Summary
5-Aminolevulinic acid (5-ALA) is an essential compound in the biosynthesis of heme, playing a critical role in various physiological processes within the human body. This review provides the thorough analysis of the latest research on the molecular mechanisms and potential therapeutic benefits of 5-ALA in managing metabolic disorders.
The ability of 5-ALA to influence immune response and inflammation, oxidative/nitrosative stress, antioxidant system, mitochondrial functions, as well as carbohydrate and lipid metabolism, is mediated by molecular mechanisms associated with the suppression of the transcription factor NF-κB signaling pathway, activation of the transcription factor Nrf2/heme oxygenase-1 (HO-1) system leading to the formation of heme-derived reaction products (carbon monoxide, ferrous iron, biliverdin, and bilirubin), which may contribute to HO-1-dependent cytoprotection through antioxidant and immunomodulatory effects. Additionally, it regulates the expression of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, cytochrome c oxidase subunit IV, uncoupling proteins UCP1 and UCP2, glucose transporters GLUT1 and GLUT2, and sterol regulatory element-binding protein 1c in relevant tissues. Randomized controlled trials have confirmed the effects of 5-ALA on glucose control in both prediabetic and diabetic patients, noting its safety and tolerability, as well as the safety of its combined use with oral hypoglycemic agents. Only minor side effects have been reported. However, the impact of 5-ALA on markers of systemic inflammation, oxidative and nitrosative stress, and dyslipidemia was not evaluated in these studies. At the same time, preparations of 5-ALA may potentially be effective not only in the treatment of prediabetes and type 2 diabetes mellitus (T2DM), but also in other conditions associated with systemic inflammation, oxidative or nitrosative stress, mitochondrial dysfunction, as well as disorders of carbohydrate and lipid metabolism.
It has been concluded that the promising advancement of formulations containing 5-ALA may pave the way for new strategies in preventing and treating these diseases, with subsequent preclinical and clinical trials likely to follow.
Subject areas: health sciences, medicine, medical specialty, therapeutics, natural sciences, biological sciences, physiology, endocrinology
Graphical abstract
Health sciences; Medicine; Medical specialty; Therapeutics; Natural sciences; Biological sciences; Physiology; Endocrinology.
Introduction
5-Aminolevulinic acid (5-ALA), also known as δ-aminolevulinic acid or 5-amino-4-oxopentanoic acid (IUPAC name), is an intriguing oxygen- and nitrogen-containing hydrocarbon that plays pivotal roles in biological systems. It serves as the universal precursor for a range of tetrapyrrole compounds, including essential molecules such as chlorophyll, heme, and vitamin B12.1,2 Synthesized across diverse kingdoms of life, including animals, plants, bacteria, and fungi, 5-ALA exhibits a versatile presence in nature. The abundance and ubiquity of 5-ALA are underscored by its endogenous nature, making it non-toxic to both humans and animals. Furthermore, its tendency to degrade easily in the environment, leaving behind no harmful residues, has garnered significant attention in recent scientific discourse.
There are two primary categories of 5-ALA applications in medical and healthcare settings. The first involves its use in combination with irradiation techniques, such as photoactivation, sonoactivation, or radioactivation, for treating malignant and inflammatory diseases. This approach leverages the accumulation of protoporphyrinogen IX, derived from 5-ALA, within the cytoplasm and mitochondria under conditions of anaerobic or aerobic glycolysis that are uncoupled with mitochondrial oxidative phosphorylation.3,4 Recent studies have collectively demonstrated the therapeutic efficacy of photodynamic therapy (PDT) with 5-ALA in treating a wide range of conditions, including keratosis, onychomycosis, lymphomas, meningiomas, malignant gliomas, basal cell carcinoma, Barrett’s esophagus, bladder cancer, brain tumors, gastric and other tumors, and in cosmetic procedures, shedding light on its potential as a cornerstone in medical interventions.4,5,6,7,8,9,10,11
Another approach to 5-ALA applications involves the production of heme in the presence of iron and its subsequent degradation by heme oxygenase (HO)-1.12,13 Recent studies have highlighted potential mechanisms through which 5-ALA may exert beneficial effects in metabolic disorders, particularly prediabetes and type 2 diabetes mellitus (T2DM),14,15,16,17,18,19 obesity,20 and atherosclerosis.21,22 These include its ability to mitigate chronic low-grade systemic inflammation and oxidative or nitrosative stress-induced damage, improve mitochondrial functions and insulin sensitivity, enhance glucose uptake in peripheral tissues, as well as decrease levels of circulating triglycerides, thus improving lipid profiles.
