Abstract
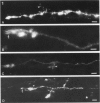
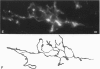
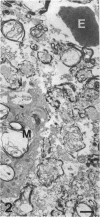
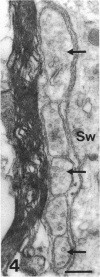
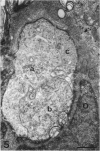
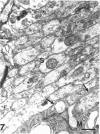
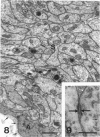
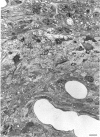
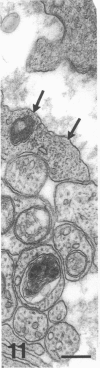
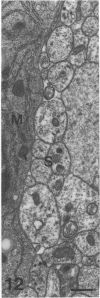
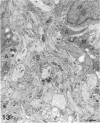
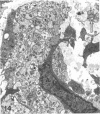
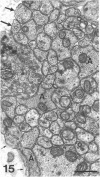
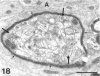
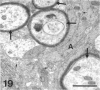
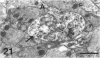
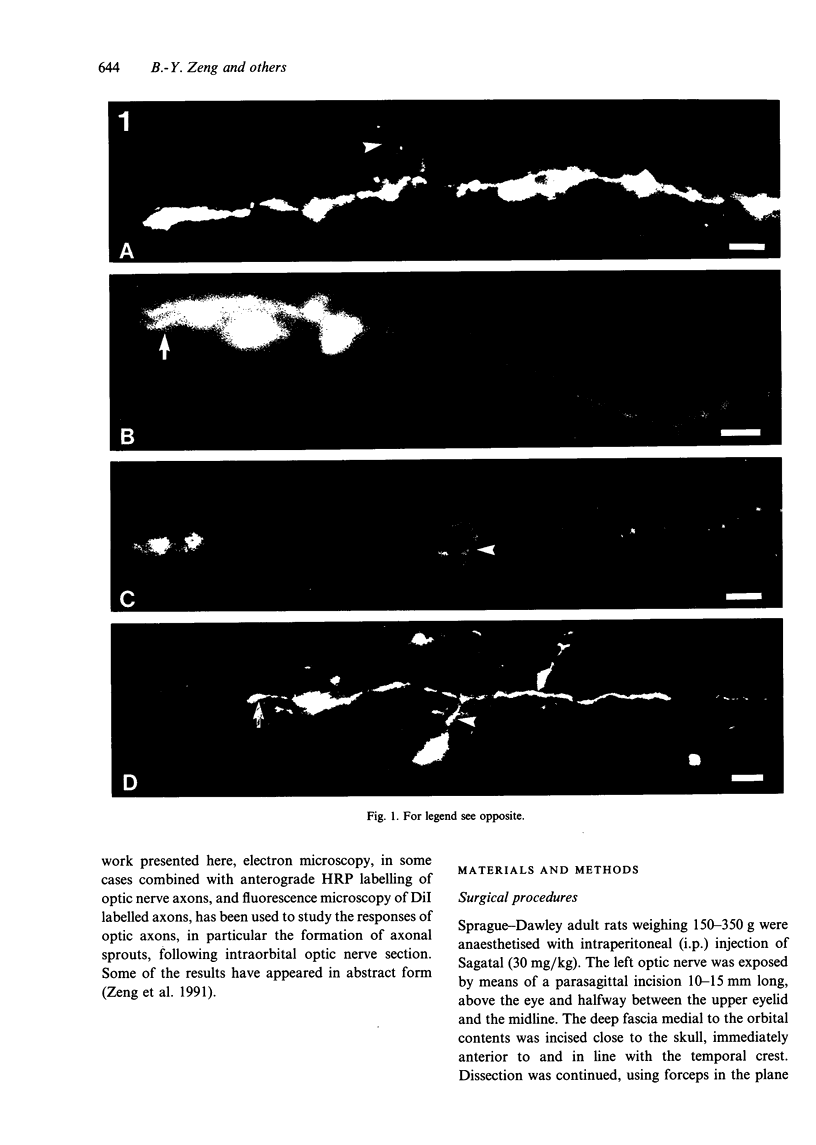
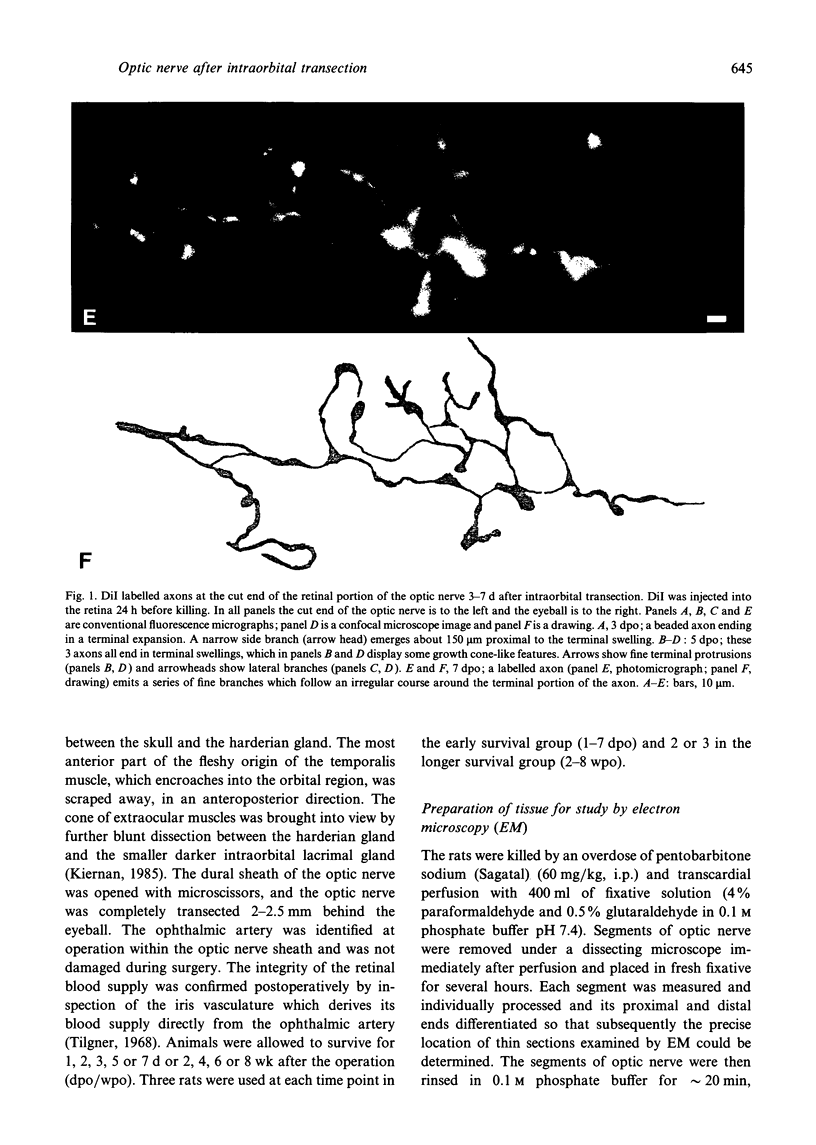
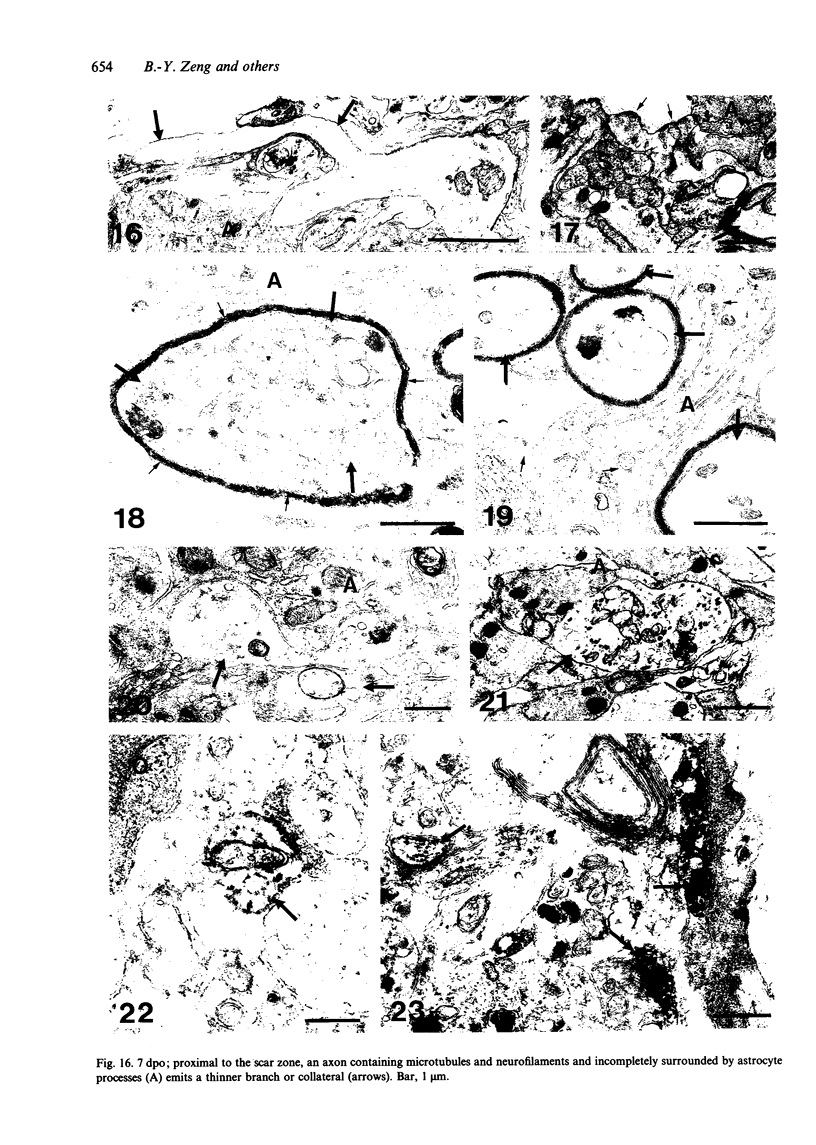
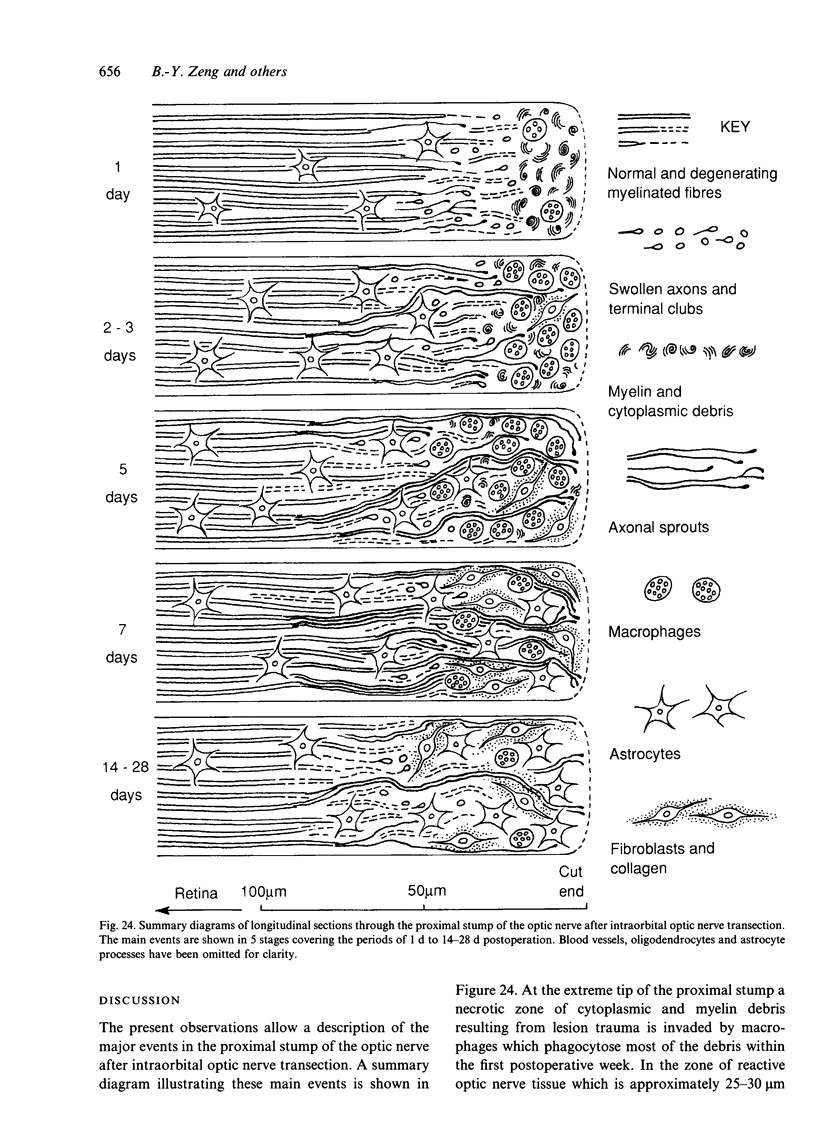
The proximal stump of the optic nerve was examined by electron microscopy from 1 d to 8 wk (dpo/wpo) after intraorbital transection. At 1 dpo a layer of axonal, cytoplasmic and myelin debris approximately 15 microns thick was present at the cut end. A zone approximately 25 microns thick of abnormal and partly degenerate tissue composed of many swollen axons filled with organelles of predominantly abnormal appearance lay between the zone of debris and more proximal levels of the optic nerve, which retained a normal appearance. The earliest putative axonal sprouts were seen at 1 dpo in this zone. By 2 dpo, bundles of small nonmyelinated axons containing microtubules, almost certainly axonal sprouts, had grown out from more proximal regions of the proximal stump and extended as far as its cut end. By 3 dpo, large numbers of axonal sprouts, as well as large numbers of macrophages and newly formed blood vessels, were seen close to the cut end of the proximal stump. Glial cells were not seen to accompany these early outgrowing bundles of axonal sprouts. By 5 dpo, the number of sprouts and macrophages had increased; many bundles of sprouts were now in contact with the surface of astrocytes, which were partly covered by basal lamina. At 7 dpo most of the macrophages