Abstract
Background
Chemotherapy is crucial in the management of tumors, but challenges such as chemoresistance and adverse reactions frequently lead to therapeutic delays or even premature cessation. A growing body of research underscores a profound connection between the gut microbiota (GM) and cancer chemotherapy (CC). This paper aims to pinpoint highly influential publications and monitor the current landscape and evolving trends within the realm of GM/CC research.
Methods
On October 1st, 2024, a comprehensive search for GM/CC publications spanning the past 20 years from 2004 to 2023 was conducted utilizing the Web of Science Core Collection (WoSCC). The scope encompassed both articles and reviews, and the data was subsequently extracted. To gain insights into the evolution and dynamics of this research field, we employed bibliometric analysis tools such as the Bibliometrix R package, VOSviewer, and Microsoft Excel to visualize and analyze various dimensions, including prominent journals, leading authors, esteemed institutions, contributing countries/regions, highly cited papers, and frequently occurring keywords.
Results
A total of 888 papers were obtained. The number of publications about GM/CC studies has increased gradually. China and the United States published the largest number of papers. The INSERM was in the leading position in publishers. The most productive authors were Zitvogel L from France. Cancers had the largest number of papers. Citation analysis explained the historical evolution and breakthroughs in GM/CC research. Highly cited papers and common keywords illustrated the status and trends of GM/CC research. Four clusters were identified, and the hot topics included the role of the GM in the efficacy and toxicity of CC, the targeting of the GM to improve the outcome of CC, the mechanism by which the GM affects CC, and the correlation of the GM with carcinogenesis and cancer therapy. Metabolism, GM-derived metabolites, tumor microenvironment, immunity, intestinal barrier, tumor microbiota and Fusobacterium nucleatum may become the new hotspots and trends of GM/CC research.
Conclusion
This study analyzed global publications and bibliometric characteristics of the links between GM and CC, identified highly cited papers in GM/CC, provided insight into the status, hotspots, and trends of global GM/CC research, and showed that the GM can be used to predict the efficacy and toxicity of CC and modifying the GM can improve the outcomes of chemotherapeutics, which may inform clinical researchers of future directions.
Keywords: Chemotherapy, Gut microbiota, Cancer, Research trends, Highly cited papers, Bibliometrics
Introduction
Cancer continues to pose a significant global public health concern, with an estimated 19.3 million new cases and nearly 10 million deaths worldwide in 2020, and this trend is showing an increasing pattern [1]. Over the past century, significant advancements have been achieved in cancer chemotherapy (CC), leading to substantial improvements in patient survival rates. Nonetheless, a subset of cancer patients may encounter drug resistance and adverse effects during CC, thereby impeding the effective utilization of chemotherapy medications. The gut microbiota (GM), a complex community of microorganisms residing in the human intestine, has emerged as a crucial factor influencing various physiological and pathological processes, including cancer development and progression. In recent decades, an increasing number of studies, including preclinical and clinical studies, have shown that the GM and its metabolites can modulate the efficacy and toxicity of chemotherapeutics [2, 3]. The GM can serve as a biomarker to predict the therapeutic response and prognosis of cancer patients [4]. This crosstalk between GM and CC has the potential to revolutionize our understanding of cancer therapy and patient outcomes. As such, a comprehensive and systematic analysis of the existing research in this field is both timely and crucial.
Bibliometrics has been widely used in medical research, which can evaluate the quality and recognition of papers and determine the discipline construction and development trend of a field. Many related areas have been well-researched through bibliometric analysis [5, 6]. However, currently, no studies on the bibliometric analysis of interactions between the GM and CC have been published. In this analysis, we aim to map out the landscape of research on the crosstalk between GM and CC. By employing bibliometric methods, we will examine trends in publication output, citation patterns, key researchers, and institutions that have contributed to this field. Additionally, we will explore the most frequently used keywords and research themes, providing insights into the evolving focus and priorities of GM/CC research. By shedding light on the interconnectedness between gut microbiota and cancer chemotherapy, we hope to pave the way for future research that can harness this crosstalk to improve cancer treatment outcomes and enhance patient quality of life. Specifically, we aim to address the following issues: (1) How have research efforts in this field evolved over time, and what are the key milestones and breakthroughs that have shaped the current landscape? (2) What are the potential mechanisms by which the GM may influence the efficacy and toxicity of CC? (3) What are the main limitations and challenges in the current research on GM/CC, and how can these be addressed in future studies? (4) What are the clinical implications of these findings, and how can they be translated into improved patient outcomes?
Materials and methods
Data sources and search methods
The WoS database is renowned and reliable as a citation index, attributed to its rigorous criteria for evaluating and selecting journals, as well as its ability to furnish accurate and trustworthy information [7]. The Science Citation Index Expanded (SCIE) of WoSCC includes the most influential academic journals and was used as the search source. This study used a similar method to Gholampour et al.'s method to identify articles relevant to the study, so the search strategy used in this study reflects the approach of Gholampour et al. [5, 6]. All searches were performed, and the data were retrieved on the same day (Oct 1st, 2024). The “advanced search” was used, and the search terms used were TI or AK or AB = “chemotherapy” and “gut microbiome” and “cancer”, and their corresponding synonyms (Table 1). The selection criteria were as follows: (1) The search period ranged from January 1, 2004, to December 31, 2023, when the most recent research progress and trends were obtained, and the accuracy and reliability of the citation data were ensured to carry out citation analysis and evaluation better. (2) The paper types were “article” and “review”. We browsed the title and abstract of the articles and excluded articles unrelated to chemotherapy, such as cancer immunotherapy and cancer-targeted therapy. The search and data extraction were independently carried out by two researchers (SY and SH). After that, we refined the key information and saved it in text format.
Table 1.
Search query and refinement procedure
| Set | Results | Refinement |
|---|---|---|
| 1 | 1226 |
Query formulation: Step 1: #1 TI OR AB OR AK = (“Tumor*” OR “Tumour*” OR “Cancer*” OR “Neoplasia*” OR “Neoplasm*” OR “Malignanc*” OR “Carcinoma*” OR “Oncolog*” OR “Melanoma*” OR “Lymphoma*” OR “Scrcoma*” OR “Adenocarcinoma*” OR “Leukemia*” OR “Myeloma*” OR “Blastoma*” OR “Glioma*”) Step 2: #2 TI OR AB OR AK = (“Gut Microb*” OR “Gut Microflora” OR “Gut Flora” OR “Gut Microbial Flora” OR “Gut Microecology” OR “Intestinal Microb*” OR “Intestinal Microflora” OR “Intestinal Flora” OR “Intestinal Microbial Flora” OR “Intestinal Microecology” OR “Gastrointestinal Microb*” OR “Gastrointestinal Microflora” OR “Gastrointestinal Flora” OR “Gastrointestinal Microbial Flora” OR “Gastrointestinal Microbial Communit*” OR “Gastrointestinal Microecology” OR “Fecal Microb*” OR “Fecal Microflora” OR “Fecal Flora” OR “Fecal Microbial Flora” OR “Faecal Microb*” OR “Faecal Microflora” OR “Faecal Flora” OR “Faecal Microbial Flora” OR “Gut Bacteri*” OR “Intestinal Bacteri*” OR “Gastrointestinal Bacteri*” OR “Gut Dysbiosis” OR “Intestinal Dysbiosis” OR “Gastrointestinal Dysbiosis” OR “Fecal Bacteri*” OR “Faecal Bacteri*” OR “Enteric Bacteri*” OR “Commensal Bacteri*” OR “Commensal Fungi” OR “Commensal Microb*” OR “Microb* Metaboli*” OR “Bacteri* Metaboli*”) Step 3: #3 TI OR AB OR AK = (“Chemothera*” OR “Chemical therap*” OR “Chemical treat*” OR “Alkylating agent*” OR “Cyclophosphamide” OR “Isophosphamide” OR “Temozolomide”OR “Platinum” OR “Cisplatin” OR “Oxaliplatin” OR “Carboplatin” OR “Nedaplatin” OR “Lobaplatin” OR “Anthracycline” OR “Doxorubicin” OR “Adriamycin” OR “Epirubicin” OR “Epidoxorubicin” OR “Bleomycin” OR “Mitomycin” OR “Fluoropyrimidine*” OR “Fluorouracil” OR “Flurouracil” OR “Floxuridine” OR “5-FU” OR “Capecitabine” OR “Cyclocytidine” OR “Cytarabine” OR “Gemcitabine” OR “Fludarabine” OR “Clartrobine” OR “Paclitaxel” OR “Docetaxel” OR “Vincristine” OR “Vinblastine” OR “Vindesine” OR “Vinorelbine” OR “Camptothecin” OR “Topotecan” OR “Irinotecan” OR “CPT-11” OR “Etoposide” OR “Teniposide” OR “Methotrexate” OR “Raltitrexed” OR “Pemetrexed” OR “Cytotoxic drug*” OR “Cytotoxic therap*” OR “Cytotoxic treat*” OR “Cytotoxic agent*” OR “Antimetabolite*” OR “Chemoresistance” OR “Chemosensitivit*”) Step 4: #1 AND #2 AND #3 Indexes = SCI-EXPANDED |
| 2 | 1149 | Refined by document types: (articles and review articles) |
| 3 | 1053 | Exclude irrelevant literature (such as cancer immunotherapy, targeted therapy, etc.) |
| 4 | 888 | Refined by publication years: (2004–2023) |
Data analysis and parameter query
Bibliometric analysis was conducted with the aid of the Bibliometrix (https://www.bibliometrix.org), VOSviewer 1.6.18 (Centre for Science and Technology Studies, Leiden University, the Netherlands), and Microsoft Excel 2021. VOSviewer was used for visualizing relational networks and for performing a cluster analysis of keywords. Bibliometrix and Excel were used for quantitative research. We used the following variables: annual paper production, journal dynamics, most local impact journals according to the H-index (the H-index of a journal is defined as the largest number h such that the journal has published h papers that have each been cited at least h times) or total citations (TC), which can indicate the academic importance of the journals, top journal production over time, top authors and their production and impact, top affiliations and funding agencies, country production and country collaboration network, historical direct citation network, highly cited papers, keywords and their cluster analysis (use statistical methods to discover latent themes or topics within a corpus of documents, aiding in the exploration of research landscapes). The impact factor (IF) and journal citation report (JCR) partition (calculate the average number of citations received by articles published in a journal over a specific period, reflecting the journal's prestige and influence) of published journals in 2023 can be found in the “2023 Journal Citation Report”.
Results
Annual development trend
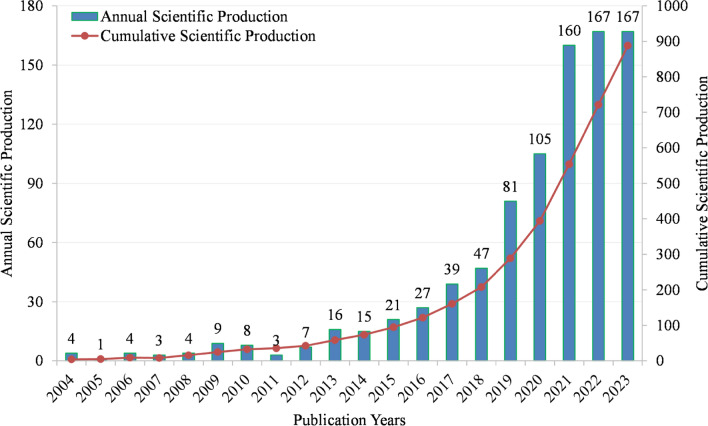
A total of 888 documents were identified, comprising 577 articles and 311 reviews. The number of papers (Np) can show the publication trend of GM/CC research. Figure 1 shows the annual and cumulative Np in GM/CC from 2004 to 2023, with an upward trend. According to the Np, we can find that the Np from 2004 to 2012 was very small, which demonstrates that relevant research is in its infancy. The Np from 2013 to 2018 increased slowly, showing that it is in the initial stage of development. From 2018 to 2021, the Np increased rapidly in the stage of rapid development. From 2021 to 2023, the growth tended to be flat.
Fig 1.
