Abstract
Animal tuberculosis (TB) has been reported in several wildlife species in the Greater Kruger Conservation Area (GKCA), South Africa. This report describes the discovery of clinical tuberculosis, caused by Mycobacterium bovis (M. bovis), in free-ranging vervet monkeys (Chlorocebus pygerythrus). The “One Health” concept is especially relevant to TB since this is a multi-host disease with zoonotic potential and is endemic in GKCA. Vervet monkeys have become habituated to humans in tourist areas and may be a source of infection through close contact. Indirect transmission of M. bovis through environmental sources has also been suspected to present a risk of spread between host species. Clinically diseased monkeys present in two tourist areas in the GKCA, that died (n = 1) or were euthanized (n = 5), were submitted for diagnostic necropsies. The presence of pathological lesions, Ziehl-Neelsen-stained impression smears, Xpert® MTB/RIF Ultra (GXU) assay, mycobacterial culture and speciation by genomic regions of difference PCR, were used to confirm the diagnosis of M. bovis infection in these monkeys. The finding of multiple cases necessitates further investigation of TB in monkey troops living within the GKCA tourist areas to determine the source of infection and assess the risk of transmission to other animals and humans.
Keywords: animal tuberculosis, Chlorocebus pygerythrus, Mycobacterium bovis, one health, vervet monkeys, wildlife
1. Introduction
Vervet monkeys (Chlorocebus pygerythrus) occupy diverse habitats across southern and eastern Africa, predominantly open woodland and savannahs, but also commonly found in several peri-urban areas (1, 2). As habitats are modified by development, this can result in competition between wildlife and humans for resources and space. Due to their flexibility in foraging strategies and absence of major threats, vervet monkeys often come into conflict with humans, because of shared human-dominated landscapes, which also holds true for game reserves and parks (3, 4). Vervet monkeys are therefore exposed to anthropogenic risks, including infectious diseases (5). However, they may also present zoonotic threats to humans, especially in areas where endemic diseases are present (6, 7).
There are numerous reports of Mycobacterium tuberculosis outbreaks in captive and free-ranging Old World primates, usually that have either direct or indirect contact with humans (8–10), including free-ranging Chacma baboons (Papio ursinus) in the Cape Peninsula, South Africa (11). Natural infections with two different M. tuberculosis strains were found in two vervet monkeys at a rehabilitation centre in South Africa following deaths of several individuals (12, 13). Generally, Old World monkeys kept under intensive captive conditions appear to be susceptible to infection by any route and can develop fulminating, fatal disease (14, 15).
In comparison, there is less knowledge about Mycobacterium bovis infection in Old World monkeys. Mycobacterium bovis infection and disease, as well as infection with other MTBC, such as Mycobacterium orygis and Mycobacterium caprae, has been sporadically reported in captive non-human primates (16–21). However, studies of M. bovis infection in free-ranging African primates are more limited, although they have been focused on M. bovis endemic areas. For example, wild chacma baboons were screened at a human-wildlife interface in the Kafue Flats, Zambia, where M. bovis-infected cattle (Bos taurus) and lechwe (Kobus leche) populations were present. Gross lesions consistent with tuberculosis (TB) in the lungs and associated lymph nodes were found in four adult male baboons (22). The M. bovis spoligotypes found in these baboons, SB0120, was the same strain reported in humans, livestock, and wildlife in the Kafue ecosystem. Opportunistic sampling of wildlife carcasses in Ruaha ecosystem, Tanzania, identified M. bovis from tissues of two vervet monkeys and one yellow baboon (Papio cynocephalus), although no gross lesions were observed (23). Spoligotyping of the isolates revealed the same M. bovis strain, SB0133, found in local livestock. These cases highlight the risk of infection to non-human primates in ecosystems with other M. bovis infected hosts.
Animal TB, caused by M. bovis infection, has been diagnosed in various wildlife species in the Greater Kruger Conservation Area (GKCA) in South Africa, since the first detection in 1990, and is now considered endemic (24, 25). Cases in non-human primates have included a TB outbreak in 1996 in a chacma baboon troop that frequented the Skukuza tourist area in GKCA (26). A second outbreak was recorded in 2010 in the same area, within a baboon troop also displaying unusual roosting behavior, by accessing a closed workshop roof at night. A program of testing and euthanasia was applied, as previously described by Keet et al. (26), and the disease was eradicated in the Skukuza baboon troop (27). However, despite continued surveillance using necropsy findings, no cases of M. bovis or M. tuberculosis infection have previously been found in vervet monkeys, although troops occupy the same areas as the baboons. Therefore, this case series describes the first report of clinical TB in free-ranging vervet monkeys in the GKCA, South Africa.
