets on asthma in clinical trials.
| Form of n-3 PUFAs |
Intervention | Participants | Outcomes | Reference |
|---|---|---|---|---|
| EPA, DHA and DPA in diet | Median dietary intakes: 0.24 g/1900 kcal and 0.27 g/1900 kcal at 8 and 16 y | 1992 children born between 1994 and 1996 Swedish population cohort |
High plasma n-3 PUFAs at 8 y was inversely associated with prevalent asthma at 24 y suggesting protection from lower dietary intake in childhood | (89) |
| Diet with enriched DHA or EPA | 9.0 to 20.3 mg/100 kcal for DHA and 2.1 to 3.3 mg/100 kcal for EPA | 8389 formula-fed infants from ELFE cohort at 2 mouths 36% had DHA and ARA supplementation and 11% had DHA, ARA and EPA supplementation | High DHA, ARA and EPA content supplementation was associated with a lower use of asthma medications from aged 2 months to 5.5 y | (90) |
| n-3 PUFAs in diet estimated by food questionnaire | 1.6 - 2.6 g/d total n-3 PUFA | 412 mother-child dyads (22% with active asthma in pregnancy) |
Lower n-3 PUFA intake during pregnancy was significantly associated with risk of childhood asthma to age 4 y (p < 0.03), more apparent in female children | (91) |
| n-3 PUFA-rich fish oil | (55% EPA + 37% DHA) 2.4 g/day and placebo in 3rd trimester, double-blind and randomized | 695 pregnant women and their children followed up till age 6 y | n-3 PUFA supplementation in 3rd trimester reduced risk of wheeze or asthma by 30% at 6 y with 73% reduction in non-atopic asthma | (92) |
| n-3 LCPUFA-rich fish oil | (800 mg DHA + 100 mg EPA)/d double-blind randomized multicenter recruited from 21 weeks of pregnancy onwards | 706 Australian children with a family history of allergic disease followed up till 6y of age | No difference in the percentage of children with allergic disease (RR 1.04 p = 0.73) | (93) |
| n-3 PUFA-rich fish oil | Fish oil dose < 250 mg in 11, Fish oil dose < 250 and < 500 mg in 9 and Fish oil dose > 500 mg in 26 | 46 pregnant women in 1st and 3rd trimester | Prenatal fish oil or fish oil supplementation in the first trimester reduced asthma risk among offspring at age 3 y | (94) |
| n-3 PUFA-rich fish oil | Fish oil capsules (900 mg of LCPUFA) vegetable oil control group | 706 children with familial risk of allergy | No difference between fish oil and controls in allergic symptoms or sensitization at 1, 3 and 6 y | (95) |
| n-3 PUFA-rich fish oil | Fish oil capsules (3.2 g EPA + 2.2 g DHA)/d and placebo | 10 athletes with exercise-induced bronchoconstriction (EIB) and 10 athletes without EIB | FEV1 decreased were inhibited (3 ± 2% vs 14.5 ± 5%) at 15 minutes postexercise,TNF-α and IL-1β decreased | (96) |
| n-3 PUFA-rich fish oil | Fish oil capsules (3.2 g EPA + 2.2 g DHA)/d and placebo | 7 male and 15 female atopic and nonsmoking asthma patients (aged 18 - 42 y) | After 2 to 7 h of dietary supplementation with Max-EPA, the late asthmatic response was significantly attenuated (p < 0.05), the EPA content in neutrophil were increased to 10-fold, and neutrophil chemotactic responses were depressed by approximately 50%, 47% inhibition of leukotriene B generation | (97) |
Table 3.
Effects of n-3 PUFAs on inflammation in animal and cellular ‘asthma’ models.
| Models | Administration | Effects | Models | Reference |
|---|---|---|---|---|
| OVA-induced asthma in BALB/c mice and control groups | LCPUFAs (1000 mg/kg/d; 50% EPA and 50% DHA) by gavage from days 21 - 28 | Airway response and BALF eosinophils were decreased by LCPUFAs (p < 0.05), IL-5, IL-4, IL-13 levels and remodeling were also decreased (p < 0.05) | OVA-induced asthma in BALB/c mice and control groups | (98) |
| HDM-induced chronic asthma model in C57BL/6 mice and controls | LCPUFA:1000 mg/kg EPA + 229.6 mg/kg DHA + 246.0 mg/kg GLA + 200.9 mg/kg SDA/day compared to1000 mg/kg/day EPA from days 11 - 35 | In asthmatic mouse lung and blood cells, AA and DHA were increased (p < 0.001 and p < 0.01) while DGLA was decreased. (p < 0.05) in lung cells. Combination n-3 and n-6 LCPUFAs decreased AA and increased EPA, DPA (all p < 0.001), and DHA (p < 0.01) and reversed the lack of DGLA (p < 0.05) | HDM-induced chronic asthma model in C57BL/6 mice and controls | (69) |
| HDM-induced asthma model in C57BL/6 mice and controls | LCPUFA combination reduced AHR, decreased the relative amount of eosinophils, reduced IL-5, IL-4, IL-13, IL-6, IL-10 and IFN-γ, IL-6, and increased the release of EPA derived E-series resolvins (RvEs), and DPA-derived SPMs and D-series resolvins (RvDs) in BALF | HDM-induced asthma model in C57BL/6 mice and controls | (99) | |
| PM2.5-induced lung injury in male C57BL/6N and control mice | ω-3 PUFAs-enriched diet (EPA/DHA = 3:2) 21 g/kg for 6 weeks, with/without intratracheal PM2.5 | n-3 fatty acid group showed reduced alveolar septal thickness and inflammatory cells, with decreased levels of TNF-α, IL-1β, IL-6, and IL-17 (p < 0.05 - 0.01) in serum and BALF | PM2.5-induced lung injury in male C57BL/6N and control mice | (100) |
| HDM-induced asthma model in female adult mice | OmeGo (enzymatically liberated salmon oil; 20 and 60 μg, vehicle and positive control (apolipoprotein) | vehicle and positive total cell and eosinophil countsin BALF (p < 0.01) and eosinophils in spleen (p < 0.001) | HDM-induced asthma model in female adult mice | (101) |
| Intraperitoneal eosinophilia polymyxin B model in guinea pig | OmeGo (30 mg/kg, 300 mg/kg), sea cod (cod liver oil/omega 3) (30 mg/kg, 300 mg/kg), also fevipiprant 5 and 20 mg/kg, and Linoleic acid (LA) 300 mg/kg | 300 mg/kg OmeGo attenuated eosinophil chemotaxis (50.7%, p < 0.002) and chemokinesis (55.7% p < 0.005) to leukotriene B4 compared to LA control | Intraperitoneal eosinophilia polymyxin B model in guinea pig | |
| LPS-induced acute lung inflammation in male Wistar rats | O3FFA:31.6% EPA, 31.6% DHA, and 15.4% DPA.EE: fish oil concentrate with 22% DHA and 33% EPA and saline and LPS controls | O3FFA and O3EE reduced LPS induced alveolar histiocytosis and decreased BALF IL-6, TNF-α, TGF-β, and IL-10 (p < 0.05) | LPS-induced acute lung inflammation in male Wistar rats | (102) |
| Human DC2 - T-cell model and LPS matured DCs as a control |
100 µM of LA, ALA, AA, DHA, or EPA | DHA (p < 0.005) and EPA (p < 0.0001), LA and AA decreased IL-12/IL-23 and IL-23production by DC2s and lowered IL-13, IFN-γ and IL10 production by DC2-induced effector T-cells | Human DC2 - T-cell model and LPS matured DCs as a control | (103) |
OVA ovalbumin, HDM, house dust mite; BALF, bronchoalveolar lavage fluid; DGLA, dihomo-linoleic acid; PM2.5, particulate matter 2.5; AHR, airway responsiveness (to metacholine); LPS, lipopolysaccharide; DC2, dendritic cells; SPM, specialized pro-resolving mediators.
There is evidence suggesting that n-3 PUFAs and marine oils have protective effects against asthma and allergies, as demonstrated in both animal studies and clinical trials (104). As summarized in Table 1 , n-3 PUFAs were supplemented in a Swedish cohort of children, a French longitudinal study of pregnant women and a small study in children with asthma. The key constituents, particularly DHA and EPA, were often reported in combination, in clinical trials or studies. Generally, n-3 PUFAs were beneficial in improving asthma-induced pathologic changes (85, 86), in reducing levels of inflammatory cytokines (86), and in the reduction in usage of asthma medications (90). In addition, prenatal n-3 PUFAs played a role in prevention of asthma risk in offspring (91, 92, 94). However, n-3 PUFA treatment did not lead to positive or significant results in some clinical reports and the effectiveness and mechanisms of action of n-3 PUFAs require further study. As shown by a meta-analysis, fish intake and maternal n-3 PUFA supplement lowered the asthma risk in childhood, but had no significant effect in adult asthma (105), while in a Cochrane review, including 9 clinical trials, no consistent effect of n-3 PUFAs on asthma was demonstrated, apart from one study indicating a reduction of asthma medication (106). A systematic review of 14 studies reported benefit effects of n-3 PUFAs on T2 inflammation (107). A further review of the effects of n-3 PUFAs on asthma pathology, cytokines and asthma exacerbations also reached similar inconsistent conclusions (108).
As shown in Table 3 , combined use of different n-3 PUFAs was documented in cell and animal experiments, with attention paid to downstream inflammatory products and signaling mechanisms. Broadly similar results in these animal and cellular experiments were seen to those in clinical trials suggesting protective effects of combined-n-3 PUFAs on pathologic changes in asthma, with a reduction in airway responsiveness (99), reduction in remodeling (100) and attenuation of eosinophil chemotaxis and chemokinesis (101) etc. Inflammatory cytokines, important in asthma, were generally decreased by n-3 PUFAs, particularly the Th2-type cytokines IL-5, IL-13 (98, 103) and those produced by Th1/Th17 cells such as TNF-α, IL-1β, IL-6, IL-17, and IL-23 (100).
7. The anti-asthma activity of DHA and DHA-derived lipid mediators: resolvins, maresins and protectin
DHA is the most significant fatty acid of the n-3 family, with much evidence suggesting beneficial effects on airway inflammation and in asthma prevention (76). In a clinical investigation of 91 healthy infants, born between 37- and 42-weeks gestation, fed with 0.32, 0.64, or 0.96% DHA or 0.64% arachidonic acid (ARA) as dietary supplements, a lower incidence of wheezing/asthma resulted, despite the mothers having a history of allergies (109). In the Etude Longitudinale Francaise depuis l’Enfance (ELFE) cohort of 8389 formula-fed infants, a high DHA content resulted in a low risk of wheezing and lower respiratory tract infections, with a lower use of asthma medications (90). DHA in human milk may also reduce allergy risk in the offspring (110).
