ABSTRACT
Newly diagnosed follicular lymphoma (FL) patients usually received first‐line rituximab‐based immunochemotherapy (R‐chemo). Recently, rituximab plus lenalidomide (R2) emerged as an alternative chemo‐free immunotherapy. We performed a comparative analysis of positron emission tomography/computed tomography (PET/CT) in FL undergoing R‐chemo or R2. With data of sequential PET/CT at the baseline, interim, and end‐of‐induction, treatment responses and survival outcomes were analyzed using Deauville scores at the interim and end‐of‐induction. Additionally, correlations between interim Deauville scores and baseline PET/CT parameters were explored. Conclusively, we revealed that Deauville 1–3 at the interim and end‐of‐induction showed lower disease progression within 24 months (POD24) and superior progression‐free survival (PFS) in R‐chemo and R2 cohorts. Also, patients with interim Deauville 1–3 exhibited reduced POD24 and favorable PFS as compared to those with interim Deauville 4–5/end‐of‐induction Deauville 1–3. Furthermore, total lesion glycolysis of baseline PET‐CT surpassed standardized uptake value and total metabolic tumor volume in predicting interim Deauville 1–3, with different optimal cutoffs of 2600 and 600 mL in the R‐chemo and R2 cohort. These findings underscored the potential of PET‐CT‐adapted strategies to achieve durable remission in FL undergoing rituximab‐based immunochemotherapy or immunotherapy.
Keywords: follicular lymphoma, immunochemotherapy, immunotherapy, interim PET/CT, total lesion glycolysis
1. Introduction
Follicular lymphoma (FL) is the most prevalent indolent lymphoma, accounting for approximately 35% of all non‐Hodgkin lymphomas (NHLs) in Western countries, with a relatively lower proportion in China [1, 2]. The standard first‐line treatment for FL involves the combination of rituximab with chemotherapy (R‐chemo), typically including rituximab plus bendamustine (BR) and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP) [3]. Recently, the immunomodulatory agent lenalidomide plus rituximab (R2) has shown promising responses in newly diagnosed FL, which exhibits comparable clinical efficacy to immunochemotherapy R‐chemo as a chemo‐free approach while mitigating chemotherapy‐related adverse effects [3, 4]. Therefore, conducting a comparative analysis between R‐chemo and R2 is essential in determining the optimal patient population for FL treatment.
Accumulating evidence underscores the critical significance of 18F‐fluorodeoxyglucose (18F‐FDG) positron emission tomography‐computed tomography (PET/CT) in managing FL patients treated with R‐chemo [5]. Baseline PET/CT demonstrates a remarkable sensitivity in detecting additional sites, particularly in patients initially diagnosed with limited‐stage disease through CT scans, impacting the timing of treatment initiation and the selection of therapies [6]. It is worth noting that in FL patients treated with R‐chemo, quantitative biomarkers of baseline PET/CT correlate with poor progression‐free survival (PFS) such as increased maximum standardized uptake value (SUVmax), total metabolic tumor volume (TMTV), and total lesion glycolysis (TLG) [7, 8, 9].
Regarding treatment assessment, PET/CT has gained prominence over contrast‐enhanced CT in FL undergoing R‐chemo therapy, especially in evaluating metabolic response at the end‐of‐induction using the 2014 Lugano Deauville 5‐point scoring system (Deauville 1–5) [10]. Deauville 1–3 at the end‐of‐induction refers to a complete metabolic response (CMR) with favorable PFS, while Deauville 4–5 represents an inadequate response with poor outcomes [10, 11]. In aggressive lymphomas, including Hodgkin lymphoma and primary mediastinal B‐cell lymphoma, patients with the end‐of‐induction Deauville 4 demonstrated superior outcomes than patients with the end‐of‐induction Deauville 5 [12, 13, 14]. Nevertheless, the differentiated prognosis between the end‐of‐induction Deauville 4 and Deauville 5 needs further investigation in FL [12]. Moreover, the research about the interim PET/CT in FL remained limited [15, 16].
Since the prognostic value of PET/CT has been predominantly established in FL treated with R‐chemo rather than those with R2, we aimed to gain full insights into the PET/CT‐guided treatment strategy in both immunochemotherapy and immunotherapy cohorts of FL, especially for the interim PET/CT.
2. Materials and Methods
2.1. Study Population
The study was approved by the ethics committee and institutional review board of Shanghai Ruijin Hospital. Written informed consent was obtained from all participants included in the study in accordance with the Declaration of Helsinki. From January 1, 2013, to December 31, 2021, 402 patients with newly diagnosed FL I‐IIIA were retrospectively enrolled, treated with rituximab‐based therapies, and had baseline PET/CT examinations. Histological diagnoses were reviewed by two independent pathologists based on the World Health Organization classification. Clinical and pathological characteristics of patients were collected at diagnosis. Disease outcomes, including progression disease within 24 months (POD24) and PFS were collected [17].
2.2. PET/CT Technique and Analysis
18F‐FDG PET/CT‐related parameters included PET/CT before treatment (baseline PET/CT), PET/CT after three cycles of induction treatment (interim PET/CT), and PET/CT at the end of six cycles of induction treatment (end‐of‐induction PET/CT). All the examinations were retrospectively reviewed by an independent nuclear medicine physician, blinded to patient clinical data and outcomes, and was reviewed by a second independent nuclear medicine physician. Nuclear medicine physicians were asked to conduct reproducibility analysis without interference.
Examinations were performed according to international guidelines for fasting (6 h before acquisition) and blood glucose limit (no more than 11 mmol/L) [18]. Radiotracer injected dose depended on each PET/CT system, from 3 to 5 MBq/kg. In each patient, imaging was started 60 ± 10 min after administration of radiotracer. The acquisition included a whole‐body scan from vertex to mid‐thigh (1.5–2 min per bed position depended on each PET/CT system). Suboptimal 18F‐FDG PET/CT examinations, especially in patients with high blood glucose or movements during acquisition resulting in poor‐quality images, were excluded from the analysis.
All 18F‐FDG PET/CT examinations were displayed on a workstation equipped with syngo.via VB2.0 (Siemens Healthineers, Erlangen, Germany) [19]. TMTV was determined automatically by a threshold of more than 1.5 × liver mean + 2 SDs, as suggested by PET/CT Response Criteria in Solid Tumors (PERCIST, version 1.0) criteria [20]. Furthermore, all the SUV‐derived parameters, such as SUVmax, SUVpeak, and SUVmean, were recorded within the metabolic volume of interest. TLG (MTV multiplied by SUVmean) was also automatically calculated. Deauville 5‐point scale was determined by visual assessment of the interim PET/CT and end‐of‐induction PET/CT study [10].