2. Case descriptions and clinical findings
Between June 2023 and May 2024, free-ranging vervet monkeys, found in tourist areas in Skukuza (n = 5) and a private lodge in the Sabie Game Reserve (n = 1), were reported with signs of depression, weakness, and emaciation. Both these areas are part of the GKCA but approximately 12 km apart, and are considered endemic for M. bovis (24, 25, 28). The case in Sabie Game Reserve died after observing clinical signs. The animals in Skukuza were captured using a baited trap and then immobilized by pole syringe or plastic projectile dart, using a combination of tiletamine-zolazepam [Zoletil®; Virbac RSA, (Pty) Ltd., Centurion, South Africa] and ketamine [Kyron Laboratories (Pty) Limited, Benrose, South Africa], both at 10 mg/kg IM. Animals were humanely euthanized while anesthetized, with sodium pentobarbitone (Euthapent; Kyron Laboratories) at 200 mg/kg IV, followed by a thorough postmortem examination and tissue sample collection. This included spleen, lung, and lymph nodes (pooled head, thoracic, abdominal, and peripheral lymph nodes), as well as other organs with lesions consistent with TB. Fresh samples were frozen, and a second set of tissues was stored in 10% buffered formalin for histopathological examination.
3. Diagnostic assessment
Frozen tissues were processed in a BSL3 laboratory for initial screening with a rapid qPCR assay (GXU; Xpert MTB/RIF Ultra; Cepheid Sunnyvale, CA, USA) for detection of Mycobacterium tuberculosis complex DNA, and mycobacterial culture using the BD BACTEC™ MGIT™ 960 system (Becton Dickinson, Franklin Lakes, NJ, USA), as previously described (29). Cultures positive for growth were genetically speciated by genomic regions of difference PCR (30) to confirm infection with M. bovis.
All six vervet monkeys (2 males, 4 females, including 3 adults >2 years of age; 2 sub-adults 1–2 years; 1 juvenile <1 year) had gross lesions consistent with TB in multiple organs, with spleen and lung lesions present in all six clinical cases (Table 1). Lungs were generally bilaterally affected with multiple necrogranulomatous lesions, varying from 1 mm to almost confluent throughout the parenchyma (Figure 1). The dorso-caudal lung lobes were more severely affected compared to the middle and cranial lobes, while only one adult female monkey had a unilateral left caudal tuberculous pneumonia. Necrogranulomatous lesions were also a common feature in the spleens of diseased monkeys, with multiple granulomas varying in size from 1 to 8 mm in diameter (Figures 2, 3). Impression smears from lung and spleen of each case were used for Ziehl-Neelsen staining. All six cases were confirmed with M. bovis infection, based on mycobacterial culture and speciation, GXU, macro- and micro- histopathological techniques. Results are summarized in Table 1.
Table 1.
Summary of demographic information and Mycobacterium bovis test results of six free-ranging vervet monkeys (Chlorocebus pygerythrus) sampled in the Greater Kruger Conservation Area, South Africa.
| Case | Age | Sex | Sample date | Clinical signs | Macroscopic lesions present | Histological lesions present | Ziehl-Neelsen stain (cytology) | GXU®# | Speciation |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Sub-adult | Female | 28 Jun 2023 | Depressed, cough, emaciated, weakness, incoordination | S, P, M, L | S, P, K | ++ | MTBC detected – medium; no RIF resistance | M. bovis |
| 2 | Adult | Female | 1 Aug 2023 | Depressed, emaciated, weakness | S, P, H, M | NA | +++ | MTBC detected – high; no RIF resistance | M. bovis |
| 3 | Adult | Female | 1 Feb 2024 | Depressed, emaciated, weakness, visibly swollen lymph nodes | S, P, M, A | S (only tissue examined) | ++ | MTBC detected – high; no RIF resistance | M. bovis |
| 4 | Juvenile | Male | 30 Mar 2024 | Depressed, emaciated, weakness, visibly swollen lymph nodes, incoordination, dyspnoea | S, P, H, M, A, L | S, P, H, E | +++ | MTBC detected – high; no RIF resistance | M. bovis |
| 5 | Adult | Female | 30 Apr 2024 | Depressed, emaciated, weakness, dyspnoea incoordination | S, P, M | S, P, K | + | MTBC detected – high; no RIF resistance | M. bovis |
| 6 | Sub-adult | Male | 4 May 2024 | Depressed, emaciated, weakness, dyspnoea | S, P, H, M, K | S, P, L, M, K | + | MTBC detected – medium; no RIF resistance | M. bovis |
S, Spleen; P, Lungs & pulmonary lymph nodes (lnn), H, Head lnn; M, Mesenteric lnn; A, Mammary/axillary lnn; L, Liver; K, Kidney; E, enteritis; NA, not available; GXU, Cepheid Xpert MTB/RIF Ultra qPCR assay; RIF, rifampicin; *histopathologic analyses performed; #the levels of MTBC detected (very low/low/medium/high) by the Cepheid Xpert MTB/RIF Ultra qPCR assay were based on preprogrammed rpoB cycle threshold (Ct) values; very low (Ct > 28), low (Ct 22–28), medium (Ct 16–22) or high (Ct < 16).