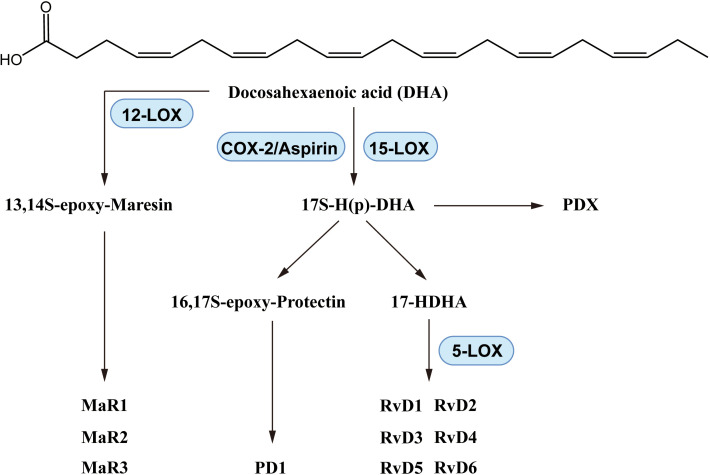
DHA reduced the pathologic changes of asthma in a mouse model (111), and inhibited prostaglandin F2α-induced tracheal smooth muscle contraction (112). In dust-induced lung inflammation in mice, DHA increased levels of Resolvin D (RvD)1, one of the DHA-derived lipid mediators, and inhibited neutrophil and macrophage recruitment (113). In a mouse agricultural dust study, DHA reduced lung neutrophil, macrophage and lymphocyte counts and IL-6 and TNF-α levels in bronchoalveolar lavage fluid (BALF), with increased RvD1 and RvD2 as well as altered macrophage polarization (114). These effects indicated that DHA significantly inhibited macrophage factors induced by lipopolysaccharide (LPS) or SiO2, reducing levels of proinflammatory eicosanoids including prostaglandins, leukotrienes, and thromboxane (115). In an agricultural dust-induced BEAS-2B inflammatory cell model DHA reduced levels of IL-6, IL-8 and TNF-α and promoted production of RvD1, amphiregulin and cell injury repair (116). SPMs including resolvins, maresins and protectins are produced from DHA via enzyme mediated biosynthesis as shown in Figure 5 .
Figure 5.
Biosynthesis of DHA-derived lipid mediators.
Resolvins, including RvD 1 - 6, were discovered after 2002, synthesized (117–122), and some have been produced on a commercial scale (123). The systemic anti-inflammatory activity of resolvins has been widely reported (124, 125). In asthma, characterized by chronic inflammation, resolvins have also shown beneficial effects. In ovalbumin (OVA)-induced murine asthma, RvD1 reduced BALF eosinophils and lymphocytes, alleviated AHR, and lowered IL-5 and IL-23 levels while enhancing allergen phagocytosis by lung macrophages (126). In children with moderate and severe asthma, RvD1 levels were typically reduced, suggesting that RvD1 might be a potential indicator of asthma severity (127). RvD1 ameliorated LPS-induced lung injury by decreasing neutrophil infiltration and lung TNF-α concentrations (128). RvD1 and RvD2 decreased IL-8 and other factors and promoted IL-10 production, and activated the glycogen synthase kinase-3β anti-inflammatory axis in human monocytes (129). RvD1 and RvD2 inhibited the differentiation of Th1/Th17 cells and promoted production of Tregs through the signature transcription factors T-bet and Rorc (130). An epimer of RvD1, AT-RvD1, has been reported to possess potential anti-asthma activity. AT-RvD1 was found to downregulate TNF-α in the peripheral blood mononuclear cells (PBMCs) from both severe asthma patients and healthy individuals (131). In addition to RvD1 and RvD2, the other RvDs also showed anti-inflammatory activity. RvD3 protected against epithelial lung injury (132) and RvD4 promoted neutrophil apoptosis and neutrophil, monocyte and macrophage phagocytosis (133). RvD5 down-regulated levels of IL-6 and the C-C motif chemokine ligand (CCL)5 in LPS-stimulated THP-1 cells (134). Furthermore, the D-series Resolvins D1-5 activated Phospholipase D, a potential target in phagocytes (135).
Research into mechanisms suggested that the proresolving actions of RvD1 on macrophages, neutrophils and leukocytes were associated with two G protein-coupled receptors (GPR) the formyl peptide receptor 2 (ALX/FPR2) and GPR 32 (136, 137), as with regulation of macrophage polarization into the anti-inflammatory type-M2 type (138, 139). ALX/FPR2 receptors were identified in T cells, macrophages and neutrophils (140, 141), and through the Gi/O family transduction mechanisms, ALX/FPR2 regulated Ca2+ flux by a CD38- dependent cyclic ADP-ribose (142), and influenced the expression of nuclear factor kappa-B (NF-κB) (140). RvD1 and RvD2 inhibited neutrophil apoptosis and promotion of macrophage phagocytosis, and these effects were reversed by GPR32 and ALX/FPR2 antibodies in a mouse LPS model of lung inflammation (143).Furthermore, an ALX/FPR2 inhibitor prevented the RvD1-reduction of TNF-α by preventing the RvD1 stimulation of type-M2 macrophages (144). Additional supportive evidence from a clinical study in severe pediatric asthma reported reduction of lipoxin A4 levels and FPR2/ALX expression (133). AT-RvD promoted phagocytosis of apoptotic neutrophils and downregulated NF-kB; anti-inflammatory effects also mediated by ALX/FRP2 receptors (145).
Maresins exhibit significant anti-inflammatory effects in lung disease. In an OVA-induced asthma model, maresin (MaR)1 alleviated inflammatory cell infiltration, reducing neutrophil and eosinophil counts, and decreasing T2-cytokines by NF-κB inhibition (146). MaR1 reduced levels of IL-5 and IL-13 in lung and ILC2 cells in OVA-induced allergic BALB/c mice. MaR1 lowered IL-6, TNF-α and the production of Tregs in an acute lung injury model (147). In pancreatitis-related lung injury, MaR1 reduced levels of IL-1β, IL-6 and TNF-α and increased IL-10 level in lung tissues (148). The anti-inflammatory activity of MaR1 was associated with the receptor retinoic acid-related orphan receptor α (RORα) and human leucine-rich repeat containing G protein-coupled receptor 6 (LGR6) (149, 150). The effects of MaR2 on Tregs and ILC2 cells were related to LGR6; LGR6-knockout mice showed IL-13 increasing and MaR1 inhibiting effects (151). In human and mouse phagocytes, MaR1 increased phagocytosis which was significantly enhanced by LGR6 overexpression (152). MaR2 decreased the chemokines CCL2, CCL3, CCL17 and other factors in LPS-injured mice (153), and conjugates of MaR1 and MaR3 reduced lung injury (154) and AHR (155).
There is less published research on protectins compared to that on resolvins and maresins, but existing studies have suggested a relationship with asthma and inflammation. Protectin D1 (PD1) administration improved AHR and mucus texture, decreased eosinophil and T-lymphocyte counts, and attenuated lung inflammation in murine asthma (156). An etiological study in infants (157) reported that particulate air pollutants increased asthma susceptibility and decreased PD1 levels. PD1 synthesis was inhibited in eosinophils of patients with severe asthma (158). PD1 downregulated IFN-γ and TNF-α in patients with severe asthma (159), and PD1 alleviated infiltration and extracellular traps of neutrophils with decreased IL-6 and TNF-α in LPS-induced acute lung injury (160). Serhan’s group reported that PD1 promoted leukocyte ingestion and macrophage phagocytosis, and facilitated phagocyte removal in inflammation resolution (161). The PD1 isomer, protectin DX (PDX), was also reported to have anti-inflammatory activity in lung (162). PDX alleviated the symptoms of lung injury in mice (163), increased alveolar fluid clearance in rats (164), and promoted alveolar epithelial cell proliferation (165). PDX inhibited BALF macrophage and neutrophil recruitment in a mouse lung injury model via the TNF-α signaling pathway (166). Protectin conjugates in tissue regeneration (PCTR1) played a protective role in acute LPS lung injury in mice, reduced IL-1β, IL-6 and TNF-α (167). In general, the anti-asthma and anti-inflammatory activity of DHA have been reported in research, with the DHA-derived lipid mediators, including resolvins, maresins and protectins potentially showing beneficial effects in both Th2 and Th1/Th17 immune mechanisms.
8. EPA and resolvin Es in asthma
EPA, a key component of n-3 fatty acids, has been studied extensively in asthma and inflammation research. In a double-blind, randomized clinical trial of 35 mild to moderate atopic asthmatics, a medical food emulsion containing EPA and gamma-linolenic acid (GLA) was reported to show improved asthma status in 19% patients with a 23% reduction in rescue medication use (168). In an uncontrolled second study on 65 patients, there was a significant improvement in quality of life questionnaires in asthma patients (p < 0.001). EPA may have beneficial effects on mesenchymal stromal cells in asthma, with reduction in levels of IL-4 and IL-13 and increase in the anti-inflammatory mediator IL-10 (169). The EPA derivative, monoacylglyceride (MAG)-EPA, may reduce bronchial hyperresponsiveness and Ca2+ hypersensitivity of bronchial smooth muscle in asthmatic guinea-pigs with reduced eosinophils and lymphocytes and lower transcript counts of eotaxin and related factors (170). Following EPA supplementation, EPA and DPA showed an increase in mice (69).
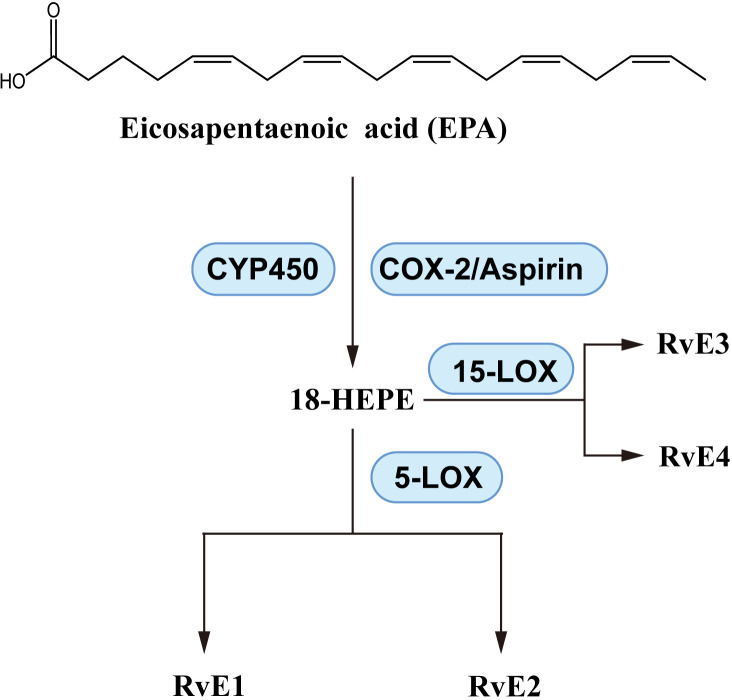
As shown in Figure 6 , EPA produces pro-resolvin mediators called E-series resolvins, which consist of RvE1, RvE2, etc. A variety of enzymes play catalytic roles in the production of resolvins, including aspirin-induced acetylated cyclooxygenase-2 (COX-2), cytochrome P450, 5-lipoxygenase (LOX) and 15-LOX (171). EPA is a substrate for E-series resolvins, and supplementation with EPA upregulates the levels of RvEs (99, 172). The laboratory of CN Serhan has been instrumental in elucidating the structure and biosynthesis of RvE1 (173), RvE2 (174), RvE3 (175), RvE4 (176). With reports of anti-inflammatory activity of RvEs (161, 177), there is evidence of beneficial effects of RvEs on asthma and inflammation. In OVA-induced BALB/c mice, RvE1 reduced IL-6, IL-17, IL-23 and improved AHR (178). Even at a dose of 200 ng/day, RvE1 reduced IL-17A and related factors, effectively reduced the eosinophil, macrophage and lymphocyte counts (179). These effects suggest that RvE1 inhibits Th1/Th17 cytokine imbalance of. Targeted research on the effect of RvE1 on Th17 differentiation further elaborated the mechanism, RvE1 suppressed the activation of DCs and T cell, inhibited IL-17 expression with reduction in the levels of IL-17A, IL-21, IL-2 and IL-6 (180).
