Abstract
The Qinghai Lake National Nature Reserve (QLNNR), renowned for its abundant natural resources and diverse ecological habitats, serves as an ideal environment for ticks, thereby increasing the risk of various tick-borne pathogens (TBPs) transmission. This study aimed to investigate the prevalence of TBPs in ticks collected from Przewalski's gazelle and Tibetan sheep within the QLNNR. A total of 313 tick samples were collected from the vicinity of Qinghai Lake. Tick species identification was conducted using both morphological and molecular biology techniques. Polymerase chain reaction (PCR) amplification was performed to detect the presence of spotted fever group (SFG) Rickettsia, Coxiella burnetii, Anaplasma phagocytophilum, Babesia microti, Theileria spp, Borrelia burgdorferi, Brucella spp, and Anaplasma ovis was performed using specific primers. Positive samples were sequenced and analyzed using BLASTn, followed by phylogenetic tree construction. The ticks collected from the Qinghai Lake area were identified as Dermacentor nuttalli. The overall prevalence rates of ticks carrying SFG Rickettsia and C. burnetii were 42.8 % (134/313) and 4.8 % (15/313), respectively. Three SFG Rickettsia species were detected, including R. raoultii 33.9 % (106/313), R. slovaca 3.8 % (12/113) and R. sibirica 7.7 % (24/113), with R. raoultii being the predominant species. The prevalence rates of SFG Rickettsia and C. burnetii in ticks from Tibetan sheep was 44.7 % (115/257) and 4.7 % (12/257), respectively,and in ticks from Przewalski's gazelle were 33.9 % (19/56) and 5.4 % (3/56). Furthermore, the study revealed a positive linear relationship between the abundance of Przewalski's gazelle and the number of ticks, as well as the prevalence of TBPs. The current study has identified Dermacentor nuttalli as the predominant tick vector species within the QLNNR region. The detection of SFG Rickettsia and C. burnetii has augmented our understanding of the epidemiological profile of ticks and TBPs in this area, thereby providing a robust theoretical foundation for the implementation of effective prevention and control strategies against TBPs.
Keywords: Tick-borne pathogens, Przewalski's gazelle, Tibetan sheep, Dermacentor nuttalli, Qinghai lake national nature reserve
Graphical abstract
Highlights
-
•
This study focuses on the prevalence of tick-borne pathogens in ticks obtained from the globally endangered Przewalski's gazelle and from Tibetan sheep residing in the Qinghai Lake area.
-
•
The findings have revealed the presence of Spotted fever group Rickettsia (42.8 %, 134/313) and Coxiella burnetii (4.8 %, 15/313) in tick vectors within the protected area.
Abbreviations
- TBPs
Tick-borne pathogens
- QTP
Qinghai-Tibet Plateau
- QLNNR
Qinghai Lake National Nature Reserve
- SFG
spotted fever group
- EDTA
Ethylene Diamine Tetraacetic Acid
- DNA
deoxyribonucleic acid
- PCR
polymerase chain reaction
- RNA
ribonucleic acid
- 16S rRNA
16S ribosomal ribonucleic acid
- 18S rRNA
18S ribosomal ribonucleic acid
- dNTPs
deoxyribonucleotide triphosphate
1. Introduction
Ticks, as ectoparasitic arthropods, are known to parasitize a wide range of hosts, including humans, domestic animals, and various wildlife species. Their significance lies not only in their parasitic nature but also in their capacity to transmit a multitude of pathogens to their hosts, a role second only to that of mosquitoes [1,2]. Dermacentor nuttalli is primarily distributed in the southern regions of Russia and throughout Mongolia, including Selenge aimag, Tov aimag, and Dornogovi. In China, it is commonly found in the northwestern regions, including Xinjiang, Gansu, and Qinghai, and notably dominant on the Qinghai-Tibet Plateau (QTP) [[3], [4], [5], [6], [7]]. Dermacentor nuttalli is capable of transmitting a diverse array of pathogens, such as Rickettsia raoultii, Candidatus Rickettsia tarasevichiae, Babesia venatorum, and Coxiella burnetii, among others [[8], [9], [10]].
Rickettsia, a globally prevalent intracellular parasitic, gram-negative prokaryotic microorganism, is categorized into four principal groups based on interspecific evolutionary relationships: the Rickettsia bellii group, the Rickettsia Canadensis group, the spotted fever group (SFG) Rickettsia, and the typhus group Rickettsia (TGR) [11,12]. SFG Rickettsia, prevalent in ticks, can be transmitted vertically to the next generation via eggs and horizontally to host through bites, causing a spectrum of natural human-veterinary diseases with fever as the primary symptom [13]. Advancements in cultivation and molecular diagnostic technologies have confirmed the existence of at least 28 SFG Rickettsia species, with new species or genotypes continually being isolated from ticks [14]. In recent years, several novel tick-borne Rickettsia species have been identified in China, including Candidutus Rickettsia jiaonani, Candidutus Rickettsia longicornii, Rickettsia yunnanensis, and Rickettsia erhaii [[15], [16], [17], [18]]. The emergence and reemergence of additional tick-borne pathogens (TBPs) also pose a significant threat to public health safety in China.
Since the large-scale outbreak of Q fever in the Netherlands from 2007 to 2010, new outbreaks of Q fever have continued to be reported globally [19]. In 2018 to 2019, a Q fever outbreak was experienced in Zhuhai City, China, with 138 patients diagnosed with Q fever, marking the first confirmed Q fever epidemic in modern Chinese cities [20]. In 2021, the outbreak of Q fever in northern Italy led to 14 tourists being infected [21]. Therefore, the prevention of Q fever was increasingly crucial. Q fever is one of the globally distributed zoonotic diseases caused by C.burnetii [22] C. burnetii can be transmitted not only through tick bites on the host but also through aerosol methods, infecting humans and animals [23,24]. The main clinical manifestations of Q fever are fever, headache, myalgia, and other flu-like symptoms. In severe cases, it can lead to complications such as chronic hepatitis, pneumonia, and myocarditis [25]. Pregnant females infected with C. burnetii may exhibit reproductive disorders such as abortion, stillbirths and sterility [26]. Most human infections with Q fever are associated with domestic animals, and individuals such as farmers, veterinarians, abattoir workers, and laboratory personnel are all at risk of infection [27,28].
The Qinghai Lake National Nature Reserve (QLNNR), situated in the northeast quadrant of the Qinghai-Tibetan Plateau (QTP), boasts a rich tapestry of natural resources and a diverse array of ecological species, creating an ideal habitat for ticks [29]. This sanctuary is home to the indigenous fauna of the QTP, including the Tibetan sheep, and the QTP-endemic and critically endangered Przewalski's gazelle, which is classified as a national first-level protected animal in China. Przewalski's gazelle predominantly inhabits the Bird Island, Chala Beach, and Xiaobei Lake regions in the northeast and west of Qinghai Lake, and is recognized as one of the world's most imperiled ungulates [30]. Research by La et al. has identified the presence of parasites such as Melophagus ovinus, Pediculus, Caenorhabditis elegans, Moniezia expanda, Cysticercus cellulosae in the Przewalski's gazelle population of the Ganzi River area. Additionally, Wang et al. have discovered a novel species of Eimeria within the intestines of these gazelles [31,32]. However, the study of ectoparasites, particularly ticks, on wild Przewalski's gazelle remains relatively unexplored. Consequently, this investigation centers on the surface ticks of the Tibetan sheep and Przewalski's gazelle, indigenous to the QTP, to ascertain the tick species prevalent on these mammals within the QLNNR and to evaluate the prevalence of tick-borne pathogens (TBPs). The findings aim to provide a scientific foundation for the regional management and control of ticks and TBPs.