+ 0–50 AFB per field; ++ 50–100 AFB per field; +++ > 100 AFB per field.
Figure 1.
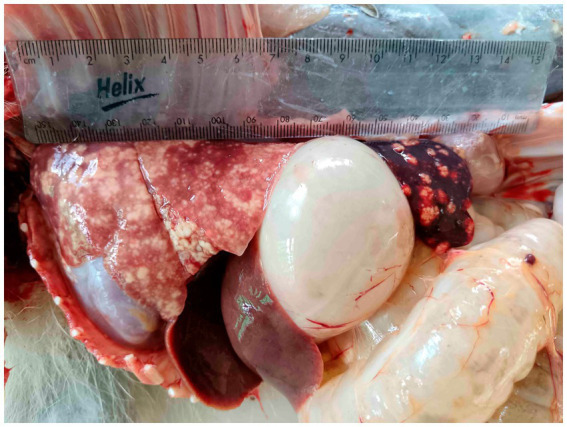
Macroscopic lesions in lung and spleen of a juvenile male vervet monkey (Chlorocebus pygerythrus), that was euthanized in 2024 from the Skukuza tourist camp in Greater Kruger Conservation Area, South Africa. Lesions were similar to other tuberculosis cases in vervet monkeys in this area, associated with Mycobacterium bovis infection. Mycobacterium tuberculosis complex DNA was detected in this case using qPCR (Cepheid Xpert MTB/RIF Ultra qPCR assay).
Figure 2.
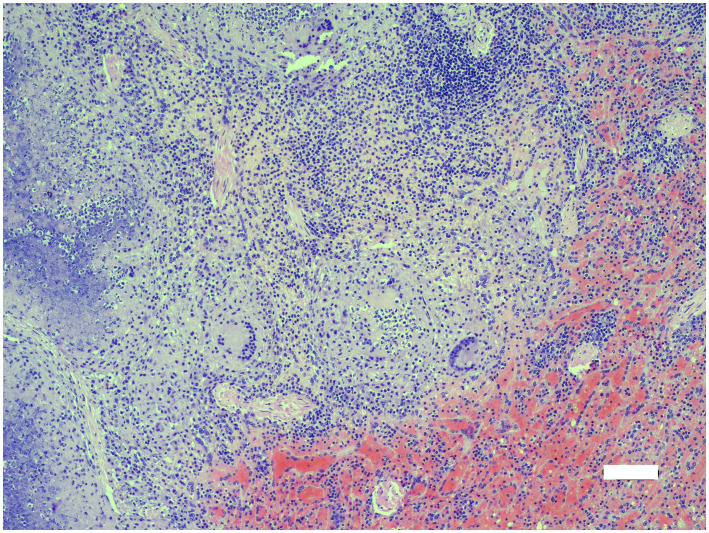
Microscopic lesions in spleen of adult female vervet monkey (Chlorocebus pygerythrus); necrogranulomatous splentitis with large multinucleate giant cells and marked splenic congestion (lower right). H&E x100. Bar = 100 μm.
Figure 3.
