ABSTRACT
The aim of this investigation was to comparatively assess the antioxidant and polyphenol compounds in fresh moringa pods sourced from two different regions in Australia, namely Queensland (QLD) and Western Australia (WAU). Total polyphenol content varied between 1.64 and 5.97 mg GAE/g in moringa pod samples from QLD, while it ranged from 2.84 to 4.31 mg GAE/g in WAU samples. Total flavonoid content in QLD and WAU samples averaged 4.62 and 4.24 mg QE/g, respectively. Total condensed tannin content in QLD and WAU samples averaged 2.07 and 1.60 mg CE/g, respectively. The QLD samples had higher DPPH (2.87 vs. 2.74 mg AAE/g), ABTS (15.0 vs. 12.9 mg AAE/g), and total antioxidant capacity (2.34 vs. 1.46 mg AAE/g) than WAU samples. LC‐ESI‐QTOF‐MS/MS analysis identified 111 polyphenol compounds in moringa pod samples, including phenolic acids, flavonoids, and tannins. Some compounds were prevalent across most samples, such as 3‐sinapoylquinic acid and theaflavin. The study revealed that moringa pods contain a high concentration of polyphenols with strong antioxidant capacity. These findings highlight the substantial influence of regional effects on the polyphenol content and bioactive properties of moringa pods.
Keywords: antioxidant activity, nutritional composition, polyphenol characterization
This study examines the influence of environmental conditions on the antioxidant potential of fresh moringa pods cultivated in two Australian states. The pods are a rich source of polyphenols with potent antioxidant properties.
Abbreviations
- ABTS
2,2′‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulfonic acid)
- DPPH
2,2′‐diphenyl‐2‐picrylhydrazyl
- FICA
ferrous ion chelating activity
- FRAP
ferric reducing antioxidant power
- LC‐ESI‐QTOF‐MS/MS
liquid chromatography‐electrospray ionization quadrupole time‐of‐flight mass spectrometry
- QLD
samples from Queensland
- RPA
reducing power assay
- TAC
total antioxidant capacity
- TFC
total flavonoid content
- TCT
total condensed tannin
- TPC
total polyphenol content
- WAU
samples from Western Australia
- •OH‐RSA
hydroxyl radical scavenging activity
1. Introduction
Moringa is commonly grown in subtropical and tropical regions and is known for its significant nutritional and therapeutic benefits (Du, Wu et al. 2021). Its leaves, pods, and seeds are used as food, pharmaceuticals and cosmetics for their health benefits (Ogunsina, Radha, and Govardhan Singh 2010; Xu, Chen, and Guo 2019). In recent years, moringa has gained attention in scientific research due to its remarkable nutritional profile and its potential as a source of pharmacological compounds.
Polyphenol compounds represent a class of chemical constituents recognized for their bioactive potential. The spectrum of polyphenol compounds in moringa is particularly diverse and includes an array of substances such as flavonoids and phenolic acids, including gallic acid, vanillin, kaempferol, chlorogenic acid, myricetin, quercetin, luteolin, and rutin (Lin, Zhang, and Chen 2018). These compounds are indispensable for their role in fortifying the plant against environmental challenges and their significant contributions to human health (Al Juhaimi, Ghafoor, Ahmed et al. 2017). The complex structures of these polyphenol compounds facilitate a wide range of biological activities, conferring protective benefits against oxidative stress and cellular damage (Athira et al. 2021; Vonghirundecha et al. 2022). Moringa is rich in polyphenols, including flavonoids, phenolic acids, and tannins, which are important for their antioxidant properties. These components can scavenge and neutralize free radicals, which are volatile molecules that may disrupt cellular function, accelerate the aging process, and potentially trigger some diseases (Zhu, Yin, and Yang 2020).
Although considerable research has been undertaken on the composition of moringa, there is still a crucial need for further investigation into its antioxidant potential under different environmental conditions, such as climate and geographical location which may influence the composition and concentration of bioactive compounds in moringa pods, leaves or seeds. Existing studies indicate variations in the nutritional and phytochemical attributes of moringa, contingent upon factors such as climate, altitude, and soil type (Iqbal and Bhanger 2006; Kim et al. 2021). The antioxidant activity of moringa may be affected by extraction methods, crop conditions, harvest time, and storage conditions (Vázquez‐León et al. 2017). The plant age, environmental conditions, and the specific parts of the plant collected may significantly alter the amount of polyphenol compounds and their antioxidant (Al Juhaimi, Ghafoor, Babiker et al. 2017; Qadir et al. 2022). Previous investigations have predominantly concentrated on moringa leaves and seeds' biochemical properties and health benefits. The pods of moringa are a notable source of polyphenol compounds with potent antioxidant properties. Despite this, research comparing the polyphenol content and bioactivity of moringa cultivated in different regions of Australia remains limited. Thus, this study was designed to assess the antioxidant activities and polyphenol characterization of moringa pods collected from two Australian states: Queensland and Western Australia.
2. Materials and Methods
2.1. Chemical and Reagents
Vanillin, gallic acid, Folin‐Ciocalteu reagent, L‐ascorbic acid, sodium phosphate, iron chloride hexahydrate, hexahydrate aluminium chloride, hydrated sodium acetate, ammonium molybdate, hydrochloric acid, sodium carbonate anhydrous, catechin, quercetin, 2,4,6‐tripyridyl‐s‐triazine (TPTZ), 2,2′‐diphenyl‐2‐picrylhydrazyl (DPPH), and 2,2′‐azinobis‐(3‐ethylbenzothiazoline‐6‐sulfonic acid) (ABTS) were procured from Chem‐Supply Ltd. (Melbourne, VIC, Australia). Sulfuric acid (H2SO4) was sourced from RCI Labscan Ltd. (Bangkok, Thailand). Caffeic acid, p‐hydroxybenzoic acid, caftaric acid, protocatechuic acid, sinapinic acid, chlorogenic acid, syringic acid, ferulic acid, coumaric acid, quercetin‐3‐galactoside, diosmin, quercetin‐3‐glucuronide, quercetin‐3‐glucosidekaempferol, kaempferol‐3‐glucoside, and epicatechin gallate were acquired from Sigma‐Aldrich (Castle Hill, NSW, Australia). The reagents used for liquid chromatography‐mass spectrometry (LC‐MS/MS), including acetonitrile, methanol, ethanol, glacial acetic acid, and formic acid, were sourced from Thermo Fisher Scientific Inc. (Scoresby, Victoria, Australia).
2.2. Sample Preparation and Extraction
Samples of moringa pods were collected from two different regions (Queensland [QLD] and Western Australia [WAU]) in Australia: Queensland (QLD1, QLD2, QLD3, QLD4, QLD5, QLD6, QLD7) and Western Australia (WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, and WAU32). The moringa plants belong to the PKM variety, and pods are used for human consumption. Pods were collected as they were available in QLD and WAU. Pods from Queensland and Western Australia were collected in December 2022 and April 2023, respectively. In farms from both regions, seven trees were randomly chosen, and seven young pods were randomly collected from each tree. Pods collected from each tree were separately wrapped in a polythene bag, and all bags containing fresh pods were packed in a large ice chest with ice packs. Fresh pods were taken directly via flight to Melbourne, Victoria. The following day, they were taken to the University of Melbourne's Food laboratory, and each fresh pod was cut in half. One portion of each half fresh pods was further cut into small cubes (~1 × 1 × 1 cm3), weighed, and dried at 60°C for 72 h. Upon recording the dry weights, samples from each tree were separately ground using a UDY Cyclone grinder (Fort Collins, CO, USA) fitted with a 1 mm mesh screen and stored in airtight plastic containers. All ground samples of fresh pods with the containers were kept in dark, refrigerated conditions. The remaining half of each pod was wrapped in separate polythene bags for each tree and maintained under frozen conditions for 3 months, which were not used for this study.
From all ground samples prepared using fresh pods (halves), homogeneous samples were used to analyze polyphenol compounds and antioxidant activity. The extraction process involved mixing 1 g of the sample with a solution comprising ethanol (70%) and formic acid (0.1%). Then, the samples underwent homogenization (10,000 rpm for 30 s) using a homogenizer (Staufen, IKA, Germany). The process continued with a 16‐h incubation period at 10°C and a shaking speed of 120 rpm, utilizing a shaking incubator (Ashwood, VIC, Australia). Afterwards, the samples underwent centrifugation at 4°C and 8000 rpm for 15 min (Tuttlingen, BW, Germany), and filtered through a 0.22 μm nylon membrane filter (Thermo Fisher Scientific Inc., USA). The supernatant was carefully collected and stored at −20°C, awaiting subsequent analysis. For LC‐MS/MS analysis, the extract was filtered through a syringe filter with a pore size of 0.45 μL.
2.3. Polyphenol Content and Antioxidant Activity
The modified methodologies from Gu et al. (2019) and Suleria, Barrow, and Dunshea (2020) were used for quantification of polyphenol compounds. Seven antioxidant assays, including ferric reducing antioxidant power (FRAP), DPPH, ABTS, total antioxidant capacity (TAC), reducing power assay (RPA), hydroxyl radical scavenging activity (•OH‐RSA), and ferrous ion chelating activity (FICA), were used in this investigation. All assays were conducted in triplicates by Multiskan Go microplate photometer (Waltham, MA, USA). Standard curves were generated with an R‐squared value exceeding 0.995.
2.3.1. Total Phenolic Acid Content (TPC) Assessment
The TPC was determined spectrophotometrically as per the methodology reported by Samsonowicz, Regulska (Samsonowicz et al. 2019). A mixture of 25 μL of the extracts, 25 μL of Folin–Ciocalteu reagent (diluted 1:3 with water), and 200 μL of water were added to a 96‐well plate. Samples were incubated at 25°C for 5 min, with an additional 1‐h incubation at the same temperature after adding 25 μL 10% (w/w) sodium carbonate. Absorbance was measured (Waltham, MA, USA) at 765 nm. Varying concentrations of gallic acid, ranging from 0 to 200 μg/mL were used for standard curve creation.
2.3.2. Total Flavonoid Content (TFC) Assay
The aluminum chloride method reported by Stavrou, Christou, and Kapnissi‐Christodoulou (2018) was used for TFC measurement. A mixture of 2% aluminum chloride (80 μL) and 50 g/L sodium acetate (120 μL) was added to a 96‐well plate. The mixture was allowed to incubate for 2.5 h at 25°C. Absorbance was read at 440 nm. A standard curve was constructed using a methanolic solution of quercetin (with concentrations ranging from 0 to 50 μg/mL) and presented as mg quercetin equivalents (QE)/g of sample.
2.3.3. Total Condensed Tannin (TCT) Assay
Quantification of TCT was undertaken according to the methodology reported by Peng et al. (2019) with some modifications. In brief, 25 μL of the extract and 150 μL 4% (w/v) vanillin solution were mixed, followed by the addition of 25 μL sulfuric acid (32%). The solution was incubated at room temperature for 15 min. Absorbance was read at 500 nm. A standard curve was created using a catechin solution with concentrations between 0 and 1000 μg/mL. The results were reported as mg catechin equivalents (CE)/g of the sample.
2.3.4. DPPH Evaluation
The DPPH activity was determined using the procedure of Vella, Cautela, and Laratta (2019). In brief, the extract (40 μL) was mixed with 0.1 M DPPH solution in methanol (260 μL) and allowed to incubate at 25°C for 30 min. Absorbance was measured at 517 nm. A standard curve was created by employing various doses of ascorbic acid dissolved in an aqueous solution, with a range of 0–50 μg/mL. The results were presented as ascorbic acid equivalent (AAE)/g of the sample.
