Abstract
Background
Intraductal papillary neoplasms (IPNs) often have a similar clinical and imaging presentation, making them difficult to diagnose. We designed this study to refine and compare intraductal papillary neoplasms' clinical and imaging characteristics.
Methods
This study included a total of 154 patients with a postoperative diagnosis of IPNs and collected their clinical, imaging, and pathological data. We compared the clinical and imaging characteristics of benign, atypical hyperplasia, and malignant lesions. We also compared the diagnostic efficacy of ultrasound and mammography.
Results
The mean age of malignant patients was 57 years old, which was significantly higher than that in the other groups (48 years in the benign group and 47 years in the atypical hyperplasia group). The proportion of patients with malignant lesions clinically presenting as palpable masses (31.3%) was significantly higher than that of benign lesions (8.6%) (P < 0.05). The proportion of malignant lesions presenting in the periphery (≥ 3 cm from the nipple) was 40.6% compared to 22.4% for benign (P < 0.05). In ultrasonography, characteristics that showed statistically significant differences between benign and malignant lesions were the shape of the mass and calcification (P < 0.05). On mammography, differences were found in mass shape, calcification, and density of masses and glands (P < 0.05).
Conclusions
Clinical features such as age, symptoms, lesion location, and imaging characteristics such as shape, calcification, mass, and density may help to differentiate the classifications of IPNs.
Trial registration: This study was registered at ClinicalTrials.gov on 12/06/2020 (identifier: NCT04429269).
Keywords: Breast, Intraductal papillary neoplasms (IPNs), Clinical characteristics, Imaging characteristics
Background
Intraductal papillary neoplasms (IPNs) are a group of difficult-to-diagnose heterogeneous breast lesions that include a wide range of pathologic characteristics [1, 2]. They can occur anywhere in the ductal system and are broadly classified into central and peripheral types [3] IPNs include various categories such as benign intraductal papilloma, intraductal papilloma with atypical hyperplasia, intraductal papillary carcinoma, and intraductal carcinoma with or without infiltration [2, 4, 5]. The malignancy rate of intraductal papilloma is 5%-12% [6, 7]. Intraductal carcinoma accounts for 12–15% of new breast cancer diagnoses in the United States each year [8]. Intraductal papillomas are relatively common tumors with an incidence of 2–3% [3]. Pathologically, the main manifestation is a highly vascular tumor, with a distinct vascular tip found at the center of the intraductal papillary neoplasm [3]. In other words, there are dendritic vessels within the mass. Therefore, IPNs usually tend to bleed spontaneously, and patients often present clinically with bloody papillary discharge. However, some patients may be asymptomatic.
Because of the similarity of different categories of IPNs clinically, imaging, and histologically, the final diagnosis of the lesion becomes a challenge [9, 10]. Secondly, the risk of sampling error on core-needle biopsy can be up to 25% [11]. For example, benign results on core-needle biopsy do not completely exclude the possibility of malignancy as the lesion may be a focal carcinoma in situ [11]. It has also been shown that there is an increased risk of upgrading the lesion to malignancy after a core-needle biopsy [12, 13]. Thus, it is necessary to differentiate between benign and malignant neoplasms based on clinical or imaging characteristics. A small number of articles have been published on the imaging presentation of IPNs [12, 14, 15]. Our study compared clinical signs, ultrasound, and mammography among different classifications of IPNs and preliminarily analyzed whether different imaging presentations could help to specifically identify the classifications of lesions.
Methods
Patient selection
We recruited 2737 consecutive Chinese women aged 35 to 70 years between November 2019 and November 2021, recruited based on at least one suspicious breast lesion detected by clinical examination or imaging and the final decision to undergo complete surgical excision of the lesions for pathological biopsy at our institution. All participants underwent standard mammography and ultrasound within 3 months prior to surgery, and all images were independently and dually interpreted by two experienced doctors who were unaware of the results of the other modalities. A total of 2844 breast lesions were identified and biopsied out of 2737 participants. This study included all patients with a postoperative diagnosis of IPNs and ultimately identified a total of 154 patients with 160 lesions. We collected breast imaging findings, pathological findings, clinical characteristics, and surgical records from these patients. Each case report was carefully reviewed and the location of the intraductal papillary lesions identified in the pathology report was compared with the imaging manifestations and surgical records to determine the exact location of the lesions. This was done by a clinician, a pathologist and two radiologists. Based on the pathological results, we finally classified the included cases into the groups of intraductal papillary lesions, intraductal papillary lesions with atypical hyperplasia and malignant lesions. We also collected prospective data on patients' age, body mass index (BMI), lesion location and clinical symptoms (including nipple discharge, palpable masses and pain). A distance of 3 cm from the nipple was used as the cut-off value between central and peripheral lesions.
Imaging analysis
Based on the location of the identified lesions, we independently evaluated the test images of both detection methods based on the 2013 version of the Breast Imaging Reporting and Data System (BI-RADS) [16]. Each lesion was diagnosed independently by two radiologists with more than 5 years of experience.
