Abstract
Background
Activated Phosphoinositide 3-Kinase (PI3K) δ Syndrome (APDS), an inborn error of immunity due to upregulation of the PI3K pathway, leads to recurrent infections and immune dysregulation (lymphoproliferation and autoimmunity).
Methods
Clinical and genetic data of 28 APDS patients from 25 unrelated families were collected from fifteen Italian centers.
Results
Patients were genetically confirmed with APDS-1 (n = 20) or APDS-2 (n = 8), with pathogenic mutations in the PIK3CD or PIK3R1 genes. The median age at diagnosis was 15.5 years, with a median follow-up of 74 months (range 6-384). The main presenting symptoms were respiratory tract infections alone (57%) or associated with lymphoproliferation (17%). Later, non-clonal lymphoproliferation was the leading clinical sign (86%), followed by respiratory infections (79%) and gastrointestinal complications (43%). Malignant lymphoproliferative disorders, all EBV-encoding RNA (EBER)-positive at the histological analysis, occurred in 14% of patients aged 17–19 years, highlighting the role of EBV in lymphomagenesis in this disorder. Diffuse large B-cell lymphoma was the most frequent. Immunological work-up revealed combined T/B cell abnormalities in most patients. Treatment strategies included immunosuppression and PI3K/Akt/mTOR inhibitor therapy. Rapamycin, employed in 36% of patients, showed efficacy in controlling lymphoproliferation, while selective PI3Kδ inhibitor leniolisib, administered in 32% of patients, was beneficial on both infections and immune dysregulation. Additionally, three patients underwent successful HSCT due to recurrent infections despite ongoing prophylaxis or lymphoproliferation poorly responsive to Rapamycin.
Conclusions
This study underscores the clinical heterogeneity and challenging diagnosis of APDS, highlighting the importance of multidisciplinary management tailored to individual needs and further supporting leniolisib efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10875-024-01835-1.
Keywords: Activated phosphoinositide 3-kinase δ syndrome; APDS; Leniolisib; Lymphoproliferation; PI3Kδ inhibitor, EBV; Lymphoma
Introduction
Activated Phosphoinositide 3-Kinase δ Syndrome (APDS) is an Inborn Error of Immunity (IEI) characterized by dysregulation of the phosphoinositide 3-kinase (PI3K) signaling pathway [1, 2]. PI3K pathway genes are categorized into three classes based on their structure and function. Class I PI3Ks, which include the p110α, p110β, p110γ, and p110δ isoforms, are the most studied and are crucial for regulating various cellular activities. Among these, the tumor suppressor gene PTEN (phosphatase and tensin homolog) plays a pivotal role by antagonizing the PI3K pathway. PTEN acts as a lipid and protein phosphatase, dephosphorylating the 3-position phosphate of phosphoinositides, thus inhibiting downstream signaling. Activation of PI3K leads to phosphorylation of phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3), by the catalytic subunit of PI3K (p110δ). PIP3 then recruits and activates AKT, a kinase acting as secondary intracellular signal to modulate survival and proliferation. AKT targets mTOR (mammalian target of rapamycin), a critical regulator of cell growth and protein synthesis. mTOR is a component of two complexes, mTORC1 and mTORC2, controlling different cellular functions. In APDS, mutations in PIK3CD or PIK3R1 cause an over activation of this pathway, particularly affecting immune cells like B and T lymphocytes [3].
APDS type 1 (APDS1) is caused by heterozygous gain-of-function (GOF) mutations in the PIK3CD gene, encoding the catalytic subunit p110δ of PI3Kδ, an isoform primarily expressed in leukocytes that is crucial for the regulation of immune cell activation and differentiation. This leads to dysregulated B cell development, impaired class-switch recombination, defective T cell regulation, and altered innate immunity [1, 2]. Patients typically present with recurrent respiratory tract infections (RTI), chronic viral infections, autoimmune manifestations, and non-clonal lymphoproliferation with the related risk of developing lymphoma [4].
APDS type 2 (APDS2) is caused by heterozygous loss-of-function mutations in the PIK3R1 gene, encoding the regulatory subunit p85α of PI3Kδ. APDS2 may present a higher prevalence of autoimmune manifestations than APDS1, a lower incidence and/or severity of respiratory infections, and possible syndromic features (short stature, mild intellectual disability, facial dysmorphia) [1, 5, 6].
The severity of APDS phenotype varies widely and diagnosis relies on identifying the mutations in the PI3K pathway genes. Management of APDS includes anti-infectious prophylaxis and immunosuppression for immune dysregulation [1]. In the most severe cases, hematopoietic stem cell transplantation (HSCT) is a potential cure, nevertheless, it remains a matter of debate [7]. Emerging therapies targeting the PI3K pathway offer promising avenues for treating APDS, aiming to restore immune-homeostasis [6, 8–11]. The U.S. Food and Drug Administration (FDA) recently approved leniolisib as the first tailored therapy for this disease [12].
Since the APDS discovery in 20134, several reports on patients with APDS have been published worldwide [6, 13–22]. Here, we carefully describe an Italian APDS cohort of 28 patients. Given the heterogeneity of APDS, these findings may expand the disease knowledge and improve awareness among pediatric and adult non-immunologists, which is crucial to guarantee accurate diagnosis and tailored treatment for affected individuals.
Patients and Methods
Fifteen Italian centers collected data on 28 APDS patients (16 females and 12 males) from 25 unrelated families. Sixteen of them have been reported previously [13, 14, 23–32].
Diagnosis of APDS-1 and APDS-2 were confirmed by identifying pathogenic variants in PIK3CD and PIK3R1, respectively, by either targeted sequencing or whole exome sequencing. Previously undescribed variants were validated by phospho-S6 Kinase or phospho-Akt Ser 473 pathway assays as recently reported in literature [14, 24].
The study was conducted in accordance with the Declaration of Helsinki. The participants or their parents provided written informed consent for the publication of any data included in this article, which was approved by the local ethical committee of each centre.
Statistical analysis was carried out using GraphPad Prism Software. For statistical comparison, a nonparametric Wilcoxon test was used. A p-value ≤ 0.05 was considered statistically significant.
Results
Twenty-six patients were alive at the time of data collection, and had a median age of 19.9 years (3.6–57.5 years). The median follow-up time was 74 months (6–384 months).Two patients died, at the age of 49: p13 due to SARS-CoV-2 infection and non-Hodgkin lymphoma (NHL); p27 following a heart attack, not related to the underlying disease.
Genetics
Twenty out of 28 APDS patients analyzed have been genetically diagnosed with APDS1. Eleven patients carried the heterozygous E1021K mutation, and one patient had the E525A mutation in the PIK3CD gene. The mutations Y524D, R108L, and P658L have been proven pathogenic by S6 phosphorylation assay [14]. P4 carried the E525G mutation in PIK3CD, pathogenicity was confirmed by hyperphosphorylation in the S473 residue of AKT [24]. Of note, p13 had both the pathogenic mutation E525K in PIK3CD and an additional heterozygous rare VUS in PIK3R1 (V179A).