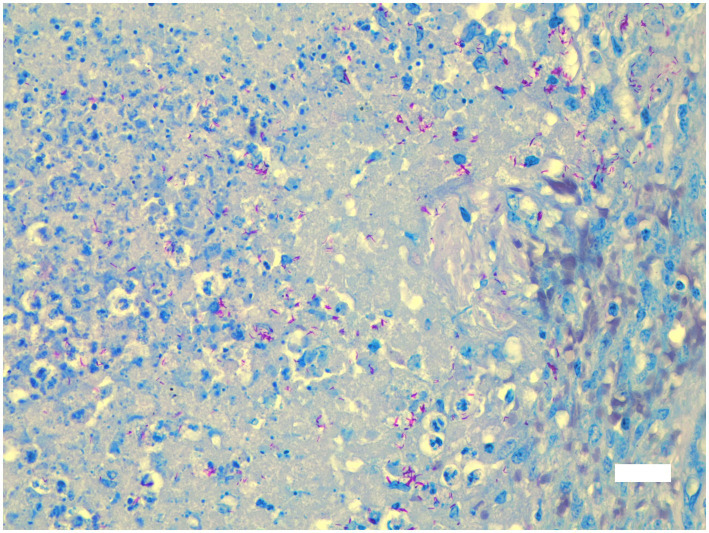
Microscopic lesions in spleen of adult female vervet monkey (Chlorocebus pygerythrus); necrogranulomatous splenitis with large numbers of fine acid-fast (red) bacilli in necrotic debris. Ziehl Neelsen x 400. Bar = 30 μm.
Histopathological examination was performed on selected tissues from five monkeys (cases 1, 3–6; no formalin fixed tissues were available for case 2). Spleen, lung and/or lymph nodes were virtually effaced by unencapsulated coalescing necrogranulomas in all five monkeys. Additional lesions included mild to moderate necrogranulomatous nephritis (cases 1, 4–6), hepatitis (cases 5, 6) and enteritis (case 4), often with numerous multinucleate giant cells. Only spleen was submitted from case 3. Mild to moderate lymphohistiocytic perivascular interstitial pneumonia was also present in the four monkeys for which lung was submitted. Rare acid-fast organisms (Ziehl-Neelsen) were seen in multinucleate giant cells, but organisms were more common in necrotic debris and macrophages in splenic granulomas, macrophages in neutrophil-rich bronchiolar exudate and in tissue impression smears (Table 1).
Mycobacterium bovis infection was confirmed by culture and speciation from multiple tissues in all six vervet monkeys. The tissues with positive culture isolation included lung, liver, spleen, kidney, and thoracic, mesenteric, and head lymph nodes. In addition, urine was collected from the bladder post-mortem from case 6 and confirmed to contain viable bacilli. Similar to culture, samples from multiple tissues were positive using the GXU, but not for the urine sample. However, none of the samples showed rifampicin resistance, based on the GXU readout.
4. Discussion
This report describes the first known outbreak of clinical TB, caused by M. bovis infection, in free-ranging vervet monkeys in the GKCA, South Africa. However, susceptibility of non-human primates to TB, caused by M. tuberculosis infection, has been recognized for nearly five decades (31–33). The similarities of TB in non-human primates and humans, especially macaques, has led to their use as an animal model for investigating TB pathogenesis, drugs, and vaccines (34, 35). However, in an experimental infection study, vervet monkeys appeared to be more susceptible to M. tuberculosis, than rhesus (Macaca mulatta) or cynomolgus (Macaca fascicularis) macaques (35).
The wildlife populations in the GKCA and Hluhluwe-iMfolozi Park (HiP), in KwaZulu-Natal province (South Africa), are considered endemic for M. bovis (24). Although the primary M. bovis maintenance host in both areas is African buffalo (Syncerus caffer), spillover to numerous other species, including lions (Panthera leo), greater kudu (Tragelaphus strepsiceros), white rhinoceros (Ceratotherium simum), and banded mongooses (Mungos mungo), has been reported (9, 36). Cases of TB in chacma baboons have occurred in GKCA (26, 37) and HiP (24). Prevalence during the 1996 outbreak reached approximately 50%, with all 14 M. bovis isolates sharing the same genotype (26). Infected baboons exhibited granulomatous pneumonia with lesions also found in pulmonary and mesenteric lymph nodes, and spleen. Similarly, all six vervet monkeys in the current case series had extensive macroscopic lesions in the lungs, pulmonary lymph nodes, spleen, and mesenteric lymph nodes. Lesions in the spleen and mesenteric lymph nodes suggest that monkeys were infected through the oral route. Pulmonary lesions may represent potential aerosol transmission but have also been found in animals infected with M. bovis orally (38, 39). Although a source was not identified in either the baboon or the current vervet monkey outbreaks, transmission of M. bovis from other members of the troop or wildlife species in the GKCA may have occurred either through direct contact or potentially indirectly from exposure to contaminated environments or scavenging (26, 27). Further investigations of potential sources are required since observations of the prior behavior of affected monkeys was limited.