Additionally, 5-ALA is used as a farm animal feed additive to enhance blood iron levels, improve immune responses and disease resistance, stimulate growth, and promote egg production and quality, as well as milk composition.23,24,25,26,27 These studies highlight the potential benefits of supplementing with 5-ALA to enhance animal well-being and productivity, ultimately benefiting the welfare and economic sustainability of farming practices.
This review dives into the latest research on the molecular mechanisms and potential therapeutic benefits of 5-ALA in managing metabolic disorders.
A comprehensive search was conducted through electronic databases such as PubMed, Scopus, Web of Science, and the Cochrane Library to identify relevant literature sources. Traditional manual searching was also performed to determine relevant reference lists using key terms and phrases associated with the research question: “aminolevulinic acid”; “5-amino-4-oxopentanoic acid”; “aminolaevulinate”; “heme metabolism”; “5-aminolevulinic acid synthase”; “oxidative stress” or “nitrosative stress” or “immune response” or “inflammation” or “mitochondrial” or “transcription factors” or “pathogenesis” or “pathophysiology” AND “metabolic syndrome” or “diabetes mellitus” or “atherosclerosis” or “metabolic disorder,” etc. All experimental and clinical studies, alongside review articles, editorials, patent documentation, and case reports, were included without any restrictions on publication dates. Additionally, textbooks and other reference materials in the fields of biochemistry, molecular biology, pharmacology, pharmacy, endocrinology, clinical and molecular medicine were consulted. The vector graphics editor CorelDRAW 11 was used for creating the figures.
Biochemical pathways of 5-aminolevulinic acid
The biochemical pathways governing the synthesis and metabolism of 5-aminolevulinic acid (5-ALA) are fundamental to understanding its role in cellular physiology and its potential therapeutic applications. As a crucial precursor in heme biosynthesis, 5-ALA occupies a central position in cellular metabolism, influencing various physiological processes.
Enzymatic reactions in 5-aminolevulinic acid synthesis
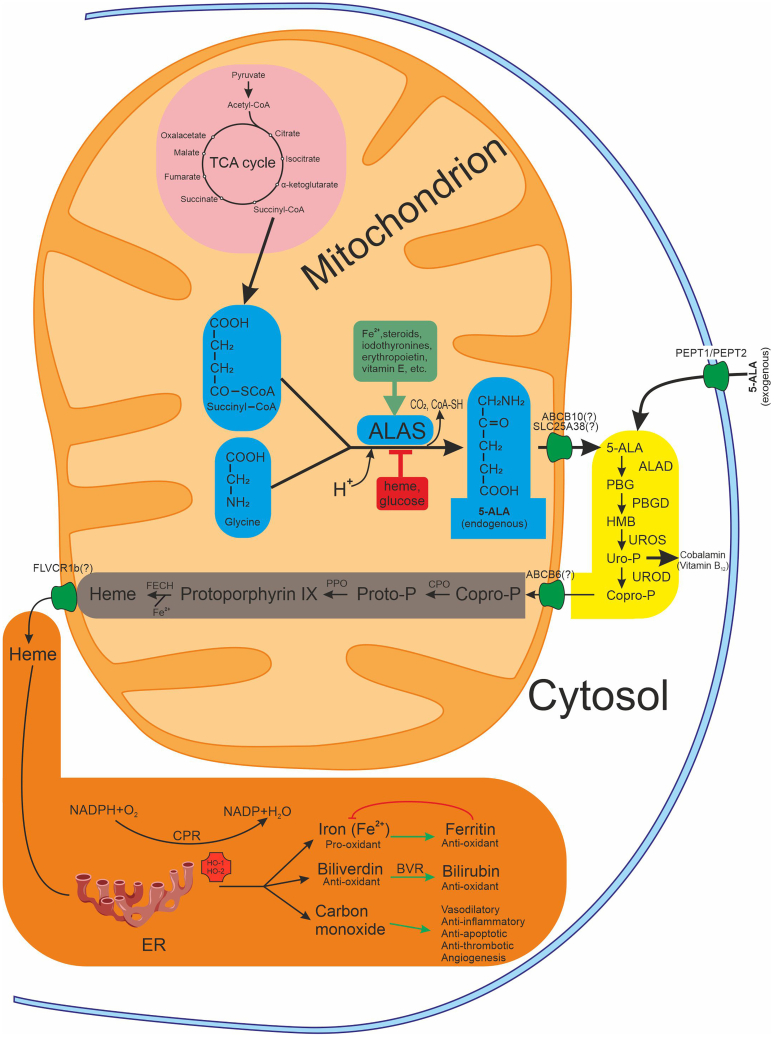
