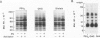Abstract
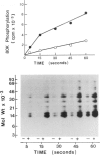
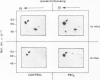
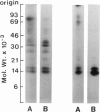
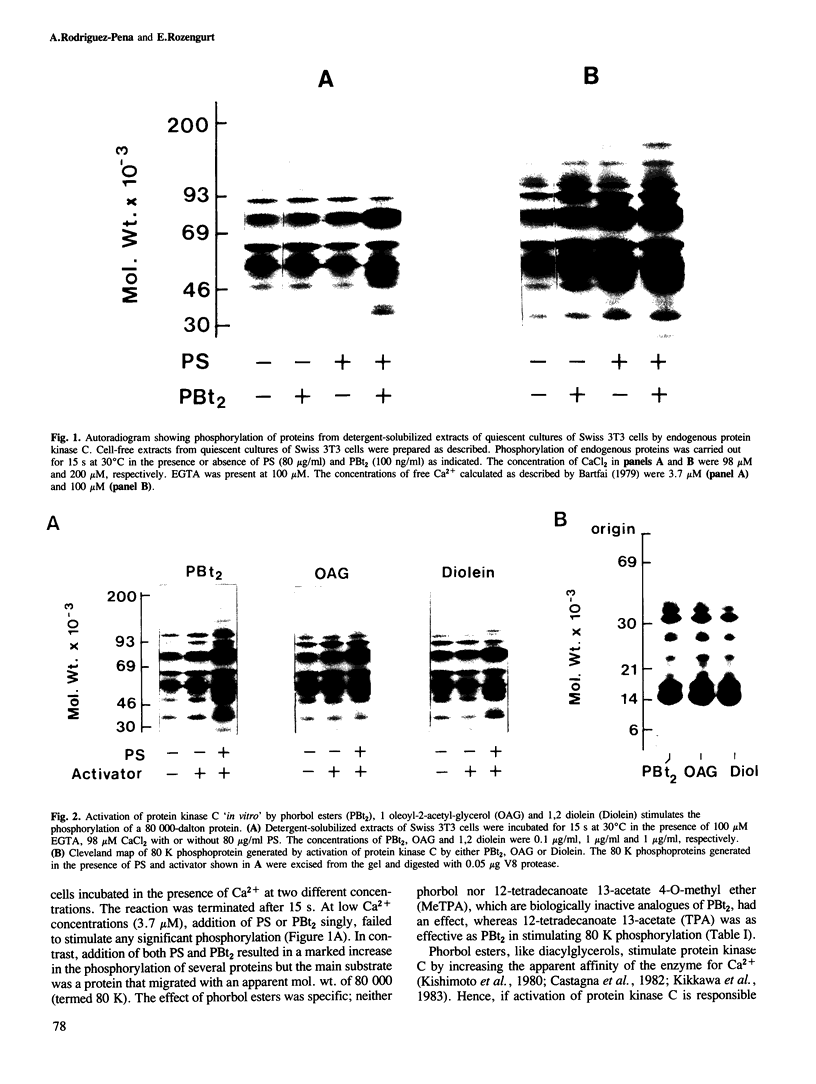
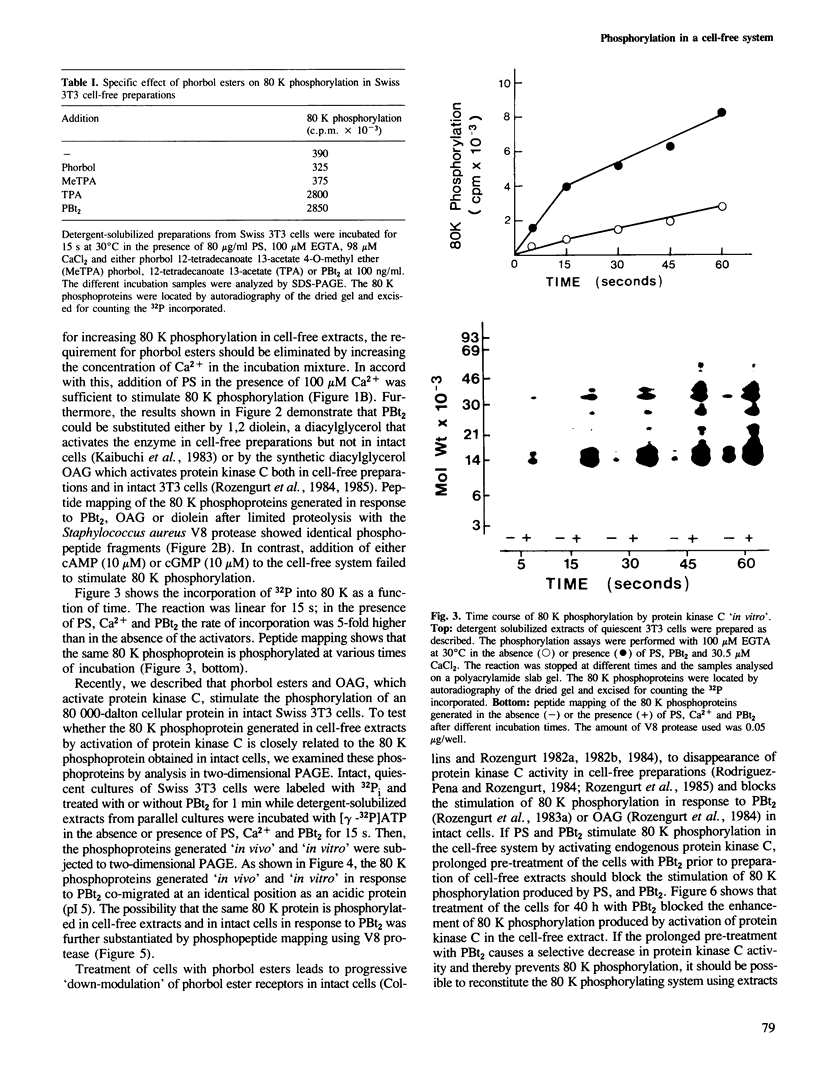
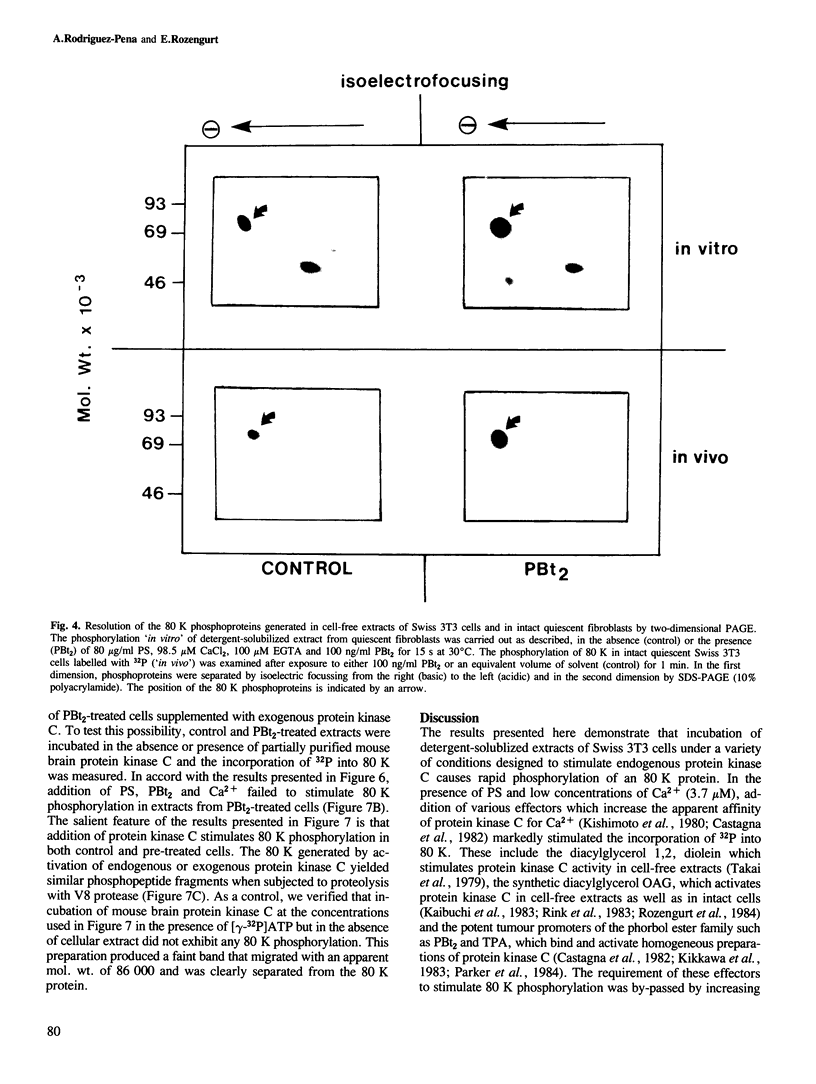
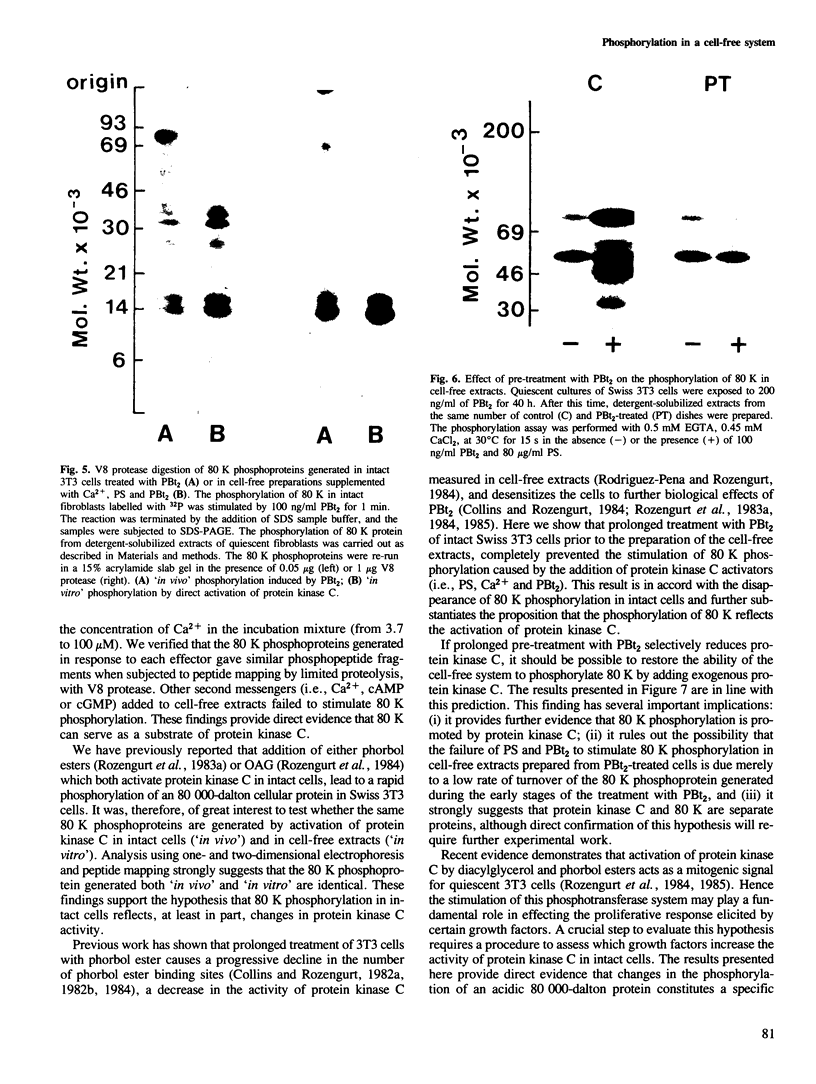
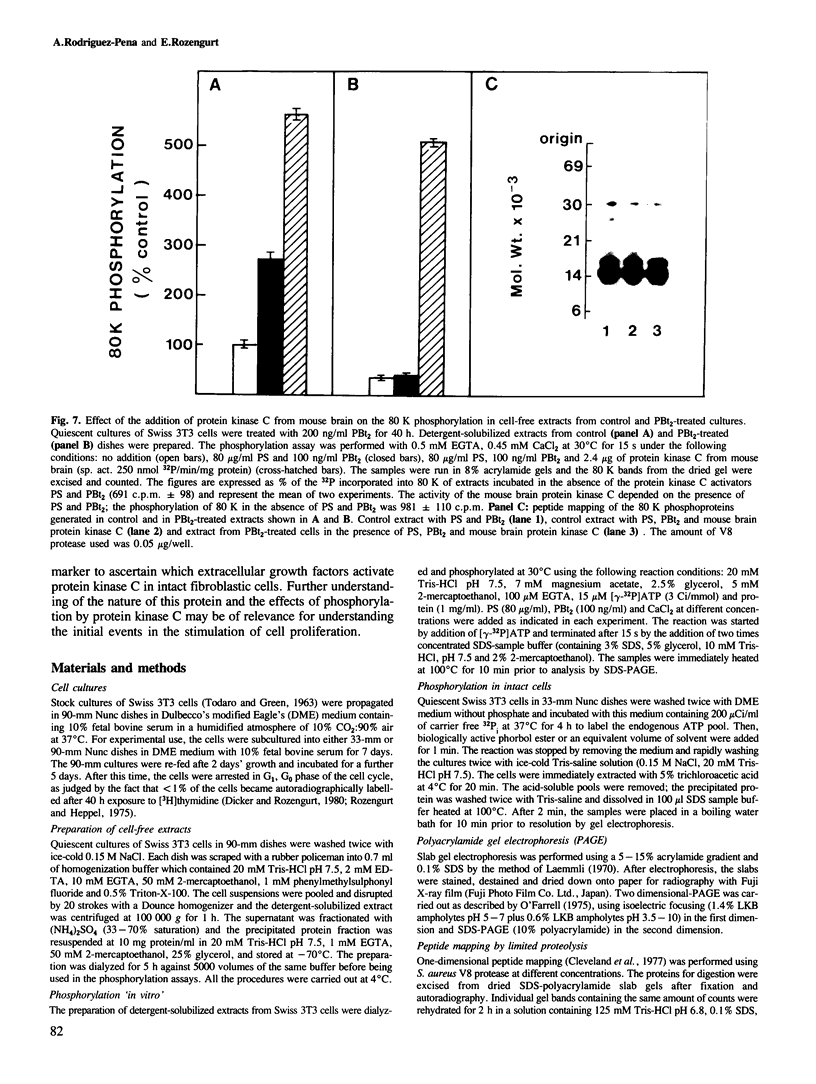
Activation of the endogenous Ca2+-activated phospholipid-dependent protein kinase (protein kinase C) by Ca2+, phosphatidylserine (PS) and phorbol dibutyrate (PBt2) in detergent-solubilized extracts of Swiss 3T3 cells resulted in a very rapid increase (detectable within seconds) in the phosphorylation of an 80 000 mol. wt. protein (termed 80 K). Neither cyclic AMP nor Ca2+ had any effect on 80 K phosphorylation. The 80 K phosphoproteins generated after activation of protein kinase C, both in cell-free conditions and in intact fibroblasts, are identical as judged by one and two-dimensional polyacrylamide slab gel electrophoresis and peptide mapping. Prolonged treatment of cells with phorbol esters causes a selective decrease in protein