2. Methods
2.1. Sample collection and DNA extraction
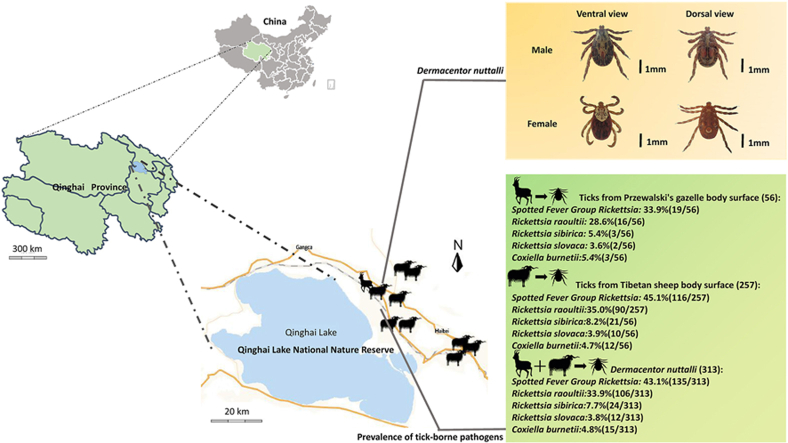
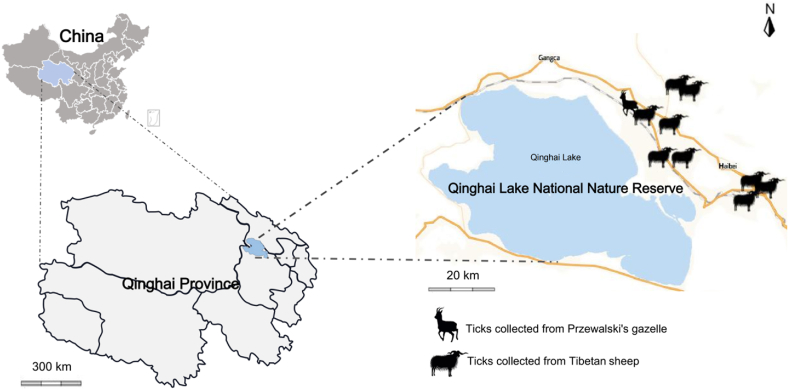
In the current investigation, a comprehensive collection of 313 tick specimens was procured from 10 designated sampling locations within the Qinghai Lake National Nature Reserve (QLNNR: Yongfeng: 101°07'65''E, 36°91'11''N, Reshui: 100°60'85''E, 37°15'89''N, Gahai: 100°47'95''E, 37°14'20''N, Dayu: 100°83'90''E, 36°98'40''N, Rixiang: 100°81'27''E, 37°01'21''N, Dongda: 101°05'39''E, 36°88'13''N, Xicha: 100°59'12''E, 36°53'58''N, Wendu: 100°65'41''E, 36°96'17''N, Dezhou: 100°59'14''E, 36°06'19''N). From April 1st to April 14th, 2022, ticks were collected from animal surfaces. Dermacentor silvarum and D. nuttalli are morphologically similar. PCR-RFLP method was carried out to identify D. silvarum and D. nuttalli through Spe I existed in their ITS2 seqeunces. Previous studies have shown that no Spe I site in ITS2 sequence of D. silvarum but one in D. nuttalli, which suggested that PCR-RFLP based on ITS2 sequences can distinguish D. silvarum from D. nuttalli.Among these, 56 tick samples were meticulously gathered from the eight captive populations of Przewalski's gazelle by the dedicated personnel of the Qinghai Lake Wildlife Rescue and Testing Center on April 14, 2022, as depicted in Fig. 1. Owing to the 2.44 km2 expanse of the anthropogenically protected area, the indigenous traits of the wild Przewalski's gazelle populace have been largely conserved. The gender of the Przewalski's gazelle was determined through the distinctive phenotypic markers: males are characterized by a pair of ebony horns, whereas females are hornless. The acquired ticks were meticulously preserved in sterile collection vessels, hermetically sealed with breathable cotton fabric to maintain their respiratory functions, and promptly transported to the laboratory for further analysis. Prior to experimentation, the ticks underwent a thorough cleansing process with 70 % ethanol and 10 × PBS to eliminate potential environmental contaminants. Each sanitised tick was then placed in a 1.5 ml centrifuge tube and immersed in liquid nitrogen for a duration of 2 min to facilitate pulverization. Subsequently, the ticks were reduced to a fine powder using a pestle and mortar, to which protease K (manufactured by Magen, China) was added for an extensive overnight digestion. To conclude, the Hipure Insect DNA kit (also provided by Magen, China) was employed for the extraction of DNA, which was then stored at a temperature of −80 °C to ensure its integrity for subsequent use.
Fig. 1.
Map of the Qinghai Lake National Nature Reserve the sampling sites. The Przewalski's gazelle and Tibetan sheep icon indicates the location of the sample collection in this study.
2.2. Identification of tick species
Ticks were identified through a combination of morphological characteristics, polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) techniques and phylogenetic analysis [33,34]. Morphological identification, mainly based on the characteristic of the anal groove, was carried out by an experienced acarologist to determine the tick species initially [8]. The peritremes of male ticks is punctate, while the peritremes of female ticks is elliptical.The PCR amplification targeted the tick's internal transcribed spacer 2 (ITS2) gene within a reaction volume of 10 μl, which included 1 μl of DNA template, 0.5 μl each of forward and reverse primers (100 μM), 0.1 μl of Taq polymerase (0.5 U; New England BioLab, USA), 0.2 μl of deoxyribonucleotide triphosphate (200 μM; New England BioLab, USA), 1 μl of 10 × ThermoPol Reaction Buffer (New England BioLab, USA), and 6.7 μl of double-distilled water. Subsequently, the PCR products were digested with the restriction endonuclease Spe I (TransGen, China) and separated on a 1 % agarose gel (TransGen, China), with the results visualized under ultraviolet light.
2.3. Detection of tick borne pathogens
The extracted tick DNA served as a template for PCR amplification with synthesized primers (Table 1). The PCR reaction, with a volume of 10 μl, contained 3 μl of DNA template, 0.5 μl each of forward and reverse primers (100 μM), 0.1 μl of Taq polymerase (0.5 U; New England BioLab, USA), 0.2 μl of deoxyribonucleotide triphosphate (200 μM; New England BioLab, USA), 1 μl of 10 × ThermoPol Reaction Buffer (New England BioLab, USA), and 4.7 μl of double-distilled water. The DNA samples from blood of animals infected with the respective pathogens were used as positive control and were collected and stored properly from previous studies. ddH2O was used as a negative control, and the PCR products were subsequently electrophoresed on a 1.5 % agarose gel.
Table 1.
Primers used in this study to detect tick-borne pathogens (TBPs) infections in ticks on the Qinghai Lake National Nature Reserve.
| Organism | Target gene | Primer sequence (5’∼3’) | Fragment (bp) | Note | References |
|---|---|---|---|---|---|
| Tick | ITS2 | CGAGACTTGGTGTGAATTGCA | 137–1695 | [33] | |
| TCCCATACACCACATTTCCCG | |||||
| SFG Rickettsia | ompA | GCTTTATTCACCACCTCAAC | 209/212 | [34] | |
| TRATCACCACCGTAAGTAAAT | |||||
| Sca4 | CGATGGTAGCATTAAAAGCT | 624 | [35] | ||
| CTTGCTTTTCAGCAATATCAC | |||||
| A. phagocytophilum | 16S rRNA | CACATGCAAGTCGAACGGATTATTC | 932 | 1st PCR | [36] |
| TTCCGTTAAGAAGGATCTAATCTCC | |||||
| AACGGATTATTCTTTATAGCTTGCT | 546/565 | Nested PCR | |||
| GGCAGTATTAAAAGCAGCTCCAGG | |||||
| B. microti | 18S rRNA | CTTAGTATAAGCTTTTATACAGC | 238 | 1st PCR | [37] |
| ATAGGTCAGAAACTTGAATGATACA | |||||
| GTTATAGTTTATTTGATGTTCGTTT | 154 | Nested PCR | |||
| AAGCCATGCGATTCGCTAAT | |||||
| Theileria spp. | 18S rRNA | GAAACGGCTACCACATCT | 778 | 1st PCR | [38] |
| AGTTTCCCCGTGTTGAGT | |||||
| TTAAACCTCTTCCAGAGT | 581 | Nested PCR | |||
| TCAGCCTTGCGACCATAC | |||||
| B. burgdorferi | 23S rRNA | AGAAGTGCTGGAGTCGA | 261 | [39] | |
| TAGTGCTCTACCTCTATTAA | |||||
| C. burnetii | htpB | GCGGGTGATGGTACCACAACA | 501 | 1st PCR | [40] |
| GGCAATCACCAATAAGGGCCG | |||||
| TTGCTGGAATGAACCCCA | 325 | Nested PCR | |||
| TCAAGCTCCGCACTCATG | |||||
| Brucella spp. | omp22 | TGATGGGAGGGACCGACTA | 494 | [41] | |
| TGGTTCTTCAGGTTGTTACGC | |||||
| F. tularensis | 16S rRNA | CAGGCCTAACACATGCAAGTC | 1300 | [42] | |
| GGGCGGWGTGTACAAGGC |
2.4. Sequencing and phylogenetic analyses
The purified PCR products were obtained using the EasyPure Quick Gel Extraction Kit (manufactured by TransGen, China) and subsequently cloned into the Escherichia coli DH5α strain with the PMDTM 19-T Vector Cloning Kit (provided by TaKaRa, Japan). Positive clones were then selected and sent for sequencing at Sangon Biotech (Shanghai) Co., Ltd. Check for any misreading in the sequencing peak map and correct it to ensure the accuracy of the target gene fragment.The gene fragments obtained from the sequencing were spliced and trimmed using DNAStar and CmSuite8 software, and their nucleotide sequence homology was determined through GenBank BLASTn analysis (available at https://blast.ncbi.nl-m.nih.gov/Blast.cgi). Select high-quality, gene-specific, and sequence length similar sequences from GenBank. Phylogenetic trees were constructed using the neighbor-joining method and p-distance model in MEGA 11.0, with Bootstrap set to 1000, and the constructed phylogenetic trees were enhanced using tvBOT (available at https://www.chiplot.online/tvbot.html) [43].
2.5. Statistical analysis
A chi-square test was performed to evaluate between the infection rate of SFG Rickettsia and C.burnetii the different hosts, with observed differences considered statistically significant when p-value <0.05 (calculator available at https://www.mathsisfun.com/data/chisquare-calculator.html).