Annual and cumulative manufacturing output in GM/CC (the bar chart shows annual scientific production, whereas the line chart shows cumulative production)
Main journals in GM/CC research
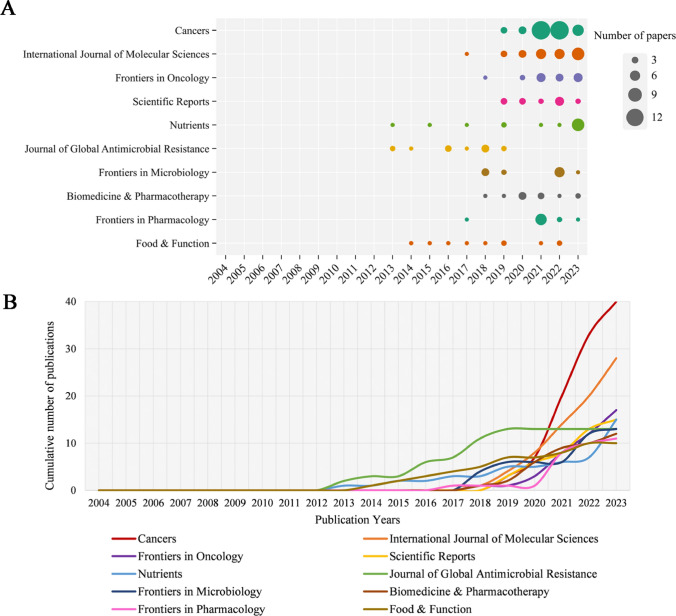
A total of 412 journals published papers on GM/CC research. Table 2 shows the top 10 prolific journals in the Np. Cancers had the highest Np (n = 40), followed by the International Journal of Molecular Sciences (n = 28), Frontiers in Oncology (n = 12), Nutrients (n = 15), and Scientific Reports (n = 15). Moreover, in the top 10 highly prolific journals, Cancers had the highest H-index (H = 17), followed by International Journal of Molecular Sciences (H = 15), the Journal of Global Antimicrobial Resistance (H = 10), and Biomedicine & Pharmacotherapy (H = 10). Figure 2A depicts the annual Np of the top 10 prolific journals, of which Nutrients and the Journal of Global Antimicrobial Resistance started earliest, and Cancers had the fastest growth rate. Figure 2B summarizes the cumulative Np in the top 10 prolific journals. There were 174 papers in these journals, accounting for approximately 19.59% of all the papers.
Table 2.
Top 10 productive journals in GM/CC
| No | Journals | Np | H-index | TC | IF | JCR | Countries |
|---|---|---|---|---|---|---|---|
| 1 | Cancers | 40 | 17 | 1107 | 4.5 | Q1 | Switzerland |
| 2 | International Journal of Molecular Sciences | 28 | 15 | 1637 | 4.9 | Q1 | Switzerland |
| 3 | Frontiers in Oncology | 17 | 8 | 227 | 3.5 | Q2 | Switzerland |
| 4 | Scientific Reports | 15 | 9 | 370 | 3.8 | Q1 | England |
| 5 | Nutrients | 15 | 8 | 327 | 4.8 | Q1 | Switzerland |
| 6 | Journal of Global Antimicrobial Resistance | 13 | 10 | 207 | 3.7 | Q2 | England |
| 7 | Frontiers in Microbiology | 13 | 8 | 416 | 4 | Q2 | Switzerland |
| 8 | Biomedicine & Pharmacotherapy | 12 | 10 | 431 | 6.9 | Q1 | France |
| 9 | Frontiers in Pharmacology | 11 | 9 | 322 | 4.4 | Q1 | Switzerland |
| 10 | Food & Function | 10 | 9 | 471 | 5.1 | Q1 | England |
Fig 2.
A The top 10 journals’ annual publications in GM/CC (the size of the circle represents the number of publications; a larger circle indicates a greater quantity of publications). B The top 10 journals’ cumulative publications in GM/CC
Main authors in GM/CC research
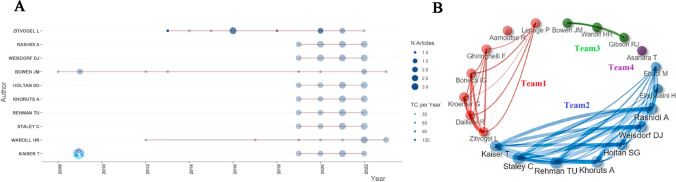
The dataset includes 5455 authors. Table 3 lists the top 10 prolific authors, of which Zitvogel Laurence (n = 12), Weisdorf Daniel J. (n = 11), Rashidi Armin (n = 11), Staley Christopher (n = 10), Wardill Hannah R. (n = 10), Bowen Joanne M. M. (n = 10), Rehman Tauseef Ur (n = 10), Khoruts Alexander (n = 10) and Holtan Shernan G. (n = 10) had no less than 10 publications. Zitvogel L had the largest Np, the highest H-index and TC, indicating her great impact on GM/CC research. Figure 3A shows the change in scientific productivity of the top 10 authors’ annual Np. Most of the authors’ papers were published in 2020. Figure 3B shows the links of the top 20 prolific authors. Each node represents an author, and the lines represent collaboration networks between authors. We can find that Zitvogel L, Daillere R, and Kroemer G (team 1), Rashidi A, Weisdorf DJ, and Holtan SG (team 2), as well as Bowen J and Wardill HR (team 3) all exhibited the closest internal collaborations within their respective teams.
Table 3.
The top 10 productive authors in GM/CC
| No | Authors | Np | H-index | TC | Main Affiliations | Countries |
|---|---|---|---|---|---|---|
| 1 | Zitvogel, Laurence | 12 | 11 | 3,004 | Gustave Roussy | France |
| 2 | Rashidi, Armin | 11 | 8 | 174 | University of Minnesota | USA |
| 3 | Weisdorf, Daniel J | 11 | 8 | 174 | University of Minnesota | USA |
| 4 | Holtan, Shernan G | 10 | 8 | 163 | University of Minnesota | USA |
| 5 | Khoruts, Alexander | 10 | 8 | 163 | University of Minnesota | USA |
| 6 | Rehman, Tauseef-ur | 10 | 8 | 163 | NIH National Cancer Institute (NCI) | USA |
| 7 | Staley, Christopher | 10 | 8 | 163 | University of Minnesota | USA |
| 8 | Bowen, Joanne | 10 | 8 | 697 | University of Adelaide | Australia |
| 9 | Wardill, Hannah R | 10 | 8 | 216 | University of Adelaide | Australia |
| 10 | Kaiser, Thomas | 9 | 8 | 157 | University of Minnesota | USA |
Fig 3.
A The top 10 authors’ annual production in GM/CC research (the size of the circle represents the number of papers, and the larger the circle is, the greater the number of papers; the depth of the circle represents the annual citations, and the darker the color is, the more citations there are). B The top 20 authors’ cooperation networks in GM/CC research (the line represents the collaboration links between authors, and the thickness of the line represents the strength of collaboration; they are divided into different teams according to their cooperative relationship)
Main countries and affiliations in GM/CC research
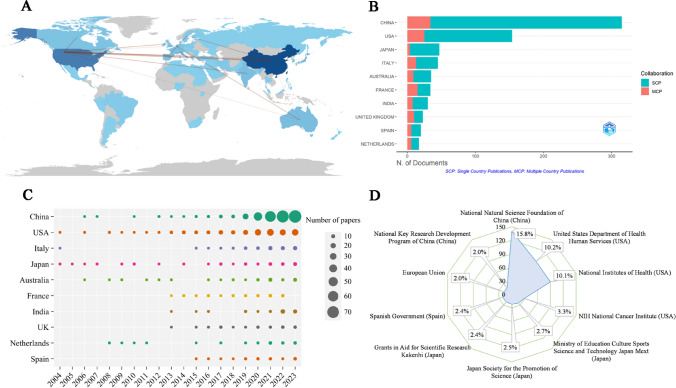
These papers were distributed across 63 countries (Fig. 4A). Table 4 shows that the papers were published mainly in China (n = 308) and the United States (n = 202), accounting for about 57.4% of the total output, followed by Italy (n = 58), Japan (n = 53), and Australia (n = 42). An international collaboration analysis of the top countries was performed (Fig. 4A), and China had the closest link with USA. Figure 4B depicts the degree of collaboration of corresponding authors from the productive country, we can find that international cooperation in France was common. Figure 4C shows the annual Np of countries in terms of time distribution. We found that the United States, Italy, Japan began to study earlier, and China developed rapidly. China experienced a surge in annual Np and has outnumbered USA in recent years, which may be related to the increased amount of attention given to the GM program and CC research. Table 5 shows the top prolific institutions, of which INSERM (n = 28), Peking Union Medical College (n = 24), Unicancer (n = 21), Chinese Academy of Sciences (n = 20) and Sun Yat-Sen University (n = 18) were among the top five. Figure 4D depicts the main funding agencies such as the National Natural Science Foundation of China, the United States Department of Health Human Services, and the National Institutes of Health, which mainly were from China, USA, Japan, and Spain.
Fig 4.
A Countries/regions scientific output and coordination relations in GM/CC (the colors in the image are composed of blue and gray, where gray represents countries/regions that have not published any documents, and blue varies in shade, with lighter blues indicating fewer published documents and darker blues indicating a higher number of published documents; the red lines depict the connections between countries, with thicker lines indicating closer connections and thinner lines indicating looser connections). B The nationality of correspondence authors and the rate of international cooperation in GM/CC (green SCP indicates the number of papers published by authors from the single country, and red MCP indicates the number of papers published by authors from multiple countries, MCP ratio shows the level of international cooperation). C The annual publications of top 10 prolific countries in GM/CC research (the size of the circle represents the number of publications; a larger circle indicates a greater quantity of publications). D The top 10 funding agencies in GM/CC research, including sources, countries and funding ratios
Table 4.
The top 10 productive countries in GM/CC
| Rank | Countries | Np | TC | H-index |
|---|---|---|---|---|
| 1 | China | 308 | 10,082 | 49 |
| 2 | USA | 202 | 14,676 | 51 |
| 3 | Italy | 58 | 2,480 | 22 |
| 4 | Japan | 53 | 1,284 | 21 |
| 5 | Australia | 42 | 2,386 | 21 |
| 6 | France | 39 | 4,510 | 22 |
| 7 | India | 38 | 626 | 15 |
| 8 | England | 34 | 1,827 | 18 |
| 9 | Netherlands | 31 | 1,594 | 16 |
| 10 | Spain | 29 | 1,785 | 14 |
Table 5.
The top 10 productive institutions in GM/CC
| Rank | Institutions | Np | TC | H-index |
|---|---|---|---|---|
| 1 | Inserm (France) | 28 | 3,868 | 19 |
| 2 | Peking Union Medical College (China) | 24 | 773 | 13 |
| 3 | Unicancer (France) | 21 | 3,280 | 16 |
| 4 | Chinese Academy of Sciences (China) | 20 | 459 | 13 |
| 5 | Sun Yat-Sen University (China) | 19 | 879 | 14 |
| 6 | Universite Paris Cite (France) | 19 | 3,165 | 15 |
| 7 | Shanghai Jiao Tong University (China) | 18 | 2,289 | 13 |
| 8 | Universite Paris Saclay (France) | 18 | 3,199 | 16 |
| 9 | University of Adelaide (Australia) | 17 | 935 | 11 |
| 10 | University of Texas System (USA) | 17 | 1,226 | 12 |
Historical direct cited papers in GM/CC research
Historiography analysis refers to the examination and interpretation of historical developments, trends, and patterns within a particular field or subject area, utilizing quantitative and qualitative methods derived from bibliometric data. This type of analysis allows researchers to understand how a field has evolved over time, identify key milestones, and recognize emerging trends or shifts in research focus. Using historiography analysis in the the Bibliometrix R package (set the number of papers to 20) to construct a chronological network of the most relevant direct citations, we found some papers in the GM/CC research (Table 6).
Table 6.
