Abstract
The emergence of pandrug-resistant (PDR) and extensive drug-resistant (XDR) methicillin-resistant and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA) isolates from bovine milk samples along with biofilm formation ability and harboring various virulence genes complicates the treatment of bovine mastitis and highlights the serious threat to public health. This study investigated for the first time the frequency, antimicrobial resistance profiles, biofilm-forming ability, virulence factors, spa and staphylococcal cassette chromosome mec (SCCmec) types of MRSA and VRSA isolated from clinical and subclinical bovine mastitis in Egypt. A total of 808 milk samples were collected from each quarter of 202 dairy animals, including 31 buffaloes and 171 cattle. The frequency of mastitis in the collected milk samples was 48.4% (60/124) in buffaloes and 29.2% (200/684) in cattle. A total of 65 Staphylococcus species isolates were recovered, including 27 coagulase-positive S. aureus (CoPS) isolates and 38 coagulase-negative staphylococci (CoNS). The CoNS included 27 mammaliicocci (20 Mammaliicoccus lentus and 7 M. sciuri) and 11 Non-aureus staphylococci (S. lugdunensis) isolates. All the CoPS isolates were mecA positive and resistant to 20–33 tested antimicrobials with multiple antibiotic resistance index ranging from 0.61 to 1. Three isolates were PDR, four were XDR, and 20 were multidrug resistant isolates. VRSA was detected in 85.2% of CoPS isolates with minimal inhibitory concentration (MIC) ranging from 64 to 1024 µg/mL. The vanA gene was found in 60.8%, vanB in 73.9%, and both genes in 43.5% of VRSA isolates. All the CoPS isolates exhibited biofilm formation ability, with 55.6% being strong, and 44.4% moderate biofilm producers, and harbored icaA (74.1%) and icaD (74.1%) biofilm-forming genes. All S. aureus isolates harbored both beta-haemolysin (hlb) and leucotoxin (lukMF) genes, while 44.4% were positive for toxic shock syndrome toxin (tsst) gene. Enterotoxin genes sea, seb, sec, sed, and see were found in 59.3%, 40.7%, 18.5%, 33.3%, and 14.8% of isolates, respectively. Additionally, 70.4% of the isolates had spa X-region gene, and exhibited eight different MRSA spa types (t127, t267, t037, t011, t843, t1081, t2663, and t1575), with spa t127 being the most common. Three SCCmec types (I, II and III) were identified, with SCCmec I being predominant, and were further classified into subtypes 1.1.1, 1.1.2, 1.n.1, and 4.1.1. The ability of MRSA and VRSA isolates to produce biofilms and resist antimicrobials highlights the serious threat these pathogens pose to bovine milk safety, animal welfare, and public health. Therefore, strict hygiene practices and antimicrobial surveillance are crucial to reduce the risk of MRSA and VRSA colonization and dissemination.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-81516-6.
Keywords: VRSA, MRSA, Pandrug-resistant, Biofilm, S. aureus, Genotyping, Coagulase-negative staphylococci, Virulence, Mastitis
Subject terms: Microbiology, Diseases
Introduction
Mastitis is one of the most prevalent diseases of bovines worldwide, causing significant economic losses by decreasing the quantity and quality of milk, increasing expenditures for drugs and veterinary services, and leading to death or early culling of affected animals1. Staphylococcus aureus (S. aureus) is one of the major causative agents of bovine mastitis, that causes clinical and subclinical infections in dairy cattle and buffalo and poses a potential health concern to humans2. Non-aureus staphylococci (NAS), considered minor mastitis pathogens, have variable effects on bovine udder health and milk production3. Recently, coagulase-negative staphylococci (CoNS) have been reclassified into NAS including S. lugdunensis, and mammaliicocci, including Mammaliicoccus sciuri and M. lentus4.
Antimicrobial therapy is crucial for mastitis control; however, S. aureus often develops resistance to multiple classes of antimicrobial agents due to the selective pressure of antimicrobials, which limiting the treatment options for clinicians and veterinarians5. The misuse of antibiotics such as using antibiotics without prescription, uncontrolled doses, or unnecessary application of drugs, has led to increased resistance of S. aureus6. The resistance to methicillin in methicillin-resistant S. aureus (MRSA) is a result of the expression of penicillin-binding protein 2a (PBP2a), which has a low affinity for β-lactam antibiotics. PBP2a is encoded by the mecA gene, which is located on the staphylococcal cassette chromosome mec (SCCmec)7. MRSA is multidrug resistant (MDR), not only resistant to β-lactam antibiotics but also to aminoglycosides, quinolones, and macrolides8.
Vancomycin, a glycopeptide antibiotic effective against Gram-positive bacteria, has been a cornerstone therapy for serious MRSA infections. However, excessive use has led to the emergence of vancomycin-resistant S. aureus (VRSA) strains9. Vancomycin resistance occurs through the horizontal transfer of a plasmid-borne transposon carrying the vanA and/or vanB genes from vancomycin-resistant Enterococcus to S. aureus crosswise the genus barrier that play a significant role in the development and dissemination of MDR7.
Besides antibiotic resistance, MRSA strains can form biofilms for survival and fitness. Strong adhesion, increased antimicrobial resistance, and decreased sanitizer efficacy are the factors that mediate this development10. Other virulence factors of S. aureus include leukotoxins, such as LukMF, the main toxin secreted in bovine mastitis and highly effective at killing bovine neutrophils11. Hemolysins (alpha, beta, gamma, delta) are major S. aureus virulence factors that facilitate invasion and evasion of the host immune response and play a crucial role in chronic mammary gland infections12. S. aureus can produce over 26 types of superantigens, such as staphylococcal enterotoxins (SEs; SEA to -E, SEG to -J, and SER to -T) and toxic shock syndrome toxin 1 (tsst−1). These toxins can overactivate specific T cells with uncontrolled release of proinflammatory cytokines, leading to various diseases in humans and animals, including mastitis in dairy cows13. Superantigens disrupt the normal immune response, impairing the body’s ability to effectively combat S. aureus infections. They can cause various diseases in humans, including food poisoning and toxic shock14. In bovine mastitis, they may facilitate S. aureus colonization and contribute to the establishment of persistent mastitis15.
Staphylococcal protein A (spa) is a key factor in S. aureus evasion of the immune system. By acting as a superantigen and disrupting the normal B-cell response, spa impairs the production of effective antibodies against S. aureus16. The spa gene, which codes for this protein, has a high degree of size variability17. The X-region of the spa gene contains repeating 24 bp repeats that vary throughout S. aureus strains, which is the source of this diversity in the gene. Therefore, spa typing can be employed as a molecular method to investigate genetic variation among S. aureus strains, thereby supporting the comparison of virulent phenotypes and epidemiological tracing of infection sources18. Moreover, SCCmec typing considered a useful tool for studying molecular epidemiology of MRSA isolates18,19.
There is limited data on the genetic diversity of VRSA and MRSA isolates causing clinical and subclinical mastitis18. Thus, the current study was designed to (i) investigate the frequency of MRSA, VRSA, and the Non-aureus staphylococci and mammaliicocci (NASM) in clinical and subclinical mastitis of cattle and buffaloes, (ii) determine the virulence determinants, biofilm formation ability of isolated MRSA and VRSA isolates, and (iii) identify the most common spa and SCCmec types of S. aureus isolates associated with bovine mastitis in Egypt.
Materials and methods
Animals and milk samples
A total of 808 milk samples were collected from each quarter of 202 lactating animals (171 cattle and 31 buffaloes) between October 2018 and April 2020. The samples were sourced from dairy farms in Sharkia and Dakahlia Governorates, Egypt, as well as from individual cases admitted to Animal Health Research Institute, Zagazig. The farms that were chosen employ extensive management strategies and hand milking. Four milk samples were collected from each animal, one from each quarter of the udder. Milk samples were aseptically placed into sterile screw-capped McCartney tubes (Thermo Fisher Scientific, Waltham, MA, USA), kept in an icebox and transferred to the microbiology laboratory for bacteriological examination. An informed consent was obtained from the owners of the farm to collect the bovine milk samples. All methods were carried out in accordance with ARRIVE guidelines (https://arriveguidelines.org) and regulations and approved by the institutional animal care and use committee at Faculty of Veterinary Medicine, Suez Canal University (Ethical approval no.: SZUC 201819).
Somatic cell counting
The somatic cell count (SCC) was determined in fresh collected milk samples within 1 h after milking using a semi-quantitative California Mastitis Test (CMT) and a somatic cell counter (MT05, Slovakia). In CMT, 2 mL of milk sample was mixed with CMT reagent (Chimertech Private Limited, Chennai, India) and gently agitated in a four-well plastic paddle. The SCC is scored on a scale of 0 to 3, with scores of 2 or 3 considered based on changes in milk viscosity20.