3. Results
3.1. Identification of ticks
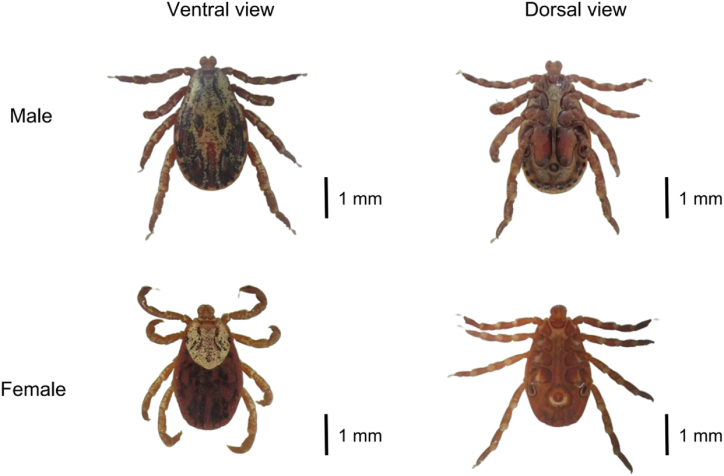
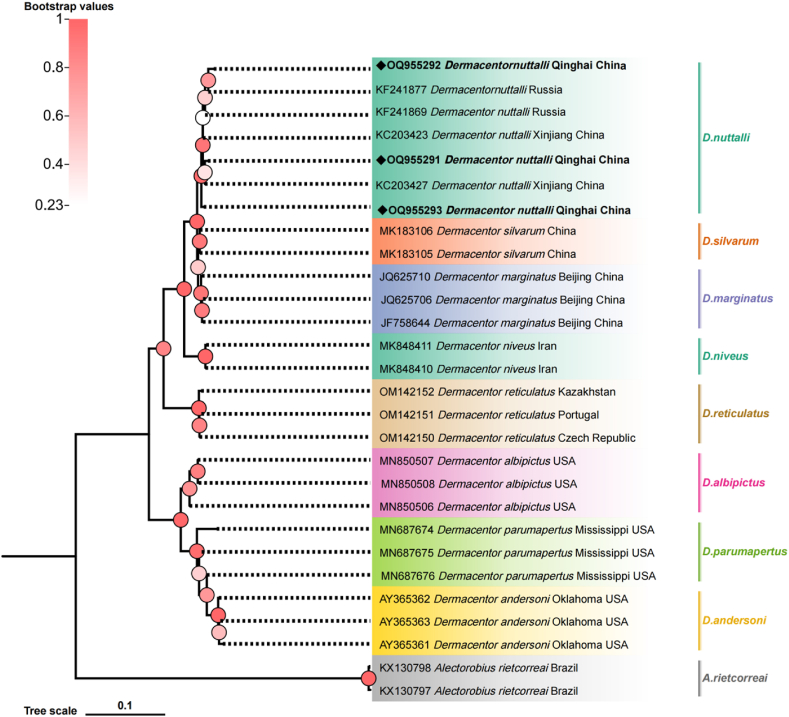
This study utilized three methods for tick species identification: morphological identification, PCR-RFLP, and phylogenetic analysis. Preliminary morphological identification of all tick samples indicated they belonged to the species D. nuttalli (Fig. 2). The basis capituli is rectangular, and the scutum is widest at the peritreme, with a strong enamel color. The peritremes of male ticks is punctate, while the peritremes of female ticks is elliptical. The PCR-RFLP results further confirmed this, as the ITS2 fragment was cut into two bands with sizes of 992 bp and 275 bp, consistent with D. nuttalli identification (Additional file 1: Fig. S1). Thirty randomly selected tick specimens were cloned and sequenced for the ITS2 gene PCR amplification products. BLASTn analysis and phylogenetic analysis revealed extensive homology between the ITS2 gene sequence of the tick samples in this study and the published sequences of D. nuttalli from Russia (KF241869) and Xinjiang, China (KC203427), clustering them into the same branch (Fig. 3).
Fig. 2.
The dorsal view and ventral view of Dermacentor nuttalli.
Fig. 3.
Phylogenetic tree based on the internal transcribed spacer 2 gene of Dermacentor spp. obtained in this study. Bootstraps analysis was performedwith 1000 replicates. The solid diamond and bolded represent the sequence of this study.Alectorobius rietcorreai (KX130798, KX130797) was used as outgroup.
3.2. Infection rates of tick-borne pathogens
The DNA extracted from the tick genome served as a template to amplify various pathogens, including spotted fever group Rickettsia, Coxiella burnetii, Anaplasma phagocytophilum, Babesia microti, Theileria spp, Borrelia burgdorferi, Brucella spp, and Anaplasma ovis. Notably, SFG Rickettsia and C. burnetii exhibited distinct electrophoretic bands that corresponded to our expectations. In our current study, the overall prevalence of SFG Rickettsia was 42.8 % (134/313), with 44.7 % (115/257) of ticks from Tibetan sheep body surface and 33.9 % (19/56) from Przewalski's gazelle body surface being positive (Table 2). Subsequent sequencing of the SFG Rickettsia-positive samples revealed the presence of three species, with Rickettsia raoultii being the most prevalent at 33.9 % (106/313), followed by Rickettsia slovaca at 3.8 % (12/113), and Rickettsia sibirica at 7.7 % (24/113). Statistical analysis using the chi-square test indicated no significant correlation between the infection rate of SFG Rickettsia and different host animals (χ2 = 0.3, df = 1, p-value >0.05). The overall prevalence of Coxiella burnetii was 4.8 % (15/313), with 4.7 % (12/257) of ticks from Tibetan sheep and 5.4 % (3/56) from Przewalski’s gazelle testing positive (Table 2). Statistical tests revealed no significant difference in the infection rate of C.burnetii between the two tick host species (χ2 = 0.2, df = 1, p-value >0.05). Among the 145 positive ticks, 8.3 % (12/145) were found to be co-infected with multiple pathogens. Notably, co-infection with R. raoultii and other pathogens was observed most frequently.
Table 2.
The positive samples of spotted fever group Rickettsia and Coxiella burnetii in ticks in this study.
| Organism | Number and prevalence (%) of individual ticks positive for single and co-infections |
||
|---|---|---|---|
| Przewalski’s Gazelle (n = 56) | Tibetan sheep (n = 257) | Total sample (n = 313) | |
| Single | |||
| R. raoultii (R.rao) | 13 (23.2) | 81 (31.5) | 94 (30.0) |
| R. sibirica (R.sib) | 2 (3.6) | 17 (6.6) | 19 (6.1) |
| R. slovaca (R.slo) | 1 (1.8) | 8 (3.1) | 9 (2.9) |
| C. burnetii (C.bur) | 2 (3.6) | 9 (3.5) | 11 (3.5) |
| Double | |||
| R. rao + R. sib | 1 (1.8) | 4 (1.6) | 5 (1.6) |
| R. rao + R. slo | 1 (1.8) | 2 (0.8) | 3 (1.0) |
| R. rao + C. bur | 1 (1.8) | 3 (1.2) | 4 (1.3) |
| Total | |||
| R. raoultii | 16 (28.6) | 90 (35.0) | 106 (33.9) |
| R. sibirica | 3 (5.4) | 21 (8.2) | 24 (7.7) |
| R. slovaca | 2 (3.6) | 10 (3.9) | 12 (3.8) |
| SFG Rickettsia | 19 (33.9) | 115 (44.7) | 134 (42.8) |
| C. burnetii | 3 (5.4) | 12 (4.7) | 15 (4.8) |
| Co-infections | 3 (5.4) | 9 (3.5) | 12 (3.8) |
3.3. Sequencing and phylogenetic analysis of spotted fever group Rickettsia ompA gene
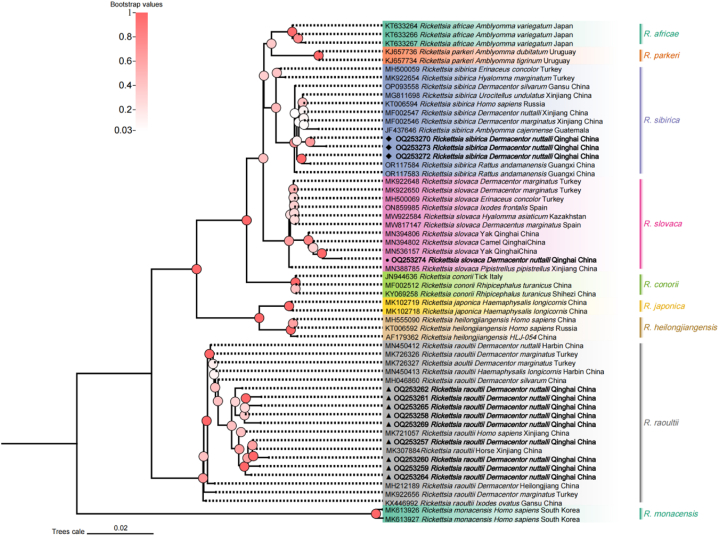
A total of 18 sequences of the ompA gene from the spotted fever group Rickettsia were successfully acquired and deposited in GenBank, where they were assigned accession numbers OQ253257-OQ253274 (refer to Table 3). The degree of identity among the sequences of R. raoultii, R. sibirica, and R. slovaca was found to be 98.6–100 %, 99.1–100 %, and 100 %, respectively. A phylogenetic tree was constructed using 13 of the uploaded SFG Rickettsia ompA gene sequences (Fig. 2). The phylogenetic analysis revealed that four sequences (OQ253257, OQ253259, OQ253260, and OQ253264) are closely related to R. raoultii (MK721057, MK307884) strains isolated from both horses and humans in Xinjiang, China. Additionally, one sequence (OQ253272) was found to be associated with R. sibirica (OR117584) isolated from Rattus andamanensis in Guangxi, China, and another sequence (OQ253274) was linked to the R. slovaca sequence (MN536157) isolated from yaks in Qinghai, China (Fig. 4).
Table 3.