2.3. Statistics
Baseline characteristics of patients were compared by Fisher's exact test. Differences in normalized data between groups were assessed by two‐sided t‐tests. The POD24 was defined as experiencing progression of disease within 2 years of first‐line therapy [17]. PFS was calculated from the diagnosis date to the date of disease progression or the date of last follow‐up. Overall survival (OS) was measured from the diagnosis date to the date of death or last follow‐up. Survival analysis was estimated using the Kaplan‐Meier method and compared by the log‐rank test. p value < 0.050 was regarded as statistically significant. Bonferroni corrections were used to adjust for a 3‐group multiple‐test, of which a p value < 0.017 was considered significant [21]. All statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS, 26.0) or GraphPad Prism 7 software.
3. Results
3.1. Patient Characteristics
Among 546 patients with newly diagnosed FL I‐IIIA, 402 patients (73.6%) who received rituximab‐based therapies and underwent baseline PET/CT assessment were enrolled (Figure 1). Of these, 232 patients were treated with R‐chemo (including BR or R‐CHOP) and 170 patients with R2. Baseline characteristics were well‐balanced across the two treatment cohorts (Table 1), with no significant difference observed between the R‐chemo and R2 cohort in terms of age, sex, ECOG (Eastern Cooperative Oncology Group) performance status, Ann Arbor stage, FLIPI (follicular lymphoma international prognostic index) score, or FLIPI‐2 score.
FIGURE 1.
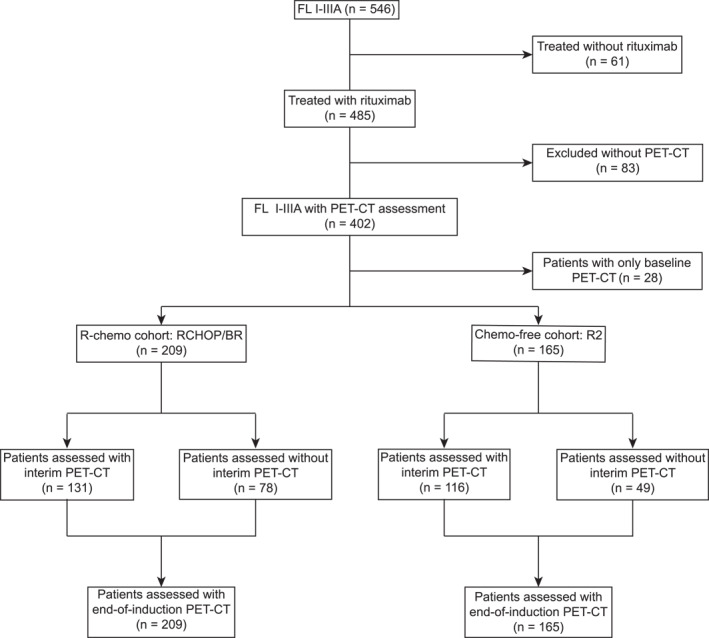
Consort diagram of the study. Among 546 FL patients screened, 402 were treated with rituximab‐based therapies and underwent baseline PET/CT assessment, with 232 in the R‐chemo arm and 170 in the R2 arm.
TABLE 1.
Baseline demographic and disease characteristics.
| Characteristic | Total (n = 402) | R‐chemo (n = 232) | R2 (n = 170) | p a |
|---|---|---|---|---|
| Demographic | ||||
| Age ≤ 60 years | 267 (66.42) | 157 (67.67) | 110 (64.71) | 0.593 |
| Male sex | 204 (50.75) | 123 (53.02) | 81 (47.65) | 0.313 |
| ECOG | 0.420 | |||
| 0, n (%) | 376 (93.53) | 219 (94.40) | 157 (92.35) | |
| 1, n (%) | 26 (6.47) | 13 (5.60) | 13 (7.65) | |
| Ann Arbor | 0.114 | |||
| I–II, n (%) | 28 (6.97) | 12 (5.17) | 16 (9.41) | |
| III–IV, n (%) | 374 (93.03) | 220 (94.83) | 154 (90.59) | |
| FLIPI score | 0.864 | |||
| Low [(0–1), n (%)] | 51 (12.69) | 29 (12.50) | 22 (12.94) | |
| Intermediate [(2), n (%)] | 165 (41.04) | 93 (40.08) | 72 (42.35) | |
| High [(3–5), n (%)] | 186 (46.27) | 110 (47.41) | 76 (44.71) | |
| FLIPI‐2 score | 0.945 | |||
| Low [(0–1), n (%)] | 181 (45.02) | 104 (44.83) | 77 (45.29) | |
| Intermediate [(2), n (%)] | 105 (26.12) | 62 (26.72) | 43 (25.29) | |
| High [(3–5), n (%)] | 116 (28.86) | 66 (28.45) | 50 (29.41) |
Baseline characteristics of patients were compared by Fisher's exact test.
With a median follow‐up of 33.8 (range 3.0–111.9) and 33.0 (range 3.1–73.7) months in the R‐chemo and R2 cohort, the proportion of patients experiencing POD24 was 18.2% and 21.1%, respectively (Figure 2a). Two‐year PFS were 82.5% and 79.2% in the R‐chemo and R2 cohort (Figure 2b). Two‐year OS were 94.9% and 96.8% in the R‐chemo and R2 cohort (Figure 2c). Above all, comparable POD24, PFS, and OS were observed in the R‐chemo and R2 cohorts.
FIGURE 2.
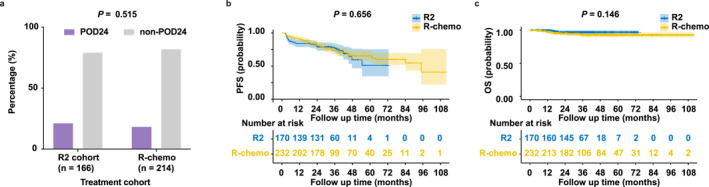
Prognosis of FL treated with the R‐chemo or R2 therapy. (a–c) POD24 (a), PFS (b), and OS (c) between different treatment cohorts: R‐chemo versus R2.