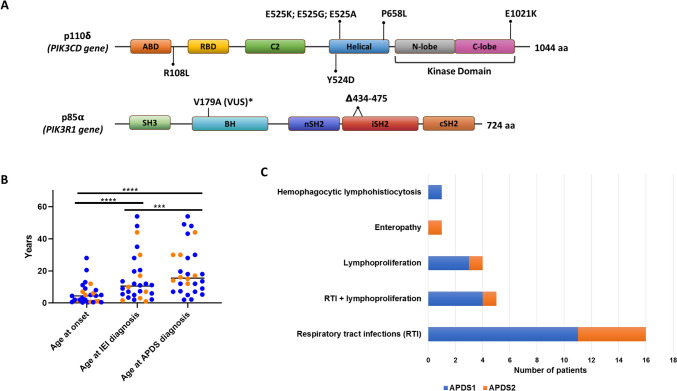
All 8 patients diagnosed with APDS2 presented splicing site mutations, either in position c.1300–2 A > G or c.1425 + 1 G > C. Independently of the position or the nucleotide change, both variants led to the deletion of 41 amino acids in the inter-SH2 domain. The distribution of the variants across the protein domains is represented in Fig. 1A.
Fig. 1.
Clinical and molecular diagnosis of APDS cohort: gene variants (A), diagnostic delay (B) and clinical signs at onset (C). Abbreviations: ABD, Adaptor-binding domain; BH, Bcl-2 homology domain; cSH2, C-terminal SH2; nSH2, N-terminal SH2; RBD, Ras-binding domain; SH, Src homology domain; VUS, Variant of Uncertain Significance; APDS, Activated PI3K-kinase Delta Syndrome; IEI, Inborn Errors of Immunity
Table S1 provides a summary of the patient’s genetic diagnosis and references for published validation assays.
Clinical Characteristics
Clinical Features at Onset
The median age of the clinical presentation of APDS was 4.4 years (3 months − 28 years), while the diagnosis of IEI arose only later in the evolution of the disease (median age 10.5 years; 1–54 years). Finally the patients reached the APDS diagnosis at a median age of 15.5 years (2–54 years) (Fig. 1B).
The main presenting symptoms were RTI alone (16/28) or associated with lymphoproliferation (5/28). Four patients presented isolated lymphoproliferation, non-clonal at the onset. Other presenting signs were enteropathy and one case of autoinflammatory symptoms with a hemophagocytic lymphohistiocytosis-like picture (Fig. 1C).
Two patients experienced adult onset: p19 had recurrent RTI from the age of 20.5; p15, at 28 years, experienced lymphoproliferation (adenopathies, tonsil hypertrophy, and hepatosplenomegaly).
One patient, diagnosed due to affected family members carrying the pathogenic mutation p.Y524D, is still asymptomatic at the age of 57 years.
Infections
Over time, up to 78.6% of patients (n = 22) presented recurrent RTI despite their absence at onset. Moreover, 57% of patients needed prolonged antibiotic therapies (> 2 weeks) suggesting severe infections.
Six patients had other infections (severe gastroenteritis, sepsis, impetigo). One patient (p22) with a CID (combined immunodeficiency) phenotype resulted positive for atypical mycobacteria on bronchoalveolar lavage; he was treated for eleven months and relapsed seven months after the therapy withdrawal.
Twelve patients (42.9%) had chronic positive EBV viremia in peripheral blood, while EBV-related symptoms were detected in only 9 patients (32% of the cohort). All the symptomatic patients had chronic viremia. Four out of six patients presenting chronic positive EBV viremia were tested for EBV serology and showed persistent positivity of IgM against the virus capsid antigen (VCA), while IgG serology was not informative due to immunoglobulin supplementation. Chronic positive CMV viremia was less common (6/28), mildly symptomatic only in one case. Other patients presented with fever and splenomegaly but completely cleared the virus after antiviral therapy.
Non-malignant Lymphoproliferation
Systemic non-clonal lymphoproliferative disorders (SNCLD) characterized 89% of patients (25/28). At a median age of 7 years (range 0.6–30), they showed multiple chronic lymphadenopathies, thoracic or gastrointestinal tract lymphoid hyperplasia, granulomatous lymphocytic interstitial lung disease (GLILD), tonsil hypertrophy, splenomegaly, and hepatomegaly.
Splenomegaly, affecting 22/28 patients, was the leading expression of SNCLD and persisted for more than six months in 19/22 of cases, associated with hepatomegaly in 8/28 individuals. Nineteen patients showed chronic lymphadenopathies, combined with thoracic and gastrointestinal lymphoid hyperplasia. Uncommon manifestations were tonsil hypertrophy (8/28) and GLILD (3/28).
Malignant Lymphoproliferation and Other Malignancies
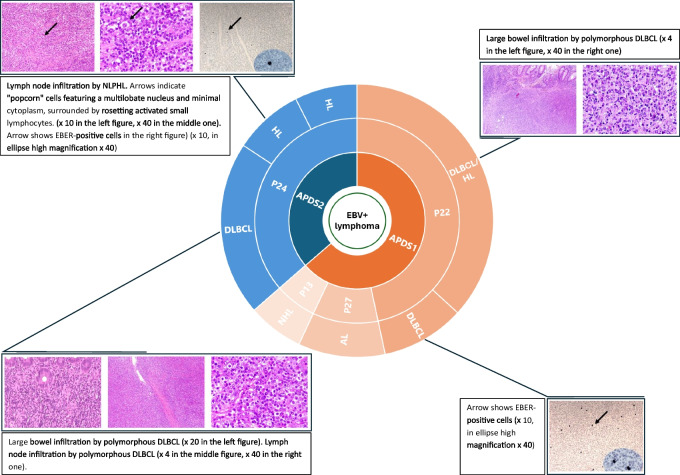
Clonal lymphoproliferative disorders occurred in 4 (14%) patients at a median age of 18 years (17–19). Two patients experienced gastrointestinal EBV-positive diffuse large B cell lymphoma (DLBCL), at the age of 19 (p22) and 18 years (p24), respectively. In the former case, a duodenal DLBCL was followed by a further caecal lymphoma with histologic features of Hodgkin lymphoma (HL)/DLCBL; in the latter, a colonic DLBCL was preceded and followed by a cervical nodular lymphocyte-predominant HL. Furthermore, there was a case of EBV-positive stage IV anaplastic lymphoma in a 17-year-old patient (p27) and a case of EBV-positive NHL in a 47-year-old patient (p13) (Fig. 2). All patients required multiple-line chemotherapies to induce remission. Nonetheless, p13 died at 49 years following SARS-CoV-2 infection and NHL relapse. Further data about malignant lymphoproliferation are reported in Tables 1 and 2.
Fig. 2.
Malignant lymphoproliferation in the APDS cohort. Abbreviations: APDS, Activated PI3K-kinase Delta Syndrome; AL, Anaplastic lymphoma; DLBCL, Diffuse Large B Cell Lymphoma; EBER, EBV-encoded RNA; EBV, Epstein-Barr virus; HL, Hodgkin Lymphoma; NHL, Non-Hodgkin Lymphoma; NLPHL, Nodular lymphocyte-predominant Hodgkin Lymphoma. Adapted from Rivalta B et al. Case Report: EBV Chronic Infection and Lymphoproliferation in Four APDS Patients: The Challenge of Proper Characterization, Therapy, and Follow-Up. Front Pediatr. 2021 Aug 27;9:703853. 10.3389/fped.2021.703853. PMID: 34540765; PMCID: PMC8448282
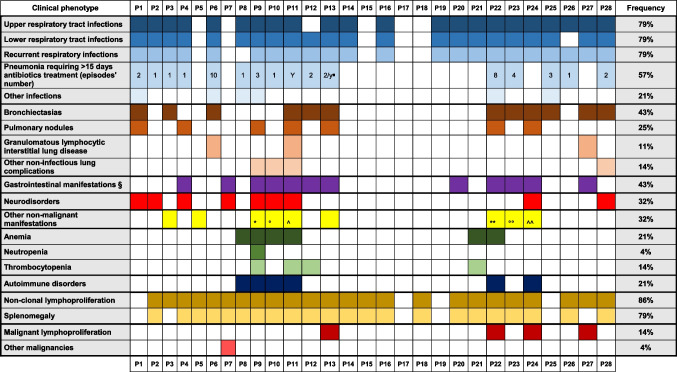
Table 1.