The 5-ALA synthesis is a critical process in cellular metabolism, serving as the precursor for heme, an essential molecule involved in oxygen transport, energy production, and numerous enzymatic reactions. The C4 (Shemin) pathway of 5-ALA biosynthesis (Figure 1) is characteristic not only of animals but also of yeast, some protozoa, and purple non-sulfur photosynthetic bacteria. Another pathway, known as the C5 pathway (also referred to as the Beale pathway), originated from the 5-ALA identification in Chlorella vulgaris.1 In the Shemin pathway, 5-aminolevulinic acid (5-ALA) is synthesized via an enzymatic reaction that occurs in the mitochondria. The synthesis of 5-ALA involves the condensation of glycine and succinyl-CoA, catalyzed by the enzyme 5-aminolevulinic acid synthase (ALAS), with pyridoxal 5′-phosphate serving as the co-factor.28 The production of succinyl-CoA is facilitated by methylmalonyl-CoA mutase, which relies on vitamin B12 as an indispensable co-factor. 5-ALA synthesis reaction represents a rate-limiting step in heme biosynthesis and is tightly regulated to maintain cellular heme homeostasis. ALAS exists in two isoforms: ALAS1, which is ubiquitously expressed and primarily responsible for heme synthesis in non-erythroid tissues, and ALAS2, which is predominantly expressed in erythroid cells.29 The activity of ALAS is regulated by various factors, including substrate availability, feedback inhibition by heme, and post-translational modifications, ensuring precise control over 5-ALA production.
Figure 1.
Biosynthetic reaction of the 5-ALA and its downstream pathways
The figure is divided into three parts: central carbon metabolic pathway—TCA cycle (pink), 5-ALA synthetic pathway (blue), cytoplasmic and mitochondrial sections of downstream pathways (yellow and gray, respectively), and heme downstream metabolic pathway (orange). The green line indicates positive regulation, the red line indicates inhibition. Abbreviations: ABCB6, ATP-binding cassette subfamily B member 6; ABCB10, ATP-binding cassette subfamily B member 10; 5-ALA, 5-aminolevulinic acid; ALAD, 5-aminolevulinic acid dehydratase; ALAS, 5-aminolevulinic acid synthase; BVR, biliverdin reductase; CoA-SH, coenzyme A; Copro-P, coproporphyrinogen; CPO, coproporphyrinogen oxidase; CPR, cytochrome P450 reductase; ER, endoplasmaic reticulum; FECH, ferrochelatase; FLVCR1b, feline leukemia virus subgroup C cellular receptor 1b; HMB, hydroxymethylbiline; HO-1, heme oxygenase-1; HO-2, heme oxygenase-2; NADP, nicotinamide adenine dinucleotide phosphate; PBG, porphobilinogen; PBGD, porphobilinogen deaminase; PEPT1, peptide transporter 1; PEPT2, peptide transporter 2; PPO, protoporphyrinogen oxidase; Proto-P, protoporphyrinogen; SLC25A38, solute carrier family 25 member 38; TCA, tricarboxylic acid; UROD, uroporphyrinogen decarboxylase; Uro-P, uroporphyrinogen; UROS, uroporphyrinogen III synthase.