2.3.5. Ferric Reducing Antioxidant Power Assay
The FRAP assay was undertaken using a method outlined by Sogi et al. (2013), with slight modifications. In brief, the FRAP reagent was freshly made by combining a 300 mM solution of sodium acetate (with a pH of 3.6), a 10 mM solution of TPTZ (2,4,6‐tripyridyl‐s‐triazine), and a 20 mM solution of ferric chloride in a volume ratio of 10:1:1, respectively. The extract (25 μL) was mixed with the FRAP reagent (280 μL). The mixture was allowed to incubate for 30 min at 37°C. Absorbance was measured at 593 nm. Ascorbic acid (0–50 μg/mL) was used for standard curve creation. The results were presented as mg of AAE/g of the sample.
2.3.6. ABTS Evaluation
The ABTS assay, as described by Sulastri, Zubair (Vella, Cautela, and Laratta 2019) was used with modifications. A freshly prepared ABTS+ dye was prepared by mixing 1.25 mL of a 7 mmol/L ABTS solution with 22 μL of 140 mmol/L potassium persulfate solutions, followed by a 16‐h incubation period in darkness at room temperature to facilitate radical formation. The ABTS reagent (290 μL) was combined with 10 μL of the sample solution. The mixture was incubated for 6 min in darkness (25°C). Absorbance was recorded at 734 nm. Ascorbic acid (0–150 μg/mL) was used for standard curve creation. The results were presented as mg AAE/g of the sample.
2.3.7. Reducing Power Assay
The RPA assay was conducted according to the method reported by Ali et al. (2021). The extract (20 μL) was mixed with 20 μL of 1% potassium ferricyanide K3[Fe (CN)6] and 50 μL of 0.2 M phosphate buffer. The mixture was heated in a water bath at 25°C for 20 min. Following this, 20 μL of 10% trichloroacetic acid was added. The solution was then centrifuged at 3000 rpm for 10 min. A 50 μL portion of the supernatant was collected and mixed with distilled water (50 μL) and 0.1% FeCl3 (10 μL). Absorbance was measured at 750 nm. A standard curve was established using ascorbic acid concentrations ranging from 0 to 300 μg/mL, with results presented as mg AAE/g.
2.3.8. Hydroxyl Radical Scavenging Activity
The •OH‐RSA assay was performed according to the method reported by Smirnoff and Cumbes (1989). A mixture of 50 μL extract, 50 μL of FeSO4·7H2O (6 mM), and 50 μL of 6 mM H2O2 (30%) were added in sequence. This solution was incubated at 25°C for 10 min. Thereafter, 50 μL of 3‐hydroxybenzoic acid (6 mM) was added. Absorbance was read at 510 nm. Ascorbic acid (0–300 μg/mL) was used for a standard curve creation. The results were reported as mg AAE/g of the sample.
2.3.9. Ferrous Ion Chelating Activity
The FICA value was quantified using a method adapted from Dinis, Madeira, and Almeida (1994). Initially, the extract (15 μL) was combined with distilled water (85 μL). Then, 50 μL of a 1:15 diluted 2 mM ferrous chloride solution and 50 μL of a 1:6 diluted 5 mM ferrozine solution were added to this mixture. The resulting solution was incubated at room temperature for 10 min, and the absorbance was measured at 562 nm. A standard curve was created using ethylenediaminetetraacetic acid (EDTA). The results were presented as mg EDTA equivalent/g of the sample.
2.3.10. Total Antioxidant Capacity
The assessment of TAC in the samples was conducted through the modified phosphomolybdate method, as reported by Du et al. (2021). Briefly, a phosphomolybdate reagent was formulated, which consisted of sulfuric acid (0.6 M), ammonium molybdate (4 mM), and sodium phosphate (20 mM). Then, 40 μL of the extract was dispensed into 260 μL of the reagent and subjected to an incubation period of 90 min at 90°C. Absorbance was read at 695 nm. The standard curve was created using ascorbic acid (0–200 μg/mL). The results were presented as mg AAE/g of the sample.
2.4. LC‐ESI‐QTOF‐MS/MS Analysis
This analysis was performed according to the methodology described by Zhong et al. (2020). An HPLC system was connected to an Agilent 6520 LC‐ESI‐QTOF‐MS/MS platform (Agilent Technologies, CA, USA). Chromatographic separation was achieved using a Synergi Hydro‐RP 80 Å reverse phase column (250 × 4.6 mm; particle size = 4 μm) and a protected C18 ODS guard column. Eluent A was a mix of water and acetic acid (98:2, v/v). Eluent B was a combination of acetonitrile, water, and acetic acid (50:49.5:0.5, v/v/v). The elution protocol was initiated with a degassing of both mobile phases for 15 min at 21°C. The elution gradient commenced at 10% eluent B, progressively increasing to 25% at 20 min, 35% at 30 min, 40% at 40 min, then advancing to 55% at 70 min, peaking at 80% by 75 min, and 100% B from 77 to 79 min, 10% B from 82 to 85 min.
Ionization was enhanced with precisely set capillary (3.5 kV) and nozzle (500 V) voltages, nitrogen gas at 45 psi nebulizing and drying at 300°C, and sheath gas at 11 L/min and 250°C. Mass spectrometric analysis covered a broad m/z range (50–1300 amu) using automated MS/MS fragmentation at 10, 15, and 30 eV to identify positive and negative ion peaks. The MassHunter workstation software (Agilent Technologies, Santa Clara, CA, USA) was utilized for instrument control, data acquisition, and processing during the experiment.
2.5. Statistical Analysis
Each assay was replicated three times and presented as mean ± standard deviation. Data analysis was performed via Minitab 19 (Minitab for Windows Release 19, Minitab Inc., Chicago, USA) utilizing a one‐way analysis of variance (ANOVA). Tukey's HSD post hoc test was used for means comparison. The significance level was established at p < 0.05.
3. Results and Discussion
3.1. Polyphenol Compound Concentration
Samples sourced from QLD had generally higher TPC, TFC, and TCT values than samples from WAU (Table 1). Among them, QLD1 displayed the greatest TPC (5.97 ± 0.55 mg GAE/g), which is significantly higher than the value reported by Gharsallah et al. (2023) from 1.1 to 2.1 mg GAE/g dry weight. However, Shih et al. (2011) reported a higher value from 71.9 to 134.4 mg GAE/g. Geographical location and weather conditions between the two regions can potentially result in significant differences. For example, total polyphenol compounds were much higher in winter than in summer samples. Iqbal and Bhanger (2006) reported the same results and explained that this could be due to the degradation of polyphenols due to the increase in mono‐linear oxygen under UV irradiation. Moreover, Sulastri et al. (2018) reported that different altitudes also resulted in changes in polyphenol content in moringa. Moringa cultivated at medium altitudes exhibited higher total polyphenol, flavonoid, and quercetin contents compared to moringa plants cultivated at very low and high altitudes (15–150 m above sea level). Therefore, the different geographical locations and climates of Queensland and Western Australia may have contributed to this significant difference.
TABLE 1.
Estimation of polyphenol compounds from moringa pods in Queensland (QLD) and Western Australia (WAU).
| Samples | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) |
|---|---|---|---|
| QLD1 | 5.97 ± 0.55a | 8.88 ± 0.49a | 1.95 ± 0.10c |
| QLD2 | 5.00 ± 0.15b,c | 4.84 ± 0.08c | 2.07 ± 0.06bc |
| QLD3 | 4.58 ± 0.37b,cd | 3.98 ± 0.13def | 2.65 ± 0.14a |
| QLD4 | 3.46 ± 0.15fg | 4.44 ± 0.09cd | 2.34 ± 0.14ab |
| QLD5 | 2.32 ± 0.09h | 4.44 ± 0.15cd | 2.38 ± 0.15ab |
| QLD6 | 1.72 ± 0.10h | 2.57 ± 0.10h | 1.57 ± 0.04d |
| QLD7 | 1.64 ± 0.07h | 3.41 ± 0.09efg | 1.55 ± 0.09d |
| QLD average | 3.53 ± 0.21 | 4.62 ± 0.16 | 2.07 ± 0.10 |
| WAU5 | 2.84 ± 0.13g | 7.98 ± 0.59b | 2.15 ± 0.20bc |
| WAU7 | 3.91 ± 0.27def | 4.31 ± 0.05cd | 1.51 ± 0.12de |
| WAU10 | 3.48 ± 0.24g | 3.27 ± 0.18de | 1.42 ± 0.14de |
| WAU15 | 3.07 ± 0.27cde | 4.08 ± 0.28h | 1.49 ± 0.03c |
| WAU17 | 4.31 ± 0.25fg | 2.68 ± 0.14fgh | 1.92 ± 0.07de |
| WAU20 | 3.48 ± 0.24b | 3.27 ± 0.18gh | 1.42 ± 0.14e |
| WAU32 | 3.14 ± 0.32efg | 3.13 ± 0.05cd | 1.19 ± 0.09d |
| WAU average | 3.48 ± 0.26 | 4.24 ± 0.20 | 1.60 ± 0.10 |
Note: Values are mean of 3 replications ± standard deviation. a‐hMeans within the same column with dissimilar superscript letters differ (p < 0.05).
The TFC value ranged from 2.57 to 8.88 mg QE/g in QLD samples and 2.68 to 7.98 mg QE/g in WAU samples. The highest TPC content was quantified in QLD1 at 8.88 mg GAE/g, which agrees with the findings of Braham et al. (2020), reporting that the TPC value ranged from 3.7 to 9.1 mg QUE/g depending on the extraction solvent. The authors also demonstrated that flavonoid content in moringa was also strongly influenced by the extraction solvent, explaining the large difference between the results of this experiment and those reported in the literature. Sulastri et al. (2018) reported the influence of agroclimatic conditions on TFC and TPC in moringa leaves sourced from various regions and noted a correlation between the geographical location and the TFC, similar to the trend observed for TPC. The authors suggested that this correlation might stem from the polyphenol properties of flavonoids. Hani et al. (2017) also highlighted the importance of factors such as maturity stage, climate, post‐harvest handling, and solvent type on TPC and TFC measurements. In support, our current experiment demonstrated consistently elevated levels of both TPC and TFC in the samples obtained from Queensland.
The TCT analysis showed that the samples originating from QLD had significantly higher values than those originating from WAU. On average, the QLD samples had a value of 2.07 ± 0.10 mg CE/g versus an average of 1.60 ± 0.10 mg CE/g in WAU samples. Additionally, the individual samples from QLD mostly had values that exceeded those of all the WAU samples. The average tannins level in this experiment was significantly lower than previously reported which reported at 4.9 mg catechin/g (Adisakwattana and Chanathong 2011). Tannins possess anticancer, anti‐inflammatory, and anti‐hepatotoxic properties (Vergara‐Jimenez, Almatrafi, and Fernandez 2017). However, consuming tannins in high doses can be toxic and may lead to adverse side effects such as abdominal pain, vomiting, nausea, and liver damage (Baldwin and Booth 2022). Therefore, the moderate tannin content of moringa pods makes them more suitable for use in the food industry.
3.2. Antioxidant Activity
The DPPH and ABTS assay results consistently showed that the samples from QLD had higher mean values (Table 2). The DPPH and ABTS values for QLD samples averaged 2.87 and 15.0 mg AAE/g, respectively. Specifically, the highest antioxidant activity, as determined by the DPPH and ABTS tests, was quantified in QLD6 and QLD5 samples, respectively. Conversely, the mean values of DPPH and ABTS for WAU samples were 2.74 and 12.9 AAE/g, respectively. A review of the existing literature reveals discrepancies in the antioxidant activities observed in the current experiment. For instance, Nobossé, Fombang, and Mbofung (2018) documented ABTS values ranging from 3.44 to 3.86 mg AAE/g in moringa samples. These variations in results are likely attributed to factors such as the tree's age and the solvents used for extraction.
TABLE 2.