Figure 6.
E-series resolvins; biosynthesis pathways.
In asthmatic FVB mice, RvE1 has been reported to decrease IL-13 and immunoglobulin E (IgE) and improve AHR (181). Another study showed similar effects on Th2-type cytokines, decreased IgE, eosinophils and lymphocytes, and related factors in lung and BALF (182). A more comprehensive examination of cytokine levels demonstrated effects of RvE1 on IL-4, IL-5, IL-1β, IL-6, IL-9, IL-13, IL-17, granulocyte macrophage colony-stimulating factor, IFN-γ and CCL family members CCL4, CCL5 and CCL11, restoring BALF cytokine levels to near baseline levels 24 - 36 h after RvE1 administration and also induced Th2 cell differentiation (183). Although relatively little research has been reported, a number of other RvEs have also demonstrated anti-asthma or anti-inflammatory activity. In asthma-susceptible neonatal BALB/c mice, RvE2 reduced eosinophil counts and IL-4, IL-5 and IL-13 levels, suggesting that RvE2 may prevent asthma risk (184). In house dust mite (HDM)-induced allergic mice, RvE3 reduced eosinophils, decreased IL-23 and IL-17 levels in BALF, and downregulated ribonucleic acid (RNA) expression in lung and peri-bronchial lymph nodes (185). In addition, anti-inflammatory activities of RvE3 and RvE4 have been reported in cell experiments (186, 187).
9. DPA and DPA-derived resolvinsn-3 DPA, protectinn-3 DPA and Maresinsn-3 DPA
As shown in Tables 1 , 2 , the combination of DPA with other fatty acids has been used in clinical and animal studies related to asthma. There are very few studies on the use of DPA alone in the treatment of asthma, but there is some literature on the anti-inflammatory effects of DPA and its derivatives. In a model of colitis, DPA inhibited the RNA expression of TNF-α, IL-1β and IL-6 and increased the amount of IL-10 (188). Increased levels of DPA induced by n-3 fatty acids improved TNF-α related apoptosis-inducing ligand and reduced allergic symptoms in infantile mice (189). MAG-DPA, a glycerol esterification product of DPA, downregulated mRNA expression of the TNF-α/NF-κB and COX-2 pathways and controlled the Ca2+ sensitivity and airway overactivity in a guinea pig AHR model (190). In experimental pulmonary hypertension, MAG-DPA showed similar anti-inflammatory activity and downregulated NF-κB expression (191). In addition, DPA derivatives were found to decrease TNF-α activity (192, 193).
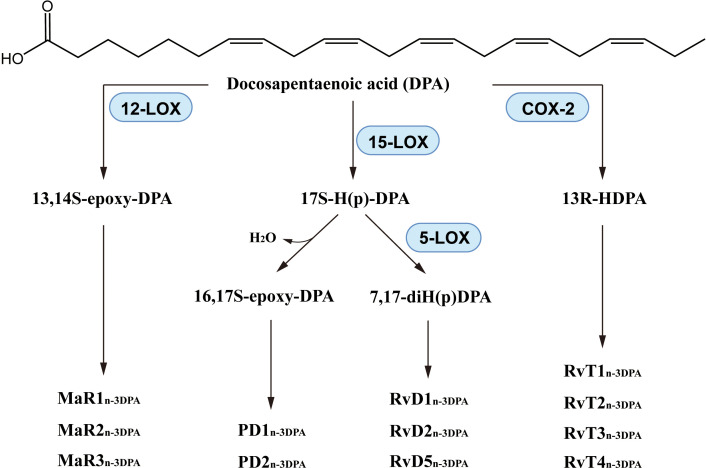
Through reactions catalyzed by 5-LOX, 15-LOX or other enzymes (194), DPA produces lipid mediators including resolvinsn-3 DPA, protectinsn-3 DPA and Maresinsn-3 DPA ( Figure 7 ). It is also worth noting that the production processes of these DPA-derived SPMs is somewhat similar to that of DHA-derived SPMs. In recent years, the synthesis pathway of DPA-derived SPMs has been described (195–200) and the anti-inflammatory activity of these lipid mediators has demonstrated. RvD1n-3 DPA decreased neutrophil numbers (195) and reduced NF-κB expression (201). The neutrophil activation marker CD11b was downregulated when plasma RvD1n-3 DPA was increased (202). RvD5n-3 DPA increased the amount of IL-10 and IL-10R and enhanced phagocytosis of neutrophils and macrophages in murine inflammatory arthritis by a mechanism that may be related to the receptor GPR101 (197, 203, 204). PD1n-3 DPA reduced the number of neutrophils and promoted phagocytosis and excretion by macrophages in mice with peritonitis (199). PD1n-3 DPA and PD2n-3 DPA regulated human monocyte differentiation and macrophage phenotype, and also stimulated phagocytosis in phagocytes, as in mice (205). In addition, PD1n-3 DPA and its analogs were protective against neuroinflammation (206) and neuropathic pain (207). 13-series resolvins (also called RvTs) had potent anti-inflammatory effect, that was substantially produced in the initiation phase of inflammation, down-regulating expression of caspase-1 and IL-1β of apoptotic neutrophils and macrophage exudation, RvTs inhibited neutrophil infiltration and improved macrophage uptake of neutrophil extracellular traps, in which the cAMP-PKA-AMPK pathway may be involved (208, 209). RvTs also have a likely treatment role in inflammatory arthritis, and the anti-inflammatory effects of therapeutic agents such as atorvastatin and pravastatin are markedly impaired when the RvT biosynthesis initiating enzyme, COX-2, is inhibited (210).
Figure 7.
Biosynthesis of DPA-derived lipid mediators.
10. Conclusions
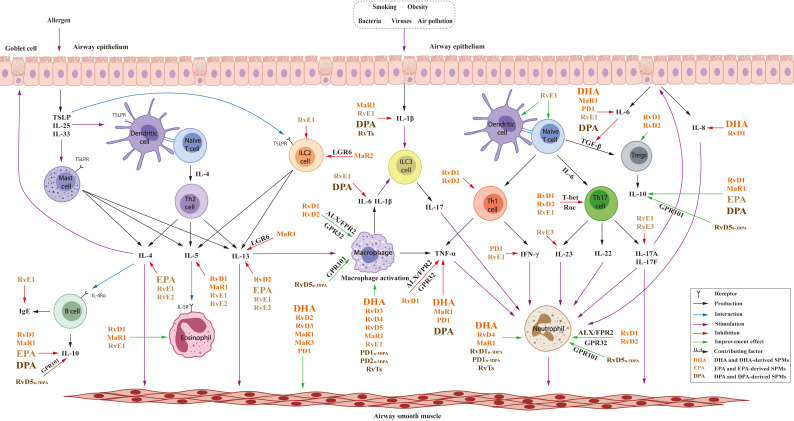
As shown in Tables 1 – 3 , the clinical trials and animal experiments indicated the anti-asthma and anti-inflammatory effects of n-3 PUFAs. The combination of n-3 PUFAs and n-3 PUFA-rich diets improved asthma-induced pathologic changes, lowered asthma risk and the use of asthma medication. As summarized in Figure 8 , DHA, EPA and DPA regulated immune cells including macrophage and neutrophils with effects on the Th2-type cytokines IL-4, IL-13 and cytokines produced by Th1/Th17 including TNF-α, IL-1β, IL-6, IL-8, IL-10 etc. However, there are some different opinions regarding the effects of n-3 PUFAs because of the inconsistent results of some clinical studies. In addition, although n-3 PUFA supplements in pregnancy and early childhood have generally decreased asthma risk, their effects in adults were less obvious. These results suggest the importance of life stages for n-3 PUFA supplementation, and further studies are required to elucidate the mechanisms of action and potential role of n-3 PUFAs in anti-asthma effects.
Figure 8.
Effects of n-3PUFAs and their lipid mediators in T2 and Th1/Th17 immune.
Further research on n-3 PUFA-derived lipid mediators may offer more insight into their anti-asthma effects. DHA-generated resolvins, maresins and protectins demonstrate similar, but more comprehensive, anti-inflammatory activity compared to DHA, with regulation of IFN-γ, TGF-β and differentiation of Th1 and Th17 cells. The G protein-coupled receptors ALX/FPR2 and GPR32 play important roles in the mechanism of action of RvDs, since the antibody to, and the inhibitor of, these receptors suppressed the anti-inflammatory effects of RvDs and DHA. The anti-inflammatory targets of EPA and RvEs, with effects on IL-4, IL-5, IL-13, are similar but there are some differences. RvEs exert effects on the Th1/Th17 cytokines TNF-α, IL-23, IL-17, and EPA regulate the level of IL-10. Fewer studies on DPA-derived SPMs were reported in the regulation of macrophages and neutrophils. However, the similarities between effects of n-3 PUFAs and their lipid mediators indicate that the lipid mediators may be the active substances, and their inflammation resolution activity may lead to their application in asthma therapy and prevention. In general, supplementation with n-3 PUFAs has been shown to be beneficial as adjunctive therapy for asthma although further study is needed, and SPMs are promising, potential adents for the treatment of asthma.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Jilin Provincial Science and Technology Agency (YDZJ202201ZYTS628, recipient: Wei-Yu Zhang).