Regulation of 5-aminolevulinic acid synthesis
The synthesis of 5-ALA is subject to intricate regulatory mechanisms that ensure its production is finely tuned to cellular demands.1,28,30 Several factors influence the ALAS activity, including substrate availability, feedback inhibition by the end-product heme, and hormonal and metabolic signals (Figure 1). The ALAS expression is regulated at the transcriptional and post-transcriptional levels by various factors, including transcription factors, microRNAs, and epigenetic modifications. The synthesis of ALAS, which acts as a rate-limiting enzyme, is highly regulated through feedback mechanisms involving its hemA and hemT genes.28
The decrease in ALAS is associated with the ability of heme to suppress mRNA synthesis of the gene for this enzyme through the inhibition of RNA polymerase activity. It is recognized that glucose can impact heme metabolism. High glucose load has been observed to effectively treat acute attacks of inducible hepatic porphyria. This beneficial impact of glucose is believed to occur through the down-regulation of ALAS1 facilitated by the peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α).31 The decrease in 5-ALA production may also result from an imbalance between the 5-ALA biosynthetic pathway and the tricarboxylic acid (TCA) cycle.32 Succinyl-CoA, a precursor of 5-ALA biosynthesis, acts as an intermediate in this cycle and plays a vital role in numerous metabolic processes.
Stimulators of ALAS induction include Fe2+ iron ions, steroid hormones (especially sex hormones), iodothyronines, vitamin E, and some drugs (barbiturates, estrogens, progestins, etc.).1,28,30,33,34 Most inducers affect ALAS synthesis similarly to lipophilic hormones (steroids), forming a ligand-receptor complex in the cytosol, which then translocates to the nucleus. Within the nucleus, the ligand-receptor complex acts as a transcriptional activator on the ALAS2 gene locus.
Physiological factors, such as blood oxygen saturation, also influence heme synthesis. Hypoxia-inducible factor-1-mediated ALAS2 upregulation has been demonstrated to enhance erythropoiesis, meeting the organism’s demands during conditions of low oxygen levels. This could be facilitated through elevated heme levels and an interaction between ALAS2 and erythropoietin.35
Numerous mechanisms regulate ALAS1 activity in response to heme levels: (1) transcriptional repression through a heme-responsive element; (2) post-transcriptional destabilization of ALAS1 mRNA; (3) post-translational inhibition via a heme regulatory motif; (4) direct inhibition of the activity of the enzyme, and (5) breakdown of ALAS1 protein via heme-mediated induction of the mitochondrial Lon peptidase 1.29
In erythroid cells, ALAS2 acts as a gatekeeper for producing large amounts of heme necessary for hemoglobin synthesis. Its synthesis rate transiently increases during active heme synthesis, regulated by the trans-activation of nuclear factor GATA1, CACC box, and NF-E2-binding sites in the promoter regions. ALAS2 mRNA translation is also controlled by the iron-responsive element/iron regulatory proteins binding system36 and microRNAs.37
The activity of ALAS is intricately regulated by post-translational modifications. For instance, the turnover of ALAS2 is controlled by the ubiquitin-proteasome system in the cytosol and by the caseinolytic mitochondrial matrix peptidase chaperone subunit X (CLPXP) in the mitochondria.38 Under normoxic conditions (21% O2), post-translational hydroxylation downregulates ALAS2 activity by promoting its ubiquitination and subsequent proteasomal degradation. The prolyl-4-hydroxylase/von Hippel-Lindau E3 ubiquitin ligase pathway is implicated in this process.39 Both ALAS proteins have mitochondrial targeting sequences that are believed to include heme-binding motifs. Two heme-binding motifs within the leader sequence, and one in the N-terminus of mature ALAS-1, are involved in heme-regulated translocation of ALAS1 in vivo.40 High heme concentrations promote its degradation by the CLPXP proteolytic complex and Lon protease within the mitochondria.30,38 Thus, post-translational modifications ensure precise regulation of ALAS activity, aligning heme biosynthesis with the cell’s physiological needs and stress responses.41
Transmembrane transportation of endogenous and exogenous 5-ALA
Once synthesized in the mitochondrial matrix, 5-ALA is transported to the cytosol. While there are several candidate proteins believed to be crucial for 5-ALA transport, none have been directly confirmed in vitro to possess 5-ALA transport activity.38 Evidence suggests that the protein SLC25A38 (solute carrier family 25 member 38) may act as a transporter of glycine and 5-ALA across the mitochondrial inner membrane.42,43 This transport mechanism constitutes a critical and rate-determining step within the heme biosynthetic pathway.43 Previously, it was hypothesized that the protein ABCB10 is involved in the export of 5-ALA from the mitochondria to the cytosol. This hypothesis was supported by the observation that the addition of 5-ALA rescues defects observed in cardiomyocytes.44 However, a subsequent study has refuted the hypothesis that ABCB10 transports 5-ALA.45