DNA sequences of TBPs obtained in this study.
| Obtained sequences |
The closest Blastn match |
|||||
|---|---|---|---|---|---|---|
| Organism | Target |
Accession |
Length |
Identity |
Pathogen isolate | Accession number (host, country) |
| gene | number | (bp) | (%) | |||
| R. raoultii | ompA | OQ253257 | 209 | 100 | R. raoultii | MN450413 Haemaphysalis longicornis China |
| ompA | OQ253258 | 209 | 100 | R. raoultii | MN536158 yak China | |
| ompA | OQ253259 | 209 | 100 | R. raoultii | MN450412 Dermacentor nuttalli China | |
| ompA | OQ253260 | 209 | 99.52 | R. raoultii | MN450413 Haemaphysalis longicornis China | |
| ompA | OQ253261 | 209 | 99.52 | R. raoultii | MN536158 yak China | |
| ompA | OQ253262 | 209 | 100 | R. raoultii | MN394799 yak China | |
| ompA | OQ253263 | 209 | 99.52 | R. raoultii | MN450413 Haemaphysalis longicornis China | |
| ompA | OQ253264 | 209 | 100 | R. raoultii | MK307883 horse China | |
| ompA | OQ253265 | 209 | 99.52 | R. raoultii | MN536158 yak China | |
| ompA | OQ253266 | 209 | 99.52 | R. raoultii | MN450413 Haemaphysalis longicornis China | |
| ompA | OQ253267 | 209 | 99.52 | R. raoultii | MN450413 Haemaphysalis longicornis China | |
| ompA | OQ253268 | 209 | 99.52 | R. raoultii | MN450413 Haemaphysalis longicornis China | |
| ompA | OQ253269 | 209 | 99.52 | R. raoultii | MN536158 yak China | |
| R. sibirica | ompA | OQ253270 | 212 | 100 | R. sibirica | MG811698 Urocitellus undulatus China |
| ompA | OQ253271 | 212 | 100 | R. sibirica | MN394806 yak China | |
| ompA | OQ253272 | 212 | 100 | R. sibirica | MG598412 Dermacentor nuttalli China | |
| ompA | OQ253273 | 212 | 99.52 | R. sibirica | MG811698 Urocitellus undulatus China | |
| R. slovaca | ompA | OQ253274 | 216 | 100 | R. slovaca | MN394806 yak China |
| R. raoultii | Sca4 | OQ253275 | 624 | 99.04 | R. raoultii | KP768191 Dermacentor reticulatus Ukraine |
| Sca4 | OQ253276 | 624 | 99.20 | R. raoultii | KP768191 Dermacentor reticulatus Ukraine | |
| Sca4 | OQ253277 | 624 | 99.36 | R. raoultii | KP768191 Dermacentor reticulatus Ukraine | |
| Sca4 | OQ253278 | 624 | 100 | R. raoultii | AY331397 Dermacentor sinicus China | |
| Sca4 | OQ253279 | 624 | 99.84 | R. raoultii | CP098324 Dermacentor silvarum China | |
| Sca4 | OQ253280 | 624 | 99.84 | R. raoultii | CP098324 Dermacentor silvarum China | |
| Sca4 | OQ253281 | 624 | 100 | R. raoultii | CP098324 Dermacentor silvarum China | |
| R. sibirica | Sca4 | OQ253282 | 624 | 99.84 | R. sibirica | AY331397 Dermacentor sinicus China |
| Sca4 | OQ253283 | 624 | 99.68 | R. sibirica | MN394810 yak China | |
| R. slovaca | Sca4 | OQ253284 | 624 | 100 | R. slovaca | MN581997 Dermacentor Pakistan |
| Sca4 | OQ253285 | 624 | 99.84 | R. slovaca | MN581997 Dermacentor Pakistan | |
| C. burnetii | htpB | OQ865125 | 325 | 99.08 | C. burnetii | MK416231 Hyalomma impeltatum Tunisia |
| htpB | OQ865126 | 325 | 100 | C. burnetii | MK416231 Hyalomma impeltatum Tunisia | |
| htpB | OQ865127 | 325 | 99.69 | C. burnetii | MK416231 Hyalomma impeltatum Tunisia | |
| htpB | OQ865128 | 325 | 99.08 | C. burnetii | MK416231 Hyalomma impeltatum Tunisia | |
Fig. 4.
Phylogenetic tree based on ompA particle sequences of spotted fever group Rickettisa obtained in this study. Bootstraps analysis was performed with 1000 replicates. The solid triangle indicates sequences from Rickettsia raoultii, the solid circle indicates sequences from Rickettsia slovaca, and the solid diamond indicate sequences from Rickettsia sibirica. All sequences from this study are bolded.
3.4. Sequencing and phylogenetic analysis of spotted fever group Rickettsia sca4 gene
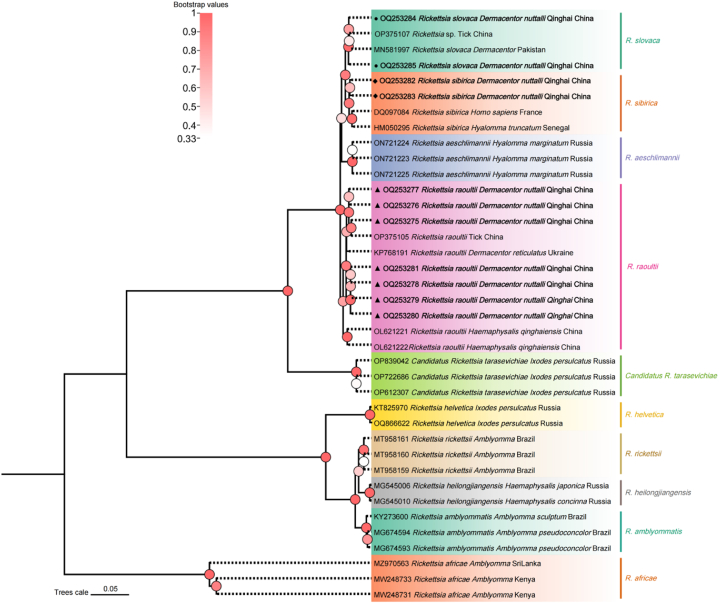
Furthermore, the study also obtained 11 sequences of the sca4 gene from SFG Rickettsia (OQ253275-OQ253285). The identity among the sequences of R. raoultii, R. sibirica, and R. slovaca was 98.6–100 %, 99.5–100 %, and 99.8–100 %, respectively. The phylogenetic analysis of the sca4 gene of SFG Rickettsia demonstrated that one sequence (OQ253277) was grouped with R. raoultii (KP768191) isolated from Dermacentor reticulatus in Ukrainian. Two sequences (OQ253282, OQ253283) were associated with R. sibirica (HM050295, DQ097084) isolated from Hyalomma truncatum in Senegal and Homo sapiens in France, and two sequences (OQ253284, OQ253285) were linked to the R. slovaca sequence (MN581997) isolated from Dermacentor in Pakistan (Fig. 5).
Fig. 5.
Phylogenetic tree based on sca4 particle sequences of spotted fever group Rickettisa obtained in this study. Bootstraps analysis was performed with 1000 replicates. The solid triangle indicates sequences from Rickettsia raoultii, the solid circle indicates sequences from Rickettsia slovaca, and the solid diamond indicate sequences from Rickettsia sibirica. All sequences from this study are bolded.
3.5. Sequencing and phylogenetic analysis of Coxiella burnetii htpB gene
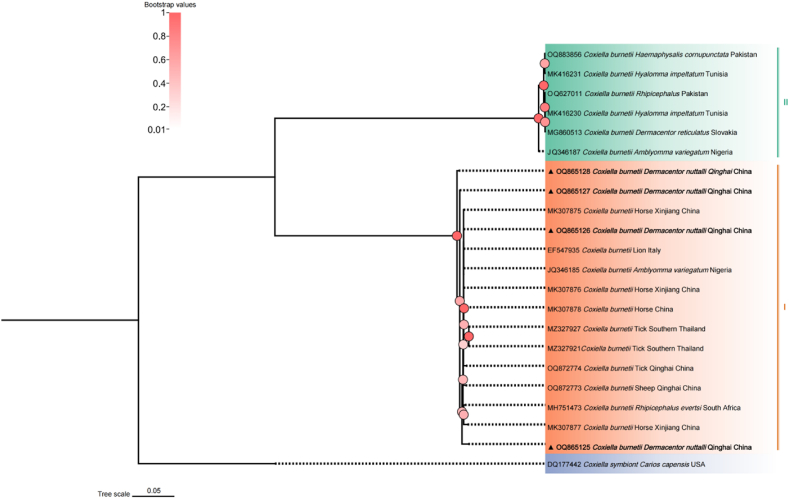
In this study, a total of four Coxiella burnetii htpB gene sequences (OQ865125 - OQ865128) were obtained, and the identity between each sequence was 98.2–100 %, The OQ865126 sequence was clustered on the same branch as the sequence of C. burnetii (MK307875, EF547935, OQ872774, OQ872773, JQ346185, MH751473) isolated from horse, lion, tick, sheep, Amblyomma variegatum, and Rhipicephalus evertsi in China’s Xinjiang, Italy, China’s Qinghai, Nigeria, and South Africa, with a identity of 99.39–100 % (Fig. 6).
Fig. 6.
Phylogenetic tree based on htpB particle sequences of Coxiella burnetii obtained in this study. Bootstraps analysis was performed with 1000 replicates. The solid triangle and bolded represent the sequence of this study. Coxiella symbiont (DQ177442) was used as outgroup.