kinase C activity and prevents the stimulation of 80 K phosphorylation in intact fibroblasts. We now show that extracts from PBt2-treated cultures fail to stimulate 80 K phosphorylation after the addition of the protein kinase C activators. This effect was due to the lack of protein kinase C activity since the addition of exogenous protein kinase C from mouse brain stimulated 80 K phosphorylation in both control and PBt2-treated preparations. The 80 K phosphoprotein generated by activation of endogenous and exogenous protein kinase C yielded similar phosphopeptide fragments after peptide mapping by limited proteolysis. We conclude that the detection of changes in the phosphorylation of 80 K provides a useful approach to ascertain which extracellular ligands activate protein kinase C in intact cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T. Preparation of metal-chelate complexes and the design of steady-state kinetic experiments involving metal nucleotide complexes. Adv Cyclic Nucleotide Res. 1979;10:219–242. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Binding of phorbol esters to high-affinity sites on murine fibroblastic cells elicits a mitogenic response. J Cell Physiol. 1982 Jul;112(1):42–50. doi: 10.1002/jcp.1041120108. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Homologous and heterologous mitogenic desensitization of Swiss 3T3 cells to phorbol esters and vasopressin: role of receptor and postreceptor steps. J Cell Physiol. 1984 Feb;118(2):133–142. doi: 10.1002/jcp.1041180205. [DOI] [PubMed] [Google Scholar]
- Collins M., Rozengurt E. Stimulation of DNA synthesis in murine fibroblasts by the tumour promoter teleocidin: relationship to phorbol esters and vasopressin. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1159–1166. doi: 10.1016/0006-291x(82)91372-9. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kent C. Stimulation of phospholipid metabolism in embryonic muscle cells treated with phospholipase C. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4474–4478. doi: 10.1073/pnas.76.9.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Serum, like phorbol esters, rapidly activates protein kinase C in intact quiescent fibroblasts. EMBO J. 1985 Jan;4(1):71–76. doi: 