Breast ultrasound examination was performed with color Doppler with a maximum frequency of at least 12 MHz and a high-resolution transducer for bilateral breast scans in the transverse and the sagittal planes. Ultrasound findings were classified as masses, non-masses and negative findings. In lesions presenting as masses, we recorded the shape (round/oval, lobular or irregular), margins (circumscribed, not circumscribed), and echo pattern (hypoechoic, isoechoic, hyperechoic and mixed echogenic) of the masses. For all breast lesions found, we also evaluated characteristics such as posterior echogenic features (absent, enhancement or shadowing), whether the ducts were dilated, presence of vascularity and calcifications.
We performed dual-view mammography using a GE digital mammography machine. The visibility of lesions, lesion type, density, structural distortion, and calcification, as well as mass shape, margins, and glandular density were assessed by the American College of Radiology (ACR) BI-RADS [16].
Statistical analysis
One-way ANOVA was used to compare continuous variables, such as patient age and BMI, between the three groups. Chi-square tests or Fisher exact tests were used to assess the statistical significance of categorical variables, including clinical presentation and imaging characteristics. Finally, the McNemar test using paired binary data was used for statistical comparison and exact p-value calculation for detection rates. The area under the receiver operating characteristic curve (AUC) was plotted to compare the diagnostic accuracy of ultrasound with that of mammography. Pairwise comparison of AUC was performed using Medcalc according to De Long et al. [17]. All statistical analyses were performed using SPSS Statistics (IBM Corp., Armonk, NY, USA) with R version 26.0.0.0 (The R Project, Vienna, Austria). Reported P values and 95% confidence intervals are two-sided, and a 0.05 threshold was used for statistical significance assessment.
Results
Demographics and clinical characteristics
Of the 154 female patients included in the study, 160 study lesions were detected, including 6 patients with bilateral lesions. Of the total 160 breast events, 32 (20%) were malignant breast cancers, 12 (7.5%) presented with atypical hyperplasia, and 116 (72.5%) presented with benign intraductal papillomas. Table 1 lists the demographic data of the included participants, including the age and BMI of the participants, and the clinical presentation including symptoms and signs (Table 1). The mean age in the malignant group was 58 years, which was higher than the 48 years in the benign group and the 47 years in the atypical hyperplasia group, with a statistically significant difference (P < 0.001). In the benign intraductal papilloma group, the clinical presentation was asymptomatic in 61 cases (52.6%), nipple discharge in 41 cases (35.3%), palpable lesion of the breast in 10 cases (8.6%), and pain in the breast area in 4 cases (3.4%). In contrast, in the malignant group, there were 17 cases (53.1%) with asymptomatic manifestations, 5 cases (15.6%) with nipple discharge and 10 cases (31.3%) with palpable lesion, and the difference between the two groups was statistically significant (P < 0.05). As shown in Table 1, there were 90 cases (77.6%) of central lesions and 26 cases (22.4%) of peripheral lesions in the benign group. In contrast, there were 19 cases (59.4%) of central lesions and 13 cases (40.6%) of peripheral lesions in the malignant group, with a significant difference between the two groups (P < 0.05).
Table 1.
Characteristics of patients with intraductal papillary neoplasms of breasts
| Benign (n = 116) | Atypical (n = 12) | Malignant (n = 32) | P | |
|---|---|---|---|---|
| Age, years | < 0.001 | |||
| Mean ± s.d | 47.74 ± 9.11 | 47.08 ± 10.72 | 57.72 ± 11.35 | |
| Body mass index, kg/m2 | 0.459 | |||
| Mean ± s.d | 23.26 ± 3.18 | 24.45 ± 2.02 | 23.35 ± 3.05 | |
| Symptoms (%) | 0.005* | |||
| Asymptomatic | 61 (52.6) | 7 (58.3) | 17(53.1) | |
| Nipple discharge | 41 (35.3) | 3 (25.0) | 5(15.6) | |
| Palpable lesion | 10 (8.6) | 2 (1.7) | 10(31.3) | |
| Breast pain | 4 (3.4) | 0 (0) | 0 (0) | |
| Lesion location (%) | 0.044* | |||
| Central (< 3 cm from the nipple) | 90 (77.6) | 6 (50.0) | 19 (59.4) | |
| Peripheral (≥ 3 cm from the nipple) | 26 (22.4) | 6 (50.0) | 13 (40.6) | |
Data are given as means ± standard deviations or number of lesions (% of each pathology subgroups). *P-value < 0.05 obtained by analyzing benign vs malignant
Ultrasound features of intraductal papillary lesions
Ultrasound can identify three main structures of IPNs, intracapsular masses (Fig. 1a), intraductal masses with or without ductal dilatation (Fig. 2a), and solid masses that manifest after the masses have completely filled the ducts (Fig. 3a). Ultrasound can also identify partial lesions that present only as calcifications (Fig. 4a). Table 2 summarizes the ultrasound features of intraductal papillary lesions. Three of these characteristics were statistically different (P < 0.05) in the comparison between the benign and malignant groups, namely mass shape and calcification. In benign masses, 48.6% showed a regular shape such as oval or round, while only 14.8% of malignant masses were likely to have a regular morphology. Malignant lesions had a significantly higher proportion of both lobulated and irregular morphology compared to benign lesions (22.2% vs 7.6%, 63.0% vs 43.8%) (P < 0.05). In addition, we found that calcification was detectable on ultrasound in 40.6% of malignant lesions, while only 12.9% of benign lesions were likely to contain calcification, with a significant difference (p = 0.001). In contrast, the nonsignificant features of ultrasonography included lesion type, margin, mass echogenicity versus posterior echogenicity, ductal dilatation, and vascularity signal (Table 2).