3.6. Analysis of the prevalence of ticks and TBPs in different populations of Przewalski’s gazelle
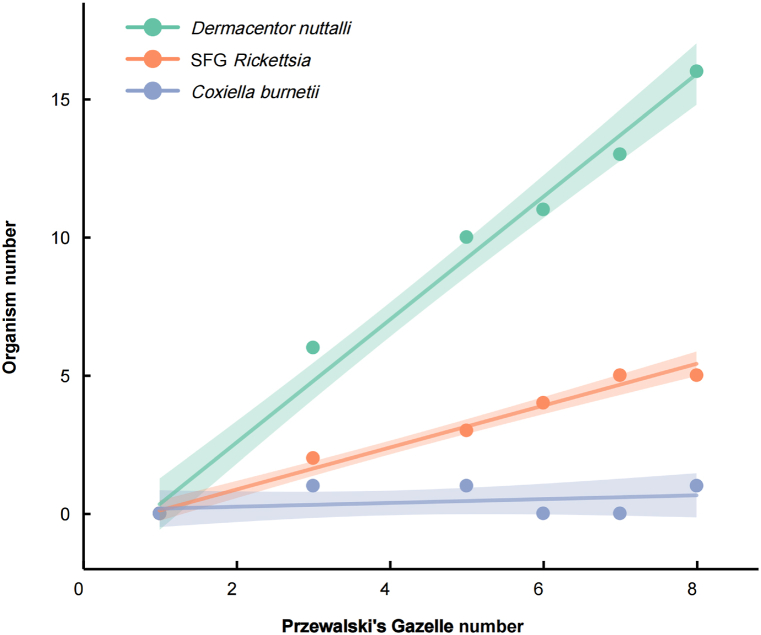
This research has uncovered substantial disparities in the prevalence of ticks and tick-borne pathogens among the female and male populations of Przewalski's gazelle across eight distinct populations. A detailed examination of the collected samples indicated that the majority of ticks were retrieved from female specimens, with a significantly lower number collected from males (refer to Table 4). During the non-breeding period in April, it is observed that male Przewalski's gazelles typically lead solitary lives, while the females congregate in groups. It is only during the breeding season in December that both sexes integrate, thereby amplifying the population's size. The study has also established a positive linear correlation between the population abundance of Przewalski's gazelle and the incidence of ticks, as well as the prevalence of tick-borne pathogens (refer to Fig. 7).
Table 4.
The number and prevalence of ticks and TBPs among different populations of Przewalski’s Gazelle in Qinghai Lake National Nature Reserve.
| Przewalski’s Gazelle | Cluster type | Number | Collecting ticks | SFG Rickettsia prevalence (%) | C. burnetii prevalence (%) |
|---|---|---|---|---|---|
| Group 1 | Male | 1 | N (0) | 0 (0.0) | 0 (0.0) |
| Group 2 | Female | 6 | Y (10) | 3 (30.0) | 0 (0.0) |
| Group 3 | Female | 5 | Y (13) | 5 (38.5) | 1 (7.7) |
| Group 4 | Female | 7 | Y(11) | 4 (36.4) | 0 (0.0) |
| Group 5 | Male | 3 | Y(6) | 2 (33.3) | 1 (16.7) |
| Group 6 | Male | 1 | N (0) | 0 (0.0) | 0 (0.0) |
| Group 7 | Male | 1 | N (0) | 0 (0.0) | 0 (0.0) |
| Group 8 | Female | 8 | Y(16) | 5 (31.3) | 1 (6.3) |
Fig. 7.
The relationship between the number of individuals in the Przewalski gazelle population and the prevalence of ticks and tick borne diseases.
4. Discussion
This research delineates the collection of ticks from the body surfaces of the globally endangered Przewalski's gazelle, situated around Qinghai Lake on the Qinghai-Tibet Plateau, encompassing their identification and an epidemiological survey of tick-borne pathogens (TBPs). As global research intensifies, an increasing array of pathogens, including severe fever with thrombocytopenia syndrome virus (SFTSV), Heartland virus (HRTV), and Bourbon virus (BRBV), are being identified as tick-transmitted [1,44]. This phenomenon poses an escalating global health threat to both humans and animals. Notably, the Przewalski's gazelle population in the Qinghai Lake region has been significantly impacted by external parasitic infections, contributing to mortality and posing conservation challenges [45]. The Qinghai Lake area is recognized as a hotspot for parasitic diseases [31], with local inhabitants and grazing yaks and Tibetan sheep potentially engaging in cross-infections with Przewalski's gazelle, thereby imperiling the gazelle population, as well as human and livestock health [30]. To investigate potential disparities in tick species and TBPs harbored on the body surfaces of Przewalski's gazelle and local grazing Tibetan sheep, this study extended to collect ticks from the latter and conducted a comprehensive identification and epidemiological survey of TBPs.
In the present study, the prevalence rates of spotted fever group Rickettsia and Coxiella burnetii in ticks sampled from Tibetan sheep determined to be 44.7 % and 4.7 %, respectively. Correspondingly, the prevalence rates in ticks collected from Przewalski's gazelle were 33.9 % and 5.4 %. Overall, the quantity of ticks harvested from Tibetan sheep was greater, and the infection rate of TBPs was higher compared to those found in Przewalski's gazelle. This discrepancy is thought to be attributed to differences in host behavior and habitat use. The smaller herd size of Przewalski's gazelle, typically consisting of only two to eight individuals per group during the non-mating season [46,47]. The unique cluster behavior of Przewalski's gazelle, where the population of Proctor's gazelle has reached its maximum during the mating season, which occurs annually in November to January of the coming year, may contribute to a reduced exposure to ticks. Furthermore, the solitary behavior of females prior to giving birth further diminishes the cluster size. Previous study have shown tick abundance was positively associated with deer abundance [48]. It is hypothesized that this behavioral adaptation effectively reduces the rate of mutual infection of ticks among individuals of Przewalski's gazelle.
In the present study, we observed a notable occurrence of mixed infections by two pathogens within Dermacentor nuttalli, as detailed in Table 2. Prior investigations have demonstrated that D.nuttalli is capable of harboring multiple pathogens concurrently, with the most prevalent being the dual co-infection of R. raoultii and the Coxiella-like endosymbiont (CLE), as well as the triple co-infection involving R. raoultii, Anaplasma spp., and CLE in Inner Mongolia, China [49]. The tendency for R. raoultii to be co-infected with other pathogen aligns with our findings, as evidenced by the discovery of mixed infections between R. raoultii and R. sibirica, R. slovaca, and C. burnetii in our study. This correlation may be attributed to the significantly higher infection rate of R. raoultii compared to the other three pathogens. These findings corroborate recent research indicating an upward trend in the detection rate of spotted fever group (SFG) Rickettsia in ticks, thereby increasing the likelihood of mixed infections with other pathogens [[50], [51], [52], [53]].
Over the past quarter-century, the global prevalence of tick-borne pathogens has been on the rise, underscoring their growing significance as a public health concern [1]. To date, over 30 SFG Rickettsia species have been reported globally, with 21 confirmed as pathogenic [54]. Notably, D. nuttalli from various regions in China, including Xinjiang, Yunnan, Gansu, Shanxi, Heilongjiang, Jilin, and Inner Mongolia, have been identified as carriers of SFG Rickettsia species such as R. raoultii, R. sibirica, and R. slovaca [[55], [56], [57], [58]]. Moreover, the presence of SFG Rickettsia in ticks has been documented in border areas between China and its neighboring countries. Specifically, R. raoultii and R. sibirica have been detected on ticks from long-tailed ground squirrels in proximity to the China-Kazakhstan border [59]. SFG Rickettsia has been detected on the body surfaces of wild rodents' ticks in the northwestern border region of China [60]. Additionally, R. raoultii and R. sibirica have been identified as being carried by D.nuttalli in neighboring countries, Mongolia and Russia [61,62]. As personnel and trade exchanges with neighboring countries intensify, the risk of transmission of TBPs continues to escalate. It is noteworthy that our previous research isolated R. raoultii from yaks, Tibetan sheep, and Haemaphysalis qinghaiensis on the QTP, with respective positivity rates of 5.9 %, 0.3 %, and 54.1 % [63]. However, the positive rate of R. raoultii infection in D. nuttalli in the present study was 33.9 %, which is relatively lower compared to the infection rate in Haemaphysalis qinghaiensis. This discrepancy may be due to differences in environmental conditions, tick species. QTP It is evident that both Haemaphysalis qinghaiensis and D. nuttalli in the region are infected with R. raoultii at a relatively high prevalence. Consequently, the local livestock economy on the QTP is significantly impacted by ticks and TBPs.
Since the initial discovery of Q fever in Australia in 1937, reports of the disease have been on the rise both domestically and internationally, making it one of the most extensively distributed zoonotic diseases today [64]. In the border area of the Xinjiang Uygur Autonomous Region in China, the infection rate of C. burnetii in D. nuttalli was found to be 58.91 % (205/348) [9]. Moreover, C.burnetii was detected in ticks collected from goats (7.7 %) and sheep (31.0 %) in Pakistan [65]. Additionally, D. nuttalli in Mongolia, ticks in Pakistan, and Hyalomma dromedarii collected from camels in Egypt were found to carry SFG Rickettsia, with varying infection rates of 90.4 %, 5.4 %, and 57.1 %, respectively [[66], [67], [68]]. In this study, the infection rates of SFG Rickettsia and C. burnetii carried by D. nuttalli in QLNNR were 42.8 % and 4.8 %, respectively. These findings suggest the presence of similar TBPs in Mongolia, Pakistan, Egypt, Xinjiang, and Qinghai, China, all of which are located along the East Africa-West Asia migratory route. QLNNR serves as a habitat for migratory birds, and the tick-bird association contributes to the dissemination of ticks and TBPs. Although tick infection in migratory birds does not necessarily imply the establishment of a new species in a new territory, global warming may alter the boundaries of their original distribution range and increase the risk of introducing new pathogens [69]. Therefore, further investigations are necessary to better understand the role of migratory birds in the introduction and spread of TBPs in the QLNNR.