had disappeared from the most distal part of proximal stump and bundles of axonal sprouts, associated with astrocytes, which in some cases had penetrated and were fasciculating such bundles, were present at the cut end. The regenerating axonal sprouts in the scar-like tissue at the distal end of the proximal stump of the optic nerve declined in numbers sharply at 2 wpo and only a few sprout-like axonal profiles were present by 8 wpo. Thus while ultimately abortive the early regenerative response is vigorous and involves the outgrowth of a large number of axonal sprouts in the first week after injury.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. N., Woodham P., Turmaine M. Peripheral nerve regeneration through optic nerve grafts. Acta Neuropathol. 1989;77(5):525–534. doi: 10.1007/BF00687255. [DOI] [PubMed] [Google Scholar]
- Barron K. D., Dentinger M. P., Krohel G., Easton S. K., Mankes R. Qualitative and quantitative ultrastructural observations on retinal ganglion cell layer of rat after intraorbital optic nerve crush. J Neurocytol. 1986 Jun;15(3):345–362. doi: 10.1007/BF01611437. [DOI] [PubMed] [Google Scholar]
- Berry M., Hall S., Follows R., Rees L., Gregson N., Sievers J. Response of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J Neurocytol. 1988 Dec;17(6):727–744. doi: 10.1007/BF01216702. [DOI] [PubMed] [Google Scholar]
- Bohn R. C., Reier P. J., Sourbeer E. B. Axonal interactions with connective tissue and glial substrata during optic nerve regeneration in Xenopus larvae and adults. Am J Anat. 1982 Dec;165(4):397–419. doi: 10.1002/aja.1001650405. [DOI] [PubMed] [Google Scholar]
- Campbell G., Lieberman A. R., Anderson P. N., Turmaine M. Regeneration of adult rat CNS axons into peripheral nerve autografts: ultrastructural studies of the early stages of axonal sprouting and regenerative axonal growth. J Neurocytol. 1992 Nov;21(11):755–787. doi: 10.1007/BF01237903. [DOI] [PubMed] [Google Scholar]
- Crespo D., Fernandez Viadero C. The microvascular system of the optic nerve in control and enucleated rats. Microvasc Res. 1989 Nov;38(3):237–242. doi: 10.1016/0026-2862(89)90002-2. [DOI] [PubMed] [Google Scholar]
