Abstract
As the size of the chemical industry increases, chemical accidents continue to occur as the handling volume of chemicals also increases. Currently, in the case of a chemical accident, the prediction of the scope of influence mainly analyzes the scope of the impact on a single substance in the accident and does not consider the scope of the decomposition and reaction products. Nitric acid, one of the many chemical accidents, produces nitrogen dioxide, which is harmful when decomposed. In this research, we at first assumed an outflow accident of nitric acid. After conducting our research using the Chemical Accident Response Information System (CARIS), we discovered that the impact range and breakdown of nitric acid were comparable to that of nitrogen dioxide. Furthermore, we were able to identify the potential ramifications associated with such occurrences. In this study, the impact scenarios were evaluated according to the worst-case scenario, the alternative scenario, the size of the leak hole diameter, atmospheric temperature, atmospheric wind speed, and atmospheric stability. The results showed that the end point concentration distance of nitrogen in ERPG-1, 2 and 3 was larger than nitric acid in most conditions, with differences of up to 5.45 times in the worst-case scenario, 5.96 times in the alternative scenario, up to 6.952 times in the leak hole diameter scenario, up to 10 times in the atmospheric temperature scenario, up to 13 times in the wind speed-specific scenario, and up to 4 times in the atmospheric stability scenario. In the event of a nitric acid spill chemical accident, the response agency should set up a boundary area at a greater distance in consideration of the impact of nitrogen dioxide when setting up a boundary area such as the Hot zone and the Warm zone. Nitric acid handling sites should install additional detectors for nitrogen dioxide, which is decomposition products, to monitor the diffusion of decomposition products into the atmosphere when installing detection alarm equipment in preparation for leakage accidents. It is believed that the government should continuously secure and study data on other decomposable chemicals besides nitric acid to provide material information and disaster prevention methods to chemical accident response agencies and communities for effective and early treatment of chemical accidents.
Keywords: Chemical accident, Nitric acid, CARIS, ERPG, Evacuation scope, Boundary area
1. Introduction
As industries become more advanced and human life becomes more enriched, the types and amounts of chemicals used in cutting-edge technologies have continued to increase, and the scale of the related chemical industry is also growing. The chemical industry is divided into two types: The petrochemical industry and the fine chemical industry. In particular, in the case of the fine chemical industry, which is a capital-, knowledge- and technology-intensive chemical industry that creates high added value, the market for materials for the electronic information industry, carbon fiber, and fuel cells is showing rapid growth worldwide, and the fine chemical industry market accordingly is growing rapidly. The size is expected to increase by an average of 4.3 % per year from $1.878 trillion in 2015 to approximately $2.87 trillion in 2025 [1].
As the scale of the chemical industry grows, the amount of chemical substances handled increases, and chemical accidents continue to occur. A major chemical accident that occurred in Korea was the hydrogen fluoride leak that occurred in Gumi, Gyeongsangbuk-do in September 2012. At that time, the accident resulted in 5 deaths, 18 injuries, and caused extensive damage with compensation and recovery costs of approximately 42.1 billion won [2]. The Gumi accident increased public interest and concerns about chemical accidents, which led to the need to strengthen regulations related to chemical substances. Accordingly, the Chemical Substances Control Act (CSCA), which completely revised the existing Toxic Chemicals Control Act, as well as established a hazardous chemical prevention and.
Management system and a rapid response system to chemical accidents, was implemented in January 1, 2015. The main purposes of the CSCA are guaranteeing the public's right to know about chemical substances, specifying standards for handling hazardous chemicals, establishing a prevention system (such as an off-site impact assessment system for chemical accidents), a risk management plan, and a hazardous chemical business license system, and immediately reporting chemical accidents. This also includes assigning duties and dispatching on-site coordination coordinators [3].
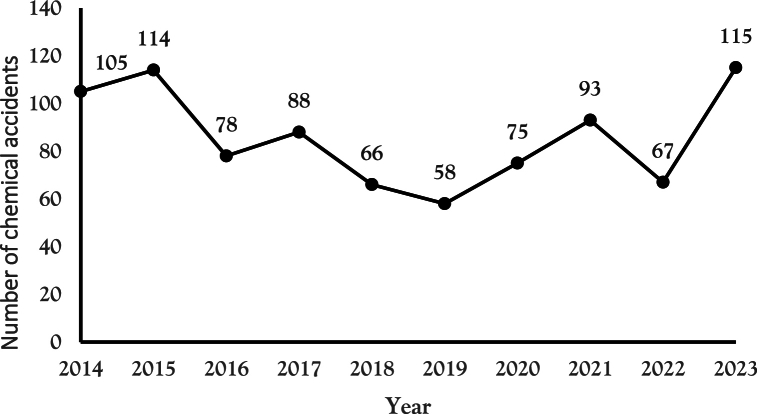
Meanwhile, looking at statistics on the occurrence of chemical accidents over the past ten years, there has been a increasing trend every year from 105 in 2014 and 114 in 2015 to 78 in 2016, 88 in 2017, 66 in 2018, 58 in 2019, 75 in 2020, 93 in 2021, 67 in 2022 and 115 in 2023, leading to the implementation of the CSCA [Fig. 1] [4].
Fig. 1.
Number of chemical accidents by year (2014–2023).
However, as can be seen in the case of the Gumi hydrogen fluoride (HF) leak, chemical accidents cause large-scale damage to life, property, and the environment, so response agencies must be prepared at all times and respond quickly when an accident occurs.
The first priority in response to and management of chemical accidents is to evacuate residents around the workplace where the accident occurred to minimize casualties. To this end, the Ministry of Environment is using the Chemical Accident Response Information System (CARIS) to predict the extent of damage in the event of a chemical accident. CARIS is a program that provides material information, handles company information, and predicts the extent of damage to responding agencies when a chemical accident occurs [5].
Utilizing the results of predicting the extent of chemical accident damage enables effective and rapid responses to chemical accidents. However, the results have mainly predicted the extent of damage to a single accidental substance and do not take into account damage due to decomposition and reaction products. Accordingly, in this study, nitric acid, a substance with a high history of chemical accidents, was selected, and the damage range of decomposition products was predicted using CARIS. After comparing the prediction with the expected damage range of nitric acid, we were able to establish alert areas and evacuation ranges for residents. We also notified response agencies to prepare for potential chemical accidents. The findings of this study will undoubtedly aid in enhancing our chemical accident response capabilities.
2. Background
2.1. Nitric acid handling status
Looking at the status of nitric acid handling by classifying only hazardous chemicals in the recently released 2016 chemical substance statistical survey, the handling volume of nitric acid was approximately 2.21 million tons, ranking 15th [Table 1], and the number of handling companies was 1,910, ranking 10th [Table 2]. When it comes to accident preparation materials, nitric acid isn't the most commonly handled substance. In fact, it ranks 10th in terms of handling volume and 7th in terms of handling companies. However, it still ranks relatively high in both categories, indicating that it's still a substance that requires careful handling and preparation. It's important to be aware of nitric acid's potential hazards and to take all necessary precautions when working with it [6].
Table 1.
2016 Statistical surveys on Chemicals (Order of Hazardous chemical handling volume).
| Order | Chemical | Volume of chemicals handled(ton/year) | Number of chemicals handled companies |
|---|---|---|---|
| 1 | p-Xylene | 16,776,272.30 | 1085 |
| 2 | Sulfuric acid | 15,264,671.70 | 3075 |
| 3 | Sodium hydroxide | 15,098,343.60 | 5820 |
| 4 | Benzene | 14,913,117.90 | 302 |
| 5 | Hydrochloric acid | 10,685,649.30 | 2600 |
| 6 | Toluene | 8,286,443.80 | 4852 |
| 7 | Methyl alcohol | 8,037,416.30 | 3531 |
| 8 | 1,3-Butdadiene | 5,529,646.90 | 80 |
| 9 | Xylene | 4,738,683.60 | 4252 |
| 10 | Chlorine | 2,784,055.20 | 249 |
| 11 | 1,2-Dichloroethane | 2,775,021.90 | 59 |
| 12 | Ammonia | 2,326,615.2 | 628 |
| 13 | Vinyl chloride | 2,727,777.30 | 30 |
| 14 | Ammonium nitrate | 2,230,666.90 | 121 |
| 15 | Nitric acid | 2,217,264.20 | 1910 |
Table 2.
