Abstract
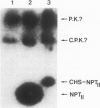
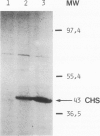
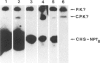
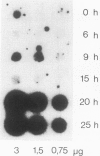
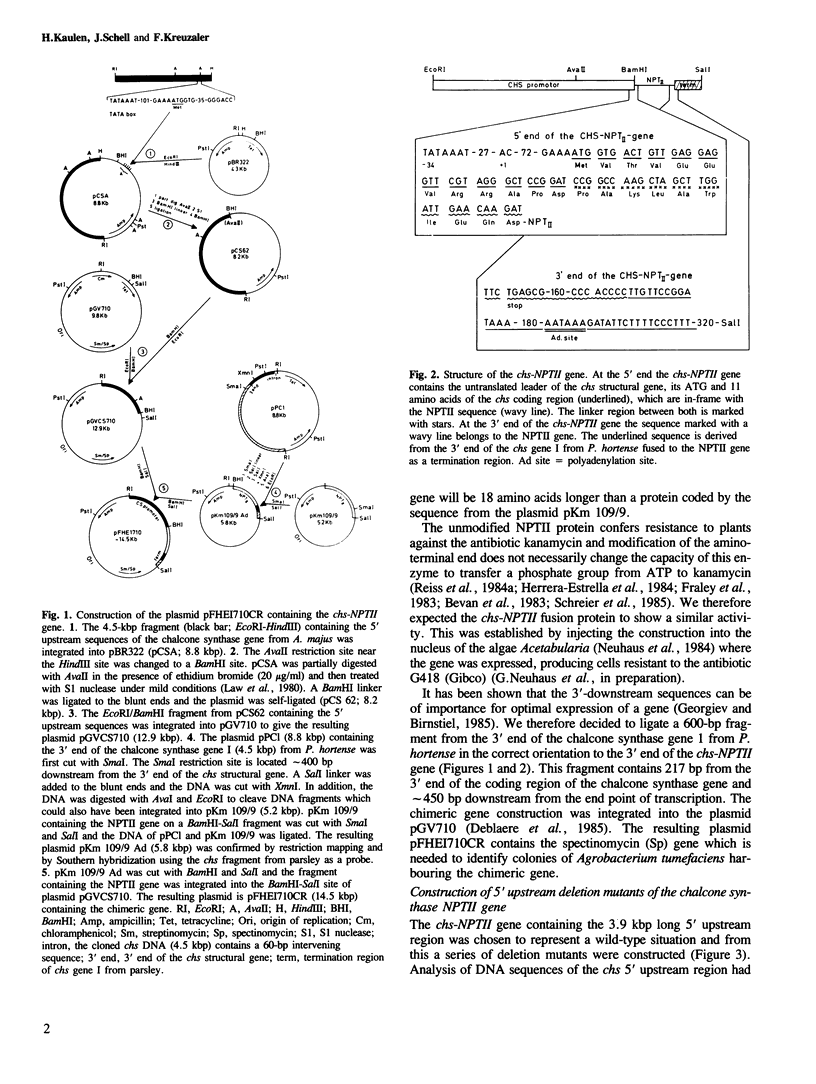
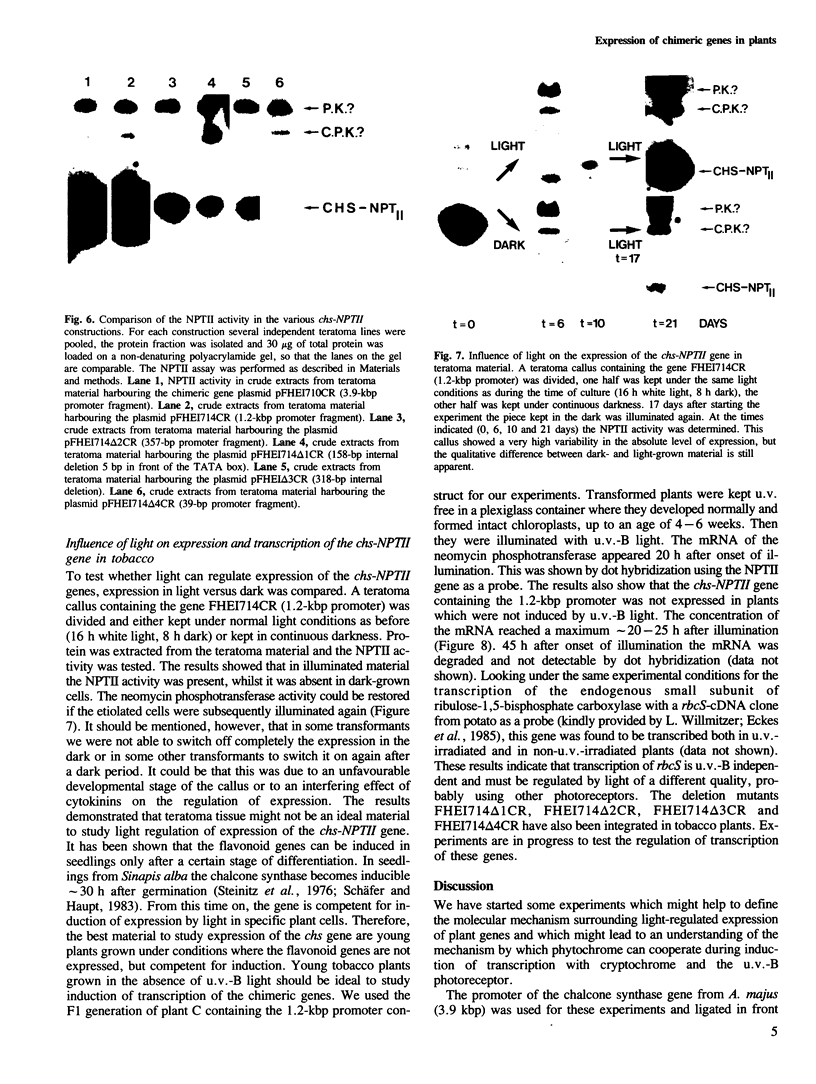
A chimeric gene was constructed containing the light-inducible chalcone synthase (chs) promoter from Antirrhinum majus, the neomycin phosphotransferase structural sequence from Tn5 as a reporter gene (NPTII) and the termination region from chs gene 1 from Petroselinum hortense. This gene was introduced into tobacco plants with the help of Ti plasmid-derived plant vectors and NPTII expression was measured. Analysis of the chs promoter sequence indicated the position of several possible regulatory regions. These were deleted to test their influence on the expression of the chs-NPTII gene. The different chimeric genes were all shown to be active after transfer to tobacco with the exception of one, in which the entire 5' upstream sequence from −1200 to −39 was deleted. The transcription of a chimeric gene with a 1.2-kbp 5' upstream promoter sequence was shown to be light inducible in tobacco plants. The analysis of various deletions of this 5' upstream sequence indicates that a number of sequence motifs have a quantitative effect on gene expression. One of these sequence motifs (−564 to −661) contains a direct repeat of 47 bp and the sequence GTGGTTAG which corresponds to the consensus core sequences observed in animal gene enhancer sequences. Deletion of a fragment containing this direct repeat resulted in a reduction of NPTII expression by a factor of 5.
Keywords: chalcone synthase-NPTII gene, light induction, promoter analysis, Ti plasmid
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaese P., De Greve H., Decraemer H., Schell J., Van Montagu M. Rapid mapping of transposon insertion and deletion mutations in the large Ti-plasmids of Agrobacterium tumefaciens. Nucleic Acids Res. 1979 Dec 11;7(7):1837–1849. doi: 10.1093/nar/7.7.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. J., Siegelman H. W. Photocontrol of Anthocyanin Synthesis in Milo Seedlings. Plant Physiol. 1963 Jan;38(1):25–30. doi: 10.1104/pp.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev O., Birnstiel M. L. The conserved CAAGAAAGA spacer sequence is an essential element for the formation of 3' termini of the sea urchin H3 histone mRNA by RNA processing. EMBO J. 1985 Feb;4(2):481–489. doi: 10.1002/j.1460-2075.1985.tb03654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W., Hahlbrock K. Highly purified "flavanone synthase" from parsley catalyzes the formation of naringenin chalcone. Arch Biochem Biophys. 