Clinical features of the APDS cohort
∎Two episodes per year
§ Gastrointestinal non-neoplastic disorders were: intestinal lymphoid hyperplasia, inflammatory colitis, Crohn's disease, Crohn-like enteropathy, indeterminate colitis, eosinophilic enteropathy, celiac disease combined with an EBV-positive anaplastic intestinal lymphoma, gastro-esophageal reflux, anal fistula
*Premature ovarian failure, mild growth retardation, and facial dysmorphisms
°Type 2 diabetes, hypertension, overweight, cerebral stroke
^C3 glomerulopathy
**Nasal polyposis
°°Vernal conjunctivitis
^^Premature ovarian failure
Table 2.
Detailed features of the malignant lymphoproliferative manifestations in the cohort
| P13 | P22 | P24 | P27 | |
|---|---|---|---|---|
| Sex | Male | Male | Female | Female |
| ADPS-related variant |
PIK3CD c.1573G > A (PIK3R1 c.536T > C VUS) |
PIK3CD p.E1021K | PIK3R1 c.1425 + 1G > T | PIK3CD c.G3061A p.E1021K |
| Age at APDS onset | 5 years | 6 months | 8 months | 13 years |
| Age at APDS diagnosis | 48 years | 18 years | 30 years | 49 years |
| Age at first malignant lymphoproliferation diagnosis | 47 years | 19 years | 18 years | 17 years |
| Type of malignant lymphoproliferation | NHL | • DLBCL | • NLPHL | Anaplastic lymphoma |
| • HL/DLBCL | • DLBCL | |||
| • NLPHL | ||||
| Localization of malignant lymphoproliferation | N.A. | Duodenum -> caecum | Cervical -> colon -> cervical | Bowel |
| EBER association with malignancy | Yes | Yes | Yes | Yes |
| Treatment | N.A. | • 3 R-CHOP + 2 CHOP cycles (DLBCL) | • 3 ABVD cycles + radiotherapy (NLPHL) | Chemotherapy and autologous HSCT |
| • Rituximab + Ibrutinib + Bendamustine (HL/DLBCL) | • 5 R-CHOP cycles + 2 Rituximab infusions (DLBCL) | |||
| • NLPHL | ||||
| Outcome | Relapse | Remission | Remission | Remission |
| EBV chronic viremia | Yes | Yes | Yes | No |
| Main clinical features | • Recurrent respiratory infections | • Recurrent respiratory infections | • Recurrent respiratory infections | • Recurrent respiratory infections |
| • Hepatosplenomegaly | • Hepatosplenomegaly | • Hepatosplenomegaly | • GLILD | |
| • Colitis | • AIH + AIHA | • Autoimmune thyroiditis | • Celiac disease | |
| • Colitis | • IBD | |||
| Immunological phenotype | CID-like | CID-like | CID-like | T effector skewed differentiation + humoral defect |
| Status | Deceased | Alive | Alive | Deceased |
| Age of death | 49 years | NA | NA | 49 years |
| Cause of death | COVID disease and lymphoma reactivation | NA | NA | Heart attack |
ABVD Adriamycin, Bleomycin, Vinblastine, Dacarbazine; AIH Autoimmune hepatitis; AIHA Autoimmune hemolytic anemia; APDS Activated PI3K-kinase Delta Syndrome; CHOP Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone; CID Combined Immunodeficiency; DLBCL Diffuse Large B Cell Lymphoma; EBER EBV-encoded RNA; EBV Epstein-Barr virus; GLILD Granulomatous Lymphocytic Interstitial Lung Disease; HL Hodgkin Lymphoma; HSCT Hematopoietic Stem Cell Transplantation; IBD Inflammatory Bowel Disease; NA Not Applicable; NHL Non-Hodgkin Lymphoma; NLPHL, Nodular lymphocyte-predominant Hodgkin Lymphoma; R-CHOP, Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisolone; VUS, Variant of uncertain significance
Of note, patient (p9) was initially diagnosed at the age of 20 with HL requiring multiple therapies: six-cycle ABVD, two-cycle IEV, and autologous HSCT. Fourteen years later, the first lymph node biopsy was reviewed, recanting the previous HL diagnosis in favor of non-malignant hyperplastic lymphadenopathy with relevant activation of the interfollicular area by CD30+, mostly mononucleated cells. This was superimposable to a follow-up biopsy performed ten years later, after therapies.
The only other malignancy was an ovarian dysgerminoma occurring in a 14-year-old female (p7).
Lung Complications
Non-infectious lung complications affected 54% of patients, aged between 5 and 40 years (median 10 years). CT scans identified bronchiectasis, nodules, and GLILD in 43%, 25%, and 11% of patients, respectively. Additional lung complications were thoracic lymphadenopathies (3 cases) and partial lung dystelectasis with aspecific inflammatory reaction (1 patient).
Gastrointestinal Benign Manifestations
Gastrointestinal benign disorders involved 43% of patients (7/20 APDS1 vs. 5/8 APDS2), at a median age of 18.5 years (range 2–45). Intestinal lymphoid hyperplasia and inflammatory colitis were the most frequent, affecting 4/28 individuals, the latter associated in 2 cases with an intestinal DLBCL. Other gastrointestinal non-neoplastic disorders are specified in Table 1.
Autoimmune Disorders
Autoimmune manifestations occurred in the 21% (6/28) of patients, with a median onset age of 19.5 years (5–42 years). The leading manifestation was autoimmune hemolytic anemia (AIHA), affecting 4/28 patients, combined in one case with autoimmune hepatitis (p22), in another with a systemic lupus erythematosus (SLE)-like condition (p9), followed by immune thrombocytopenia with renal disease (C3-glomerulopathy in p11), and thyroiditis with inflammatory bowel disease (IBD) (p24).
A quarter of patients presented cytopenia, with anemia being the most common (21%; n = 6), followed by thrombocytopenia (4 cases) and neutropenia (1 case). Immune cytopenias were more frequent in APDS2 than APDS1 patients (4/8 vs. 2/20). Multiple causes of cytopenia, such as autoimmunity, concomitant infection, or chronic blood loss, could be recognized; often contemporary present in a single patient .
Neurological Disorders
Nine patients (32%) displayed some neurological manifestations, most commonly identified at a median age of 6.7 years (range 3–31). Mild cognitive impairment was reported in 7 patients by physicians during clinical evaluation, although it was not confirmed by intellectual quotient evaluation in the majority of cases. Associated features were anxiety and facial paresthesia, epilepsy, and posterior reversible encephalopathy syndrome (the latter probably related to kidney disease).