Fig. 1.
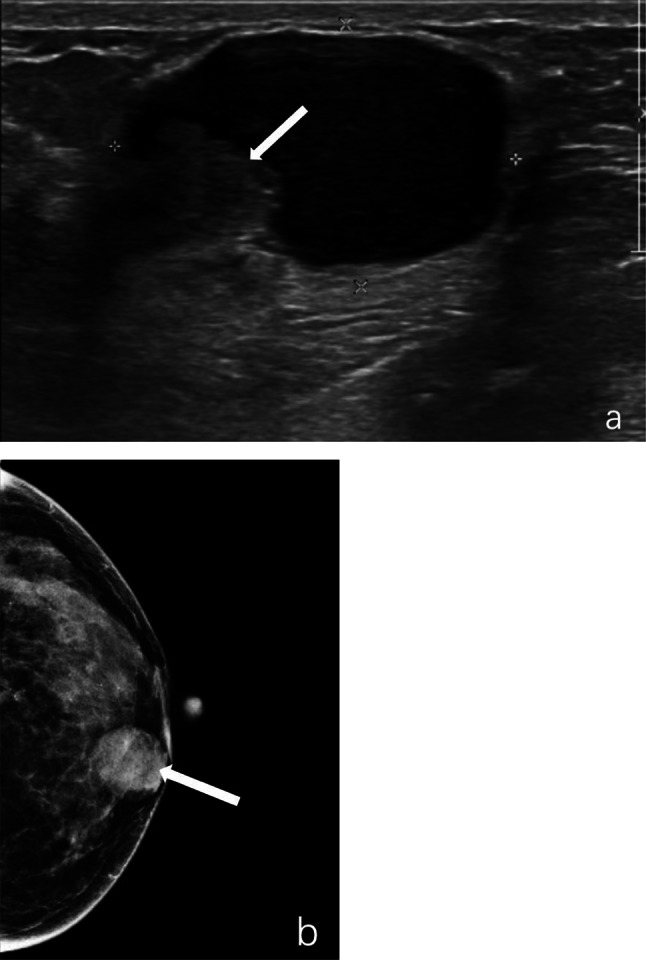
Intraductal papilloma with hemorrhage of the left breast in a 50 year-old woman. a, Ultrasound revealed a 3.1 × 2.0 cm complex echogenic mass with circumscribed margin and a 1.2 × 0.9 cm hypoechoic mass visible in the wall of it (marked by arrow). b, A high-density mass of approximately 2.5 cm in diameter with circumscribed margin and round shape was seen in the inner lower quadrant on mammography (marked by arrow)
Fig. 2.
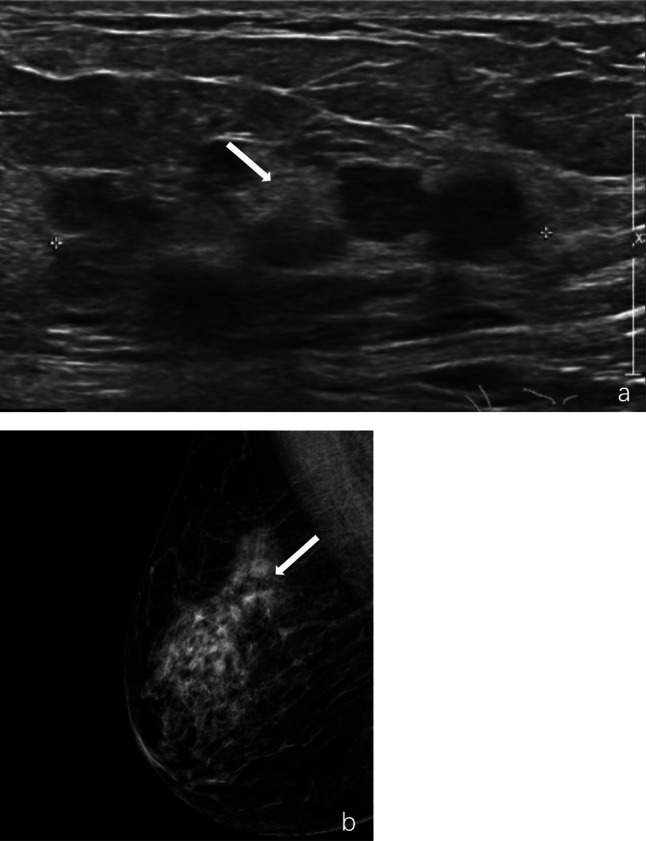
Intraductal papillary carcinoma of the right breast in a 68 year-old woman. a, Ultrasound revealed a complex echogenic mass of approximately 5.3 × 2.3 cm in extent at 9–10 o’clock with multiple ductal dilatations and multiple nodules of variable size with indistinct margin (marked by arrow). b, Mammography showed a high-density mass of approximately 3.5 × 1.3 cm in the outer upper quadrant with indistinct margin and irregular morphology (marked by arrow)
Fig. 3.