Abbreviations
PUFAs, Polyunsaturated fatty acids; SPMs, Specialized pro-resolving mediators; n-3, Omega-3; DHA, Docosahexaenoic acid; EPA, Eicosapentaenoic acid; AHR, Airway hyper-responsiveness; Th, T-helper; T2, Type 2; DCs, Dendritic cells; ILC2s, Type 2 innate lymphoid cells; IL, Interleukin; TGF-β, Transforming growth factor-β; ILC, Innate lymphoid cells; NGF, Nerve growth factor; ALA, Alpha-linoleic acid; DPA, Docosapentaenoic acid; ARA, Arachidonic acid; ELFE, Etude Longitudinale Francaise depuis l’Enfance; RvD, Resolvin D; TNF-α, Tumor necrosis factor-α; IFN-γ, Interferon-gamma; OVA, Ovalbumin; LPS, Lipopolysaccharide; FPR2/ALX, Formyl peptide receptor 2; GPR, G-protein-coupled receptor; PBMCs, Peripheral blood mononuclear cells; NF-κB, Nuclear factor kappa-B; CCL, C-C motif chemokine ligand; MaR, Maresin; RORα, Receptor retinoic acid-related orphan receptor α (RORα); LGR6, leucine-rich repeat containing G protein-coupled receptor 6; PD1, Protectin D1; PDX, Protectin DX; PCTR1, Protectin conjugates in tissue regeneration; GLA, Gamma-linolenic acid; MAG, Monoacylglyceride; COX-2, Cyclooxygenase-2; LOX, Lipoxygenase; IgE, Immunoglobulin E; BALF, Bronchoalveolar lavage fluid; HDM, House dust mite; RNA, Ribonucleic acid.
Author contributions
YT: Writing – original draft. J-MS: Writing – review & editing. D-MJ: Writing – review & editing. W-YZ: Writing – review & editing.
Conflict of interest
Author D-MJ is employed by Shouyao Holdings Beijing Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet (British edition). (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asher I, Bissell K, Chiang C, El Sony A, Ellwood P, García-Marcos L, et al. Calling time on asthma deaths in tropical regions—how much longer must people wait for essential medicines? Lancet Respir Med. (2019) 7:13–5. doi: 10.1016/S2213-2600(18)30513-7 [DOI] [PubMed] [Google Scholar]
- 3. Cullinan P, Vandenplas O, Bernstein D. Assessment and management of occupational asthma. J Allergy Clin Immunology: In Pract. (2020) 8:3264–75. doi: 10.1016/j.jaip.2020.06.031 [DOI] [PubMed] [Google Scholar]
- 4. Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors, Semin. Immunopathol. (2020) 42:5–15. doi: 10.1007/s00281-020-00785-1 [DOI] [PubMed] [Google Scholar]
- 5. Wang J, Zhou Y, Zhang H, Hu L, Liu J, Wang L, et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal transduction targeted Ther. (2023) 8:138. doi: 10.1038/s41392-023-01344-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chappus-Mccendie H, Chevalier L, Roberge C, Plourde M. Omega-3 PUFA metabolism and brain modifications during aging. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 94:109662. doi: 10.1016/j.pnpbp.2019.109662 [DOI] [PubMed] [Google Scholar]
- 7. Oikonomou E, Vogiatzi G, Karlis D, Siasos G, Chrysohoou C, Zografos T, et al. Effects of omega-3 polyunsaturated fatty acids on fibrosis, endothelial function and myocardial performance, in ischemic heart failure patients. Clin Nutr. (2019) 38:1188–97. doi: 10.1016/j.clnu.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 8. Calder PC. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem Soc T. (2017) 45:1105–15. doi: 10.1042/BST20160474 [DOI] [PubMed] [Google Scholar]
- 9. Lee-Sarwar K, Kelly RS, Lasky-Su J, Kachroo P, Zeiger RS, O'Connor GT, et al. Dietary and plasma polyunsaturated fatty acids are inversely associated with asthma and atopy in early childhood. J Allergy Clin Immunol Pract. (2019) 7:529–38. doi: 10.1016/j.jaip.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manson JE, Bassuk SS, Lee I, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin Trials. (2012) 33:159–71. doi: 10.1016/j.cct.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Basil MC, Levy BD. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation, Nature reviews. Immunology. (2016) 16:51–67. doi: 10.1038/nri.2015.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Global Initiative For Asthma . Global strategy for asthma management and prevention, 2023. USA: GINA; (2023). Available at: www.ginasthma.org/reports. [Google Scholar]
- 13. Khalfaoui L, Symon FA, Couillard S, Hargadon B, Chaudhuri R, Bicknell S, et al. Airway remodelling rather than cellular infiltration characterises both type2 cytokine biomarker-high and -low severe asthma. Allergy. (2022) 77:2974–86. doi: 10.1111/all.15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. (2018) 391:783–800. doi: 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- 15. Ricciardolo FLM, Bertolini F, Carriero V, Sprio AE. Asthma phenotypes and endotypes: A systematic review. Minerva Med. (2021) 112:547–63. doi: 10.23736/S0026-4806.21.07498-X [DOI] [PubMed] [Google Scholar]
- 16. Asher MI, Rutter CE, Bissell K, Chiang C, Sony AE, Ellwood E, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. (2021) 398:1569–80. doi: 10.1016/S0140-6736(21)01450-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su K, Yan D, Ou L, Lin L, Wu C, Huang S, et al. Prevalence, associated factors, and impact of adolescent asthma in Taiwan: Global Asthma Network phase I survey. World Allergy Organ J. (2023) 16:100794. doi: 10.1016/j.waojou.2023.100794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunasekera KD, Amarasiri WADL, Undugodage UCM, Silva HKMS, Sadikeen A, Gunasinghe W, et al. Prevalence of asthma and its symptoms in Sri Lankan adults. BMC Public Health. (2022) 22:2330. doi: 10.1186/s12889-022-14793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benfante A, Tomasello A, Gianquinto E, Cicero MN, Scichilone N. Diagnostic and therapeutic approaches for elderly asthma patients: The importance of multidisciplinary and multidimensional management. Expert Rev Resp Med. (2023) 17:459–68. doi: 10.1080/17476348.2023.2215432 [DOI] [PubMed] [Google Scholar]
- 20. Horvat JC, Kim RY, Weaver N, Augood C, Brown AC, Donovan C, et al. Characterization and inhibition of inflammasome responses in severe and non-severe asthma. Resp Res. (2023) 24:303. doi: 10.1186/s12931-023-02603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shailesh H, Bhat AA, Janahi IA. Obesity-Associated Non-T2 mechanisms in obese asthmatic individuals. Biomedicines. (2023) 11:2797. doi: 10.3390/biomedicines11102797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: Assessment and identification using induced sputum. Respirology. (2006) 11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- 23. Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: Moving toward precision medicine. J Allergy Clin Immunol. (2019) 144:1–12. doi: 10.1016/j.jaci.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 24. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allerg. Immu. (2019) 56:219–33. doi: 10.1007/s12016-018-8712-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The prevalence of subtypes of type-2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol In Pract. (2021) 9:1267–75. doi: 10.1016/j.jaip.2020.09.051 [DOI] [PubMed] [Google Scholar]
- 26. Wang W, Li Y, Lv Z, Chen Y, Li Y, Huang K, et al. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP,and IL-25) response in the airways epithelium and submucosa. J Immunol. (2018) 201:2221–31. doi: 10.4049/jimmunol.1800709 [DOI] [PubMed] [Google Scholar]
- 27. Deckers J, De Bosscher K, Lambrecht BN, Hammad H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol Rev. (2017) 278:131–44. doi: 10.1111/imr.12542 [DOI] [PubMed] [Google Scholar]
- 28. Howell I, Howell A, Pavord ID. Type 2 inflammation and biological therapies in asthma: Targeted medicine taking flight. J Exp Med. (2023) 220(7):e20221212. doi: 10.1084/jem.20221212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harker JA, Lloyd CM. T helper 2 cells in asthma. J Exp Med. (2023) 220(6):e20221094. doi: 10.1084/jem.20221094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mcknight CG, Potter C, Finkelman FD. IL-4Rα expression by airway epithelium and smooth muscle accounts for nearly all airway hyperresponsiveness in murine allergic airway disease. Mucosal Immunol. (2020) 13:283–92. doi: 10.1038/s41385-019-0232-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: A critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. (1997) 186:1737–47. doi: 10.1084/jem.186.10.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor ␣ 1 and the type II IL-4 receptor in asthma pathogenesis. P. Natl Acad Sci USA. (2008) 105:7240–5. doi: 10.1073/pnas.0802465105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Busse WW, Kraft M, Rabe KF, Deniz Y, Rowe PJ, Ruddy M, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J. (2021) 58:2003393. doi: 10.1183/13993003.03393-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luo J, Chen W, Liu W, Jiang S, Ye Y, Shrimanker R, et al. IL-5 antagonism reverses priming and activation of eosinophils in severe eosinophilic asthma. Mucosal Immunol. (2024) 17(4):524–36. doi: 10.1016/j.mucimm.2024.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaur D, Doe C, Woodman L, Wan H, Sutcliffe A, Hollins F, et al. Mast cell-airway smooth muscle crosstalk: The role of thymic stromal lymphopoietin. Chest. (2012) 142:76–85. doi: 10.1378/chest.11-1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdo M, Pedersen F, Kirsten AM, Veith V, Biller H, Trinkmann F, et al. Longitudinal impact of sputum inflammatory phenotypes on small airway dysfunction and disease outcomes in asthma. J Allergy Clin Immunol Pract. (2022) 10:1545–53. doi: 10.1016/j.jaip.2022.02.020 [DOI] [PubMed] [Google Scholar]
- 37. Kyriakopoulos C, Gogali A, Bartziokas K, Kostikas K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. (2021) 7:309–2020. doi: 10.1183/23120541.00309-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Niessen NM, Fricker M, Mcdonald VM, Gibson PG. T2-low: What do we know? – Past, present, and future of biologic therapies in non-eosinophilic asthma. Ann Allergy Asthma Immunol. (2022) 129:150–9. doi: 10.1016/j.anai.2022.04.020 [DOI] [PubMed] [Google Scholar]
- 39. Ramakrishnan RK, Al Heialy S, Hamid Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev Resp Med. (2019) 13:1057–68. doi: 10.1080/17476348.2019.1666002 [DOI] [PubMed] [Google Scholar]
- 40. De Volder J, Vereecke L, Joos G, Maes T. Targeting neutrophils in asthma: A therapeutic opportunity? Biochem Pharmacol. (2020) 182:114292. doi: 10.1016/j.bcp.2020.114292 [DOI] [PubMed] [Google Scholar]
- 41. Choy DF, Arron JR. Beyond type 2 cytokines in asthma - new insights from old clinical trials. Expert Opin Ther Tar. (2020) 24:463–75. doi: 10.1080/14728222.2020.1744567 [DOI] [PubMed] [Google Scholar]