Immunological Profile
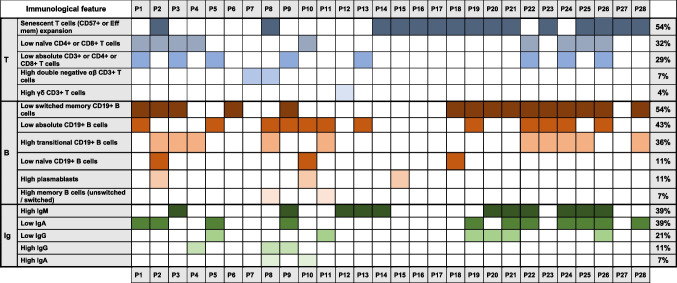
The immunological assessment of this patients’ cohort reveals a wide range of cellular and humoral abnormalities (Table 3). Eight out of 28 (29%) showed low absolute counts of T cells. Expansion of senescent CD57 + CD3 + T cells or effector memory subsets was the most common T cell alteration, detected in 54% of patients, usually associated with normal T cell counts, except for 1 patient with T cell lymphopenia. Similarly, 32% of patients showed a reduction of naïve T cell (CD4 + or CD8+). Double-negative αβ T cells and γδ T cells were increased in a minority of cases (2 and 1 out of 28, respectively).
Table 3.
Immunological features of the APDS cohort
B cells lymphopenia occurred in 43% of cases. Independently from the total B cell count, 54% of patients displayed low switched memory B cells (in the majority of cases coupled with an abnormal T cell phenotype). Moreover, 36% of patients presented high transitional B cell counts. In the B cell compartment, less common features were a reduction of naïve B cells and increase of plasmablasts (11% each).
In line with the B cell phenotype observed, humoral abnormalities were frequent: high IgM levels (39%), low IgA levels (39%), low IgG levels (21%), while elevated IgG and IgA levels were less frequent.
As illustrated in Table 3, most patients showed variable combinations of T and B cells defects (20/28, 71%). Few cases presented with immunological profiles resembling other primary immunodeficiencies, such as: combined immunodeficiency (4/28), autoimmune lymphoproliferative syndrome (1/28), common variable immunodeficiency (1/28), hyper-IgM syndrome (1/28). This heterogeneous immunological profile highlights both T and B cell dysregulation as hallmark features of APDS, particularly affecting memory and naïve B cell populations, as well as senescent T cell expansion in over half of the patients.
Treatment
Supportive/Symptomatic Treatment
Most patients (n = 19) received Ig supplementation, all of them suffered from recurrent RTI. Eight out of 19 had hypogammaglobulinemia. The remaining 11 patients received Ig supplementation despite normal to high IgG serum levels: 7/11 due to lack of specific antibody response; 3/11 due to RTI and considering the molecular diagnosis of APDS; one patient started supplementation just for clinical indication due to RTI and benefited.
Thirteen patients underwent additional prophylaxis with either trimethoprim/sulfamethoxazole or azithromycin. One patient received azithromycin without immunoglobulins due to recurrent RTI and bronchiectasis.
Compared to the onset of infectious episodes, prophylaxis was introduced later. Median delay time was: 5.5 years for Ig supplementation (range 0.1–33); 6 years for antimicrobial prophylaxis (range 0–39).
Steroids
Only 5/28 patients received steroid immunosuppressive therapy starting at a median age of 21 years (range 3–40). None of the patients showed a complete and persistent clinical benefit from steroids alone. Two patients needed multiple immunosuppressants to partially control immune dysregulation (SLE and IBD, respectively) (Table 2S).
PI3K/Akt/mTOR Inhibitor Treatment
Rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR), can modulate the dysregulated PI3Kδ-signaling pathway [33, 34], reducing lymphoproliferation [34, 35] and improving immune dysregulation even in the long-term [6]. Rapamycin was introduced at a median age of 14 (3–31 years) in 10/28 patients (36%). Two patients received rapamycin after other immunosuppressants, in 8 patients it was first-line therapy. Eight (80%) patients showed remarkable improvement of non-clonal lymphoproliferation, benefit was absent or partial in only two cases. Overall, rapamycin was well tolerated without any significant side effects. P28 showed partial improvement with theophylline administration, which seems to inhibit mTOR pathway [26] (Table 2S).
Selective PI3Kδ Inhibitors
Selective PI3Kδ inhibitors are a promising therapeutic approach for APDS. In Italy, leniolisib is only available for Compassionate Drug Use (CDU).
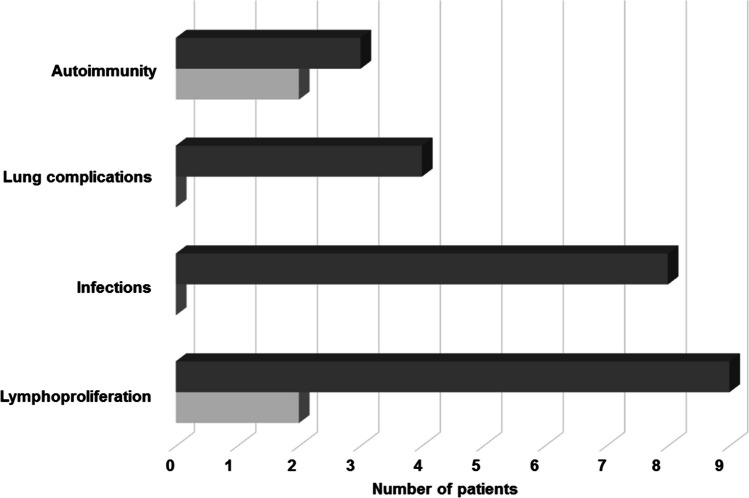
Nine patients (32%) accessed the selective PI3Kδ inhibitor leniolisib as tailored therapy with a high safety profile and improvements: reduction of infection rate, lymphoproliferation, lung complications and autoimmunity (Fig. 3). The median follow-up under therapy is 4.5 years (range 0.2–5). Retrospective data collection does not allow a comprehensive analysis, however, for 4 patients more detailed information are available. Patient p6 did not show EBV reactivation or thrombocytopenia relapses, and a significant reduction of the spleen and liver size was documented; p9 did not have a recurrence of infections or cytopenias and improved erythema pernio as well as lymphocyte typing; p26 normalized transitional and naive B cells and reduced hyper-IgM; p28 normalized naive B cells and slightly increased memory unswitched and switched B cells (Table 3S). The first clinical trial on selective PI3Kδ inhibitors efficacy was conducted in 2015–2016 on patients older than 12: the median age at starting treatment in our cohort was 22 years (range 12–33 years). Before receiving leniolisib, p25 and p28 took part in phase 1b, an open-label, multicenter, exploratory study aimed to assess the safety, tolerability, pharmacokinetics, and efficacy of seletalisib (2015–002900-10) and its extension study (2015–005541). Even with this compound, patients improved clinically with favorable risk-benefit profiles [9]. Patients p6, p14, p18, p26, and (after the end of seletalisib clinical trial) p25, participated in a randomized, placebo-controlled phase 3 trial on leniolisib (#NCT02435173) in 2015–2022 and its extension study (#NCT02859727) currently ongoing (Table 2S) [32, 36].
Fig. 3.