2016 Statistical surveys on Chemicals (Order of Hazardous chemical handling companies).
| Order | Chemical | Volume of chemicals handled(ton/year) | Number of chemicals handled companies |
|---|---|---|---|
| 1 | Sodium hydroxide | 15,098,343.60 | 5820 |
| 2 | Toluene | 8,286,443.80 | 4852 |
| 3 | Xylene | 4,738,683.60 | 4252 |
| 4 | Methyl alcohol | 8,037,416.30 | 3531 |
| 5 | Sulfuric acid | 15,264,671.70 | 3075 |
| 6 | Ethyl acetate | 987,039.90 | 2717 |
| 7 | Methyl ethyl ketone | 1,192,814.20 | 2700 |
| 8 | Hydrochloric acid | 10,685,649.30 | 2600 |
| 9 | Talc | 1,256,875.90 | 2451 |
| 10 | Nitric acid | 2,217,264.20 | 1910 |
| 11 | Potassium hydroxide | 732,477.00 | 1638 |
| 12 | Hydrogen peroxide | 828,333.80 | 1486 |
| 13 | m-xylene | 892,779.80 | 1306 |
| 14 | p-xylene | 16,776,272.30 | 1085 |
| 15 | Ammonium hydroxide | 66,478.50 | 970 |
2.2. Nitric acid properties
In this study, nitric acid with a concentration of 70 %, which is mainly used in industry, was selected. Nitric acid (CAS No. 7697-37-2) is a highly corrosive and oxidizing substance. Pure nitric acid is colorless at room temperature, but over time it decomposes into nitrogen oxide and water, gradually turning into a yellow liquid. It is a non-flammable material and does not burn on its own, but decomposes when heated, thereby producing corrosive and toxic fumes. 70 % nitric acid has a molecular weight of 63.01, specific gravity of 1.4, boiling point of approximately 122 °C, melting point of approximately −42 °C, and vapor pressure of 6.4 kPa (at 20 °C) [4,7].
In addition, the definition of ERPG and the standards for nitric acid in the ERPG published by the American Industrial Hygiene Association (AIHA) are [Table 3] and [Table 4], respectively [8].
Table 3.
ERPG-1, 2, 3 definitions [9].
| ERPG tiers | Definition |
|---|---|
| ERPG-1 | The maximum airborne concentration below which nearly all individuals could be exposed for up to 1 h without experiencing more than mild, transient adverse health effects or without perceiving a clearly defined objectionable odor. |
| ERPG-2 | The maximum airborne concentration below which nearly all individuals could be exposed for up to 1 h without experiencing or developing irreversible or other serious health effects or symptoms which could impair an individual's ability to take protective action. |
| ERPG-3 | The maximum airborne concentration below which it is believed that nearly all individuals could be exposed for up to 1 h without experiencing or developing life-threatening health effects. |
Table 4.
ERPG value of Nitric acid.
| ERPG tiers | Value(ppm) |
|---|---|
| ERPG-1 | 1 |
| ERPG-2 | 10 |
| ERPG-3 | 78 |
The National Fire Protection Association (NFPA) hazard code for nitric acid is classified as health hazard 3, reaction hazard 1, and special hazard oxidizing material. The time-weighted average exposure standard (TWA), which is based on 8 h a day and does not have any adverse health effects on almost all workers, is 2 ppm [10]. The Acute Exposure Guideline Levels (AEGLs), which are critical exposure limits for the general public created by the U.S. Environmental Protection Agency (EPA), are listed in Table 5 [11].
Table 5.
AEGLs value of Nitric acid.
| Exposure time | AEGL-1 Value(ppm) | AEGL-2 Value(ppm) | AEGL-3 Value(ppm) |
|---|---|---|---|
| 10 min | 0.16 | 43 | 170 |
| 30 min | 0.16 | 30 | 120 |
| 60 min | 0.16 | 24 | 92 |
| 4 h | 0.16 | 6 | 23 |
| 8 h | 0.16 | 3 | 11 |
2.3. Decomposition reaction of nitric acid
Nitric acid is known to be decomposed by heat to produce nitrogen dioxide (NO2), water (H2O), and oxygen (O2) [12], and the reaction formula is as shown in (1). The decomposition of nitric acid can be promoted, not only by temperature, but also by light, an equivalent energy source [13].
| (1) |
Nitrogen dioxide, which is produced from the decomposition reaction of nitric acid, has a red to brown color at room temperature. It is also observed as a colored cloud that spreads to the surrounding area when a nitric acid spill chemical accident occurs. Based on the photodecomposition test of nitric acid, it has been discovered that 99.9 % nitric acid has a 25 ± level of decomposition. Further analysis revealed that around 1 % of the nitric acid was decomposed at a temperature of 2 °C and within the wavelength range of 2650-2537 Å. These findings highlight the importance of carefully monitoring the conditions under which nitric acid is stored and handled to ensure its stability and effectiveness [14]. This shows that not only nitric acid, but also nitrogen dioxide, must be considered when predicting the extent of damage spreading into the atmosphere in the event of a nitric acid leak.
2.4. Physical and chemical properties of nitrogen dioxide
Nitrogen dioxide (CAS No. 10102-44-0) exists as a red to brown gas with a pungent odor above 21.1 °C. The standards of the Emergency Response Planning Guidelines (ERPG) published by the American Industrial Hygiene Association (AIHA) are as shown in [Table 6] [8]. The National Fire Protection Association (NFPA) hazard code is classified as Health Hazard 3. The time weighted average (TWA) exposure standard for 8 h a day, which does not have any adverse health effects on almost all workers, is 3 ppm [10].
Table 6.
ERPG value of Nitrogen dioxide.
| ERPG tiers | Value(ppm) |
|---|---|
| ERPG-1 | 1 |
| ERPG-2 | 15 |
| ERPG-3 | 30 |
The Acute Exposure Guideline Levels (AEGLs) announced by the U.S. Environmental Protection Agency (EPA) are listed in [Table 7] [15].
Table 7.
AEGLs value of Nitrogen dioxide.
| Exposure time | AEGL-1 Value(ppm) | AEGL-2 Value(ppm) | AEGL-3 Value(ppm) |
|---|---|---|---|
| 10 min | 0.5 | 20 | 34 |
| 30 min | 0.5 | 15 | 25 |
| 60 min | 0.5 | 12 | 20 |
| 4 h | 0.5 | 8.2 | 14 |
| 8 h | 0.5 | 6.7 | 11 |
In the Hazardous Substance Emergency Response Handbook, the initial separation distance, protective activity distance, and ERG response guideline number for nitric acid and nitrogen dioxide are shown in Table 8 [16].
Table 8.
ERG information of Nitric acid and Nitrogen dioxide.
| Division | Nitric acid | Nitrogen dioxide | |
|---|---|---|---|
| Initial separation distance when leaking | Small spills (less than 200L) | 30m | 30m |
| Large spills (over 200L) | 150m | 400m | |
| Distance of protective action in case of spill | Small spills (less than 200L) | Day: 0.1 km, | Day: 0.1 km, |
| Night: 0.1 km | Night: 0.4 km | ||
| Large spills (over 200L) | Day: 0.2 km, | Day: 1.2 km, | |
| Night: 0.4 km | Night: 3.0 km | ||
| ERG Guide Number | 157 | 124 | |
In the initial separation distance, if it is small, the distance between the two substances is the same, but if it is large, nitrogen dioxide is about 2.67 times larger. The protective action distance for nitrogen dioxide in the event of a leak is 4 times that of a small-scale leak at night, 6 times that of a large-scale daytime leak, and 7.5 times that of a large-scale night leak. Based on the accident response information on the ERG, it is judged that in the case of a chemical accident with a large amount of nitric acid leakage, a wider initial separation distance and protective activity distance should be set in consideration of the conversion rate of nitrogen dioxide.