- 42. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. (2007) 8:967–74. doi: 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- 43. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. (2006) 441:235–8. doi: 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- 44. Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma. Chest. (2001) 119:1329–36. doi: 10.1378/chest.119.5.1329 [DOI] [PubMed] [Google Scholar]
- 45. Jonckheere A, Bullens DMA, Seys SF. Innate lymphoid cells in asthma: Pathophysiological insights from murine models to human asthma phenotypes. Curr Opin Allergy Clin Immunol. (2019) 19:53–60. doi: 10.1097/ACI.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 46. Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Resp Crit Care. (2018) 197:22–37. doi: 10.1164/rccm.201611-2232PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. (2014) 20:54–61. doi: 10.1038/nm.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lynch JP, Werder RB, Curren BF, Sikder MAA, Ullah A, Sebina I, et al. Long-lived regulatory T cells generated during severe bronchiolitis in infancy influence later progression to asthma. Mucosal Immunol. (2020) 13:652–64. doi: 10.1038/s41385-020-0268-8 [DOI] [PubMed] [Google Scholar]
- 49. Kawano H, Kayama H, Nakama T, Hashimoto T, Umemoto E, Takeda K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int Immunol. (2016) 28):489–501. doi: 10.1093/intimm/dxw012 [DOI] [PubMed] [Google Scholar]
- 50. Tedder TF, Matsushita T. Regulatory B cells that produce IL-10: A breath of fresh air in allergic airway disease. J Allergy Clin Immunol. (2010) 125:1125–7. doi: 10.1016/j.jaci.2010.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Machura E, Mazur B, Rusek-Zychma M, Barc-Czarnecka M. Cytokine production by peripheral blood CD4+ and CD8+ T cells in atopic childhood asthma. Clin Dev Immunol. (2010) 2010:606139. doi: 10.1155/2010/606139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Demarche S, Schleich F, Henket M, Paulus V, Van Hees T, Louis R. Detailed analysis of sputum and systemic inflammation in asthma phenotypes: Are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm. Med. (2016) 16:46. doi: 10.1186/s12890-016-0208-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. (2014) 133:1557–63. doi: 10.1016/j.jaci.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Braun A, Quarcoo D, Schulte-Herbruggen O, Lommatzsch M, Hoyle G, Renz H. Nerve growth factor induces airway hyperresponsiveness in mice. Int Arch Allergy Immunol. (2001) 124:205–7. doi: 10.1159/000053711 [DOI] [PubMed] [Google Scholar]
- 55. Chen J, Miller M, Unno H, Rosenthal P, Sanderson MJ, Broide DH. Orosomucoid-like 3 (ORMDL3) upregulates airway smooth muscle proliferation, contraction, and Ca(2+) oscillations in asthma. J Allergy Clin Immunol. (2018) 142:207–18. doi: 10.1016/j.jaci.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balenga NA, Jester W, Jiang M, Panettieri RJ, Druey KM. Loss of regulator of G protein signaling 5 promotes airway hyperresponsiveness in the absence of allergic inflammation. J Allergy Clin Immunol. (2014) 134:451–9. doi: 10.1016/j.jaci.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khan I, Hussain M, Jiang B, Zheng L, Pan Y, Hu J, et al. Omega-3 long-chain polyunsaturated fatty acids: Metabolism and health implications. Prog Lipid Res. (2023) 92:101255. doi: 10.1016/j.plipres.2023.101255 [DOI] [PubMed] [Google Scholar]
- 58. Bhatt DL, Budoff MJ, Mason RP. A revolution in omega-3 fatty acid research. J Am Coll Cardiol. (2020) 76:2098–101. doi: 10.1016/j.jacc.2020.09.005 [DOI] [PubMed] [Google Scholar]
- 59. Djuricic I, Calder PC. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients. (2021) 13:2421. doi: 10.3390/nu13072421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - a review. Life Sci. (2018) 203:255–67. doi: 10.1016/j.lfs.2018.04.049 [DOI] [PubMed] [Google Scholar]
- 61. Kalish BT, Fallon EM, Puder M. A tutorial on fatty acid biology. JPEN J Parenter Enteral Nutr. (2012) 36:380–8. doi: 10.1177/0148607112449650 [DOI] [PubMed] [Google Scholar]
- 62. Harnack K, Andersen G, Somoza V. Quantitation of alpha-linolenic acid elongation to eicosapentaenoic and docosahexaenoic acid as affected by the ratio of n6/n3 fatty acids. Nutr Metab (Lond). (2009) 6:8. doi: 10.1186/1743-7075-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood). (2008) 233:674–88. doi: 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- 64. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. (2002) 56:365–79. doi: 10.1016/s0753-3322(02)00253-6 [DOI] [PubMed] [Google Scholar]
- 65. Oddy WH, de Klerk NH, Kendall GE, Mihrshahi S, Peat JK. Ratio of omega-6 to omega-3 fatty acids and childhood asthma. J Asthma. (2004) 41:319–26. doi: 10.1081/jas-120026089 [DOI] [PubMed] [Google Scholar]
- 66. Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. (1997) 65:1011–7. doi: 10.1093/ajcn/65.4.1011 [DOI] [PubMed] [Google Scholar]
- 67. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. (2011) 93:950–62. doi: 10.3945/ajcn.110.006643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bolte G, Kompauer I, Fobker M, Cullen P, Keil U, Mutius E, et al. Fatty acids in serum cholesteryl esters in relation to asthma and lung function in children. Clin Exp Allergy. (2006) 36:293–302. doi: 10.1111/j.1365-2222.2006.02441.x [DOI] [PubMed] [Google Scholar]
- 69. Fussbroich D, Zimmermann K, Gopel A, Eickmeier O, Trischler J, Zielen S, et al. A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma. Lipids Health Dis. (2019) 18:16. doi: 10.1186/s12944-018-0947-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou J, Chen L, Liu Z, Sang L, Li Y, Yuan D. Changes in erythrocyte polyunsaturated fatty acids and plasma eicosanoids level in patients with asthma. Lipids Health Dis. (2018) 17:206. doi: 10.1186/s12944-018-0853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sokolowska M, Rovati GE, Diamant Z, Untersmayr E, Schwarze J, Lukasik Z, et al. Current perspective on eicosanoids in asthma and allergic diseases: EAACI Task Force consensus report, part I. Allergy. (2021) 76:114–30. doi: 10.1111/all.14295 [DOI] [PubMed] [Google Scholar]
- 72. Innes JK, Calder PC. Omega-6 fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. (2018) 132:41–8. doi: 10.1016/j.plefa.2018.03.004 [DOI] [PubMed] [Google Scholar]
- 73. Godson C. Balancing the effect of leukotrienes in asthma. N Engl J Med. (2020) 382:1472–5. doi: 10.1056/NEJMcibr2000118 [DOI] [PubMed] [Google Scholar]
- 74. Lee K, Lee SH, Kim TH. The biology of prostaglandins and their role as a target for allergic airway disease therapy. Int J Mol Sci. (2020) 21:1851. doi: 10.3390/ijms21051851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dogne JM, de Leval X, Benoit P, Delarge J, Masereel B. Thromboxane A2 inhibition: Therapeutic potential in bronchial asthma. Am J Respir Med. (2002) 1:11–7. doi: 10.1007/BF03257158 [DOI] [PubMed] [Google Scholar]
- 76. Ghasemi Fard S, Wang F, Sinclair AJ, Elliott G, Turchini GM. How does high DHA fish oil affect health? A systematic review of evidence. Crit Rev Food Sci. (2019) 59:1684–727. doi: 10.1080/10408398.2018.1425978 [DOI] [PubMed] [Google Scholar]
- 77. Calder PC. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc Nutr Soc. (2018) 77:52–72. doi: 10.1017/S0029665117003950 [DOI] [PubMed] [Google Scholar]
- 78. Kaur G, Guo XF, Sinclair AJ. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Curr Opin Clin Nutr Metab Care. (2016) 19:88–91. doi: 10.1097/MCO.0000000000000252 [DOI] [PubMed] [Google Scholar]
- 79. Barceló-Coblijn G, Murphy EJ. Alpha-linolenic acid and its conversion to longer chain n–3 fatty acids: Benefits for human health and a role in maintaining tissue n–3 fatty acid levels. Prog Lipid Res. (2009) 48:355–74. doi: 10.1016/j.plipres.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 80. Prasad P, Anjali P, Sreedhar RV. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit Rev Food Sci Nutr. (2021) 61:1725–37. doi: 10.1080/10408398.2020.1765137 [DOI] [PubMed] [Google Scholar]
- 81. Jerzyńska A, Polańska A, Trafalska E, Jankowska A, Podlecka D, Brzozowska A. Prenatal polyunsaturated fatty acids and atopic dermatitis and food allergy in children from Polish Mother and Child Cohort study. Int J Occup. Med Env. (2023) 36:428–36. doi: 10.13075/ijomeh.1896.02222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rosa MJ, Hartman TJ, Adgent M, Gardner K, Gebretsadik T, Moore PE, et al. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J Allergy Clin Immunol. (2020) 145:800–7. doi: 10.1016/j.jaci.2019.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brigham EP, Woo H, Mccormack M, Rice J, Koehler K, Vulcain T, et al. Omega-3 and omega-6 intake modifies asthma severity and response to indoor air pollution in children. Am J Resp Crit Care. (2019) 199:1478–86. doi: 10.1164/rccm.201808-1474OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang X, Han Y, Tian Q, Du L, Chen L, Zhang Y, et al. The association between n-3 polyunsaturated fatty acid intakes and asthma in US children and adolescents: A cross-sectional study from NHANES. Pediat. Allerg. Imm.-UK. (2023) 34:e14024. doi: 10.1111/pai.14024 [DOI] [PubMed] [Google Scholar]
- 85. Williams NC, Hunter KA, Shaw DE, Jackson KG, Sharpe GR, Johnson MA. Comparable reductions in hyperpnoea-induced bronchoconstriction and markers of airway inflammation after supplementation with 6.2 and 3.1 g/d of long-chain n-3 PUFA in adults with asthma. Br J Nutr. (2017) 117:1379–89. doi: 10.1017/S0007114517001246 [DOI] [PubMed] [Google Scholar]
- 86. Farjadian S, Moghtaderi M, Kalani M, Gholami T, Hosseini Teshnizi S. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine. (2016) 85:61–6. doi: 10.1016/j.cyto.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 87. Schubert R, Kitz R, Beermann C, Rose MA, Lieb A, Sommerer PC, et al. Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int Arch Allergy Immunol. (2009) 148:321–9. doi: 10.1159/000170386 [DOI] [PubMed] [Google Scholar]
- 88. Best K, Makrides M. Possible protective effect of prenatal omega-3 long-chain polyunsaturated fatty acids supplementation on persistent wheeze and asthma in early childhood. Evid Based Med. (2017) 22:104. doi: 10.1136/ebmed-2017-110696 [DOI] [PubMed] [Google Scholar]
