Summary
Background
The absolute overall survival (OS) improvement with preoperative chemotherapy or chemoradiotherapy in locally advanced non-small cell lung cancer (NSCLC) patients is controversial and unsatisfactory. We designed this trial to explore the efficacy and safety of perioperative sintilimab plus platinum-based chemotherapy for potentially resectable stage IIIB NSCLC to facilitate further optimization of this therapeutic strategy.
Methods
Patients diagnosed with stage IIIB NSCLC through invasive staging approaches and/or PET/CT scans and evaluated as having a high probability of radical resection of the primary lesion and metastatic lymph nodes with clear pathological margins by a multidisciplinary team were enrolled in this open-label, single-arm, phase II trial at a single centre in China. The participants received two cycles of intravenous neoadjuvant treatment with PD-1 inhibitor sintilimab (200 mg), pemetrexed (500 mg/m2) for adenocarcinoma, paclitaxel (175 mg/m2) or nab-paclitaxel (260 mg/m2) for other histological subtypes, plus carboplatin (area under the curve 5) or cisplatin (75 mg/m2) on the first day of each 3-week cycle. Surgical resection was performed 28–42 days later. After recovery from surgery, two cycles of adjuvant treatment were carried out in strict conformity with the neoadjuvant regimen, and then sintilimab maintenance monotherapy were given. The primary endpoint was major pathological response (MPR). The key secondary endpoints included the objective response rate (ORR), radical resection (R0) rate, pathological complete response (pCR) rate, event-free survival (EFS), disease-free survival (DFS), OS, treatment-related adverse events (TRAEs), surgical complications, and surgery delay rate. This trial is registered with the Chinese Clinical Trial Registry (ChiCTR2000040673).
Findings
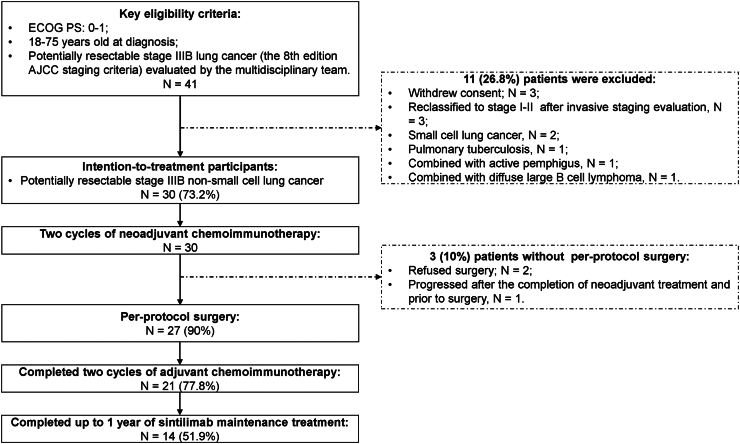
Forty-one patients were assessed for eligibility between December 2020 and August 2022; 30 patients were enrolled and given two cycles of neoadjuvant chemoimmunotherapy (neoCIT). Nineteen patients achieved a radiographic partial response, resulting in an ORR of 63.3%. Although 26 patients (86.7%) experienced TRAEs during the neoadjuvant phase, only two patients (6.7%) had ≥ grade 3 TRAEs. Surgical resection was performed on 27 patients (90%), with two patients experienced surgical delay because of coronavirus disease 2019, and the R0 rate was 96.4%. Twelve patients (44.4%) in the per-protocol (PP) population achieved an MPR, including six patients (22.2%) with a pCR. The most common postoperative complications were atrial fibrillation (6, 22.2%), pneumonitis (5, 18.5%), and heart failure (4, 14.8%); no deaths occurred within 90 days after surgery. As of October 31, 2024, the median follow-up was 34.7 months. The estimated EFS and OS rates at 36 months in the intention-to-treat population were 42.8% and 70.1%, respectively, and the estimated DFS and OS rates at 36 months in the PP population were 52.5% and 70.4%, respectively.
Interpretation
Perioperative sintilimab plus platinum-based chemotherapy is an emerging treatment option for patients with potentially resectable stage IIIB NSCLC; it has a high response rate and tolerable treatment-related toxic effects, and enables radical resection in most patients.
Funding
The Cancer Research Program of National Cancer Center (NCC201919B02), Shenzhen Clinical Research Center for Cancer (No. [2021]287), Shenzhen High-level Hospital Construction Fund, Shenzhen Key Medical Discipline Construction Fund (SZXK075) and Sanming Project of Medicine in Shenzhen (SZSM201612097) funded this study.
Keywords: Perioperative chemoimmunotherapy, Potentially resectable, IIIB, Non-small cell lung cancer, Sintilimab
Research in context.
Evidence before this study
We conducted a comprehensive search on PubMed for English-language papers published as of December 31, 2020, with no restrictions on article type, using the search terms “induction” OR “neoadjuvant” AND “stage IIIB” AND “non-small cell lung cancer”, yielding a total of 249 articles. Clinical stage IIIB non-small cell lung cancer (NSCLC) is generally regarded as an inoperable or unresectable disease and is mainly managed with definitive chemotherapy or chemoradiotherapy; as a result, the 5-year survival rate is approximately 26%. Although several population-based retrospective studies and a phase II prospective trial indicated that the addition of radical resection as a part of trimodal treatment in a selected subset of patients with technically resectable stage IIIB NSCLC trended to improve survival, the long-term outcome remained unsatisfactory, with a 5-year survival of 37–43%. At the beginning of this phase II trial, several pilot trials demonstrated that among patients with resectable NSCLC, including some with stage IIIB (T3-4N2) disease, neoadjuvant immune checkpoint inhibitors (ICIs) could elicit encouraging radiographic and pathological responses with tolerable side effects. However, owing to the small number of stage IIIB patients, subgroup analysis results, and short follow-up period, the efficacy, safety, and survival benefit of neoadjuvant chemoimmunotherapy (neoCIT) in patients with potentially resectable stage IIIB NSCLC warrant further investigation.
Added value of this study
We reported the clinical trial to evaluate the efficacy and safety of perioperative CIT specifically in patients with potentially resectable stage IIIB NSCLC. The primary endpoint was reached, while the major pathological response (MPR) rate remained comparable to that reported in previously conducted studies which more cycles of neoCIT were used. Overall, the cycles and doses of perioperative sintilimab plus platinum-based chemotherapy designed in our trial were well tolerated, and the completion rates of neoCIT, adjuvant CIT, and sintilimab maintenance therapy were quite high. In addition, among the 6 patients harboring EGFR mutations and two patients with EML4-ALK translocation who underwent per-protocol (PP) surgery after neoCIT, 3 patients achieved an MPR (37.5%).
Implications of all the available evidence
The available evidence and our present study, with respect to early efficacy and safety, uniformly demonstrate that perioperative administration of ICIs plus chemotherapy could emerge as a novel option in patients with initially unresectable or potentially resectable stage IIIB (T1-2N3 and T3-4N2) NSCLC, which may ultimately improve survival. However, randomized controlled trials conducted at multiple centers are warranted to shed further insight into potential biomarkers to identify patients who may benefit, and to assess evidence-based management of side effects and qualitative survival benefits.