The historical direct cited papers in GM/CC
| Sort | DOI | Document Type | Journals | First Author | Year | LCS | GCS | Top Papers |
|---|---|---|---|---|---|---|---|---|
| 1 | 10.4161/cbt.7.12.6940 | Animal study | Cancer Biol. Ther | Stringer, AM | 2008 | 34 | 139 | Yes |
| 2 | 10.1086/599346 | Clinical study | Clin. Infect. Dis | van Vliet, MJ | 2009 | 61 | 206 | Yes |
| 3 | 10.3181/0810-RM-301 | Animal study | Exp. Biol. Med | Stringer, AM | 2009 | 43 | 170 | Yes |
| 4 | 10.1126/science.1191175 | Animal study | Science | Wallace, BD | 2010 | 72 | 728 | Yes |
| 5 | 10.1371/journal.ppat.1000879 | Review | PLoS Pathog | van Vliet, MJ | 2010 | 66 | 307 | Yes |
| 6 | 10.1371/journal.pone.0028654 | Clinical study | PLoS One | Zwielehner, J | 2011 | 48 | 159 | Yes |
| 7 | 10.1371/journal.pone.0039764 | Animal study | PLoS One | Lin, XB | 2012 | 44 | 121 | No |
| 8 | 10.1126/science.1240527 | Animal study | Science | Iida, N | 2013 | 170 | 1584 | Yes |
| 9 | 10.1126/science.1240537 | Animal study | Science | Viaud, S | 2013 | 192 | 1454 | Yes |
| 10 | 10.1111/apt.12878 | Review | Aliment. Pharmacol. Ther | Touchefeu, Y | 2014 | 71 | 314 | Yes |
| 11 | 10.1007/s00248-013–0355-4 | Clinical study | Microb. Ecol | Montassier, E | 2014 | 34 | 115 | No |
| 12 | 10.1111/apt.13302 | Clinical study | Aliment. Pharmacol. Ther | Montassier, E | 2015 | 86 | 324 | Yes |
| 13 | 10.4238/2015.May.25.16 | Animal study | Genet. Mol. Res | Gui, QF | 2015 | 32 | 168 | Yes |
| 14 | 10.1016/j.immuni.2016.09.009 | Animal study | Immunity | Daillere, R | 2016 | 81 | 566 | Yes |
| 15 | 10.1002/cncr.30039 | Clinical study | Cancer | Galloway-Pena, JR | 2016 | 40 | 113 | No |
| 16 | 10.1016/j.cell.2017.07.008 | Clinical study | Cell | Yu, TC | 2017 | 95 | 1303 | Yes |
| 17 | 10.1038/nrc.2017.13 | Review | Nat. Rev. Cancer | Roy, S | 2017 | 65 | 615 | Yes |
| 18 | 10.1038/nrgastro.2017.20 | Review | Nat. Rev. Gastroenterol. Hepatol | Alexander, JL | 2017 | 117 | 596 | Yes |
| 19 | 10.1016/j.biopha.2018.08.165 | Animal study | Biomed. Pharmacother | Yuan, L | 2018 | 39 | 132 | No |
| 20 | 10.1186/S13046-018–0985-Y | Clinical study | J. Exp. Clin. Cancer Res | Zhang, S | 2019 | 32 | 186 | Yes |
LGS denotes local citation score in the local downloaded papers; GCS denotes global citation score in the WoSCC database; Top papers represent highly cited papers or reviews
The GM/CC correlation study initially focused on chemotherapy-induced diarrhea or mucositis. In 2008, Stringer et al. [8] showed that irinotecan increased the abundance of β-glucuronidase-producing bacteria such as Escherichia coli, Staphylococcus and Clostridium and decreased the abundance of beneficial bacteria such as Lactobacillus and Bifidobacterium, and an increase in β-glucuronidase-producing bacteria can aggravate the toxicity of irinotecan. In 2009, Stringer et al. [9] showed that 5-fluorouracil (5-FU) treatment can cause significant changes in GM and mucus secretion, which may lead to the development of CC-induced mucositis. Vliet et al. [10] reported that during chemotherapy, the total number of gut bacteria in patients decreased significantly, the balance between aerobic and anaerobic bacteria was disrupted, and these changes reduced resistance to pathogen colonization and increased the risk of gram-positive aerobic bacterial infection. In 2010, Wallace et al. [11] showed that the dose-limiting toxicity of CPT-11 (irinotecan hydrochloride) was severe diarrhea, which is caused by symbiotic bacterial β-glucuronidase, and that of CPT-11 could be reduced by bacterial β-glucuronidase inhibitors. In 2011, Zwielehner et al. [12] reported that CC patients had lower bacterial loads than healthy controls. Chemotherapy can lead to changes in the GM, which is consistent with the occurrence of Clostridium difficile infection in some patients. In addition, GM changes may have systemic effects and lead to the development of CC-related mucositis. In 2012, Lin et al. [13] showed that irinotecan chemotherapy changed the GM of rats and increased the abundance of potentially pathogenic bacteria, such as Clostridium cluster XI and Enterobacteriaceae, and that oral glutamine could partially alleviate the toxicity of irinotecan and cause temporary changes in the GM.
In 2013, Iida et al. [14] reported that disruption of the GM impairs the subcutaneous tumor response to platinum-based chemotherapy, and the efficacy of oxaliplatin depends on the ability of the GM to activate myeloid cells and release reactive oxygen species (ROS). GM dysbiosis leads to decreased ROS levels, resulting in a decrease in the efficacy of CC. Viaud et al. [15] reported that cyclophosphamide can cause gram-positive bacteria to translocate to mesenteric lymph nodes and the spleen, thereby stimulating Th1 and Th17 cells to produce immune responses, while other sterile mice treated with antibiotics for gram-positive bacteria fail to produce this response, indicating that CC can cause GM translocation. The use of some antibiotics may disrupt these gram-positive bacteria, reducing their beneficial effects. In 2014, Montassier et al. [16] showed that the alpha diversity of the GM decreased sharply after chemotherapy. During chemotherapy, the abundance of Firmicutes and Actinobacteria decreased, while that of Bacteroidetes and Proteobacteria increased. At the genus level, the abundance of Bacteroidetes and Escherichia coli increased sharply, accompanied by a decrease in the abundance of Fecalibacterium, Rothia, and Bifidobacterium. Chemotherapy-induced changes in the GM may cause serious side effects in immunosuppressive cancer patients. In 2015, Gui et al. [17] showed that in mice treated with cisplatin combined with an antibiotic mixture (ABX), the tumor size was greater than that in mice treated with cisplatin alone, and the survival rate was significantly lower. In contrast, the mice treated with cisplatin combined with Lactobacillus had smaller tumors, greater survival rates, and enhanced antitumor responses. Probiotic combination therapy can enhance the anti-growth and pro-apoptotic effects of cisplatin. The composition and functional imbalance of the GM community associated with chemotherapy-induced gastrointestinal mucositis were determined.
In 2016, Daillère et al. [18] reported that the anticancer effect of cyclophosphamide depends on intestinal bacteria, among which Enterococcus hirae (E. hirae) and Barnesiella intestinihominis (B. intestinihominis) were crucial. The former migrates from the small intestine to secondary lymphoid organs and increases the proportion of CD8 + /Treg cells, while the latter enriches the colon and promotes the infiltration of IFN-γ-producing γδT cells into tumors. Galloway-Peña et al. [19] reported that the baseline fecal alpha diversity of patients with infection during chemotherapy was significantly lower than that of patients without infection. A significant decrease in oral and fecal microbial alpha diversity was observed during chemotherapy. Microbiome analysis can help reduce the incidence of infection complications during chemotherapy. In 2017, a study [20] showed that Fusobacterium nucleatum (F. nucleatum) promoted the chemoresistance of colorectal cancer (CRC) to activate the autophagy pathway by targeting specific innate immune signals and microRNAs. Reducing the specific GM in CRC patients may improve the tumor response to CC and reduce cancer recurrence. In 2018, Yuan et al. [21] showed that GM dysbiosis may reduce the therapeutic effect of 5-fluorouracil (5-FU) on subcutaneous colon tumors. ABX administration destroyed the GM and reduced the antitumor efficacy of 5-FU, but supplementation with probiotics did not significantly improve the efficacy of 5-FU. Moreover, 5-FU treatment also reduced the alpha diversity of the GM in the mice. In 2019, Zhang et al. [22] showed that high F. nucleatum abundance is associated with chemoresistance in patients with advanced CRC who underwent standard 5-FU-based adjuvant chemotherapy, and F. nucleatum can promote 5-FU chemoresistance by upregulating BIRC3 expression in CRC.
High-cited papers in GM/CC research
Top 20 most cited articles in GM/CC research
Highly cited research holds immense significance in the academic and scientific landscape. It serves as a testament to the impact that a particular study has had on its field. Table 7 lists the top 20 highly cited articles (published in 2008–2023), and they were mainly from world-renowned journals, such as Science (n = 4), Cell (n = 3), Nature (n = 1). Such research made a meaningful contribution to the field of GM/CC study.
Table 7.
The top 20 cited original research related to the GM/CC
| No | DOI | First author | Year | Journals | IF | JCR | TC |
|---|---|---|---|---|---|---|---|
| 1 | 10.1126/science.1240527 | Iida, N | 2013 | Science | 44.7 | Q1 | 1584 |
| 2 | 10.1126/science.1240537 | Viaud, S | 2013 | Science | 44.7 | Q1 | 1454 |
| 3 | 10.1016/j.cell.2017.07.008 | Yu, TC | 2017 | Cell | 45.5 | Q1 | 1303 |
| 4 | 10.1126/science.1191175 | Wallace, BD | 2010 | Science | 44.7 | Q1 | 728 |
| 5 | 10.1016/j.immuni.2016.09.009 | Daillere, R | 2016 | Immunity | 25.5 | Q1 | 566 |
| 6 | 10.1111/apt.13302 | Montassier, E | 2015 | Aliment. Pharmacol. Ther | 6.6 | Q1 | 324 |
| 7 | 10.1016/j.cmet.2021.03.002 | He, Y | 2021 | Cell Metab | 27.7 | Q1 | 303 |
| 8 | 10.1371/journal.ppat.0040035 | Koh, AY | 2008 | PLoS Pathog | 5.5 | Q1 | 260 |
| 9 | 10.1038/s41551-019–0423-2 | Zheng, DW | 2019 | Nat. Biomed. Eng | 26.8 | Q1 | 254 |
| 10 | 10.1126/science.aax0701 | Fluckiger, A | 2020 | Science | 44.7 | Q1 | 240 |
| 11 | 10.1086/599346 | van Vliet, MJ | 2009 | Clin. Infect. Dis | 8.2 | Q1 | 206 |
| 12 | 10.1186/s13046-018–0985-y | Zhang, S | 2019 | J. Exp. Clin. Cancer Res | 11.4 | Q1 | 186 |
| 13 | 10.3181/0810-RM-301 | Stringer, AM | 2009 | Exp. Biol. Med | 2.8 | Q2 | 170 |
| 14 | 10.1016/j.cell.2017.03.040 | Scott, TA | 2017 | Cell | 45.5 | Q1 | 168 |
| 15 | 10.4238/2015.May.25.16 | Gui, QF | 2015 | Genet. Mol. Res | 0.6 | Q4 | 168 |
| 16 | 10.1016/j.cell.2017.03.046 | Garcia-Gonzalez, AP | 2017 | Cell | 45.5 | Q1 | 166 |
| 17 | 10.1126/sciadv.aba1590 | Dong, X | 2020 | Sci. Adv | 11.7 | Q1 | 164 |
| 18 | 10.1371/journal.pone.0028654 | Zwielehner, J | 2011 | PLoS One | 2.9 | Q1 | 159 |
| 19 | 10.4161/cbt.7.12.6940 | Stringer, AM | 2008 | Cancer Biol. Ther | 4.4 | Q2 | 139 |
| 20 | 10.1038/s41586-023–05728-y | Tintelnot, J | 2023 | Nature | 50.5 | Q1 | 137 |
Firstly, some articles show there is an interaction between CC and GM. On the one hand, some of the abovementioned papers depicted CC-driven GM dysbiosis [12, 23], such as the change in alpha diversity, an increase in pathogenic bacteria [10] and a decrease in beneficial bacteria [12]. On the other hand, some papers mentioned the effect of GM on CC, for example, GM can modulate anticancer effects of chemotherapeutics [14, 15, 17], chemoresistance [20], and CC-induced toxicity such as diarrhea and mucositis [8, 9].
Secondly, several papers showed that specific GM can predict the outcome of CC. For instance, E. hirae and B. intestinihominis can enhance cyclophosphamide-related immunomodulatory effects [18], while F. nucleatum can promote 5-FU chemoresistance [22]. Moreover, a study [24] showed that Candida albicans bacteremia in cancer patients was thought to develop from gastrointestinal (GI) colonization and subsequently translocate to the bloodstream after CC. After depletion of the GM and stable GI colonization of Candida albicans, cyclophosphamide led to 100% mortality, while selective neutrophil or macrophage depletion, lymphocytopenia or GI mucosal destruction alone did not lead to mortality.