3.2. Patients With the Interim Deauville 1–3 Indicated Favorable Prognosis in the R‐Chemo Cohort
In the R‐chemo cohort, the prognostic value of PET/CT scans was investigated at the interim and end‐of‐induction using the Deauville 1–5 system. As depicted in Figure S1, POD24 and PFS of patients who were treated with R‐chemo regimens were displayed in detail according to the interim and end‐of‐induction Deauville score. According to the interim PET/CT assessment, we stratified patients into three groups: Deauville 1–3, Deauville 4, and Deauville 5. Proportions of patients experiencing POD24 in the interim Deauville 1–3, Deauville 4, and Deauville 5 groups were 5.1%, 31.6%, and 55.0% (p < 0.001, Figure 3a). Similarly, the 2‐year PFS was 95.2%, 70.7%, and 45.0%, respectively (p < 0.001, Figure 3b). Furthermore, according to the end‐of‐induction PET/CT assessment, we stratified patients into three groups: Deauville 1–3, Deauville 4, and Deauville 5. Proportions of patients experiencing POD24 in the end‐of‐induction Deauville 1–3, Deauville 4, and Deauville 5 groups were 5.1%, 40.0%, and 48.6% (p < 0.001, Figure 3c). Similarly, the 2‐year PFS was 95.0%, 60.3%, and 52.5%, respectively (p < 0.001, Figure 3d). Overall, Deauville 1–3 at both interim and end‐of‐induction were significantly associated with superior POD24 and prolonged PFS in R‐chemo‐treated FL patients.
FIGURE 3.
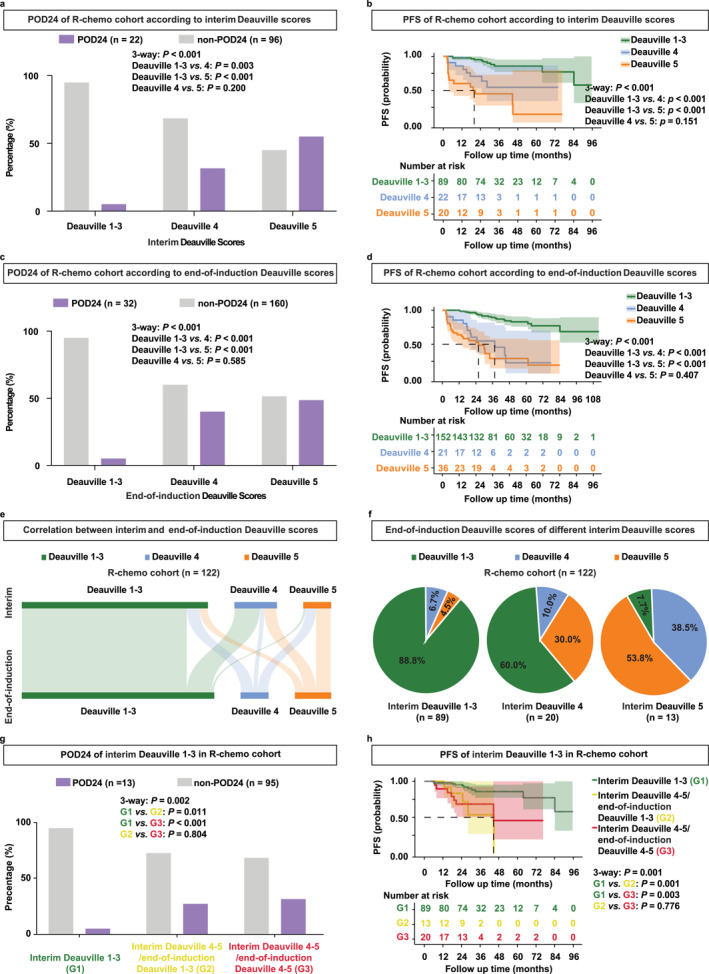
Patients with interim Deauville 1–3 indicated favorable prognosis in the R‐chemo cohort. (a, b) POD24 (a) and PFS (b) of patients according to the interim Deauville score in the R‐chemo cohort. (c, d) POD24 (c) and PFS (d) of patients according to the end‐of‐induction Deauville score in the R‐chemo cohort. (e) Sankey plot of correlation between the interim and end‐of‐induction Deauville scores in the R‐chemo cohort. (f) Detailed end‐of‐induction Deauville scores of patients with the interim Deauville 1–3, interim Deauville 4, and interim Deauville 5 in the R‐chemo cohort. (g, h) POD24 (g) and PFS (h) of patients with the interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5 in the R‐chemo cohort.
Moreover, we explored the correlation between the interim and end‐of‐induction Deauville scores. One hundred and twenty‐two FL patients, who completed six cycles of R‐chemo and underwent both interim and end‐of‐induction PET/CT were included for analysis (Figure 3e). Among 89 FL patients with the interim Deauville 1–3, 88.8% remained Deauville 1–3, 6.7% became Deauville 4%, and 4.5% became Deauville 5 when assessed at the end‐of‐induction (Figure 3f, left panel). Among 20 FL patients with the interim Deauville 4, 60.0% became Deauville 1–3, 10.0% remained Deauville 4%, and 30.0% became Deauville 5 when assessed at the end‐of‐induction (Figure 3f, middle panel). Among 13 FL patients with the interim Deauville 5, 7.7% became Deauville 1–3, 38.5% became Deauville 4%, and 53.8% remained the Deauville 5 at the end‐of‐induction (Figure 3f, right panel). Additionally, we stratified patients into three groups: interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5. Proportions of patients experiencing POD24 were 5.1%, 27.3%,and 31.6% in the interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5 subgroup, respectively (p = 0.002, Figure 3g). Consistently, the 2‐year PFS was 95.2% in the interim Deauville 1–3, 83.3% in the interim Deauville 4–5/end‐of‐induction Deauville 1%–3%, and 68.4% in the interim Deauville 4–5/end‐of‐induction Deauville 4–5 (p = 0.001, Figure 3h). Taken together, most patients with the interim Deauville 1–3 maintained Deauville 1–3 at the end‐of‐induction. In addition, patients with the interim Deauville 1–3 had a better prognosis than those with the interim Deauville 4–5/end‐of‐induction Deauville 1–3, indicating the importance of interim Deauville 1–3 for prediction of disease progression in R‐chemo‐treated FL.