Introduction
Stage IIIB non-small cell lung cancer (NSCLC) is a subgroup of locally advanced disease with adverse survival factors, including primary tumor size and/or direct invasion into adjacent organs or structures and/or regional lymph node metastasis (N2-3); as a result, the five-year survival rate is approximately 26% in the era of chemoradiotherapy (CRT).1 Recently, as a new benchmark for the standard of care, immune checkpoint inhibitors (ICIs) have been used as consolidation treatment for patients with stage III NSCLC whose disease has not progressed after CRT has been established, with a 9.5% absolute improvement in the estimated 5-year overall survival (OS) rate compared with that with the placebo (42.9% vs. 33.1%).2 Nevertheless, the therapeutic strategy for resectable NSCLC (including selected stage IIIB) has shifted toward neoadjuvant chemoimmunotherapy (neoCIT), which could increase the objective response rate (ORR) and pathological response rate, and potentially improve long-term outcomes compared with neoadjuvant chemotherapy (neoCT) alone. The phase 2 NADIM trial, three cycles of neoadjuvant nivolumab and chemotherapy administered to patients with stage IIIA, resectable NSCLC according to the 7th edition American Joint Committee on Cancer (AJCC) staging criteria.3 Among the 10 (19.6%) eligible patients with clinical T3N2M0 disease (classified as stage IIIB in the 8th edition), 6 (60%) achieved a pathological complete response (pCR), and 8 (80%) had pathological downstaging.3 Furthermore, Jianxing He et al. reported a 60.8% (31/51) surgical resection rate after at least two cycles of CIT in patients with initial unresectable stage IIIB (cT2-4N2-3M0) NSCLC, and patients who underwent subsequent resection had better recurrence/progression-free survival than those who did not undergo surgery.4 Although Sun and colleagues recently reported the efficiency (ORR: 70%; major pathological response [MPR] rate: 62.5%; pCR rate: 31.3%) and safety (grade 1–2 and ≥3 immune-related adverse events [irAEs]: 20% and 15%, respectively) of neoadjuvant sintilimab combined with chemotherapy among patients with potentially resectable stage IIIA/IIIB NSCLC, only 33.3% of the enrolled patients had clinical stage IIIB disease.5 Taken together, insights into the feasibility and effectiveness of neoCIT in patients with clinical stage IIIB NSCLC have been limited in retrospective studies4,6 and subgroup analyses of prospective trials.7,8
Sintilimab is a fully humanized, highly selective IgG4 monoclonal anti-programmed cell death (PD)-1 antibody that blocks interactions between PD-1 and its ligands (PD-L1 and PD-L2) and has been approved as a first-line treatment for locally advanced or metastatic nonsquamous NSCLC, as it was demonstrated to manageable safety profiles and greatly improve survival.9 Clinical trials regarding combination sintilimab plus chemotherapy with surgical resection to prospectively assess the safety and possible survival benefit in patients with locally advanced NSCLC are urgently needed to further support and promote this promising therapeutic strategy.
Therefore, this phase II trial (periSCOPE) was promptly designed to investigate the efficacy and safety of perioperative sintilimab plus platinum-based chemotherapy in patients with potentially resectable stage IIIB NSCLC.
Methods
Study design and participants
This open-label, single-arm, phase II trial was conducted at an integrated cancer center in southern China, approved by the institutional clinical research ethics committee (2020-186-1), performed in accordance with the Helsinki Declaration and the Good Clinical Practice guidelines, and registered with the Chinese Clinical Trial Registry (ChiCTR2000040673). Every participant signed an informed consent form before enrollment.
Eligible participants were aged 18–75 years and had histologically or cytologically diagnosed, treatment-naïve stage IIIB NSCLC (the 8th edition AJCC staging criteria), which was evaluated as having a high-probability of radical resection of the primary lesion and metastatic lymph nodes with clear pathological margins by our multidisciplinary team (MDT). The largest dimension of the measurable pulmonary tumor, according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), for included patients was greater than or equal to 2.0 cm (T1c-4). The ipsilateral (N2, including single-station, multi-station or bulky N2 disease) mediational lymph node involvement was determined by invasive staging approaches (including endobronchial ultrasound-guided transbronchial needle aspiration [EBUS-TBNA], endoscopic ultrasound [EUS]-guided biopsy, video-assisted thoracoscopic surgery [VATS] biopsy) and/or fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) scan for included patients. In addition, clinical N3 disease, specificially refer to the unilateral supraclavicular lymph node metastasis, must be confirmed through ultrasound-guided fine-needle aspiration (FNA). Included patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, adequate organ and pulmonary function, and life expectancy of more than 6 months were mandatory at participant inclusion. Although participants were enrolled regardless of their molecular gene profiles, all included patients provided pretreatment biopsy samples for detecting PD-L1 expression via the immunocytochemistry 22C3 pharmDx assay (Dako, Denmark) and next-generation sequencing of a customized 1021 gene panel containing whole exons (GenePlus Technology Co., Ltd., Beijing, China). Further information on the detection methods is presented in the Supplemental Data.
Participants were excluded if they received previous treatments regulating T-cell activities (such as anti-CTLA-4, anti-OX40, and anti-CD137 antibodies) or immune checkpoint blockade therapies (such as anti-PD-L1, anti-PD-L2, and anti-PD-1 inhibitor); were diagnosed with other malignancies within the pretreatment period of 5 years (with exceptions including bladder carcinoma, basal cell carcinoma or squamous cell carcinoma of the skin, or carcinoma in situ that had undergone resection for potential cure); received traditional Chinese medicines or immunomodulatory drugs approved for cancer treatment within the pretreatment period of two weeks; were participating in other ongoing interventional studies; were undergoing systemic immunosuppressive therapy; received solid-organ or hematopoietic stem cell transplantation; or had confirmed human immunodeficiency virus (HIV) infection, active autoimmune disease, or acute hepatitis B virus infection.
Procedures
All participants received two cycles of neoadjuvant treatment with intravenous PD-1 inhibitor sintilimab (200 mg, Innovent Biologics Co., Ltd., Suzhou, China), pemetrexed (500 mg/m2) for adenocarcinoma, paclitaxel (175 mg/m2) or nab-paclitaxel (260 mg/m2) for other histological subtypes, plus carboplatin (area under the curve 5) or cisplatin (75 mg/m2) on the first day of each three-week cycle. Surgical resection was performed 28–42 days later. Three thoracic surgeons, who all specialized in thoracic oncology surgery for more than twenty years, collectively determined the surgical approach (i.e., thoracotomy, VATS, or robot-assisted thoracoscopic surgery [RATS]) and the extent of resection (i.e., [sleeve] lobectomy, bilobectomy, or pneumonectomy). In patients with unilateral supraclavicular lymph node metastasis (N3 disease), the ipsilateral levels IV and VB neck lymph node must be additionally dissected. After recovery from surgery, two cycles of adjuvant treatment were carried out in strict conformity with the neoadjuvant regimen, and then sintilimab maintenance treatment was given once every three weeks until disease progression, death, withdrawal of consent, intolerable toxicity, or up to one year.