Thirdly, GM may regulate the efficacy and toxicity of CC by affecting metabolism and immune function. In 2017, a study [25] in Cell showed that the GM can enhance or inhibit the effect of 5-FU and ribonucleotide metabolism by transforming metabolic drugs involving the bacterial vitamins B6 and B9. A study [26] in Cell showed that bacterial metabolism affects the host response to cancer chemotherapeutics. A 2021 study [27] found that GM metabolite butyrate can enhance antitumor therapeutic efficacy by modulating cytotoxic CD8 + T cell immunity. Furthermore, A 2023 study [28] in Nature showed that the GM-derived tryptophan metabolite 3-IAA affects the chemotherapeutic efficacy in pancreatic cancer.
Phage-guided nanotechnology can change the GM to modulate the outcome of CC. A 2019 study [29] showed that phage-guided nanotechnology may inspire new ways to treat CRC. Targeted drug delivery targeting F. nucleatum-enriched tumor tissue can be achieved through phage guidance, thereby enhancing the efficacy of CC drugs and reducing drug damage to normal tissue, and phage-specific removal of F. nucleatum can reduce the resistance of CRC to chemotherapeutic drugs. A 2020 study [30] showed that enterococcal bacteriophages can enhance the efficacy of cyclophosphamide antitumor therapy. Moreover, dietary fiber protects against CRC in a microbiota- and butyrate-dependent manner [31]. Specific measures such as bacterial β-glucuronidase inhibitors can also reduce the toxicity of CC [11].
Top 10 most cited reviews in GM/CC research
The reviews can help researchers understand new progress, current problems and future trends in this field and provide timely guidance. Table 8 shows the top 10 most cited reviews. Several review articles [2, 32–34] have overviewed the role of GM in cancer and mechanisms by which GM influence cancer growth, outlined the impact of the GM on oncological pathogenesis, immune responses and treatment efficacy and toxicity, demonstrated various approaches in modulation methods of GM, and discussed current limitations, ongoing efforts, and future perspectives in cancer treatment. Two review articles [35, 36] mentioned the role of the GM in the pathogenesis of chemotherapy-induced mucositis, including the modification of intestinal barrier function and repair mechanisms and host innate immunity. There is crosstalk between the GM and immune cells. Two papers [37, 38] outlined the links between the GM and immune development and function and between the GM and anticancer immunosurveillance. A 2020 paper [39] in Nature revealed that the GM was associated with human immune cell dynamics, and changes in the concentrations of different types of immune cells in the blood may be directly related to the presence of different GMs. Furthermore, A 2018 review [40] proposed the concept of pharmacomicrobiomics and outlined the mechanisms of anticancer drug-microbiota interactions.
Table 8.
The top 10 cited reviews related to the GM/CC
| No | DOI | First author | Year | Journals | IF | JCR | TC |
|---|---|---|---|---|---|---|---|
| 1 | 10.1038/nrc.2017.13 | Roy, S | 2017 | Nat. Rev. Cancer | 72.5 | Q1 | 615 |
| 2 | 10.1038/nrgastro.2017.20 | Alexander, JL | 2017 | Nat. Rev. Gastroenterol. Hepatol | 45.9 | Q1 | 596 |
| 3 | 10.1038/s41571-018–0006-2 | Routy, B | 2018 | Nat. Rev. Clin. Oncol | 81.1 | Q1 | 361 |
| 4 | 10.3390/cancers11010038 | Vivarelli, S | 2019 | Cancers | 4.5 | Q1 | 342 |
| 5 | 10.1111/apt.12878 | Touchefeu, Y | 2014 | Aliment. Pharmacol. Ther | 6.6 | Q1 | 314 |
| 6 | 10.1371/journal.ppat.1000879 | van Vliet, MJ | 2010 | PLoS Pathog | 5.5 | Q1 | 307 |
| 7 | 10.1038/s41586-020–2971-8 | Schluter, J | 2020 | Nature | 50.5 | Q1 | 281 |
| 8 | 10.1136/gutjnl-2020–321153 | Cheng, WY | 2020 | Gut | 23 | Q1 | 193 |
| 9 | 10.1186/s40168-018–0483-7 | Panebianco, C | 2018 | Microbiome | 13.8 | Q1 | 192 |
| 10 | 10.1016/j.phrs.2012.09.002 | Bengmark, S | 2013 | Pharmacol. Res | 9.1 | Q1 | 155 |
High-frequency keywords in GM/CC research
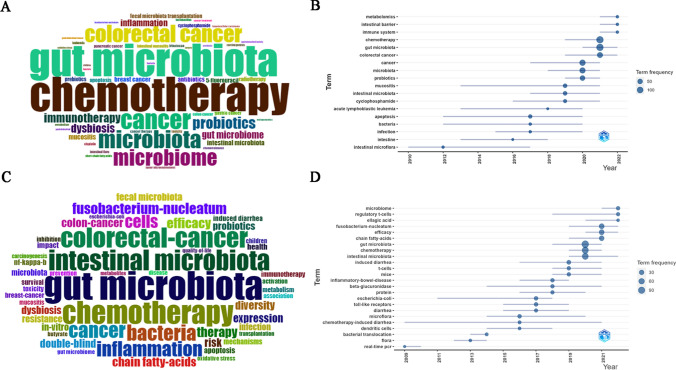
To identify hotspots in GM/CC studies, we examined important index keywords. In this study, a total of 4,294 keywords were extracted, including 1,951 authors' keywords and 2,343 keywords plus.
Figure 5A and Fig. 5C show the top 50 authors' keywords and keywords plus. Among the authors' keywords, the common terms were “chemotherapy”, “gut microbiota”, “colorectal cancer”, “probiotics”, “inflammation”, “mucositis”, “breast cancer”, etc. Among the keywords plus, the top-ranked terms were “gut microbiota”, “chemotherapy”, “colorectal-cancer”, “Fusobacterium-nucleatum”, “inflammation”, “efficacy”, “diversity”, “chain fatty acids”, “probiotics”, “resistance”, “mechanisms”, etc. The keyword evolution may reflect frontier knowledge. Figure 5B and Fig. 5D depict the trends of the authors' keywords and keywords plus, revealing that metabolomics, intestinal barrier, immune system, regulatory T cells, F. nucleatum, and chain fatty acids had attracted increased interest.
Fig 5.
A Top 50 Author's keywords in GM/CC research (the size of the font represents the frequency of occurrence). B Trend topics of Author's keywords (word minimum frequency set to 5, number of words per year set to 3) in GM/CC research (the abscissa represents the year, and the ordinate represents the hot keywords of this year). C Top 50 Keywords Plus in GM/CC research. D Trend topics of Keywords Plus (word minimum frequency set to 5, number of words per year set to 3) in GM/CC research (the abscissa represents the year, and the ordinate represents the hot keywords of that year)
In accordance with the co-occurrence keywords and the correlation, a cluster analysis was performed. Each clustered keyword was assigned a category based on the same color. Common keywords (frequency ≥ 10) were divided into four clusters (Fig. 6A).
Fig 6.
A Cluster analysis of common keywords (frequency ≥ 10) in GM/CC (different colors represent different clusters, the size of the circle represents the frequency at which the keywords appear, and the thickness of the line represents the total link strength between keywords). B Trends in keywords (frequency ≥ 10) over time based on all keywords of publications in GM/CC (the purple boxes represent the earliest keywords, and the yellow boxes represent the latest keywords, which may represent the research trend in recent years)
Cluster 1 (red boxes): This showed that the links between the GM and the efficacy of CC and the mechanism by which the GM affects CC, including tumor microenvironment, immunity (such as regulatory T cells), inflammation (such as nf-kappa-b), metabolism (such as short-chain fatty acids), apoptosis, oxidative stress, DNA-damage, pathway and protein.
Cluster 2 (green boxes): This showed that links between the GM and CC-induced toxicity (such as gastrointestinal toxicity, intestinal mucositis, gastrointestinal mucositis, diarrhea, induced diarrhea, 5-fluorouracil, capecitabine, oxaliplatin, paclitaxel and irinotecan) and the mechanisms of CC-induced toxicity (such as β-glucuronidase, toll-like receptors, pharmacokinetics and gene-expression).
Cluster 3 (blue boxes): This showed that the impact of specific bacteria (such as Fusobacterium nucleatum and Helicobacter pylori), tumor microbiome, and GM-derived metabolites (such as chain fatty-acids, short-chain fatty acids and butyrate) on carcinogenesis and cancer therapy (such as gastric cancer, colorectal cancer, pancreatic cancer, antitumor immunity and immunotherapy).
Cluster 4 (yellow boxes): This showed that the modulation methods of GM in improving cancer therapy, such as prebiotics, probiotics, synbiotics, antibiotics, and fecal microbiota transplantation.
We further used VOSviewer to analyze the trends of keywords. As shown in Fig. 6B, yellow nodes were centered around Clusters 1 and 3, and the keywords trend were expressed by the keyword “concentrated year” and “occurring” (year, time), including “efficacy” (2021, 67), “mechanisms” (2020, 33), “metabolites” (2021, 10), “short-chain fatty acids” (2021, 11), “tumor microenvironment” (2021, 15), “intestinal barrier” (2021, 15), “Fusobacterium nucleatum” (2021, 10), “tumor microbiome” (2022, 17), “pancreatic cancer” (2021, 19), “gastric cancer” (2021, 16), and “breast cancer” (2021, 30).
Discussion
Characteristics of publications
From 2004 to 2023, GM/CC research progressed through elementary stages (2004–2012), a slow development phase (2013–2018), rapid development (2018–2021), and steady development (2021–2023). The National Institutes of Health in the US launched the Human Microbiome Project in 2007. In 2008, the European Union launched the Human Intestinal Metagenome Project. In 2010, a new direction was opened for studying the human GM gene catalogue [41]. During this period (2004–2012), the research was in the initial stage of exploration, and the Np is small. In 2013, GM/CC research made breakthroughs and two studies [14, 15] published in Science found that the GM can influence the efficacy of CC. Correspondingly, GM/CC research began to gain more attention, and the Np began to increase. Subsequently, more and more countries began to pay attention to microbiome research. In 2016, the US initiated the National Microbiome Initiative. In 2017, Chinese Academy of Sciences took the lead in initiating the China Microbiome Initiative. Hereby, since 2018, GM/CC research had received increasing attention and produced many papers. Notably, the global outbreak and subsequent pandemic of COVID-19 had significantly impacted the work of scientists and publishers, leading to the interruption of some research and delays in publications. Consequently, this may have adversely affected research output and hindered continuous development in the years 2021–2023.
This study showed that Cancers, the International Journal of Molecular Sciences, and the Journal of Global Antimicrobial Resistance published the most papers on GM/CC. The publication output of a journal is directly influenced by both the number of submissions and the acceptance rate. Strategies in these journals to enhance these factors include simplifying the submission process, efficient manuscript handling and shorter review cycles further facilitate increased publication output. Additionally, implementing open access policies enhances the accessibility and visibility of research, potentially boosting the number of submissions. The most highly cited articles were mainly published in Science, followed by Cell, and Nature. These journals are renowned internationally and have high IFs and TCs, indicating that these prestigious journals are more likely to publish high-quality research in the future.
China and the United States were leading the way in GM/CC research may be related to their advanced research facilities, significant investments in research and development, collaborative research efforts, large patient populations for clinical trials, the launch of specialized microbial research programs, growing interest and awareness in the fields. The most productive institutions were mainly from China and France. Inserm, UDICE-French Research Universities and Unicancer from France and Tsinghua University, Chinese Academy of Sciences, Sun Yat-Sen University and Shanghai Jiao Tong University from China are well-known universities or research institutes. These institutions have received strong support from government funds and attracted a large number of outstanding talents due to their strong influence. The most prolific authors were mainly from Gustave Roussy and the University of Minnesota. As highly cited scholars in the world from famous cancer centers and world-class universities, they had received a lot of financial and technical support from countries, governments and institutions. Zitvogel Laurence from Gustave Roussy in France, with the highest Np and H-index, contributed to the study of GM/CC, especially the effect of GM on the anticancer effects of cyclophosphamide [15, 18] and the efficacy of chemotherapy in colon cancer [42] and breast cancer [43], and the action of the probiotic Enterococcus hirae [18, 30]. She was also at the core of the authors’ collaboration and had close cooperation with Daillere Romain. Rashidi Armin and Weisdorf Daniel J from the University of Minnesota have long been engaged in evaluating the effect of CC on the GM in acute leukemia and the key role of the GM in acute leukemia-associated infection complications [44, 45]. Staley Christopher and Rehman Tauseef also participated in the related research. Bowen Joanne M from the University of Adelaide focused on the role of the GM in CC-induced GI toxicity [46], diarrhea [47] and cognitive impairment [48].