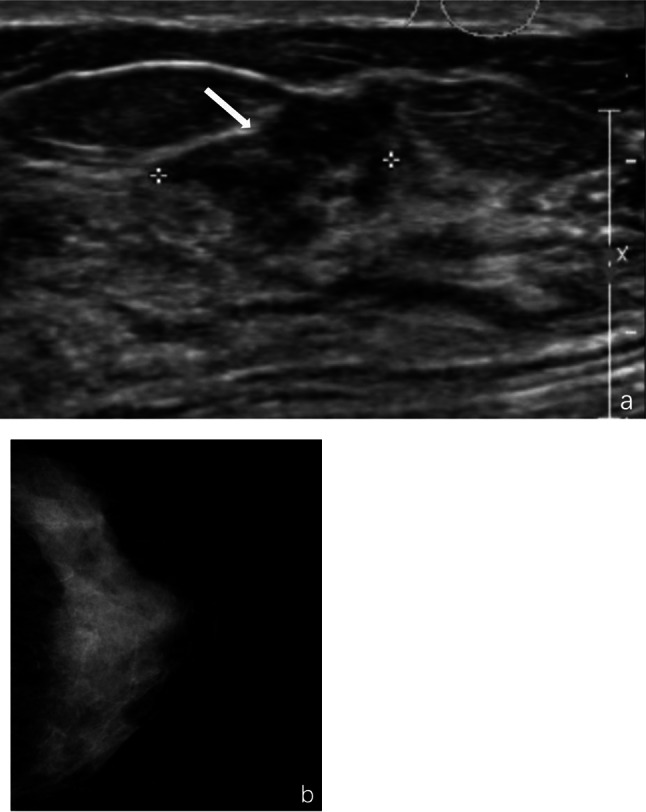
Intraductal carcinoma with micro-infiltration of the left breast in a 66 year-old woman. a, Ultrasound revealed a hypoechoic mass at 2 o'clock in the areola area, measuring approximately 1.5 × 1.3 × 0.9 cm, with irregular morphology and indistinct margin (marked by arrow). b, Mammography showed uniform breast density and clear subcutaneous tissue structure, with no significant positive findings
Fig. 4.
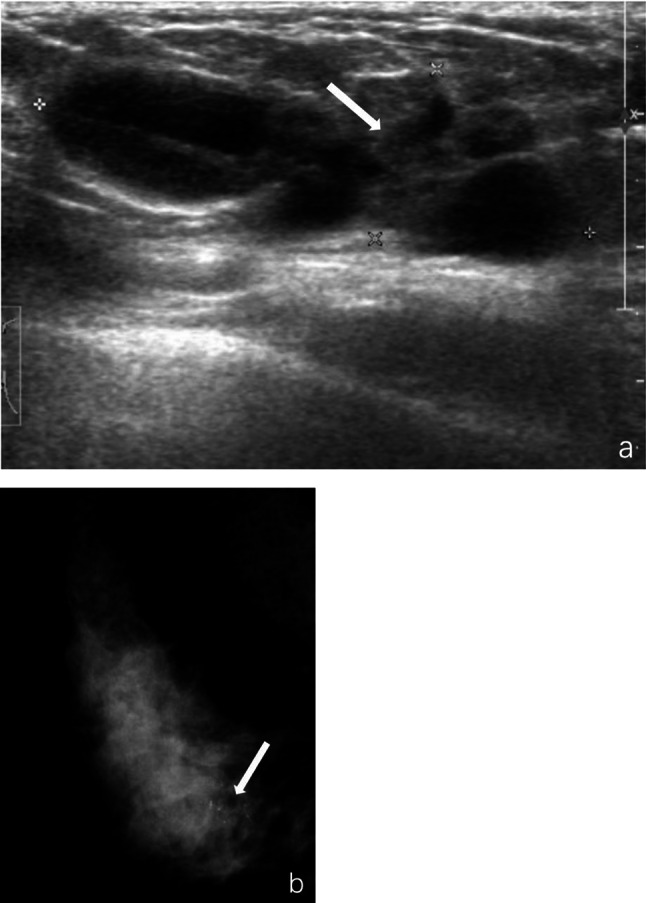
Intraductal papilloma of the right breast in a 47 year-old woman. a, Ultrasound revealed a complex echogenic mass at 5 o'clock with a size of about 4.0 × 1.8 × 1.4 cm, irregular morphology, lobulated, with circumscribed margin. Spotty, strongly echogenic calcifications in the mass were faintly visible (marked by arrow). b, Clusters of calcifications in the lower quadrant of the breast were seen on mammography (marked by arrow)
Table 2.