Assessments
Whole-body PET/CT scans and contrast-enhanced chest CT were recommended for baseline assessments. When mediastinal and/or supraclavicular lymph nodes were suspected to be positive, histological and/or cytological confirmation was performed. Magnetic resonance imaging (MRI) of the brain with contrast agent is optimal for eliminating asymptomatic brain metastasis; however, when MRI could not be performed, a head CT with contrast agent was used as an option for these patients. Chest CT with contrast agent was performed at least 28 days after the completion of neoCIT and prior to surgery, and the response status was collectively evaluated according to the RECIST by two radiologists who specialized in chest radiological diagnosis for more than ten years. During the posttreatment period, chest CT with (or without) contrast and ultrasound examinations of the neck lymph nodes were routinely performed for recurrence surveillance every three months during the first two years and every 6 months thereafter.
The surgically resected lung tumor and lymph node samples were evaluated according to the immune-related pathological response criteria (irPRC)10 and independently staged according to the 8th edition of the AJCC-pTNM system1 by two pathologists who have been involved in the pathological diagnosis of thoracic tumors for more than ten years. Discordant assessments were reevaluated together via a double-head microscope after a one-month washout period, and a consensus was reached.
The Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) was utilized for recording and grading AEs during the neoCIT, adjuvant CIT, and sintilimab maintenance periods by two blinded medical oncologists. However, complications within the 30 days of surgery were defined and graded via the Clavien-Dindo classification11 by the above three thoracic surgeons independently.
Outcomes
The primary endpoint was the proportion of participants who achieved a major pathological response (MPR), defined as 10% or less viable tumor cells in the surgically resected lung tumor regardless of the lymph node status.
The secondary endpoints included the objective response rate (ORR), radical resection (R0) rate, pCR (defined as no viable tumor cells in the entire tumor bed as well as all resected lymph nodes) rate, event-free survival (EFS, defined as the time from the beginning day of neoadjuvant treatment to the day of any disease progression impeding surgery, progression of disease in the absence of surgery, recurrence of disease after surgery, or death from any cause), disease-free survival (DFS, calculated from the date of surgery until the date of death due to any cause or disease recurrence), overall survival (OS, defined as the interval between the beginning day of neoadjuvant treatment and the day of death because of any cause), TRAEs, surgical complications during the first 30 days after surgery, and surgery delay (more than 42 days from the second cycle of neoCIT to surgery) rate.
Statistical analysis
On the basis of the optimal two-stage designs for phase II clinical trials and the related trials that selected PD-1 or PD-L1 inhibitor as neoadjuvant therapy in patients with NSCLC, we assumed that the estimated (p1) and tolerable minimum (p0) proportion of patients achieving an MPR after neoadjuvant immunotherapy were approximately 45%12 and 20%,13 respectively. Nineteen participants were enrolled in the first stage upon the optimal size of the type I (0.05) and type II (0.10) errors. If 4 or fewer MPR cases were observed, then the trial would be terminated. Otherwise, accrual continued to the second stage with the enrollment of an additional eleven participants (the optimal average sample size was 30.4 in total). Finally, more than 8 participants achieved an MPR, which was considered to reach its primary endpoint (Supplementary Figure S1). The estimated 95% confidence interval (CI) of the proportion is calculated using the Wilson score interval method.
Data for continuous variables are presented as the mean (standard deviation, SD) or median (interquartile range, IQR) and were compared via the Mann–Whitney U test. Enumeration and ranked variables are expressed as counts and corresponding percentages and were compared via the χ2 test or Fisher's exact test. The Kaplan–Meier method was used to estimate median survival and the corresponding two-sided 95% confidence interval (CI) for time to event, and a two-sided log-rank test was used to assess the differences. All the statistical analyses were performed via Stata software (version 15.0, StataCorp, Chicago, IL) and GraphPad Prism (version 9.0, GraphPad Software, Inc., La Jolla, CA). The threshold for statistical significance was set as a two-sided P value less than 0.05 for all tests.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Characteristics of the intention-to-treat population
Forty-one patients (25 patients in the first stage and 16 patients in the second stage) with potentially resectable stage IIIB (8th edition AJCC staging criteria) lung cancer were screened between December 2020 and August 2022, and 30 patients were ultimately eligible for intention-to-treat (ITT) analyses (Table 1, Fig. 1). Most patients were older than 60 years (73.3%), male patients predominated (76.7%), and 60% of patients had a history of smoking. Among the histological subtypes, 19 (63.3%) of the 30 patients had adenocarcinoma (ADC), 8 (26.7%) had squamous cell carcinoma (SCC), and three (10%) had primary pulmonary lymphoepithelioma-like carcinoma (PPLELC). The PD-L1 tumor cell proportion score (TPS) at baseline was less than 1% in 6 (20%) patients and 50% or higher in 9 (30%) patients. Eight patients (26.7%) with epidermal growth factor receptor (EGFR) mutations and two patients (6.7%) with echinoderm microtubule-associated protein-like-4-anaplastic lymphoma kinase (EML4-ALK) translocation were identified. The details of the 25 (83.3%) patients who received baseline invasive lymph node assessment included the following: nineteen underwent EBUS-TBNA, three underwent EBUS-TBNA plus ultrasound-guided FNA, two underwent station 5 and 6 lymph node dissections through uniportal VATS, and one underwent ultrasound-guided FNA. In addition, four (13.3%) patients had N2 disease according to FDG PET/CT scan alone. The remaining one (3.3%) patient received EBUS-TBNA with an eventual diagnosis of a station 7 lymph node negative tumor; nevertheless, his tumor was classified as N2 positive by MDT because of enlarged bulky lymph nodes (greater than 3 cm) at stations 5 and 6 on enhanced CT (Supplementary Tables S1 and S2).
Table 1.
Baseline demographic and clinical characteristics of the intention-to-treat population.