Current research status and hotspots
Hotspots were found by the analysis of common keywords and highly cited papers in GM/CC research; these hotspots were enriched in four aspects. (1) GM affects the efficacy and toxicity of CC. (2) The GM can serve as a biomarker for estimating the efficacy and toxicity of CC treatment. (3) Potential mechanisms involved in GM modulation of the CC. (4) Modulation of the GM can improve CC outcomes.
The GM affects the efficacy and toxicity of CC
Efficacy is the most critical factor for chemotherapeutics. Most related studies have focused on the effect of the GM on the CC, elaborating on the role of the GM in facilitating and abrogating drug efficacy [2]. Related research shows that the features of the GM are linked to chemotherapy outcomes in cancers such as gastrointestinal cancer [49] and breast cancer [50], and the composition and abundance of the GM differ between chemotherapy responders and nonresponders [51]. On the one hand, the GM and its metabolism can affect the host response to CC [15, 26]. A well-balanced GM can contribute to the anticancer response of CC [17], and its metabolites could also promote the efficacy of CC [27]. However, gut dysbiosis can promote chemotherapy resistance, including gemcitabine/paclitaxel resistance to pancreatic cancer [52], 5-FU resistance to CRC [21] and docetaxel resistance to prostate cancer [53], cisplatin resistance to epithelial ovarian cancer [54]. Moreover, pretreatment for cancer-related dysbiosis (or disease-related and medication-related dysbiosis) and chemotherapy drug-induced dysbiosis can further affect the efficacy of chemotherapy [2]. On the other hand, specific bacteria can affect the host response to CC. For example, Akkermansia muciniphila (A. muciniphila) may enhance the antitumor effect of cisplatin in lung cancer mice [55]. The abundance of A. muciniphila was positively correlated with the antitumor effect of FOLFOX in colon cancer patients [56]. Enterococcus hirae compensated for cancer-associated dysbiosis and facilitated the efficacy of cyclophosphamide [18, 57]. Conversely, Yu et al. [20] reported that F. nucleatum activated autophagy to lead to oxaliplatin chemoresistance. Zhang et al. [22] showed that F. nucleatum reduced the chemosensitivity of CRC cells to 5-FU and was associated with chemoresistance.
Chemotherapy-induced gastrointestinal toxicity, such as gastrointestinal mucositis and diarrhea, is a key factor affecting the completion rate of chemotherapeutics. Several studies [11, 58] have shown that CPT-11 is converted into active SN-38 by carboxylesterase, and the active SN-38 of CPT-11 is metabolized to the inactive metabolite SN-38 glucuronide (SN-38G) during hepatic glucuronidation and then exported to the intestine, where SN-38G is reconverted to SN-38 by β-glucuronidase secreted by gut bacteria, resulting in severe diarrhea. Related studies [11, 59] have shown that β-glucuronidase produced by the GM can regulate the level of the biologically active form of CPT-11 in the intestinal cavity, thereby affecting the toxicity of CPT-11. Certainly, irinotecan-induced diarrhea may be caused by an increase in β-glucuronidase-producing bacteria, such as Escherichia coli, but this increase may also be caused by irinotecan, further exacerbating the drug's toxicity [8, 60]. In addition, GM imbalance can be one of the etiological mechanisms underlying doxorubicin-induced cardiotoxicity [61], cisplatin-induced acute liver injury [62], oxaliplatin-induced mechanical hyperalgesia [63], chemotherapy-induced behavioral side effects [64] and cognitive impairment [48]. Reducing treatment-induced toxicity by regulating the GM may lead to improved therapeutic efficacy via adjuvant therapy. For instance, Prevotella copri was associated with carboplatin-induced gut toxicity and targeting Prevotella copri maypotentially attenuate carboplatin-induced gut mucositis [65]. A. muciniphila can attenuate 5-FU-induced gut mucositis [66]. Moreover, the GM may regulate the toxicity of chemotherapeutic drugs by affecting the microbiome, microbial enzymes and microbial metabolites [67].
GM as a biomarker for outcomes of CC
The GM can serve as a biomarker to predict the efficacy of CC treatment. For example, the alteration in the abundance of Roseburia faecalis in patients with gastrointestinal cancer might be a predictor of CC efficacy [49]. Compared with nonresponders, docetaxel responders had an increased proportion of A. muciniphila before treatment [50]. Moreover, the GM can predict the response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Responders were overrepresented in butyrate-producing GM, such as Roseburia, Dorea, and Anaerostipes, whereas non-responders were overrepresented in the Coriobacteriaceae and Fusobacterium. Ten biomarkers were used for the response-prediction classifier, yielding an area under the curve of 93.57% in the training cohort and 73.53% in the validation cohort [68]. The Shannon and Simpson indices of the GM in ovarian cancer patients revealed statistically significant differences between chemoresistant and chemosensitive individuals, and the random forest model, which included Angleakisella, Arenimonas, and Roseburia, exhibited good prediction accuracy, with an area under the receiver operating characteristic curve of 0.909 [69].
In addition, intestinal bacterial β-glucuronidase may be a predictive biomarker of irinotecan-induced diarrhea severity and may aid in selecting cancer patients for irinotecan treatment [70]. A study [71] showed that patients with bacteremia or bloodstream infection (BSI) after chemotherapy had a decrease in overall diversity and group abundance, and machine learning methods were used to develop a BSI risk index that can predict the incidence of BSI with a sensitivity of 90% and a specificity of 90%. Shi et al. [51] showed that, compared with diarrhea patients after neoadjuvant chemoradiotherapy, patients without diarrhea were richer in Bifidobacterium, Clostridia, and Bacteroides. Stringer et al. [47] showed that CC-induced diarrhea was related to the GM, which was characterized by a decrease in Lactobacillus, Bifidobacterium, Bacteroides and Enterococcus and an increase in Escherichia coli and Staphylococcus. Zhang et al. [72] showed that lung cancer patients with a higher relative abundance of specific bacterial genera, such as Prevotella, Megamonas, and Streptococcus, at baseline were more likely to have gastrointestinal reactions. A significant increase in the abundance of Prevotella was observed in carboplatin-treated mice, and the content of Prevotella was positively correlated with the severity of carboplatin-induced gut mucositis [65].
Potential mechanisms in GM modulation of CC
The GM may influence the outcomes of CC through the “TIMER” mechanism (translocation, immunomodulation, metabolism, enzyme degradation, and reduction in diversity and ecological variation) [2, 73]. (1) Translocation: Cyclophosphamide can cause intestinal villus shortening, focal accumulation of inflammatory cells and mucosal barrier damage, leading to translocation of gram-positive bacteria to the mesenteric lymph nodes and spleen, thereby stimulating immune cells to produce an immune response [15]. The anticancer effect of cyclophosphamide depended on the GM, in which E. hirae translocated from the small intestine to secondary lymphoid organs to increase the intratumoral CD8+/Treg ratio [18]. CC-induced infections may arise primarily from the GM through bacterial translocation [74]. (2) Immunomodulation: Gram-positive bacteria can stimulate the generation of a specific subset of Th17 cells and memory Th1 immune responses during cyclophosphamide treatment [15]. The GM can regulate chemotherapy-induced small bowel injury via TLR2 signaling and the drug transporter p-gp [75]. The GM mediates cisplatin hepatotoxicity through enhanced inflammatory reactions and oxidative stress [62]. Moreover, GM impacts local and systemic antitumor immune responses through the use of GM metabolites. (3) Metabolism: Gut bacteria convert the active metabolite SN-38G of irinotecan to SN-38, resulting in diarrhea [11, 58]. Anticancer fluoropyrimidine drugs can be metabolized by gut bacteria via conserved pathways found in mammalian hosts [76]. The GM can bolster or suppress the effects of fluoropyrimidines by interconverting metabolic drugs involved in bacterial vitamin B6, B9 and ribonucleotide metabolism [25]. Bacterial metabolism can affect the host response to chemotherapy, and 5-FU and FUDR can act through bacterial ribonucleotide metabolism to elicit their cytotoxic effects [26]. (4) Enzyme degradation: SN-38G is reconverted to SN-38 by β-glucuronidase secreted by intestinal bacteria, resulting in side effects of intestinal toxicity and severe diarrhea [11, 58]. Targeting gut microbial enzymes, especially β-glucuronidase, can improve chemotherapeutic outcomes [58]. Gut bacterial β-glucuronidase inhibitors ameliorate irinotecan-induced toxicity [11]. Squalene epoxidase drives cancer cell proliferation and promotes intestinal ecological imbalance to accelerate the occurrence of CRC. The inhibition of squalene epoxidase can improve the efficacy of CRC chemotherapy [77]. (5) Reduction in diversity and ecological variation: Reduced diversity and shifts in the relative abundance of GM were associated with methotrexate chemotherapy-induced gastrointestinal mucositis [78]. Moreover, the microbial diversity of rats treated with irinotecan decreased significantly, while the abundance of Fusobacteria and Proteobacteria increased, which may be related to the gastrointestinal toxicity of irinotecan [79]. Notably, some scholars [2, 40] applied the concept of pharmacomicrobiomics to exploit drug-microbiota interactions, particularly focused on the interplay between the GM and the pharmacokinetics or pharmacodynamics of cancer treatment.
GM modulation to improve CC
The GM is a potential target for improving CC outcomes [2, 32]. Modulating the GM to improve CC outcomes involves several aspects. (1) Dietary adjustments: Dietary components can affect the efficacy of CC. For instance, dietary serine-GM interactions can enhance chemotherapeutic toxicity without altering drug conversion [80]. Dietary strategies can modulate the GM to enhance the antitumor response of CC [81]. Manipulating the GM with caloric restriction prior to CC can have potential benefits [82]. Dietary restriction can alleviate lethal intestinal toxicity caused by 5-FU by preventing the translocation of opportunistic pathogens [83]. (2) Probiotics and Prebiotics: Modifying the GM by administering prebiotics that facilitate the expansion of useful bacteria and reduce pathogenic bacteria is helpful aid in cancer treatment [84]. For example, the oral administration of Bifidobacterium can enhance the tumor suppressive effect of irinotecan [85] and attenuate its intestinal and hepatic toxicity [86]. Supplementation of Lactobacillus reuteri combined with Clostridium butyricum can alleviate cisplatin-induced renal injury [87]. Bifidobacterium longum SX-1326 can mitigate gastrointestinal toxicity following irinotecan chemotherapy by modulating the P53 signaling pathway and the brain-gut axis [88]. (3) Fecal microbiota transplantation (FMT): FMT may reverse antibiotic- and chemotherapy-induced gut dysbiosis [89] and can prevent CC-induced mucosal injury and toxicity [90]. FMT could paradoxically prevent life-threatening bacteremia in CC patients [91]. (4) Antibiotics: Antibiotics play a dual role in CC. Several studies [92, 93] have shown that antibiotic use is closely related to worse clinical outcomes in CC patients. However, metronidazole pretreatment reduced the abundance of Prevotella and alleviated carboplatin-induced intestinal mucosal injury and inflammation [65].
Emerging research interests and trends
The analysis of keyword trends reveals several emerging hot topics, including the investigation of "efficacy" and the underlying "mechanisms" of various biological processes. Among these, the study of metabolites such as short-chain fatty acids (SCFAs) and the broader field of metabolomics have gained significant attention. Furthermore, there is a growing interest in tumor microenvironment (TME) and immune system and regulatory T cells, as well as the importance of the intestinal barrier in maintaining health. Additionally, F. nucleatum and tumor microbiome have emerged as key areas of focus in understanding disease pathogenesis and potential therapeutic interventions.
Metabolomics, metabolites and SCFAs
GM-derived metabolites serve as crucial links between the GM and cancer treatment [94]. For instance, GM-derived SCFAs affect the efficacy and toxicity of antitumor therapy [95]. SCFAs mainly include acetate, propionate and butyrate. Butyrate may enhance the efficacy of oxaliplatin by regulating CD8 + T-cell activity in the tumor microenvironment [27] and improve irinotecan effect [96]. Furthermore, In recent years, more and more studies have begun to focus on the effects of bacterial metabolites on CC. For example, GM-derived tryptophan metabolite 3-IAA affects the chemotherapeutic efficacy in pancreatic cancer [28]. A. muciniphila-derived pentadecanoic acid potentiates the sensitivity of gastric cancer to oxaliplatin through modulation of glycolysis [97]. Desulfovibrio desulfuricans and its metabolites impart chemoresistance resistance to CRC [98].