2.5. Current status of chemical accidents
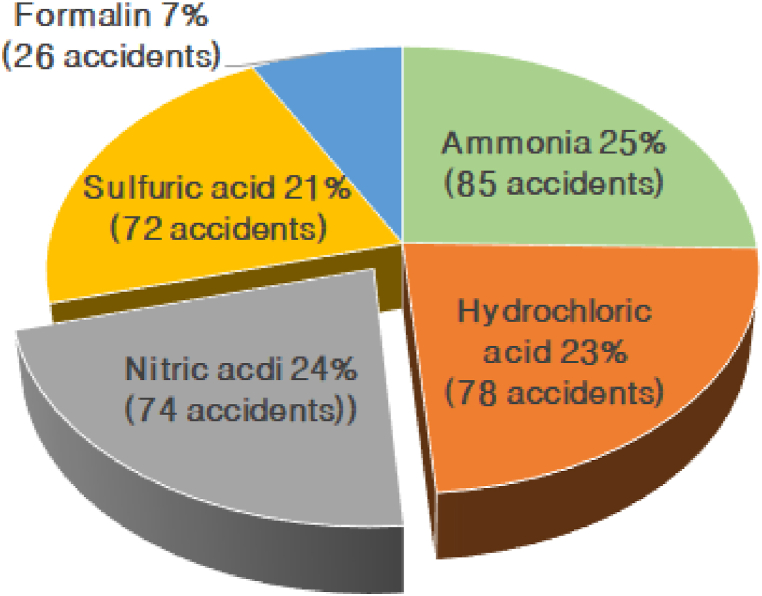
From 2014 to 2023, the total number of chemical accidents in Korea was 859. Of these, 72 cases, or about 8.38 %, were nitric acid single substance accidents in Fig. 2, and when adding mixture spill accidents containing nitric acid, 84 cases, or about 9.78 %, occurred. Looking at each year, 14 chemical accidents caused by nitric acid alone occurred in 2014, 7 in 2015, 8 in 2016, 12 in 2017, 6 in 2018, 4 in 2019, 4 in 2020, 6 in 2021, 6 in 2022, and 5 in 2023. Among the chemical accidents that occurred within the same period, nitric acid accounted for 74 cases, or 24 %, of the top 5 substances classified as single substances. Looking at Table 9, nitric acid leak accidents appear to have a higher accident frequency than other accident substances. When classifying nitric acid leak accidents by accident type, it was found that worker negligence occurred the most with 17 cases, followed by poor facility management with 12 cases, and transportation vehicle accidents with 9 cases [4]. Through the surveyed domestic chemical accident status, it can be seen that the frequency of chemical accidents caused by nitric acid leaks is higher than that of other substances. In addition, safety education, management, and supervision for workers when handling nitric acid must be strengthened.
Fig. 2.
Top 5 single chemicals causing chemical accidents (2014–2023).
Table 9.
Order by single chemicals causing chemical accidents (2018–2023).
| Order | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|---|---|
| 1st | Ammonia | Ammonia | Hydrochloric acid | Ammonia | Nitric acid | Sulfuric acid |
| 2nd | Nitric acid | Hydrochloric acid | Sulfuric acid | Sulfuric acid | Hydrochloric acid | Ammonia |
| 3rd | Hydrochloric acid | Nitric acid | Nitric acid | Hydrochloric acid | Ammonia | Hydrochloric acid |
| 4th | Sulfuric acid | Sulfuric acid | Sodium Hydroxide | Nitric acid | Sulfuric acid | Nitric acid |
| 5th | Chlorine | Hydrogen peroxide | Toluene | Hydrogen peroxide | Sodium Hydroxide | Toluene |
2.6. Utilization of CARIS (Chemical Accident Response Information System)
In order to predict the extent of damage in the event of a chemical spill, ALO- HA (Areal Location of Hazardous Atmospheres), PHAST (Process Hazard Analysis), and KORA (Korea Off-site Risk Assessment supporting tool) are used in Korea. As the Chemical Substances Management Act went into effect on January 1, 2015 and Off-site Risk Assessment (ORA) was implemented, all workplaces handling hazardous chemicals are required to conduct their own risk assessment using KORA, which was developed by the Korea Institute of Chemical Safety and Safety in 2014 [17].
Meanwhile, chemical accident response agencies are using CARIS, developed by the National Institute of Environmental Research in 2002, to predict the extent of damage. This analysis is then used to establish alert zones, send emergency disaster text messages, and select resident evacuation zones [18]. CARIS is implemented to manage spatial information and attribute information together based on the GIS (Geographic Information System). Its main functions include real-time weather information and diffusion assessment information, chemical information searches, handling company searches, and responses. It is a comprehensive chemical accident and terrorist response system equipped with tips and prevention information [19].
The scope of impact assessment using CARIS is divided into explosion, crater fire, grass fire, explosion, and toxic spread. For toxic diffusion, the scope of influence is evaluated by selecting one of either the container leakage, puddle evaporation, atmospheric inflow rate, and leakage time. The common element required when assessing the spread of toxicity is meteorological information. The meteorological information that must be entered when evaluating the scope of influence of the CARIS toxic spread is wind direction, wind speed, atmospheric temperature, atmospheric pressure, relative humidity, and atmospheric stability. Six grades of atmospheric stability are used according to Pasquill, and the grade classification is as shown in Table 10.
Table 10.
Pasquill stability class table.
| Wind speed, S(m/s) | Day |
Night |
|||
|---|---|---|---|---|---|
| Strong | Moderate | Slight | Cloudy | Clear | |
| S ≤ 2 | A | A ∼ B | B | E | F |
| 2 < S ≤ 3 | A ∼ B | B | C | E | F |
| 3 < S ≤ 5 | B | B ∼ C | C | D | E |
| 5 < S ≤ 6 | C | C ∼ D | D | D | D |
| 6 < S | C | D | D | D | D |
The diffusion models used to calculate the extent of CARIS' damage impact are the Gaussian and SLAB models. Gaussian is a model applied when diffusing light gas, and SLAB is a model applied when diffusing heavy gas. In the case of the Gaussian model mounted on CARIS, there is a limitation that the topographic conditions must be relatively flat. However, the system is very convenient and quick, so it is useful in the early stages of a chemical accident [20,21].
2.6.1. Gaussian model
For continuous leakage of light gas, the Gaussian plume model is applied. Prerequisites are '① leak rate is constant.', '② leak occurs on a flat surface without obstacles.', '③ There is no chemical reaction or thermodynamic effect.', '④ than the time it takes for the leak period to reach a certain point.', '⑤ A stable gas where a leaked substance can stay in the air for a long time.', '⑥ flume is completely reflected at the surface and mixing height,' and '⑦ leak occurs at one point.' Concentration at a certain point is predicted by calculating the diffusion coefficient according to the atmospheric stability and then calculating the effective leakage height.
2.6.2. SLAB model
For continuous leakage of light gas, the Gaussian plume model is applied. Prerequisites are '① leak rate is constant.', '② leak occurs on a flat surface without obstacles.', '③ There is no chemical reaction or thermodynamic effect.', '④ than the time it takes for the leak period to reach a certain point.', '⑤ A stable gas where a leaked substance can stay in the air for a long time.', '⑥ flume is completely reflected at the surface and mixing height,' and '⑦ leak occurs at one point.' Concentration at a certain point is predicted by calculating the diffusion coefficient according to the atmospheric stability and then calculating the effective leakage height.
3. Method
3.1. Business location
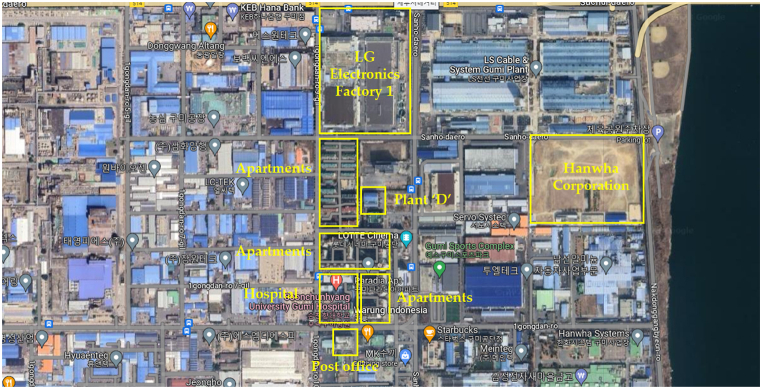
Most nitric acid handling businesses are located in industrial complexes or agricultural and industrial complexes. Since the actual workplace could not be selected for security reasons, the Gumi City Lifelong Education Center was assumed to be the location of the “D” accident workplace in Fig. 3. Plant “D’” handles 70 % nitric acid. It is located in one of the Gumi National Industrial Complexes, and there are multi-use facilities, apartments, government offices, and many businesses nearby. In the south, university hospitals, apartments, community centers, postal offices, businesses, and shopping centers are concentrated. To the west are apartments, to the north are the Gumi Tax Office and LG Electronics Factory 1, and to the east are a number of businesses, including Hanwha Corporation.
Fig. 3.
Plant ‘D’ location on the map.