Clinical phenotype of APDS patients receiving leniolisib prior to treatment and after initiation. The graph represent the variation of clinical signs before (dark grey) and after (light grey) starting leniolisib
Hematopoietic Stem Cell Transplantation
Three patients (p1, p2, p8) affected by APDS1 underwent hematopoietic stem cell transplantation (HSCT) at 9.9, 7.3, and 14.5 years of age. Indications to transplant were either recurrent infections despite ongoing prophylaxis (p1) and lymphoproliferation poorly responsive to rapamycin (p2, p8). Donors were all unrelated (9/10-HLA matched for p1 and p2; 10/10 for p8). All patients reached complete remission of the disease manifestations. Graft versus Host Disease (GVHD) was observed only in p1, experiencing grade III intestinal aGVHD at day + 21, grade II skin aGVHD at day + 30. She developed an autoimmune neurological complication 18 months after HSCT with central nervous system lesions detectable at magnetic resonance, requiring prolonged immunosuppression. Immunosuppressive drugs were progressively tapered and withdrawn except for immunoglobulins, continued for immune-modulatory purposes, without relapse. None of the other two patients is currently under immunosuppression or anti-infectious prophylaxis. Median follow-up after HSCT was 4 years at the time of data collection. All patients are currently alive and well, maintaining full donor chimerism (Table 4S).
Discussion
The Italian cohort of 28 APDS patients shows features comparable to the previously described cohorts (Table 4) and displays a narrow genetic variability, despite it reproduces the well-known phenotypic heterogeneity of the disease.
Table 4.
Comparison of APDS cohorts reported in literature
| Barzaghi et al. 2024 | Tessarin et al. 2020 |
Maccari et al. 2018 (ESID Registry) |
Maccari et al. 2023 (ESID Registry) |
Oh et al. 2021 (USIDNET) | Coulter et al. 2016 |
Elkaim et al. 2016 |
Qiu et al. 2022 |
Wang et al. 2018 |
Fekrvand et al. 2020 |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | (this study) | 13 | 5 | 12 | 18 | 15 | 16 | 14 | 19 | 17 | |
| Ethnicity | Italian | Italian | European | European | American | European, American and Japanese | European, Asian and Japanese | Chinese | Chinese | Iranian | |
| Number of patients | 28 | 8 | 77 | 170 | 40 | 53 | 36 | 40 | 15 | 15 | |
| Number of APDS1 patients (% of total APDS) | 20 (71%) | 8 (100%) | 51 (66%) | 115 (68%) | 33 (82%) | N.A. | 0 (0%) | 37 (92%) | 15 (100%) | 6 (40%) | |
| Number of APDS2 patients (% of total APDS) | 8 (29%) | 0 (0%) | 26 (34%) | 55 (32%) | 7 (18%) | N.A. | 36 (100%) | 3 (8%) | 0 (0%) | 9 (60%) | |
| Age in years at study enrollment | 19.9 (median) | 14.9 (median) | 17.9 (mean) | N.A. | Range 14–72 years | 17.2 (mean) |
18 (median) |
10 (median) |
N.A. |
22 (median) |
|
| Non-clonal lymphoproliferation | Overall non-clonal lymphoproliferation | 89% | 87% | 87% | 86% | 75% | 75% | 89% | 100% | 93% | 53% |
| Splenomegaly | 79% | 87% | 39% | 60% | 55% | 58% | 43% | 93% | 93% | 40% | |
| Hepatomegaly | 29% | 75% | N.A. | N.A. | 27% | 45% | 22% | 90% | 93% | 27% | |
| Lymphadenopathy | 36% | 87% | 69% | 80% | 53% | 64% | 75% | 97% | 93% | 33% | |
| Lymphoid hyperplasia | 18% | 0% | 32% | N.A. | 10% | 32% | 48% | 53% | N.A. | N.A. | |
| Malignant lymphoproliferation | Overall malignant lymphoproliferation | 14% | 0% | 10% | 13% | 12% | 13% | 25% | 5% | 0% | 7% |
| Hodgkin lymphoma | 25% | / | 25% | 32% | 20% | 29% | 55% | N.A. | 0% | N.A. | |
| Non-Hodgkin lymphoma | 100% | / | 88% | 68% | 80% | 71% | 55% | N.A. | / | N.A. | |
| EBV-association | 100% | / | N.A. | 50% | N.A. | 13% | 44% | 100% | / | 0% | |
| Immune dysregulation | Autoimmune disorders | 21% | 50% | N.A. | N.A. | N.A. | 42% | 17% | 67% | 80% | 27% |
| Immune cytopenias | 21% | 25% | 30% | 19% | 22% | N.A. | N.A. | N.A. | N.A. | N.A. | |
| Enteropathy | 43% | 12% | 51% | 35% | 52% | 25% | 22% | 47% | 33% | N.A. | |
| Neurodevelopmental delay | 25% | 0% | 4% | N.A. | 20% | 19% | 31% | 27% | 13% | 20% | |
Most patients experienced early onset characterized by recurrent RTI with or without lymphoproliferation. Nevertheless, the referral for the suspicion of IEI and the final diagnosis of APDS occurred with a median delay of 4 and 10 years, respectively, after onset. The delay in APDS diagnosis was significant and related to an initial delay in the suspicion of IEI, amplified by the time required for the genetic confirmation. The diagnostic gap was longer for patients experiencing the onset in adulthood or before APDS discovery (2013). Still, the diagnosis can be challenging since pathognomonic biomarkers are lacking, and genetic analysis driven by clinical suspicion is crucial.
The prevalent phenotype was recurrent RTI with significant bronchospasm, possibly related to the role of PI3Kδ in the pathogenesis of bronchial asthma [37] and severe allergic inflammation [37]. Indeed, a percentage of asthmatic individuals, poorly responsive to standard therapies, may carry PI3K pathway dysfunction and thus represent good candidates for administration of selective inhaled PI3Kδ inhibitors, demonstrated to block asthma-related inflammation in mice [10]. In some APDS cases, the dysregulation of the PI3Kδ pathway locally and the recurrent infections may contribute to increased airway remodeling, leading to chronic lung complications (such as bronchiectasis and interstitial lung disease). APDS patients with severe asthma may benefit from selective inhibitors, not only for their positive effect on the immune function but also on the airway epithelium and local leukocyte trafficking.
EBV susceptibility is another common feature among APDS patients. However, severe mononucleosis is not typical in this cohort, as in other reports [6, 13–20]. Conversely, EBV viremia may remain positive, at low levels, for a long time. Viral persistence worsens hyperactivation of the PI3K/Akt/mTOR pathway [4, 38, 39] and increases the risk of EBV-related lymphomas, similarly to other IEI [40].
Only one patient of this cohort was affected by recurrent mycobacteriosis, a susceptibility that probably stems from a monocytes-derived macrophage (MDM) impairment. As previously demonstrated, MDM from this patient failed to control the Mycobacterium bovis Bacillus Calmette Guèrin (BCG) infection in vitro. The defect was reversible by adding the selective p110δ inhibitor in vitro, suggesting a disease-specific vulnerability in exposed patients [27]. This susceptibility is usually more evident in patients from endemic areas and could suggest an immunological worsening [10].
In different APDS cohorts, including ours, SNCLD manifests with multiple-site lymph node involvement, especially in the thoracic and abdominal areas, occurring concomitantly with splenomegaly [13–20, 22]. In our patients, SNCLD occurred with a frequency comparable to other larger cohorts [6, 16, 17, 19], without differences in APDS1 vs. APDS26,18.