The relatively high levels of mycobacteria in the lungs of the vervet monkeys, detected in Ziehl-Neelsen stained impression smears and by GXU, suggest that these animals were potentially excreting M. bovis. The finding of viable M. bovis in urine from case 6 demonstrates that these monkeys could be shedding and pose a risk of further transmission. Studies in other species have linked development of generalized infection and presence of large lesions with potential excretion of M. bovis (40, 41). The social nature of vervet monkeys supports pathogen transmission within troops (42). In addition, since these animals shared environments with tourists and staff, including raiding outdoor guest tables and staff dwellings, there is a potential risk of zoonotic transmission through direct contact with infected monkeys or indirectly through excretions (26, 43). Vervet monkey bites in tourist areas in GKCA average about 37 reported occurrences per year, with two bites occurring in June 2024, during the TB outbreak [S. Midzi, J. Dabrowski, L. Mdletshe, pers. comm]. This is a significant concern, especially in South Africa where there is a high burden of HIV-AIDS in the human population (44). However, there is limited zoonotic TB surveillance in South Africa (45). This highlights the need to study prevalence in vervet monkey populations to assess the risk of transmission to other animals and humans. Active surveillance could include tuberculin skin testing, interferon gamma release assay, and direct detection of MTBC in secretions, such as respiratory fluids and feces. Therefore, with the discovery of TB in vervet monkeys in GKCA, further surveillance and efforts to control spread should be prioritized at interfaces between wildlife and humans in M. bovis endemic areas.
5. Conclusion
The TB outbreak in the vervet monkey population inside the Skukuza Rest Camp and a private lodge was surprising and alarming. Given the potential for human interaction in areas such as restaurants, shops, camping sites, dust bins, and kitchen facilities, an M. tuberculosis outbreak would have been more likely, especially considering the available published data on the susceptibility of non-human primates to the human pathogen. However, vervet monkey behavior has not been associated with scavenging (46), as was reported for baboons during the M. bovis outbreaks in the Skukuza area (26, 37). In the absence of clinical TB in the local baboon population, there must be an alternative epidemiological link to the source of infection in the vervet monkeys, such as exposure to M. bovis infected banded mongoose or warthog, also found in the same areas (25, 36). The severity of lesions and clinical signs in affected monkeys suggest that M. bovis may have spread within the troop. Future studies should include whole genome sequencing of M. bovis isolates to investigate epidemiologic links between these cases and to other hosts in the GKCA.
Acknowledgments
We wish to thank the SANParks Skukuza Rest Camp hospitality managers for alerting the State Veterinary Services to the presence of moribund vervet monkeys within the Skukuza Rest Camp and the State Veterinary technicians for their quick response in either capturing or euthanasia of the vervet monkeys.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for capture and sampling was provided by the South African Department of Agriculture, Land Reform, and Rural Development (DALRRD). Support for Stellenbosch personnel and laboratory analyses was partially funded by the South African government through the South African Medical Research Council and the National Research Foundation South African Research Chair Initiative (Grant #86949).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was performed by the Skukuza State Veterinary Services, who performed all captures, euthanasia, and postmortem examinations. Since this case series was part of a disease outbreak investigation, a waiver of the approval under section 20 of the Animal Diseases Act was granted by the South African Department of Agriculture, Land Reform and Rural Development (DALRRD), formerly the Department of Agriculture, Forestry and Fisheries (DAFF), South Africa. Ethical approval for the use of samples was granted by Stellenbosch University Animal Care and Use Committee (ACU-2021-22997). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