5-ALA cellular influx transporters PEPT1 (SLC15A1) and PEPT2 (SLC15A2) belong to the solute carrier family 15 (SLC15), also known as the proton-coupled oligopeptide transporter (POT) family.46,47,48,49 PEPT1 and PEPT2 are known to be the most well-studied transporters responsible for the uptake, distribution and reabsorption of di- and tripeptides in the body.49 The intestinal brush border membrane primarily mediates the uptake of exogenous 5-ALA via PEPT1, while PEPT2 facilitates its transport in the kidney, mammary gland, brain, lung, and other organs.46,47,48 PEPT1 and PEPT2 function as secondary active transporters using an inward-directed electrochemical proton gradient as an energy source, which provides a driving force for the transport and accumulation of substances against a concentration gradient, resulting in intracellular concentrations higher than those in the extracellular space.49
Significantly, PEPT1-mediated peptide uptake has been demonstrated to attenuate inflammatory signaling pathways, including those involving nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinases (MAPKs).50 This translates to a reduction in pro-inflammatory cytokine secretion and a decreased incidence of colitis in murine models.51
Metabolism of 5-aminolevulinic acid
Following its synthesis, 5-ALA, in the presence of ferrous iron, undergoes further enzymatic transformations to produce protoporphyrin IX, the immediate precursor of heme. This process involves a series of enzymatic reactions within the heme biosynthetic pathway, occurring sequentially in the cytoplasm and mitochondria (Figure 1). In the cytosol, 5-ALA undergoes sequential conversions to generate porphobilinogen, hydroxymethylbilane, uroporphyrinogen III, and ultimately coproporphyrinogen III. Porphyrinogens are synthesized through the condensation polymerization of eight molecules of 5-ALA. Within the mitochondrion, coproporphyrinogen III is metabolized to protoporphyrinogen IX and then to protoporphyrin IX. Iron is subsequently inserted into protoporphyrin IX through a reaction catalyzed by ferrochelatase, leading to the formation of heme.1,52
Each enzymatic step is tightly regulated, ensuring the efficient production of heme while preventing the accumulation of toxic intermediates. Notably, disturbances in heme biosynthesis can lead to various disorders, such as porphyrias, underscoring the importance of maintaining proper regulation of 5-ALA metabolism.
Heme downstream metabolic pathway
This pathway is essential for maintaining iron homeostasis and preventing the toxic accumulation of free heme (Figure 1), which can generate reactive oxygen species (ROS) and lead to cellular damage. Heme is transported out of mitochondria by FLVCR1b, a mitochondrial isoform of Feline leukemia virus subgroup C receptor 1 (FLVCR1). Overexpression of FLVCR1b stimulates heme synthesis and in vitro erythroid differentiation. Conversely, silencing FLVCR1b leads to mitochondrial heme accumulation and halted erythroid differentiation.53
The first and rate-limiting step in the heme degradation pathway is catalyzed by HO. This enzyme catalyzes the initial step in the oxidative breakdown of heme, converting it into ferrous iron (which is rapidly stored by ferritin), carbon monoxide (CO), and biliverdin-IXα, which is reduced to bilirubin-IXα by biliverdin reductase.54,55 There are two primary isoforms of heme oxygenase: HO-1 (inducible) and HO-2 (constitutive). HO-1 plays a particularly crucial role in cellular stress responses. Under conditions of oxidative stress, hypoxia, or inflammation, HO-1 expression is upregulated to protect cells from damage.56,57 HO-1 is strongly induced by a variety of stimuli, including its substrate heme, heat shock, heavy metal ions, ROS and reactive nitrogen species (RNS), inflammatory cytokines, and lipopolysaccharide (LPS).55
Oral administration of 5-ALA at 600 mg and sodium ferrous citrate (SFC) at 942 mg induced HO-1 expression in healthy human peripheral blood mononuclear cells at the 8-h time point. Neither 5-ALA nor SFC alone was able to induce HO-1 expression. Additionally, HO-1 was upregulated in blood myeloid and plasmacytoid dendritic cells following ALA+SFC treatment.58 Notably, HO-1 expression is regulated by transcription factors including NF-E2-related factor 2 (Nrf2), activator protein-1 (AP-1), and hypoxia-inducible factor, which integrate signaling pathway information at the HO-1 gene promoter.59
Recent decades have unveiled the beneficial properties of biliverdin, bilirubin, and biliverdin reductase A in a variety of biological processes, including their potent antioxidant capacities and immunomodulatory effects.55,60,61 CO, a gaseous signaling molecule, exhibits vasodilatory, anti-inflammatory, anti-apoptotic, anti-thrombotic, pro-angiogenic, and immunoregulatory properties.13,54,62,63 The levels of 5-ALA stored in the human body decline with age, resulting in diminished HO-1 expression within cells.