3.3. Patients With the Interim Deauville 1–3 Indicated Favorable Prognosis in the R2 Cohort
In the R2 cohort, the prognostic value of PET/CT scans was further investigated both at the interim and end‐of‐induction using the Deauville 1–5 system. As depicted in Figure S2, POD24 and PFS of patients treated with R2 were displayed in detail according to both the interim Deauville score and end‐of‐induction Deauville score. According to the interim PET/CT assessment, we stratified patients into Deauville 1–3, Deauville 4, and Deauville 5. Proportions of patients experiencing POD24 were 0%, 29.2%, and 64.7% in the interim Deauville 1–3, Deauville 4, and Deauville 5 group (p < 0.001, Figure 4a). Similarly, the 2‐year PFS was 100.0%, 71.9%, and 35.3% (p < 0.001, Figure 4b). Furthermore, according to the end‐of‐induction PET/CT assessment, we stratified patients into three groups: Deauville 1–3, Deauville 4, and Deauville 5. Proportions of patients experiencing POD24 were 3.7%, 26.9%, and 82.1% in the end‐of‐induction Deauville 1–3, Deauville 4, and Deauville 5 group (p < 0.001, Figure 4c). Similarly, the 2‐year PFS was 96.3%, 73.1%, and 18.7% (p < 0.001, Figure 4d). Overall, Deauville 1–3 at both interim and end‐of‐induction were significantly associated with superior POD24 and prolonged PFS in R2‐treated FL.
FIGURE 4.
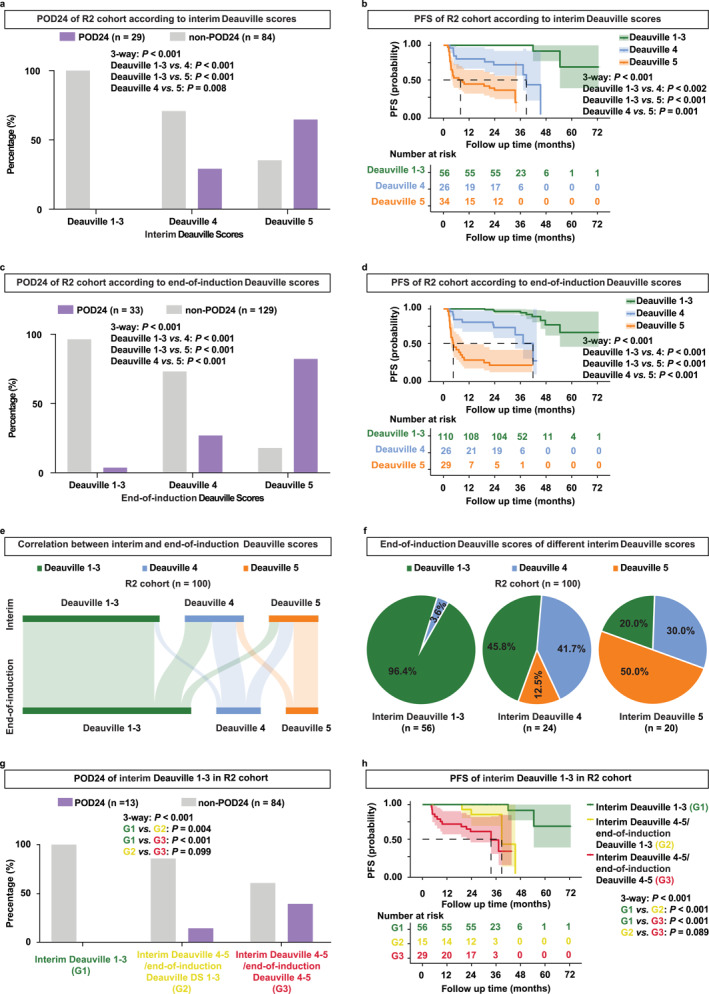
Patients with interim Deauville 1–3 indicated favorable prognosis in the R2 cohort. (a, b) POD24 (a) and PFS (b) of patients according to the interim Deauville scores in the R2 cohort. (c, d) POD24 (c) and PFS (d) of patients according to the end‐of‐induction Deauville scores in the R2 cohort. (e) Sankey plot of correlation between the interim and end‐of‐induction Deauville scores in the R2 cohort. (f) Detailed end‐of‐induction Deauville scores of patients with the interim Deauville 1–3, interim Deauville 4, and interim Deauville 5 in the R2 cohort. (g, h) POD24 (g) and PFS (h) of patients with the interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5 in the R2 cohort.
Moreover, we explored the correlation between interim and end‐of‐induction Deauville scores (Figure 4e). One hundred FL patients, who completed six cycles of R2 and underwent PET/CT assessment at both interim and end‐of‐induction were included for analysis. Among 56 FL patients with the interim Deauville 1–3, 96.4% remained as Deauville 1–3, and 3.6% became Deauville 4 when assessed by PET/CT at the end‐of‐induction (Figure 4f, left panel). Among 24 FL patients with the interim Deauville 4, 45.8% became Deauville 1–3, 41.7% remained as Deauville 4%, and 12.5% became Deauville 5 at the end‐of‐induction (Figure 4f, middle panel). Among 20 FL patients with the interim Deauville 5, 20.0% became Deauville 1–3, 30.0% became Deauville 4%, and 50.0% remained as Deauville 5 when assessed at the end‐of‐induction (Figure 4f, right panel). Subsequently, we stratified patients into three groups: interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5. Proportions of patients experiencing POD24 were 0%, 14.3%, and 39.3% in the interim Deauville 1–3, interim Deauville 4–5/end‐of‐induction Deauville 1–3, and interim Deauville 4–5/end‐of‐induction Deauville 4–5 (p < 0.001, Figure 4g). Consistently, the 2‐year PFS was 100.0%, 85.7%, and 61.1% (p < 0.001, Figure 4h). Taken together, most patients with the interim Deauville 1–3 maintained Deauville 1–3 at the end‐of‐induction. In addition, patients with the interim Deauville 1–3 had a better prognosis than those with the interim Deauville 4–5/end‐of‐induction Deauville 1–3, also indicating the importance of the interim Deauville 1–3 in R2‐treated FL.