L-MK-L: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. EM: Investigation, Methodology, Resources, Visualization, Writing – review & editing. RL: Investigation, Methodology, Project administration, Writing – review & editing. DD: Investigation, Methodology, Project administration, Writing – review & editing. NM: Data curation, Investigation, Methodology, Project administration, Resources, Writing – review & editing. LG: Data curation, Investigation, Writing – review & editing. RD-L: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. GG: Data curation, Formal analysis, Investigation, Writing – review & editing. ES: Formal analysis, Investigation, Methodology, Writing – review & editing. TK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Kindon J, Gippoliti S, Butynski TM, De Jong Y. Chlorocebus pygerythrus. In: IUCN 2012. IUCN red list of threatened species version 2012.2. (2008). Available at: www.iucnredlist.org
- 2.Isbell L, Jaffe K. Chlorocebus pygerythrus Vervet monkey. In: Butynski TM, Kingdon JS, Kalina J, editors. Mammals of Africa, Volume II. London: Bloomsbury. (2013); 277–283. [Google Scholar]
- 3.Whitaker D. Chlorocebus: species accounts In: Mittermeier RA, Rylands AB, Wilson DE, editors. Handbook of the mammals of the world, vol. 3. Primates. Barcelona: Lynx Edicions; (2013). 672–5. [Google Scholar]
- 4.Patterson LL. Urban ecology of the Vervet monkey (Chlorocebus pygerythrus) in KwaZulu-Natal, South Africa [dissertation]. Pietermaritzberg (South Africa): University of KwaZulu-Natal; (2018). [Google Scholar]
- 5.Healy A, Nijman V. Pets and pests: vervet monkey intake at a specialist south African rehabilitation Centre. Anim Welf. (2014) 23:353–60. doi: 10.7120/09627286.23.3.353 [DOI] [Google Scholar]
- 6.Kaschula VR, Van Dellen AF, De Vos V. 1978. Some infectious diseases of wild vervet monkeys (Cercopithecus aethiops pygerythrus) in South Africa. J S Afr Vet Assoc. (1978) 49:223–7. PMID: [PubMed] [Google Scholar]
- 7.Nakayima J, Hayashida K, Nakao R, Ishii A, Ogawa H, Nakamura I, et al. Detection and characterization of zoonotic pathogens of free-ranging non-human primates from Zambia. Parasit Vectors. (2014) 7:1–7. doi: 10.1186/s13071-014-0490-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avi RDT, Paul S, Muhit S, Chowdhury MM, Sen A. A report on tuberculosis in monkeys (Macaca mulatta): a case study at Chittagong zoo. IJEAB. (2017) 2:238870: 2005–9. doi: 10.22161/ijeab/2.4.58 [DOI] [Google Scholar]
- 9.Mätz-Rensing K, Lowenstine LJ. New world and old world monkeys In: Terio KA, Mcaloose D, St Leger J, editors. Pathology of wildlife and zoo animals. Cambridge, Massachusetts: Academic Press; (2018). 343–74. [Google Scholar]
- 10.Meesawat S, Aiempichitkijkarn N, Warit S, Kaewparuehaschai M, Malaivijitnond S. Non-invasive specimen collections for Mycobacterium tuberculosis detection in free-ranging long-tailed macaques (Macaca fascicularis). PLoS One. (2023) 18:e0289961. doi: 10.1371/journal.pone.0289961, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons SDC, Gous TA, Warren RM, de Villiers C, Seier JV, van Helden PD. Detection of Mycobacterium tuberculosis infection in Chacma baboons (Papio ursinus) using the QuantiFERON-TB gold (in-tube) assay. J Med Primat. (2009) 38:411–7. doi: 10.1111/j.1600-0684.2009.00367.x, PMID: [DOI] [PubMed] [Google Scholar]
- 12.Michel AL, Hlokwe TM, Espie IW, van Zijl Langhout M, Koeppel K, Lane E. Human tuberculosis in captive wild animal populations: trends in South Africa and implications for one health. Proceedings of the 13th Association of Institutions for Tropical Veterinary Medicine (AITVM) Conference, 2010 Aug 23–26. Bangkok, Thailand. Utrecht: AITVM, (2010); 91–94. [Google Scholar]
- 13.Michel AL, Hlokwe TM, Espie IW, van Zijll LM, Koeppel K, Lane E. Mycobacterium tuberculosis at the human/wildlife interface in a high TB burden country. Transbound Emerg Dis. (2013) 60:46–52. doi: 10.1111/tbed.12099 [DOI] [PubMed] [Google Scholar]