In summary, the heme downstream metabolic pathway, mediated by enzymes such as HO-1 and biliverdin reductase, plays a critical role in heme catabolism, protecting the body from heme-induced toxicity by degrading heme and generating biologically active molecules with significant physiological functions.
Physiological and pharmacological functions of 5-aminolevulinic acid
The synthesis of 5-ALA plays a crucial role in cellular metabolism and physiology, serving as the starting point for heme biosynthesis. Heme, also known as iron protoporphyrin IX, serves as a prosthetic group in various hemoproteins, including respiratory cytochromes, gas sensors, P450 enzymes (CYPs), catalase, peroxidases, nitric oxide synthase (NOS), guanylyl cyclase, and even transcription factors.52,53,54,55,56,57,58,59,60,61,62,63,64,65 Heme also functions as a regulatory molecule in various cellular processes, including gene expression, circadian rhythm regulation, and response to oxidative or nitrosative stress.66 Conversely, hemin, the oxidized form of iron protoporphyrin IX, acts as a vital regulator of gene expression and promotes the growth of hematopoietic progenitor cells.52
Effect of 5-aminolevulinic acid on immune response and inflammation
Only few clinical studies have investigated the beneficial effect of 5-ALA/SFC on biomarkers of systemic inflammatory response (SIR). Kaketani and Nakajima evaluated the efficacy of 5-ALA and SFC in treating COVID-19 patients with elevated C-reactive protein (CRP). Initial 5-ALA dosage was tailored to disease severity: 1,500 mg/day (500 mg thrice daily, approximately 25 mg/kg) for severe cases, and 750 mg/day (approximately 12.5 mg/kg) for mild cases.67
In an open-label, non-randomized pilot study, patients with moderate and severe COVID-19 received daily oral doses of 500 mg/750 mg 5-ALA and 286 mg/430 mg SFC, respectively, for the initial seven days. Subsequently, the dosage was reduced to 250 mg ALA and 143 mg SFC for the following 21 days in both groups. The severe group exhibited significantly lower levels of CRP, procalcitonin, and interleukin (IL)-6 compared to baseline. Notably, the severe group experienced a significantly shorter hospital stay (8 days) compared to the control group (16 days). Although these results are encouraging, larger-scale studies are needed to definitively establish the efficacy and safety of this treatment.68
However, most studies investigating the impact of 5-ALA on immune response and inflammation have been conducted in animal models and within the realm of veterinary medicine. Supplementation of 5-ALA in the diet of weaned castrated male pigs significantly increased levels of CD2+, CD8+, B cells, MHC-I, and MHC-II.69 5-ALA enhances white blood cell counts and granulocytes, as well as the rate of phagocytosis and mitogen-induced proliferation of peripheral blood mononuclear cells in cows.24 Additionally, it has been demonstrated that 5-ALA might be beneficial as an immunomodulator, stimulating T cell activity through mild oxidative stress in growing broiler chickens, consequently enhancing growth performance.23
5-ALA has emerged as a potential therapeutic agent for modulating LPS-induced SIR due to its anti-inflammatory properties. Studies investigating the effects of 5-ALA supplementation on inflammatory markers have yielded mixed results. Some trials have reported decreases in pro-inflammatory cytokine levels, such as IL-2 and IL-6, interferon-γ, inducible NOS, and tumor necrosis factor (TNF)-like ligand 1A mRNA expression, following LPS injection.23 The plasma ceruloplasmin oxidase activity, a SIR marker, was reported to decrease in piglets following supplementation with 5-ALA.70 Furthermore, the ingestion of 5-ALA through the diet led to a reduction in plasma cortisol and TNF-α levels 2 h after LPS challenge.71 However, other studies have shown no significant changes in various inflammatory markers, including lymphocyte counts, haptoglobin levels, and Toll-like receptors (TLR) 2, 4, and 7 mRNA expression.23,71,72 Some conflicting findings have been reported regarding the relative weight of immune organs, such as the spleen, liver, bursa of Fabricius, and thymus gland.23,73,74
In summary, the literature suggests that the effects of 5-ALA supplementation on LPS-induced systemic inflammation vary and depend on various factors. While some studies indicate potential anti-inflammatory effects of 5-ALA, others report conflicting findings. Further research is needed to elucidate the mechanisms underlying the effects of 5-ALA on LPS-induced inflammation and to determine its therapeutic potential in inflammatory conditions.