Estimation of antioxidant capacity of moringa pods in Queensland (QLD) and Western Australia (WAU).
| Samples | DPPH (mg AAE/g) | ABTS (mg AAE/g) | FRAP (mg AAE/g) | RPA (mg AAE/g) | •OH‐RSA (mg AAE/g) | FICA (mg EDTA/g) | TAC (mg AAE/g) |
|---|---|---|---|---|---|---|---|
| QLD1 | 2.65 ± 0.14cde | 14.8 ± 0.8cde | 14.6 ± 1.4bcd | 3.50 ± 0.09e | 96.3 ± 3.1bc | 0.80 ± 0.07bcd | 2.48 ± 0.16b |
| QLD2 | 2.93 ± 0.12bc | 15.1 ± 0.5bcd | 14.5 ± 0.8bcd | 5.82 ± 0.578d | 88.1 ± 2.4cd | 0.73 ± 0.04cd | 2.37 ± 0.13b |
| QLD3 | 2.84 ± 0.14bcd | 16.0 ± 0.1bc | 13.5 ± 0.5cde | 3.04 ± 0.15ef | 97.2 ± 3.4bc | 0.79 ± 0.06bcd | 2.96 ± 0.10a |
| QLD4 | 2.76 ± 0.19bcde | 16.9 ± 0.9ab | 12.7 ± 0.5de | 7.85 ± 0.26c | 84.5 ± 5.4d | 0.83 ± 0.01bcd | 2.55 ± 0.21b |
| QLD5 | 3.05 ± 0.16bc | 17.5 ± 1.5a | 14.7 ± 0.6bcd | 5.57 ± 0.16d | 95.8 ± 5.0bc | 0.83 ± 0.01bcd | 3.11 ± 0.24a |
| QLD6 | 3.23 ± 0.21ab | 12.4 ± 0.6fg | 9.30 ± 0.2f | 2.31 ± 0.20f | 100.6 ± 1.4b | 1.05 ± 0.08a | 0.44 ± 0.03e |
| QLD7 | 2.63 ± 0.14cde | 12.4 ± 0.7fg | 11.6 ± 0.4ef | 2.94 ± 0.06ef | 98.6 ± 4.0b | 0.84 ± 0.02bc | 2.44 ± 0.13b |
| QLD average | 2.87 ± 0.16 | 15.0 ± 0.74 | 13.0 ± 0.64 | 4.43 ± 0.21 | 94.4 ± 3.52 | 0.84 ± 0.04 | 2.34 ± 0.14 |
| WAU5 | 3.61 ± 0.11a | 13.6 ± 0.9def | 15.1 ± 0.7bc | 3.30 ± 0.16e | 95.7 ± 3.01bc | 1.06 ± 0.08a | 0.27 ± 0.01e |
| WAU7 | 2.36 ± 0.22de | 14.6 ± 0.2cde | 15.7 ± 1.1bc | 0.74 ± 0.07g | 119.1 ± 5.8a | 0.87 ± 0.01bc | 1.84 ± 0.03c |
| WAU10 | 2.33 ± 0.04e | 12.1 ± 0.1efg | 13.9 ± 0.2bcd | 7.81 ± 0.12a | 124.8 ± 1.5a | 0.91 ± 0.08bc | 1.71 ± 0.07b |
| WAU15 | 2.32 ± 0.18bc | 13.0 ± 0.5g | 14.6 ± 0.4a | 12.34 ± 0.23f | 124.6 ± 1.6a | 0.84 ± 0.07bcd | 1.31 ± 0.01d |
| WAU17 | 3.03 ± 0.20de | 11.4 ± 0.7fg | 18.4 ± 0.9cd | 2.44 ± 0.08c | 117.3 ± 1.0a | 0.80 ± 0.04ab | 1.82 ± 0.09c |
| WAU20 | 2.33 ± 0.04cde | 12.9 ± 0.1fg | 13.8 ± 0.2ab | 7.81 ± 0.12b | 124.8 ± 1.5a | 0.91 ± 0.08d | 1.71 ± 0.07c |
| WAU32 | 2.83 ± 0.19a | 12.3 ± 0.4defg | 16.3 ± 1.3ab | 10.2 ± 0.50c | 122.9 ± 2.4a | 0.68 ± 0.05bcd | 1.74 ± 0.14cd |
| WAU average | 2.74 ± 0.17 | 12.9 ± 0.4 | 15.8 ± 0.8 | 3.50 ± 0.09e | 118.5 ± 2.6 | 0.84 ± 0.05 | 1.46 ± 0.07 |
Note: Values are mean of 3 replications ± standard deviation. a–gMeans within the same column with dissimilar superscript letters differ (p < 0.05).
In contrast to the DPPH and ABTS assay results, the FRAP assay data indicated higher values for the samples from WAU as compared to those from QLD (15.8 ± 0.8 vs. 13.0 ± 0.64 mg AAE/g), with WAU17 exhibiting the maximum reducing power (18.4 mg AAE/g), while the lowest was observed in QLD6 (9.30 mg AAE/g). This variation may be attributed to differences in antioxidant assay procedures. The FRAP assay evaluates antioxidant ability based on the reducing power, which involves the ability of antioxidant chemicals to donate electrons and reduce Fe3+ to Fe2+ ions.
The RPA assay utilizes a colorimetric method where the reduction of the Fe3+/ferricyanide complex induces a color change upon conversion to the ferrous form. In this experiment, the RPA results mirrored the trend observed in the FRAP assay, showing generally higher values for the samples from WAU. Among these samples, WAU15 exhibited the highest reducing power (12.3 ± 0.23 mg AAE/g). The consistent findings from both assays support the reliability of the results and confirm the stronger reducing power of the samples originating from WAU.
The WAU samples had generally higher •OH‐RSA activity than the QLD samples (118.5 vs. 94.4 mg AAE/g). Several investigations have stated that flavonoids are effective scavengers of hydroxyl free radicals, implying the contribution of flavonoids to the scavenging activity (Hu et al. 2021). However, this study found no statistically significant association between TFC and •OH‐RSA. The •OH‐RSA assay quantifies the ability to scavenge the highly reactive hydroxyl radical (•OH) generated in the Fenton reaction. Chronic exposure to this radical may cause significant health concerns.
Polyphenol compounds can bind to metal ions like Fe2+, providing a means to assess their antioxidant capabilities. Both samples from QLD and WAU demonstrated significant metal chelating capabilities, likely attributed to their higher flavonoid content as flavonoids are known to be linked with their chelating capacity. The assay results showed a range of 0.73–1.06 mg EDTA/g, averaging 0.84 mg EDTA/g across all samples.
Polyphenolic compounds, including flavonoids and phenolic acids, may contribute to antioxidant ability through following mechanisms: (1) neutralization of free radicals, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), by donating hydrogen atoms or electrons to reduce oxidative stress; (2) inhibition of enzymes involved in the production of these radicals or chelating trace metals that catalyze ROS/RNS formation; and (3) regulating or enhancing endogenous antioxidant defense systems (Hassan et al. 2021). Polyphenols such as quercetin and caffeic acid determined by LC‐ESI‐QTOF‐MS in this study demonstrated strong free radical scavenging and metal‐chelating abilities, which are likely responsible for the observed antioxidant effects. These mechanisms may explain the higher antioxidant activities in the QLD samples as indicated by DPPH and ABTS assays. These findings are consistent with previous findings on how environmental factors, such as climate, soil, and UV radiation, on the synthesis and accumulation of bioactive compounds in plants (Özcan 2020).
3.3. Correlation Between Polyphenol Content and Antioxidant Assays
The TCT strongly correlated with both ABTS and •OH‐RSA antioxidant assays (Table 3). Specifically, a correlation coefficient (r) of 0.765 for TCT and ABTS indicates a substantial positive relationship, and the high correlation suggests that the condensed tannins present in moringa pods are potent radical scavengers. This is possibly owing to their polyphenol structures which can donate hydrogen atoms to neutralize free radicals. Similarly, the strong positive correlation of TCT with •OH‐RSA (r = 0.744) may indicate the effectiveness of condensed tannins in neutralizing hydroxyl radicals. The ability of moringa pod to counteract these radicals highlights its potential application as a potent natural antioxidant source within the food industry.
TABLE 3.
Pearson's correlation coefficients.
| Variables | TPC | TFC | TCT | DPPH | FRAP | ABTS | •OH‐RSA | RPA | FICA |
|---|---|---|---|---|---|---|---|---|---|
| TFC | 0.319 | ||||||||
| TCT | 0.015 | 0.346 | |||||||
| DPPH | 0.216 | 0.229 | 0.438 | ||||||
| FRAP | 0.495 | 0.124 | 0.062 | 0.074 | |||||
| ABTS | 0.001 | 0.345 | 0.765** | 0.073 | 0.136 | ||||
| •OH‐RSA | 0.105 | 0.399 | 0.744** | 0.502 | 0.482 | 0.650* | |||
| RPA | 0.085 | 0.156 | 0.297 | 0.360 | 0.145 | 0.061 | 0.362 | ||
| FICA | 0.594* | 0.132 | 0.027 | 0.415 | 0.499 | 0.153 | 0.158 | 0.384 | |
| TAC | 0.209 | 0.023 | 0.472 | 0.337 | 0.080 | 0.630* | 0.333 | 0.014 | 0.661** |
Indicates a significant correlation with p ≤ 0.05.
Indicates a significant correlation with p ≤ 0.01.
A moderate positive correlation (r = 0.594) as shown in FICA and TPC may indicate that polyphenol content is significantly related to iron‐chelating activity. Polyphenol compounds play a role in chelating ferric ions, significantly reducing oxidative stress. A robust correlation between TAC and ABTS (r = 0.630) suggests that samples with higher total antioxidant capacities exhibit higher scavenging activity against the ABTS radical cation, potentially validating the consistency between these assays in measuring the antioxidant potential.
Previous studies have reported a positive correlation between polyphenol content, suggesting that as the content of polyphenol compounds increases, the overall antioxidant capacity also increases (González‐Romero et al. 2020). This observation aligns with the findings of our study, where higher TAC values were observed in QLD samples along with elevated levels of TPC, TFC, and TCT.
Figure 1 illustrates the Principal Component Analysis (PCA) of antioxidant components, including TPC, TFC, TCT, and individual antioxidant activity assays such as DPPH, ABTS, FRAP, •OH‐RSA in the moringa pod samples originated from QLD and WAU. The ABTS, TFC, and TCT vectors were closely positioned in the loading plot, indicating a high correlation between these antioxidant compounds. These are concentrated on the negative side of principal component 1 (F1), accounting for 32.81% of the variance in the data. The antioxidant assays form a distinct cluster, reflecting their cumulative contribution to total antioxidant activity but independence from phenolic acid and flavonoid concentration.
FIGURE 1.
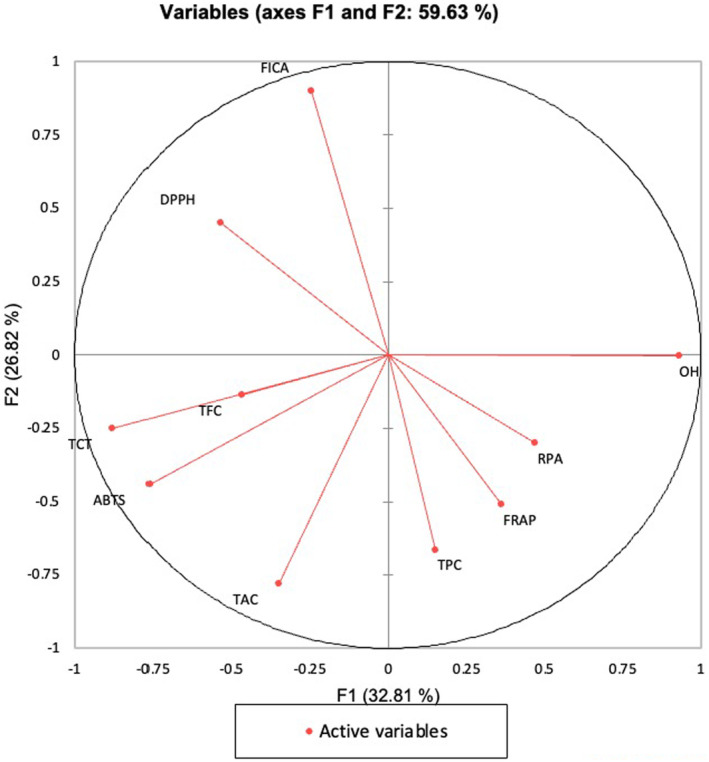
Principal component analysis of antioxidant components and polyphenol compounds.