The SCC was also measured automatically using MT05 somatic cell counter after mixing the milk sample (10 mL) with 5 mL of the 20% S4 reagent. SCC < 200,000 cells/mL of milk were considered normal, while ≥ 250,000 cells/mL were considered positive for subclinical mastitis, and those with over 1,000,000 cells/mL were classified as clinical mastitis1.
Isolation and identification of Staphylococcus spp.
Milk samples were centrifuged at 3000 rpm for 5 min, the cream layer was discarded, and sediments were streaked onto Baird-Parker agar medium supplemented with an egg yolk–tellurite emulsion (Thermo Fisher Scientific Oxoid Ltd., Basingstoke, Hampshire, UK). Presumptive staphylococci colonies were subcultured onto mannitol salt agar medium (Thermo Fisher Scientific Oxoid Ltd., Basingstoke, Hampshire, UK), blood agar, and subjected to Gram staining, catalase, and coagulase tests21. The recovered S. aureus isolates were confirmed by amplification of Sa0836 gene22 using primers listed in Table S1.
Coagulase-negative staphylococci (CoNS) isolates were further confirmed by amplification of 16S rRNA gene (Table S1)23. The VITEK® technique (BioMérieux, Marcy l’Etoile, France) was employed for identification of CoNS isolates using ID-GpVITEK® identification cards. For subsequent examination, the identified isolates were stored at −20 °C in brain heart infusion broth (BHI, Oxoid, Ltd., Basingstoke, Hampshire, UK) containing 30% glycerol.
Antimicrobial susceptibility testing of S. aureus isolates
The Kirby-Bauer disc diffusion test was employed to determine the susceptibility of S. aureus isolates to 33 antimicrobial drugs representing 21 antibiotic groups commonly used in human and veterinary medicine, according to the Clinical and laboratory standards institute (CLSI) guidelines24. The tested antimicrobial agents including: amikacin (AK, 30 µg), amoxicillin-clavulanic acid (AMC, 20 µg/10 µg), ampicillin (AM, 10 µg), ampicillin/ sulbactam (SAM, 20 µg), bacitracin (B, 10 µg), ceftaroline (CPT, 30 µg), ceftazidime (CAZ, 10 µg), cefuroxime (CXM, 30 µg), cephradine (CE, 30 µg), ciprofloxacin (CIP, 5 µg), chloramphenicol (C, 30 µg), clindamycin (DA, 2 µg), daptomycin (DAP, 30 µg), erythromycin (E, 15 µg), doxycycline (DO, 30 µg), fosfomycin (FOS, 50 µg), fusidic acid (FA, 10 µg), gentamicin (CN, 10 µg), linzolid (LNZ, 30 µg), methicillin (ME, 5 µg), neitilmicin (NET, 10 µg), nitrofurantoin (F, 300 µg), norfloxacin (NOR, 10 µg), oxacillin (OX, 30 µg), penicillin (P, 10 µg), piperacillin + tazobactam (TZP, 10 µg), quinupristin/dalfopristin (QD, 15 µg), rifampin (RA, 5 µg), spiramycin (SP, 100 µg), tetracycline (TE, 30 µg), tigecycline (TGC, 10 µg), trimethoprim-sulfamethoxazole (SXT, 1.25 µg/23.75 µg), and vancomycin (VA, 30 µg). The rationale behind the choice of these antimicrobial drugs was to monitor the MDR, extensively drug-resistant (XDR), and pandrug-resistant (PDR) isolates25, aiming to address public health concerns.
The results were interpreted according to CLSI guidelines24. S. aureus ATCC 29737 reference strain was used as a control. The number of antimicrobial agents to which the isolate exhibited resistance was divided by the total number of antimicrobials tested to calculate the multiple antibiotic resistance (MAR) index26. If the isolate originated from a high-risk source with widespread antibiotic usage, the MAR index value is more than 0.2.
In accordance with the CLSI standards24, the isolates were further assessed using the broth microdilution test to determine the minimum inhibitory concentrations (MICs) of vancomycin (Sigma-Aldrich, USA). Vancomycin susceptible S. aureus (VSSA) isolates were those with MIC ≤ 2 µg/mL, whereas those with MIC of 4–8 µg /mL were classified as vancomycin intermediate S. aureus (VISA), and isolates with MIC > 16 µg/mL were considered vancomycin-resistant S. aureus (VRSA).
Detection of biofilm formation ability and biofilm genes
Using 96-well flat-bottom polystyrene microtiter plates (Techno Plastic Products, Switzerland), the biofilm formation potential of S. aureus isolates (n= 27) was evaluated as previously described27. A sterile microtiter plate was inoculated with 200 µL of fresh cultures of each isolate in tryptic soy broth supplemented with 1% glucose (TSB, Thermo Fisher Scientific Oxoid Ltd., Basingstoke, Hampshire, UK) at 106 CFU/mL. The plate was then incubated for 24 h at 37 °C. S. aureus ATCC 25923 and TSB with 1% glucose were employed as positive and negative controls, respectively. To get rid of non-adherent cells, the wells were aspirated and cleaned three times using 200 µL of phosphate buffer saline (PBS, pH 7.3). After a 15-min air-drying, the plates were drained. The biofilms underwent 30 min of staining with 150 µL of 0.1% crystal violet (Fluka AG, Buchs, Switzerland), followed by two PBS washes and air drying.
After resolving the stain that had bound to the cells with 150 µL of 95% ethanol for 45 min, the optical density (OD) was measured using an ELISA reader (Awareness Technologies stat fax 2100, CA, USA) at a wavelength of 570 nm.
Every isolate had a triplicate test, conducted three times. For both the tested isolates and the negative controls, average optical density (OD) values and standard deviations (SD) were determined.
The cut-off value of the OD (ODc), which is used to interpret the formation of biofilms, was computed as follows: ODc = average OD of negative control + (3 SD of negative control). The isolates were divided into four categories: weak (ODc < OD ≤ 2 ODc), moderate (2 ODc < OD ≤ 4 ODc), strong biofilm producers (4 ODc < OD), and non-producer (OD ≤ ODc).
Using primers listed in Table S1, biofilm-producing isolates were further tested for biofilm-related genes (icaA and icaD)28,29.
Detection of methicillin- and Vancomycin- resistance genes
Using the QIAampDNA Mini Kit (Qiagen, GmbH, Germany) following the manufacturer’s instructions, genomic DNA was extracted from 24-h cultures of MRSA and VRSA isolates in brain heart infusion broth (BHI, Oxoid, Ltd., Basingstoke, Hampshire, UK). The NanoDropTM 1000 spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA) was used to measure the quantity and purity of DNA. Using oligonucleotide primers (Table S1), the methicillin resistance gene (mecA) and the vancomycin resistance genes (vanA and vanB) were identified by PCR as previously described29–32.
PCR amplification was performed using 5 µL of the extracted DNA, 12.5 µL of 2X EmeraldAmpGT PCR master mix (Takara, Japan), 1 µL (20 pmol) of both forward and reverse primers (Metabion, Germany), and nuclease-free water to a final volume of 25 µL in a T3 Thermal cycler (BiometraGmbH, Göttingen, Germany). Methicillin- and VAN-susceptible S. aureus ATCC 29213, VAN-resistant Enterococcus faecium ATCC 51559 and E. faecalis ATCC 51299, and MRSA ATCC 33591 were used as positive and negative controls in each PCR amplification test. The 1.5% agarose gel containing 0.5 µg/mL ethidium bromide was used for electrophoresis analysis of the PCR-amplified products, and a gel documentation system (Alpha Innotech Corp., San Leandro, CA, USA) was used to visualise the results. To determine the molecular size, a 100 bp ladder (Cat. No. SM0243, Fermentas, USA) was employed.
Detection of virulence genes
PCR amplification for staphylococcal enterotoxin genes (sea, seb, sec, sed, and see), toxic shock syndrome toxin (tsst), leukotoxin (lukF and lukM), and the hemolysin gene (hlb) (Table S1) was performed as previously described33–36. The amplification was carried out on a T3 Thermal cycler (Biometra, Germany). The PCR fragments were visualized using agarose gel electrophoresis and ethidium bromide staining.
Typing of S. aureus isolates
DNA sequencing of spa gene
The X-region of the spa gene was amplified using primers 1095 F (5´-AGACGATCCTTCGGTGAGC-3´) and 1517R (5´-GCTTTTGCAATGTCATTTACTG-3´) (Midland Certified Reagent Company, oligos, USA)37. PCR products were purified using PureLink® PCR Purification Kit (K3100-01, ThermoFisher, Waltham, MA, USA). Sequence reaction was prepared using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA). Multiple sequence alignment was conducted using ClustalW application in MEGA v7.2.5 software. spa types were determined with the Ridom StaphType software (Ridom GmbH, Wu¨rzburg, Germany) as previously described37. The sequence was submitted to GenBank using web tool BankIt of GenBank http://www.ncbi.nlm.nih.gov/WebSub/. Nucleotide sequences of spa gene were deposited in GenBank (accession nos: PP249546 - PP249564).