- 89. Ekstrom S, Sdona E, Klevebro S, Hallberg J, Georgelis A, Kull I, et al. Dietary intake and plasma concentrations of PUFAs in childhood and adolescence in relation to asthma and lung function up to adulthood. Am J Clin Nutr. (2022) 115:886–96. doi: 10.1093/ajcn/nqab427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Adjibade M, Davisse Paturet C, Bernard JY, Adel Patient K, Divaret Chauveau A, Lioret S, et al. Enrichment of infant formula with long-chain polyunsaturated fatty acids and risk of infection and allergy in the nationwide ELFE birth cohort. Allergy. (2022) 77:1522–33. doi: 10.1111/all.15137 [DOI] [PubMed] [Google Scholar]
- 91. Flom JD, Chiu YM, Cowell W, Kannan S, Ganguri HB, Coull BA, et al. Maternal active asthma in pregnancy influences associations between polyunsaturated fatty acid intake and child asthma. Ann Allergy Asthma Immunol. (2021) 127:553–61. doi: 10.1016/j.anai.2021.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bisgaard H, Mikkelsen M, Rasmussen MA, Sevelsted A, Schoos AM, Brustad N, et al. Atopic and non-atopic effects of fish oil supplementation during pregnancy. Thorax. (2023) 78:1168–74. doi: 10.1136/thorax-2022-219725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Best KP, Sullivan T, Palmer D, Gold M, Kennedy DJ, Martin J, et al. Prenatal fish oil supplementation and allergy: 6-Year follow-up of a randomized controlled trial. Pediatrics. (2016) 137:e20154443. doi: 10.1542/peds.2015-4443 [DOI] [PubMed] [Google Scholar]
- 94. Kachroo P, Kelly RS, Mirzakhani H, Lee-Sarwar K, Chawes BL, Blighe K, et al. Fish oil supplementation during pregnancy is protective against asthma/wheeze in offspring. J Allergy Clin Immunology: In Pract. (2020) 8:388–91. doi: 10.1016/j.jaip.2019.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Best KP, Sullivan TR, Palmer DJ, Gold M, Martin J, Kennedy D, et al. Prenatal omega-3 LCPUFA and symptoms of allergic disease and sensitization throughout early childhood – a longitudinal analysis of long-term follow-up of a randomized controlled trial. World Allergy Organ J. (2018) 11:10. doi: 10.1186/s40413-018-0190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. (2003) 168:1181–9. doi: 10.1164/rccm.200303-373OC [DOI] [PubMed] [Google Scholar]
- 97. Arm JP, Horton CE, Spur BW, Mencia-Huerta JM, Lee TH. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am Rev Respir Dis. (1989) 139:1395–400. doi: 10.1164/ajrccm/139.6.1395 [DOI] [PubMed] [Google Scholar]
- 98. Jiang T, Li P, Zhao J, Dai L, Sun D, Liu M, et al. Long-chain polyunsaturated fatty acids improve airway pathological features and gut microbial imbalances in BALB/c mice with ovalbumin-induced asthma. J Funct Foods. (2021) 81:104465. doi: 10.1016/j.jff.2021.104465 [DOI] [Google Scholar]
- 99. Fussbroich D, Colas RA, Eickmeier O, Trischler J, Jerkic SP, Zimmermann K, et al. A combination of LCPUFA ameliorates airway inflammation in asthmatic mice by promoting pro-resolving effects and reducing adverse effects of EPA. Mucosal Immunol. (2020) 13:481–92. doi: 10.1038/s41385-019-0245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Li J, Chen Y, Shi Q, Sun J, Zhang C, Liu L. Omega-3 polyunsaturated fatty acids ameliorate PM2.5 exposure induced lung injury in mice through remodeling the gut microbiota and modulating the lung metabolism. Environ Sci pollut R. (2023) 30:40490–506. doi: 10.1007/s11356-022-25111-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Currie C, Framroze B, Singh D, Sharma D, Bjerknes C, Hermansen E. Pharmacological evaluation of the effects of enzymatically liberated fish oil on eosinophilic inflammation in animal models. Biotechnol Appl Bioc. (2023) 70:157–63. doi: 10.1002/bab.2338 [DOI] [PubMed] [Google Scholar]
- 102. Kocherlakota C, Nagaraju B, Arjun N, Srinath A, Kothapalli KSD, Brenna JT. Inhalation of nebulized omega-3 fatty acids mitigate LPS-induced acute lung inflammation in rats: Implications for treatment of COPD and COVID-19. Prostaglandins Leukotrienes Essential Fatty Acids. (2022) 179:102426. doi: 10.1016/j.plefa.2022.102426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hoppenbrouwers T, Fogliano V, Garssen J, Pellegrini N, Willemsen LEM, Wichers HJ. Specific polyunsaturated fatty acids can modulate in vitro human moDC2s and subsequent th2 cytokine release. Front Immunol. (2020) 11:748. doi: 10.3389/fimmu.2020.00748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Willemsen L. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur J Pharmacol. (2016) 785:174–86. doi: 10.1016/j.ejphar.2016.03.062 [DOI] [PubMed] [Google Scholar]
- 105. Yang H, Xun P, He K. Fish and fish oil intake in relation to risk of asthma: A systematic review and meta-analysis. PLoS One. (2013) 8:e80048. doi: 10.1371/journal.pone.0080048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Woods RK, Thien FC, Abramson MJ. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst Rev. (2002) 3:D1283. doi: 10.1002/14651858.CD001283 [DOI] [PubMed] [Google Scholar]
- 107. Visser E, Ten BA, Sizoo D, Pepels J, Ten HL, van der Wiel E, et al. Effect of dietary interventions on markers of type 2 inflammation in asthma: A systematic review. Respir Med. (2024) 221:107504. doi: 10.1016/j.rmed.2023.107504 [DOI] [PubMed] [Google Scholar]
- 108. Hardy MS, Kekic A, Graybill NL, Lancaster ZR. A systematic review of the association between fish oil supplementation and the development of asthma exacerbations. SAGE Open Med. (2016) 4:2103738216. doi: 10.1177/2050312116666216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Foiles AM, Kerling EH, Wick JA, Scalabrin DM, Colombo J, Carlson SE. Formula with long-chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr Allergy Immunol. (2016) 27:156–61. doi: 10.1111/pai.12515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Miliku K, Richelle J, Becker AB, Simons E, Moraes TJ, Stuart TE, et al. Sex-specific associations of human milk long-chain polyunsaturated fatty acids and infant allergic conditions. Pediat. Allerg. Imm.-UK. (2021) 32:1173–82. doi: 10.1111/pai.13500 [DOI] [PubMed] [Google Scholar]
- 111. Yokoyama A, Hamazaki T, Ohshita A, Kohno N, Sakai K, Zhao G, et al. Effect of aerosolized docosahexaenoic acid in a mouse model of atopic asthma. Int Arch Allergy Imm. (2000) 123:327–32. doi: 10.1159/000053645 [DOI] [PubMed] [Google Scholar]
- 112. Obara K, Inaba R, Kawakita M, De Dios RM, Uetake T, Murata A, et al. Docosahexaenoic acid selectively suppresses u46619- and PGF(2alpha)-Induced contractions in Guinea pig tracheal smooth muscles. Biol Pharm Bull. (2022) 45:240–4. doi: 10.1248/bpb.b21-00905 [DOI] [PubMed] [Google Scholar]
- 113. Dominguez EC, Heires AJ, Pavlik J, Larsen TD, Guardado S, Sisson JH, et al. A high docosahexaenoic acid diet alters the lung inflammatory response to acute dust exposure. Nutrients. (2020) 12:2334. doi: 10.3390/nu12082334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ulu A, Burr A, Heires AJ, Pavlik J, Larsen T, Perez PA, et al. A high docosahexaenoic acid diet alters lung inflammation and recovery following repetitive exposure to aqueous organic dust extracts. J Nutr Biochem. (2021) 97:108797. doi: 10.1016/j.jnutbio.2021.108797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Favor OK, Rajasinghe LD, Wierenga KA, Maddipati KR, Lee KSS, Olive AJ, et al. Crystalline silica-induced proinflammatory eicosanoid storm in novel alveolar macrophage model quelled by docosahexaenoic acid supplementation. Front Immunol. (2023) 14:1274147. doi: 10.3389/fimmu.2023.1274147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Heires AJ, Samuelson D, Villageliu D, Nordgren TM, Romberger DJ. Agricultural dust derived bacterial extracellular vesicle mediated inflammation is attenuated by DHA. Sci Rep.-UK. (2023) 13:2767. doi: 10.1038/s41598-023-29781-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. (2007) 282:9323–34. doi: 10.1074/jbc.M609212200 [DOI] [PubMed] [Google Scholar]
- 118. Li J, Leong MM, Stewart A, Rizzacasa MA. Total synthesis of the endogenous inflammation resolving lipid resolvin D2 using a common lynchpin. Beilstein J Org Chem. (2013) 9:2762–6. doi: 10.3762/bjoc.9.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. (2013) 20:188–201. doi: 10.1016/j.chembiol.2012.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Winkler JW, Orr SK, Dalli J, Cheng CY, Sanger JM, Chiang N, et al. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Sci Rep. (2016) 6:18972. doi: 10.1038/srep18972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ogawa N, Sugiyama T, Morita M, Suganuma Y, Kobayashi Y. Total synthesis of resolvin d5. J Org Chem. (2017) 82:2032–9. doi: 10.1021/acs.joc.6b02870 [DOI] [PubMed] [Google Scholar]
- 122. Pham TL, Kakazu AH, He J, Nshimiyimana R, Petasis NA, Jun B, et al. Elucidating the structure and functions of Resolvin D6 isomers on nerve regeneration with a distinctive trigeminal transcriptome. FASEB J. (2021) 35:e21775. doi: 10.1096/fj.202100686R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Edin ML, Zeldin DC. Commercial scale production of RvD4 opens the resolving door to new research. J Leukoc Biol. (2018) 103:991–3. doi: 10.1002/JLB.3CE0118-032R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-Hayat SM, Bo S, Sahebkar A. Resolvins: Emerging players in autoimmune and inflammatory diseases. Clin Rev Allerg. Immu. (2020) 58:82–91. doi: 10.1007/s12016-019-08754-9 [DOI] [PubMed] [Google Scholar]
- 125. Park J, Roh J, Pan J, Kim YH, Park CK, Jo YY. Role of resolvins in inflammatory and neuropathic pain. Pharm (Basel). (2023) 16:1366. doi: 10.3390/ph16101366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, et al. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. (2012) 189:1983–91. doi: 10.4049/jimmunol.1101665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gagliardo R, Ferrante G, Fasola S, Di Vincenzo S, Pace E, La Grutta S. Resolvin D1 and miR-146a are independent distinctive parameters in children with moderate and severe asthma. Clin Exp Allergy. (2021) 51:350–3. doi: 10.1111/cea.13771 [DOI] [PubMed] [Google Scholar]
- 128. Zhang HW, Wang Q, Mei HX, Zheng SX, Ali AM, Wu QX, et al. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int Immunopharmacol. (2019) 76:105877. doi: 10.1016/j.intimp.2019.105877 [DOI] [PubMed] [Google Scholar]