3.4. Polyphenol Characterization
Identification of polyphenol components in the moringa pod samples from two regions was performed using qualitative analysis by LC‐ESI‐QTOF‐MS. Results with a mass inaccuracy exceeding ± 5 ppm were excluded. In general, there was a considerable diversity of antioxidant compounds within the samples from both regions. In total, 111 polyphenol compounds were identified, comprising 32 phenolic acids, 54 flavonoids, 13 other phenolic compounds, 3 lignans, and 9 stilbenes (Table 4).
TABLE 4.
Characterization of polyphenol compounds in moringa pod by LC‐ESI‐QTOF MS/MS.
| No. | Proposed compounds | Molecular formula | RT (min) | Ionization (ESI + /ESI−) | Molecular weight | Theoretical (m/z) | Observed (m/z) | MS2 Productions | Error (ppm) | Moringa samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Hydroxybenzoic acids | ||||||||||
| 1 | Protocatechuic acid | C7H6O4 | 8.601 | [M + H]+ | 154.0271 | 155.0344 | 155.0338 | 109, 139 | −3.9 | a QLD, WAU5 |
| 2 | 4‐Hydroxybenzoic acid 4‐O‐glucoside | C13H16O8 | 35.595 | [M − H]− | 300.0867 | 299.0794 | 299.0795 | 255, 137 | 0.3 | a QLD1, QLD5 |
| 3 | Gallic acid | C7H6O5 | 37.239 | [M − H]− | 170.0231 | 169.0158 | 169.0157 | 125 | −0.6 | a QLD1, QLD7 |
| 4 | Paeoniflorin | C23H28O11 | 54.258 | [M − H]− | 480.1648 | 479.1575 | 479.1562 | 449, 327 | −2.7 | a QLD1, QLD2, QLD5, WAU15, WAU20 |
| 5 | 4‐O‐Methylgallic acid | C8H8O5 | 58.44 | [M + H]+ | 184.0388 | 185.0461 | 185.0461 | 124, 170 | 0.0 | a WAU5, WAU20, WAU 32 |
| 6 | Benzoic acid | C7H6O2 | 60.026 | b [M − H]− | 122.0375 | 121.0302 | 121.0302 | 77 | 0.0 | a QLD1, QLD 2, QLD 3, QLD5, QLD 6, QLD7, WAU5, WAU7, WAU10 WAU15, WAU32 |
| 7 | Ellagic acid | C14H6O8 | 61.909 | [M − H]− | 302.0075 | 301.0002 | 301.0007 | 257 | 1.7 | QLD4 |
| 8 | Protocatechuic acid 4‐O‐glucoside | C13H16O9 | 65.464 | [M + H]+ | 316.079 | 317.0863 | 317.0856 | 153 | −2.2 | a QLD2, QLD6, WAU7 |
| 9 | 3,4‐O‐Dimethylgallic acid | C9H10O5 | 67.076 | [M + H]+ | 198.0546 | 199.0619 | 199.0619 | 153, 139, 125, 111 | 0.0 | a QLD6, QLD7, WAU20 |
| 10 | 2‐Hydroxybenzoic acid | C7H6O3 | 68.424 | b [M − H]− | 138.0322 | 137.0249 | 137.0249 | 93, 65 | 0.0 | a QLD1, QLD2, QLD4, QLD5, QLD6, QLD7, WAU10, WAU 7, WAU15 |
| Hydroxycinnamic acids | ||||||||||
| 11 | Caffeoyl glucose | C15H18O9 | 11.187 | b [M − H]− | 342.0966 | 341.0893 | 341.0891 | 179, 161 | −0.6 | a QLD1, QLD3, QLD5, QLD 6, QLD7, WAU5 WAU10, WAU15, WAU20, WAU32 |
| 12 | 1‐Sinapoyl‐2‐feruloylgentiobiose | C33H40O18 | 11.702 | b [M − H]− | 724.2205 | 723.2132 | 723.2124 | −1.1 | a QLD7, WAU5, WAU10, WAU17 | |
| 13 | 4,5‐Dicaffeoylquinic acid | C25H24O12 | 11.828 | [M − H]− | 516.1305 | 515.1232 | 515.1232 | 191 | 0.0 | a QLD3, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 |
| 14 | Ferulic acid 4‐O‐glucuronide | C16H18O10 | 13.618 | b [M − H]− | 370.0899 | 369.0826 | 369.0825 | 193, 175 | −0.3 | a QLD4, QLD5, WAU20 |
| 15 | Rosmarinic acid | C18H16O8 | 28.355 | [M − H]− | 360.086 | 359.0787 | 359.0785 | 197, 179 | −0.6 | a QLD1, QLD 4 |
| 16 | Caffeoyl C1‐glucuronide | C15H16O10 | 39.359 | b [M − H]− | 356.0742 | 355.0669 | 355.0663 | −1.7 | a QLD1, QLD4, QLD6, WAU7, WAU10, WAU32 | |
| 17 | Ferulic acid 4‐O‐glucoside | C16H20O9 | 41.558 | [M − H]− | 356.1126 | 355.1053 | 355.1045 | 193, 178, 149, 134 | −2.3 | a QLD1, QLD3, QLD5, QLD7, WAU5, WAU10, WAU15, WAU17, WAU20 |
| 18 | Caffeic acid | C9H8O4 | 41.923 | [M − H]− | 180.0438 | 179.0365 | 179.0368 | 143, 135 | 1.7 | QLD1 |
| 19 | Ferulic acid | C10H10O4 | 48.519 | [M − H]− | 194.0589 | 193.0516 | 193.0519 | 178, 149 | 1.6 | a QLD1, QLD3, QLD5 |
| 20 | m‐Coumaric acid | C9H8O3 | 50.67 | [M − H]− | 164.0462 | 163.0389 | 163.0388 | 148, 119 | −0.6 | a QLD1, QLD6, WAU17 |
| 21 | 3‐Sinapoylquinic acid | C18H22O10 | 52.062 | b [M − H]− | 398.1217 | 397.1144 | 397.1144 | 233, 179 | 0.0 | a QLD1, QLD2, QLD3, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU17, WAU 20, WAU32 |
| 22 | Feruloyl tartaric acid | C14H14O9 | 52.071 | [M − H]− | 326.063 | 325.0557 | 325.0551 | 193, 149 | −1.8 | a WAU5, WAU7, WAU10, WAU15, WAU17, WAU32, QLD2, QLD3, QLD 5, QLD6, QLD7 |
| 23 | p‐Coumaroyl tartaric acid | C13H12O8 | 52.55 | [M − H]− | 296.0505 | 295.0432 | 295.0423 | 115 | −3.1 | WAU10 |
| 24 | 5‐p‐Coumaroylquinic acid | C16H18O8 | 63.523 | b [M − H]− | 338.103 | 337.0957 | 337.0956 | 163, 191 | −0.3 | a QLD1, QLD2, QLD3, QLD4, QLD5, QLD6, QLD7, WAU5 WAU7, WAU10, WAU15, WAU32 |
| 25 | 5‐Feruloylquinic acid | C17H20O9 | 65.315 | b [M + H]+ | 368.1076 | 369.1149 | 369.1147 | 173, 191 | −0.5 | a QLD1, QLD2, QLD3, QLD4, QLD5, QLD6, QLD7, WAU7, WAU 10WAU15, WAU17, WAU32 |
| 26 | p‐Coumaric acid 4‐O‐glucoside | C15H18O8 | 66.484 | b [M + H]+ | 326.1017 | 327.109 | 327.1087 | 163 | −0.9 | a QLD1, QLD3, WAU5, WAU 32 |
| 27 | Cinnamoyl glucose | C15H18O7 | 66.526 | b [M + H]+ | 310.1037 | 311.111 | 311.111 | 147, 131, 103 | 0.0 | a QLD3, WAU15, WAU32 |
| 28 | 3‐Caffeoylquinic acid | C16H18O9 | 67.638 | b [M + H]+ | 354.0935 | 355.1008 | 355.1005 | 253, 190, 144 | −0.8 | a QLD1, QLD4, WAU7, WAU15, WAU17, WAU20 |
| 29 | p‐Coumaroyl malic acid | C13H12O7 | 68.652 | b [M + H]+ | 280.0568 | 281.0641 | 281.0641 | 163, 119 | 0.0 | a WAU10, WAU15 |
| Hydroxyphenylacetic acids | ||||||||||
| 30 | 3,4‐Dihydroxyphenylacetic acid | C8H8O4 | 42.768 | [M − H]− | 168.0438 | 167.0365 | 167.0362 | 149, 123 | −1.8 | a QLD1, QLD6, QLD7 |
| Hydroxyphenylpropanoic acids | ||||||||||
| 31 | Dihydrocaffeic acid 3‐O‐glucuronide | C15H18O10 | 9.451 | [M − H]− | 358.0935 | 357.0862 | 357.0861 | 181 | −0.3 | a QLD1, QLD2, QLD4, QLD5, QLD6, QLD7 |
| 32 | Dihydroferuloylglycine | C12H15NO5 | 31.054 | b [M − H]− | 253.0934 | 252.0861 | 252.086 | 173,151 | −0.4 | a QLD6, WAU32 |
| Flavonoids | ||||||||||
| Anthocyanins | ||||||||||
| 33 | Pelargonidin | C15H11O5 | 12.015 | [M − H]− | 271.0611 | 270.0538 | 270.0534 | 225, 215 | −1.5 | QLD5 |
| 34 | Cyanidin 3‐O‐(6″‐acetyl‐glucoside) | C23H23O12 | 63.96 | [M − H]− | 491.1195 | 490.1122 | 490.1118 | −0.8 | a QLD1, QLD2, QLD3, QLD5, QLD6 QLD7, WAU7 | |
| 35 | Petunidin 3,5‐O‐diglucoside | C28H33O17 | 68.834 | [M + H]+ | 641.1706 | 642.1779 | 642.1797 | 2.8 | QLD2 | |
| Dihydrochalcones | ||||||||||
| 36 | Dihydromyricetin 3‐O‐rhamnoside | C21H22O12 | 63.353 | [M − H]− | 466.1111 | 465.1038 | 465.1037 | 301 | −0.2 | a QLD1, WAU5, WAU32 |
| 37 | Dihydroquercetin 3‐O‐rhamnoside | C21H22O11 | 63.86 | [M − H]− | 450.1138 | 449.1065 | 449.1051 | −3.1 | WAU17 | |
| 38 | 3‐Hydroxyphloretin 2′‐O‐glucoside | C21H24O11 | 66.534 | [M − H]− | 452.1313 | 451.124 | 451.1235 | 289, 273 | −1.1 | a QLD3, QLD4, QLD5, QLD7, WAU7 |
| Flavanols | ||||||||||
| 39 | 4″‐O‐Methylepigallocatechin 3‐O‐gallate | C23H20O11 | 9.811 | [M − H]− | 472.1023 | 471.095 | 471.093 | 169, 319 | −4.2 | QLD4 |
| 40 | Prodelphinidin dimer B3 | C30H26O14 | 11.185 | [M + H]+ | 610.1344 | 611.1417 | 611.1444 | 469, 311, 291 | 4.4 | QLD1 |
| 41 | (+)‐Gallocatechin 3‐O‐gallate | C22H18O11 | 11.728 | [M − H]− | 458.0865 | 457.0792 | 457.079 | 289, 169, 125 | −0.4 | QLD5 |
| 42 | 4′‐O‐Methyl‐(−)‐epigallocatechin 7‐O‐glucuronide | C22H24O13 | 12.905 | [M − H]− | 496.1218 | 495.1145 | 495.1145 | 451, 313 | 0.0 | QLD6 |
| 43 | (+)‐Gallocatechin | C15H14O7 | 60.666 | b [M + H]− | 306.0736 | 305.0663 | 305.0657 | −2.0 | a QLD1, QLD6, QLD7, WAU5, WAU7, WAU10, WAU20, WAU32 | |
| 44 | Theaflavin | C29H24O12 | 65 | b [M + H]+ | 564.1295 | 565.1368 | 565.1368 | 0.0 | a WAU5, WAU7 WAU10, WAU15 WAU17, WAU20 WAU32, QLD4, QLD5, QLD6, QLD7 | |
| 45 | Procyanidin dimer B7 | C30H26O12 | 65.848 | b [M − H]− | 578.1434 | 577.1361 | 577.1353 | −1.4 | a QLD1, QLD7, WAU5, WAU7, WAU10, WAU15, WAU20 | |
| Flavanones | ||||||||||
| 46 | Sakuranetin | C16H14O5 | 13.922 | [M + H]+ | 286.085 | 287.0923 | 287.0923 | 255 | 0.0 | WAU5 |
| 47 | Hesperetin 3ʹ‐O‐glucuronide | C22H22O12 | 63.943 | b [M − H]− | 478.1135 | 477.1062 | 477.1054 | 301, 175, 113, 85 | −1.7 | a QLD1, QLD2, QLD3, QLD5, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU20 |
| 48 | Naringin 4ʹ‐O‐glucoside | C33H42O19 | 64.552 | b [M − H]− | 742.2303 | 741.223 | 741.2223 | 433, 271 | −0.9 | a QLD3, QLD5, WAU5, WAU10 |