In our cohort, four patients were diagnosed with lymphoma, DLBCL being the most frequent. EBV-encoded small RNA was positive in all cases in the context of EBV chronic viremia, which can often be associated with lymphoma, underscoring its role in lymphomagenesis [13, 15–17, 19]. The types and prevalence of lymphomas observed in our study align with existing literature (range 10–13%)6,13,16,17,19, mainly DLBCL and HL [6, 13, 16, 17] and mucosa-associated lymphoid tissue (MALT) lymphoma [19]. The cohorts’ age influences the prevalence of the abovementioned lymphomas, set to grow over time [6]. Indeed, median age range is 17.2–19.9 years in our work and others [6, 16, 17]. In contrast, studies including younger patients, display lower lymphoma prevalence [15, 18]. The case of p9, whose diagnosis of HL was revised years later, highlights how the knowledge of atypical phenotypes (e.g., SNCLD) in the setting of IEIs is crucial to provide accurate diagnosis. Histological revision, performed before APDS diagnosis, may help physicians in shaping an adequate long-term follow-up, avoiding inappropriate therapies. Indeed, p9, following the therapies for the supposed HL, developed cardiopathy in a complex clinical scenario dominated by severe infections, lymphopenia, arthro-myalgias, and chilblain lupus [31]. Similar cases would deserve a watchful-waiting approach and immunosuppression (such as rapamycin) or targeted therapies to contrast the lymphoproliferative drive instead of aggressive and potentially detrimental therapies.
Non-neoplastic gastrointestinal manifestations affected almost half of patients, similarly to larger cohorts [13, 15, 16, 19], and consisted mainly of intestinal lymphoid hyperplasia and inflammatory colitis. PI3K/Akt pathway plays a pivotal role in regulating intestinal epithelial proliferation: in vivo, experiments suggest that PI3K inhibitors attenuate mucosal proliferation [41]; loss-of-function mutations in phosphatase and tensin homolog (PTEN), a PI3K inhibitor, favor gastric cancer [41–43]. Hence, downregulation of the PI3K pathway may reduce proliferation and risk of malignancy [44].
Autoimmune cytopenias (mainly AIHA), showed a delayed onset over the course of the disease, as mentioned in the literature [13]. Only one patient presented nephropathy, as the first manifestation of APDS, which deserves appropriate diagnosis and follow-up, even after HSCT [45]. Non-clonal lymphoproliferation is confirmed as the dominant expression of immune dysregulation in APDS. Other immune dysregulation disorders were less common when compared to other primary immune regulatory disorders (PIRDs) [13].
Neurological disorders interested the 32% of patients, in line with literature, reporting an occurrence from 10 to 40% across various APDS cohorts [6, 13, 15–19]. In our study and others [17], neurological disturbances, especially mild retardation, skewed towards APDS2 subcohort, possibly due to the ubiquitous expression of p85α protein, encoded by PIK3R1 gene, whose mutation is also causative for SHORT syndrome, associated with neurodevelopmental abnormalities [46, 47]. This can potentially explain neurologic disease onset and progression, supporting the role of PI3Kδ signaling in axonal growth and regeneration, neuroprotection, vesicular trafficking, neuronal connectivity [48, 49], and neuroinflammation [50]. Similarly, few studies mention PI3Kδ involvement in murine psychotic behavior [51] and in PTEN-mediated human autistic disorders [52]. Despite advancements, diagnosing mild mental retardation remains challenging, particularly in patients with normal IQ scores but subtle cognitive deficits. Therefore, comprehensive neuropsychiatric evaluations are necessary to accurately diagnose and support APDS patients.
The dominant immunological pattern of skewed effector T cell differentiation associated with a humoral defect and a CID-like condition is commonly reported in APDS. These features were common in the 4 patients developing lymphoma, suggesting that a critical combination of immune deficiency (with chronic EBV viremia) and reduced immune surveillance prompts the malignant evolution of lymphoproliferation. However, an in-depth analysis was impossible being a multicentre-retrospective study: patients were tested at different ages, using variable cytofluorimetric markers, not always with extended immunophenotyping.
In our cohort, immunosuppressive drugs were used only in selected cases (complex autoimmune phenotype). Rapamycin was preferred, given the prevalence of non-clonal lymphoproliferation. The treatment was beneficial, reducing LPDs, and well tolerated, as generally reported [6, 26, 33–35]. Clinical trials on small-molecule inhibitors of the PI3K signaling [8, 9, 34, 36, 53] paved a new perspective for APDS management. The FDA has recently approved leniolisib for APDS patients older than twelve years [12], and the drug is also under review by the European Medicines Agency. One-third of the described patients are treated with leniolisib with no side effects and considerable improvements. These promising results need confirmation with longer follow-up on larger cohorts, to potentially identify PI3K selective inhibitors as long-term treatment or bridge-therapy before HSCT. For 3 patients, transplant was motivated by the clinical need to control persistent disease manifestations, the availability of an HSC donor, along with the young age at onset, and absence of suitable compound for selective PI3K-inhibition at the time, which represented a significant risk for prolonged exposure to infectious and lymphoproliferative complications.
Interestingly, only two patients in the present cohort died: one for lymphoma relapse in the context of COVID19 infection, while the other had a traumatic death (7%). A recent work reported lymphoma and HSCT complications as major causes shortening life expectancy in the 11% of patients. This is not the case of our study, although larger numbers would be desirable to make appropriate comparison [54].
Limitations
The retrospective nature of the present study carries some limitations. The number of cases may be underestimated by poor disease awareness among physicians. The suspicion of immunodeficiency was often delayed and the initial immunological work-up was usually performed years before APDS diagnosis. Thus, immunological data available were not contemporary to the onset and based on general parameters from different centers, impairing the analysis of some disease-specific features.
Conclusions
Our APDS cohort displays a classical onset dominated by recurrent respiratory infections, often mild, although frequent and associated with airway remodeling. The suspicion of IEI and the diagnosis of APDS are usually delayed. Given the typical multisystem involvement, awareness among different specialists (including pediatric and adult physicians) may improve referrals and timely recognition of the disease.
Lymphoproliferation arises and evolves overtime, requiring careful monitoring: immunodeficiency with underlying chronic EBV appears a relevant risk factor for malignant transformation. Appropriate genetic diagnosis and treatment may limit this phenomenon. Cooperation between specialists may support the appropriate histological interpretation, avoiding excessive medicalization.
Targeted therapies may guarantee lower side effects and hospitalizations, reducing the indication to HSCT as definitive therapy. Extended studies on the long-term benefits of these therapies in a larger cohort may ensure tailored treatment paths for APDS patients.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 28.0 KB)
(DOCX 20.0 KB)
(DOCX 25.4 KB)
(DOCX 16.3 KB)
Acknowledgements
We would like to thank the patients, their families, and the nurses for all their efforts. Part of the participating centers are members of the Italian Network for Primary Immunodeficiencies (IPINet), the European Society for Immunodeficiencies (ESID), and the European Reference Network for Rare Immunodeficiency, Autoinflammatory, and Autoimmune Diseases (ERN-RITA). This work has been conducted with the support of IEI-Net study (PNRR-MR1-2022-12376594), Italian Ministry of Health; Ricerca Corrente 5 × 1000 from Childrens’ Hospital Bambino Gesù, Rome, Italy, (202205_INFETT_CIFALDI and 2021105_INFETT_CANCRINI); Development of Innovative Diagnostic and Therapeutic Approaches for PID grant, Programma di rete (NET- 2011-02350069).