SCCmec typing
The isolates were further characterized by Staphylococcal cassette chromosome mec (SCCmec) typing using six multiplex PCRs to identify the mec gene complex, as described by Kondo et al.38
Data analysis
Collected information and laboratory results were merged in excel sheet before being imported into STATA version 18 for Windows (Stata Corp., USA) and R software (version 4.2.0). The Chi-square (χ2) and Fisher exact test were used to assess the differences in proportion of mastitis cases between cattle and buffaloes and other factors. A significance level of < 0.05 was used to determine statistical significance. Correlations among resistance patterns, virulence and resistance genes and biofilm forming ability were evaluated by the Spearman’s rank correlation test and R package “corrplot”. The R package “Complex-Heatmap” was used to build heatmap39.
Results
Frequency of bovine mastitis
The overall frequency of mastitis in the examined animals was 32.2%, with 5.9% being clinical cases. The proportion of mastitis was 29.2% in cattle and 78.4% in buffaloes (Table 1). The frequency of mastitis was significantly higher (P < 0.05) in animals aged ≤ 4 years, backyard animals, cattle, and native breeds (Table 2).
Table 1.
Frequency of clinical and subclinical mastitis in examined cattle and buffaloes.
| Species | No. of animals | No. of milk samples | No. (%) of | Total mastitis cases | ||
|---|---|---|---|---|---|---|
| CMT positive | SCC (> 200,000 cell/mL) | |||||
| Subclinical | Clinical | |||||
| Cattle | 171 | 684 | 50 (29.2) | 41 (23.9) | 9 (5.3) | 50 (29.2) |
| Buffaloes | 31 | 124 | 15 (48.4) | 12 (38.7) | 3 (9.7) | 15 (48.4) |
| Total | 202 | 808 | 65 (32.2) | 53 (26.3) | 12 (5.9) | 65 (32.2) |
No = number, CMT = California mastitis test, SCC = Somatic cell count.
Table 2.
Number and percentage of mastitis cases in the examined cattle and buffaloes.
| No. of examined animals | No. (%) of mastitis cases (SCC > 200,000 cell/mL) | % (95% CI) | χ² P-value |
|
|---|---|---|---|---|
| Year | ||||
| 2018 | 45 | 25 (55.6) | (40.0–70.4) | 0.000 |
| 2019 | 91 | 35 (38.5) | (28.4–49.2) | |
| 2020 | 66 | 5 (7.6) | (2.5–16.8) | |
| Governorate | ||||
| Sharkia | 147 | 43 (29.3) | (22.1–37.3) | 0.146 |
| Dakahlia | 55 | 22 (40.0) | (27.0–54.1) | |
| Source | ||||
| Backyard | 90 | 38 (42.2) | (31.9–53.1) | 0.006 |
| Farm | 112 | 27 (24.1) | (16.5–33.1) | |
| Animal species | ||||
| Cattle | 171 | 50 (29.2) | (22.5–36.7) | 0.036 |
| Buffaloes | 31 | 15 (48.4) | (30.2–66.9) | |
| Breed | ||||
| Native | 62 | 29 (46.8) | (34.0–59.9) | 0.007 |
| Holstein | 118 | 28 (23.7) | (16.4–32.4) | |
| Mixed | 10 | 5 (50.0) | (18.7–81.3) | |
| Simmental | 8 | 3 (37.5) | (8.5–75.5) | |
| Montbéliarde | 4 | 0 (0.0) | 0.0 | |
| Age | ||||
| ≤ 4 years | 107 | 26 (24.3) | (16.5–33.5) | 0.011 |
| > 4 years | 95 | 39 (41.1) | (31.1–51.6) | |
The result is significant at P < 0.05.
Staphylococcus spp. isolated from bovine mastitis
A total of 65 Staphylococcus spp. isolates were isolated from 65 mastitis cases, including 12 (18.5%) from clinical mastitis, and 53 (81.5%) from subclinical mastitis. Among these, 27 (41.5%) were identified as S. aureus, with nine (75%) from clinical mastitis and 18 (34%) from subclinical mastitis (Table 3). S. aureus isolates were β-hemolytic on blood agar, grew on Baird Parker agar media with the characteristic clear zones around black colonies, coagulase-positive and were further confirmed by PCR amplification of Sa0836 gene that yielded 573 bp amplicons.
Table 3.
Frequency of Staphylococcus spp. isolated from bovine mastitis.
| Mastitis | No. of Staphylococcus isolates | No. (%) of S. aureus isolates | No. (%) of coagulase-negative Staphylococcus isolates | |||
|---|---|---|---|---|---|---|
| Cattle | Buffaloes | Cattle | Buffaloes | Cattle | Buffaloes | |
| Clinical | 9 | 3 | 7(77.8) | 2(66.7) | 2(22.2) | 1 (33.3) |
| Subclinical | 41 | 12 | 16 (39) | 2(16.7) | 25 (61) | 10 (83.3) |
| Total | 50 | 15 | 23 (46) | 4(26.7) | 27 (54) | 11 (73.3) |
| 65 | 27 (41.5) | 38 (58.5) | ||||
No = number.
The remaining 38 (58.5%) isolates were CoNS, with three (25%) from clinical mastitis, and 35 (66%) from subclinical mastitis (Table 3). These isolates were identified by VITEK 2 system as Mammaliicoccus, including M. lentus (n = 20), M. sciuri (n = 7), and S. lugdunensis (n = 11) (Fig. 1). These isolates were further confirmed by PCR amplification of 16 S rRNA gene that yielded 285 bp amplicons. Among the 20 M. lentus isolates, one (33.33%) was from clinical mastitis, and 19 (54.3%) from subclinical mastitis. Of the seven M. sciuri isolates, two (6%) were isolated from clinical mastitis and five (14.3%) from subclinical mastitis. All the 11 (31.4%) M. lentus isolates were recovered from subclinical mastitis.
Fig. 1.
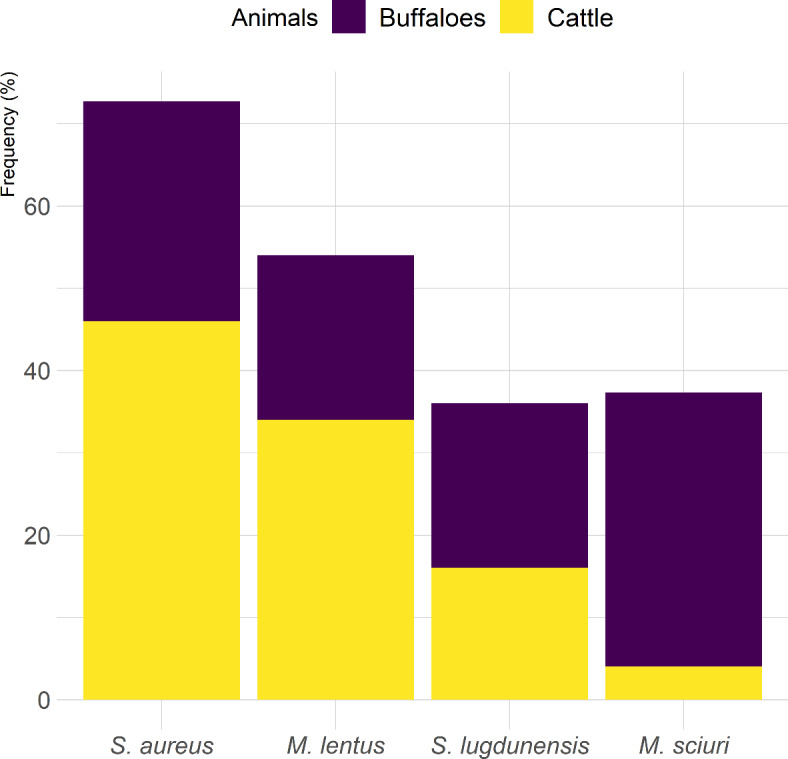
Frequency of Staphylococcus spp. and Mammaliicoccus spp. recovered from bovine mastitis cases.
Antimicrobial susceptibility of S. aureus isolates
According to the antibiogram profiles of the S. aureus isolates, 24 of the isolates had MAR index between 0.61 and 0.82 and were resistant to 20–27 antimicrobials. Twenty MDR isolates showed resistance to 19 antibiotic classes and four XDR isolates were resistant to 20 antibiotic classes with distinct antibiogram patterns. Three isolates were PDR and were resistant to all 33 tested antibiotics in 21 classes (MAR index = 1.0) (Tables 4 and Table 2S). Most MDR isolates were resistant to ampicillin, ampicillin/sulbactam, bacitracin, ceftazidime, cephradine, daptomycin, erythromycin, oxacillin, methicillin, neitilmicin, and tigecycline (100%), followed by spiramycin (96.3%), amoxicillin-clavulanic acid, clindamycin, doxycycline, and penicillin (92.6%). The resistance rates for amikacin (29.6%), nitrofurantoin (22.2%), and linzolid, ceftaroline (11.1%) were the lowest (Fig. 2).