| Characteristic | Participants (N = 30) |
|---|---|
| Age at diagnosis | |
| Mean (SD), years old | 64.4 (6.2) |
| Distribution, n (%) | |
| <60 years old | 8 (26.7%) |
| ≥60 years old | 22 (73.3%) |
| Sex, n (%) | |
| Female | 7 (23.3%) |
| Male | 23 (76.7%) |
| ECOG performance status score, n (%) | |
| 0 | 12 (40.0%) |
| 1 | 18 (60.0%) |
| Basic disease at diagnosis, n (%) | |
| None | 16 (53.3%) |
| Hypertension | 8 (26.6%) |
| Diabetes mellitus | 4 (13.3%) |
| Coronary heart disease | 1 (3.3%) |
| Paroxysmal atrial tachycardia | 1 (3.3%) |
| Smoking history | |
| Smoking Status, n (%) | |
| Never | 12 (40.0%) |
| Current or Former | 18 (60.0%) |
| Smoking index, median (IQR) | 17.5 (0–40.0) |
| Pathological subtype, n (%) | |
| Adenocarcinoma | 19 (63.3%) |
| Squamous cell carcinoma | 8 (26.7%) |
| PPLELC | 3 (10.0%) |
| Location of tumor, n (%) | |
| Left upper lobe | 11 (36.7%) |
| Left lower lobe | 3 (10.0%) |
| Right upper lobe | 11 (36.7%) |
| Right middle lobe | 2 (6.7%) |
| Right lower lobe | 3 (10.0%) |
| Clinical T staging, n (%) | |
| T1c | 1 (3.3%) |
| T2b | 2 (6.7%) |
| T3 | 8 (26.7%) |
| T4 | 19 (63.3%) |
| Primary tumor size, median (IQR), cm | 5.65 (4.28–7.40) |
| Clinical N staging, n (%) | |
| N2a | 6 (20.0%) |
| N2b | 21 (70.0%) |
| N3 (supraclavicular lymph node metastasis) | 3 (10.0%) |
| Lymph nodes evaluation, n (%) | |
| EBUS-TBNA | 19 (63.3%) |
| FDG PET/CT scan | 4 (13.3%) |
| Lymph nodes dissection under VATS | 2 (6.7%) |
| Ultrasound-guided FNA | 1 (3.3%) |
| EBUS-TBNA + ultrasound-guided FNA | 3 (10.0%) |
| Enhanced CT scan alone | 1 (3.3%) |
| PD-L1 tumor cell proportion score, n (%) | |
| <1% | 6 (20.0%) |
| 1–49% | 13 (43.3%) |
| ≥50% | 9 (30.0%) |
| Unknown | 2 (6.7%) |
| PD-L1 combined positive score, n (%) | |
| <10 | 7 (23.3%) |
| ≥10 | 21 (70.0%) |
| Unknown | 2 (6.7%) |
| EGFR mutation status, n (%) | |
| No | 21 (70.0%) |
| L858R | 2 (6.7%) |
| 19del | 2 (6.7%) |
| L858R/G721A | 1 (3.3%) |
| T790m/L861Q | 1 (3.3%) |
| G719S/S768I | 1 (3.3%) |
| A289V | 1 (3.3%) |
| Unknown | 1 (3.3%) |
| EML4-ALK translocation status, n (%) | |
| No | 27 (90.0%) |
| Yes | 2 (6.7%) |
| Unknown | 1 (3.3%) |
| Tumor mutational burden (TMB), n (%) | |
| Median (IQR), mutation/megabase (mut/Mb) | 11.67 (6.96–17.95) |
| <10 mut/Mb | 12 (40.0%) |
| ≥10 mut/Mb | 16 (53.3%) |
| Unknown | 2 (6.7%) |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PPLELC, primary pulmonary lymphoepithelioma-like carcinoma; EBUS-TBNA, endobronchial ultrasound–guided transbronchial needle aspiration; FDG PET/CT, fluorodeoxyglucose positron emission tomography/computed tomography; VATS, video-assisted thoracic surgery; FNA, fine-needle aspiration; PD-L1, programmed cell death 1-ligand 1; EGFR, epidermal growth factor receptor; EML4-ALK, echinoderm microtubule-associated protein-like-4-anaplastic lymphoma kinase.
Fig. 1.
CONSORT flow diagram. ECOG PS, Eastern Cooperative Oncology Group Performance Status; AJCC, American Joint Committee on Cancer.
Safety and efficacy of neoadjuvant chemoimmunotherapy
Among the 30 intention-to-treat participants, 28 (93.3%) completed the two planned cycles and doses of neoadjuvant sintilimab plus platinum-based chemotherapy, and the other two patients received a 30% reduction in the dose of cisplatin in the second cycle due to grade 3 decreased neutrophil count or grade 2 atrial fibrillation (Supplementary Tables S3 and S4). At presentation, the most frequent any-grade TRAEs were anemia (21, 70%), alopecia (15, 50%), fatigue (6, 20%), increased alanine aminotransferase (5, 16.7%), peripheral sensory neuropathy (5, 16.7%), anorexia (5, 16.7%), and increased aspartate aminotransferase (3, 10%). Although there were no grade 4 or 5 events, grade 3 (decreased neutrophil count (2, 6.7%), arthralgia and myalgia (1, 3.3%), fatigue (1, 3.3%), and decreased platelet count (1, 3.3%)) were observed in two patients (Supplementary Table S4).
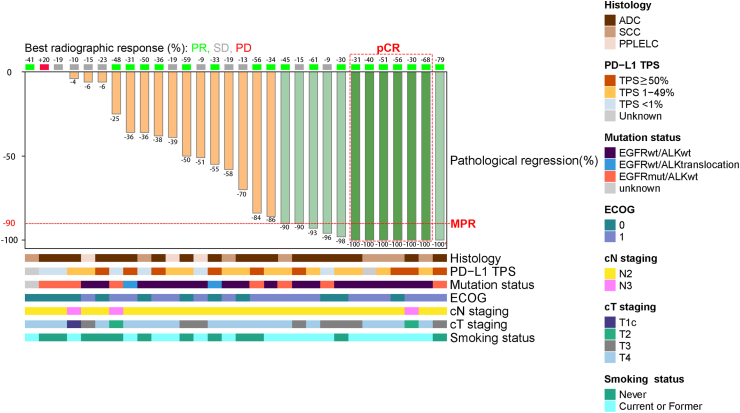
According to the RECIST (version 1.1) assessment, 63.3% (19/30; 95% CI, 45.5–78.1%) achieved a partial response (PR), 33.3% (10/30; 95% CI, 19.2–51.2%) had stable disease, and 3.3% (1/30; 95% CI, 0.6–16.7%) presented disease progression after neoadjuvant treatment (Fig. 2, Supplementary Table S3). In post-hoc analyses, radiologic objective responses were achieved regardless of PD-L1 TPS (<1% vs. ≥1%, P = 0.157; <50% vs. ≥50%, P = 0.249), EGFR mutation status (P = 0.524), or EML4-ALK translocation status (P = 0.381). However, the ORR in patients with SCC was significantly greater than that in patients with ADC and PPLELC (87.5%, 63.2%, and 0%, respectively; P = 0.027. Supplementary Figure S2A). Radiologic T downstaging and N downstaging were observed in twelve (40%) and three (10%) patients, respectively, and 10 (33.3%) patients exhibited radiologic TNM downstaging (Supplementary Table S3).
Fig. 2.
Radiographic response and pathological response in primary tumors according to clinical and pathological characteristics. ∗No residual viable tumor in the primary tumor bed, but the resected lymph nodes had remaining tumor cells; PR, partial response; SD, stable disease; PD, progress disease; pCR, pathological complete response; MPR, major pathological response; ADC, adenocarcinoma; SCC, squamous cell carcinoma; PPLELC, primary pulmonary lymphoepithelioma-like carcinoma; PD-L1 TPS, programmed cell death 1-ligand 1tumor cell proportion score; ECOG, Eastern Cooperative Oncology Group.
Of the eight patients harboring EGFR mutations, including two patients with 19del, two with L858R, one with L858R/G721A, one with T790M/L861Q, one with G719S/S768I, and one with A289V (Supplementary Table S5), four patients (L858R, n = 2; G719S/S768I, n = 1; A289V, n = 1) met the criteria for objective radiological response, one patient (T790M/L861Q) had disease progression, and the remaining three patients presented stable disease. In addition, two patients with EML4-ALK translocation had a PR according to RECIST assessment.