3.4. Baseline TLG Predicted the Interim Deauville 1–3 Differently in the R‐Chemo and R2 Cohorts
We further detected whether baseline PET/CT parameters predicted interim Deauville scores. As for SUVmax, patients with the interim Deauville 1–3 showed no statistically significant difference compared to patients with the interim Deauville 4–5 in the R‐chemo and R2 cohorts (Figure S3a,b). As for TMTV, patients with the interim Deauville 1–3 manifested a notably lower baseline TMTV than patients with the interim Deauville 4–5 in the R‐chemo and R2 cohorts (Figure S3c,d). As illustrated by receiver operating characteristic (ROC) curves, areas under the curve (AUC) were 0.687 and 0.725 in the R‐chemo and R2 cohort, with the highest accuracy of 66.7% sensitivity and 76.9% specificity, 71.1% sensitivity and 70.3% specificity (Figure S3e,f). As for TLG, patients with interim Deauville 1–3 displayed lower baseline TLG than patients with interim Deauville 4–5 in the R‐chemo cohort (p < 0.001, Figure 5a) and R2 cohort (p < 0.001, Figure 5b). When illustrated by ROC curves, AUCs were 0.803 in the R‐chemo cohort (p < 0.001, Figure 5c) and 0.842 in the R2 cohort (p < 0.001, Figure 5d). In the R‐chemo cohort, the optimal cutoff of 2600 cm3 had the highest accuracy for predicting the interim Deauville 1–3 probability, with a sensitivity of 80.0% and specificity of 83.1%. Accordingly, 90.0% of patients with baseline TLG ≤ 2600 cm3 achieved interim Deauville 1–3. However, 31.4% of patients with baseline TLG > 2600 cm3 achieved interim Deauville 1–3 (p < 0.001, Figure 5e). In the R2 cohort, the optimal cutoff of 600 cm3 had the highest accuracy for predicting the probability of interim Deauville 1–3, with a sensitivity of 86.8%, and specificity of 78.4%. Consistently, 85.3% of patients with baseline TLG ≤ 600 cm3 were scored as interim Deauville 1–3. However, 19.5% of patients with baseline TLG > 600 cm3 were scored as interim Deauville 1–3 (p < 0.001, Figure 5f). Therefore, TLG of baseline PET/CT was a better predictor of the interim Deauville 1–3 than SUVmax or TMTV, with different optimal cutoffs of 2600 and 600 cm3 in the R‐chemo and R2 cohorts.
FIGURE 5.
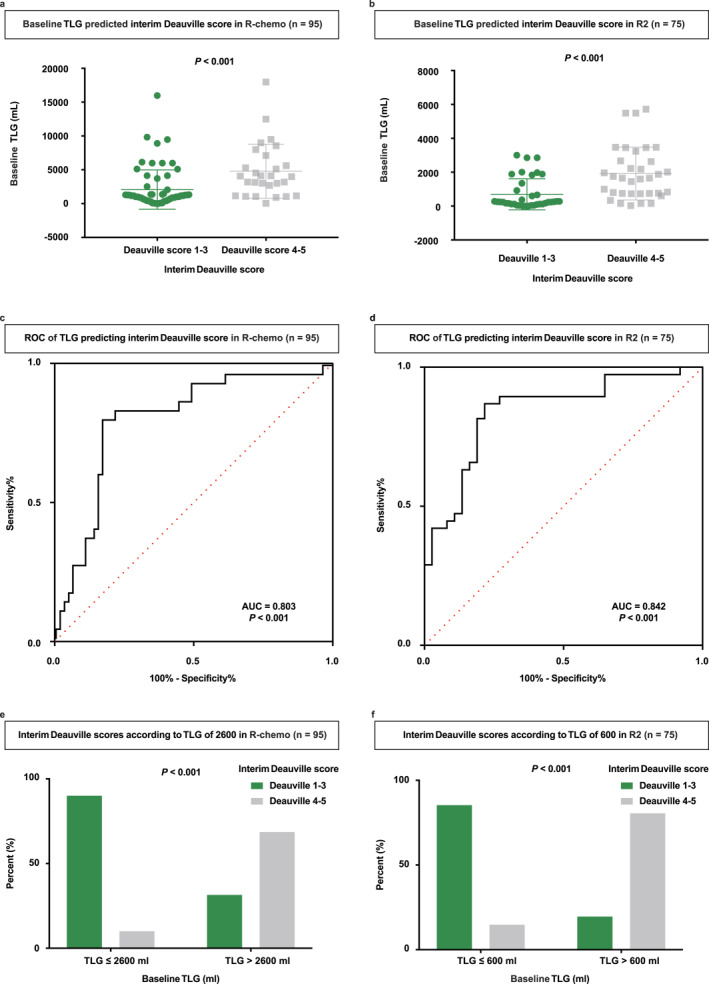
Baseline TLG predicted the interim Deauville 1–3 differently in the R‐chemo and R2 cohorts. (a) Baseline TLG of the interim Deauville 1–3 versus Deauville 4–5 patients in the R‐chemo cohort. (b) Baseline TLG of the interim Deauville 1–3 versus Deauville 4–5 patients in the R2 cohort. (c) ROC curve of baseline TLG for predicting the interim Deauville 1–3 versus Deauville 4–5 in the R‐chemo cohort. (d) ROC curve of baseline TLG for predicting the interim Deauville 1–3 versus Deauville 4–5 in the R2 cohort. (e) Percent of patients with the interim Deauville 1–3 or Deauville 4–5 according to baseline TLG 2600 cm3 in the R‐chemo cohort. (f) Percent of patients with the interim Deauville 1–3 or Deauville 4–5 according to baseline TLG 600 cm3 in the R2 cohort.
3.5. Differential Prognosis Based on Baseline TLG in the R‐Chemo and R2 Cohorts
The optimal cutoffs in predicting interim Deauville 1–3 were further analyzed. Patients with baseline PET/CT were categorized into three groups: low TLG (TLG ≤ 600 cm3), intermediate TLG (600 < TLG ≤ 2600 cm3), and high TLG (TLG > 2600 cm3). In the R‐chemo cohort, proportions of patients experiencing POD24 were 10.5% in the low TLG group, 10.9% in the intermediate TLG group, and 40.0% in the high TLG group (p < 0.001, Figure 6a). As for PFS, the 2‐year PFS was 89.7% and 89.6% for patients with low TLG and intermediate TLG, which were superior to 67.3% for patients with high TLG (p < 0.001, Figure 6b). Thus, patients with low‐intermediate TLG were associated with superior POD24 and prolonged PFS, compared to patients with high TLG in the R‐chemo cohort.
FIGURE 6.