- David S., Bouchard C., Tsatas O., Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990 Oct;5(4):463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- Doster S. K., Lozano A. M., Aguayo A. J., Willard M. B. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991 Apr;6(4):635–647. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- Dyson S. E., Harvey A. R., Trapp B. D., Heath J. W. Ultrastructural and immunohistochemical analysis of axonal regrowth and myelination in membranes which form over lesion sites in the rat visual system. J Neurocytol. 1988 Dec;17(6):797–808. doi: 10.1007/BF01216707. [DOI] [PubMed] [Google Scholar]
- Forrester J., Peters A. Nerve fibres in optic nerve of rat. Nature. 1967 Apr 15;214(5085):245–247. doi: 10.1038/214245a0. [DOI] [PubMed] [Google Scholar]
- Giulian D., Chen J., Ingeman J. E., George J. K., Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 1989 Dec;9(12):4416–4429. doi: 10.1523/JNEUROSCI.09-12-04416.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S., Berry M. Electron microscopic study of the interaction of axons and glia at the site of anastomosis between the optic nerve and cellular or acellular sciatic nerve grafts. J Neurocytol. 1989 Apr;18(2):171–184. doi: 10.1007/BF01206660. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hausmann B., Sievers J., Hermanns J., Berry M. Regeneration of axons from the adult rat optic nerve: influence of fetal brain grafts, laminin, and artificial basement membrane. J Comp Neurol. 1989 Mar 15;281(3):447–466. doi: 10.1002/cne.902810309. [DOI] [PubMed] [Google Scholar]
- Hunter A., Bedi K. S. A quantitative morphological study of interstrain variation in the developing rat optic nerve. J Comp Neurol. 1986 Mar 8;245(2):160–166. doi: 10.1002/cne.902450203. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. Axonal and vascular changes following injury to the rat's optic nerve. J Anat. 1985 Aug;141:139–154. [PMC free article] [PubMed] [Google Scholar]