| 49 | Isoxanthohumol | C21H22O5 | 64.943 | b [M + H]+ | 354.1471 | 355.1544 | 355.1529 | −4.2 | a QLD2, QLD7, WAU7, WAU10, WAU15, WAU17 | |
| 50 | Narirutin | C27H32O14 | 64.996 | b [M + H]+ | 580.1777 | 581.185 | 581.1834 | 271 | −2.8 | a QLD4, QLD6, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20 |
| 51 | 8‐Prenylnaringenin | C20H20O5 | 65.491 | [M + H]+ | 340.1311 | 341.1384 | 341.1383 | 285 | −0.3 | a QLD3, QLD5, QLD6, QLD7 |
| 52 | Neohesperidin | C28H34O15 | 68.158 | b [M + H]+ | 610.1897 | 611.197 | 611.194 | −4.9 | WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 | |
| Flavones | ||||||||||
| 53 | Nepetin | C16H12O7 | 3.082 | b [M + H]+ | 316.0561 | 317.0634 | 317.0624 | −3.2 | a QLD2, QLD5, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 | |
| 54 | Luteolin 7‐O‐(2‐apiosyl‐glucoside) | C26H28O15 | 9.172 | [M + H]+ | 580.1392 | 581.1465 | 581.146 | −0.9 | QLD4 | |
| 55 | Apigenin 6,8‐di‐C‐glucoside | C27H30O15 | 11.409 | [M − H]− | 594.164 | 593.1567 | 593.157 | 503, 473 | 0.5 | a WAU10, WAU15, WAU20, WAU 32 |
| 56 | Nobiletin | C21H22O8 | 14.049 | b [M − H]− | 402.1305 | 401.1232 | 401.1221 | −2.7 | a QLD1, QLD4, WAU5, WAU7, WAU10, WAU15, WAU32 | |
| 57 | Apigenin 6‐C‐glucoside | C21H20O10 | 51.706 | [M − H]− | 432.1066 | 431.0993 | 431.0974 | 413, 341, 311 | −4.4 | QLD5 |
| 58 | Neodiosmin | C28H32O15 | 51.756 | b [M − H]− | 608.1728 | 607.1655 | 607.1626 | −4.8 | a QLD2, QLD4, QLD5, WAU7, WAU 32 | |
| 59 | Gardenin B | C19H18O7 | 52.095 | b [M − H]− | 358.1052 | 357.0979 | 357.0965 | 344, 329, 311 | −3.9 | a QLD1, QLD2, QLD 5, WAU10, WAU15, WAU17, WAU20, WAU32 |
| 60 | Isorhoifolin | C27H30O14 | 55.222 | b [M − H]− | 578.1689 | 577.1616 | 577.1588 | −4.9 | a WAU5, WAU7, WAU15, WAU20, WAU32 | |
| 61 | Apigenin 7‐O‐apiosyl‐glucoside | C26H28O14 | 63.862 | [M + H]+ | 564.145 | 565.1523 | 565.152 | −0.5 | QLD1 | |
| 62 | Apigenin 7‐O‐diglucuronide | C27H26O17 | 64.477 | [M − H]− | 622.1174 | 621.1101 | 621.1105 | 0.6 | a QLD4, QLD5 | |
| 63 | Scutellarein | C15H10O6 | 64.981 | b [M + H]+ | 286.0479 | 287.0552 | 287.0554 | 0.7 | a QLD1, QLD2, QLD3, QLD5, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU 32 | |
| Flavonols | ||||||||||
| 64 | Myricetin 3‐O‐arabinoside | C20H18O12 | 12.27 | [M − H]− | 450.0769 | 449.0696 | 449.0707 | 317 | 2.4 | WAU20 |
| 65 | Kaempferol 7‐O‐glucoside | C21H19O11 | 50.987 | [M − H]− | 447.0948 | 446.0875 | 446.0869 | 162 | −1.3 | a WAU5, WAU17, WAU 20 |
| 66 | Quercetin 3ʹ‐O‐glucuronide | C21H18O13 | 60.34 | b [M + H]+ | 478.0767 | 479.084 | 479.0838 | 301 | −0.4 | a QLD4, WA15 |
| 67 | Spinacetin 3‐O‐(2″″‐p‐coumaroylglucosyl) (1‐ > 6)‐ [apiosyl (1‐ > 2)]‐glucoside | C43H48O24 | 61.84 | b [M + H]+ | 948.2526 | 949.2599 | 949.2594 | −0.5 | a WAU5, WAU15, WAU32 | |
| 68 | Myricetin 3‐O‐galactoside | C21H20O13 | 63.032 | b [M − H]− | 480.0871 | 479.0798 | 479.0802 | 317 | 0.8 | a QLD3, QLD4, WAU 5, WAU7, WAU15, WAU17 |
| 69 | Quercetin 3‐O‐glucosyl‐xyloside | C26H28O16 | 63.487 | b [M − H]− | 596.1381 | 595.1308 | 595.1293 | 265, 138, 116 | −2.5 | a QLD4, WAU5, WAU10 |
| 70 | Quercetin 3‐O‐rhamnoside | C21H20O11 | 64.808 | b [M + H]+ | 448.1011 | 449.1084 | 449.1085 | 0.2 | a WAU5, WAU7, WAU17, WAU20 | |
| 71 | Quercetin 3‐O‐xylosyl‐glucuronide | C26H26O17 | 64.86 | [M + H]+ | 610.1192 | 611.1265 | 611.1256 | 479, 303, 285, 239 | −1.5 | a QLD2, QLD4, WAU10 |
| 72 | Quercetin 3‐O‐rutinoside | C27H30O16 | 65.399 | [M − H]− | 610.1524 | 609.1451 | 609.1437 | −2.3 | a QLD7, WAU17 | |
| 73 | Patuletin 3‐O‐glucosyl‐(1‐ > 6)‐ [apiosyl (1‐ > 2)]‐glucoside | C33H40O22 | 65.472 | [M − H]− | 788.2056 | 787.1983 | 787.1983 | 625, 463, 301, 271 | 0.0 | a QLD2, QLD3 |
| 74 | Quercetin 3‐O‐(6″″‐malonyl‐glucoside) | C24H22O15 | 67.477 | [M − H]− | 550.0988 | 549.0915 | 549.0915 | 0.0 | QLD3, WAU5, WAU10, WAU20 | |
| Isoflavonoids | ||||||||||
| 75 | Tectoridin | C22H22O11 | 9.017 | b [M + H]+ | 462.114 | 463.1213 | 463.1216 | 0.6 | a QLD1, QLD3, QLD7, WAU5, WAU7, WAU10 | |
| 76 | Dalbergin | C16H12O4 | 11.335 | [M − H]− | 268.0742 | 267.0669 | 267.0667 | 252, 224, 180 | −0.7 | WAU5 |
| 77 | Formononetin 7‐O‐glucuronide | C22H20O10 | 11.731 | [M − H]− | 444.1082 | 443.1009 | 443.1015 | 267, 252 | 1.4 | a WAU17, WAU20 |
| 78 | Violanone | C17H16O6 | 41.17 | b [M − H]− | 316.0967 | 315.0894 | 315.0899 | 300, 285, 135 | 1.6 | a QLD1, QLD2, QLD5, QLD6, WAU10, WAU17, WAU32 |
| 79 | 2′‐Hydroxyformononetin | C16H12O5 | 42.303 | b [M − H]− | 286.0829 | 285.0756 | 285.076 | 1.4 | a QLD1, QLD2, QLD5, WAU15, WAU20, WAU32 | |
| 80 | 3′‐O‐Methylviolanone | C18H18O6 | 49.183 | b [M − H]− | 330.1096 | 329.1023 | 329.1023 | 0.0 | a QLD2, QLD7, WAU10, WAU15, WAU20, WAU32 | |
| 81 | 6″‐O‐Acetylgenistin | C23H22O11 | 50.88 | b [M − H]− | 474.1184 | 473.1111 | 473.1115 | 0.8 | a QLD1, QLD2, QLD3, WAU7, WAU10, WAU20 | |
| 82 | Prunetin | C16H12O5 | 51.938 | b [M − H]− | 284.0671 | 283.0598 | 283.0597 | −0.4 | a QLD2, WAU7, WAU20 | |
| 83 | 6″‐O‐Malonylgenistin | C24H22O13 | 58.952 | b [M + H]+ | 518.1058 | 519.1131 | 519.1133 | 271 | 0.4 | a QLD3, WAU15, WAU17, WAU20 |
| 84 | Glycitin | C22H22O10 | 63.998 | b [M − H]− | 446.1226 | 445.1153 | 445.114 | 285 | −2.9 | a QLD5, WAU20, WAU32 |
| 85 | 5,6,7,3′,4′‐Pentahydroxyisoflavone | C15H10O7 | 68.651 | b [M − H]− | 302.0432 | 301.0359 | 301.0357 | 285, 257 | −0.7 | a QLD1, QLD2, QLD3, QLD4, QLD5, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 |
| 86 | 2‐Dehydro‐O‐desmethylangolensin | C15H12O4 | 68.901 | [M − H]− | 256.0756 | 255.0683 | 255.0686 | 135, 119 | 1.2 | a QLD3, QLD4, QLD6, WAU15, WAU17, WAU20, WAU32 |
| Other polyphenols | ||||||||||
| Alkylphenols | ||||||||||
| 89 | 4‐Vinylphenol | C8H8O | 9.489 | b [M − H]− | 120.0576 | 119.0503 | 119.0504 | 0.8 | a QLD2, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU 32 | |
| Hydroxybenzoketones | ||||||||||
| 87 | 2‐Hydroxy‐4‐methoxyacetophenone 5‐sulfate | C9H10O7S | 42.431 | [M − H]− | 262.0173 | 261.01 | 261.0103 | 181, 97 | 1.1 | QLD6 |
| 88 | 2,3‐Dihydroxy‐1‐guaiacylpropanone | C10H12O5 | 68.427 | [M − H]− | 212.0692 | 211.0619 | 211.062 | 167, 123, 105, 93 | 0.5 | a QLD2, QLD3, QLD4, WAU5, WAU7, WAU10, WAU15, WAU 32 |
| Curcuminoids | ||||||||||
| 90 | Demethoxycurcumin | C20H18O5 | 56.969 | b [M − H]− | 338.1121 | 337.1048 | 337.1047 | 217 | −0.3 | a WAU10, WAU15, WAU17, WAU32 |
| 91 | Curcumin | C21H20O6 | 60.583 | b [M − H]− | 368.1226 | 367.1153 | 367.1153 | 217 | 0.0 | a WAU5, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 |
| 92 | Bisdemethoxycurcumin | C19H16O4 | 65.359 | [M + H]+ | 308.1041 | 309.1114 | 309.111 | 291, 263 | −1.3 | WAU15 |
| Tyrosols | ||||||||||
| 93 | Demethyloleuropein | C24H30O13 | 11.644 | b [M + H]+ | 526.1669 | 527.1742 | 527.1742 | 495 | 0.0 | a QLD2, QLD4, QLD5, QLD6, QLD7, WAU5, WAU7, WAU10, WAU15, WAU17, WAU20 |
| Phenolic terpenes | ||||||||||
| 94 | Epirosmanol | C20H26O5 | 17.762 | [M + H]+ | 346.1772 | 347.1845 | 347.1847 | 0.6 | a QLD4, WAU5 | |
| Hydroxyphenylpropenes | ||||||||||
| 95 | Eugenol | C10H12O2 | 68.08 | b [M − H]− | 164.0831 | 163.0758 | 163.0755 | −1.8 | a WAU5, WAU20 | |
| Other polyphenols | ||||||||||
| 96 | Salvianolic acid B | C36H30O16 | 57.92 | [M − H]− | 718.154 | 717.1467 | 717.1452 | 519, 339, 321, 295 | −2.1 | WAU5 |
| 97 | Salvianolic acid C | C26H20O10 | 65.311 | [M − H]− | 492.1074 | 491.1001 | 491.1012 | 311, 267, 249 | 2.2 | QLD2 |
| Hydroxycoumarins | ||||||||||
| 98 | Scopoletin | C10H8O4 | 13.489 | [M − H]− | 192.0414 | 191.0341 | 191.0341 | 174 | 0.0 | a QLD1, QLD2, QLD3, QLD5, QLD6, WAU5, WAU10, WAU15, WAU17, WAU20 |
| Hydroxybenzaldehydes | ||||||||||
| 99 | Vanillin | C8H8O3 | 69.298 | b [M + H]+ | 152.0459 | 153.0532 | 153.0532 | 136,122 | 0.0 | a QLD5, QLD6, WAU5, WAU7, WAU10, WAU17 |
| Stilbenes | ||||||||||
| 100 | Resveratrol 5‐O‐glucoside | C20H22O8 | 32.334 | [M − H]− | 390.1305 | 389.1232 | 389.1225 | 227 | −1.8 | a WAU7, WAU15 |
| 101 | 4‐Hydroxy‐3,5,4′‐trimethoxystilbene | C17H18O4 | 58.99 | b [M + H]+ | 286.1211 | 287.1284 | 287.1293 | 271, 241, 225 | 3.1 | a QLD3, QLD5, QLD6, WAU15 |