Ultrasonic features of intraductal papillary neoplasms
| Benign (n = 116) | Atypical (n = 12) | Malignant (n = 32) | P | |
|---|---|---|---|---|
| Lesion type (%) | 0.176 | |||
| Mass | 105 (90.5) | 11 (91.7) | 27 (84.4) | |
| Non-mass | 10 (8.6) | 0 (0) | 5 (15.6) | |
| Negative findings | 1 (0.9) | 1 (8.3) | 0 (0) | |
| Shape of mass (%) | 0.003* | |||
| Round/Oval | 51 (48.6) | 4 (36.4) | 4 (14.8) | |
| Lobular | 8 (7.6) | 1 (9.1) | 6 (22.2) | |
| Irregular | 46 (43.8) | 6 (54.5) | 17 (63.0) | |
| Margin of mass (%) | 0.165 | |||
| Circumscribed | 58 (55.2) | 4 (36.4) | 10 (37.0) | |
| Not circumscribed | 47 (44.8) | 7 (63.6) | 17 (63.0) | |
| Echo pattern of mass (%) | 0.573 | |||
| Hypo-echoic | 84 (80.0) | 10 (90.9) | 26 (96.3) | |
| Hyper-echoic | 2 (1.9) | 0 (0) | 0 (0) | |
| Iso-echoic | 2 (1.9) | 0 (0) | 0 (0) | |
| Complex | 17 (16.2) | 1 (9.1) | 1 (3.7) | |
| Posterior features of lesion (%) | 0.195 | |||
| Absent | 112 (97.4) | 10 (90.9) | 31 (96.9) | |
| Enhancement | 0 (0) | 0 (0) | 1 (3.1) | |
| Shadowing | 3 (2.6) | 1 (9.1) | 0 (0) | |
| Duct dilatation of lesion (%) | 0.828 | |||
| Absent | 49 (42.6) | 5 (45.5) | 15 (46.9) | |
| Present | 66 (57.4) | 6 (54.5) | 17 (53.1) | |
| Vascularity of lesion (%) | 0.083 | |||
| Absent | 34 (29.6) | 4 (36.4) | 4 (12.5) | |
| Present | 81 (70.4) | 7 (63.6) | 28 (87.5) | |
| Calcifications (%) | 0.001* | |||
| Absent | 101 (87.1) | 9 (75.0) | 19 (59.4) | |
| Present | 15 (12.9) | 3 (25.0) | 13 (40.6) | |
*P-value < 0.05 obtained by analyzing benign vs malignant
Mammographic features of intraductal papillary lesions
Most large cystic or solid masses may be visualized on mammography (Fig. 1b, Fig. 2b), however, when the mass is small, the most common presentation on mammography is asymmetric increase in glandular density, or even negative finding (Fig. 3b). In addition, mammography has great ability to identify lesions only present as calcifications (Fig. 4b). As shown in Table 3, mammography showed significant differences in findings of mass shape, lesion density, calcification and glandular density. Fifty percent of the malignant masses also showed an irregular pattern on mammography, significantly higher than the 11.6% of benign ones (P < 0.05). Only 13% of malignant lesions were equal density, however the percentage of benign lesions that appeared equal or low density was 27.8% (P < 0.05). The proportion of malignant lesions found to be calcified on mammography was 33.3%, significantly higher than the 15.5% of benign lesions (P < 0.05). In breast density, 28.1% of malignant lesions were found in non-dense breasts, significantly higher than that of benign lesions, which was 4.3% (P < 0.001). The remaining features demonstrated no significant differences between the groups (Table 3).
Table 3.