Recent findings indicate that 5-ALA has emerged as a promising therapeutic approach for a range of conditions, including inflammatory diseases, autoimmune disorders, and transplantation. This potential is attributed to its anti-inflammatory, immunoregulatory and antioxidant properties, which are mediated through the induction of HO-1.12,13,75 Numerous studies have demonstrated the safety of typical doses of 5-ALA supplementation in both animals and humans, along with its anti-inflammatory properties.13,68,76
Recent studies by Chinese researchers have demonstrated that supplementing with 5-ALA aids liver regeneration by enhancing anti-inflammatory macrophage activity and promoting tissue repair. In mouse models of hepatic ischemia-reperfusion, 5-ALA enhanced liver metabolism and decreased inflammatory responses by shifting macrophages toward an anti-inflammatory M2 phenotype.77 Additionally, the suppression of the C-X3-C motif chemokine receptor 1 (CX3CR1) boosts this regenerative effect by increasing levels of insulin-like growth factor 1 and hepatocyte growth factor, further supporting liver recovery. The combined treatment with applying 5-ALA and CX3CR1 inhibition showed superior outcomes in promoting liver regeneration compared to 5-ALA alone, highlighting a potential therapeutic strategy for liver transplant and resection patients.78
The 5-ALA combined with SFC may alleviate ischemia-reperfusion injury in the mouse fatty liver model by reducing the expression of TLR 2 and 4, NF-κB, inflammatory cytokines, and ROS production in Kupffer cells.79 In an experiment involving LPS-challenged broiler chickens, dietary supplementation of 5-ALA significantly mitigated the increase in mRNA expression levels of hepatic TLR4, IL-1β, and IL-2.27 Another study demonstrated that the ability of 5-ALA to inhibit NF-κB activation was accompanied by a reduction in inducible NOS and cyclooxygenase-2 protein expression, subsequently leading to decreased production of nitric oxide and prostaglandin E2.80,81 The authors attribute this effect to the ability of 5-ALA/SFC to suppress LPS-induced phosphorylation of IκB kinase (IKK), degradation of the inhibitory protein IκBα, and the NF-κB subunit, thereby preventing further translocation of NF-κB into the nucleus.
These findings suggest that 5-ALA has significant potential as a therapeutic tool for managing inflammatory diseases and their complications by modulating key signaling pathways involved in SIR. Its capacity to induce HO-1 and inhibit NF-κB activation makes it a strong candidate for treating various inflammatory conditions.
Effect of 5-aminolevulinic acid on oxidative/nitrosative stress and antioxidant system
Numerous studies have investigated the effects of 5-ALA on oxidative and nitrosative stress parameters in various experimental models.25,27 One research study observed a decrease in plasma ceruloplasmin oxidase activity, which typically elevates due to deficiency of antioxidant enzymes during iron deficiency, following 5-ALA administration (with or without iron).70 This suggests that 5-ALA may help regulate antioxidant activity, potentially mitigating the effects of iron deficiency. Another investigation found significantly lower levels of thiobarbituric acid reactive substances, lipid peroxidation products, in swine loin meat treated with a combination of 5-ALA and oriental medicinal plants compared to the control group.82 This implies that 5-ALA might aid in preserving tissues by mitigating oxidative damage.