Table 4.
Antimicrobial susceptibility of S. aureus isolates causing bovine mastitis.
| Class | Antimicrobial agent | Number (%) of S. aureus isolates (n = 27) | ||
|---|---|---|---|---|
| R | I | S | ||
| Aminoglycosides | AK | 8 (29.6) | 9 (33.3) | 10 (37.1) |
| CN | 24 (88.9) | 2 (7.4) | 1 (3.7) | |
| NET | 27 (100) | 0.0 | 0.0 | |
| Ansamycin | RA | 19 (70.4) | 3 (11.1) | 5 (18.5) |
| Cephalosporins | CAZ | 27 (100) | 0.0 | 0.0 |
| CE | 27 (100) | 0.0 | 0.0 | |
| CPT | 3 (11.1) | 0.0 | 24 (88.9) | |
| CXM | 17 (63.0) | 5 (18.5) | 5 (18.5) | |
| Fluoroquinolones | CIP | 19 (70.4) | 6 (22.2) | 2 (7.4) |
| NOR | 14 (51.9) | 1 (3.7) | 12 (44.4) | |
| Fucidanes | FA | 20 (74.1) | 1 (3.7) | 6 (22.2) |
| Glycopeptides | VA | 23 (85.2) | 0.0 | 4 (14.8) |
| Glycylcyclines | TGC | 27 (100) | 0.0 | 0.0 |
| Lincosamides | DA | 25 (92.6) | 0.0 | 2 (7.4) |
| Lipopeptides | DAP | 27 (100) | 0.0 | 0.0 |
| Macrolides | E | 27 (100) | 0.0 | 0.0 |
| SP | 26 (96.3) | 0.0 | 1 (3.7) | |
| Nitrofurans | F | 6 (22.2) | 1 (3.7) | 20 (74.1) |
| Oxazolidinones | LNZ | 3 (11.1) | 0.0 | 24 (88.9) |
| Penicillinase – labile Penicillins | AM | 27 (100) | 0.0 | 0.0 |
| P | 25 (92.6) | 1 (3.7) | 1 (3.7) | |
| Penicillinase - stable Penicillins | ME | 27 (100) | 0.0 | 0.0 |
| OX | 27 (100) | 0.0 | 0.0 | |
| Penicillin + β lactamase Inhibitors | AMC | 25 (92.6) | 0.0 | 2 (7.4) |
| SAM | 27 (100) | 0.0 | 0.0 | |
| TZP | 22 (81.5) | 0.0 | 5 (18.5) | |
| Phenicols | C | 15 (55.6) | 10 (37.0) | 2 (7.4) |
| Phosphonic acid | FOS | 21 (77.8) | 6 (22.2) | 0.0 |
| Polypeptides | B | 27 (100) | 0.0 | 0.0 |
| Streptogramins | QD | 12 (44.5) | 5 (18.5) | 10 (37.0) |
| Sulfonamides | SXT | 24 (88.9) | 1 (3.7) | 2 (7.4) |
| Tetracyclines | DO | 25 (92.6) | 2 (7.4) | 0.0 |
| TE | 19 (70.4) | 2 (7.4) | 6 (22.2) | |
n, number of isolates; R, resistant; I, intermediate; S, sensitive.
AK, amikacin; AM, ampicillin; AMC, amoxicillin/clauvalanic acid; B, bacitracin; C, chloroamphenicol; CAZ, ceftazidime; CE, cephradine; CIP, ciprofloxacin; CN, gentamycin; CPT, ceftarolline; CXM, cefuroxime; DA, clindamycin; DAP, daptomycin; DO, doxycycline; E, erythromycin; F, nitrofurantoin; FD, fusidic acid; FOS, fosfomycin; LNZ, linezolid; ME, methicillin; NET, neitilmicin; NOR, norfloxacin; OX, oxicillin; P, penicillin; QD, quinopristin/ dalfopristin; RA, rifampin; SAM, ampicillin + sulbctam; SP, spiramycin; SXT, trimethoprim/Sulfamethoxazole; TE, tetracycline; TGC, tigecycline; TZP, pipracillin + tazobactam; VA, vancomycin.
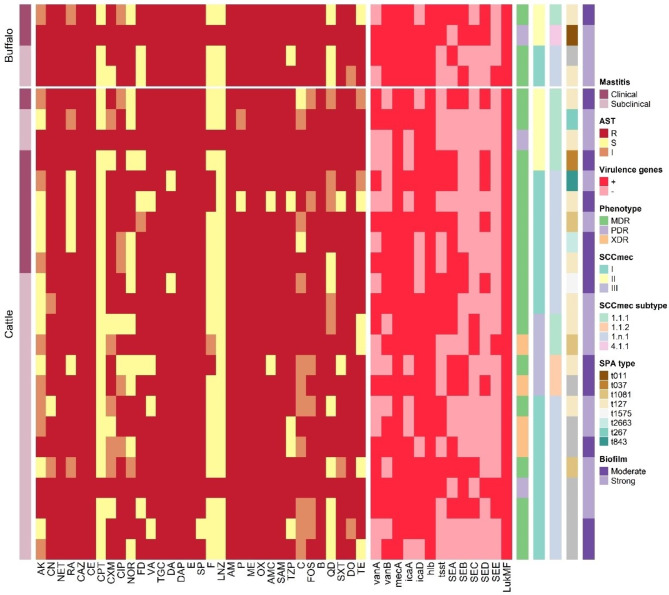
Fig. 2.
Heatmap representing antibiograms, biofilm formation ability, resistance, and virulence genes clustering of MRSA and VRSA isolates causing bovine mastitis.
Twenty seven isolates were MRSA. Of them, 23 (85.2%) isolates had resistance to vancomycin (VRSA), with MIC values ranging from 64 to 1024 µg/mL (Table 4 and Table 2S).
The ability of MRSA and VRSA isolates to produce biofilms
All 27 MRSA isolates (100%) were biofilm producers, with 15 (55.6%) classified as strong and 12 (44.4%) as moderate biofilm producers (Fig. 2 and Table 2S). Additionally, all 23 VRSA isolates were biofilm producers, with 14 being strong and 9 moderate biofilm producers. Both icaA and icaD biofilm-producing genes were detected in 51.9% (14/27) of the isolates, while one isolate had neither gene. The icaA and icaD genes were each found in 74% of the isolates.
Resistance and virulence genes in MRSA and VRSA isolates
All 27 MRSA isolates harbored the mecA gene. The resistance genes vanA, vanB, and both were found in 60.8%, 73.9%, and 43.5% of the 23 VRSA isolates, respectively.
Twelve out of 27 MRSA isolates (44.4%) harbored tsst gene, while the hlb (beta-haemolysin) and lukMF (leukotoxins) genes were detected in all isolates (100%). The enterotoxin genes sea, seb, sec, sed, and see were detected in 16 (59.3%), 11 (40.7%), 5 (18.5%), 9 (33.3%), and 4 (14.8%) of the 27 MRSA isolates, respectively (Table 2S and Fig. 2).
Correlation between phenotypic and genotypic traits of S. aureus isolates
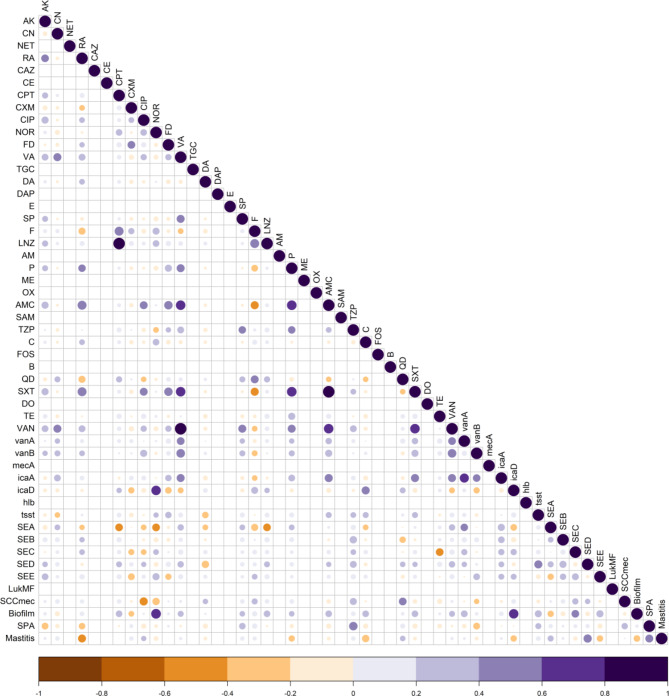
Antimicrobial resistance has been correlated in different ways with the capacity of MRSA and VRSA isolates to form biofilms and to harbor virulence genes (Table 2S). Correlation matrix analysis was used to determine the relationships between phenotypic and genotypic traits, virulence and biofilm-forming abilities of the isolates (Fig. 3). Significant positive (P < 0.05) correlations were identified between biofilm formation and the presence of icaD gene and resistance to norfloxacin. Similarly, a positive correlation was observed between the presence of vanA and icaA genes. Additionally, resistance to antibiotics such as vancomycin, linzolid, and ceftaroline showed significant positive correlations. Moreover, clinical mastitis was positively correlated with the presence of the spa gene and the virulence gene sed. Conversely, few significant negative correlations were observed between the presence of SCCmec and ciprofloxacin, and between virulence gene sea and ceftaroline, and norfloxacin and linzolid. These correlations indicate the co-occurrence of resistance and the presence of MDR, XDR, and PDR isolates.