Immune system, regulatory T cells and TME
The influence of the GM on the initiation of innate and adaptive immune responses that are beneficial to the host in the context of effective anticancer therapies has recently been highlighted. A study shows that GM is associated with human immune cell dynamics [39]. T and B cells of the immune system interact with the GM to influence the CC [99]. GM and its metabolites can enhance or suppress anti-tumor immune responses. The role of regulatory T cells in TME is crucial in the formation of immune regulation and tolerance, and the induction of their normal immune regulation function is also regulated by GM. The local microbiome composition influences CC efficacy in colon cancer by modulating tolerogenic versus immunogenic ileal epithelial cell death, which in turn influences follicular helper T-cell priming [42].
Intestinal barrier
Intestinal barrier can prevent systemic or local infection caused by bacterial translocation. Tight junction is a major component of the intestinal barrier and is essential for maintaining barrier integrity. Chemotherapy can destroy the intestinal barrier and cause GM imbalance. For instance, mucosal damage may be required for Candida albicans dissemination [24]. GM imbalance causes abnormal expression of transmembrane proteins, which in turn destroys barrier function and increases paracellular permeability, causing the infiltration of pro-inflammatory factors in the intestinal cavity, leading to persistent inflammation and intestinal tissue damage. Butyrate can influence immune system function, preserve the integrity of the gut barrier, and minimize the risk of chemotherapy-induced mucositis, which could be effective as a part of cancer therapy [100].
Tumor microbiota and F. nucleatum
Several noted studies [101, 102] have explored the correlation between tumor microbiota and chemotherapy, and found that intratumor bacteria can promote metastatic colonization, and mediate tumor response and tumor resistance to gemcitabine by metabolizing gemcitabine to an inactive form. Notably, GM may modulate tumor microbiota. In addition, some studies showed that intratumoral F. nucleatum can alter autophagy to promote chemoresistance against CRC [20] and ESCC [103]. F. nucleatum can induce chemoresistance in CRC by inhibiting pyroptosis via the Hippo pathway [104]. By utilizing biomimetic nanovehicles for targeted depletion of intratumoral F. nucleatum, a synergistic effect is achieved in combination with PD-L1 blockade against breast cancer [105].
Key research gaps and opportunities for future investigation
Certainly, there are key some research gaps in GM/CC research. By addressing these research gaps and seizing these opportunities for future investigation, the field of GM/CC can advance significantly, leading to improved treatment outcomes and better quality of life for cancer patients.
The key research gaps in the GM/CC research include: (1) Which specific bacterial species within the GM are most closely associated with positive or negative CC outcomes in different cancer types? This question aims to identify key bacterial biomarkers that could predict treatment response and toxicity. (2) What methods should be applied to further research in the field of GM/CC? Employing high-throughput sequencing technologies to perform metagenomic profiling and metabolomic analyses of the GM can provide a comprehensive view of the microbial communities and their metabolic activities. (3) What are the mechanisms underlying the crosstalk between specific gut bacteria and CC? Identifying these mechanisms could reveal new targets for therapeutic interventions that enhance drug delivery, metabolism, or elimination. (4) How do specific interventions alter the GM composition and subsequently impact the efficacy and toxicity of CC? Understanding the modulatory effects of these interventions could lead to novel adjuvant therapies that improve patient outcomes.
The opportunities for future investigation in GM/CC research include: (1) Identifying Specific Microbial Species and Their Roles: Specific microbial species play critical roles in modulating the efficacy and toxicity of CC or predicting outcomes of CC. Understanding these species and their interactions with CC could lead to the development of more targeted and effective treatment strategies. (2) Mechanisms of Microbial-Drug Interactions: Further research is required to elucidate the mechanisms underlying the interactions between GM and CC. This includes understanding how microbial metabolites, such as SCFAs, affect drug metabolism, absorption, and efficacy. (3) Cross-Disciplinary Collaboration: Cross-disciplinary collaboration among microbiologists, oncologists, immunologists, and other experts is important in mechanism research. This collaboration can lead to innovative approaches and insights that are not possible when working in isolation. (4) Personalized Microbiota-Based Therapies: Develop personalized microbiota-based therapies can enhance the effectiveness of CC while minimizing adverse effects. Efforts should be made to translate findings from preclinical studies into clinical trials, and ultimately, into routine clinical practice. Some related clinical trials from the International Clinical Trials Registry Platform in manipulating the GM via dietary adjustments (NCT06015087, NCT06376604), probiotics (NCT03642548, ChiCTR2100046237, ChiCTR1800016824), or FMT (NCT06403111, ChiCTR2400087820, ACTRN12624000455561, ACTRN12624001104549) have also gradually increased in recent years.
Limitations of the article
This paper has several limitations. First, the publications included in the SCIE of WoSCC cannot cover all studies in different languages, so we may have missed out on valuable contributions from researchers working in different linguistic contexts and using different databases. However, it is worth noting that the Web of Science is a highly reputable and widely used database that covers a significant portion of scholarly literature. Despite these considerations, we acknowledge that our results may not fully capture the diversity of research perspectives and methodologies that exist globally. Second, a highly competitive publication environment in a journal might result in lower acceptance rates, thereby impacting the final Np. We analyzed highly cited papers to compensate for this flaw. Third, this study is only an analysis of previous studies. Some recent publications may have a greater impact but may be less cited now. In addition, the evolution of microbial analysis platforms and sequencing technologies may also have an impact on the results of the GM/CC research, but we did not analyze the different microbial analysis platforms and sequencing methods that may be involved in different studies.
Conclusion
In conclusion, the number of publications in GM/CC had seen a gradual increase over time, with China and the United States contributing the highest number of papers. Among the publishers, Cancers had the largest number of papers. INSERM emerged as a prominent leader. Zitvogel L from France stood out as the most productive author. Four distinct research hotspots were identified, focusing on the GM's role in CC efficacy and toxicity, strategies for targeting the GM to enhance CC outcomes, the mechanisms underlying GM's influence on CC, and the relationships between the GM, carcinogenesis, and cancer therapy. Looking ahead, metabolism, GM-derived metabolites, tumor microenvironment, immunity, intestinal barrier, tumor microbiota, and the bacterium F. nucleatum are poised to become the new hotspots and trends in GM/CC research. Notably, by conducting more in-depth research, specific microbial species and their roles may be identified, along with the mechanisms governing microbial-drug interactions. This may lay the groundwork for the development of personalized microbiota-based therapies, offering the potential for tailored treatment plans. In the future, manipulating the GM to enhance the effectiveness of cancer treatments and adverse side effects will become possible. In all, this study preliminarily shows the status of publications on GM/CC studies, provides researchers with a clearer impression of the knowledge map of GM/CC, and provides references for GM/CC research by summarizing current hotspots and future directions. By leveraging these insights, relevant researchers, theorists, and stakeholders can effectively utilize the findings to drive advancements in healthcare and personalized medicine.
Acknowledgements
Not applicable.
Abbreviations
- GM
Gut microbiota
- CC
Cancer chemotherapy
- WoSCC
The Web of Science Core Collection
- IF
Impact factor
- JCR
Journal citation report
- 5-FU
5-Fluorouracil
- GI
Gastrointestinal
- CRC
Colorectal cancer
- ESCC
Esophageal squamous cell carcinoma
- ROS
Reactive oxygen species
- SCP
Single country publication
- MCP
Multiple countries publications
- TC
Total citations
- H-index
Hirsch index
- SCFAs
Short-chain fatty acids
- TME
Tumor microenvironment
- TM
Tumor microbiota
Author contributions
SY and SH were responsible for the manuscript preparation, conduct of the investigation, and figure development. HY and XZ oversaw the methodology, provided supervision, and conceptualized the research. All authors made significant contributions to the article and have approved the submitted version.
Funding
This study was funded by grants from the National Natural Science Foundation of China (Grant No. 81803910 and 81973615), the Capital's Funds for Health Improvement and Research (Grant No. 2022-2-4077 and 2022-2-40711), and the National High-Level Hospital Clinical Research Funding Scientific Research Seed Fund of Peking University First Hospital (Grant No. 2024SF49) and the Qi-Huang Scholar Chief Scientist Program of the National Administration of TCM Leading Talents Support Program (2021).
Data availability
The datasets used and analyzed in this study are available from the corresponding author on reasonable request. Note: query link: https://webofscience.clarivate.cn/wos/woscc/summary/59b34ff3-5e4e-47db-b39c-5312e31ed302-010e528e0c/times-cited-descending/1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hui Ye, Email: yehui@pkufh.cn.