| 102 | 3′‐Hydroxy‐3,4,5,4′‐tetramethoxystilbene | C17H18O5 | 68.956 | b [M + H]+ | 302.1146 | 303.1219 | 303.1218 | 229, 201, 187, 175 | −0.3 | a QLD3, QLD6, WAU17 |
| Lignans | ||||||||||
| 103 | 7‐Hydroxymatairesinol | C20H22O7 | 9.447 | b [M + H]+ | 374.1376 | 375.1449 | 375.1451 | 343, 313, 298, 285 | 0.5 | a QLD1, QLD2, QLD 3, QLD4, WAU15 |
| 104 | Schisandrin C | C22H24O6 | 50.981 | b [M − H]− | 384.1595 | 383.1522 | 383.1527 | 370, 315, 300 | 1.3 | a QLD1, QLD5, QLD6, QLD7, WAU5, WAU20 |
| 105 | Schisandrin | C24H32O7 | 55.146 | b [M − H]− | 432.2136 | 431.2063 | 431.2064 | 0.2 | a WAU10, WAU15, WAU20, WAU32 | |
| 106 | Episesamin | C20H18O6 | 56.537 | b [M − H]− | 354.108 | 353.1007 | 353.099 | −4.8 | a QLD2, QLD5, QLD6, WAU7, WAU10, WAU15, WAU17, WAU20, WAU32 | |
| 107 | 7‐Oxomatairesinol | C20H20O7 | 59.041 | b [M + H]+ | 372.1204 | 373.1277 | 373.1276 | 358, 343, 328, 325 | −0.3 | a QLD4, QLD6, QLD7, WAU5, WAU 10, WAU15, WAU20, WAU32 |
| 108 | Schisandrol B | C23H28O7 | 60.35 | b [M − H]− | 416.1822 | 415.1749 | 415.174 | 224, 193, 165 | −2.2 | QLD2, WAU5 |
| 109 | Enterolactone | C18H18O4 | 65.059 | [M + H]+ | 298.1217 | 299.129 | 299.1291 | 281, 187, 165 | 0.3 | WAU5 |
| 110 | Pinoresinol | C20H22O6 | 67.031 | b [M + H]+ | 358.1407 | 359.148 | 359.1473 | 342, 327, 313, 221 | −1.9 | a QLD1, QLD2, QLD3, QLD5, QLD 7, WAU15, WAU10 |
| 111 | Secoisolariciresinol‐sesquilignan | C30H38O10 | 67.814 | [M − H]− | 558.2486 | 557.2413 | 557.2419 | 539, 521, 509, 361 | 1.1 | QLD4 |
Abbreviations: QLD, samples from Queensland; RT, retention time; WAU, samples from Western Australia.
Compound was detected in more than one sample. Data presented in this table are from asterisk sample.
Compounds were detected in both negative [M − H]− and positive [M + H]+ mode of ionization, while only single‐mode data are presented.
3.4.1. Phenolic Acids
Thirty‐two phenolic acids were detected including hydroxybenzoic acids (10), hydroxycinnamic acids (19), hydroxyphenylacetic acid (1), and hydroxyphenylpropanoic acids (2).
Compound 3 was detected as gallic acid, as evidenced by the precursor ion [M − H]− observed at m/z 169.0157. Further confirmation was achieved through MS/MS analysis, which revealed a peak fragment at m/z 125 resulting from the loss of a CO2 unit (44 Da) (Chou et al. 2021). Gallic acid is one of the most significant phenolic acids present in moringa pods and leaves (Amaglo et al. 2010). Gallic acid can neutralize free radicals that can cause cellular damage, potentially reducing the risk of chronic diseases (Kerdsomboon, Chumsawat, and Auesukaree 2021). Gallic acid may exhibit a wide range of biological activities, including anti‐inflammatory, anti‐tumor, antiviral, and antimicrobial activities (Prakash et al. 2007).
Compound 4 was identified to be paeoniflorin based on the detected m/z of 497.1562 in negative mode, which was subsequently verified through an MS/MS analysis, revealing the consecutive elimination of CH2O (30 Da) and benzoic acid (122 Da) (Liu, Agar, and Imran 2024). Paeoniflorin is a bioactive constituent commonly found in plants, which has been the subject of extensive research because of its favorable pharmacological properties. It has been demonstrated that paeoniflorin possesses antioxidant properties and exerts various bioactive functions. Its pharmacological effects include anti‐inflammatory, anti‐thrombotic, and immunomodulatory activities, rendering it a compound of significant interest for therapeutic applications (Zhou et al. 2020).
Compound 6 was identified as benzoic acid in both positive and negative ionization modes, with a tentative identification based on the precursor ion [M − H]− observed at m/z 121.0302. The peak fragmentation at m/z 77 [M − H]− indicates a loss of CO2 (44 Da), further supporting its identification as benzoic acid. This compound was detected in most samples from both regions (QLD1, QLD2, QLD4, QLD5, QLD6, and QLD7 and WAU5, WAU7, WAU10, WAU15 and WAU32).
Compound 7, identified as ellagic acid, was detected in negative ionization mode with a precursor ion observed at m/z 301.0007. This identification was corroborated by the fragments observed at m/z 257 in MS/MS analysis, indicating a loss of CO2 (44 Da) from the precursor ion. El‐Shehawi et al. (2021) also reported the presence of this substance in moringa leaves. Shakeri, Zirak, and Sahebkar (2018) reported that ellagic acid possesses antioxidant energy, anticancer potential, and hepatoprotection activity. Rauha et al. (2000) also reported the antimicrobial activity of ellagic acid.
Compound 18, detected in negative ionization mode, was identified as caffeic acid with a precursor ion at m/z 179.0368. Further confirmation of the compound was achieved through MS/MS analysis, which revealed product ions at m/z 143 and m/z 135, indicating the loss of 2 units of H2O and CO2, respectively. A study by Asgari‐Kafrani, Fazilati, and Nazem (2020) demonstrated that caffeic acid may play a role in reducing triglycerides and LDL cholesterol levels.
Ferulic acid, identified as compound 19, was detected using mass spectrometry in positive ionization mode with a precursor ion at m/z 193.0519. Confirmation through MS/MS analysis revealed fragment peaks at m/z 178 and 194. Ferulic acid is noted as one of the most prevalent phenolic acids (Stohs and Hartman 2015), possessing the capability to enhance antioxidant enzymes, inhibit the formation of ROS, and scavenge free radicals.
3.4.2. Flavonoids
Flavonoids are secondary metabolites, characterized by their structure as plant polyphenol molecules that consist of two benzene rings. Flavonoids represent the primary polyphenol compounds in moringa (Oldoni et al. 2019). Samples from both regions demonstrated a broad spectrum of flavonoids, including dihydrochalcones (3), flavanols (7), flavanones (7), flavones (22), and isoflavonoids (12).
8‐Prenylnaringenin (compound 51) was detected under positive ionization mode. The compound exhibited a precursor ion at m/z 341.1383 and distinctive product ions at m/z 285. 8‐Prenylnaringenin is a flavonoid compound found in hops and is an essential ingredient in brewing beer, and is gaining interest because of its potential bioactivity, especially estrogenic effects (Paoletti et al. 2009). This constituent was widely detected in samples originating from QLD.
Compound 63 was recognized as Scutellarin with a precursor ion at [M − H]+ m/z 287.0554, featuring a distinctive fragment marked by the loss of an O2 unit (32 Da). Scutellarin, known for its strong antioxidant properties, was detected in samples from both QLD and WAU. Scutellarin has been widely studied as a natural medicine and has been experimentally shown to be helpful in the treatment of heart disease (Gao and Gu 2006).
Compound 65 was tentatively identified in a negative ionization mode with a precursor ion at m/z 446.0869. An MS/MS analysis revealed product ions at m/z 103 and m/z 163, indicating the presence of Kaempferol 7‐O‐glucoside owing to the loss of a glucose unit (284 Da). This compound has demonstrated antiviral properties as reported by Gansukh et al. (2016).
Quercetin 3′‐O‐glucuronide (compound 66) was detected in both positive and negative ionization modes, with a precursor ion observed at m/z 479.0838. The confirmation of its identity was established through MS/MS analysis, where a peak fragment at m/z 301 was observed, indicating the loss of a glucuronic acid moiety (C6H10O7), which is commonly attached to flavonoids through glucuronidation (Zhu et al. 2022). This substance, reported in both wine and lotus flowers, is believed to exhibit sedative, anticonvulsant, and anxiety‐relieving properties (Kim et al. 2021).
Compound 68 was identified as Myricetin 3‐O‐galactoside with precursor ion at both negative and positive ionization modes with m/z at 479.0802. Myricetin 3‐O‐galactoside is a flavonoid glycoside derived from the flavonol myricetin and was only identified in samples from WAU, and it was reported as an active compound with medicinal potential (Xu et al. 2020). This compound has exhibited antioxidant, anti‐inflammatory, and antigenotoxic properties (de Oliveira Azevedo et al. 2015).