Fig. 3.
Correlation between resistance to antimicrobials, resistance genes, virulence genes, biofilm formation ability, and typing of MRSA and VRSA isolates.
spa and SCCmec types
Nineteen isolates (70.4%) harbored the spa X-region gene. Based on spa typing, eight different spa types (t127, t267, t037, t011, t843, t1081, t2663, and t1575) were identified, with spa t127 being the predominant type (Table 5 and Table 2S).
Table 5.
spa types of MRSA and VRSA isolates recovered from bovine clinical and subclinical mastitis.
| spa type | N | No. (%) of spa types in | |||||
|---|---|---|---|---|---|---|---|
| Farm | Backyard | Cattle | Buffalo | Clinical | Subclinical | ||
| t127 | 10 | 5 (50) | 5 (50) | 8 (80) | 2 (20) | 4 (40) | 6 (60) |
| t267 | 1 | 0 | 1 (100) | 1 (100) | 0 | 0 | 1 (100) |
| t037 | 1 | 0 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 |
| t011 | 1 | 0 | 1 (100) | 0 | 1 (100) | 1 (100) | 0 |
| t843 | 1 | 0 | 1 (100) | 1 (100) | 0 | 1 (100) | 0 |
| t1081 | 3 | 2 (66.7) | 1 (33.3) | 3 (100) | 0 | 1 (33.3) | 2 (66.7) |
| t2663 | 1 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 |
| t1575 | 1 | 1 (100) | 0 | 1 (100) | 0 | 0 | 1 (100) |
| Total | 19 | 9 (47.4) | 10 (52.6) | 16 (84.2) | 3 (15.8) | 9 (47.4) | 10 (52.6) |
Table 6 shows that three SCCmec types (I, II and III) were identified to detect the essential genetic components of mecA, with SCCmec type I being the predominant type (n = 17), and were further classified into subtypes (1.1.1, 1.1.2, 1.n.1, and 4.1.1). SCCmec type II (1.1.1 and 4.1.1) and type III (1.1.1 and 1.1.2) were found in six and four isolates, respectively.
Table 6.
SCCmec types of MRSA and VRSA isolates recovered from bovine clinical and subclinical mastitis.
| SCCmec | N | No. (%) of SCCmec types in | ||||||
|---|---|---|---|---|---|---|---|---|
| Type | Subtype | Farm | Backyard | Cattle | Buffalo | Clinical | Subclinical | |
| I | 1.n.1 | 17 | 9 (52.9) | 8 (47.1) | 15 (88.2) | 2 (11.8) | 5 (29.4) | 12 (70.6) |
| II | 1.1.1 | 5 | 0 | 5 (100) | 4 (80) | 1 (20) | 3 (60) | 2 (40) |
| 4.1.1 | 1 | 0 | 1 (100) | 0 | 1 (100) | 1 (100) | 0 | |
| III | 1.1.1 | 2 | 2 (100) | 0 | 2 (100) | 0 | 0 | 2 (100) |
| 1.1.2 | 2 | 2 (100) | 0 | 2 (100) | 0 | 0 | 2 (100) | |
| Total | 27 | 13 (48.1) | 14 (51.9) | 23 (85.2) | 4 (14.8) | 9 (33.3) | 18 (66.7) | |
Discussion
Bovine mastitis is a disease that seriously impairs cattle productivity and costs the livestock business money globally, including Egypt40. In this study, we aimed to provide detailed insights into the frequency of MRSA, VRSA, and NASM in clinical and subclinical bovine mastitis in Egypt, along with genotyping, resistance traits, and virulence profiles of MRSA and VRSA isolates. The inclusion of subclinical mastitis cases is important because this milk is often consumed by farmers or sold for human consumption without pasteurization.
In the milk samples taken from each quarter, the overall frequency of mastitis was 32.2%; this included 48.4% in buffaloes and 29.2% in cattle. This was further classified into 9.7% clinical, and 38.7% subclinical mastitis in buffaloes, and 5.3% clinical, and 23.9% subclinical mastitis in cattle. These results are similar to the 39.9% reported in Ethiopia41, and 44% in buffaloes and 52.1% in cattle reported in Egypt42. However, higher frequencies were also reported in Ethiopia (63.02%43), and Uganda (86.2%44).
The frequency of S. aureus from total mastitis cases was 41.5%. This finding is similar to the 46.2% reported previously in China45, 41% in France46, and 47.2% in Italy47. However, higher isolation rates of S. aureus from milk samples were recorded in India (79.71%48) and Bangladesh (100%49).
Non-aureus staphylococci and mammaliicocci (NASM) are the most frequently isolated bacterial group from bovine milk samples, comprising a heterogeneous group of numerous species50. Recently, NAS are reclassified into CoNS including S. lugdunensis, and mammaliicocci including M. sciuri and M. lentus4. They are commonly associated with subclinical mastitis rather than clinical mastitis51,52. In this study, the frequency of NAS isolates in milk samples was 58.5% mainly from subclinical mastitis of both cattle and buffaloes, including M. lentus, M. sciuri, and CoNS S. lugdunensis. Similarly, Freu et al.53 reported that M. sciuri (3.75%), S. lugdunensis (0.05%), and M. lentus (0.01%) were the most frequent NASM species isolated from bovine clinical mastitis cases.
MRSA frequency in our study was 100%, while in China was varied from 15.5 to 47.6%45,54, in Korea55 was 13.9%, while in Tunisia was 6.6%56and 48.7% in India57. Elfaramawy et al.58 reported that 67.4% of S. aureus bovine mastitis isolates in Egypt were MRSA and showed resistance to several antimicrobials classes. These variations in frequencies may be due to differences in sample sizes, seasons, and geographical factors56.
MRSA and VRSA are particularly concerning because they are often resistant to multiple antibiotics, making them difficult to treat. This can lead to serious infections that are difficult to manage and can be life-threatening6,10. Therfore, the increasing prevalence of these resistant strains, including MDR, XDR, and PDR phenotypes is a major public health concern. For instance, among the 27 MRSA isolates in this study, a significant number were categorized into these resistant phenotypes. PCR verified the presence of the mecA gene in all detected MRSA strains. This aligns with the findings of Ito et al.59who found that most MRSA isolates have an MDR phenotype and carry multiple resistance determinants. Glycopeptide antibiotics, including vancomycin, were frequently utilized as a treatment option for MRSA infections after the emergence of MRSA strains. However, VRSA has emerged10. The excessive usage of the vancomycin derivative (avoparcin) as a growth promoter or antibiotic use in food-producing animals may contribute to decreased vancomycin susceptibility60.
In the current study, all VRSA isolates were MRSA. This aligns with Cong et al.10 who reported that that pre-existing MRSA infection is typically the source of S. aureus isolates that have lower vancomycin susceptibility. It is worth mentioning that 85.2% of S. aureus isolates from milk samples were resistant to vancomycin (MIC ≥ 64 µg/mL) which represents a significant public health hazard since milk can serve as a vehicle for the acquisition of vancomycin resistance to humans.
We detected vanA gene in 60.8% of VRSA strains, that was nearly similar to those reported by Shady et al.61 who detected vanA gene in 50% of VRSA strains. However, other studies reported different percentages (86% and 27%) of vanA gene in VRSA strains62,63. Additionally, 74% of VRSA strains harbored vanB gene, which is higher than the 36% reported by Shindia et al.63. Only 10 VRSA isolates harbored both vanA and vanB genes (MIC ≥ 1024 µg/mL). Two isolates tested negative for van genes despite displaying high vancomycin MIC values of 64 and 128 µg/mL. The lack of vanA/B genes in these isolates does not revoke VRSA status64. According to the hypothesis that these isolates’ development of vancomycin resistance is caused by thicker cell walls, the dense accumulation of vancomycin molecules within the thicker cell wall considerably slows down the inhibition of cell wall synthesis by impeding vancomycin molecules’ ability to efficiently pass through the thicker cell-wall layers65. Previous studies have recorded the emergence of VRSA in clinical samples without the presence of van genes66.