Author Contributions
Study design: F.B., F.C., C.P. Data interpretation: F.B., M.Mo., G.P., F.C. Patients’ recruitment, management, and data collection: F.B., M.Mo., G.P., B.R., L.A.B., M.C., A.M., D.M., M.Ma., G.C., S.R., L.L., B.M., C.M., A.Tr., A.To., R.B., C.C., V.L., C.P., F.C. Manuscript writing: F.B., M.Mo., G.P., F.C. Critical review of the manuscript: B.R., C.C., S.R., L.L., V.L., C.P. Work coordination: F.C., F.B. All authors have read the manuscript and agree to all its contents.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Compliance with Ethical Standards
The study was conducted in strict adherence to the Helsinki Declaration: participants or their parents provided written informed consent for the collection and publication of any data included in this article, which was approved by the local ethical committee of each centre.
Competing Interests
Authors V.L., C.C., F.C., A.To., A.Tr., R.B., M.Z., F.B., C.P. received an honorarium from Pharming Group N.V. to participate in the APDS advisory board. The other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jamee M, Moniri S, Zaki-Dizaji M, et al. Clinical, immunological, and genetic features in patients with activated PI3Kδ syndrome (APDS): a systematic review. Clin Rev Allergy Immunol. 2020;59(3):323–33. 10.1007/s12016-019-08738-9. [DOI] [PubMed] [Google Scholar]
- 2.Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–71. 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–35. 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating, germline mutations in phosphoinositide 3-Kinase p110δ cause T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen T, Lau A, Bier J, et al. Human PIK3R1 mutations disrupt lymphocyte differentiation to cause activated PI3Kδ syndrome 2. J Exp Med. 2023;220(6): e20221020. 10.1084/jem.20221020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccari ME, Abolhassani H, Aghamohammadi A, et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-Kinase δ syndrome: the European society for immunodeficiencies-activated phosphoinositide 3-Kinase δ syndrome registry. Front Immunol. 2018;9. 10.3389/fimmu.2018.00543. [DOI] [PMC free article] [PubMed]
- 7.Vanselow S, Hanitsch L, Hauck F, et al. Future directions in the diagnosis and treatment of APDS and IEI: a survey of German IEI centers. Front Immunol. 2023;14: 1279652. 10.3389/fimmu.2023.1279652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cant AJ, Chandra A, Munro E, Rao VK, Lucas CL. PI3Kδ pathway dysregulation and unique features of its inhibition by Leniolisib in activated PI3Kδ syndrome and beyond. J Allergy Clin Immunol Pract. 2024;12(1):69–78. 10.1016/j.jaip.2023.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz N, Juarez M, Cancrini C, et al. Seletalisib for activated PI3Kδ syndromes: open-label phase 1b and extension studies. J Immunol Baltim Md 1950. 2020;205(11):2979–87. 10.4049/jimmunol.2000326. [DOI] [PubMed] [Google Scholar]
- 10.Gunerka P, Gala K, Banach M, et al. Preclinical characterization of CPL302-253, a selective inhibitor of PI3Kδ, as the candidate for the inhalatory treatment and prevention of asthma. PLoS ONE. 2020;15(7). 10.1371/journal.pone.0236159. [DOI] [PMC free article] [PubMed]
- 11.Khindri S, Cahn A, Begg M, et al. A multicentre, randomized, double-blind, placebo-controlled, crossover study to investigate the efficacy, safety, tolerability, and pharmacokinetics of repeat doses of inhaled nemiralisib in adults with persistent, uncontrolled asthma. J Pharmacol Exp Ther. 2018;367(3):405–13. 10.1124/jpet.118.249516. [DOI] [PubMed]
- 12.Mullard A. FDA approves PI3K inhibitor for a rare immune disorder. Nat Rev Drug Discov. 2023;22(5):342–342. 10.1038/d41573-023-00061-5. [DOI] [PubMed] [Google Scholar]
- 13.Maccari ME, Wolkewitz M, Schwab C, et al. Activated phosphoinositide 3-kinase δ syndrome: update from the ESID Registry and comparison with other autoimmune-lymphoproliferative inborn errors of immunity. J Allergy Clin Immunol. 2023;152(4):984-e99610. 10.1016/j.jaci.2023.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Tessarin G, Rossi S, Baronio M, et al. Activated phosphoinositide 3-Kinase delta syndrome 1: clinical and immunological data from an Italian cohort of patients. J Clin Med. 2020;9(10): 3335. 10.3390/jcm9103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu L, Wang Y, Tang W, et al. Activated phosphoinositide 3-Kinase δ syndrome: a large pediatric cohort from a single center in China. J Clin Immunol. 2022;42(4):837–50. 10.1007/s10875-022-01218-4. [DOI] [PubMed] [Google Scholar]
- 16.Coulter TI, Chandra A, Bacon CM, et al. Clinical spectrum and features of activated phosphoinositide 3-kinase δ syndrome: a large patient cohort study. J Allergy Clin Immunol. 2017;139(2):597-e6064. 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elkaim E, Neven B, Bruneau J, et al. Clinical and immunologic phenotype associated with activated phosphoinositide 3-kinase δ syndrome 2: a cohort study. J Allergy Clin Immunol. 2016;138(1):210-e2189. 10.1016/j.jaci.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Fekrvand S, Delavari S, Chavoshzadeh Z, et al. The first Iranian cohort of pediatric patients with activated phosphoinositide 3-Kinase-δ (PI3Kδ) syndrome (APDS). Immunol Invest. 2022;51(3):644–59. 10.1080/08820139.2020.1863982. [DOI] [PubMed] [Google Scholar]
- 19.Oh J, Garabedian E, Fuleihan R, Cunningham-Rundles C. Clinical manifestations and outcomes of activated phosphoinositide 3-Kinase δ syndrome from the USIDNET cohort. J Allergy Clin Immunol Pract. 2021;9(11):4095–102. 10.1016/j.jaip.2021.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Wang W, Liu L, et al. Report of a Chinese cohort with activated phosphoinositide 3-Kinase δ syndrome. J Clin Immunol. 2018;38(8):854–63. 10.1007/s10875-018-0568-x. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed AA, El Shahaway AA, Hussien SA. Activated PI3K-delta syndrome in an Egyptian pediatric cohort with primary immune deficiency. Allergol Immunopathol (Madr). 2020;48(6):686–93. 10.1016/j.aller.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Elgizouli M, Lowe DM, Speckmann C, et al. Activating PI3Kδ mutations in a cohort of 669 patients with primary immunodeficiency. Clin Exp Immunol. 2016;183(2):221–9. 10.1111/cei.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saettini F, Pelagatti MA, Sala D, et al. Early diagnosis of PI3Kδ syndrome in a 2 years old girl with recurrent otitis and enlarged spleen. Immunol Lett. 2017;190:279–81. 10.1016/j.imlet.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Marzollo A, Bresolin S, Colavito D, et al. Case report: intestinal nodular lymphoid hyperplasia as first manifestation of activated PI3Kδ syndrome due to a novel PIK3CD variant. Front Pediatr. 2021;9: 703056. 10.3389/fped.2021.703056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivalta B, Amodio D, Milito C, et al. Case report: EBV chronic infection and lymphoproliferation in four APDS patients: the challenge of proper characterization, therapy, and follow-up. Front Pediatr. 2021;9: 703853. 