3.4.3. Other Polyphenols
A total of 13 other polyphenol compounds were detected in moringa samples grown in Australia. These compounds were 1 Alkylphenol, 2 Hydroxybenzoketones, 3 Curcuminoids, 1 Tyrosol, 1 Phenolic terpene, 1 Hydroxyphenylpropene, 2 other polyphenols, 1 Hydroxycoumarin, and 1 Hydroxybenzaldehyde.
In the negative ion mode ([M − H]−), compound 98 was identified tentatively as a component of the hydroxycoumarins group, specifically scopoletin, across samples from both studied regions. The characterization of scopoletin was achieved with mass‐to‐charge ratios recorded as m/z = 191.0341 and MS/MS analysis confirmed it by the peak fragment at m/z174 because of loss of H₂O (18 Da). Scopoletin, also recognized as 7‐hydroxycoumarin, is a natural coumarin derivative commonly found in various plants. It is an aromatic compound known for its diverse biological activities. Scopoletin exhibits antioxidant, antimicrobial, anti‐inflammatory, and anticancer properties, highlighting its potential therapeutic applications (Jamuna et al. 2015).
Vanillin (compound 99) was identified in both negative and positive ionization modes and was tentatively identified with at m/z 153.0532. This substance was detected in samples from both WAU and QLD. In support of this observation, this compound has been reported to be present in (Bhattacharya et al. 2018) and is thought to contribute to the antioxidant capacity of moringa.
3.4.4. Stilbenes and Lignans
Three stilbenes and nine lignans derivatives were identified in the moringa pod samples. Compound 100 only observed in WAU samples with a precursor ion at 389.1225 was tentatively identified as Resveratrol 5‐O‐glucoside. Compounds 104 and 105 were detected as schisandrin derivatives in the negative model at m/z 383.1527 and 431.2064, respectively, presented in both two regions. Compound 106 with [M − H]− at m/z 353.099 was tentatively identified as Episesamin, which was present in both QLD and WAU samples.
3.5. Venn Graphing of Polyphenol Compounds Distribution
The Venn diagram provided a visualized representation of the distribution and overlap of antioxidant components in the moringa pod samples from QLD and WAU. An analysis of LC‐ESI‐QTOF‐MS/MS data showed distinct variations in the polyphenol profiles between QLD and WAU (Figure 2). The QLD samples exhibited a higher diversity of total polyphenol compounds than WAU samples, with 28 unique compounds identified in QLD and 26 in WAU, while 111 compounds were shared between both regions. These regional differences in polyphenol compound profiles can be attributable to the difference in environmental factors, such as soil composition, climate, and agricultural practices in QLD and WAU, potentially influencing the biosynthesis of polyphenols in moringa pods.
FIGURE 2.
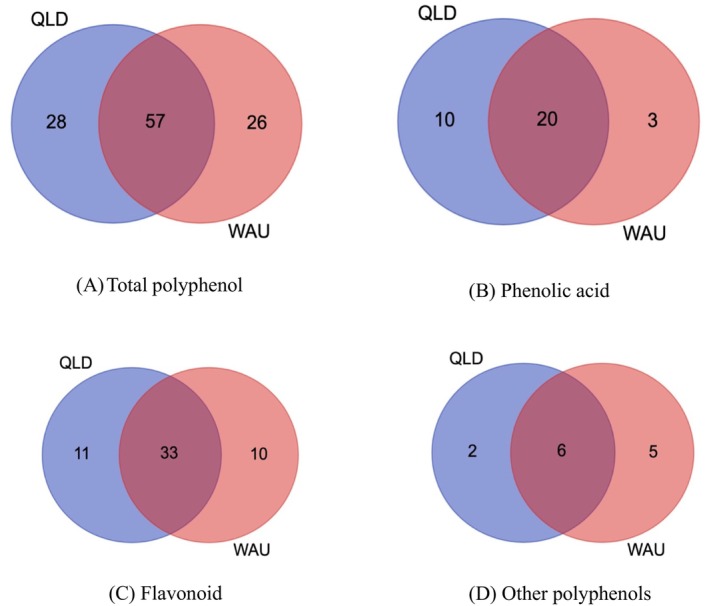
Venn diagram depicting the distribution of polyphenol compounds among moringa pod samples collected from two regions in Australia. Panel (A) illustrates the overlap of total polyphenol compounds across moringa pod samples from different regions. Panel (B) shows the correlation of phenolic acids among these samples. Panel (C) displays the relationship between flavonoids. Panel (D) highlights the connections of other polyphenol compounds within the moringa samples.
Figure 2B presents the profile of phenolic acids in moringa samples from both regions. QLD samples showed a greater variety of phenolic acids, with 30 compounds identified, compared to 23 phenolic acids identified in WAU samples. Notably, 20 phenolic acids were shared between samples from both regions. As illustrated in Figure 2C, 54 flavonoids were detected in QLD samples versus 43 flavonoids identified in WAU samples. Among them, 33 flavonoids were shared between samples from both regions. Samples originating from QLD were found to contain 8 other phenolic compounds, while 11 were identified in WAU samples, with 6 of these compounds being similar between both regions (Figure 2D).
4. Conclusions
This study identified significant differences in polyphenol compounds and antioxidant properties of moringa pods sourced from Queensland and Western Australia, with samples originating from Queensland showing higher TPC, TFC, and TCT. Seven different methods were used to detect antioxidant properties. LC‐ESI‐QTOF‐MS2 analysis identified 111 compounds, which included phenolic acids, flavonoids, and other polyphenols, with polyphenol types being more abundant in samples from Queensland. Overall, these findings demonstrate that moringa pods could be viewed as a rich source of natural antioxidants with high antioxidant capacity for developing functional foods. More experiments are warranted to assess Australia's most suitable growing environment targeting accumulation of polyphenol compounds in moringa pods.
Author Contributions
Rongjia Xie: formal analysis (equal), investigation (equal), methodology (equal), visualization (equal), writing – original draft (equal). Eric N. Ponnampalam: investigation (equal), project administration (equal), resources (equal), writing – review and editing (equal). Farhad Ahmadi: investigation (equal), supervision (equal), writing – review and editing (equal). Frank R. Dunshea: resources (equal), validation (equal), writing – review and editing (equal). Hafiz A. R. Suleria: conceptualization (equal), funding acquisition (equal), methodology (equal), resources (equal), supervision (equal), validation (equal), writing – review and editing (equal).
Ethics Statement
This study does not involve any animal or human experimentation. Moringa pods were collected from two farms in the Queensland and Western Australian regions.
Acknowledgments
The authors thank the Queensland company Moringa Bowen and the Western Australian Department of Primary Industries and Regional Development for providing the Moringa pods used in this study. The transformation of Australian‐grown Moringa into a high‐value feed ingredient for human and animal consumption project has been funded by AgriFutures Australia as part of the Emerging Industries Program, which focuses on new and emerging industries with high growth potential.
Funding: This work was supported by Agrifutures Australia.
Contributor Information
Eric N. Ponnampalam, Email: eponnampalam@unimelb.edu.au.
Hafiz A. R. Suleria, Email: hafiz.suleria@unimelb.edu.au.
Data Availability Statement
Data is available for sharing upon request.
References
- Adisakwattana, S. , and Chanathong B.. 2011. “Alpha‐Glucosidase Inhibitory Activity and Lipid‐Lowering Mechanisms of Moringa oleifera Leaf Extract.” European Review for Medical and Pharmacological Sciences 15, no. 7: 803–808. [PubMed] [Google Scholar]
- Al Juhaimi, F. , Ghafoor K., Ahmed I. A. M., Babiker E. E., and Özcan M. M.. 2017. “Comparative Study of Mineral and Oxidative Status of Sonchus oleraceus , Moringa Oleifera and Moringa Peregrina Leaves.” Journal of Food Measurement and Characterization 11, no. 4: 1745–1751. [Google Scholar]
- Al Juhaimi, F. , Ghafoor K., Babiker E. E., Matthäus B., and Özcan M. M.. 2017. “The Biochemical Composition of the Leaves and Seeds Meals of Moringa Species as Non‐Conventional Sources of Nutrients.” Journal of Food Biochemistry 41, no. 1: e12322. [Google Scholar]
- Ali, A. , Wu H., Ponnampalam E. N., Cottrell J. J., Dunshea F. R., and Suleria H. A. R.. 2021. “Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds Through LC‐ESI‐QTOF‐MS2 and Their Antioxidant Potential.” Antioxidants 10, no. 5: 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaglo, N. K. , Bennett R. N., Lo Curto R. B., et al. 2010. “Profiling Selected Phytochemicals and Nutrients in Different Tissues of the Multipurpose Tree Moringa oleifera L., Grown in Ghana.” Food Chemistry 122, no. 4: 1047–1054. [Google Scholar]
- Asgari‐Kafrani, A. , Fazilati M., and Nazem H.. 2020. “Hepatoprotective and Antioxidant Activity of Aerial Parts of Moringa oleifera in Prevention of Non‐Alcoholic Fatty Liver Disease in Wistar Rats.” South African Journal of Botany 129: 82–90. [Google Scholar]
- Athira, N. D. , James T. J., Sreelatha S. L., Kariyil B. J., and Nair S. N.. 2021. “ Moringa oleifera (Lam.): A Natural Remedy for Ageing?” Natural Product Research 35, no. 24: 6216–6222. [DOI] [PubMed] [Google Scholar]
- Baldwin, A. , and Booth B. W.. 2022. “Biomedical Applications of Tannic Acid.” Journal of Biomaterials Applications 36, no. 8: 1503–1523. [DOI] [PubMed] [Google Scholar]
- Bhattacharya, A. , Tiwari P., Sahu P. K., and Kumar S.. 2018. “A Review of the Phytochemical and Pharmacological Characteristics of Moringa oleifera .” Journal of Pharmacy & Bioallied Sciences 10, no. 4: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braham, F. , Carvalho D. O., Almeida C. M. R., et al. 2020. “Online HPLC‐DPPH Screening Method for Evaluation of Radical Scavenging Phenols Extracted From Moringa oleifera Leaves.” South African Journal of Botany 129: 146–154. [Google Scholar]
- Chou, O. , Ali A., Subbiah V., Barrow C. J., Dunshea F. R., and Suleria H. A. R.. 2021. “LC‐ESI‐QTOF‐MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential.” Fermentation 7, no. 2: 73. [Google Scholar]
- Dinis, T. C. , Madeira V. M., and Almeida L. M.. 1994. “Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5‐Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers.” Archives of Biochemistry and Biophysics 315, no. 1: 161–169. [DOI] [PubMed] [Google Scholar]
- Du, J. , Zhong B., Subbiah V., Barrow C. J., Dunshea F. R., and Suleria H. A. R.. 2021. “LC‐ESI‐QTOF‐MS/MS Profiling and Antioxidant Activity of Phenolics From Custard Apple Fruit and By‐Products.” Separations 8, no. 5: 62. [Google Scholar]
- Du, Q.‐H. , Wu Y.‐H., Tang S.‐Q., Ren M.‐H., and Fu Z.. 2021. “Influences of Ultrasonic Treatment on Structure and Functional Properties of Salt‐Soluble Protein From Moringa oleifera Seeds.” International Journal of Food Science & Technology 56, no. 11: 5871–5880. [Google Scholar]
- El‐Shehawi, A. M. , Alkafafy M., El‐Shazly S., et al. 2021. “ Moringa oleifera Leaves Ethanolic Extract Ameliorates High Fat Diet‐Induced Obesity in Rats.” Journal of King Saud University, Science 33, no. 6: 101552. [Google Scholar]
- Gansukh, E. , Kazibwe Z., Pandurangan M., Judy G., and Kim D. H.. 2016. “Probing the Impact of Quercetin‐7‐O‐Glucoside on Influenza Virus Replication Influence.” Phytomedicine 23, no. 9: 958–967. [DOI] [PubMed] [Google Scholar]