Surgical details and outcomes
As presented in Table 2, 27 patients (90%) completed the in-protocol surgery, of whom two experienced surgical delay as defined in the protocol, because of coronavirus disease 2019 (COVID-19). The median duration between the date of the second cycle of NeoCIT and the date of surgery was 34 (IQR, 32–40) days. R0 resection was reached in 26 out of 27 patients (96.3%), while one patient underwent R(un) resection (systematic lymph node sampling) because of the dense fibrosis of the right paratracheal, subcarinal and hilar zones observed intraoperatively. Minimally invasive approaches, including VATS (uniportal and two-port: 22 cases [81.5%] and 1 case [3.7%], respectively) and RATS (3 cases, 11.1%), were more common, and 6 cases were converted to thoracotomy during the uniportal VATS procedure (conversion rate: 23.1%). The most common extent of resection was lobectomy (21 cases [77.8%] included four bronchial sleeve lobectomies), followed by right sided bilobectomy (3 cases, 11.1%) and left sided pneumonectomy (3 cases, 11.1%). Surgical complications were observed in 10 patients (37.0%; Supplementary Table S6). The major complications were atrial fibrillation (6, 22.2%), pneumonitis (5, 18.5%), and heart failure (4, 14.8%), and one patient required postoperative intensive care unit management due to grade III heart failure. However, none of the surgical complications led to unplanned reoperation or 90-day mortality.
Table 2.
Surgical details of the participants who underwent surgical resection.
| Characteristic | Participants (N = 27) |
|---|---|
| Duration of neoCIT and surgery, median (IQR), daysa | 34.0 (32.0–40.0) |
| R0 resection, n (%) | 26 (96.3%) |
| Incision approach, n (%) | |
| Video-assisted thoracoscopic surgery (VATS) | 17 (63.0%) |
| Robot-assisted thoracoscopic surgery (RATS) | 3 (11.1%) |
| Thoracotomy | 7 (25.9%) |
| Extent of resection, n (%) | |
| Lobectomy | 21 (77.8%) |
| Bilobectomy | 3 (11.1%) |
| Pneumonectomy | 3 (11.1%) |
| Operative time, median (IQR), minute | 225.0 (195.0–272.0) |
| Estimated blood loss, median (IQR), ml | 50.0 (20.0–200.0) |
| Intraoperative blood transfusion, n (%) | 1 (3.3%) |
| Postoperative blood transfusion, n (%) | 1 (3.3%) |
| Length of postoperative hospital stay, mean (SD), day | 8.4 (3.3) |
| Length of tube drainage, median (IQR), day | 5.0 (4.0–7.0) |
| Drainage volume in the first day, median (IQR), ml | 90.0 (10.0–210.0) |
| Drainage volume in the first three days, mean (SD), ml | 583.7 (298.9) |
| Total drainage volume, median (IQR), ml | 1180.0 (570.0–1550.0) |
| Intensive care unit stay, n (%) | 1 (3.7%) |
CIT, chemoimmunotherapy; IQR, interquartile range; SD, standard deviation.
Duration of neoCIT and surgery >42 days occurred in two patients all due to coronavirus disease 2019 (COVID-19) infection.
Among the 27 per-protocol patients, 12 (44.4%, 95% CI, 27.6–62.7%; 7 in the first stage and 5 in the second stage) achieved an MPR, of whom 6 reached a pCR (22.2%, 95% CI, 10.6–40.8%; Fig. 2, Supplementary Tables S7 and S8). MPR status (Table 3) was not related to the PD-L1 TPS (<1% vs. ≥1%, P = 0.614; <50% vs. ≥50%, P = 0.873), EGFR mutation status (P = 0.756), EML4-ALK translocation status (P = 0.487), tumor mutational burden (TMB) score (<10 vs. ≥10 mut/Mb, P = 0.441), or histological subtype (P = 0.103, Supplementary Table S9). However, the postoperative pathological findings were significantly correlated with the preoperative radiographic response (Pearson's correlation = 0.391, P = 0.044; Supplementary Figure S2B). Pathological TNM downstaging was achieved in 23 (85.2%) patients, including 7 patients (25.9%) whose tumors were downstaged to ypT0 (Supplementary Figure S3A) and 10 patients (37.0%) who achieved nodal clearance (Supplementary Figure S3B).
Table 3.
Association between clinical, radiographic, and pathological characteristics and a major pathological response (MPR) in the per-protocol population.
| Characteristic | Participants with MPR (N = 12) | Participants without MPR (N = 15) | P value |
|---|---|---|---|
| Age at diagnosis, n (%) | |||
| <60 years old | 2 (16.7%) | 5 (33.3%) | 0.408 |
| ≥60 years old | 10 (83.3%) | 10 (66.7%) | |
| Sex, n (%) | |||
| Female | 0 (0%) | 6 (40.0%) | 0.020 |
| Male | 12 (100%) | 9 (60.0%) | |
| ECOG performance status score, n (%) | |||
| 0 | 3 (25.0%) | 6 (40.0%) | 0.683 |
| 1 | 9 (75.0%) | 9 (60.0%) | |
| Smoking status, n (%) | |||
| Never | 2 (16.7%) | 8 (53.3%) | 0.107 |
| Current or former | 10 (83.3%) | 7 (46.7%) | |
| Pathological subtype, n (%) | |||
| Adenocarcinoma | 7 (58.3%) | 10 (66.7%) | 0.103 |
| Squamous cell carcinoma | 5 (41.7%) | 2 (13.3%) | |
| PPLELC | 0 (0%) | 3 (20.0%) | |
| Clinical T staging, n (%) | |||
| T1–2 | 1 (8.3%) | 2 (13.3%) | 0.681 |
| T3–4 | 11 (91.7%) | 13 (86.7%) | |
| Clinical N staging, n (%) | |||
| N2 | 11 (91.7%) | 13 (86.7%) | 0.681 |
| N3 | 1 (8.3%) | 2 (13.3%) | |
| Radiographic response status, n (%) | |||
| Partial response | 10 (83.3%) | 8 (53.3%) | 0.217 |
| Stable disease | 2 (16.7%) | 7 (46.7%) | |
| PD-L1 tumor cell proportion score, n (%) | |||
| <1% | 1 (8.3%) | 3 (20.0%) | 0.598 |
| 1–49% | 6 (50.0%) | 7 (46.7%) | |
| ≥50% | 4 (33.3%) | 5 (33.3%) | |
| Unknown | 1 (8.3%) | 0 (0%) | |
| PD-L1 combined positive score, n (%) | |||
| <10 | 2 (16.7%) | 3 (20.0%) | 0.519 |
| ≥10 | 9 (75.0%) | 12 (80.0%) | |
| Unknown | 1 (8.3%) | 0 (0%) | |
| EGFR mutations status, n (%) | |||
| No | 9 (75.0%) | 12 (80.0%) | 0.756 |
| Yes | 3 (25.0%) | 3 (20.0%) | |
| EML4-ALK translocation status, n (%) | |||
| No | 12 (100%) | 13 (86.7%) | 0.487 |
| Yes | 0 (0%) | 2 (13.3%) | |
| TMB, n (%) | |||
| <10 mut/Mb | 4 (33.3%) | 8 (53.3%) | 0.441 |
| ≥10 mut/Mb | 8 (66.7%) | 7 (46.7%) | |
ECOG, Eastern Cooperative Oncology Group; PPLELC, primary pulmonary lymphoepithelioma-like carcinoma; PD-L1, programmed cell death 1-ligand 1; EGFR, epidermal growth factor receptor; EML4-ALK, echinoderm microtubule-associated protein-like-4-anaplastic lymphoma kinase; TMB, tumor mutational burden; mut/Mb, mutation/megabase.
Six patients with EGFR mutations (L858R, n = 2; 19del, n = 1; L858R/G721A, n = 1; G719S/S768I, n = 1; A289V, n = 1) and two patients with EML4-ALK translocation completed the in-protocol surgery, and the corresponding MPR rates were 50.0% and 0%, respectively (Table 2).