10.3389/fped.2021.703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valencic E, Grasso AG, Conversano E, et al. Theophylline as a precision therapy in a young girl with PIK3R1 immunodeficiency. J Allergy Clin Immunol Pract. 2018;6(6):2165–7. 10.1016/j.jaip.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 27.Chiriaco M, Brigida I, Ariganello P, et al. The case of an APDS patient: defects in maturation and function and decreased in vitro anti-mycobacterial activity in the myeloid compartment. Clin Immunol Orlando Fla. 2017;178:20–8. 10.1016/j.clim.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Milito C, Lougaris V, Giardino G, et al. Clinical outcome, incidence, and SARS-CoV-2 infection-fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract. 2021;9(7):2904-e29062. 10.1016/j.jaip.2021.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conti F, Pacillo L, Amodio D, et al. SARS-CoV-2 infection and treatment in a cohort of patients with inborn errors of immunity. Pediatr Allergy Immunol off Publ Eur Soc Pediatr Allergy Immunol. 2022;33(8): e13833. 10.1111/pai.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivalta B, Amodio D, Giancotta C, et al. Case report: successful treatment with monoclonal antibodies in one APDS patient with prolonged SARS-CoV-2 infection not responsive to previous lines of treatment. Front Immunol. 2022;13: 891274. 10.3389/fimmu.2022.891274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conti F, Catelli A, Cifaldi C, et al. Case report: Hodgkin lymphoma and refractory systemic lupus erythematosus unveil activated phosphoinositide 3-Kinase-δ syndrome 2 in an adult patient. Front Pediatr. 2021;9: 702546. 10.3389/fped.2021.702546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao VK, Webster S, Šedivá A, et al. A randomized, placebo-controlled phase 3 trial of the PI3Kδ inhibitor leniolisib for activated PI3Kδ syndrome. Blood. 2023;141(9):971–83. 10.1182/blood.2022018546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marc E, Rothenberg MD, PhD J, Bousquet MD. News beyond our pages. J Allergy Clinical Immunol. 2016; 138(1):1–210.1016/j.jaci.2016.05.013.
- 34.Rao VK, Webster S, Dalm VASH, et al. Effective activated PI3Kδ syndrome-targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130(21):2307–16. 10.1182/blood-2017-08-801191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15(2):128–35. 10.1038/ni.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao VK, Kulm E, Šedivá A, et al. Interim analysis: open-label extension study of leniolisib for patients with APDS. J Allergy Clin Immunol. 2024;153(1):265-e2749. 10.1016/j.jaci.2023.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeong JS, Kim JS, Kim SR, Lee YC. Defining bronchial asthma with phosphoinositide 3-Kinase delta activation: towards endotype-driven management. Int J Mol Sci. 2019;20(14): 3525. 10.3390/ijms20143525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carpier JM, Lucas CL. Epstein-barr virus susceptibility in activated PI3Kδ syndrome (APDS) immunodeficiency. Front Immunol. 2017;8: 2005. 10.3389/fimmu.2017.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JI. Herpesviruses in the activated phosphatidylinositol-3-Kinase-δ syndrome. Front Immunol. 2018;9:9. 10.3389/fimmu.2018.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herber M, Mertz P, Dieudonné Y, et al. Primary immunodeficiencies and lymphoma: a systematic review of literature. Leuk Lymphoma. 2020;61(2):274–84. 10.1080/10428194.2019.1672056. [DOI] [PubMed] [Google Scholar]
- 41.Sheng H, Shao J, Townsend CM, Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52(10):1472–8. 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: epigenetics and beyond. Life Sci. 2020;262: 118513. 10.1016/j.lfs.2020.118513. [DOI] [PubMed] [Google Scholar]
- 43.Morgos DT, Stefani C, Miricescu D, et al. Targeting PI3K/AKT/mTOR and MAPK signaling pathways in gastric cancer. Int J Mol Sci. 2024;25(3): 1848. 10.3390/ijms25031848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komiya T, Blumenthal GM, DeChowdhury R, et al. A pilot study of sirolimus in subjects with Cowden syndrome or other syndromes characterized by germline mutations in PTEN. Oncologist. 2019;24(12):1510-e1265. 10.1634/theoncologist.2019-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimitrova D, Nademi Z, Maccari ME, et al. International retrospective study of allogeneic hematopoietic cell transplantation for activated PI3K-delta syndrome. J Allergy Clin Immunol. 2022;149(1):410-e4217. 10.1016/j.jaci.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bravo García-Morato M, García-Miñaúr S, Molina Garicano J, et al. Mutations in PIK3R1 can lead to APDS2, SHORT syndrome or a combination of the two. Clin Immunol Orlando Fla. 2017;179:77–80. 10.1016/j.clim.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Moreno-Corona N, Chentout L, Poggi L, et al. Two monogenetic disorders, activated PI3-Kinase-δ syndrome 2 and Smith–Magenis syndrome, in one patient: case report and a literature review of neurodevelopmental impact in primary immunodeficiencies associated with disturbed PI3K signaling. Front Pediatr. 2021;9. 10.3389/fped.2021.688022. [DOI] [PMC free article] [PubMed]
- 48.Sánchez-Alegría K, Flores-León M, Avila-Muñoz E, Rodríguez-Corona N, Arias C. PI3K signaling in neurons: a Central Node for the control of multiple functions. Int J Mol Sci. 2018;19(12):3725. 10.3390/ijms19123725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eickholt BJ, Ahmed AI, Davies M, et al. Control of axonal growth and regeneration of sensory neurons by the p110delta PI 3-kinase. PLoS ONE. 2007;2(9):e869. 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chu E, Mychasiuk R, Hibbs ML, Semple BD. Dysregulated phosphoinositide 3-kinase signaling in microglia: shaping chronic neuroinflammation. J Neuroinflammation. 2021;18(1):276. 10.1186/s12974-021-02325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Law AJ, Wang Y, Sei Y, et al. Neuregulin 1-ErbB4-PI3K signaling in schizophrenia and phosphoinositide 3-kinase-p110δ inhibition as a potential therapeutic strategy. Proc Natl Acad Sci U S A. 2012;109(30):12165–70. 10.1073/pnas.1206118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spinelli L, Black FM, Berg JN, Eickholt BJ, Leslie NR. Functionally distinct groups of inherited PTEN mutations in autism and tumour syndromes. J Med Genet. 2015;52(2):128–34. 10.1136/jmedgenet-2014-102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Begg M, Amour A, Jarvis E, et al. An open label trial of nemiralisib, an inhaled PI3 kinase delta inhibitor for the treatment of activated PI3 kinase delta syndrome. Pulm Pharmacol Ther. 2023;79: 102201. 10.1016/j.pupt.2023.102201. [DOI] [PubMed] [Google Scholar]
- 54.Hanson J, Bonnen PE. Systematic review of mortality and survival rates for APDS. Clin Exp Med. 2024;24(1):17. 10.1007/s10238-023-01259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28.0 KB)
(DOCX 20.0 KB)
(DOCX 25.4 KB)
(DOCX 16.3 KB)
Data Availability Statement
No datasets were generated or analysed during the current study.