- Lanners H. N., Grafstein B. Early stages of axonal regeneration in the goldfish optic tract: an electron microscopic study. J Neurocytol. 1980 Dec;9(6):733–751. doi: 10.1007/BF01205016. [DOI] [PubMed] [Google Scholar]
- Linden R., Perry V. H. Massive retinotectal projection in rats. Brain Res. 1983 Aug 1;272(1):145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- Liuzzi F. J., Lasek R. J. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science. 1987 Aug 7;237(4815):642–645. doi: 10.1126/science.3603044. [DOI] [PubMed] [Google Scholar]
- Liuzzi F. J. Proteolysis is a critical step in the physiological stop pathway: mechanisms involved in the blockade of axonal regeneration by mammalian astrocytes. Brain Res. 1990 Apr 2;512(2):277–283. doi: 10.1016/0006-8993(90)90637-Q. [DOI] [PubMed] [Google Scholar]
- Madison R., Moore M. R., Sidman R. L. Retinal ganglion cells and axons survive optic nerve transection. Int J Neurosci. 1984 Mar;23(1):15–32. doi: 10.3109/00207458408985342. [DOI] [PubMed] [Google Scholar]
- McConnell P., Berry M. Regeneration of ganglion cell axons in the adult mouse retina. Brain Res. 1982 Jun 10;241(2):362–365. doi: 10.1016/0006-8993(82)91079-4. [DOI] [PubMed] [Google Scholar]
- McKeon R. J., Schreiber R. C., Rudge J. S., Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991 Nov;11(11):3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L., Essagian C., Aguayo A. J. Temporal changes in beta-tubulin and neurofilament mRNA levels after transection of adult rat retinal ganglion cell axons in the optic nerve. J Neurosci. 1993 Jun;13(6):2617–2626. doi: 10.1523/JNEUROSCI.13-06-02617.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuarrie I. G. Effect of conditioning lesion on axonal sprout formation at nodes of Ranvier. J Comp Neurol. 1985 Jan 8;231(2):239–249. doi: 10.1002/cne.902310211. [DOI] [PubMed] [Google Scholar]
- Murakami M., Ide C., Kanaya H. Regeneration in the rat optic nerve after cold injury. J Neurosurg. 1989 Aug;71(2):254–265. doi: 10.3171/jns.1989.71.2.0254. [DOI] [PubMed] [Google Scholar]