Safety of adjuvant chemoimmunotherapy and maintenance treatment
Among the 27 patients who underwent surgery, 22 patients (85.2%) completed two cycles of adjuvant CIT, 3 patients (11.1%) discontinued the second cycle of adjuvant CIT most commonly because of grade 3–4 adjuvant treatment-related AEs, one patient (3.7%) completed two cycles of adjuvant chemotherapy alone because of persistent grade 2 hypothyroidism, and the remaining one patient refused any adjuvant therapy (Supplementary Table S10). Across the adjuvant CIT phase, the pooled incidence rates of any grade, grade 1–2, and grade 3–4 TRAEs in 24 patients were 92.3%, 69.2%, and 15.4%, respectively (Supplementary Table S11).
In the per-protocol population, 14 patients (51.9%) completed sintilimab maintenance treatment once every three weeks for up to one year, and three patients (11.1%) refused this treatment at the beginning (Supplementary Table S10). Disease recurrence (5 patients, 18.5%) was the most common reason for maintenance treatment discontinuation, followed by TRAEs (4 patients, 14.8%) and COVID-19 infection (1 patient, 3.3%). The median number of cycles for maintenance treatment was 13 (interquartile range [IQR], 5.5–16.5). The sintilimab maintenance treatment-related AEs are summarized in Supplementary Table S12. Notably, one patient died from a potentially immune-mediated myocarditis after 7 cycles of maintenance treatment.
Follow-up
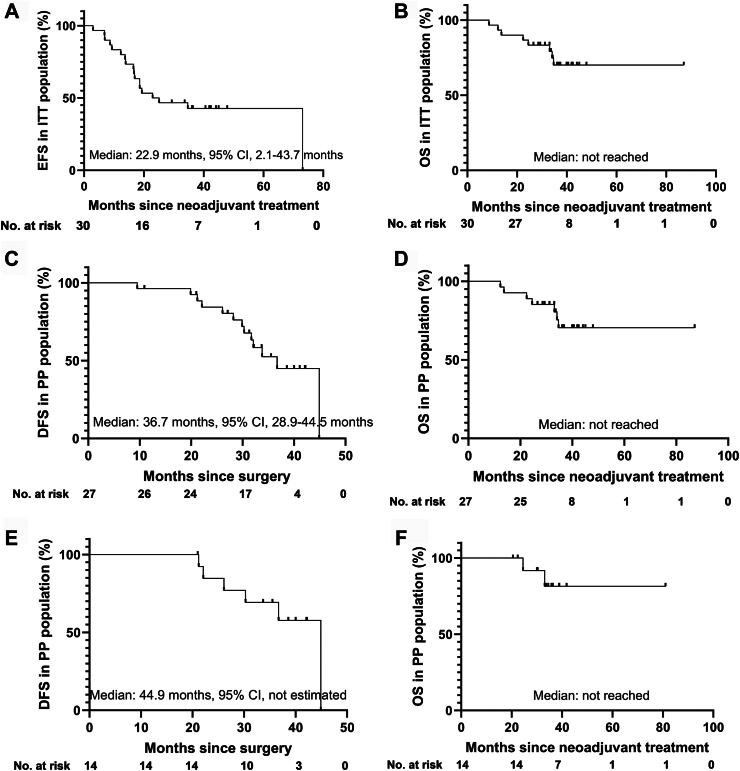
As of October 31, 2024, the median follow-up of the entire ITT population was 34.7 months (IQR, 29.1–40.9 months). A total of 18 patients (60.0%) experienced an event or died, and most of the primary events were disease progression or recurrence (16/18, 88.9%, Supplementary Table S13). The estimated EFS rates at 12, 24, and 36 months were 83.3%, 50.0%, 42.8%, respectively (Fig. 3A), and the estimated median EFS was 34.7 months in patients who underwent surgery and 6.8 months in patients who did not undergo surgery (P < 0.001). Five patients died from lung cancer, one patient died because of immune-mediated myocarditis, one patient died due to acute myocardial infarction, and one patient died from COVID-19 (Supplementary Table S13). The estimated 12-month, 24-month, and 36-month OS rates were 96.7%, 86.7%, and 70.1%, respectively (Fig. 3B).
Fig. 3.
Event-free survival (EFS, A) and overall survival (OS, B) in the intention-to-treat (ITT) population population, respectively; disease-free survival (DFS, C) and OS (D) in the per-protocol (PP) population, respectively; DFS (E) and OS (F) in patients who completed the scheduled perioperative treatment regimen.
In the per-protocol population, 13 patients (48.1%) experienced disease progression including 6 (46.2%) with distant metastases alone, 6 (46.2%) with distant and simultaneous locoregional metastases, and one (7.6%) with separate lung metastasis alone (Supplementary Table S13). Among the 12 patients who achieved MPR, disease recurrence was recorded in four patients (33.3%), and death occurred in two patient (16.7%). The calculated 12-, 24- and 36-month rates of DFS and OS were 96.3%, 84.4% and 52.9%, 100%, 88.9% and 70.4%, respectively (Fig. 3C and D). In addition, the median DFS and OS of the 14 patients who completed the scheduled perioperative treatment regimen were 44.9 months and not reached (Fig. 3E and F). No significant differences were observed between the MPR and non-MPR groups in terms of median DFS (46.0 vs. 37.2 months, P = 0.180) or OS (all not reached, P = 0.324; Supplementary Figure S4).
Discussion
This is a distinctive clinical trial to evaluate the safety and efficacy of perioperative CIT specifically in patients with potentially resectable stage IIIB NSCLC. The neoadjuvant regimen of two cycles of sintilimab plus platinum-based chemotherapy did not increase the frequency (any grade, 86.7%) or severity (≥3 grade, 6.7%) of TRAEs, which was similar to the findings in patients treated with the same number of cycles of neoCIT (any grade, 23.0–96.4%; and ≥3 grade, 4.5–31.0%).6,12,14,15 No previously unreported toxic effects were documented, and TRAEs did not impede the planned surgery. Furthermore, 74.1% and 77.8% of the per-protocol patients received minimally invasive surgery and lobectomy, respectively. This trial reached its primary endpoint with 7 and 5 patients achieved an MPR in the first and second stage, respectively. In addition, 21 (77.8%) and 14 (51.9%) patients completed the planned cycles and doses of adjuvant CIT and sintilimab maintenance treatment, respectively.