The genes predominantly employed for species identification within the Rickettsia genus encompass ompA, Sca4, ompB, 16S rRNA, and gltA [70]. Notably, the 5' end of the ompA gene exhibits high specificity and has been extensively utilized in the identification and classification of SFG Rickettsia genotypes or subtypes [71]. The Sca4 gene has also been employed to effectively differentiate members of the genus Rickettsia [36]. In prior studies, the ompA and Sca4 genes were specifically utilized to detect SFG Rickettsia [[72], [73], [74], [75]]. In the present study, these genes were targeted to detect SGF Rickettsia in ticks, with the research indicating the presence of SGF Rickettsia in ticks collected from the body surfaces of Przewalski’s gazelle and Tibetan sheep. Detected species included R.raoultii (33.9 %), R. slovaca (3.8 %), and R.sibirica (7.7 %), with R. raoultii being the predominant species. This finding aligns with previous research on Qinghai Dermacentor nuttalli and Haemaphysalis qinghaiensis in China [61,76]. For the identification of Coxiella burnetii, genes such as IS1111, icd, scvA, p1, GroEL, and htpB genes are commonly utilized [77]. The htpB gene, in particular, is highly conserved and considered universal across various C. burnetii types [78]. Consequently, it is frequently used as a target for identifying C. burnetii. This study successfully obtained four C. burnetii sequences, with one sequence (OQ865126) sharing 99.39 %–100 % homology with C. burnetii sequences isolated from regions including Xinjiang, Italy, Qinghai, Nigeria, and South Africa. This suggests a widespread geographical distribution of C. burnetii across the globe.
In summary, the research conducted has identified the presence of spotted fever group Rickettsia and Coxiella burnetii within Dermacentor nuttalli collected from Przewalski's gazelle and Tibetan sheep. The study has also demonstrated a positive linear correlation between the population density of Przewalski's gazelle and the prevalence of ticks, as well as the incidence of TBPs. However, the potential impact of seasonal variations in the population size and composition of Przewalski's gazelle on the dissemination of ticks and TBPs requires further examination.
5. Conclusions
This study focuses on the prevalence of tick-borne pathogens in ticks obtained from the globally endangered Przewalski's gazelle and from Tibetan sheep residing in the Qinghai Lake area. The findings have revealed the presence of SFG Rickettsia (42.8 %, 134/313) and C. burnetii (4.8 %, 15/313) in tick vectors within the protected area. Individuals residing near Qinghai Lake, along with yaks, Tibetan sheep, and the endangered Przewalski's gazelle, are at heightened risk of tick-borne infections, posing a threat to the Przewalski's gazelle population and to human and livestock health. Furthermore, the QLNNR, serving as a habitat for migratory birds, creates favorable conditions for the propagation of ticks and TBPs. Consequently, there is an urgent need to establish a robust monitoring system within the QLNNR to gain a comprehensive understanding of the diversity of tick species and the prevalence of TBPs. Notably, the current study has been limited by the small number of body tick samples collected from Przewalski's gazelle. It is anticipated that future research will expand the scope of detection to provide a more detailed insight into the diversity of tick species and TBPs in the QLNNR, thereby enhancing the effectiveness of tick and TBP prevention and control strategies in the region.
CRediT authorship contribution statement
Qiang Chen: Writing – original draft, Visualization, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Zengkui Li: Writing – review & editing, Supervision, Methodology. Ming Kang: Writing – review & editing, Supervision, Methodology. Guangwei Hu: Writing – review & editing, Supervision, Methodology. Jinshan Cai: Writing – review & editing, Supervision, Methodology. Jing Li: Writing – review & editing, Supervision, Methodology. Xiaoling Han: Writing – review & editing, Supervision, Methodology. Changjiang Chen: Writing – review & editing, Supervision, Methodology. Shunfu He: Writing – review & editing, Supervision, Methodology. Xiaoyu Hu: Methodology. Yongcai He: Visualization, Validation, Methodology, Formal analysis. Zhongyu Li: Supervision, Methodology. Jiyong Chen: Supervision, Methodology. Pengcheng Geng: Supervision, Methodology. Shuo Jiang: Methodology. Jinghua Ma: Methodology. Xiao Zhang: Methodology. Ximei Tai: Methodology. Ying Li: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis.
Availability of data and materials
All data generated or analyzed during this study were included in this published.
Rickettsia raoultii OmpA data have been deposited at Genbank repository with accession numbers OQ 253257 - OQ 253269. Rickettsia sibirica OmpA data have been deposited at Genbank repository with accession numbers OQ 253270 - OQ 253273. Rickettsia slovaca OmpA data have been deposited at Genbank repository with accession numbers OQ 253274. Rickettsia raoultii Sca4 data have been deposited at Genbank with accession numbers OQ 253275 - OQ 253281. Rickettsia sibirica Sca4 data have been deposited at Genbank repository with accession numbers OQ 253282 - OQ 253283. Rickettsia slovaca Sca4 data have been deposited at Genbank repository with accession numbers OQ 253284 - OQ 253285. Dermacentor nuttalli ITS2 data have been deposited at Genbank repository with accession numbers OQ 955291 - OQ 955293. Coxiella burnetii htpB data have been deposited at Genbank repository with accession numbers OQ 865125 - OQ 865128.
Ethics statement
The study was conducted in compliance with the ethical policies of the journal and the rules of the ethics committee of Qinghai University.
Funding
This project support was provided by the Regular Assistance Project of National Natural Science Foundation of China (32060806) and the special project for scientific and technological international cooperation of the Science and Technology Department, Qinghai Province (2021-HZ-801).
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40205.
Supplementary information
Additional file 1:Fig. S1: PCR amplification and electrophoresis results of the ITS2 gene fragment of Dermacentor nuttalli before and after Spe I digestion. Note: M: DL 2000 DNA Marker; A-J: The size of the ITS2 gene fragment before enzyme digestion; A-j: The size of ITS2 gene fragments after enzyme digestion; Kk: Negative control.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- 1.Madison-Antenucci S., Kramer L.D., Gebhardt L.L., Kauffman E. Emerging tick-borne diseases. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulanger N., Boyer P., Talagrand-Reboul E., Hansmann Y. Ticks and tick-borne diseases. Med Mal Infect. 2019;49(2):87–97. doi: 10.1016/j.medmal.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Kholodilov I., Belova O., Burenkova L., Korotkov Y., Romanova L., Morozova L., et al. Ixodid ticks and tick-borne encephalitis virus prevalence in the South Asian part of Russia (Republic of Tuva) Ticks Tick Borne Dis. 2019;10(5):959–969. doi: 10.1016/j.ttbdis.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 4.von Fricken M.E., Qurollo B.A., Boldbaatar B., Wang Y.W., Jiang R.R., Lkhagvatseren S., et al. Genetic diversity of Anaplasma and ehrlichia bacteria found in dermacentor and ixodes ticks in Mongolia. Ticks Tick Borne Dis. 2020;11(1) doi: 10.1016/j.ttbdis.2019.101316. [DOI] [PubMed] [Google Scholar]
- 5.Sheng J., Jiang M., Yang M., Bo X., Zhao S., Zhang Y., et al. Tick distribution in border regions of Northwestern China. Ticks Tick Borne Dis. 2019;10(3):665–669. doi: 10.1016/j.ttbdis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu H., Li Q., Zhang X., Li Z., Wang Z., Song M., et al. Characterization of rickettsiae in ticks in northeastern China. Parasit Vectors. 2016;9(1):498. doi: 10.1186/s13071-016-1764-2. Published 2016 Sep. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y. Jilin Agricultural University; 2019. Distribution of Tick Species and Epidemiological Background Investigation of Three Tick Borne Bacteria and Protozoa in Qinghai Province. in Chinese. [Google Scholar]
- 8.Jiao J., Lu Z., Yu Y., Ou Y., Fu M., Zhao Y., et al. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasit Vectors. 2021;14(1):287. doi: 10.1186/s13071-021-04740-3. Published 2021 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni J., Lin H., Xu X., Ren Q., Aizezi M., Luo J., et al. Coxiella burnetii is widespread in ticks (Ixodidae) in the Xinjiang areas of China. BMC Vet. Res. 2020;16:317. doi: 10.1186/s12917-020-02538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speck S., Derschum H., Damdindorj T., Dashdavaa O., Jiang J., Kaysser P., et al. Rickettsia raoultii, the predominant Rickettsia found in Mongolian Dermacentor nuttalli. Ticks Tick Borne Dis. 2012;3(4):227–231. doi: 10.1016/j.ttbdis.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Li H., Li X.M., Du J., Zhang X.A., Cui N., Yang Z.D., et al. Candidatus Rickettsia xinyangensis as cause of spotted fever group rickettsiosis, xinyang, China, 2015. Emerg. Infect. Dis. 2020;26(5):985–988. doi: 10.3201/eid2605.170294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merhej V., Angelakis E., Socolovschi C., Raoult D. Genotyping, evolution and epidemiological findings of Rickettsia species. Infect. Genet. Evol. 2014;25:122–137. doi: 10.1016/j.meegid.2014.-03.014. [DOI] [PubMed] [Google Scholar]