- 129. Gu Z, Lamont GJ, Lamont RJ, Uriarte SM, Wang H, Scott DA, et al. resolvin D2 and maresin 1 activate the GSK3beta anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. (2016) 22:186–95. doi: 10.1177/1753425916628618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. (2016) 8:111r–353r. doi: 10.1126/scitranslmed.aaf7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zambalde ÉP, Teixeira MM, Favarin DC, de Oliveira JR, Magalhães ML, Cunha MM, et al. The anti-inflammatory and pro-resolution effects of aspirin-triggered RvD1 (AT-RvD1) on peripheral blood mononuclear cells from patients with severe asthma. Int Immunopharmacol. (2016) 35:142–8. doi: 10.1016/j.intimp.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 132. Colby JK, Abdulnour RE, Sham HP, Dalli J, Colas RA, Winkler JW, et al. Resolvin d3 and Aspirin-Triggered resolvin d3 are protective for injured epithelia. Am J Pathol. (2016) 186:1801–13. doi: 10.1016/j.ajpath.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Libreros S, Nshimiyimana R, Lee B, Serhan CN. Infectious neutrophil deployment is regulated by resolvin D4. Blood. (2023) 142:589–606. doi: 10.1182/blood.2022019145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chun HW, Lee J, Pham TH, Lee J, Yoon JH, Lee J, et al. Resolvin d5, a lipid mediator, inhibits production of interleukin-6 and CCL5 via the ERK-NF-kappaB signaling pathway in Lipopolysaccharide-Stimulated THP-1 cells. J Microbiol Biotechnol. (2020) 30:85–92. doi: 10.4014/jmb.1907.07033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Ganesan R, Henkels KM, Shah K, de la Rosa X, Libreros S, Cheemarla NR, et al. D-series Resolvins activate Phospholipase D in phagocytes during inflammation and resolution. FASEB J. (2020) 34:15888–906. doi: 10.1096/fj.201903025RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U.S.A. (2010) 107:1660–5. doi: 10.1073/pnas.0907342107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN. Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol. (2012) 180:2018–27. doi: 10.1016/j.ajpath.2012.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gemperle C, Tran S, Schmid M, Rimann N, Marti-Jaun J, Hartling I, et al. Resolvin D1 reduces inflammation in co-cultures of primary human macrophages and adipocytes by triggering macrophages. Prostaglandins Leukot Essent Fatty Acids. (2021) 174:102363. doi: 10.1016/j.plefa.2021.102363 [DOI] [PubMed] [Google Scholar]
- 139. Uleman JF, Mancini E, Al-Shama R, Te VA, Kraneveld AD, Castiglione F. A multiscale hybrid model for exploring the effect of Resolvin D1 on macrophage polarization during acute inflammation. Math Biosci. (2023) 359:108997. doi: 10.1016/j.mbs.2023.108997 [DOI] [PubMed] [Google Scholar]
- 140. Bisicchia E, Sasso V, Catanzaro G, Leuti A, Besharat ZM, Chiacchiarini M, et al. Resolvin d1 halts remote neuroinflammation and improves functional recovery after focal brain damage via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol Neurobiol. (2018) 55:6894–905. doi: 10.1007/s12035-018-0889-z [DOI] [PubMed] [Google Scholar]
- 141. Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo . Pharmacol Rev. (2006) 58:463–87. doi: 10.1124/pr.58.3.4 [DOI] [PubMed] [Google Scholar]
- 142. Gilbert NC, Newcomer ME, Werz O. Untangling the web of 5-lipoxygenase-derived products from a molecular and structural perspective: The battle between pro- and anti-inflammatory lipid mediators. Biochem Pharmacol. (2021) 193:114759. doi: 10.1016/j.bcp.2021.114759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gao J, Su Y, Wang Z. Lung inflammation resolution by RvD1 and RvD2 in a Receptor-Dependent manner. Pharmaceutics. (2023) 15:1527. doi: 10.3390/pharmaceutics15051527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gagliardo R, Gras D, La Grutta S, Chanez P, Di Sano C, Albano GD, et al. Airway lipoxin A4/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J Allergy Clin Immunol. (2016) 137:1796–806. doi: 10.1016/j.jaci.2015.11.045 [DOI] [PubMed] [Google Scholar]
- 145. de Oliveira JR, Favarin DC, Tanaka SCSV, Balarin MAS, Silva Teixeira DN, Levy BD, et al. AT-RvD1 modulates CCL-2 and CXCL-8 production and NF-κB, STAT-6, SOCS1, and SOCS3 expression on bronchial epithelial cells stimulated with IL-4. BioMed Res Int. (2015) 2015:1–8. doi: 10.1155/2015/178369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ou G, Liu Q, Yu C, Chen X, Zhang W, Chen Y, et al. The protective effects of maresin 1 in the OVA-Induced asthma mouse model. Mediat. Inflammation. (2021) 2021:1–11. doi: 10.1155/2021/4131420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, et al. Cutting edge: Maresin-1 engages regulatory t cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol. (2015) 194):863–7. doi: 10.4049/jimmunol.1402534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Munir F, Jamshed MB, Shahid N, Muhammad SA, Bhandari A, Zhang Q. Protective effects of maresin 1 against inflammation in experimentally induced acute pancreatitis and related lung injury. Am J Physiol Gastrointest Liver Physiol. (2019) 317:G333–41. doi: 10.1152/ajpgi.00078.2019 [DOI] [PubMed] [Google Scholar]
- 149. Im D. Maresin-1 resolution with RORα and LGR6. Prog Lipid Res. (2020) 78:101034. doi: 10.1016/j.plipres.2020.101034 [DOI] [PubMed] [Google Scholar]
- 150. Zhao M, Li C, Zhang J, Yin Z, Zheng Z, Wan J, et al. Maresin-1 and its receptors RORα/LGR6 as potential therapeutic target for respiratory diseases. Pharmacol Res. (2022) 182:106337. doi: 10.1016/j.phrs.2022.106337 [DOI] [PubMed] [Google Scholar]
- 151. Krishnamoorthy N, Walker KH, Brüggemann TR, Tavares LP, Smith EW, Nijmeh J, et al. The Maresin 1–LGR6 axis decreases respiratory syncytial virus-induced lung inflammation. Proc Natl Acad Sci. (2023) 120(2):e2206480120. doi: 10.1073/pnas.2206480120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chiang N, Libreros S, Norris PC, de la Rosa X, Serhan CN. Maresin 1 activates LGR6 receptor promoting phagocyte immunoresolvent functions. J Clin Invest. (2019) 129:5294–311. doi: 10.1172/JCI129448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Fattori V, Zaninelli TH, Ferraz CR, Brasil-Silva L, Borghi SM, Cunha JM, et al. Maresin 2 is an analgesic specialized pro-resolution lipid mediator in mice by inhibiting neutrophil and monocyte recruitment, nociceptor neuron TRPV1 and TRPA1 activation, and CGRP release. Neuropharmacology. (2022) 216:109189. doi: 10.1016/j.neuropharm.2022.109189 [DOI] [PubMed] [Google Scholar]
- 154. Han J, Li H, Bhandari S, Cao F, Wang XY, Tian C, et al. Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury. J Cell Mol Med. (2020) 24:4736–47. doi: 10.1111/jcmm.15146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Säfholm J, Abma W, Bankova LG, Boyce JA, Al-Ameri M, Orre A, et al. Cysteinyl-maresin 3 inhibits IL-13 induced airway hyperresponsiveness through alternative activation of the CysLT1 receptor. Eur J Pharmacol. (2022) 934:175257. doi: 10.1016/j.ejphar.2022.175257 [DOI] [PubMed] [Google Scholar]
- 156. Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin d1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. (2007) 178):496–502. doi: 10.4049/jimmunol.178.1.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kumar M, Yano N, Fedulov AV. Gestational exposure to titanium dioxide, diesel exhaust, and concentrated urban air particles affects levels of specialized pro-resolving mediators in response to allergen in asthma-susceptible neonate lungs. J Toxicol Environ Health A. (2022) 85:243–61. doi: 10.1080/15287394.2021.2000906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immun. (2013) 131:353–60. doi: 10.1016/j.jaci.2012.07.048 [DOI] [PubMed] [Google Scholar]
- 159. Ariel A, Li P, Wang W, Tang W, Fredman G, Hong S, et al. The docosatriene protectin d1 is produced by TH2 skewing and promotes human t cell apoptosis via lipid raft clustering. J Biol Chem. (2005) 280:43079–86. doi: 10.1074/jbc.M509796200 [DOI] [PubMed] [Google Scholar]
- 160. Wu Z, Zhang L, Zhao X, Li Z, Lu H, Bu C, et al. Protectin D1 protects against lipopolysaccharide−induced acute lung injury through inhibition of neutrophil infiltration and the formation of neutrophil extracellular traps in lung tissue. Exp Ther Med. (2021) 22:1074. doi: 10.3892/etm.2021.10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. (2007) 447:869–74. doi: 10.1038/nature05877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lagarde M, Guichardant M, Bernoud-Hubac N. Anti-inflammatory and anti-virus potential of poxytrins, especially protectin DX. Biochimie. (2020) 179:281–4. doi: 10.1016/j.biochi.2020.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Xia H, Ge Y, Wang F, Ming Y, Wu Z, Wang J, et al. Protectin DX ameliorates inflammation in sepsis-induced acute lung injury through mediating PPARγ/NF-κB pathway. Immunol Res. (2020) 68:280–8. doi: 10.1007/s12026-020-09151-7 [DOI] [PubMed] [Google Scholar]
- 164. Zhuo X, Hao Y, Cao F, Yan S, Li H, Wang Q, et al. Protectin DX increases alveolar fluid clearance in rats with lipopolysaccharide-induced acute lung injury. Exp Mol Med. (2018) 50:1–13. doi: 10.1038/s12276-018-0075-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yang JX, Li M, Hu X, Lu JC, Wang Q, Lu SY, et al. Protectin DX promotes epithelial injury repair and inhibits fibroproliferation partly via ALX/PI3K signalling pathway. J Cell Mol Med. (2020) 24:14001–12. doi: 10.1111/jcmm.16011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ye Y, Zhang HW, Mei HX, Xu HR, Xiang SY, Yang Q, et al. PDX regulates inflammatory cell infiltration via resident macrophage in LPS-induced lung injury. J Cell Mol Med. (2020) 24:10604–14. doi: 10.1111/jcmm.15679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lv Y, Chen D, Tian X, Xiao J, Xu C, Du L, et al. Protectin conjugates in tissue regeneration 1 alleviates sepsis-induced acute lung injury by inhibiting ferroptosis. J Transl Med. (2023) 21:293. doi: 10.1186/s12967-023-04111-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Surette ME, Stull D, Lindemann J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr Med Res Opin. (2008) 24:559–67. doi: 10.1185/030079908X273011 [DOI] [PubMed] [Google Scholar]
- 169. Abreu SC, Lopes-Pacheco M, Da Silva AL, Xisto DG, de Oliveira TB, Kitoko JZ, et al. Eicosapentaenoic acid enhances the effects of mesenchymal stromal cell therapy in experimental allergic asthma. Front Immunol. (2018) 9:1147. doi: 10.3389/fimmu.2018.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Morin C, Fortin S, Cantin AM, Rousseau É. MAG-EPA resolves lung inflammation in an allergic model of asthma. Clin Exp Allergy. (2013) 43:1071–82. doi: 10.1111/cea.12162 [DOI] [PubMed] [Google Scholar]