Xuezhi Zhang, Email: zhang.xuezhi@263.net.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14(6):356–65. 10.1038/nrgastro.2017.20. [DOI] [PubMed] [Google Scholar]
- 3.Park EM, Chelvanambi M, Bhutiani N, Kroemer G, Zitvogel L, Wargo JA. Targeting the gut and tumor microbiota in cancer. Nat Med. 2022;28(4):690–703. 10.1038/s41591-022-01779-2. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Lee HK. Potential role of the gut microbiome in colorectal cancer progression. Front Immunol. 2021;12: 807648. 10.3389/fimmu.2021.807648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gholampour B, Noruzi A, Elahi A, Barranco Gil D, Gholampour S. Who are the key figures in Grand Tours cycling events publications? A systematic review of main themes. Global Knowl Memory Commun. 2024. 10.1108/GKMC-12-2023-0472. [Google Scholar]
- 6.Gholampour S, Gholampour B, Elahi A, Noruzi A, Saboury AA, Hassan S, Ahmed F, Nawaz R, Terason S. From mega-events hosting to scientific leadership: a seven-decade scientometric analysis of pioneer countries. Cogent Soc Sci. 2023;9(1):2210398. 10.1080/23311886.2023.2210398. [Google Scholar]
- 7.Gholampour B, Gholampour S, Noruzi A. Research trend analysis of information science in france based on total, cited and uncited publications: a scientometric and altmetric analysis. 2022;1:7-26
- 8.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Keefe DM. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther. 2008;7(12):1919–25. 10.4161/cbt.7.12.6940. [DOI] [PubMed] [Google Scholar]
- 9.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh AS, Hamilton J, Keefe DM. Gastrointestinal microflora and mucins may play a critical role in the development of 5-Fluorouracil-induced gastrointestinal mucositis. Exp Biol Med (Maywood). 2009;234(4):430–41. 10.3181/0810-RM-301. [DOI] [PubMed] [Google Scholar]
- 10.van Vliet MJ, Tissing WJ, Dun CA, Meessen NE, Kamps WA, de Bont ES, Harmsen HJ. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis. 2009;49(2):262–70. 10.1086/599346. [DOI] [PubMed] [Google Scholar]
- 11.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh LA, Mani S, Redinbo MR. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831–5. 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS ONE. 2011;6(12): e28654. 10.1371/journal.pone.0028654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin XB, Dieleman LA, Ketabi A, Bibova I, Sawyer MB, Xue H, Field CJ, Baracos VE, Gänzle MG. Irinotecan (CPT-11) chemotherapy alters intestinal microbiota in tumour bearing rats. PLoS ONE. 2012;7(7): e39764. 10.1371/journal.pone.0039764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iida N, Dzutsev A, Stewart CA, Smith L, Bouladoux N, Weingarten RA, Molina DA, Salcedo R, Back T, Cramer S, Dai RM, Kiu H, Cardone M, Naik S, Patri AK, Wang E, Marincola FM, Frank KM, Belkaid Y, Trinchieri G, Goldszmid RS. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Bérard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Doré J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6. 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montassier E, Batard E, Massart S, Gastinne T, Carton T, Caillon J, Le Fresne S, Caroff N, Hardouin JB, Moreau P, Potel G, Le Vacon F, de La Cochetière MF. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microb Ecol. 2014;67(3):690–9. 10.1007/s00248-013-0355-4. [DOI] [PubMed] [Google Scholar]
- 17.Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14(2):5642–51. 10.4238/2015.May.25.16. [DOI] [PubMed] [Google Scholar]
- 18.Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong C, Flament C, Lepage P, Roberti MP, Routy B, Jacquelot N, Apetoh L, Becharef S, Rusakiewicz S, Langella P, Sokol H, Kroemer G, Enot D, Roux A, Eggermont A, Tartour E, Johannes L, Woerther PL, Chachaty E, Soria JC, Golden E, Formenti S, Plebanski M, Madondo M, Rosenstiel P, Raoult D, Cattoir V, Boneca IG, Chamaillard M, Zitvogel L. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–43. 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Galloway-Peña JR, Smith DP, Sahasrabhojane P, Ajami NJ, Wadsworth WD, Daver NG, Chemaly RF, Marsh L, Ghantoji SS, Pemmaraju N, Garcia-Manero G, Rezvani K, Alousi AM, Wargo JA, Shpall EJ, Futreal PA, Guindani M, Petrosino JF, Kontoyiannis DP, Shelburne SA. The role of the gastrointestinal microbiome in infectious complications during induction chemotherapy for acute myeloid leukemia. Cancer. 2016;122(14):2186–96. 10.1002/cncr.30039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, Chen Y, Chen H, Hong J, Zou W, Fang JY. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548-63.e16. 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, Shi Y, An C, Xu J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–93. 10.1016/j.biopha.2018.08.165. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma Y, Cai S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019;38(1):14. 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley Varannes S des, Massart S, Moreau P, Potel G, de La Cochetière MF, Batard E, Knights D. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42(5):515–28. 10.1111/apt.13302. [DOI] [PubMed]
- 24.Koh AY, Köhler JR, Coggshall KT, Van Rooijen N, Pier GB. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 2008;4(2): e35. 10.1371/journal.ppat.0040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung KY, Herrera-Dominguez L, Sudiwala S, Pessia A, Clayton PT, Bryson K, Velagapudi V, Mills PB, Typas A, Greene N, Cabreiro F. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169(3):442-56.e18. 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout A. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169(3):431-41.e8. 10.1016/j.cell.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, Dong X, Huang J, Wang Q, Mackay CR, Fu YX, Chen Y, Guo X. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988-1000.e7. 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Tintelnot J, Xu Y, Lesker TR, Schönlein M, Konczalla L, Giannou AD, Pelczar P, Kylies D, Puelles VG, Bielecka AA, Peschka M, Cortesi F, Riecken K, Jung M, Amend L, Bröring TS, Trajkovic-Arsic M, Siveke JT, Renné T, Zhang D, Boeck S, Strowig T, Uzunoglu FG, Güngör C, Stein A, Izbicki JR, Bokemeyer C, Sinn M, Kimmelman AC, Huber S, Gagliani N. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615(7950):168–74. 10.1038/s41586-023-05728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng DW, Dong X, Pan P, Chen KW, Fan JX, Cheng SX, Zhang XZ. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–28. 10.1038/s41551-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 30.Fluckiger A, Daillère R, Sassi M, Sixt BS, Liu P, Loos F, Richard C, Rabu C, Alou MT, Goubet AG, Lemaitre F, Ferrere G, Derosa L, Duong C, Messaoudene M, Gagné A, Joubert P, De Sordi L, Debarbieux L, Simon S, Scarlata CM, Ayyoub M, Palermo B, Facciolo F, Boidot R, Wheeler R, Boneca IG, Sztupinszki Z, Papp K, Csabai I, Pasolli E, Segata N, Lopez-Otin C, Szallasi Z, Andre F, Iebba V, Quiniou V, Klatzmann D, Boukhalil J, Khelaifia S, Raoult D, Albiges L, Escudier B, Eggermont A, Mami-Chouaib F, Nistico P, Ghiringhelli F, Routy B, Labarrière N, Cattoir V, Kroemer G, Zitvogel L. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369(6506):936–42. 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 31.Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, Godfrey V, Heise MT, Threadgill DS, Han A, Swenberg JA, Threadgill DW, Bultman SJ. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov. 2014;4(12):1387–97. 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–85. 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 33.Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers (Basel). 2019. 10.3390/cancers11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe. Gut. 2020;69(10):1867–76. 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6(5): e1000879. 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40(5):409–21. 10.1111/apt.12878. [DOI] [PubMed]
- 37.Bengmark S. Gut microbiota, immune development and function. Pharmacol Res. 2013;69(1):87–113. 10.1016/j.phrs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15(6):382–96. 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- 39.Schluter J, Peled JU, Taylor BP, Markey KA, Smith M, Taur Y, Niehus R, Staffas A, Dai A, Fontana E, Amoretti LA, Wright RJ, Morjaria S, Fenelus M, Pessin MS, Chao NJ, Lew M, Bohannon L, Bush A, Sung AD, Hohl TM, Perales MA, van den Brink M, Xavier JB. The gut microbiota is associated with immune cell dynamics in humans. Nature. 2020;588(7837):303–7. 10.1038/s41586-020-2971-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6(1):92. 10.1186/s40168-018-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberti MP, Yonekura S, Duong C, Picard M, Ferrere G, Tidjani Alou M, Rauber C, Iebba V, Lehmann C, Amon L, Dudziak D, Derosa L, Routy B, Flament C, Richard C, Daillère R, Fluckiger A, Van Seuningen I, Chamaillard M, Vincent A, Kourula S, Opolon P, Ly P, Pizzato E, Becharef S, Paillet J, Klein C, Marliot F, Pietrantonio F, Benoist S, Scoazec JY, Dartigues P, Hollebecque A, Malka D, Pagès F, Galon J, Gomperts Boneca I, Lepage P, Ryffel B, Raoult D, Eggermont A, Vanden Berghe T, Ghiringhelli F, Vandenabeele P, Kroemer G, Zitvogel L. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–31. 10.1038/s41591-020-0882-8. [DOI] [PubMed] [Google Scholar]
- 43.Terrisse S, Derosa L, Iebba V, Ghiringhelli F, Vaz-Luis I, Kroemer G, Fidelle M, Christodoulidis S, Segata N, Thomas AM, Martin AL, Sirven A, Everhard S, Aprahamian F, Nirmalathasan N, Aarnoutse R, Smidt M, Ziemons J, Caldas C, Loibl S, Denkert C, Durand S, Iglesias C, Pietrantonio F, Routy B, André F, Pasolli E, Delaloge S, Zitvogel L. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021;28(9):2778–96. 10.1038/s41418-021-00784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rashidi A, Weisdorf DJ. Microbiota-based approaches to mitigate infectious complications of intensive chemotherapy in patients with acute leukemia. Transl Res. 2020;220:167–81. 10.1016/j.trsl.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, Ramamoorthy S, Holtan SG, Khoruts A, Weisdorf DJ, Staley C. Altered microbiota-host metabolic cross talk preceding neutropenic fever in patients with acute leukemia. Blood Adv. 2021;5(20):3937–50. 10.1182/bloodadvances.2021004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Secombe KR, Coller JK, Gibson RJ, Wardill HR, Bowen JM. The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int J Cancer. 2019;144(10):2365–76. 10.1002/ijc.31836. [DOI] [PubMed] [Google Scholar]
- 47.Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radzuan M, Logan RM, Mayo B, Keefe DM, Gibson RJ. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer. 2013;21(7):1843–52. 10.1007/s00520-013-1741-7. [DOI] [PubMed] [Google Scholar]
- 48.Subramaniam CB, Bowen JM, Gladman MA, Lustberg MB, Mayo SJ, Wardill HR. The microbiota-gut-brain axis: An emerging therapeutic target in chemotherapy-induced cognitive impairment. Neurosci Biobehav Rev. 2020;116:470–9. 10.1016/j.neubiorev.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Li N, Bai C, Zhao L, Sun Z, Ge Y, Li X. The relationship between gut microbiome features and chemotherapy response in gastrointestinal cancer. Front Oncol. 2021;11: 781697. 10.3389/fonc.2021.781697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bawaneh A, Wilson AS, Levi N, Howard-McNatt MM, Chiba A, Soto-Pantoja DR, Cook KL. Intestinal microbiota influence doxorubicin responsiveness in triple-negative breast cancer. Cancers (Basel). 2022. 10.3390/cancers14194849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi W, Shen L, Zou W, Wang J, Yang J, Wang Y, Liu B, Xie L, Zhu J, Zhang Z. The gut microbiome is associated with therapeutic responses and toxicities of neoadjuvant chemoradiotherapy in rectal cancer patients-a pilot study. Front Cell Infect Microbiol. 2020;10: 562463. 10.3389/fcimb.2020.562463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kesh K, Mendez R, Abdelrahman L, Banerjee S, Banerjee S. Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb Cell Fact. 2020;19(1):75. 10.1186/s12934-020-01330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong W, Wu K, Long Z, Zhou X, Zhong C, Wang S, Lai H, Guo Y, Lv D, Lu J, Mao X. Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome. 2022;10(1):94. 10.1186/s40168-022-01289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chambers LM, Esakov Rhoades EL, Bharti R, Braley C, Tewari S, Trestan L, Alali Z, Bayik D, Lathia JD, Sangwan N, Bazeley P, Joehlin-Price AS, Wang Z, Dutta S, Dwidar M, Hajjar A, Ahern PP, Claesen J, Rose P, Vargas R, Brown JM, Michener CM, Reizes O. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer. Cancer Res. 2022;82(24):4654–69. 10.1158/0008-5472.CAN-22-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in lewis lung cancer mice. J Immunol Res. 2020;2020:2969287. 10.1155/2020/2969287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou X, Zhang P, Du H, Chu W, Sun R, Qin S, Tian Y, Zhang Z, Xu F. Akkermansia muciniphila potentiates the antitumor efficacy of FOLFOX in colon cancer. Front Pharmacol. 2021;12: 725583. 10.3389/fphar.2021.725583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goubet AG, Wheeler R, Fluckiger A, Qu B, Lemaître F, Iribarren K, Mondragón L, Tidjani Alou M, Pizzato E, Durand S, Derosa L, Aprahamian F, Bossut N, Moya-Nilges M, Derrien D, Chen G, Leduc M, Joseph A, Pons N, Le Chatelier E, Segata N, Yonekura S, Iebba V, Kepp O, Raoult D, André F, Kroemer G, Boneca IG, Zitvogel L, Daillère R. Multifaceted modes of action of the anticancer probiotic Enterococcus hirae. Cell Death Differ. 