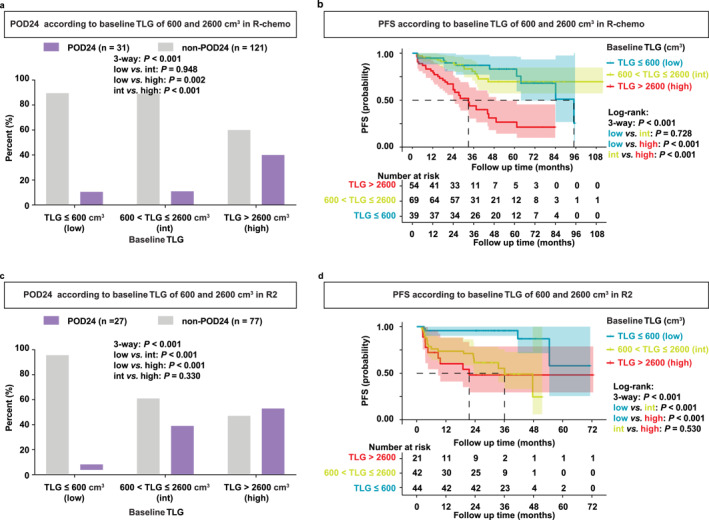
Differential prognosis based on baseline TLG in the R‐chemo and R2 cohorts. (a, b) POD24 (a) and PFS (b) of patients with baseline TLG ≤ 600 cm3 (low TLG), 600 < TLG ≤ 2600 cm3 (intermedia TLG), and TLG > 2600 cm3 (high TLG) in the R‐chemo cohort. (c, d) POD24 (c) and PFS (d) of patients with baseline TLG ≤ 600 cm3 (low TLG), 600 < TLG ≤ 2600 cm3 (intermedia TLG), and TLG > 2600 cm3 (high TLG) in the R2 cohort.
In the R2 cohort, proportions of patients experiencing POD24 were 4.3% in the low TLG group, 39.0% in the intermediate TLG group, and 52.9% in the high TLG (p < 0.001, Figure 6c). As for PFS, the 2‐year PFS was 95.7% for patients with low TLG, which was superior to 61.4% for patients with intermediate TLG, or 48.1% for patients with high TLG (p < 0.001, Figure 6d). Therefore, patients with low TLG were associated with superior POD24 and prolonged PFS, compared to patients with intermediate‐high TLG in the R2 cohort.
4. Discussion
This study constituted a comprehensive, integrated analysis concerning the prognostic implications of sequential PET/CT scans, encompassing PET/CT assessments at the baseline, interim, and end‐of‐induction for newly diagnosed FL patients treated with rituximab‐based immunochemotherapy and immunotherapy.
The significance of PET/CT evaluation at the end‐of‐induction in FL undergoing R‐chemo had been extensively documented [10, 11]. A pooled analysis of patients receiving R‐chemo regimens as first‐line induction therapy from the PRIMA study, the PET‐Folliculaire study, and the FOLL05 study, reported that patients exhibiting a positive PET/CT scan at the end‐of‐induction (defined as Deauville 4–5) had a 4‐year PFS rate of 23.2%, in contrast to 63.4% observed in those with a negative PET/CT scan (defined as Deauville 1–3) [11]. Similarly, an analysis of data from the GALLIUM study involving R‐chemo or O‐chemo (obinutuzumab plus CHOP) regimens demonstrated that PET/CT outperformed contrast‐enhanced CT as an imaging modality for evaluating R‐chemo response in FL, yielding a 2.5‐year PFS of 54.9% in the end‐of‐induction Deauville 4–5 patients, with even higher rates of 87.4% in the end‐of‐induction Deauville 1–3 responders [22]. Our study consistently underscored the prognostic significance of the end‐of‐induction PET/CT in FL patients undergoing R‐chemo regimens, with a remarkably low POD24 of 5.1% and a robust 2‐year PFS of 95.0% in patients with the end‐of‐induction Deauville 1–3. Meanwhile, as for R2 treatment in FL, patients achieving the best response of CMR in the NCT00695786 trial had a longer PFS than those without CMR [23]. In our R2 cohort, patients who reached Deauville 1–3 at the end‐of‐induction also experienced lower POD24 probability and a longer PFS.
In addition, patients not achieving Deauville 1–3 at the end‐of‐induction were further investigated. The GALLIUM study indicated that disparities of PFS between the end‐of‐induction Deauville 4 and Deauville 5 patients did not attain statistical significance [12]. In our R‐chemo cohort, no statistically significant differences were found in terms of POD24 and PFS across the end‐of‐induction Deauville 4 and Deauville 5 patients. However, in our R2 cohort, the end‐of‐induction Deauville 4 patients demonstrated superior outcomes compared to Deauville 5 patients, consistent with the different treatment response for patients with interim Deauville 4 in our R‐chemo and R2 cohorts, possibly attributed to the immunomodulatory effects of lenalidomide in disease control [24].
The prognostic role of interim PET/CT has been recently emphasized, especially in several FDG‐avid aggressive lymphomas where it served as a robust surrogate marker for precision treatment [25, 26]. However, the reports about the prognostic value of interim PET/CT in FL remained limited, with some suggesting that a positive interim PET/CT is associated with inferior PFS, and others concluding that interim PET/CT scans were not prognostic [15, 16]. In the scope of our study, we further investigated the prognostic value of interim PET/CT assessments. Interestingly, patients with the interim Deauville 1–3 demonstrated a diminished incidence of POD24 and a superior PFS, as compared to their counterparts with the interim Deauville 4–5. They also outperformed patients exhibiting the interim Deauville 4–5/end‐of‐induction Deauville 1–3. This trend persisted irrespective of the specific treatment regimens employed, encompassing both the R‐chemo and R2 cohorts. It underscored the pivotal role of interim PET/CT in evaluating the sensitivity of R‐chemo or R2, providing indispensable insights into early‐stage treatment response, and necessary support for further discussion of response‐adapted strategies in FL, aiming at improving outcomes in patients with the interim Deauville 4–5.