- 171. Serhan CN, Libreros S, Nshimiyimana R. E-series resolvin metabolome, biosynthesis and critical role of stereochemistry of specialized pro-resolving mediators (SPMs) in inflammation-resolution: Preparing SPMs for long COVID-19, human clinical trials, and targeted precision nutrition. Semin Immunol. (2022) 59:101597. doi: 10.1016/j.smim.2022.101597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Calder PC. Eicosapentaenoic and docosahexaenoic acid derived specialised proresolving mediators: Concentrations in humans and the effects of age, sex, disease and increased omega-3 fatty acid intake. Biochimie. (2020) 178:105–23. doi: 10.1016/j.biochi.2020.08.015 [DOI] [PubMed] [Google Scholar]
- 173. Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. (2000) 192:1197–204. doi: 10.1084/jem.192.8.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Eric Tjonahen SFO, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, et al. Resolvin e2: Identification and Anti-Inflammatory actions: Pivotal role of human 5-Lipoxygenase in resolvin e series biosynthesis. Chem Biol. (2006) 13:1193–202. doi: 10.1016/j.chembiol.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 175. Isobe Y, Arita M, Matsueda S, Iwamoto R, Fujihara T, Nakanishi H, et al. Identification and structure determination of novel anti-inflammatory mediator resolvin e3,17,18-Dihydroxyeicosapentaenoic acid. J Biol Chem. (2012) 287:10525–34. doi: 10.1074/jbc.M112.340612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Norris PC, Libreros S, Serhan CN. Resolution metabolomes activated by hypoxic environment. Sci Adv. (2019) 5:x4895. doi: 10.1126/sciadv.aax4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Xu Z, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins as new fascinating drug candidates for inflammatory diseases, Nat. Med. (2010) 16:592–7. doi: 10.1038/nm.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ishizuka T, Hisada T, Aoki H, Mori M. Resolvin E1: A novel lipid mediator in the resolution of allergic airway inflammation. Expert Rev Clin Immu. (2008) 4:669–72. doi: 10.1586/1744666X.4.6.669 [DOI] [PubMed] [Google Scholar]
- 179. Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin-23, interferon-γ and lipoxin A4 to promote resolution of allergic airway inflammation. Nat Immunol. (2008) 9:873–9. doi: 10.1038/ni.1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Oner F, Alvarez C, Yaghmoor W, Stephens D, Hasturk H, Firatli E, et al. Resolvin e1 regulates th17 function and t cell activation. Front Immunol. (2021) 12:637983. doi: 10.3389/fimmu.2021.637983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Bioph. Res Co. (2008) 367:509–15. doi: 10.1016/j.bbrc.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 182. Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, et al. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem Bioph. Res Co. (2010) 400:128–33. doi: 10.1016/j.bbrc.2010.08.025 [DOI] [PubMed] [Google Scholar]
- 183. Flesher RP, Herbert C, Kumar RK. Resolvin E1 promotes resolution of inflammation in a mouse model of an acute exacerbation of allergic asthma. Clin Sci. (2014) 126:805–18. doi: 10.1042/CS20130623 [DOI] [PubMed] [Google Scholar]
- 184. Ramar M, Yano N, Fedulov AV. Intra-Airway treatment with synthetic lipoxin a4 and resolvin e2 mitigates neonatal asthma triggered by maternal exposure to environmental particles. Int J Mol Sci. (2023) 24:6145. doi: 10.3390/ijms24076145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sato M, Aoki-Saito H, Fukuda H, Ikeda H, Koga Y, Yatomi M, et al. Resolvin E3 attenuates allergic airway inflammation via the interleukin-23–interleukin-17A pathway. FASEB journal: Off Publ Fed Am Societies Exp Biol. (2019) 33:12750–9. doi: 10.1096/fj.201900283R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Fukuda H, Ikeda H, Muromoto R, Hirashima K, Ishimura K, Fujiwara K, et al. Synthesis of resolvin e3, a proresolving lipid mediator, and its deoxy derivatives:identification of 18-Deoxy-resolvin e3 as a potent Anti-Inflammatory agent. J organic Chem. (2020) 85:14190–200. doi: 10.1021/acs.joc.0c01701 [DOI] [PubMed] [Google Scholar]
- 187. Libreros S, Shay AE, Nshimiyimana R, Fichtner D, Martin MJ, Wourms N, et al. A new E-Series resolvin: RvE4 stereochemistry and function in efferocytosis of Inflammation-Resolution. Front Immunol. (2021) 11:631319. doi: 10.3389/fimmu.2020.631319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zheng Z, Dai Z, Cao Y, Shen Q, Zhang Y. Docosapentaenoic acid (DPA, 22:5n-3) ameliorates inflammation in an ulcerative colitis model. Food Funct. (2019) 10:4199–209. doi: 10.1039/c8fo02338g [DOI] [PubMed] [Google Scholar]
- 189. Hirata SI, Nagatake T, Sawane K, Hosomi K, Honda T, Ono S, et al. Maternal omega3 docosapentaenoic acid inhibits infant allergic dermatitis through TRAIL-expressing plasmacytoid dendritic cells in mice. Allergy. (2020) 75:1939–55. doi: 10.1111/all.14217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Khaddaj-Mallat R, Hiram R, Sirois C, Sirois M, Rizcallah E, Marouan S, et al. MAG-DPA curbs inflammatory biomarkers and pharmacological reactivity in cytokine-triggered hyperresponsive airway models. Pharmacol Res Perspect. (2016) 4:e263. doi: 10.1002/prp2.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Morin C, Hiram R, Rousseau E, Blier PU, Fortin S. Docosapentaenoic acid monoacylglyceride reduces inflammation and vascular remodeling in experimental pulmonary hypertension. Am J Physiol.-Heart C. (2014) 307:H574–86. doi: 10.1152/ajpheart.00814.2013 [DOI] [PubMed] [Google Scholar]
- 192. Choi MK, Kim J, Park HM, Lim CM, Pham TH, Shin HY, et al. The DPA-derivative 11S, 17S-dihydroxy 7,9,13,15,19 (Z,E,Z,E,Z)-docosapentaenoic acid inhibits IL-6 production by inhibiting ROS production and ERK/NF-kappaB pathway in keratinocytes HaCaT stimulated with a fine dust PM(10). Ecotoxicol Environ Saf. (2022) 232:113252. doi: 10.1016/j.ecoenv.2022.113252 [DOI] [PubMed] [Google Scholar]
- 193. Wang L, Choi HS, Su Y, Ju JH, Heo SY, Yi JJ, et al. The docosahexaenoic acid derivatives, diHEP-DPA and TH-DPA, synthesized via recombinant lipoxygenase, ameliorate disturbances in lipid metabolism and liver inflammation in high fat diet-fed mice. Life Sci. (2022) 291:120219. doi: 10.1016/j.lfs.2021.120219 [DOI] [PubMed] [Google Scholar]
- 194. Drouin G, Rioux V, Legrand P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie. (2019) 159:36–48. doi: 10.1016/j.biochi.2019.01.022 [DOI] [PubMed] [Google Scholar]
- 195. Tungen JE, Gerstmann L, Vik A, De Matteis R, Colas RA, Dalli J, et al. Resolving inflammation: Synthesis, configurational assignment, and biological evaluations of RvD1(n-3 DPA). Chemistry. (2019) 25:1476–80. doi: 10.1002/chem.201806029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Reinertsen AF, Primdahl KG, De Matteis R, Dalli J, Hansen TV. Stereoselective synthesis, configurational assignment and biological evaluations of the lipid mediator RvD2(n-3 DPA). Chemistry. (2022) 28:e202103857. doi: 10.1002/chem.202103857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Ervik K, Reinertsen AF, Koenis DS, Dalli J, Hansen TV. Stereoselective synthesis, pro-resolution, and anti-inflammatory actions of RvD5(n-3 DPA). J Nat Prod. (2023) 86:2546–53. doi: 10.1021/acs.jnatprod.3c00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sonderskov J, Tungen JE, Palmas F, Dalli J, Serhan CN, Stenstrom Y, et al. Stereoselective synthesis of mar2(n-3 DPA). Tetrahedron Lett. (2020) 61(7):151510. doi: 10.1016/j.tetlet.2019.151510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Aursnes M, Tungen JE, Vik A, Colas R, Cheng CY, Dalli J, et al. Total synthesis of the lipid mediator PD1n-3 DPA: Configurational assignments and anti-inflammatory and pro-resolving actions. J Nat Prod. (2014) 77:910–6. doi: 10.1021/np4009865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Weylandt KH. Docosapentaenoic acid derived metabolites and mediators - the new world of lipid mediator medicine in a nutshell. Eur J Pharmacol. (2016) 785:108–15. doi: 10.1016/j.ejphar.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 201. Balta MG, Schreurs O, Halder R, Kuntziger TM, Saetre F, Blix I, et al. RvD1(n-3 DPA) downregulates the transcription of Pro-Inflammatory genes in oral epithelial cells and reverses nuclear translocation of transcription factor p65 after TNF-alpha stimulation. Int J Mol Sci. (2022) 23:14878. doi: 10.3390/ijms232314878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Colas RA, Souza PR, Walker ME, Burton M, Zaslona Z, Curtis AM, et al. Impaired production and diurnal regulation of vascular RvD(n-3 DPA) increase systemic inflammation and cardiovascular disease. Circ Res. (2018) 122:855–63. doi: 10.1161/CIRCRESAHA.117.312472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Flak MB, Colas RA, Munoz-Atienza E, Curtis MA, Dalli J, Pitzalis C. Inflammatory arthritis disrupts gut resolution mechanisms, promoting barrier breakdown by Porphyromonas gingivalis. JCI Insight. (2019) 4(13):e125191. doi: 10.1172/jci.insight.125191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Flak MB, Koenis DS, Sobrino A, Smith J, Pistorius K, Palmas F, et al. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J Clin Invest. (2020) 130:359–73. doi: 10.1172/JCI131609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Pistorius K, Souza PR, De Matteis R, Austin-Williams S, Primdahl KG, Vik A, et al. PD(n-3 DPA) pathway regulates human monocyte differentiation and macrophage function. Cell Chem Biol. (2018) 25:749–60. doi: 10.1016/j.chembiol.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Frigerio F, Pasqualini G, Craparotta I, Marchini S, van Vliet EA, Foerch P, et al. N-3 Docosapentaenoic acid-derived protectin D1 promotes resolution of neuroinflammation and arrests epileptogenesis. Brain. (2018) 141:3130–43. doi: 10.1093/brain/awy247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Nesman JI, Chen O, Luo X, Ji RR, Serhan CN, Hansen TV. A new synthetic protectin D1 analog 3-oxa-PD1(n-3 DPA) reduces neuropathic pain and chronic itch in mice. Org Biomol Chem. (2021) 19:2744–52. doi: 10.1039/d0ob02136a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Dalli J, Chiang N, Serhan CN. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat Med. (2015) 21:1071–5. doi: 10.1038/nm.3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chiang N, Sakuma M, Rodriguez AR, Spur BW, Irimia D, Serhan CN. Resolvin T-series reduce neutrophil extracellular traps. Blood. (2022) 139:1222–33. doi: 10.1182/blood.2021013422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Walker ME, Souza PR, Colas RA, Dalli J. 13-Series resolvins mediate the leukocyte-platelet actions of atorvastatin and pravastatin in inflammatory arthritis. FASEB J. (2017) 31:3636–48. doi: 10.1096/fj.201700268 [DOI] [PMC free article] [PubMed] [Google Scholar]