3.2. Meteorological data
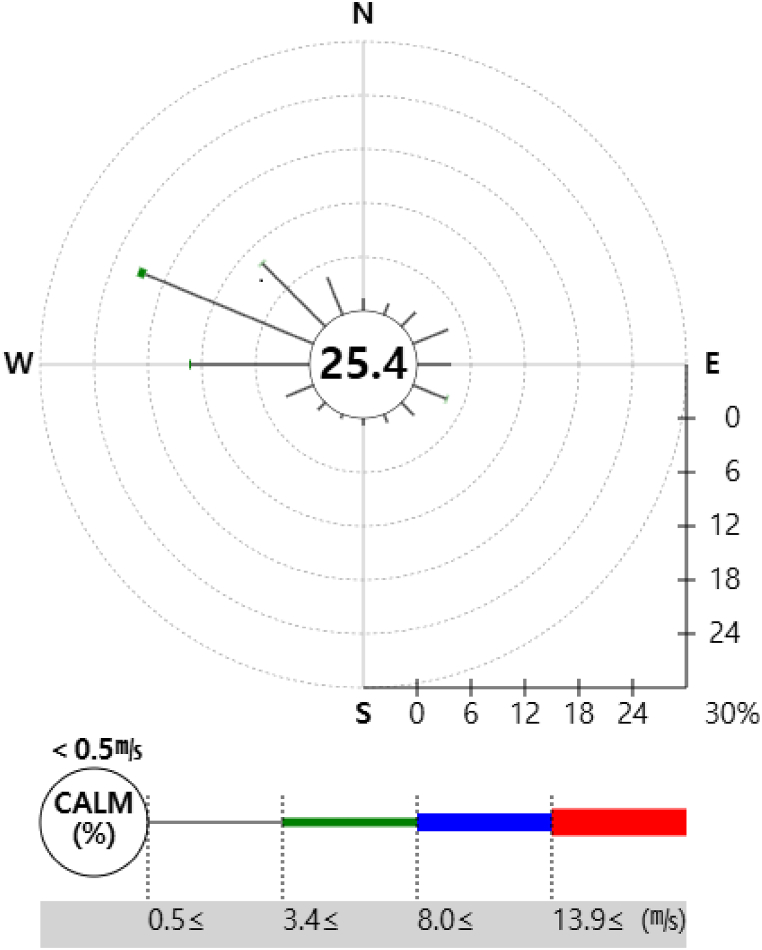
As there was no meteorological data for the “D” workplace, i.e., the virtual workplace of this study, ground observation data from the Gumi Meteorological Station, i.e., the nearest meteorological station, was used for the dates January 2019 to December 2019 [Table 11]. In 2019, the local pressure averaged at 1010.53 hPa, while the average temperature stood at 14.15 °C. The highest temperature recorded during the year was 25.94 °C. The relative humidity averaged at 70.50 %, and the average wind speed was 1.05 m/s. The maximum average wind speed was 4.72 m/s, with the wind direction being westerly. The maximum average wind direction was west, which is worth noting [22]. Based on [Table 11], the wind rose is represented as Fig. 4.
Table 11.
Weather data of Plant “D” Area.
| Date (Year-Month) | Average Atmosphere (hPa) | Temperature (°C) |
Average relative humidity (%) | Wind |
||||
|---|---|---|---|---|---|---|---|---|
| Average | Maximum | Average wind speed (m/s) | Maximum |
Most |
||||
| Wind speed (m/s) | Wind direction | Wind direction | ||||||
| 2019–01 | 1025.6 | 1.0 | 6.5 | 55 | 1.3 | 5.1 | W | W |
| 2019–02 | 1023.8 | 3.4 | 9.0 | 56 | 1.3 | 5.7 | W | W |
| 2019–03 | 1017.0 | 8.9 | 15.4 | 56 | 1.4 | 5.8 | W | W |
| 2019–04 | 1015.1 | 13.2 | 19.4 | 61 | 1.1 | 4.9 | W | W |
| 2019–05 | 1011.8 | 20.0 | 27.1 | 55 | 1.2 | 5.0 | W | W |
| 2019–06 | 1007.1 | 22.7 | 28.5 | 74 | 1.0 | 3.8 | ENE | W |
| 2019–07 | 1006.7 | 25.2 | 29.7 | 87 | 0.9 | 3.6 | W | W |
| 2019–08 | 1007.2 | 26.4 | 31.4 | 85 | 1.0 | 4.0 | W | W |
| 2019–09 | 1015.2 | 21.9 | 26.6 | 90 | 0.8 | 4.5 | E | W |
| 2019–10 | 1019.0 | 15.7 | 21.5 | 84 | 0.8 | 4.2 | W | W |
| 2019–11 | 1022.7 | 8.8 | 15.3 | 75 | 0.8 | 5.2 | W | W |
| 2019–12 | 1025.6 | 2.6 | 8.3 | 68 | 1.0 | 4.8 | W | W |
| Average | 1010.53 | 14.15 | 25.94 | 70.50 | 1.05 | 4.72 | W | W |
Fig. 4.
Wind roses based on [Table 11].
3.3. Accident facility
The “D” workplace is a business that transports and sells nitric acid in tank trucks to the Gumi National Industrial Complex. The nitric acid (70 %) storage facility is located outdoors and is assumed to be a vertical cylinder with a diameter of 4m and a height of 6m. The storage volume is 67.86m3, which is 90 % of the maximum capacity of the storage tank (75.40m3) [Table 12].
Table 12.
Nitric acid storage facility design value.
| Division | Value |
|---|---|
| Diameter(d) | 4m |
| Height(h) | 6m |
| Volume | 75.40m3 |
| Distance from storage facility to embankment | 2m |
| Height of embankment | 1.5m |
| Horizontal length of embankment | 8m |
| longitudinal length of embankment | 18m |
| Area within the embankment | 51.34m2 |
The CSCA clearly specifies the standards for hazardous chemical handling facilities. Before 2015, there were no special regulations on discharge walls in the Toxic Chemicals Control Act, but the CSCA established new regulations related to discharge walls to reduce the spread of hazardous chemicals due to leakage [3]. Outdoor storage facilities in accordance with the Chemical Substances Management Act must install a discharge wall to reduce oil and leakage damage. The internal capacity of the discharge wall must be more than 110 % of the storage facility capacity, and the height of the discharge wall must be more than 0.5m.
The distance from the storage facility side plate to the discharge wall is at least 1.5m from the tank side plate. If the tank diameter is less than 15m, it is a distance of more than 1/3 of the tank height, and if the tank diameter is 15m or more, it is a distance of more than 1/2 of the tank height. You must keep your distance. The outdoor storage facility for nitric acid at the “D” workplace is equipped with a square discharge wall, and the distance between the storage facility and the discharge wall is assumed to be 2m.
3.4. Accident scenario selection
In this study, some of the accident scenario conditions from the Korea Chemical Safety Institute’s Technical Guidelines for Accident Scenario Selection were used. “Accident scenario” refers to either a description of an accident in which the scope of impact due to a fire, explosion, or leak or spill extends to the outside of the workplace, or an accident that does not affect the outside of the workplace, but may have a serious impact on workers [23]. In addition, this study aimed to calculate and compare the range of influence by selecting the toxicity assessment of nitric acid as well as the leakage and the toxicity assessment of nitrogen dioxide, a decomposition product.The nitric acid storage facility operates at room temperature and pressure. It was assumed that the external level gauge of the 15 mm diameter storage tank was damaged and the entire storage amount (67.86 m3, 95.00 tons) leaked inside the discharge wall. The amount of nitrogen dioxide leaked was assumed to be 1 % (approximately 485.6 kg) of the leaked amount converted to nitrogen dioxide, considering the amount of nitrogen dioxide present in the storage facility and the amount that reacts when exposed to temperature and light as it leaks. Nitric acid uses puddle evaporation during toxic diffusion, and the puddle diameter was calculated by converting the inner area of the discharge walls into a circular area (8.092m). Nitrogen dioxide was used in container leaks during toxic diffusion. The endpoint concentration standards for the range of influence were ERPG-1, 2, and 3 for each substance.The “worst case scenario” refers to a case where the maximum amount that can be retained in individual unit facilities or piping that handles hazardous chemicals is retained. An accident scenario can have a maximum range of impact on people and the environment due to various factors such as fire, explosion, leakage, or seepage. These can cause serious harm and damage, and it is important to take all necessary precautions to prevent such incidents from occurring. It is crucial to be aware of potential hazards and to have a plan in place to handle emergencies in a safe and effective manner. Safety should always be the top priority in any situation, and everyone should do their part to ensure that accidents are minimized and that people and the environment are protected [23]. To calculate the maximum range of influence, the wind speed was set to 1.5 m/s, and the atmospheric stability was set to F (very stable). The air temperature was set to 25 °C, the relative humidity was set to 50 %, and the terrain was urban. The temperature of the leaked material was used as the operating temperature. The wind direction was west, which was the most frequent direction in 2019. In addition, to compare the range of influence of nitric acid and nitrogen dioxide by major input factors, diffusion was evaluated according to the size of the leak hole’s diameter, temperature, wind speed, and atmospheric stability.“Alternative accident scenario” refers to a scenario in which the scope of impact on people and the environment extends beyond the workplace among accident scenarios that are realistically more likely to occur than the worst accident scenario [23]. The average meteorological data referring to [Table 11] was used as the meteorological data, the wind speed was set to 1.05 m/s, and the atmospheric stability was set to D (neutral). The air temperature was set at 14.15 °C, the relative humidity was set at 70.5 %, and the terrain was selected as urban terrain. Operating temperature was used as the flow rate of the leaked material, and westerly wind was used as the wind direction.