Beyond being resistant to antimicrobial agents, MRSA isolates produce biofilms, which help them survive in the infection niche67. All 27 MRSA isolates in this study produced biofilm, categorized as strong (55.6%) and moderate (44.4%) biofilm producers. Similarly, all 23 VRSA isolates produced biofilm, with 14 strong and 9 moderate producers. Phenotypic resistance and biofilm formation were significantly correlated, with vancomycin resistance being enhanced by the biofilm microenvironment68. Of the isolates that produced biofilm, 74% had biofilm genes found in them; icaA and icaD were equally prevalent, and just one isolate had no genes. Our results are in line with previous studies69 that identified the ica genes in all isolate, particularly the icaA and icaD genes70. However, 75.3% of isolates have ica genes71, with a lesser percentage having both icaA and icaD.
Staphylococcal enterotoxin genes (sea, seb, sec, sed, and see) have been detected in 46.9% of S. aureus isolated from bovine mastitis72. These enterotoxins are stable at high temperatures and retain their biological activity in milk even after pasteurization15. The sea gene was the most prevalent (59.3%), followed by seb gene (40.7%), sed gene (33.3%), sec gene (18.5%), and see gene (14.8%). S. aureus isolates from bovine mastitis harbored these enterotoxin-related genes at different percentages. Grispoldi et al.73 found sea (35.3%), sed (29.4%), seb and sec genes (5.9%, each) in S. aureus isolates. Moreover, Algammal et al.42 reported that 30% and 10% of isolates harbored sea and sec genes, respectively. Monistero et al.74 found the sea gene in 65.6% of isolates, and Neelam et al.48 found the seb in 9.1%, sec in 1.8%, and sed in 7.3%. This variation in the frequency of enterotoxin-related genes among S. aureus isolated from bovine mastitis may be linked to the geographical origin of the isolates and various clinical signs of infection. Acute infections have been reported, although most infections are chronic13.
Protein A is a cell wall component of S. aureus that hinders phagocytosis by neutrophils and the polymorphic X-region of the protein A gene (spa) was used for molecular typing of MRSA strains75. In this study, the spa gene (X-region) was detected in 70.4% of S. aureus isolates, showing remarkable polymorphism with amplicon sizes of 220 bp, 315 bp and 610 bp. Algammal et al.42 found 26 out of 30 MRSA strains (86.6%) were positive for the spa gene, showing polymorphism with amplicon sizes of 140 bp, 270 bp and 290 bp. The variations in the amplicon size of the X-region of the spa gene could be due to deletions of repeats and might give evidence for evolutionary changes76. Similarly, Kalorey et al.77 reported that the amplification of the polymorphic spa gene segment encoding the X-region was detected in 70.3% of S. aureus isolates from subclinical mastitis in India. In addition, Momtaz et al.78 found that 25.6% of isolates from bovine clinical and subclinical mastitis in Iran contained the X-region of the protein A gene. spa gene was not found in all isolates because spa typing can be unreliable for S. aureus strains with mutations in the IgG binding domain, as these mutations can disrupt the primer binding site79. Additionally, some strains may lack spa entirely due to mutations in the spa gene’s 5’ untranslated region80. This absence of spa can result in increased capsule production, which paradoxically makes the bacteria more vulnerable to phagocytosis and killing by the host immune system16.
Eight different spa types were identified in this study including: t267, t037, t011, t834, t2663, t1575, t1081, and t127. MRSA strains belonged to spa types t267, t037, t011, t834, t2663, and t1575 (n = 1) and t1081 (n= 3). Huber et al.81 detected spa types t011 (n = 7), t034 (n = 11), and t127 (n = 1) in mastitis milk samples in Switzerland. The spa type t011 was also found in dairy cows in Germany82. Cvetnić et al.83 isolated t267 (7.33%) from milk of cows with subclinical mastitis in Croatia. The predominant spa type detected in this study was t127 (52.6%), which aligns with previous studies by Juhász-Kaszanyitzky et al.84 and Basanisi et al.85 which found t127 to be common in MRSA isolates from bovine mastitis in Hungary and Italy. On the other hand, Vanderhaeghen et al.86 reported that t011 was the most widely distributed spa type associated with clinical and subclinical mastitis in Belgian cows.
Two necessary gene complexes constitute a SCCmec element: the mec complex and the cassette chromosomal recombinase (ccr) complex, responsible for the movements of SCCmec87,88. SCCmec I, II or III are usually resistant to multiple drugs, and SCCmec subtypes can be classified by differences in the J regions89. In this study, SCCmec typing revealed SCCmec I (n = 17), SCCmec II (n = 6) and SCCmec III (n = 4), and were classified into four subtypes (1.1.1, 1.1.2, 1.n.1, and 4.1.1). SCCmec I 1.n.1 was the predominant type identified from clinical and subclinical mastitis cases on farms and backyards. MRSA isolates from different areas carried SCCmec, such as SCCmec III or SCCmec XII in China, SCCmec V in Malaysia, SCCmec IX in Thailand, and SCCmec IVb, or V in Hong Kong17,90,91.
Conclusions
The emergence of MRSA and VRSA in cattle complicates the treatment of bovine mastitis caused by S. aureus and poses a potential risk of animal-to-human transmission. Screening milk from bovine mastitis cases and the genotypic analysis of the isolated strains are essential to estimate MRSA spread and understand transmission mechanisms.
The high prevalence of MDR and XDR isolates in the research area has been linked to inadequate infection control and preventive procedures as well as the overuse and reckless use of antibiotics in cattle husbandry. Therefore, to stop MRSA and VRSA from spreading further, both individuals and governments need to take action.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, M.E.E., Y.H.T., and A.S.E-D.; Collection of clinical data and sampling, A.S.E-D and N.I.I.; methodology, Y.H.T., A.S.E-D, and N.I.I.; Data analysis, I.E.; Interpretation of data, M.E.E, Y.H.T., N.I.I., A.S.E.-D, N.H.E, R.M.A., Y.M., and I.E.; writing—original draft preparation, N.I.I. and Y.H.T.; writing—review and editing, Y.H.T. and I.E.; All authors have read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data supporting the findings of this study are available within the article and its supplementary materials. Sequence data that support the findings of this study have been deposited in the GenBank with accession nos: PP249546 - PP249564.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
An informed consent was obtained from the owners of the farm to collect the bovine milk samples. All methods were carried out in accordance with ARRIVE guidelines (https://arriveguidelines.org) and regulations and approved by the institutional animal care and use committee at Faculty of Veterinary Medicine, Suez Canal University (Ethical approval no.: SZUC 201819).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Romero, J., Benavides, E. & Meza, C. Assessing Financial impacts of subclinical mastitis on Colombian dairy farms. Front. Vet. Sci.5, 273–273. 10.3389/fvets.2018.00273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rollin, E., Dhuyvetter, K. C. & Overton, M. W. The cost of clinical mastitis in the first 30 days of lactation: an economic modeling tool. Prev. Vet. Med.122, 257–264 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Valckenier, D., Piepers, S., De Visscher, A., Bruckmaier, R. M. & De Vliegher, S. Effect of intramammary infection with non-aureus staphylococci in early lactation in dairy heifers on quarter somatic cell count and quarter milk yield during the first 4 months of lactation. J. Dairy. Sci.102, 6442–6453. 10.3168/jds.2018-15913 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Madhaiyan, M., Wirth, J. S. & Saravanan, V. S. Phylogenomic analyses of the Staphylococcaceae family suggest the reclassification of five species within the genus Staphylococcus as heterotypic synonyms, the promotion of five subspecies to novel species, the taxonomic reassignment of five Staphylococcus species to Mammaliicoccus gen. nov., and the formal assignment of Nosocomiicoccus to the family Staphylococcaceae. Int. J. Sys Evol. Microbiol.70, 5926–5936. 10.1099/ijsem.0.004498 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Gomes, F. & Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol.72, 377–382. 10.1007/s00284-015-0958-8 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Guo, Y., Song, G., Sun, M., Wang, J. & Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol.17, 10:107–doi. 10.3389/fcimb.2020.00107 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu, K. et al. Multi-drug-resistant Staphylococcus aureus and future chemotherapy. J. Infect. Chemother.20, 593–601. 10.1016/j.jiac.2014.08.001 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard, M., Frees, D. & Ingmer, H. In Gram-Positive Pathogens747–765 (ASM, 2019). [Google Scholar]
- 9.Cong, Y., Yang, S. & Rao, X. Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J. Adv. Res.21, 169–176. 10.1016/j.jare.2019.10.005 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craft, K. M., Nguyen, J. M., Berg, L. J. & Townsend, S. D. Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. MedChemComm10, 1231–1241. 10.1039/c9md00044e (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrieling, M. et al. LukMF′ is the major secreted leukocidin of bovine Staphylococcus aureus and is produced in vivo during bovine mastitis. Sci. Rep.6, 37759. 10.1038/srep37759 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez, V. K. C. et al. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Glob Antimicrob. Resist.22, 792–802. 10.1016/j.jgar.2020.06.010 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Wilson, G. J. et al. Bovine Staphylococcus aureus Superantigens Stimulate the Entire T Cell Repertoire of Cattle. Infect Immun. 86, e00505-18; doi: (2018). 10.1128/IAI.00505-18 14. [DOI] [PMC free article] [PubMed]
- 14.Stach CS, Herrera A, Schlievert PM. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 59, 177 – 81, doi: 10.1007/s12026-014-8539-7 (2014). [DOI] [PMC free article] [PubMed]
- 15.Artursson, K., Söderlund, R., Liu, L., Monecke, S. & Schelin, J. Genotyping of Staphylococcus aureus in bovine mastitis and correlation to phenotypic characteristics. Vet. Microbiol.25,193, 156–161. 10.1016/j.vetmic.2016.08.012 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Bear, A. et al. The immune evasion roles of Staphylococcus aureus protein A and impact on vaccine development. Front. Cell. Infect. Microbiol.27, 13:1242702. 10.3389/fcimb.2023.1242702 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandt, K. M. et al. Evaluation of multiple-locus variable number of tandem repeats analysis for typing livestock-associated methicillin-resistant Staphylococcus aureus. PloS One. 8, e54425–e54425. 10.1371/journal.pone.0054425 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, T. et al. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front. Cell. Infect. Microbiol.7, 127–127. 10.3389/fcimb.2017.00127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uehara, Y. Current status of Staphylococcal Cassette chromosome mec (SCCmec). Antibiot. (Basel Switzerland). 11, 86. 10.3390/antibiotics11010086 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisira, V. et al. Comparative analysis of methods for somatic cell counting in cow’s milk and relationship between somatic cell count and occurrence of intramammary bacteria. Vet. Sci.10, 468. 10.3390/vetsci10070468 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn, P. J., Hartigan, F. C. L. P., Markey, B. K. & Fanning, S. E. S. Fitzpatrick Veterinary Microbiology and Microbial Disease 2nd edn 179–187 (Wiley-Blackwell, 2011). [Google Scholar]
- 22.Liu, D., Lawrence, M. L. & Austin, F. W. Evaluation of PCR primers from putative transcriptional regulator genes for identification of Staphylococcus aureus. Lett. Appl. Microbiol.40, 69–73. 10.1111/j.1472-765x.2004.01629.x (2005). [DOI] [PubMed] [Google Scholar]
- 23.Edwards, K. J., Kaufmann, M. E. & Saunders, N. A. Rapid and accurate identification of coagulase-negative staphylococci by real-time PCR. J. Clin. Microbiol.39, 3047–3051. 10.1128/jcm.39.9.3047-3051.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, USA. (2022).