1980 Apr 1;200(2):617–619. doi: 10.1016/0003-9861(80)90395-1. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella L., Van den Broeck G., Maenhaut R., Van Montagu M., Schell J., Timko M., Cashmore A. Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature. 1984 Jul 12;310(5973):115–120. doi: 10.1038/310115a0. [DOI] [PubMed] [Google Scholar]
- Koncz C., Kreuzaler F., Kalman Z., Schell J. A simple method to transfer, integrate and study expression of foreign genes, such as chicken ovalbumin and alpha-actin in plant tumors. EMBO J. 1984 May;3(5):1029–1037. doi: 10.1002/j.1460-2075.1984.tb01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler F., Hahlbrock K. Enzymatic synthesis of aromatic compounds in higher plants. Formation of bis-noryangonin (4-hydroxy-6[4-hydroxystyryl]2-pyrone) from p-coumaroyl-CoA and malonyl-CoA. Arch Biochem Biophys. 1975 Jul;169(1):84–90. doi: 10.1016/0003-9861(75)90319-7. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Hahlbrock K. Enzymic synthesis of an aromatic ring from acetate units. Partial purification and some properties of flavanone synthase from cell-suspension cultures of Petroselinum hortense. Eur J Biochem. 1975 Aug 1;56(1):205–213. doi: 10.1111/j.1432-1033.1975.tb02223.x. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Fautz E., Kuhn D. N., Hahlbrock K. UV-induction of chalcone synthase mRNA in cell suspension cultures of Petroselinum hortense. Proc Natl Acad Sci U S A. 1983 May;80(9):2591–2593. doi: 10.1073/pnas.80.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Heller W., Tesch R., Witt I., Hammer D., Hahlbrock K. Flavanone synthase from Petroselinum hortense. Molecular weight, subunit composition, size of messenger RNA, and absence of pantetheinyl residue. Eur J Biochem. 1979 Aug 15;99(1):89–96. doi: 10.1111/j.1432-1033.1979.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S., Tamoaki T., Kreuzaler F., Dugaiczyk A. Molecular cloning of DNA complementary to a mouse alpha-fetoprotein mRNA sequence. Gene. 1980 Jun;10(1):53–61. doi: 10.1016/0378-1119(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Neuhaus-Url G., Gruss P., Schweiger H. G. Enhancer-controlled expression of the simian virus 40 T-antigen