4. Results and discussion
4.1. Comparison of scope of impact according to worst-case scenario
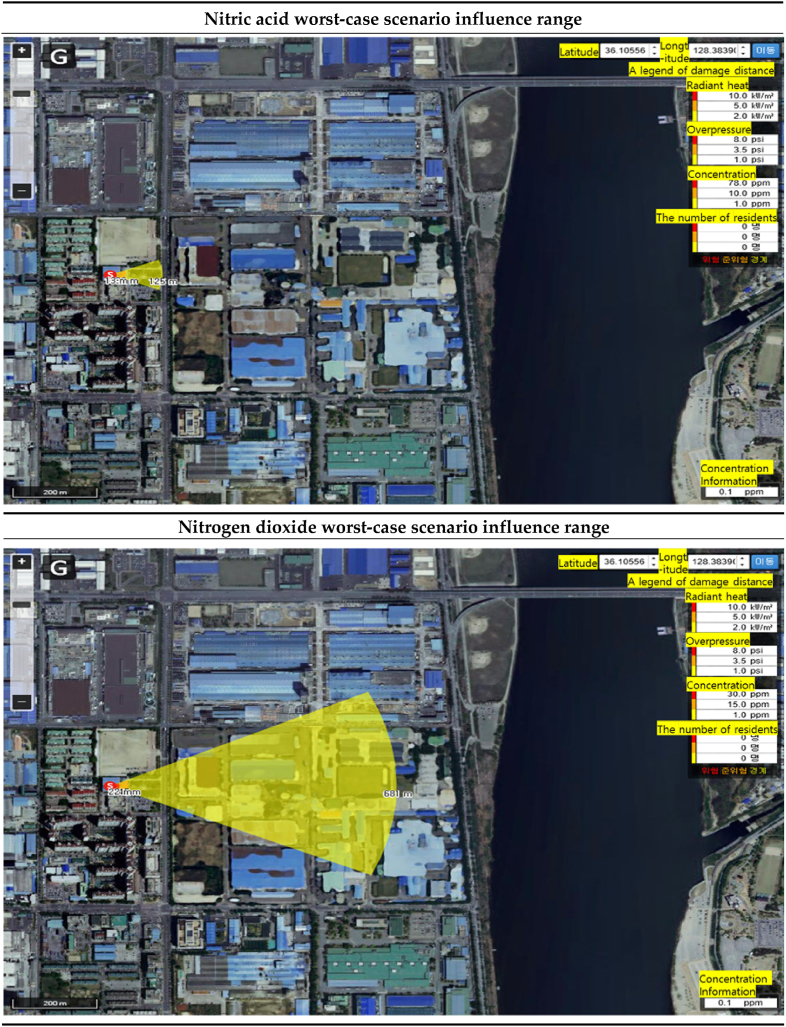
In the “worst case scenario”, the range of influence of nitric acid is 13m, with ERPG-3 (78ppm), the maximum airborne concentration at which almost all people are exposed to life-threatening or health effects after being exposed for 1 h from the accident point, and almost all people are exposed for 1 h. ERPG-2 (10ppm), the maximum concentration in the air that does not cause irreversible serious health effects or cause serious adverse health effects, even if exposed for a long time, is 38m. Almost all people can perceive an unpleasant odor without adverse health effects after being exposed for 1 h. The maximum concentration that can be measured, ERPG-1 (1ppm), was found to be 125m [Table 13]. The range of influence of nitrogen dioxide was found to be 22m for the ERPG-3 concentration (30ppm), 45m for the ERPG-2 concentration (15ppm), and 681m for the ERPG-3 concentration (1ppm) from the accident site. The difference in the influence range was that the ERPG-3 endpoint concentration distance of nitrogen dioxide was 9m, the ERPG-2 endpoint concentration distance was 7m, and the ERPG-1 endpoint concentration distance was 556m greater than the influence range of nitric acid [Table 14].
Table 13.
Worst-case scenario influence range prediction results.
| ERPG tiers | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|
| ERPG-1 | 125 | 681 | 556 |
| ERPG-2 | 38 | 45 | 7 |
| ERPG-3 | 13 | 22 | 9 |
Table 14.
Worst-case scenario influence range modeling results.
4.2. Comparison of scope of influence according to alternative scenarios
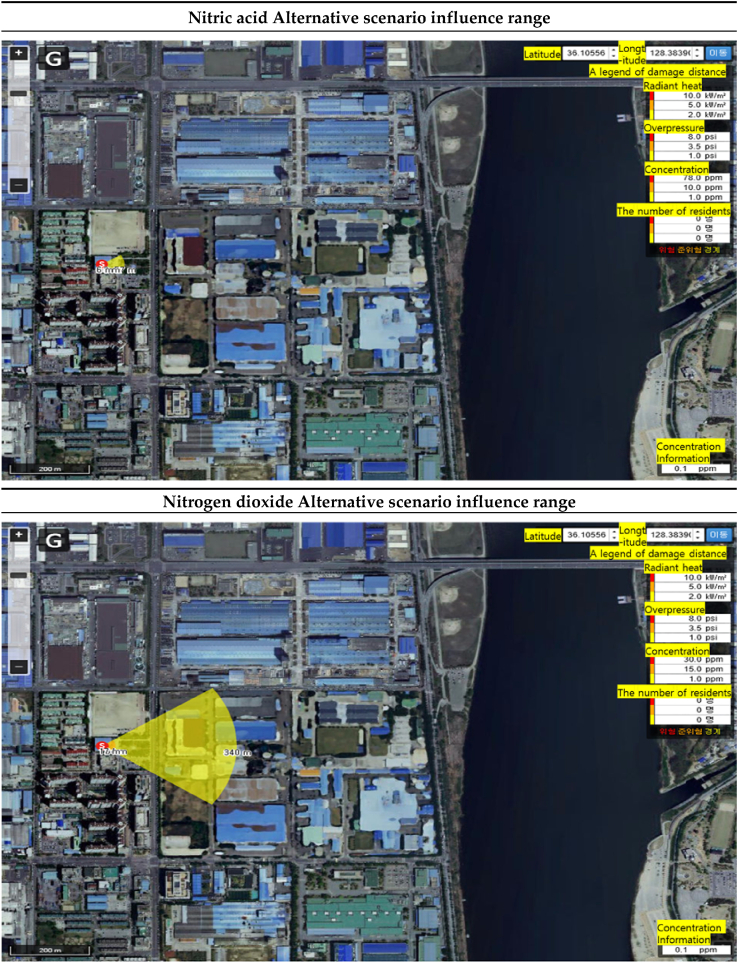
In the “alternative scenario”, the range of influence of nitric acid was found to be 6m for the ERPG-3 concentration (78ppm), 18m for the ERPG-2 concentration (10ppm), and 57m for the ERPG-1 concentration (1ppm) from the accident site. The range of influence of nitrogen dioxide was found to be 17m for the ERPG-3 concentration (30ppm), 34m for the ERPG-2 concentration (15ppm), and 340m for the ERPG-3 concentration (1ppm) from the accident site [Table 15]. The difference in the influence range was that the ERPG-3 end point concentration distance of nitrogen dioxide was 11 m, the ERPG-2 end point concentration distance was 16 m, and the ERPG-1 end point concentration distance was 283 m greater than the influence range of nitric acid [Table 16].
Table 15.
Alternative scenario influence range prediction results.
| ERPG tiers | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|
| ERPG-1 | 6 | 17 | 11 |
| ERPG-2 | 18 | 34 | 16 |
| ERPG-3 | 57 | 340 | 283 |
Table 16.
Alternative scenario influence range modeling results.