Although stage IIIB NSCLC is generally regarded as inoperable or unresectable disease and is managed mainly with definitive chemotherapy or chemoradiotherapy, several population-based retrospective studies and a phase II prospective trial have indicated that the addition of radical resection as a part of trimodal treatment in highly selected patients with technically resectable stage IIIB NSCLC could improve survival, with a median OS ranging from 15.1 to 40.0 months and a 3-year OS rate ranging from 15% to 56%.16, 17, 18 However, owing to the lack of significant advances in shrinking or downstaging primary lesions and regional metastatic lymph nodes in recent years, fewer than 10% of patients with stage IIIB disease receive surgical treatment and the 5-year survival rate remains unsatisfactory (approximately 26%).1 Recently, numerous randomized phase III trials comparing perioperative immune checkpoint inhibitors (ICIs) with placebo in combination with chemotherapy for resectable NSCLC have enrolled patients with stage IIIB (T3-4N2M0) disease. Subgroup analyses revealed that patients with technically resectable stage III or IIIB disease after perioperative ICI therapy not only exhibited good radiographic response (ORR, 64.0%–72.1%)3,8 but also had greater magnitudes of oncological benefit (EFS and pCR) than those with stage II disease, which probably translated into survival improvement in the follow-up/surveillance period.19,20 In addition, a retrospective cohort study reported that 31 (60.8%) of 51 patients with initial unresectable stage IIIB NSCLC (T3-4N2M0 or T2-4N3M0) underwent curative resection after at least 2 cycles of ICIs plus platinum-based chemotherapy, and the treatment strategy of induction CIT followed by surgery should be considered in these locally advanced NSCLC patients to prolong the median DFS of 27.5 months.4 Similarly, the 3-year DFS rate of 52.5% and OS rate of 70.4% in the per-protocol population from our present trial were among the best results reported in patients with stage IIIB NSCLC to date.2,4,5,17,18
A phase 1b study reported that two cycles of neoadjuvant sintilimab in adults with treatment-naïve, surgically resectable NSCLC (stage IA-IIIB) achieved an ORR of 20.0%, an MPR rate of 40.5%, and a pCR rate of 16.2%, which were highly similar to results from the earliest trial regarding neoadjuvant PD-1 blockade in resectable NSCLC (the ORR was 10.0%, the MPR rate was 45%, and the pCR rate was 14.3%).12,21 Simultaneously, two trials of at least three cycles of ICIs plus chemotherapy in the neoadjuvant setting revealed that 63.3–76.1% of patients achieved an objective response, 56.7–82.9% achieved an MPR, and 33.3–63.4% achieved a pCR. Although combination regimens and increased doses could result in better radiological and pathological responses in numerical terms, the increased incidences of any (93% vs. 22.7–52.5%) and ≥3 grade (30% vs. 5–10%) TRAEs and the decreased rate of completion of scheduled surgery (86.7–92.5% vs. 89–97%) should be noted.3,13 Therefore, the optimal number of cycles and regimen of neoadjuvant treatment to balance efficacy and toxicity were fully considered during our study design phase. As we expected, two cycles of sintilimab plus platinum-based chemotherapy in the present study were well tolerated, and any and ≥3 grade TRAEs were observed in 86.7% and 6.7% of participants, respectively. Moreover, no TRAEs related to surgical delay or cancellation occurred, and postoperative complications were common and manageable. The ORR, MPR rate and pCR rate reached 63.3%, 40% and 20%, respectively, in our study, which was consistent with the findings of the recently published neoSCORE trial, which explored the outcomes of two (50.0%, 26.9% and 19.2%) vs. three (55.2%, 41.4% and 24.1%) cycles of neoadjuvant sintilimab plus chemotherapy in patients with resectable IB-IIIA NSCLC.15 In addition, the interim analysis results of the LCMC3 trial indicated that patients who received adjuvant atezolizumab as maintenance therapy tended to have improved survival.22 Thus, patients with locally advanced NSCLC in the current study received implemented sintilimab combined with chemotherapy for 2 cycles before surgery and 2 cycles after surgery, followed by sintilimab monotherapy for up to 1 year. In recent years, the above-described perioperative approaches have been explored in four large-scale phase III trials and are expected to become the standard of care for patients with resectable NSCLC.20,23, 24, 25
Considering the low efficacy, limited survival benefit, and high cost of ICIs in locally advanced or metastatic NSCLC accompanied by common targetable gene mutations, almost all neoadjuvant immunotherapy trials have excluded patients with resectable disease with known gene mutations.9,19,20,24, 25, 26 However, in a pilot trial to test the activity of atezolimuab with carboplatin and nab-paclitaxel as preoperative treatments for resectable NSCLC, Catherine et al. reported that 2 of the 4 patients with EGFR mutations achieved a pCR.13 Given the nonimproved ORR (54.1% vs. 34.3%, P = 0.092) and low MPR rate (9.7% vs. 0%) in patients with stage IIIA(N2) NSCLC who received neoadjuvant EGFR tyrosine kinase inhibitor (EGFR-TKI) or chemotherapy,27 patients with potentially resectable locally advanced NSCLC regardless of their molecular gene status were given perioperative CIT in our study with the intent of improving radiological and pathological responses. An MPR was achieved in three (50.0%) of the six patients harboring EGFR mutations, which was comparable to that reported in the real-world study (42.1%) and the NEOTIDE trial (44.4%).28,29 Moreover, the R0 resection and N downstaging rates of patients with EGFR mutations were 100% and 33.3%, respectively, after neoadjuvant CIT in the current study; and 73.0% and 10.8%, respectively, after neoadjuvant EGFR-TKI treatment in the EMERGING-CTONG1103 study. In addition, consistent with the post-hoc analysis results from the NADIM study demonstrated that patients with EGFR mutations or EML4-ALK translocation had no difference in MPR rates (37.5% vs. 47.4%, P = 0.696) but had worse DFS (median: 29.9 vs. 44.9 months, P = 0.041) than those without mutations. Although none of the two patients with EML4-ALK rearrangement in the current study achieved an MPR, they all had been observed radiological objective response and pathological downstaging. Similarly, no tumor with ALK rearrangements demonstrated an MPR in the LCMC3 (0/6) and neoSCORE (0/1) studies.15,22 Taken together, it remains controversial whether patients with targetable gene mutations are candidates for neoadjuvant immunotherapy because of the small number of patients enrolled. In future trials, driver gene alterations, TMB, and the tumor immune microenvironment should be comprehensively considered and explored to guide personalized neoadjuvant and adjuvant strategies.
This phase II trial had several limitations, and the novel perioperative approach for potentially resectable stage IIIB NSCLC should be practiced with caution. First, it was an open-label, single-arm study with a small number of patients and a short follow-up period. Second, in contrast with the inclusion criteria of other studies, patients were enrolled in the present study regardless of gene mutation status, resulting in a high proportion of patients with EGFR mutations (26.7%) or EML4-ALK translocation (6.7%). Third, mediastinoscopy was not routinely used as an option for evaluating the mediastinal lymph nodes at baseline, which means that those lymph nodes far from the bronchi were not accurately evaluated pathologically. Fourth, PET/CT scans were not performed after the completion of neoCIT or prior to surgery to evaluate the response status because of the high cost. Nevertheless, the findings of promising radiographic and pathological downstaging, comparable radical resection rates, and tolerable side effects in our study have a considerable impact on future large-scale, randomized controlled trials designed to confirm the role of perioperative CIT in downstaging tumors, decreasing recurrence risk, and curing locally advanced NSCLC.
In conclusion, perioperative sintilimab plus platinum-based chemotherapy treatment of potentially resectable stage IIIB NSCLC yielded an MPR of 44.4%, included few toxic effects, facilitated a high radical resection rate, and exhibited promising long-term benefits.
Contributors
Jie He, Zhentao Yu and Kai Ma: conceptualization, design, funding acquisition, and supervision; Xiangyang Yu, Chujian Huang, Longde Du, Chunguang Wang, Yikun Yang, Xin Yu and Shengcheng Lin: data collection, follow-up, and data analysis; Chenglin Yang, Hongbo Zhao, Songhua Cai and Zhe Wang: data validation, investigation, and formal analysis; Lixu Wang, Xiaotong Guo and Baihua Zhang: data validation, formal analysis, and data interpretation; Xiangyang Yu, Longde Du and Chujian Huang: data access, data verification, and writing (original manuscript); all authors: writing (review and editing).