- Ng S. C., de la Monte S. M., Conboy G. L., Karns L. R., Fishman M. C. Cloning of human GAP-43: growth association and ischemic resurgence. Neuron. 1988 Apr;1(2):133–139. doi: 10.1016/0896-6273(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Oestreicher A. B., Devay P., Isaacson R. L., Gispen W. H. Changes in the distribution of the neuron-specific B-50, neurofilament protein and glial fibrillary acidic proteins following an unilateral mesencephalic lesion in the rat. Brain Res Bull. 1988 Nov;21(5):713–722. doi: 10.1016/0361-9230(88)90037-8. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Brown M. C., Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987 Apr 1;165(4):1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Vaughn J. E. Microtubules and filaments in the axons and astrocytes of early postnatal rat optic nerves. J Cell Biol. 1967 Jan;32(1):113–119. doi: 10.1083/jcb.32.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience. 1985 Jan;14(1):237–254. doi: 10.1016/0306-4522(85)90176-9. [DOI] [PubMed] [Google Scholar]
- Reier P. J., Webster H. F. Regeneration and remyelination of Xenopus tadpole optic nerve fibres following transection or crush. J Neurocytol. 1974 Nov;3(5):591–618. doi: 10.1007/BF01097626. [DOI] [PubMed] [Google Scholar]
- Richardson P. M., Issa V. M., Shemie S. Regeneration and retrograde degeneration of axons in the rat optic nerve. J Neurocytol. 1982 Dec;11(6):949–966. doi: 10.1007/BF01148310. [DOI] [PubMed] [Google Scholar]
- Rio J. P., Repérant J., Ward R., Peyrichoux J., Vesselkin N. A preliminary description of the regeneration of optic nerve fibers in a reptile, Vipera aspis. Brain Res. 1989 Feb 6;479(1):151–156. doi: 10.1016/0006-8993(89)91345-0. [DOI] [PubMed] [Google Scholar]
- Rudge J. S., Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci. 1990 Nov;10(11):3594–3603. doi: 10.1523/JNEUROSCI.10-11-03594.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L., Schwab M. E. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990 Jan 18;343(6255):269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988 Jul;8(7):2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Bray D. Movement and extension of isolated growth cones. Exp Cell Res. 1977 Jan;104(1):55–62. doi: 10.1016/0014-4827(77)90068-4. [DOI] [PubMed] [Google Scholar]