4.3. Comparison of influence range according to leak hole diameter
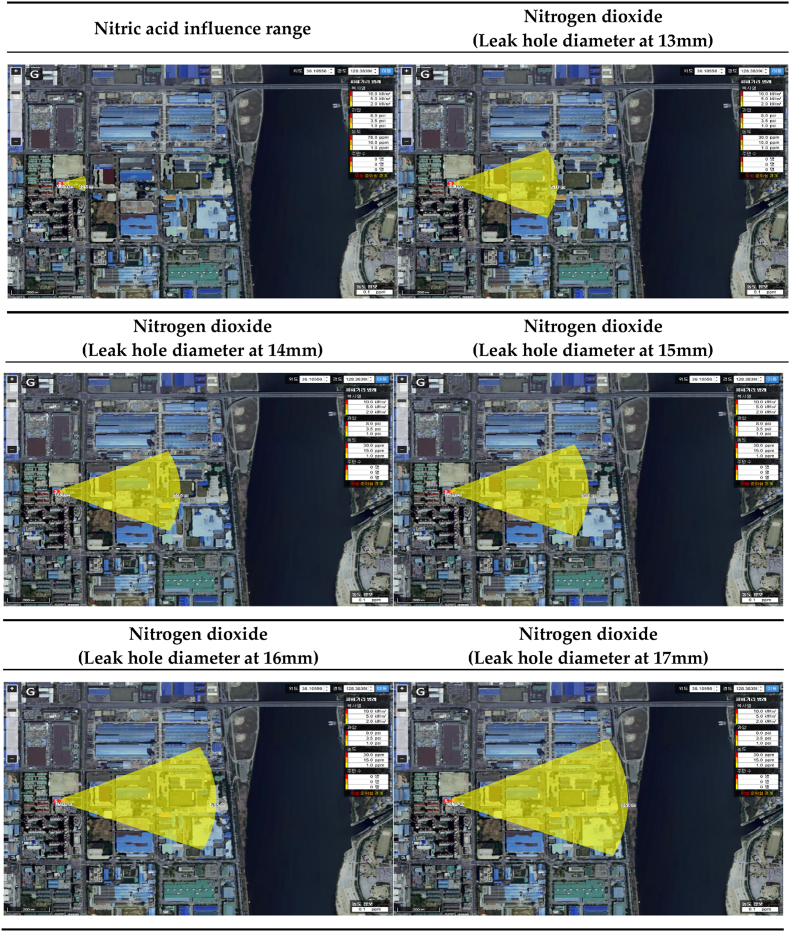
The range of influence of nitric acid and nitrogen dioxide according to the diameter of the storage tank leak hole is shown in Table 17. In the worst-case scenario conditions, the leak hole diameter was categorized into 13–17 mm, and the range of influence was compared. In the case of nitric acid puddle evaporation, the diameter of the leak hole was not affected [Table 17]. The range of influence of nitrogen dioxide according to the diameter of the leak hole increased as the diameter increased. In addition, the range of influence of nitrogen dioxide was found to be larger than that of nitric acid in all conditions, except for the ERPG-2 range of influence under the condition of a leak hole diameter of 13 mm [Table 18].
Table 17.
Prediction results of influence range according to leak hole diameter.
| ERPG tiers | Leak hole diameter(mm) | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|---|
| ERPG-1 | 13 | 125 | 510 | 385 |
| 14 | 595 | 470 | ||
| 15 | 681 | 556 | ||
| 16 | 766 | 641 | ||
| 17 | 869 | 744 | ||
| ERPG-2 | 13 | 38 | 34 | −4 |
| 14 | 39 | 1 | ||
| 15 | 45 | 7 | ||
| 16 | 51 | 13 | ||
| 17 | 58 | 20 | ||
| ERPG-3 | 13 | 13 | 16 | 3 |
| 14 | 19 | 6 | ||
| 15 | 22 | 9 | ||
| 16 | 25 | 12 | ||
| 17 | 28 | 15 |
Table 18.
Influence range modeling results according to leak hole diameter.
4.4. Comparison of influence range according to air temperature
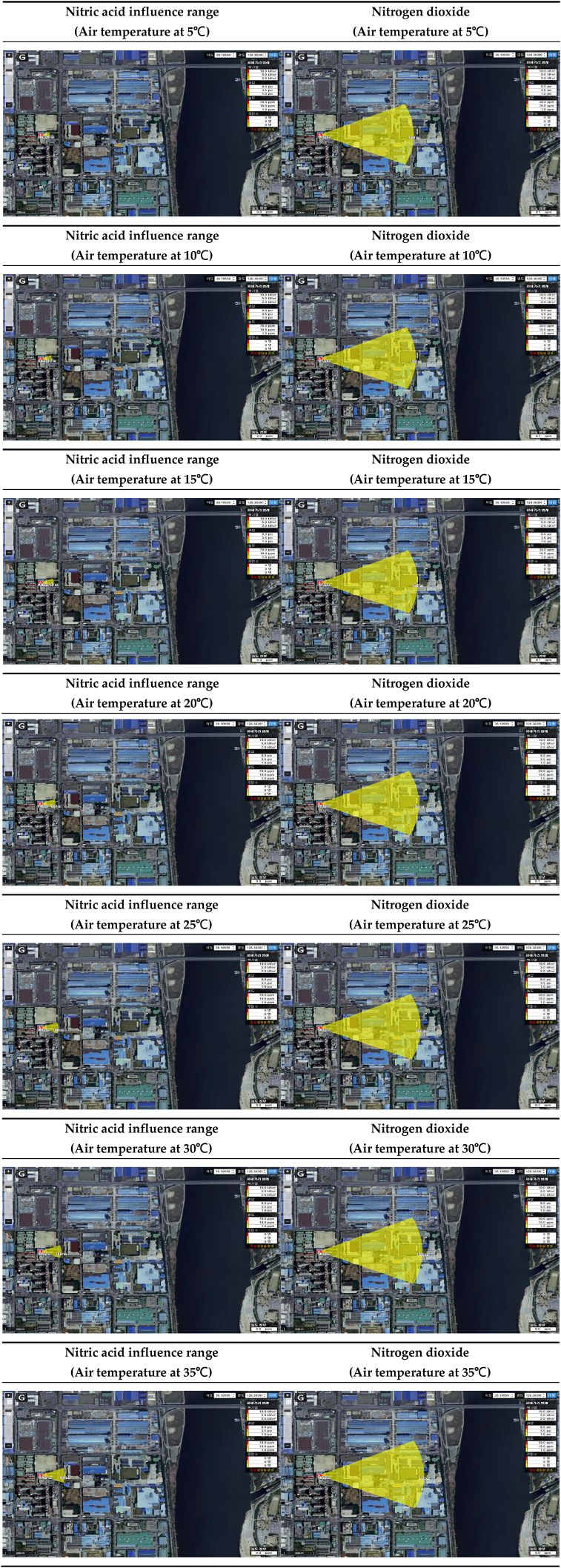
The range of influence of nitric acid and nitrogen dioxide according to air temperature is shown in Table 19. Conditions were classified into the worst-case scenario conditions, with air temperature ranging from 5 to 35 °C, and the range of influence was compared. As a result of predicting the range of influence, the higher the air temperature, the larger the range of influence of nitric acid and nitrogen dioxide. It was confirmed that the range of influence of nitrogen dioxide was larger than that of nitric acid in all conditions, except the ERPG-2 range of influence at an air temperature of 35 °C [Table 20].
Table 19.
Prediction results of influence range according to air temperature.
| ERPG tiers | Air temperature(°C) | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|---|
| ERPG-1 | 5 | 63 | 631 | 568 |
| 10 | 76 | 644 | 568 | |
| 15 | 90 | 657 | 567 | |
| 20 | 106 | 670 | 564 | |
| 25 | 125 | 681 | 556 | |
| 35 | 145 | 693 | 548 | |
| 35 | 169 | 704 | 535 | |
| ERPG-2 | 5 | 20 | 42 | 22 |
| 10 | 23 | 43 | 20 | |
| 15 | 28 | 43 | 15 | |
| 20 | 32 | 44 | 12 | |
| 25 | 38 | 45 | 7 | |
| 35 | 44 | 45 | 1 | |
| 35 | 51 | 46 | −5 | |
| ERPG-3 | 5 | 7 | 21 | 14 |
| 10 | 8 | 21 | 13 | |
| 15 | 10 | 21 | 11 | |
| 20 | 11 | 22 | 11 | |
| 25 | 13 | 22 | 9 | |
| 35 | 16 | 23 | 7 | |
| 35 | 18 | 23 | 5 |
Table 20.
Influence range modeling results according to air temperature.