- 25.Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect.18, 268–281. 10.1111/j.1469-0691.2011.03570.x (2012). [DOI] [PubMed] [Google Scholar]
- 26.Krumperman, P. H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol.46, 165–170. 10.1128/aem.46.1.165-170.1983 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StepanoviĆ, S. et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS115, 891–899. 10.1111/j.1600-0463.2007.apm_630.x (2007). [DOI] [PubMed] [Google Scholar]
- 28.Vasudevan, P., Nair, M. K. M., Annamalai, T. & Venkitanarayanan, K. S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol.92, 179–185. 10.1016/s0378-1135(02)00360-7 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Rohde, H., Knobloch, J. K., Horstkotte, M. A. & Mack, D. Correlation of Staphylococcus aureusicaADBC genotype and biofilm expression phenotype. J. Clin. Microbiol.39, 4595–4596. 10.1128/JCM.39.12.4595-4596.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spanu, T. et al. Identification of methicillin-resistant isolates of Staphylococcus aureus and coagulase-negative staphylococci responsible for bloodstream infections with the Phoenix™ system. Diagn. Microbiol. Infect. Dis.48, 221–227. 10.1016/j.diagmicrobio.2003.11.004 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Saha, B., Singh, A. K., Ghosh, A. & Bal, M. Identification and characterization of a Vancomycin-resistant Staphylococcus aureus isolated from Kolkata (South Asia). J. Med. Microbiol.57, 72–79. 10.1099/jmm.0.47144-0 (2008). [DOI] [PubMed] [Google Scholar]
- 32.Clark, N. C., Cooksey, R. C., Hill, B. C., Swenson, J. M. & Tenover, F. C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob. Agents Chemother.37, 2311–2317. 10.1128/AAC.37.11.2311 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehrotra, M., Wang, G. & Johnson, W. M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol.38, 1032–1035. 10.1128/JCM.38.3.1032-1035.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Løvseth, A., Loncarevic, S. & Berdal, K. G. Modified multiplex PCR method for detection of pyrogenic exotoxin genes in staphylococcal isolates. J. Clin. Microbiol.42, 3869–3872. 10.1128/JCM.42.8.3869-3872.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoekstra, J. et al. High production of LukMF’ in Staphylococcus aureus Field strains is Associated with clinical bovine mastitis. Toxins10, 200. 10.3390/toxins10050200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miruka, S. A., Aboge, G. O., Macharia, R. W., Obiero, G. O. & Omwenga, I. M. Beta hemolysin gene of Staphylococcus phage 3AJ_2017 genome is a suitable molecular marker for identification and characterization of pathogenic Staphylococcus aureus. Vet. Med. Sci.8, 845–851. 10.1002/vms3.676 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harmsen, D. et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol.41, 5442–5448. 10.1128/JCM.41.12.5442-5448.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondo, Y. et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother.51, 264–274. 10.1128/AAC.00165-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics32, 2847–2849. 10.1093/bioinformatics/btw313 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Stevens, M., Piepers, S. & De Vliegher, S. Mastitis prevention and control practices and mastitis treatment strategies associated with the consumption of (critically important) antimicrobials on dairy herds in Flanders, Belgium. J. Dairy. Sci.99, 2896–2903. 10.3168/jds.2015-10496 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Kitila, G., Kebede, B. & Wakgari, M. Prevalence, aetiology and risk factors of mastitis of dairy cows kept under extensive management system in west wollega, western Oromia, Ethiopia. Vet. Med. Sci.7, 1593–1599. 10.1002/vms3.503 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Algammal, A. M. et al. Antimicrobial Resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens9, 362. 10.3390/pathogens9050362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakew, B. T., Fayera, T. & Ali, Y. M. Risk factors for bovine mastitis with the isolation and identification of Streptococcus agalactiae from farms in and around Haramaya district, eastern Ethiopia. Trop. Anim. Health Prod.51, 1507–1513. 10.1007/s11250-019-01838-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrahmsén, M., Persson, Y., Kanyima, B. M. & Båge, R. Prevalence of subclinical mastitis in dairy farms in urban and peri-urban areas of Kampala, Uganda. T Trop. Anim. Health Prod.46, 99–105. 10.1007/s11250-013-0455-7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, W. et al. Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with Mastitis in Beijing, China. Front. Microbiol.9, 1123–1123. 10.3389/fmicb.2018.01123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poutrel, B., Bareille, S., Lequeux, G. & Leboeuf, F. Prevalence of Mastitis pathogens in France: Antimicrobial susceptibility of Staphylococcus aureus, Streptococcus uberis and Escherichia coli. J. Vet. Sci. Technol.0910.4172/2157-7579.1000522 (2018). [Google Scholar]
- 47.Cortimiglia, C. et al. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus clonal complexes in bulk tank milk from dairy cattle herds in Lombardy Region (Northern Italy). Epidemiol. Infect.144, 3046–3051. 10.1017/S0950268816001576 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neelam et al. Virulence and antimicrobial resistance gene profiles of Staphylococcus aureus associated with clinical mastitis in cattle. PloS One. 17, e0264762–e0264762. 10.1371/journal.pone.0264762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jahan, M. et al. Isolation and characterization of Staphylococcus aureus from raw cow milk in Bangladesh. J. Adv. Vet. Anim. Res.210.5455/javar.2015.b47 (2015). [Google Scholar]
- 50.Taponen, S., Myllys, V. & Pyörälä, S. Somatic cell count in bovine quarter milk samples culture positive for various Staphylococcus species. Acta vet. Scand.64, 32–32. 10.1186/s13028-022-00649-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Addis, M. F. et al. Non-aureus staphylococci and mammaliicocci isolated from bovine milk in Italian dairy farms: a retrospective investigation. Vet. Res. Commun.48, 547–554. 10.1007/s11259-023-10187-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruiz-Romero, R. A. & Vargas-Bello-Pérez, E. Non-aureus staphylococci and mammaliicocci as a cause of mastitis in domestic ruminants: current knowledge, advances, biomedical applications, and future perspectives - a systematic review. Vet. Res. Commun.47, 1067–1084. 10.1007/s11259-023-10090-5 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freu, G. et al. Frequency of non-aureus staphylococci and mammaliicocci species isolated from quarter clinical mastitis: a 6-year retrospective study. J. Dairy. Sci.107, 3813–3823. 10.3168/jds.2023-24086 (2024). [DOI] [PubMed] [Google Scholar]
- 54.Wang, D. et al. Bovine mastitis Staphylococcus aureus: antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China. Infect. Genet. Evol.31, 9–16. 10.1016/j.meegid.2014.12.039 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Song, J. W. et al. Genotypic and phenotypic characterization of methicillin-resistant Staphylococcus aureus isolated from bovine mastitic milk in Korea. J. Food Prot.79, 1725–1732. 10.4315/0362-028x.jfp-16-067 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Klibi, A., Maaroufi, A., Torres, C. & Jouini, A. Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia. Int. J. Antimicrob. Agents. 52, 930–935. 10.1016/j.ijantimicag.2018.07.026 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Mistry, H. et al. Prevalence and characterization of oxacillin susceptible mecA-positive clinical isolates of Staphylococcus aureus causing bovine mastitis in India. PloS One. 11, e0162256–e0162256. 10.1371/journal.pone.0162256 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ElFaramaway, R., Abdeen, E., Tawab, A. & Mousa, W. Antibiogram profile and molecular characterization of coa and spa genes of methicillin-resistant Staphylococcus aureus (MRSA) from clinical mastitis. Alex J. Vet. Sci.110.5455/ajvs.41507 (2019). [Google Scholar]