- 14.Mätz-Rensing K, Hartmann T, Wendel GM, Frick JS, Homolka S, Richter E, et al. Outbreak of tuberculosis in a colony of rhesus monkeys (Macaca mulatta) after possible indirect contact with a human TB patient. J Compar Pathol. (2015) 153:81–91. doi: 10.1016/j.jcpa.2015.05.006, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Sapolsky R, Else J. Bovine tuberculosis in a wild baboon population: epidemiological aspects. J Med Primatol. (1987) 16:229–35. doi: 10.1111/j.1600-0684.1987.tb00331.x [DOI] [PubMed] [Google Scholar]
- 16.Frost PA. Tuberculosis in nonhuman primates with an emphasis on Mycobacterium bovis In: Thoen CO, Steele JH, Gilsdorf MJ, editors. Mycobacterium bovis infection in animals and humans. 2nd ed. Hoboken: Wiley; (2006). 271–84. [Google Scholar]
- 17.Garcia MA, Bouley DM, Larson MJ, Lifland B, Moorhead R, Simkins MD, et al. Outbreak of Mycobacterium bovis in a conditioned colony of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp Med. (2004) 54:578–84. [PubMed] [Google Scholar]
- 18.Panarella ML, Bimes RSA. Naturally occurring outbreak of tuberculosis in a group of imported Cynomolgus monkeys (Macaca fascicularis). J Am Assoc Lab Anim Sci. (2010) 49:221–5. PMID: [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson P, Weavers E, West B, Taylor M, Kavanagh J, Jones P. Mycobacterium bovis infection in primates in Dublin zoo: epidemiological aspects and implications for management. Lab Anim. (1984) 18:383–7. doi: 10.1258/002367784780865351 [DOI] [PubMed] [Google Scholar]
- 20.Rahim Z, Thapa J, Fukushima Y, Van Der Zanden AG, Gordon SV, Suzuki Y, et al. Tuberculosis caused by Mycobacterium orygis in dairy cattle and captured monkeys in Bangladesh: a new scenario of tuberculosis in South Asia. Transbound Emerg Dis. (2017) 64:1965–9. doi: 10.1111/tbed.12596 [DOI] [PubMed] [Google Scholar]
- 21.Weber K, Mayoral FJ, Vallejo C, Sanchez R, Hartelust R, Mendoza P, et al. Natural outbreak of Mycobacterium caprae infection in imported laboratory cynomolgus macaques (Macaca fascicularis): diagnostic pitfalls and management of safety precautions. J Toxicol Pathol. (2024) 37:197–206. doi: 10.1293/tox.2024-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Squarre D, Chizimu J, Nakajima C, Muma JB, Hang’ombe BM, Simulundu E, et al. First report of Mycobacterium bovis in wild chacma baboons (Papio ursinus) at the human–wildlife interface area in Zambia. Transbound Emerg Dis. (2022) 69:1659–62. doi: 10.1111/tbed.14124, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Clifford DL, Kazwala RR, Sadiki H, Roug A, Muse EA, Coppolillo PC, et al. Tuberculosis infection in wildlife from the Ruaha ecosystem Tanzania: implications for wildlife, domestic animals, and human health. Epidemiol Infect. (2013) 141:1371–81. doi: 10.1017/S0950268813000836, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel AL, Bengis RG, Keet DF, Hofmeyr M, De KLM, Cross PC, et al. Wildlife tuberculosis in south African conservation areas: implications and challenges. Vet Microbiol. (2006) 112:91–100. doi: 10.1016/j.vetmic.2005.11.035, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Bernitz N, Kerr TJ, Goosen WJ, Chileshe J, Higgitt RL, Roos EO, et al. Review of diagnostic tests for detection of Mycobacterium bovis infection in south African wildlife. Front Vet Sci. (2021) 8:588–697. doi: 10.3389/fvets.2021.588697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keet DF, Kriek NPJ, Bengis RG, Grobler DG, Michel A. The rise and fall of tuberculosis in a free-ranging chacma baboon troop in the Kruger National Park. Onderstepoort J Vet Res. (2000) 67:115–22. PMID: [PubMed] [Google Scholar]
- 27.De Klerk-Lorist L. The epidemiology of bovine tuberculosis in chacma baboons (Papio ursinus) in the Kruger National Park. Proceedings of the International Wildlife Tuberculosis Conference, (2012). Skukuza, South Africa. [Google Scholar]
- 28.Hlokwe TM, Michel AL, Mitchel E, Gcebe N, Reininghaus B. First detection of Mycobacterium bovis infection in giraffe (Giraffa camelopardalis) in the greater Kruger National Park complex: role and implications. Transbound Emerg Dis. (2019) 66:2264–70. doi: 10.1111/tbed.13275, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Kerr TJ, Goosen WJ, Gumbo R, de Klerk-Lorist LM, Pretorius O, Buss PE, et al. Diagnosis of Mycobacterium bovis infection in free-ranging common hippopotamus (Hippopotamus amphibius). Transbound Emerg Dis. (2022) 69:378–84. doi: 10.1111/tbed.13989 [DOI] [PubMed] [Google Scholar]