- Sievers J., Hausmann B., Berry M. Fetal brain grafts rescue adult retinal ganglion cells from axotomy-induced cell death. J Comp Neurol. 1989 Mar 15;281(3):467–478. doi: 10.1002/cne.902810310. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981 Apr;89(1):96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So K. F., Aguayo A. J. Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res. 1985 Mar 4;328(2):349–354. doi: 10.1016/0006-8993(85)91047-9. [DOI] [PubMed] [Google Scholar]
- Stoll G., Müller H. W. Macrophages in the peripheral nervous system and astroglia in the central nervous system of rat commonly express apolipoprotein E during development but differ in their response to injury. Neurosci Lett. 1986 Dec 23;72(3):233–238. doi: 10.1016/0304-3940(86)90519-7. [DOI] [PubMed] [Google Scholar]
- Turner J. E., Singer M. The ultrastructure of regeneration in the severed newt optic nerve. J Exp Zool. 1974 Dec;190(3):249–268. doi: 10.1002/jez.1401900302. [DOI] [PubMed] [Google Scholar]
- Vidal-Sanz M., Bray G. M., Villegas-Pérez M. P., Thanos S., Aguayo A. J. Axonal regeneration and synapse formation in the superior colliculus by retinal ganglion cells in the adult rat. J Neurosci. 1987 Sep;7(9):2894–2909. doi: 10.1523/JNEUROSCI.07-09-02894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Bray G. M., Aguayo A. J. Influences of peripheral nerve grafts on the survival and regrowth of axotomized retinal ganglion cells in adult rats. J Neurosci. 1988 Jan;8(1):265–280. doi: 10.1523/JNEUROSCI.08-01-00265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Pérez M. P., Vidal-Sanz M., Rasminsky M., Bray G. M., Aguayo A. J. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993 Jan;24(1):23–36. doi: 10.1002/neu.480240103. [DOI] [PubMed] [Google Scholar]
- Wujek J. R., Reier P. J. Astrocytic membrane morphology: differences between mammalian and amphibian astrocytes after axotomy. J Comp Neurol. 1984 Feb 1;222(4):607–619. doi: 10.1002/cne.902220411. [DOI] [PubMed] [Google Scholar]