- 13.Harris E.K., Verhoeve V.I., Banajee K.H., Macaluso J.A., Azad A.F., Macaluso K.R. Comparative vertical transmission of Rickettsia by Dermacentor variabilis and Amblyomma maculatum. Ticks Tick Borne Dis. 2017;8(4):598–604. doi: 10.1016/j.ttbdis.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev., 26(4), 657–702. [DOI] [PMC free article] [PubMed]
- 15.Qin X.R., Han H.J., Han F.J., Zhao F.M., Zhang Z.T., Xue Z.F., et al. Rickettsia japonica and novel Rickettsia species in ticks, China. Emerg. Infect. Dis. 2019;25(5):992–995. doi: 10.3201/eid2505.171745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan T.T., Du C.H., Xia L.Y., Que T.C., von Fricken M.E., Jiang B.G., et al. Molecular evidence of Candidatus Rickettsia longicornii and a novel Rickettsia strain from ticks in Southern China. Ticks Tick Borne Dis. 2021;12(3) doi: 10.1016/j.ttbdis.2021.101679. [DOI] [PubMed] [Google Scholar]
- 17.Xu J., Gao L., Zhu P., Chen S., Chen Z., Yan Z., Lin W., et al. Isolation, identification, and pathogenicity analysis of newly emerging gosling astrovirus in South China. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1112245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang L.Q., Liu K., Li X.L., Liang S., Yang Y., Yao H.W., et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect. Dis. 2015;15(12):1467–1479. doi: 10.1016/S1473-3099(15)00177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roest H.I., Tilburg J.J., van der Hoek W., Vellema P., van Zijderveld F.G., et al. The Q fever epidemic in The Netherlands: history, onset, response and reflection. Epidemiol. Infect. 2011;139(1):1–12. doi: 10.1017/S0950268810002268. [DOI] [PubMed] [Google Scholar]
- 20.Huang M., Ma J., Jiao J., Li C., Chen L., Zhu Z., et al. The epidemic of Q fever in 2018 to 2019 in Zhuhai city of China determined by metagenomic next-generation sequencing. PLoS Negl Trop Dis. 2021;15(7) doi: 10.1371/journal.pntd.0009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiesa A., Onza C., Sulcaj N., Torggler A., Morosetti G., Conforti F., et al. Coxiella burnetii outbreak, Northern Italy 2021. One Health. 2023;17 doi: 10.1016/j.onehlt.2023.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw E.I., Voth D.E. Coxiella burnetii: a pathogenic intracellular acidophile. Microbiology. 2019;165(1):1–3. doi: 10.1099/mic.0.000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.España P.P., Uranga A., Cillóniz C., Torres A. Q fever (coxiella burnetii) Semin. Respir. Crit. Care Med. 2020;41(4):509–521. doi: 10.1055/s-0040-1710594. [DOI] [PubMed] [Google Scholar]
- 24.Kazar J. Coxiella burnetii infection. Ann. N. Y. Acad. Sci. 2005;1063:105–114. doi: 10.1196/annals.1355.018. [DOI] [PubMed] [Google Scholar]
- 25.Marrie T.J. Coxiella burnetii (Q fever) pneumonia. Clin. Infect. Dis. 1995;21(Suppl 3):S253–S264. doi: 10.1093/clind/21.supplement_3.s253. [DOI] [PubMed] [Google Scholar]
- 26.Roest H.I., Bossers A., van Zijderveld F.G., Rebel J.M. Clinical microbiology of Coxiella burnetii and relevant aspects for the diagnosis and control of the zoonotic disease Q fever. Vet. Q. 2013;33(3):148–160. doi: 10.1080/01652176.2013.843809. [DOI] [PubMed] [Google Scholar]
- 27.Clark N.J., Magalhaes R.J.S. Airborne geographical dispersal of q fever from livestock holdings to humancommunities: a systematic review and critical appraisal of evidence. BMC Infect. Dis. 2018;18 doi: 10.1186/s12879-018-3135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plummer P.J., McClure J.T., Menzies P., Morley P.S., Van den Brom R., Van Metre D.C. Manage-ment of coxiella burnetii infection in livestock populations and the associated zoonotic risk: a consensus statement. J. Vet. Intern. Med. 2018:1–14. doi: 10.1111/jvim.15229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J., Lu F., Wu Y., Zhang H. Survey and research on the habitat of biro and Qinghai lake Przewalski's przewalskii. Qinghai Prataculture. 2011;20(1):41–48. in Chinese. [Google Scholar]
- 30.Ping X., Chun W., Chun L., Tang S., Fang H., Cui S., et al. The distribution, population and conservation status of Przewalski's gazelle, Procapra przewalskii. Biodivers. Sci. 2018;26(2):177–184. in Chinese. [Google Scholar]
- 31.La M., Zhang L., Ma Q., He S. Investigation and analysis of the causes of death of Przewalski's gazelle in the Ganzi River area of Qinghai Lake. Heilongjiang Animal Husbandry Veterinary. 2017;(2):200–201. in Chinese. [Google Scholar]
- 32.Wang Y., Du S., Yang Y., Zhang X., Duszynski D.W., Bian J., et al. Intestinal parasites in the critically endangered Przewalski's gazelle (Procapra przewalskii) in China, with the description of a new species of Eimeria (apicomplexa: eimeriidae) J. Wildl. Dis. 2016;52(4):945–948. doi: 10.7589/2016-03-062. [DOI] [PubMed] [Google Scholar]
- 33.Chitimia L., Lin R.Q., Cosoroaba I., Braila P., Song H.Q., Zhu X.Q. Molecular characterization of hard and soft ticks from Romania by sequences of the internal transcribed spacers of ribosomal DNA. Parasitol. Res. 2009;105(4):907–911. doi: 10.1007/s00436-009-1474-1. [DOI] [PubMed] [Google Scholar]
- 34.Guo D.H. Heilongjiang bayi agricultural university; 2019. Differentiation of Dermacentor Silvarum and D.Nuttalli by PCR-RFLP and Studies Complete Mitochondrial Genome Sequences of D.Silvarum. in Chinese. [Google Scholar]
- 35.Kidd L., Maggi R., Diniz P.P., Hegarty B., Tucker M., Breitschwerdt E. Evaluation of conventional and real-time PCR assays for detection and differentiation of Spotted Fever Group Rickettsia in dog blood. Vet. Microbiol. 2008;129(3–4):294–303. doi: 10.1016/j.vetmic.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Sekeyova Z., Roux V., Raoult D. Phylogeny of Rickettsia spp. inferred by comparing sequences of 'gene D', which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 2001;51(Pt 4):1353–1360. doi: 10.1099/00207713-51-4-1353. [DOI] [PubMed] [Google Scholar]
- 37.Massung R.F., Slater K., Owens J.H., Nicholson W.L., Mather T.N., Solberg V.B., et al. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998;36(4):1090–1095. doi: 10.1128/jcm.36.4.1090-1095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Persing D.H., Mathiesen D., Marshall W.F., Telford S.R., Spielman A., Thomford J.W., et al. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 1992;30(8):2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao S., Zhang S., Jia L., Xue S., Yu L., Kamyingkird K., et al. Molecular detection of Theileria species in sheep from northern China. J. Vet. Med. Sci. 2013;75(9):1227–1230. doi: 10.1292/jvms.13-0028. [DOI] [PubMed] [Google Scholar]
- 40.Chang Y.F., Novosol V., McDonough S.P., Chang C.F., Jacobson R.H., Divers T., et al. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet. Pathol. 2000;37(1):68–76. doi: 10.1354/vp.37-1-68. [DOI] [PubMed] [Google Scholar]
- 41.To H., Kako N., Zhang G.Q., Otsuka H., Ogawa M., Ochiai O., et al. Q fever pneumonia in children in Japan. J. Clin. Microbiol. 1996;34(3):647–651. doi: 10.1128/jcm.34.3.647-651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H., Wang B., Yi J., Wang Z., Zhang J., Wang Y., et al. Brucella suis S2 isolated from aborted sheep fetuses in Northwestern China. Kafkas Universitesi Veteriner Fakultesi Dergisi. 2019;25(6):869–872. [Google Scholar]
- 43.Xie J., Chen Y., Cai G., Cai R., Hu Z., Wang H. Tree Visualization by One Table (tvBOT): a web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023 Jul 5;51(W1):W587–W592. doi: 10.1093/nar/gkad359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen S., Duan X., Wang B., Zhu L., Zhang Y., Zhang J., et al. A novel tick-borne phlebovirus, closely related to severe fever with thrombocytopenia syndrome virus and heartland virus, is a potential pathogen. Emerg Microbes Infect. 2018;7(1):95. doi: 10.1038/s41426-018-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C., He J., Li W. Infection status and control ,measures of Dermacentor abaensis in Procapra przewalskii around Qinghai lake area. Chin. J. Zool. 2018;53(4):668–670. in Chinese. [Google Scholar]
- 46.Jiang Z, Li D, Wang Z, Zhu S, Wei W, et al. Population structurt of the Przewalski's gazelle around the Qinghai LakeLake China (in Chinese). Acta Zool. Sin.,2001(2):158-162.
- 47.Li Z, Jiang Z. Group patterns of sympatric Przewalskiʾs gazelle and the Tibetan gazelle during the green grass period in upper buha river tianjun county, Qinghai province (in Chinese). Zool. Res. 2006(4):396-402.