10.1002/j.1460-2075.1985.tb02319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Collins M. Molecular aspects of growth factor action: receptors and intracellular signals. J Pathol. 1983 Nov;141(3):309–331. doi: 10.1002/path.1711410310. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena A., Coombs M., Sinnett-Smith J. Diacylglycerol stimulates DNA synthesis and cell division in mouse 3T3 cells: role of Ca2+-sensitive phospholipid-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5748–5752. doi: 10.1073/pnas.81.18.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Peña A., Sinnett-Smith J. Signalling mitogenesis in 3T3 cells: role of Ca2+-sensitive, phospholipid-dependent protein kinase. Ciba Found Symp. 1985;116:66–86. doi: 10.1002/9780470720974.ch5. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Stroobant P., Waterfield M. D., Deuel T. F., Keehan M. Platelet-derived growth factor elicits cyclic AMP accumulation in Swiss 3T3 cells: role of prostaglandin production. Cell. 1983 Aug;34(1):265–272. doi: 10.1016/0092-8674(83)90157-5. [DOI] [PubMed] [Google Scholar]
- Sano K., Takai Y., Yamanishi J., Nishizuka Y. A role of calcium-activated phospholipid-dependent protein kinase in human platelet activation. Comparison of thrombin and collagen actions. J Biol Chem. 1983 Feb 10;258(3):2010–2013. [PubMed] [Google Scholar]
- Shier W. T., Durkin J. P. Role of stimulation of arachidonic acid release in the proliferative response of 3T3 mouse fibroblasts to platelet-derived growth factor. J Cell Physiol. 1982 Aug;112(2):171–181. doi: 10.1002/jcp.1041120204. [DOI] [PubMed] [Google Scholar]
- Sleight R., Kent C. Regulation of phosphatidylcholine biosynthesis in mammalian cells. I. Effects of phospholipase C treatment on phosphatidylcholine metabolism in Chinese hamster ovary cells and LM mouse fibroblasts. J Biol Chem. 1983 Jan 25;258(2):824–830. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Valentine K. A., Ngai P. K., Carruthers C. A., Hollenberg M. D. Ca2+-dependent hydrophobic-interaction chromatography. Isolation of a novel Ca2+-binding protein and protein kinase C from bovine brain. Biochem J. 1984 Nov 15;224(1):117–127. doi: 10.1042/bj2240117. [DOI] [PMC free article] [PubMed] [Google Scholar]