Mammographic features of intraductal papillary neoplasms
| Benign (n = 116) | Atypical (n = 12) | Malignant (n = 32) | P | |
|---|---|---|---|---|
| Visibility (%) | 0.475 | |||
| Visible | 82 (70.7) | 8 (66.7) | 26 (81.25) | |
| Invisible | 34 (29.3) | 4 (33.3) | 6 (18.75) | |
| Lesion type (visible) (%) | 0.232 | |||
| Mass | 43 (52.4) | 3 (37.5) | 10 (38.5) | |
| (Focal) asymmetry | 29 (35.4) | 2 (25.0) | 13 (50.0) | |
| Calcification only | 10 (12.2) | 3 (37.5) | 3 (11.5) | |
| Shape of mass (%) | 0.003* | |||
| Round/Oval | 38 (88.4) | 2 (66.7) | 4 (40.0) | |
| Lobular | 0 (0) | 0 (0) | 1 (10.0) | |
| Irregular | 5 (11.6) | 1 (33.3) | 5 (50.0) | |
| Margin of mass (%) | 0.174 | |||
| Circumscribed | 24 (55.8) | 0 (0) | 4 (40.0) | |
| Not circumscribed | 19 (44.2) | 3 (100.0) | 6 (60.0) | |
| Lesion density (%) | 0.025 | |||
| Low density | 2 (2.8) | 2 (40.0) | 0 (0) | |
| Equal density | 18 (25.0) | 1 (20.0) | 3 (13.0) | |
| High density | 52 (72.2) | 2 (40.0) | 20 (87.0) | |
| Structural distortion (%) | 0.321 | |||
| Absent | 109 (94.0) | 11 (91.7) | 28 (87.5) | |
| Present | 7 (6.0) | 1 (8.3) | 4 (12.5) | |
| Calcification (%) | 0.024* | |||
| Absent | 98 (84.5) | 8 (66.7) | 21 (63.6) | |
| Present | 18 (15.5) | 4 (33.3) | 11 (33.3) | |
| Density (%) | < 0.001* | |||
| Non-dense breast (a–b) | 5 (4.3) | 0 (0) | 9 (28.1) | |
| Dense breast (c–d) | 111 (95.7) | 12 (100.0) | 23 (71.9) | |
*P-value < 0.05 obtained by analyzing benign vs malignant
Comparison of the detection results between the two modalities
The results of BI-RADS for IPNs in the breast on both ultrasound and mammography modalities have been summarized in Table 4. For benign lesions, the percentage of ultrasound grade 4a and below was 77.6% and the percentage of mammography grade 4a and below was 84.5%. For malignant lesions, the proportion of ultrasound grade 4a and below was 34.4% and grade 4b and above was 65.7%; the proportion of mammography grade 4a and below was 53.2% and grade 4b and above was 46.9%. Table 5 shows the detection rate and accuracy results for both modalities. We defined lesions with BI-RADS results of 4a and above as detected lesions. A total of 141 lesions were detected by ultrasound while the number of lesions detected by mammography was 73. The detection rate of IPNs by ultrasound was 88.1%, which was significantly higher than that of mammography at 45.6% (P < 0.001). However, the diagnostic accuracy of both modalities was comparable and the difference was not statistically significant.
Table 4.
BI-RADS categories of intraductal papillary neoplasms on ultrasound and mammography
| BI-RADS | Ultrasound (%) | Mammography (%) | ||||
|---|---|---|---|---|---|---|
| Benign (n = 116) | Atypical (n = 12) | Malignant (n = 32) | Benign (n = 116) | Atypical (n = 12) | Malignant (n = 32) | |
| 1 | 1 (0.9) | 1 (8.3) | 0 (0) | 10 (8.6) | 1 (8.3) | 0 (0) |
| 2 | 0 (0) | 0 (0) | 0 (0) | 25 (21.6) | 5 (41.7) | 4 (12.5) |
| 3 | 11 (9.5) | 3 (25.0) | 3 (9.4) | 39 (33.6) | 2 (16.7) | 3 (9.4) |
| 4a | 78 (67.2) | 5 (41.7) | 8 (25.0) | 24 (20.7) | 1 (8.3) | 10 (31.3) |
| 4b | 21 (18.1) | 2 (1.7) | 14 (43.8) | 19 (16.4) | 3 (25.0) | 11 (34.4) |
| 4c | 5 (4.3) | 1 (8.3) | 6 (18.8) | 1 (0.9) | 0 (0) | 4 (12.5) |
| 5 | 0 (0) | 0 (0) | 1 (3.1) | 0 (0) | 0 (0) | 0 (0) |
Table 5.
Comparison of the detection rate and diagnostic accuracy between ultrasound and mammography
| Ultrasound | Mammography | P | |
|---|---|---|---|
| Detection rate | < 0.001 | ||
| No./total | 141/160 | 73/160 | |
| % (95%CI) | 88.1 (81.8–92.5) | 45.6 (37.8–53.7) | |
| Diagnostic accuracy (AUC) | 0.789 | ||
| Value | 0.711 | 0.729 | |
| % (95%CI) | 71.1(63.4–78.0) | 72.9(65.3–79.6) | |
CI confidence interval, AUC area under the receiver operating characteristic curve
Discussion
Because IPNs have a certain rate of malignancy, patients are often clinically advised to undergo surgical resection to prevent the formation of malignant tumors [18, 19]. This can often lead to overtreatment, making the use of appropriate modalities to identify malignant tumors particularly important [4]. As reported in previous studies, we found that patients with malignant tumors were nearly 10 years older than patients with benign or atypical hyperplasia of IPNs [12, 15]. We also found a higher rate of malignant escalation in patients older than 50 years, which is the same as the findings of Youk et al. [13] And unlike the results of Kuzmiak et al. [15] study, there was no significant difference in the mean age between patients with high-risk lesions (atypical hyperplasia) and benign lesions in our study. In terms of clinical symptoms, almost all types of IPNs were more than 50% more likely to be asymptomatic, with malignant IPNs more likely to present as palpable masses and benign IPNs more likely to present as nipple discharge and pain. In addition, as in previous studies, we also found that malignant lesions were more likely to be found in the peripheral areas of the breast, while benign lesions were more likely to be found in the central region of the breast [4]. The probability of malignancy in peripheral papillary lesions has been reported to be 10–30% [3]. In our study 13 of 45 cases of peripheral IPNs were malignant, which is consistent with a malignancy rate of 28.9%.