- 30.Warren RM, Gey van Pittius NC, Barnard M, Hesseling A, Engelke E, de Kock M, et al. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int J Tuberc Lung Dis. (2006) 10:818–22. PMID: [PubMed] [Google Scholar]
- 31.Fourie BP, Kleeberg HH. Tuberculosis in laboratory animals. J S Afr Vet Assoc. (1978) 49:219–21. PMID: [PubMed] [Google Scholar]
- 32.Anderson DC. Tuberculosis control in nonhuman primate colonies In: Kaufmann AF, Montali RJ, editors. Mycobacterial infection of zoo animals. Washington, D.C.: Smithsonian Institution Press; (1978). 227–34. [Google Scholar]
- 33.Fourie PB, Odendaal MW. Mycobacterium tuberculosis in a closed colony of baboons (Papio ursinus). Lab Anim. (1983) 17:125–8. doi: 10.1258/002367783780959376, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Peña JC, Wen-Zhe H. Monkey models of tuberculosis: lessons learned. Infect Immun. (2015) 83:852–62. doi: 10.1128/IAI.02850-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scanga CA, Flynn JL. Modeling tuberculosis in nonhuman primates. Cold Spring Harb Perspect Med. (2014) 4:a018564. doi: 10.1101/cshperspect.a018564, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brüns AC, Tanner M, Williams MC, Botha L, O’Brien A, Fosgate GT, et al. Diagnosis and implications of Mycobacterium bovis infection in banded mongooses (Mungos mungo) in the Kruger National Park. South Africa J Wildl Dis. (2017) 53:19–29. doi: 10.7589/2015-11-318, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Keet DF, Kriek NPJ, Penrith ML, Michel A, Huchzermeyer H. Tuberculosis in buffaloes (Syncerus caffer) in the Kruger National Park: spread of the disease to other species. Onderstpoort J Vet Res. (1996) 63:239–44. [PubMed] [Google Scholar]
- 38.Palmer MV, Waters WR, Whipple DL. Investigation of the transmission of Mycobacterium bovis from deer to cattle through indirect contact. Am J Vet Res. (2004) 65:1483–9. doi: 10.2460/ajvr.2004.65.1483, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Serrano M, Sevilla IA, Fuertes M, Geijo M, Risalde MÁ, Ruiz-Fons JF, et al. Different lesion distribution in calves orally or intratracheally challenged with Mycobacterium bovis: implications for diagnosis. Vet Res. (2018) 49:1. doi: 10.1186/s13567-018-0566-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Hernando MP, Höfle U, Vicente J, Ruiz-Fons F, Vidal D, Barral M, et al. Lesions associated with Mycobacterium tuberculosis complex infection in the European wild boar. Tuberculosis. (2007) 87:360–7. doi: 10.1016/j.tube.2007.02.003, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Corner LA, Murphy D, Gormley E. Mycobacterium bovis infection in the Eurasian badger (Meles meles): the disease, pathogenesis, epidemiology and control. J Compar Pathol. (2011) 144:1–24. doi: 10.1016/j.jcpa.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 42.McFarland R, Henzi SP, Barrett L, Bonnell T, Fuller A, Young C, et al. Fevers and the social costs of acute infection in wild vervet monkeys. Proc Natl Acad Sci USA. (2021) 118:e2107881118. doi: 10.1073/pnas.2107881118, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michel AL, Müller B, van Helden PD. Mycobacterium bovis at the animal–human interface: a problem, or not? Vet Microbiol. (2010) 140:371–81. doi: 10.1016/j.vetmic.2009.08.029, PMID: [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization , (2021). WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025: Background document.
- 45.Goosen WJ, Moodley S, Ghielmetti G, Moosa Y, Zulu T, Smit T, et al. Identification and molecular characterization of Mycobacterium bovis DNA in GeneXpert® MTB/RIF ultra-positive, culture-negative sputum from a rural community in South Africa. One Health. (2024) 18:100702. doi: 10.1016/j.onehlt.2024.100702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Struhsaker TT. Ecology of vervet monkeys (Cercopithecus aethiops) in the Masai-Amboseli game reserve. Kenya Ecol. (1967) 48:891–904. doi: 10.2307/1934531 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.