Considering that baseline PET/CT measurements have been demonstrated to predict clinical outcomes in FL, including SUVmax, TMTV, and TLG [7, 8, 9], we embarked on an exploration of the utility of baseline PET/CT parameters in distinguishing interim Deauville 1–3 versus Deauville 4–5. Our findings indicated that TLG surpassed SUVmax and TMTV in predicting interim Deauville scores, as evidenced by AUC‐ROC analysis. Interestingly, the optimal thresholds for TLG in predicting interim Deauville 1–3 diverged between the R‐chemo and R2 cohorts, with a value of TLG 2600 and 600 cm3, respectively. Although unlike reports that classified patients into high and low groups based on TLG for predicting survival of R‐chemo‐treated patients [7, 27], we divided patients into three groups based on the two thresholds for predicting the interim Deauville score in R‐chemo and R2 cohorts. In line with previous literature [7, 28], patients with the lowest TLG (TLG ≤ 600 cm3) demonstrated favorable outcomes in both R‐chemo and R2 cohorts, while those with the highest TLG (TLG > 2600 cm3) experienced poor outcomes. On the contrary, patients with the intermediate TLG (600 cm3 < TLG ≤ 2600 cm3) exhibited different patterns between the R‐chemo and R2 cohorts. This suggested a difference in prognosis between R‐chemo and R2 in certain TLG‐based subgroups of FL patients, possibly because TLG represents tumor glucose metabolism [29]. Higher glucose metabolism of tumor cells has been reported to result in immune suppression [30]. In contrast, the tumor immune microenvironment was an essential target of lenalidomide [24], which could make the efficacy of R2 more dependent on different TLG.
5. Conclusion
In summary, our findings underscored the prognostic potential of PET/CT scans in FL patients undergoing rituximab‐based immunochemotherapy and immunotherapy. Interim Deauville 1–3 was emerged as a pivotal predictor of prognosis, which could be effectively predicted by TLG of baseline PET/CT, providing an alternative individualized approach for newly diagnosed FL.
Author Contributions
All authors contributed to the study conception and design. Nan Wang, Xin‐Yun Huang, and Xu‐Feng Jiang collected and analyzed the data, performed the formal analysis, wrote the original draft, and were responsible for methodology. Nan Wang, Li Wang, Shu Cheng, and Peng‐Peng Xu recruited patients. Lei Dong and Bin‐Shen Ou‐Yang reviewed the histopathologic diagnoses. Rong‐Ji Mu gave statistical advice for this manuscript. Chen Li, Yan Zhao, Yan Feng, and Hong‐Jing Dou gave technical support. Zhong Zheng and Wei‐Li Zhao conceived the study, directed, and supervised research and reviewed and edited the manuscript.
Ethics Statement
The study was approved by the ethics committee and institutional review board of Shanghai Ruijin Hospital. Written informed consent was obtained from all participants included in the study in accordance with the Declaration of Helsinki.
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/hon.70012.
Supporting information
Supporting Information S1
Acknowledgments
We thank the Multicenter Hematology‐Oncology Programs Evaluation System (M‐HOPES), Network & Information Center at Shanghai Jiao Tong University, Collaborative Innovation Center of Systems Biomedicine, and the Samuel Waxman Cancer Research Foundation, and all the patients involved for allowing us to analyze their clinical data.
Funding: This work was supported by the National Key R&D Program of China (2022YFC2502600), National Natural Science Foundation of China (82130004, 81830007, 81900193, and 82400230), and Clinical Research Plan of Shanghai Hospital Development Center (SHDC2020CR1032B).
Nan Wang, Xin‐Yun Huang, and Xu‐Feng Jiang equally contributed to this work.
Contributor Information
Zhong Zheng, Email: zheng_zhong89@163.com.
Wei‐Li Zhao, Email: zhao.weili@yahoo.com.
Data Availability Statement
Any data not published within the article will be shared in an anonymized format by request from any qualified investigator. If desired, please contact the corresponding author of this article.
References
- 1. Jacobsen E., “Follicular Lymphoma: 2023 Update on Diagnosis and Management,” American Journal of Hematology 97, no. 12 (2022): 1638–1651, 10.1002/ajh.26737. [DOI] [PubMed] [Google Scholar]
- 2. Liu W., Ji X., Song Y., et al., “Improving Survival of 3760 Patients With Lymphoma: Experience of an Academic Center Over Two Decades,” Cancer Medicine 9, no. 11 (2020): 3765–3774, 10.1002/cam4.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morschhauser F., Nastoupil L., Feugier P., et al., “Six‐Year Results From RELEVANCE: Lenalidomide Plus Rituximab (R(2)) Versus Rituximab‐Chemotherapy Followed by Rituximab Maintenance in Untreated Advanced Follicular Lymphoma,” Journal of Clinical Oncology 40, no. 28 (2022): 3239–3245, 10.1200/jco.22.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morschhauser F., Fowler N. H., Feugier P., et al., “Rituximab Plus Lenalidomide in Advanced Untreated Follicular Lymphoma,” New England Journal of Medicine 379, no. 10 (2018): 934–947, 10.1056/nejmoa1805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barrington S. F. and Trotman J., “The Role of PET in the First‐Line Treatment of the Most Common Subtypes of Non‐Hodgkin Lymphoma,” Lancet Hematology 8, no. 1 (2021): e80–e93, 10.1016/s2352-3026(20)30365-3. [DOI] [PubMed] [Google Scholar]
- 6. Brady J. L., Binkley M. S., Hajj C., et al., “Definitive Radiotherapy for Localized Follicular Lymphoma Staged by (18)F‐FDG PET‐CT: A Collaborative Study by ILROG,” Blood 133, no. 3 (2019): 237–245, 10.1182/blood-2018-04-843540. [DOI] [PubMed] [Google Scholar]
- 7. Zhou Y., Zhao Z., Li J., et al., “Prognostic Values of Baseline, Interim and End‐Of Therapy (18)F‐FDG PET/CT in Patients With Follicular Lymphoma,” Cancer Management and Research 11 (2019): 6871–6885, 10.2147/cmar.s216445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meignan M., Cottereau A. S., Versari A., et al., “Baseline Metabolic Tumor Volume Predicts Outcome in High‐Tumor‐Burden Follicular Lymphoma: A Pooled Analysis of Three Multicenter Studies,” Journal of Clinical Oncology 34, no. 30 (2016): 3618–3626, 10.1200/jco.2016.66.9440. [DOI] [PubMed] [Google Scholar]