- Gao, M. , Gu M., and C.‐Z. L. 2006. “Two‐Step Purification of Scutellarin From Erigeron Breviscapus (Vant.) Hand. Mazz. By High‐Speed Counter‐Current Chromatography.” Journal of Chromatography B 838, no. 2: 139–143. [DOI] [PubMed] [Google Scholar]
- Gharsallah, K. , Rezig L., Rajoka M. S. R., Mehwish H. M., Ali M. A., and Chew S. C.. 2023. “ Moringa oleifera : Processing, Phytochemical Composition, and Industrial Application.” South African Journal of Botany 160: 180–193. [Google Scholar]
- González‐Romero, J. , Arranz‐Arranz S., Verardo V., García‐Villanova B., and Guerra‐Hernández E. J.. 2020. “Bioactive Compounds and Antioxidant Capacity of Moringa Leaves Grown in Spain Versus 28 Leaves Commonly Consumed in Pre‐Packaged Salads.” Processes 8, no. 10: 1297. [Google Scholar]
- Gu, C. , Howell K., Dunshea F. R., and Suleria H. A. R.. 2019. “LC‐ESI‐QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol‐Rich Fruits and Vegetables and Their Potential Antioxidant Activities.” Antioxidants 8, no. 9: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hani, N. M. , Torkamani A. E., Azarian M. H., Mahmood K. W. A., and Ngalim S. H.. 2017. “Characterisation of Electrospun Gelatine Nanofibres Encapsulated With Moringa oleifera Bioactive Extract.” Journal of the Science of Food and Agriculture 97, no. 10: 3348–3358. [DOI] [PubMed] [Google Scholar]
- Hassan, M. A. , Xu T., Tian Y., et al. 2021. “Health Benefits and Phenolic Compounds of Moringa oleifera Leaves: A Comprehensive Review.” Phytomedicine 93: 153771. [DOI] [PubMed] [Google Scholar]
- Hu, T. , Subbiah V., Wu H., et al. 2021. “Determination and Characterization of Phenolic Compounds From Australia‐Grown Sweet Cherries ( Prunus avium L.) and Their Potential Antioxidant Properties.” ACS Omega 6, no. 50: 34687–34699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal, S. , and Bhanger M.. 2006. “Effect of Season and Production Location on Antioxidant Activity of Moringa oleifera Leaves Grown in Pakistan.” Journal of Food Composition and Analysis 19, no. 6–7: 544–551. [Google Scholar]
- Jamuna, S. , Karthika K., Paulsamy S., Thenmozhi K., Kathiravan S., and Venkatesh R.. 2015. “Confertin and Scopoletin From Leaf and Root Extracts of Hypochaeris radicata Have Anti‐Inflammatory and Antioxidant Activities.” Industrial Crops and Products 70: 221–230. [Google Scholar]
- Kerdsomboon, K. , Chumsawat W., and Auesukaree C.. 2021. “Effects of Moringa oleifera Leaf Extracts and Its Bioactive Compound Gallic Acid on Reducing Toxicities of Heavy Metals and Metalloid in Saccharomyces cerevisiae .” Chemosphere 270: 128659. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Hong K. B., Jo K., and Suh H. J.. 2021. “Quercetin‐3‐O‐Glucuronide in the Ethanol Extract of Lotus Leaf ( Nelumbo nucifera ) Enhances Sleep Quantity and Quality in a Rodent Model via a GABAergic Mechanism.” Molecules 26, no. 10: 3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. , Zhang J., and Chen X.. 2018. “Bioactive Flavonoids in Moringa Oleifera and Their Health‐Promoting Properties.” Journal of Functional Foods 47: 469–479. [Google Scholar]
- Liu, H. , Agar O. T., and Imran A.. 2024. “LC‐ESI‐QTOF‐MS/MS Characterization of Phenolic Compounds in Australian Native Passion Fruits and Their Potential Antioxidant Activities.” Food Science & Nutrition 52: 102331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobossé, P. , Fombang E. N., and Mbofung C. M.. 2018. “Effects of Age and Extraction Solvent on Phytochemical Content and Antioxidant Activity of Fresh Moringa oleifera L. Leaves.” Food Science & Nutrition 6, no. 8: 2188–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunsina, B. S. , Radha C., and Govardhan Singh R. S.. 2010. “Physicochemical and Functional Properties of Full‐Fat and Defatted Moringa oleifera Kernel Flour.” International Journal of Food Science & Technology 45, no. 11: 2433–2439. [Google Scholar]
- Oldoni, T. L. C. , Merlin N., Karling M., et al. 2019. “Bioguided Extraction of Phenolic Compounds and UHPLC‐ESI‐Q‐TOF‐MS/MS Characterization of Extracts of Moringa oleifera Leaves Collected in Brazil.” Food Research International 125: 108647. [DOI] [PubMed] [Google Scholar]
- de Oliveira Azevedo, A. , Campos J. J., de Souza G. G., et al. 2015. “Antinociceptive and Anti‐Inflammatory Effects of Myricetin 3‐O‐β‐Galactoside Isolated From Davilla Elliptica: Involvement of the Nitrergic System.” Journal of Natural Medicines 69: 487–493. [DOI] [PubMed] [Google Scholar]
- Özcan, M. M. 2020. “Moringa spp.: Composition and Bioactive Properties.” South African Journal of Botany 129: 25–31. [Google Scholar]
- Paoletti, T. , Fallarini S., Gugliesi F., Minassi A., Appendino G., and Lombardi G.. 2009. “Anti‐Inflammatory and Vascularprotective Properties of 8‐Prenylapigenin.” European Journal of Pharmacology 620, no. 1–3: 120–130. [DOI] [PubMed] [Google Scholar]
- Peng, D. , Zahid H. F., Ajlouni S., Dunshea F. R., and Suleria H. A. R.. 2019. “Lc‐Esi‐Qtof/Ms Profiling of Australian Mango Peel By‐Product Polyphenols and Their Potential Antioxidant Activities.” Processes 7, no. 10: 764. [Google Scholar]
- Prakash, D. , Suri S., Upadhyay G., and Singh B. N.. 2007. “Total Phenol, Antioxidant and Free Radical Scavenging Activities of Some Medicinal Plants.” International Journal of Food Sciences and Nutrition 58, no. 1: 18–28. [DOI] [PubMed] [Google Scholar]
- Qadir, R. , Anwar F., Bashir K., Tahir M. H., Alhumade H., and Mehmood T.. 2022. “Variation in Nutritional and Antioxidant Attributes of Moringa oleifera L. Leaves at Different Maturity Stages.” Frontiers in Energy Research 10: 888355. [Google Scholar]
- Rauha, J.‐P. , Remes S., Heinonen M., et al. 2000. “Antimicrobial Effects of Finnish Plant Extracts Containing Flavonoids and Other Phenolic Compounds.” International Journal of Food Microbiology 56, no. 1: 3–12. [DOI] [PubMed] [Google Scholar]
- Samsonowicz, M. , Regulska E., Karpowicz D., and Leśniewska B.. 2019. “Antioxidant Properties of Coffee Substitutes Rich in Polyphenols and Minerals.” Food Chemistry 278: 101–109. [DOI] [PubMed] [Google Scholar]
- Shakeri, A. , Zirak M. R., and Sahebkar A.. 2018. “Ellagic Acid: A Logical Lead for Drug Development?” Current Pharmaceutical Design 24, no. 2: 106–122. [DOI] [PubMed] [Google Scholar]
- Shih, M.‐C. , Chang C. M., Kang S. M., and Tsai M. L.. 2011. “Effect of Different Parts (Leaf, Stem and Stalk) and Seasons (Summer and Winter) on the Chemical Compositions and Antioxidant Activity of Moringa oleifera .” International Journal of Molecular Sciences 12, no. 9: 6077–6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff, N. , and Cumbes Q. J.. 1989. “Hydroxyl Radical Scavenging Activity of Compatible Solutes.” Phytochemistry 28, no. 4: 1057–1060. [Google Scholar]
- Sogi, D. S. , Siddiq M., Greiby I., and Dolan K. D.. 2013. “Total Phenolics, Antioxidant Activity, and Functional Properties of ‘Tommy Atkins’ Mango Peel and Kernel as Affected by Drying Methods.” Food Chemistry 141, no. 3: 2649–2655. [DOI] [PubMed] [Google Scholar]
- Stavrou, I. J. , Christou A., and Kapnissi‐Christodoulou C. P.. 2018. “Polyphenols in Carobs: A Review on Their Composition, Antioxidant Capacity and Cytotoxic Effects, and Health Impact.” Food Chemistry 269: 355–374. [DOI] [PubMed] [Google Scholar]
- Stohs, S. J. , and Hartman M. J.. 2015. “Review of the Safety and Efficacy of Moringa oleifera .” Phytotherapy Research 29, no. 6: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulastri, E. , Zubair M. S., Anas N. I., et al. 2018. “Total Phenolic, Total Flavonoid, Quercetin Content and Antioxidant Activity of Standardized Extract of Moringa oleifera Leaf From Regions With Different Elevation.” Pharmacognosy Journal 10, no. 6s: s104–s108. [Google Scholar]
- Suleria, H. A. , Barrow C. J., and Dunshea F. R.. 2020. “Screening and Characterization of Phenolic Compounds and Their Antioxidant Capacity in Different Fruit Peels.” Food 9, no. 9: 1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez‐León, L. A. , Páramo‐Calderón D. E., Robles‐Olvera V. J., et al. 2017. “Variation in Bioactive Compounds and Antiradical Activity of Moringa oleifera Leaves: Influence of Climatic Factors, Tree Age, and Soil Parameters.” European Food Research and Technology 243, no. 9: 1593–1608. [Google Scholar]
- Vella, F. M. , Cautela D., and Laratta B.. 2019. “Characterization of Polyphenolic Compounds in Cantaloupe Melon By‐Products.” Food 8, no. 6: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara‐Jimenez, M. , Almatrafi M. M., and Fernandez M. L.. 2017. “Bioactive Components in Moringa oleifera Leaves Protect Against Chronic Disease.” Antioxidants 6, no. 4: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonghirundecha, P. , Chusri S., Meunprasertdee P., and Kaewmanee T.. 2022. “Microencapsulated Functional Ingredients From a Moringa oleifera Leaf Polyphenol‐Rich Extract: Characterization, Antioxidant Properties, In Vitro Simulated Digestion, and Storage Stability.” LWT 154: 112820. [Google Scholar]
- Xu, Y.‐B. , Chen G.‐L., and Guo M.‐Q.. 2019. “Antioxidant and Anti‐Inflammatory Activities of the Crude Extracts of Moringa oleifera From Kenya and Their Correlations With Flavonoids.” Antioxidants 8, no. 8: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. , He W. Q., Liu C. S., and Kong J. Q.. 2020. “Enzymatic Synthesis of Myricetin 3‐O‐Galactoside Through a Whole‐Cell Biocatalyst.” Chinese Herbal Medicines 12, no. 4: 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, B. , Robinson N. A., Warner R. D., Barrow C. J., Dunshea F. R., and Suleria H. A. R.. 2020. “LC‐ESI‐QTOF‐MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential.” Marine Drugs 18, no. 6: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y.‐X. , Gong X. H., Zhang H., and Peng C.. 2020. “A Review on the Pharmacokinetics of Paeoniflorin and Its Anti‐Inflammatory and Immunomodulatory Effects.” Biomedicine & Pharmacotherapy 130: 110505. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Yin Q., and Yang Y.. 2020. “Comprehensive Investigation of Moringa oleifera From Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC‐ESI‐MS/MS.” Molecules 25, no. 3: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Z. , Zhong B., Yang Z., et al. 2022. “LC‐ESI‐QTOF‐MS/MS Characterization and Estimation of the Antioxidant Potential of Phenolic Compounds From Different Parts of the Lotus ( nelumbo nucifera ) Seed and Rhizome.” ACS Omega 7, no. 17: 14630–14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available for sharing upon request.