- 48.Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia. 2010 Jan;162(1):217–225. doi: 10.1007/s00442-009-1430-x. [DOI] [PubMed] [Google Scholar]
- 49.Zhao B., Hou H., Gao R., Tian B., Deng B. Mononucleosis-like illnesses due to co-infection with severe fever with thrombocytopenia syndrome virus and spotted fever group rickettsia:a case report. BMC Infect. Dis. 2021;21:829. doi: 10.1186/s12879-021-06434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matei I.A., Corduneanu A., Sándor A.D., Ionică A.M., Panait L., Kalmár Z., et al. Rickettsia spp. in bats of Romania: high prevalence of Rickettsia monacensis in two insectivorous bat species. Parasites Vectors. 2021;14:107. doi: 10.1186/s13071-021-04592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gui Z., Cai H., Qi D.D., Zhang S., Fu S.Y., Yu J.F., et al. Identification and genetic diversity analysis of Rickettsia in Dermacentor nuttalli within inner Mongolia, China. Parasit Vectors. 2022;15(1):286. doi: 10.1186/s13071-022-05387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martínez-Sánchez E.T., Cardona-Romero M., Ortiz-Giraldo M., Tobón-Escobar W.D., Moreno-López D., et al. Rickettsia spp. in ticks (Acari: ixodidae) from wild birds in Caldas, Colombia. Acta Trop. 2021;213 doi: 10.1016/j.actatropica.2020.105733. [DOI] [PubMed] [Google Scholar]
- 53.Matulis G.A., Sakolvaree J., Boldbaatar B., Cleary N., Takhampunya R., Poole-Smith B.K., et al. Applying next generation sequencing to detect tick-pathogens in Dermacentor nuttalli, Ixodes persulcatus, and Hyalomma asiaticum collected from Mongolia. Ticks Tick Borne Dis. 2023 Sep;14(5) doi: 10.1016/j.ttbdis.2023.102203. [DOI] [PubMed] [Google Scholar]
- 54.Shao J.W., Zhang X.L., Li W.J., Huang H.L., Yan J. Distribution and molecular characterization of rickettsiae in ticks in Harbin area of Northeastern China. PLoS Negl Trop Dis. 2020;14(6):e00–e8342. doi: 10.1371/journal.pntd.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin X., Guo S., Ding C., Cao M., Kawabata H., Sato K., et al. Spotted fever group rickettsiae in inner Mongolia, China, 2015-2016. Emerg. Infect. Dis. 2018;24(11):2105–2107. doi: 10.3201/eid2411.162094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Q., Pan Y.S., Jiang B.G., Ye R.Z., Chang Q.C., Shao H.Z., et al. Prevalence of multiple tick-borne pathogens in various tick vectors in northeastern China. Vector Borne Zoonotic Dis. 2021;21(3):162–171. doi: 10.1089/vbz.2020.2712. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Wen X., Li M., Moumouni P.F.A., Galon E.M., Guo Q., et al. Molecular detection of tick-borne pathogens harbored by ticks collected from livestock in the Xinjiang Uygur Autonomous Region, China. Ticks Tick Borne Dis. 2020;11(5) doi: 10.1016/j.ttbdis.2020.101478. [DOI] [PubMed] [Google Scholar]
- 58.Song S., Chen C., Yang M., Zhao S., Wang B., Hornok S., et al. Diversity of Rickettsia species in border regions of northwestern China. Parasit Vectors. 2018;11(1):634. doi: 10.1186/s13071-018-3233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao S., Yang M., Jiang M., Yan B., Zhao S., Yuan W., et al. Rickettsia raoultii and Rickettsia sibirica in ticks from the long-tailed ground squirrel near the China-Kazakhstan border. Exp. Appl. Acarol. 2019;77(3):425–433. doi: 10.1007/s10493-019-00349-5. [DOI] [PubMed] [Google Scholar]
- 60.Fischer T., Myalkhaa M., Krücken J., Battsetseg G., Batsukh Z., Baumann M.P.O., et al. Molecular detection of tick-borne pathogens in bovine blood and ticks from Khentii, Mongolia. Transbound Emerg Dis. 2020;67(Suppl 2):111–118. doi: 10.1111/tbed.13315. [DOI] [PubMed] [Google Scholar]
- 61.Moore T.C., Pulscher L.A., Caddell L., von Fricken M.E., Anderson B.D., Gonchigoo B., et al. Evidence for transovarial transmission of tick-borne rickettsiae circulating in Northern Mongolia. PLoS Negl Trop Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Fricken M.E., Voorhees M.A., Koehler J.W., Asbun C., Lam B., Qurollo B., et al. Molecular characteristics of Rickettsia in ticks collected along the southern border of Mongolia. Pathogens. 2020;9(11):943. doi: 10.3390/pathogens9110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Y.C., Li J.X., Sun Y.L., Kang M., He H.X., Guo Y.H., et al. Spotted fever group Rickettsia infecting ticks (Acari: ixodidae), yak (Bos grunniens), and Tibetan sheep (Ovis aries) in the Qinghai-Tibetan plateau area, China. Front. Vet. Sci. 2022;8 doi: 10.3389/fvets.2021.779387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baca O.G., Paretsky D. Q fever and Coxiella burnetii: a model for host-parasite interactions. Microbiol. Rev. 1983;47(2):127–149. doi: 10.1128/mr.47.2.127-149.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pascucci I., Di Domenico M., Capobianco Dondona G., Di Gennaro A., Polci A., Capobianco Dondona A., et al. Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: an Italian experience. Ticks Tick Borne Dis. 2019 Oct;10(6) doi: 10.1016/j.ttbdis.2019.101272. [DOI] [PubMed] [Google Scholar]
- 66.Abdel-Shafy S., Allam N.A., Mediannikov O., Parola P., Raoult D. Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis. 2012 May;12(5):346–359. doi: 10.1089/vbz.2010.0241. [DOI] [PubMed] [Google Scholar]
- 67.Shehla S., Almutairi M.M., Alouffi A., Tanaka T., Chang S.C., Chen C.C., et al. Molecular survey of Rickettsia raoultii in ticks infesting livestock from Pakistan with notes on pathogen distribution in palearctic and oriental regions. Vet Sci. 2023 Oct 29;10(11):636. doi: 10.3390/vetsci10110636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ullah Q., El-Adawy H., Jamil T., Jamil H., Qureshi Z.I., Saqib M., et al. Serological and molecular investigation of coxiella burnetii in small ruminants and ticks in Punjab, Pakistan. Int J Environ Res Public Health. 2019 Nov 4;16(21):4271. doi: 10.3390/ijerph16214271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pascucci I., Di Domenico M., Capobianco Dondona G., Di Gennaro A., Polci A., Capobianco Dondona A., et al. Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: an Italian experience. Ticks Tick Borne Dis. 2019 Oct;10(6) doi: 10.1016/j.ttbdis.2019.101272. [DOI] [PubMed] [Google Scholar]
- 70.Jiang L., Meng Z., Cui Q., Tong W., Ling F., Wang Z., et al. Detection of rOmpA and gltA genes of spotted fever group rickettsiae from tick specimens in Zhejiang province. China J Vector Biol Control. 2010;21(4):350–352. in Chinese. [Google Scholar]
- 71.Fournier P.E., Roux V., Raoult D. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 1998;48(Pt 3):839–849. doi: 10.1099/00207713-48-3-839. [DOI] [PubMed] [Google Scholar]
- 72.Taylor A.J., Vongphayloth K., Vongsouvath M., Grandadam M., Brey P.T., Newton P.N., et al. Large-scale survey for tickborne bacteria, khammouan province, Laos. Emerg. Infect. Dis. 2016;22(9):1635–1639. doi: 10.3201/eid2209.151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gui Z., Yu J.F., Mu L. Detection of DNA in spotted fever group Rickettsia carried by Dermacentor nuttalli in partial areas of Inner Mongolia and its distribution of genotypes. J. Jilin Univ. - Med. Ed. 2021;47(1):210–215. in Chinese. [Google Scholar]
- 74.Nadim A., Khanjani M., Hosseini-Chegeni A., Telmadarraiy Z. Identity and microbial agents related to Dermacentor marginatus Sulzer (Acari: ixodidae) with a new record of Rickettsia slovaca (Rickettsiales: rickettsiaceae) in Iran. Syst. Appl. Acarol. 2021;26:367–378. [Google Scholar]
- 75.Kooshki H., Goudarzi G., Faghihi F., Telmadarraiy Z., Edalat H., HosseiniChegeni A. vol. 25. Syst ApplAcarol; 2020. pp. 1611–1617. (The First Record of Rickettsia Hoogstraalii (Rickettsiales:Rickettsiaceae) from Argas Persicus (Acari: Argasidae) in Iran). [Google Scholar]
- 76.Ma H., Ai J., Kang M., Li J., Sun Y. The life cycle of Dermacentor nuttalli from the Qinghai-Tibetan Plateau under laboratory conditions and detection of spotted fever group Rickettsia spp. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1126266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elsa J., Duron O., Séverine B., González-Acuña D., Sidi-Boumedine K. Molecular methods routinely used to detect Coxiella burnetii in ticks cross-react with Coxiella-like bacteria. Infect. Ecol. Epidemiol. 2015;5 doi: 10.3402/iee.v5.29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yuasa Y., Yoshiie K., Takasaki T., Yoshida H., Oda H. Retrospective survey of chronic Q fever in Japan by using PCR to detect Coxiella burnetii DNA in paraffin-embedded clinical samples. J. Clin. Microbiol. 1996;34(4):824–827. doi: 10.1128/jcm.34.4.824-827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study were included in this published.
Rickettsia raoultii OmpA data have been deposited at Genbank repository with accession numbers OQ 253257 - OQ 253269. Rickettsia sibirica OmpA data have been deposited at Genbank repository with accession numbers OQ 253270 - OQ 253273. Rickettsia slovaca OmpA data have been deposited at Genbank repository with accession numbers OQ 253274. Rickettsia raoultii Sca4 data have been deposited at Genbank with accession numbers OQ 253275 - OQ 253281. Rickettsia sibirica Sca4 data have been deposited at Genbank repository with accession numbers OQ 253282 - OQ 253283. Rickettsia slovaca Sca4 data have been deposited at Genbank repository with accession numbers OQ 253284 - OQ 253285. Dermacentor nuttalli ITS2 data have been deposited at Genbank repository with accession numbers OQ 955291 - OQ 955293. Coxiella burnetii htpB data have been deposited at Genbank repository with accession numbers OQ 865125 - OQ 865128.