2021;28(7):2276–95. 10.1038/s41418-021-00753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng KW, Tseng CH, Tzeng CC, Leu YL, Cheng TC, Wang JY, Chang JM, Lu YC, Cheng CM, Chen IJ, Cheng YA, Chen YL, Cheng TL. Pharmacological inhibition of bacterial β-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol Res. 2019;139:41–9. 10.1016/j.phrs.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 59.Wallace BD, Roberts AB, Pollet RM, Ingle JD, Biernat KA, Pellock SJ, Venkatesh MK, Guthrie L, Neal SK, Robinson SJ, Dollinger M, Figueroa E, McShane SR, Cohen RD, Jin J, Frye SV, Zamboni WC, Pepe-Ranney C, Mani S, Kelly L, Redinbo MR. Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem Biol. 2015;22(9):1238–49. 10.1016/j.chembiol.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stringer AM, Gibson RJ, Bowen JM, Logan RM, Ashton K, Yeoh AS, Al-Dasooqi N, Keefe DM. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int J Exp Pathol. 2009;90(5):489–99. 10.1111/j.1365-2613.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang J, Wei S, Jiang C, Xiao Z, Liu J, Peng W, Zhang B, Li W. Involvement of abnormal gut microbiota composition and function in doxorubicin-induced cardiotoxicity. Front Cell Infect Microbiol. 2022;12: 808837. 10.3389/fcimb.2022.808837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gong S, Feng Y, Zeng Y, Zhang H, Pan M, He F, Wu R, Chen J, Lu J, Zhang S, Yuan S, Chen X. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J Transl Med. 2021;19(1):147. 10.1186/s12967-021-02814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neurosci. 2017;20(9):1213–6. 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant CV, Loman BR, Bailey MT, Pyter LM. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav Immun. 2021;95:401–12. 10.1016/j.bbi.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu C, Zhou B, Xia X, Chen S, Deng Y, Wang Y, Wu L, Tian Y, Zhao B, Xu H, Yang L. Prevotella copri is associated with carboplatin-induced gut toxicity. Cell Death Dis. 2019;10(10):714. 10.1038/s41419-019-1963-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S, Qian K, Zhang G, Zhang M. Akkermansia muciniphila and its outer membrane protein Amuc_1100 prophylactically attenuate 5-fluorouracil-induced intestinal mucositis. Biochem Biophys Res Commun. 2022;614:34–40. 10.1016/j.bbrc.2022.04.135. [DOI] [PubMed] [Google Scholar]
- 67.Luo Y, Zhou T. Connecting the dots: targeting the microbiome in drug toxicity. Med Res Rev. 2022;42(1):83–111. 10.1002/med.21805. [DOI] [PubMed] [Google Scholar]
- 68.Yi Y, Shen L, Shi W, Xia F, Zhang H, Wang Y, Zhang J, Wang Y, Sun X, Zhang Z, Zou W, Yang W, Zhang L, Zhu J, Goel A, Ma Y, Zhang Z. Gut microbiome components predict response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a prospective. Longitudinal Study Clin Cancer Res. 2021;27(5):1329–40. 10.1158/1078-0432.CCR-20-3445. [DOI] [PubMed] [Google Scholar]
- 69.Gong TT, He XH, Gao S, Wu QJ. Application of machine learning in prediction of chemotherapy resistant of ovarian cancer based on gut microbiota. J Cancer. 2021;12(10):2877–85. 10.7150/jca.46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chamseddine AN, Ducreux M, Armand JP, Paoletti X, Satar T, Paci A, Mir O. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacol Ther. 2019;199:1–15. 10.1016/j.pharmthera.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 71.Montassier E, Al-Ghalith GA, Ward T, Corvec S, Gastinne T, Potel G, Moreau P, de la Cochetiere MF, Batard E, Knights D. Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 2016;8(1):49. 10.1186/s13073-016-0301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang M, Zhou H, Xu S, Liu D, Cheng Y, Gao B, Li X, Chen J. The gut microbiome can be used to predict the gastrointestinal response and efficacy of lung cancer patients undergoing chemotherapy. Ann Palliat Med. 2020;9(6):4211–27. 10.21037/apm-20-2183. [DOI] [PubMed] [Google Scholar]
- 73.Singh A, Nayak N, Rathi P, Verma D, Sharma R, Chaudhary A, Agarwal A, Tripathi YB, Garg N. Microbiome and host crosstalk: a new paradigm to cancer therapy. Semin Cancer Biol. 2021;70:71–84. 10.1016/j.semcancer.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 74.Yang J, Liu KX, Qu JM, Wang XD. The changes induced by cyclophosphamide in intestinal barrier and microflora in mice. Eur J Pharmacol. 2013;714(1–3):120–4. 10.1016/j.ejphar.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Frank M, Hennenberg EM, Eyking A, Rünzi M, Gerken G, Scott P, Parkhill J, Walker AW, Cario E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol. 2015;194(4):1983–95. 10.4049/jimmunol.1402481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spanogiannopoulos P, Kyaw TS, Guthrie B, Bradley PH, Lee JV, Melamed J, Malig Y, Lam KN, Gempis D, Sandy M, Kidder W, Van Blarigan EL, Atreya CE, Venook A, Gerona RR, Goga A, Pollard KS, Turnbaugh PJ. Host and gut bacteria share metabolic pathways for anti-cancer drug metabolism. Nat Microbiol. 2022;7(10):1605–20. 10.1038/s41564-022-01226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C, Wang Y, Liu D, Wong CC, Coker OO, Zhang X, Liu C, Zhou Y, Liu Y, Kang W, To KF, Sung JJ, Yu J. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut. 2022;71(11):2253–65. 10.1136/gutjnl-2021-325851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fijlstra M, Ferdous M, Koning AM, Rings EH, Harmsen HJ, Tissing WJ. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer. 2015;23(6):1513–22. 10.1007/s00520-014-2487-6. [DOI] [PubMed] [Google Scholar]
- 79.Forsgård RA, Marrachelli VG, Korpela K, Frias R, Collado MC, Korpela R, Monleon D, Spillmann T, Österlund P. Chemotherapy-induced gastrointestinal toxicity is associated with changes in serum and urine metabolome and fecal microbiota in male Sprague-Dawley rats. Cancer Chemother Pharmacol. 2017;80(2):317–32. 10.1007/s00280-017-3364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ke W, Saba JA, Yao CH, Hilzendeger MA, Drangowska-Way A, Joshi C, Mony VK, Benjamin SB, Zhang S, Locasale J, Patti GJ, Lewis N, O’Rourke EJ. Dietary serine-microbiota interaction enhances chemotherapeutic toxicity without altering drug conversion. Nat Commun. 2020;11(1):2587. 10.1038/s41467-020-16220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rinninella E, Raoul P, Cintoni M, Palombaro M, Pulcini G, Gasbarrini A, Mele MC. Nutritional interventions targeting gut microbiota during cancer therapies. Microorganisms. 2021. 10.3390/microorganisms9071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu T, Wu Y, Wang L, Pang X, Zhao L, Yuan H, Zhang C. A more robust gut microbiota in calorie-restricted mice is associated with attenuated intestinal injury caused by the chemotherapy drug cyclophosphamide. MBio. 2019. 10.1128/mBio.02903-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang D, Qiu R, Qiu X, Sun M, Su M, Tao Z, Zhang L, Tao S. Dietary restriction rescues 5-fluorouracil-induced lethal intestinal toxicity in old mice by blocking translocation of opportunistic pathogens. Gut Microbes. 2024;16(1):2355693. 10.1080/19490976.2024.2355693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kahouli I, Tomaro-Duchesneau C, Prakash S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J Med Microbiol. 2013;62(Pt 8):1107–23. 10.1099/jmm.0.048975-0. [DOI] [PubMed] [Google Scholar]
- 85.Ren Z, Chen S, Lv H, Peng L, Yang W, Chen J, Wu Z, Wan C. Effect of Bifidobacterium animalis subsp. lactis SF on enhancing the tumor suppression of irinotecan by regulating the intestinal flora. Pharmacol Res. 2022;184:106406. 10.1016/j.phrs.2022.106406. [DOI] [PubMed] [Google Scholar]
- 86.Zhu H, Lu C, Gao F, Qian Z, Yin Y, Kan S, Chen D. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed Pharmacother. 2021;143: 112192. 10.1016/j.biopha.2021.112192. [DOI] [PubMed] [Google Scholar]
- 87.Hsiao YP, Chen HL, Tsai JN, Lin MY, Liao JW, Wei MS, Ko JL, Ou CC. Administration of Lactobacillus reuteri combined with clostridium butyricum attenuates cisplatin-induced renal damage by gut microbiota reconstitution, increasing butyric acid production, and suppressing renal inflammation. Nutrients. 2021. 10.3390/nu13082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yue F, Zeng X, Wang Y, Fang Y, Yue M, Zhao X, Zhu R, Zeng Q, Wei J, Chen T. Bifidobacterium longum SX-1326 ameliorates gastrointestinal toxicity after irinotecan chemotherapy via modulating the P53 signaling pathway and brain-gut axis. BMC Microbiol. 2024;24(1):8. 10.1186/s12866-023-03152-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Bastard Q, Ward T, Sidiropoulos D, Hillmann BM, Chun CL, Sadowsky MJ, Knights D, Montassier E. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci Rep. 2018;8(1):6219. 10.1038/s41598-018-24342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chang CW, Lee HC, Li LH, Chiang Chiau JS, Wang TE, Chuang WH, Chen MJ, Wang HY, Shih SC, Liu CY, Tsai TH, Chen YJ. Fecal microbiota transplantation prevents intestinal injury, upregulation of toll-like receptors, and 5-fluorouracil/oxaliplatin-induced toxicity in colorectal cancer. Int J Mol Sci. 2020. 10.3390/ijms21020386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perales-Puchalt A, Perez-Sanz J, Payne KK, Svoronos N, Allegrezza MJ, Chaurio RA, Anadon C, Calmette J, Biswas S, Mine JA, Costich TL, Nickels L, Wickramasinghe J, Rutkowski MR, Conejo-Garcia JR. Frontline science: microbiota reconstitution restores intestinal integrity after cisplatin therapy. J Leukoc Biol. 2018;103(5):799–805. 10.1002/JLB.5HI1117-446RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu C, Lai R, Li J, Zhang J, Zhao Y, Zhang X, Zhao Y, Guo Z. Antibiotics modulate chemotherapy efficacy in patients with esophageal cancer. Cancer Manag Res. 2020;12:4991–7. 10.2147/CMAR.S248130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iida N, Mizukoshi E, Yamashita T, Terashima T, Arai K, Seishima J, Kaneko S. Overuse of antianaerobic drug is associated with poor postchemotherapy prognosis of patients with hepatocellular carcinoma. Int J Cancer. 2019;145(10):2701–11. 10.1002/ijc.32339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Q, Wang B, Zheng Q, Li H, Meng X, Zhou F, Zhang L. A review of gut microbiota-derived metabolites in tumor progression and cancer therapy. Adv Sci (Weinh). 2023;10(15): e2207366. 10.1002/advs.202207366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut microbiota-derived short-chain fatty acids: impact on cancer treatment response and toxicities. Microorganisms. 2022. 10.3390/microorganisms10102048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Encarnação JC, Pires AS, Amaral RA, Gonçalves TJ, Laranjo M, Casalta-Lopes JE, Gonçalves AC, Sarmento-Ribeiro AB, Abrantes AM, Botelho MF. Butyrate, a dietary fiber derivative that improves irinotecan effect in colon cancer cells. J Nutr Biochem. 2018;56:183–92. 10.1016/j.jnutbio.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 97.Xu Q, Gao J, Zhao R, Li H, Cui H, Yuan Z, Ren H, Cao B, Wei B. Akkermansia muciniphila-derived pentadecanoic acid enhances oxaliplatin sensitivity in gastric cancer by modulating glycolysis. Pharmacol Res. 2024;206: 107278. 10.1016/j.phrs.2024.107278. [DOI] [PubMed] [Google Scholar]
- 98.Li G, Liu H, Yu Y, Wang Q, Yang C, Yan Y, Wang F, Mao Y. Desulfovibrio desulfuricans and its derived metabolites confer resistance to FOLFOX through METTL3. EBioMedicine. 2024;102: 105041. 10.1016/j.ebiom.2024.105041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 100.Russo M, Guida F, Paparo L, Trinchese G, Aitoro R, Avagliano C, Fiordelisi A, Napolitano F, Mercurio V, Sala V, Li M, Sorriento D, Ciccarelli M, Ghigo A, Hirsch E, Bianco R, Iaccarino G, Abete P, Bonaduce D, Calignano A, Berni Canani R, Tocchetti CG. The novel butyrate derivative phenylalanine-butyramide protects from doxorubicin-induced cardiotoxicity. Eur J Heart Fail. 2019;21(4):519–28. 10.1002/ejhf.1439. [DOI] [PubMed] [Google Scholar]
- 101.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357(6356):1156–60. 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang N, Sun T, Xu J. Tumor-related microbiome in the breast microenvironment and breast cancer. J Cancer. 2021;12(16):4841–8. 10.7150/jca.58986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y, Baba Y, Ishimoto T, Tsutsuki H, Zhang T, Nomoto D, Okadome K, Yamamura K, Harada K, Eto K, Hiyoshi Y, Iwatsuki M, Nagai Y, Iwagami S, Miyamoto Y, Yoshida N, Komohara Y, Ohmuraya M, Wang X, Ajani JA, Sawa T, Baba H. Fusobacterium nucleatum confers chemoresistance by modulating autophagy in oesophageal squamous cell carcinoma. Br J Cancer. 2021;124(5):963–74. 10.1038/s41416-020-01198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang N, Zhang L, Leng XX, Xie YL, Kang ZR, Zhao LC, Song LH, Zhou CB, Fang JY. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes. 2024;16(1):2333790. 10.1080/19490976.2024.2333790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Geng S, Guo P, Li X, Shi Y, Wang J, Cao M, Zhang Y, Zhang K, Li A, Song H, Zhang Z, Shi J, Liu J, Yang Y. Biomimetic nanovehicle-enabled targeted depletion of intratumoral Fusobacterium nucleatum synergizes with PD-L1 blockade against breast cancer. ACS Nano. 2024;18(12):8971–87. 10.1021/acsnano.3c12687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author on reasonable request. Note: query link: https://webofscience.clarivate.cn/wos/woscc/summary/59b34ff3-5e4e-47db-b39c-5312e31ed302-010e528e0c/times-cited-descending/1.