4.5. Comparison of influence range according to wind speed
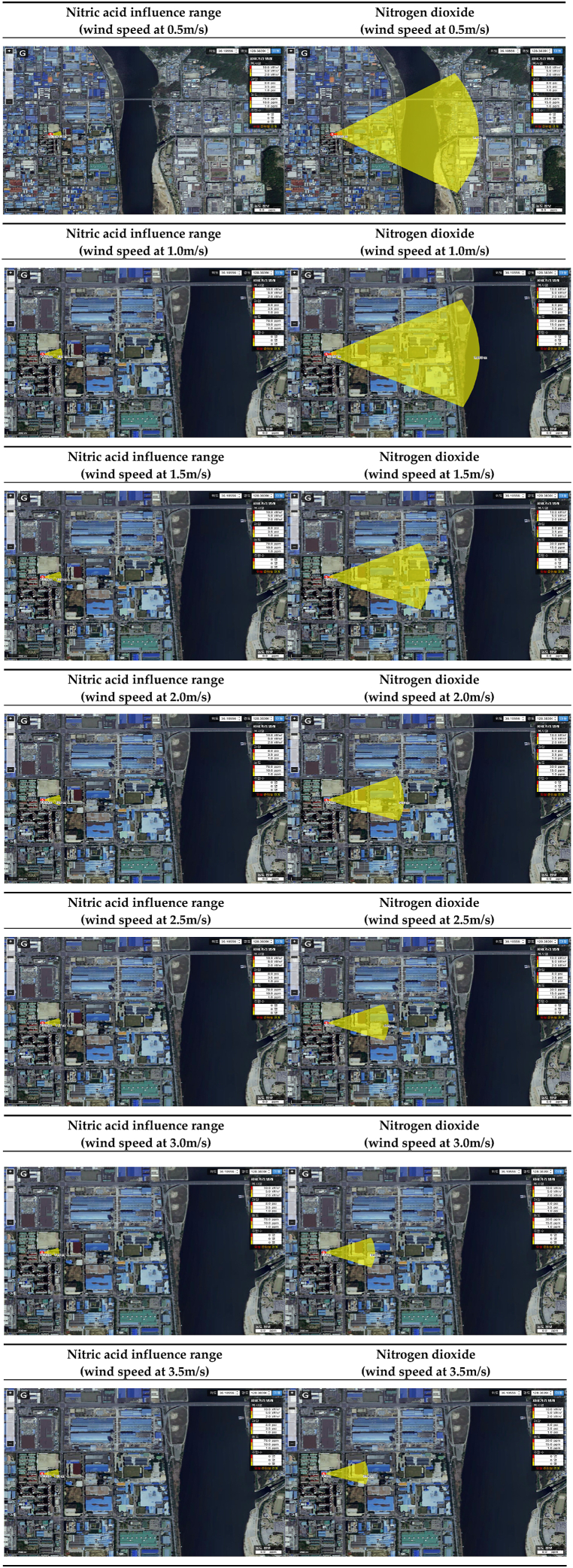
The range of influence of nitric acid and nitrogen dioxide according to wind speed is shown in Table 21. Conditions were classified into wind speeds of 0.5–3.5 m/s under the worst-case scenario conditions, and the range of influence was compared. As a result of predicting the range of influence, the lower the wind speed, the smaller the difference in the range of influence between nitric acid and nitrogen dioxide, but it was found that the range of influence of nitrogen dioxide was larger under most conditions [Table 22].
Table 21.
Prediction results of influence range according to wind speed.
| ERPG tiers | Wind speed(m/s) | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|---|
| ERPG-1 | 0.5 | 142 | 1,940 | 1798 |
| 1.0 | 131 | 1,008 | 877 | |
| 1.5 | 125 | 681 | 556 | |
| 2.0 | 120 | 510 | 390 | |
| 2.5 | 117 | 409 | 292 | |
| 3.0 | 115 | 340 | 225 | |
| 3.5 | 113 | 294 | 181 | |
| ERPG-2 | 0.5 | 43 | 132 | 89 |
| 1.0 | 40 | 67 | 27 | |
| 1.5 | 38 | 45 | 7 | |
| 2.0 | 37 | 34 | −3 | |
| 2.5 | 36 | 26 | −10 | |
| 3.0 | 35 | 22 | −13 | |
| 3.5 | 35 | 19 | −16 | |
| ERPG-3 | 0.5 | 15 | 63 | 48 |
| 1.0 | 14 | 33 | 19 | |
| 1.5 | 13 | 22 | 9 | |
| 2.0 | 13 | 17 | 4 | |
| 2.5 | 13 | 12 | −1 | |
| 3.0 | 12 | 11 | −1 | |
| 3.5 | 12 | 9 | −3 |
Table 22.
Influence range modeling results according to wind speed.
4.6. Comparison of influence range according to atmospheric stability
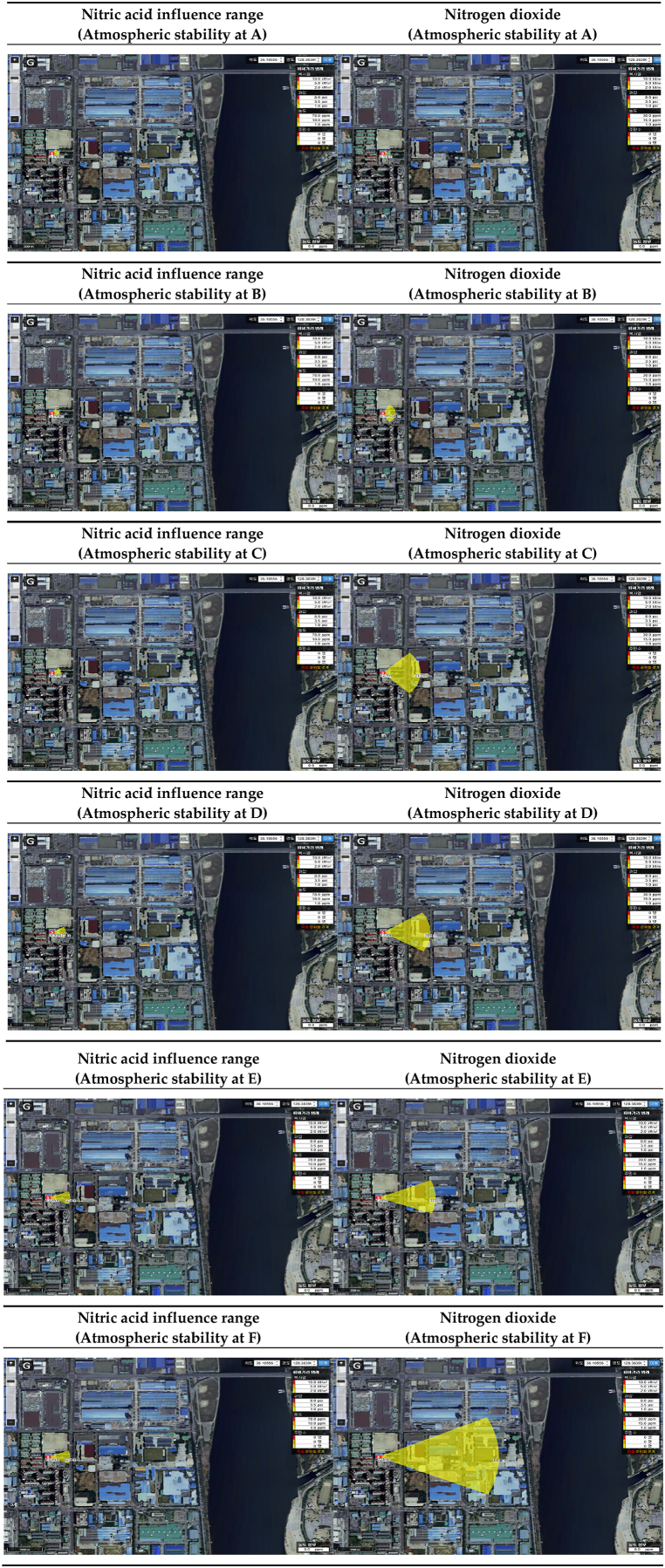
The range of influence of nitric acid and nitrogen dioxide according to atmospheric stability is shown in Table 23. Conditions were classified into A to F for atmospheric stability under worst-case scenario conditions, and the range of influence was compared. As a result of predicting the range of influence, the more stable the atmospheric stability, the larger the range of influence of nitric acid and nitrogen dioxide. In most conditions, the range of influence of nitrogen dioxide was found to be larger than that of nitric acid [Table 24].
Table 23.