The results of previous studies have shown that there is an overlap in the imaging characteristics of different types of IPNs [12, 14, 20]. Therefore, we evaluated the findings on ultrasound and mammography to assess the presence of characteristics that could distinguish benign, high-risk (atypical hyperplasia), and malignant lesions. The results revealed significant differences in the imaging results of lesions of different natures between the two examination modalities. On ultrasound, irregular shape and the presence of calcifications suggested a malignant tendency of the lesion. Kim et al. [12] found that 76.4% of benign papillary lesions and 57.1% of malignant lesions had circumscribed margins and the difference was not statistically significant. Similarly, our study found that unlike the imaging presentation of invasive breast cancer, the presence or absence of circumscribed margins in the lesions did not significantly differentiate between benign and malignant IPNs. In addition, because of the presence of dendritic vessels in IPNs, abundant blood flow signals may also be seen in benign IPNs [11, 21]. Our findings suggest that ultrasound and mammography have similar findings in terms of mass shape and calcification. We also found that 29.3% of benign lesions were not visible on mammography and that hypodense and isodense lesions accounted for 27.8% of the visible benign lesions. Malignant lesions tended to be denser than benign lesions (87% vs. 72.2%). This may be related to the density of the breast itself, as we found a significantly higher proportion of patients with dense mammary glands (c–d) in the benign group than in the malignant group (p < 0.001), as in the study by Woods et al [22]. Dense mammary glands may mask the presence of a lesion, and the mass is more likely to appear dense in non-dense mammary glands [15]. On mammography, some IPNs may show only an asymmetric increase in density or appear only negative, making them difficult to identify. Because IPNs are located in the ducts, they are difficult to detect if they are small and if they lack calcifications or fibrosis [23]. For both ultrasound and mammography, we found that ultrasound has a higher detection rate for IPNs and is more suitable for the early diagnosis of IPNs, which is consistent with the findings of Brookes et al. [24].
Admittedly, this study had some limitations as the incidence of IPNs is lower than that of invasive breast cancer, and the sample size included is relatively small, especially the number of atypical groups is so small that the difference between it and the other two groups is not significant. The sample size could be increased by extending the statistical year appropriately in the future. Besides, the patients included in this study were all from our hospital, which may not cover all institutions.
Conclusions
Clinical symptoms and analysis of ultrasound and mammography findings can be recommended for patients with suspected IPNs, and some clinical and imaging characteristics can be used to initially identify malignant and benign IPNs. Clinical features such as age, symptoms, and lesion site of the patients in this study, the shape and calcification of the masses on ultrasound and mammography, and the density of the masses and breasts on mammography can help in the characterization of IPNs. Ultrasound had a significantly higher detection rate than mammography for IPNs and could be used for the initial diagnosis, but there was no difference in the accuracy of diagnosis between the two.
Author contributions
Conception and design: YW, SS Collection and assembly of data: YW, YL, YQ, YZ, QS, SS Data analysis and interpretation: YW, YL, YQ, YZ, QS, SS Manuscript writing: YW, SS Final approval of manuscript: YW, YL, YQ, YZ, QS, SS Accountable for all aspects of the work: YW, YL, YQ, YZ, QS, SS.
Funding
This study was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019XK320033), National Natural Science Foundation of China (52173149), Beijing Natural Science Foundation (7222129), and National High Level Hospital Clinical Research Funding (2022-PUMCH-B-038).