- 59.Ito, T., Okuma, K., Ma, X. X., Yuzawa, H. & Hiramatsu, K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist. Updates. 6, 41–52. 10.1016/s1368-7646(03)00003-7 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Aqib, A. I. & Alsayeqh, A. F. Vancomycin drug resistance, an emerging threat to animal and public health. Front. Vet. Sci.9, 1010728. 10.3389/fvets.2022.1010728 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shady, A., El-Essawy, A. K., El-Ayesh, A. J. A. & Salama & J. o. B. Detection and molecular characterization of Vancomycin resistant Staphylococcus aureus from clinical isolates. Afr. J. Biotechnol.11, 16494–16503 (2012). [Google Scholar]
- 62.Thati, V., Shivannavar, C. T. & Gaddad, S. M. Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad. Indian J. Med. Res.134, 704–708. 10.4103/0971-5916.91001 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shindia, A. A. & Nasrat, R. F. Emergence of high-level Vancomycin-resistant Staphylococcus aureus in the critical care patients Cairo University Hospitals. Aust J. Basic. Appl. Sci.5, 1281–1290 (2011). [Google Scholar]
- 64.Palazzo, I. C. V., Araujo, M. L. C. & Darini, A. L. C. First report of Vancomycin-resistant staphylococci isolated from healthy carriers in Brazil. J. Clini Microbiol.43, 179–185. 10.1128/JCM.43.1.179-185.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gardete, S. & Tomasz, A. Mechanisms of Vancomycin resistance in Staphylococcus aureus. J. Clin. Invest.124, 2836–2840. 10.1172/JCI68834 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moses, A., Uchenna, U. & Nworie, O. Epidemiology of Vancomycin resistant Staphylococcus aureus among clinical isolates in a Tertiary Hospital in Abakaliki, Nigeria. Am. J. Epidemiol. Infect. Dis.1, 24–26. 10.12691/ajeid-1-3-2 (2013). [Google Scholar]
- 67.Archer, N. K. et al. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence2, 445–459. 10.4161/viru.2.5.17724 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weigel, L. M. et al. High-level Vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob. Agents Chemother.51, 231–238. 10.1128/AAC.00576-06 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen, Q. et al. Biofilm formation and prevalence of adhesion genes among Staphylococcus aureus isolates from different food sources. MicrobiologyOpen9, e00946–e00946. 10.1002/mbo3.946 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai, J. et al. Prevalence and characterization of Staphylococcus aureus isolated from pasteurized milk in China. Front. Microbiol.10, 641. 10.3389/fmicb.2019.00641 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bissong, M. E. A. & Ateba, C. N. Genotypic and phenotypic evaluation of biofilm production and antimicrobial resistance in Staphylococcus aureus isolated from milk, North West Province, South Africa. Antibiot. (Basel Switzerland). 9, 156. 10.3390/antibiotics9040156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Günaydın, B., Aslantaş, Ö. & Demir, C. Detection of superantigenic toxin genes in Staphylococcus aureus strains from subclinical bovine mastitis. Trop. Anim. Health Prod.43, 1633–1637. 10.1007/s11250-011-9882-5 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Grispoldi, L. et al. Short communication: characterization of enterotoxin-producing Staphylococcus aureus isolated from mastitic cows. J. Dairy. Sci.102, 1059–1065. 10.3168/jds.2018-15373 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Monistero, V. et al. Different distribution of antimicrobial resistance genes and virulence profiles of Staphylococcus aureus strains isolated from clinical mastitis in six countries. J. Dairy. Sci.103, 3431–3446. 10.3168/jds.2019-17141 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Mathema, B., Mediavilla, J. & Kreiswirth, B. N. in Bacterial Pathogenesis 285–305Humana Press, (2008).
- 76.Annemüller, C., Lämmler, C. & Zschöck, M. Genotyping of Staphylococcus aureus isolated from bovine mastitis. Vet. Microbiol.69, 217–224. 10.1016/s0378-1135(99)00117-0 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Kalorey, D. R., Shanmugam, Y., Kurkure, N. V., Chousalkar, K. K. & Barbuddhe, S. B. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from bovine subclinical mastitis cases. J. Vet. Sci.8, 151–154. 10.4142/jvs.2007.8.2.151 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Momtaz, H., Tajbakhsh, E., Rahimi, E. & Momeni, M. Coagulase gene polymorphism of Staphylococcus aureus isolated from clinical and sub-clinical bovine mastitis in Isfahan and Chaharmahal Va Bakhtiari provinces of Iran. Comp. Clin. Path. 20, 519–522. 10.1007/s00580-010-1029-y (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baum, C. et al. Non-spa-typeable clinical Staphylococcus aureus strains are naturally occurring protein A mutants. J. Clin. Microbiol.47, 3624–3629. 10.1128/JCM.00941-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brignoli, T. et al. Absence of protein A expression is associated with higher capsule production in staphylococcal isolates. Front. Microbiol. 10, 863. 10.3389/fmicb.2019.00863 (2019). [DOI] [PMC free article] [PubMed]
- 81.Huber, H., Koller, S., Giezendanner, N., Stephan, R. & Zweifel, C. Prevalence and characteristics of meticillin-resistant Staphylococcus aureus in humans in contact with farm animals, in livestock, and in food of animal origin, Switzerland, 2009. Eurosurveillance15, 10.2807/ese.15.16.19542-en (2010). [PubMed]
- 82.Spohr, M. et al. Methicillin-Resistant Staphylococcus aureus (MRSA) in three dairy herds in Southwest Germany. Zoonoses Public. Health. 58, 252–261. 10.1111/j.1863-2378.2010.01344.x (2011). [DOI] [PubMed] [Google Scholar]
- 83.Cvetnić, L. et al. Multi locus sequence typing and spa typing of Staphylococcus aureus isolated from the milk of cows with subclinical mastitis in Croatia. Microorganisms9, 725. 10.3390/microorganisms9040725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Juhász-Kaszanyitzky, E. et al. MRSA transmission between cows and humans. Emerg. Infect. Dis.13, 630–632. 10.3201/eid1304.060833 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basanisi, M. G., La Bella, G., Nobili, G., Franconieri, I. & La Salandra, G. Genotyping of methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and dairy products in South Italy. Food Microbiol.62, 141–146. 10.1016/j.fm.2016.10.020 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Vanderhaeghen, W. et al. Methicillin-resistant Staphylococcus aureus (MRSA) ST398 associated with clinical and subclinical mastitis in Belgian cows. Vet. Microbiol.144, 166–171. 10.1016/j.vetmic.2009.12.044 (2010). [DOI] [PubMed] [Google Scholar]
- 87.Katayama, Y., Ito, T. & Hiramatsu, K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother.44, 1549–1555. 10.1128/AAC.44.6.1549-1555.2000 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ito, T. et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemoth. 45, 1323–1336. 10.1128/AAC.45.5.1323-1336.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vandenesch, F. et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. dis.9, 978–984. 10.3201/eid0908.030089 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang, H. W., Chiang, P. H. & Huang, Y. C. Livestock-associated methicillin-resistant Staphylococcus aureus ST9 in pigs and related personnel in Taiwan. PloS One. 9, e88826–e88826. 10.1371/journal.pone.0088826 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chuang, Y. Y. & Huang, Y. C. Livestock-associated meticillin-resistant Staphylococcus aureus in Asia: an emerging issue? Int. J. Antimicrob. Agents. 45, 334–340. 10.1016/j.ijantimicag.2014.12.007 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available within the article and its supplementary materials. Sequence data that support the findings of this study have been deposited in the GenBank with accession nos: PP249546 - PP249564.