Prediction results of influence range according to atmospheric stability.
| ERPG tiers | Atmospheric stability | Nitric acid influence range(m) | Nitrogen dioxide influence range(m) | Difference of influence range(m) |
|---|---|---|---|---|
| ERPG-1 | A | 40 | 25 | −15 |
| B | 40 | 74 | 34 | |
| C | 53 | 213 | 160 | |
| D | 79 | 269 | 190 | |
| E | 125 | 324 | 199 | |
| F | 125 | 681 | 556 | |
| ERPG-2 | A | 13 | 4 | −9 |
| B | 13 | 10 | −3 | |
| C | 17 | 23 | 6 | |
| D | 24 | 25 | 1 | |
| E | 38 | 26 | −12 | |
| F | 38 | 45 | 7 | |
| ERPG-3 | A | 5 | 2 | −3 |
| B | 5 | 6 | 1 | |
| C | 6 | 12 | 6 | |
| D | 8 | 13 | 5 | |
| E | 13 | 13 | 0 | |
| F | 13 | 22 | 9 |
Table 24.
Influence range modeling results according to atmospheric stability.
5. Conclusions
In this study, the range of influence of nitric acid and the range of influence of nitrogen dioxide according to the decomposition of nitric acid were analyzed, assuming the situation of a nitric acid spill accident. The end point concentration distance values of ERPG-1, 2, and 3 were compared and evaluated in the accident of completely leaking from the storage facility using CARIS, a program for predicting the impact range of nitric acid and nitrogen dioxide, and the conclusions were drawn as follows. First, in most scenarios, nitrogen dioxide's impact range was found to be greater than nitric acid. Looking at each scenario, it was found that there was a difference of up to 5.45 times in the worst-case scenario, about 5.96 times in the alternative scenario, up to about 6.952 times in the leak hole diameter condition of 16 mm in the leak hole diameter scenario, up to about 10 times in the atmospheric temperature condition of 5 °C in the atmospheric temperature scenario, up to about 13 times in the wind speed condition of 0.5 m/s in the wind speed scenario, and up to about 4 times in the atmospheric stability 'C' condition in the atmospheric stability scenario.Second, in the event of a nitric acid spill chemical accident, response agencies such as fire, police, and local governments need to set up a boundary area at a greater distance in consideration of the impact of nitrogen dioxide when setting boundary areas such as the Hot Zone and the Warm Zone. Third, it will be effective for emergency response at nitric acid handling sites to monitor the diffusion of decomposition products in the atmosphere by installing additional detectors for nitrogen dioxide, which is decomposition products, when installing detection alarm equipment in preparation for leakage accidents. Fourth, the government should continuously secure and study data on other decomposable chemicals besides nitric acid to provide material information and disaster prevention methods to chemical accident response agencies and communities to deal with effective and early chemical accidents.
CRediT authorship contribution statement
Ji Ung Choi: Writing – review & editing, Investigation, Conceptualization. Woo Sub Shim: Writing – review & editing, Writing – original draft, Validation, Supervision, Conceptualization.
Informed consent statement
Not applicable.
Institutional review board statement
Not applicable.
Data availability statement
Some or all data used in this research are available from the corresponding author by request.
Funding
This work was partially supported by the Ministry of Environment, South Korea and Ministry of Employment and Labor, South Korea.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ji Ung Choi, Email: cju1128@korea.kr.
Woo Sub Shim, Email: shimws0720@korea.kr.
Abbreviations(alphabetical order)
- AEGLs
Acute Exposure Guideline Levels
- AIHA
American Industrial Hygiene Association
- ALOHA
Areal Location of Hazardous Atmospheres
- CARIS
Chemical Accident Response Information System
- CSCA
Chemical Substances Control Act
- EPA
Environmental Protection Agency
- ERPG
Emergency Response Planning Guideline
- GIS
Geographic Information System
- KORA
Korea Off-site Risk Assessment
- NFPA
National Fire Protection Association
- PHAST
Process Hazard Analysis
- TWA
Time-Weighted Average exposure standard
References
- 1.Korea Labor Institute Plan to train and secure human resources in the chemical field (petrochemical, fine chemical, bio) Research Report, Sejong, Republic of Korea. 2018 [Google Scholar]
- 2.Gumi-si HF changes Gumi, Huve Global Co., Ltd. Hydrofluoric acid leak accident white paper. Research Report, Gyeongsangbuk-do, Republic of Korea. 2013 [Google Scholar]
- 3.Ministry of Environment . Korea Ministry of Government Registration; Sejong, Republic of Korea: 2021. Chemical Substances Control Act. [Google Scholar]
- 4.Chemical substance comprehensive information system. Sep.03, 2023. https://icis.me.go.kr/main.do
- 5.National Institute of Environmental Research . 2010. CARIS Enhanced Security and Improved Functionality, Research Report, Incheon, Republic of Korea. [Google Scholar]
- 6.Information on chemical emissions and movements. https://icis.me.go.kr/prtr/main.do
- 7.Chemistry encyclopedia. https://terms.naver.com/entry.nhn?cid=62802&docId=5827788&categoryId=62802
- 8.AIHA Guideline Foundation . 2016. 2016 ERPG/WEEL Handbook. Virginia, USA. [Google Scholar]
- 9.Cavender F., Phillips S., Holland M. Development of emergency of response planning guidelines(ERPGs) J. Med. Toxicol. 2018;4:127–131. doi: 10.1007/BF03160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Employment and Labor . Korea Ministry of Government Registration; Sejong, Republic of Korea: 2020. Exposure Limits for Chemicals and Physical agents(Notice No. 2020-48) [Google Scholar]
- 11.National Research Council . vol. 14. National Academies Press; Washington DC, USA: 2013. (Division on Earth and Life Studies, Board on Environmental Studies and Toxicology, Committee on Toxicology, Committee on Acute Exposure Guideline Levels, Acute Exposure Guideline Levels for Selected Airborne Chemicals). [PubMed] [Google Scholar]
- 12.Glenn D.R., David M.M., William H.C. The kinetics of the thermal decomposition of nitric acid in the liquid phase. J. Phys. Chem. 1955;59:683–690. doi: 10.1021/j150530a004. [DOI] [Google Scholar]
- 13.National Institute of Chemical Safety Hazardous chemical reactivity prediction research (Ⅱ), Korea Ministry of Government Registration: Research Report, Cheongju-si, Chungcheongbuk-do, Republic of Korea. 2019 [Google Scholar]
- 14.Bérces T., Förgeteg S. Kinetics of photolysis of nitric acid vapour. Part 1. Direct photolysis at low conversion. Trans. Faraday Soc. 1970;66:633–639. [Google Scholar]
- 15.Committee on Acute Exposure Guideline Levels . vol. 11. National Academies Press; Washington DC, USA: 2012. (Committee on Toxicology, National Research Council, Acute Exposure Guideline Levels for Selected Airborne Chemicals). [PubMed] [Google Scholar]
- 16.National Institute of Chemical safety Hazardous substance emergency response Handbook (emergency response guidebook), korea Ministry of government registration: cheongju-si. Chungcheongbuk-do, Republic of Korea. 2016 [Google Scholar]
- 17.Shin C.H., Park J.H. An evaluation of the off-site risk of spill from a storage tank of nitric acid. Crisisonomy. 2016;12:187–200. doi: 10.14251/crisisonomy.2016.12.3.187. [DOI] [Google Scholar]
- 18.Park Y.K. Graduate School Kumoh National Institute of Technology; 2015. Evaluation of Hazardous Chemical Substance Releases in Industrial Complex. [Google Scholar]
- 19.National Institute of Environmental Research Chemical accident response information system 2012 user manual, korea Ministry of government registration: cheongju-si. Chungcheongbuk-do, Republic of Korea. 2013 [Google Scholar]
- 20.Lee H.S., Lee T.H., Park K.S., Kim J.G. A study on the evaluation of effects of chemical accident toxicity using CARIS & ALOHA. Journal of Korean Society of Environmental Engineers. 2019;20:8–15. doi: 10.26511/JKSET.20.1.2. [DOI] [Google Scholar]
- 21.Jung W.Y., Lim D.Y., Choi J.W., Seo J.M. Development of chemical material behavior analysis system considering to domestic topography and environmental characteristics. Korean Journal of Hazardous Materials. 2019;7:71–78. doi: 10.31333/kihm.2019.7.1.71. [DOI] [Google Scholar]
- 22.Korea meteorological administration meteorological data open portal. https://data.kma.go.kr/cmmn/main.do
- 23.National Institute of Chemical Safety . Korea Ministry of Government Registration: Cheongju-Si. Chungcheongbuk-do, Republic of Korea; 2018. Technical guidelines for accident scenario selection. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data used in this research are available from the corresponding author by request.