in the green alga Acetabularia. EMBO J. 1984 Sep;3(9):2169–2172. doi: 10.1002/j.1460-2075.1984.tb02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold U., Kröger M., Kreuzaler F., Hahlbrock K. Coding and 3' non-coding nucleotide sequence of chalcone synthase mRNA and assignment of amino acid sequence of the enzyme. EMBO J. 1983;2(10):1801–1805. doi: 10.1002/j.1460-2075.1983.tb01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Schaller H. Protein fusions with the kanamycin resistance gene from transposon Tn5. EMBO J. 1984 Dec 20;3(13):3317–3322. doi: 10.1002/j.1460-2075.1984.tb02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Will H., Schaller H. A new sensitive method for qualitative and quantitative assay of neomycin phosphotransferase in crude cell extracts. Gene. 1984 Oct;30(1-3):211–217. doi: 10.1016/0378-1119(84)90122-7. [DOI] [PubMed] [Google Scholar]
- Rüdiger W. Uber die Struktur des Phytochrom-Chromophors und seine Protein-Bindung. Justus Liebigs Ann Chem. 1969;723:208–212. doi: 10.1002/jlac.19697230124. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Seftor E. A., Schell J., Bohnert H. J. The use of nuclear-encoded sequences to direct the light-regulated synthesis and transport of a foreign protein into plant chloroplasts. EMBO J. 1985 Jan;4(1):25–32. doi: 10.1002/j.1460-2075.1985.tb02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Siegelman H. W., Hendricks S. B. Purification and properties of phytochrome: a chromoprotein regulating plant growth. Fed Proc. 1965 Jul-Aug;24(4):863–867. [PubMed] [Google Scholar]
- Siegelman H. W., Turner B. C., Hendricks S. B. The chromophore of phytochrome. Plant Physiol. 1966 Oct;41(8):1289–1292. doi: 10.1104/pp.41.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten J., Velten L., Hain R., Schell J. Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1984 Dec 1;3(12):2723–2730. doi: 10.1002/j.1460-2075.1984.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann E., Schopfer P. Phytochrome-mediated de Novo Synthesis of Phenylalanine Ammonia-Lyase in Cell Suspension Cultures of Parsley. Plant Physiol. 1975 May;55(5):822–827. doi: 10.1104/pp.55.5.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuhashi H., Hashimoto T., Shimizu S. Ultraviolet action spectrum for anthocyanin formation in broom sorghum first internodes. Plant Physiol. 1982 Sep;70(3):735–741. doi: 10.1104/pp.70.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]