Data sharing statement
Datasets generated during the current study are available upon reasonable request.
Declaration of interests
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest, and no competing interests.
Acknowledgements
We wish to express our sincere gratitude to the patients and their families who kindly participated in the study. In addition, we also would like to thank all nurses who participated in this study for their spontaneous cooperation, Dr. Jianhua Chang and Dr. Shi Jin for recording, grading and managing the TRAEs, Dr. Dehong Luo and Dr. Zhou Liu for evaluating the radiographic response status, Dr. Jiping Da and Dr. Xiaoliang Wang for diagnosing the surgically resected specimens. This is an investigator-initiated trial, and sintilimab (PD-1 inhibitor) applied in the current study is provided by Innovent Biologics Co., Ltd, Suzhou, China.
Part result of this study was present at the 104th Annual Meeting of the American Association of Thoracic Surgery (AATS, Toronto, Canada, April 27-30, 2024) during the late breaking prospective clinical trials session.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102997.
Contributor Information
Jie He, Email: prof.jiehe@gmail.com.
Kai Ma, Email: makai@cicams-sz.org.cn.
Appendix A. Supplementary data
References
- 1.Goldstraw P., Chansky K., Crowley J., et al. The IASLC lung cancer staging Project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Spigel D.R., Faivre-Finn C., Gray J.E., et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Provencio M., Nadal E., Insa A., et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(11):1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 4.Deng H., Liu J., Cai X., et al. Radical minimally invasive surgery after immuno-chemotherapy in initially-unresectable stage IIIB non-small cell lung cancer. Ann Surg. 2022;275(3):e600–e602. doi: 10.1097/SLA.0000000000005233. [DOI] [PubMed] [Google Scholar]
- 5.Sun C., Liu Y., Zhang P., et al. Interim analysis of the efficiency and safety of neoadjuvant PD-1 inhibitor (sintilimab) combined with chemotherapy (nab-paclitaxel and carboplatin) in potentially resectable stage IIIA/IIIB non-small cell lung cancer: a single-arm, phase 2 trial. J Cancer Res Clin Oncol. 2023;149(2):819–831. doi: 10.1007/s00432-021-03896-w. [DOI] [PubMed] [Google Scholar]
- 6.Huang X., Zhu L., Liu J., et al. Safety and efficacy of tislelizumab plus chemotherapy as preoperative treatment in potentially resectable locally advanced non-small cell lung cancer patients. Interdiscip Cardiovasc Thorac Surg. 2024;38(1) doi: 10.1093/icvts/ivad157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provencio M., Serna-Blasco R., Nadal E., et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non-small-cell lung cancer (NADIM phase II trial) J Clin Oncol. 2022;40(25):2924–2933. doi: 10.1200/JCO.21.02660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei J., Zhao J., Gong L., et al. Neoadjuvant camrelizumab plus platinum-based chemotherapy vs chemotherapy alone for Chinese patients with resectable stage IIIA or IIIB (T3N2) non-small cell lung cancer: the TD-FOREKNOW randomized clinical trial. JAMA Oncol. 2023;9(10):1348–1355. doi: 10.1001/jamaoncol.2023.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y., Wang Z., Fang J., et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11) J Thorac Oncol. 2020;15(10):1636–1646. doi: 10.1016/j.jtho.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell T.R., Thompson E.D., Forde P.M., et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann Oncol. 2018;29(8):1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forde P.M., Chaft J.E., Smith K.N., et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu C.A., Gainor J.F., Awad M.M., et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–795. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y., Li X., Liu B., et al. Association of early immune-related adverse events with treatment efficacy of neoadjuvant Toripalimab in resectable advanced non-small cell lung cancer. Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1135140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao M., Yao J., Wang Y., et al. Two vs three cycles of neoadjuvant sintilimab plus chemotherapy for resectable non-small-cell lung cancer: neoSCORE trial. Signal Transduct Targeted Ther. 2023;8(1):146. doi: 10.1038/s41392-023-01355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitz C.C., Maas K.W., Van Swieten H.A., de la Rivière A.B., Hofman P., Schramel F.M. Surgery as part of combined modality treatment in stage IIIB non-small cell lung cancer. Ann Thorac Surg. 2002;74(1):164–169. doi: 10.1016/s0003-4975(02)03647-0. [DOI] [PubMed] [Google Scholar]
- 17.Collaud S., Provost B., Besse B., et al. Should surgery be part of the multimodality treatment for stage IIIB non-small cell lung cancer? J Surg Oncol. 2018;117(7):1570–1574. doi: 10.1002/jso.25042. [DOI] [PubMed] [Google Scholar]
- 18.Stupp R., Mayer M., Kann R., et al. Neoadjuvant chemotherapy and radiotherapy followed by surgery in selected patients with stage IIIB non-small-cell lung cancer: a multicentre phase II trial. Lancet Oncol. 2009;10(8):785–793. doi: 10.1016/S1470-2045(09)70172-X. [DOI] [PubMed] [Google Scholar]
- 19.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heymach J.V., Harpole D., Mitsudomi T., et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672–1684. doi: 10.1056/NEJMoa2304875. [DOI] [PubMed] [Google Scholar]
- 21.Gao S., Li N., Gao S., et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2020;15(5):816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Kwiatkowski D.J., Rusch V.W., Chaft J.E., et al. Neoadjuvant atezolizumab in resectable non-small cell lung cancer (NSCLC): interim analysis and biomarker data from a multicenter study (LCMC3) J Clin Oncol. 2019;37(15) suppl.8503. [Google Scholar]
- 23.Wakelee H., Liberman M., Kato T., et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491–503. doi: 10.1056/NEJMoa2302983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cascone T., Awad M.M., Spicer J.D., et al. Perioperative nivolumab in resectable lung cancer. N Engl J Med. 2024;390(19):1756–1769. doi: 10.1056/NEJMoa2311926. [DOI] [PubMed] [Google Scholar]
- 25.Lu S., Zhang W., Wu L., et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201–211. doi: 10.1001/jama.2023.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisberg A., Cummings A., Goldman J.W., et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol. 2018;13(8):1138–1145. doi: 10.1016/j.jtho.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong W.Z., Chen K.N., Chen C., et al. Erlotinib versus gemcitabine plus cisplatin as neoadjuvant treatment of stage IIIA-N2 EGFR-mutant non-small-cell lung cancer (EMERGING-CTONG 1103): a randomized phase II study. J Clin Oncol. 2019;37(25):2235–2245. doi: 10.1200/JCO.19.00075. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C., Chen H.F., Yan S., et al. Induction immune-checkpoint inhibitors for resectable oncogene-mutant NSCLC: a multicenter pooled analysis. NPJ Precis Oncol. 2022;6(1):66. doi: 10.1038/s41698-022-00301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., Sun Y.X., Yi D.C., et al. Neoadjuvant sintilimab plus chemotherapy in EGFR-mutant NSCLC: phase 2 trial interim results (NEOTIDE/CTONG2104) Cell Rep Med. 2024;5 doi: 10.1016/j.xcrm.2024.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.