Data availability
Data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was performed by the Declaration of Helsinki and was approved by the institutional review board of Peking Union Medical College Hospital (JS-2367). Prior to enrollment, all participants provided written informed consent for participation in the study and the subsequent publication of its results.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu QL, Zhang J, Lai XJ, Wang HY, Xiao MS, Jiang YX. Characterisation of breast papillary neoplasm on automated breast ultrasound. Br J Radiol. 2013;86(1029):20130215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fadzli F, Rahmat K, Ramli MT, et al. Spectrum of imaging findings of papillary breast disease: A radiopathological review in a tertiary center. Medicine. 2021;100(16): e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganesan S, Karthik G, Joshi M, Damodaran V. Ultrasound spectrum in intraductal papillary neoplasms of breast. Br J Radiol. 2006;79(946):843–9. [DOI] [PubMed] [Google Scholar]
- 4.Choi SH, Jo S, Kim DH, et al. Clinical and imaging characteristics of papillary neoplasms of the breast associated with malignancy: a retrospective cohort study. Ultrasound Med Biol. 2014;40(11):2599–608. [DOI] [PubMed] [Google Scholar]
- 5.Ueng SH, Mezzetti T, Tavassoli FA. Papillary neoplasms of the breast: a review. Arch Pathol Lab Med. 2009;133(6):893–907. [DOI] [PubMed] [Google Scholar]
- 6.Gu M, Yan J, Shi K. Overview of intraductal papilloma of the breast. Chin Practical J Rural Doctor. 2020;27(11):17–8. [Google Scholar]
- 7.Wen W, Du Y, Huang H, Chen L. A report of 103 cases of breast intraductal papillomatosis (BIDP). Med J Chin PLA. 2005;30(1):75–6. [Google Scholar]
- 8.Su X, Lin Q, Cui C, et al. Non-calcified ductal carcinoma in situ of the breast: comparison of diagnostic accuracy of digital breast tomosynthesis, digital mammography, and ultrasonography. Breast Cancer. 2017;24(4):562–70. [DOI] [PubMed] [Google Scholar]
- 9.Bode MK, Rissanen T, Apaja-Sarkkinen M. Ultrasonography-guided core needle biopsy in differential diagnosis of papillary breast tumors. Acta Radiol. 2009;50(7):722–9. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo M, Lund MJ, Oprea G, Schniederjan M, Wood WC, Mosunjac M. Surgical follow-up and clinical presentation of 142 breast papillary lesions diagnosed by ultrasound-guided core-needle biopsy. Ann Surg Oncol. 2008;15(4):1040–7. [DOI] [PubMed] [Google Scholar]
- 11.Collins LC, Schnitt SJ. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology. 2008;52(1):20–9. [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Kang DK, Kim SY, Lee EJ, Jung YS, Yim H. Sonographic differentiation of benign and malignant papillary lesions of the breast. J Ultrasound Med. 2008;27(1):75–82. [DOI] [PubMed] [Google Scholar]
- 13.Youk JH, Kim EK, Kwak JY, Son EJ, Park BW, Kim SI. Benign papilloma without atypia diagnosed at US-guided 14-gauge core-needle biopsy: clinical and US features predictive of upgrade to malignancy. Radiology. 2011;258(1):81–8. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Kim HH, Kim SM, et al. Papillary lesions of the breast diagnosed at percutaneous sonographically guided biopsy: comparison of sonographic features and biopsy methods. AJR Am J Roentgenol. 2008;190(3):630–6. [DOI] [PubMed] [Google Scholar]
- 15.Kuzmiak CM, Lewis MQ, Zeng D, Liu X. Role of sonography in the differentiation of benign, high-risk, and malignant papillary lesions of the breast. J Ultrasound Med. 2014;33(9):1545–52. [DOI] [PubMed] [Google Scholar]
- 16.American College of Radiology BIRC. ACR BI-RADS atlas : breast imaging reporting and data system. 5th ed. Reston, VA: American College of Radiology. 2013.
- 17.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 18.Mercado CL, Hamele-Bena D, Oken SM, Singer CI, Cangiarella J. Papillary lesions of the breast at percutaneous core-needle biopsy. Radiology. 2006;238(3):801–8. [DOI] [PubMed] [Google Scholar]
- 19.Sydnor MK, Wilson JD, Hijaz TA, Massey HD, Shaw de Paredes ES. Underestimation of the presence of breast carcinoma in papillary lesions initially diagnosed at core-needle biopsy. Radiology. 2007;242(1):58–62. [DOI] [PubMed] [Google Scholar]
- 20.Skandarajah AR, Field L, Yuen Larn Mou A, et al. Benign papilloma on core biopsy requires surgical excision. Ann Surg Oncol. 2008;15(8):2272–7. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi S, Bendinelli B, Saladino V, et al. Non-malignant breast papillary lesions - b3 diagnosed on ultrasound–guided 14-gauge needle core biopsy: analysis of 114 cases from a single institution and review of the literature. Pathol Oncol Res. 2015;21(3):535–46. [DOI] [PubMed] [Google Scholar]
- 22.Woods RW, Sisney GS, Salkowski LR, Shinki K, Lin Y, Burnside ES. The mammographic density of a mass is a significant predictor of breast cancer. Radiology. 2011;258(2):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang WT, Suen M, Metreweli C. Sonographic features of benign papillary neoplasms of the breast: review of 22 patients. J Ultrasound Med. 1997;16(3):161–8. [DOI] [PubMed] [Google Scholar]
- 24.Brookes MJ, Bourke AG. Radiological appearances of papillary breast lesions. Clin Radiol. 2008;63(11):1265–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.