- 9. Liang J. H., Zhang Y. P., Xia J., et al., “Prognostic Value of Baseline and Interim Total Metabolic Tumor Volume and Total Lesion Glycolysis Measured on 18F‐FDG PET‐CT in Patients With Follicular Lymphoma,” Cancer Research Treatment 51, no. 4 (2019): 1479–1487, 10.4143/crt.2018.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrington S. F., Mikhaeel N. G., Kostakoglu L., et al., “Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group,” Journal of Clinical Oncology 32, no. 27 (2014): 3048–3058, 10.1200/jco.2013.53.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trotman J., Luminari S., Boussetta S., et al., “Prognostic Value of PET‐CT After First‐Line Therapy in Patients With Follicular Lymphoma: A Pooled Analysis of Central Scan Review in Three Multicentre Studies,” Lancet Hematology 1, no. 1 (2014): e17–e27, 10.1016/s2352-3026(14)70008-0. [DOI] [PubMed] [Google Scholar]
- 12. Barrington S. F., Mir F., El‐Galaly T. C., et al., “Follicular Lymphoma Treated With First‐Line Immunochemotherapy: A Review of PET/CT in Patients Who Did Not Achieve a Complete Metabolic Response in the GALLIUM Study,” Journal of Nuclear Medicine 63, no. 8 (2022): 1149–1154, 10.2967/jnumed.121.262869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barrington S. F., Phillips E. H., Counsell N., et al., “Positron Emission Tomography Score Has Greater Prognostic Significance Than Pretreatment Risk Stratification in Early‐Stage Hodgkin Lymphoma in the UK RAPID Study,” Journal of Clinical Oncology 37, no. 20 (2019): 1732–1741, 10.1200/jco.18.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceriani L., Martelli M., Gospodarowicz M. K., et al., “Positron Emission Tomography/Computed Tomography Assessment After Immunochemotherapy and Irradiation Using the Lugano Classification Criteria in the IELSG‐26 Study of Primary Mediastinal B‐Cell Lymphoma,” International Journal of Radiation Oncology, Biology, Physics 97, no. 1 (2017): 42–49, 10.1016/j.ijrobp.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 15. Procházka V., “Interim PET in Follicular Lymphoma: More Patience, Please,” Annals of Hematology 102, no. 7 (2023): 1953–1954, 10.1007/s00277-023-05238-x. [DOI] [PubMed] [Google Scholar]
- 16. Merryman R. W., Michaud L., Redd R., et al., “Interim Positron Emission Tomography During Frontline Chemoimmunotherapy for Follicular Lymphoma,” Hemasphere 7, no. 2 (2023): e826, 10.1097/hs9.0000000000000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casulo C., Byrtek M., Dawson K. L., et al., “Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study,” Journal of Clinical Oncology 33, no. 23 (2015): 2516–2522, 10.1200/jco.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boellaard R., Delgado‐Bolton R., Oyen W. J., et al., “FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0,” European Journal of Nuclear Medicine and Molecular Imaging 42, no. 2 (2015): 328–354, 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Louarn N., Galicier L., Bertinchamp R., et al., “First Extensive Analysis of (18)F‐Labeled Fluorodeoxyglucose Positron Emission Tomography‐Computed Tomography in a Large Cohort of Patients With HIV‐Associated Hodgkin Lymphoma: Baseline Total Metabolic Tumor Volume Affects Prognosis,” Journal of Clinical Oncology 40, no. 12 (2022): 1346–1355, 10.1200/jco.21.01228. [DOI] [PubMed] [Google Scholar]
- 20. Blanc‐Durand P., Jégou S., Kanoun S., et al., “Fully Automatic Segmentation of Diffuse Large B Cell Lymphoma Lesions on 3D FDG‐PET/CT for Total Metabolic Tumour Volume Prediction Using a Convolutional Neural Network,” European Journal of Nuclear Medicine and Molecular Imaging 48, no. 5 (2021): 1362–1370, 10.1007/s00259-020-05080-7. [DOI] [PubMed] [Google Scholar]
- 21. Longhini F., Pan C., Xie J., et al., “New Setting of Neurally Adjusted Ventilatory Assist for Noninvasive Ventilation by Facial Mask: A Physiologic Study,” Critical Care 21, no. 1 (2017): 170, 10.1186/s13054-017-1761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trotman J., Barrington S. F., Belada D., et al., “Prognostic Value of End‐Of‐Induction PET Response After First‐Line Immunochemotherapy for Follicular Lymphoma (GALLIUM): Secondary Analysis of a Randomised, Phase 3 Trial,” Lancet Oncology 19, no. 11 (2018): 1530–1542, 10.1016/s1470-2045(18)30618-1. [DOI] [PubMed] [Google Scholar]
- 23. Strati P., Jain P., Johnson R. J., et al., “Long‐term Follow‐Up of Lenalidomide and Rituximab as Initial Treatment of Follicular Lymphoma,” Blood 137, no. 8 (2021): 1124–1129, 10.1182/blood.2020007994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gribben J. G., Fowler N., and Morschhauser F., “Mechanisms of Action of Lenalidomide in B‐Cell Non‐Hodgkin Lymphoma,” Journal of Clinical Oncology 33, no. 25 (2015): 2803–2811, 10.1200/jco.2014.59.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaCasce A. S., Dockter T., Ruppert A. S., et al., “Positron Emission Tomography‐Adapted Therapy in Bulky Stage I/II Classic Hodgkin Lymphoma: CALGB 50801 (Alliance),” Journal of Clinical Oncology 41, no. 5 (2023): 1023–1034, 10.1200/jco.22.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shi Q., He Y., Yi H. M., et al., “Positron Emission Tomography‐Adapted Therapy in Low‐Risk Diffuse Large B‐Cell Lymphoma: Results of a Randomized, Phase III, Non‐Inferiority Trial,” Cancer Communications 43, no. 8 (2023): 896–908, 10.1002/cac2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H., Wang M., Zhang Y., et al., “Prediction of Prognosis and Pathologic Grade in Follicular Lymphoma Using (18)F‐FDG PET/CT,” Frontiers in Oncology 12 (2022): 943151, 10.3389/fonc.2022.943151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pacella S., “Prognostic Role of Metabolic Parameters (MTV and TLG) of Staging PET/CT in the Pediatric Population With Hodgkin's Lymphoma: An Open Discussion,” European Journal of Nuclear Medicine and Molecular Imaging 50, no. 1 (2022): 12–13, 10.1007/s00259-022-05990-8. [DOI] [PubMed] [Google Scholar]
- 29. Viglianti B. L., Wale D. J., Wong K. K., et al., “Effects of Tumor Burden on Reference Tissue Standardized Uptake for PET Imaging: Modification of PERCIST Criteria,” Radiology 287, no. 3 (2018): 993–1002, 10.1148/radiol.2018171356. [DOI] [PubMed] [Google Scholar]
- 30. Arner E. N. and Rathmell J. C., “Metabolic Programming and Immune Suppression in the Tumor Microenvironment,” Cancer Cell 41, no. 3 (2023): 421–433, 10.1016/j.ccell.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Any data not published within the article will be shared in an anonymized format by request from any qualified investigator. If desired, please contact the corresponding author of this article.