A placebo-controlled, double-blind trial conducted on healthy volunteers revealed that supplementation with 5-ALA might enhance redox balance during high-intensity aerobic exercise.83 Specifically, the biological antioxidant potential and its ratio to diacron reactive oxygen metabolite showed significant differences in the onset of blood lactate accumulation state at week 4 in the group receiving 5-ALA compared to the placebo. A previous study conducted by Shinshu University in Japan demonstrated a decline in blood lactate concentrations among elderly female participants who took 5-ALA and SFC supplements.84,85
Some researchers suggest that 5-ALA, accumulated in acquired conditions like lead poisoning and inherited disorders such as intermittent acute porphyria, may serve as contributing sources of oxyradicals and oxidative stress in these diseases.86 However, having thoroughly examined various literature sources, Hendawy et al.25 concluded that the antioxidant properties of 5-ALA may be linked to its ability to induce a mild level of oxidative stress, leading to cellular preconditioning against ROS/RNS. Specifically, ROS/RNS induction supports the generation of antioxidants by activating Nrf2, enhances autophagy, and strengthens intracellular defenses against pathogens. LPS treatment is known to elevate ROS and RNS production, while the production of HO-1 increases following exposure to various stress stimuli. Moreover, in addition to their antioxidant properties, both HO-1 and Nrf2 inhibit oxidative/nitrosative stress and SIR by suppressing NF-κB.87,88,89,90,91,92
Given its antioxidant properties, 5-ALA holds promise as a potential therapeutic approach for preventing and managing a range of oxidative stress-related conditions, such as cardiovascular diseases, neurodegenerative disorders, metabolic syndrome, and T2DM.
Effect of 5-aminolevulinic acid on mitochondrial functions
5-ALA is an important precursor of heme, essential for the activities of mitochondrial respiratory complexes II, III, and IV, as well as cytochrome c. It exerts an influence on mitochondrial energy metabolism by enhancing adenosine triphosphate (ATP) production and promoting mitochondrial respiration. It has also been revealed that the heme synthetic enzyme ALAS, also known as Hem1 in yeast, along with guanosine triphosphatases (GTPases) that control mitochondrial dynamics machinery (Mgm1 and Dnm1) and endoplasmic reticulum contact sites (Gem1), play a role in regulating the flow of heme between the mitochondria and nucleus.93
Several studies have documented an elevation in ATP levels post-5-ALA treatment, indicating its ability to stimulate mitochondrial energy generation. Recently, it has been demonstrated that administering a combination of 5-ALA hydrochloride and SFC augments the activities of complexes II and IV, boosts ATP production, and alleviates defective phenotypes associated with complexes I deficiency in Drosophila.94
In another study, the effects of different concentrations of 5-ALA on fibroblasts from 8 individuals with mitochondrial diseases and healthy controls were investigated. Results showed that in normal fibroblasts, 5-ALA upregulated the expression levels of oxidative phosphorylation complex subunits and corresponding genes.95 Moreover, treatment with 5-ALA led to increased oxygen consumption rate and ATP levels in normal fibroblasts, as well as enhanced the levels of HO-1 protein and mRNA in all fibroblasts, and increased the relative mitochondrial DNA (mtDNA) copy number. These findings suggest that 5-ALA effectively enhances oxidative phosphorylation, HO-1 protein, and mtDNA.
It is noteworthy that the use of 5-ALA is considered a novel approach to anti-atherosclerotic therapy. Previous research identified mitochondrial mutations linked to atherosclerosis, leading to the hypothesis that targeting mitochondria burdened with these mutations could be beneficial. As a result, 5-ALA is being considered as a promising agent for this purpose.22 By targeting key aspects of mitochondrial biology, 5-ALA holds promise as a therapeutic agent for mitigating mitochondrial dysfunction-associated disorders, including metabolic diseases.
Effect of 5-aminolevulinic acid on carbohydrate and lipid metabolism
5-ALA has garnered significant attention in recent years due to its potential effects on carbohydrate metabolism. Research indicates that 5-ALA may play a role in modulating key pathways involved in glucose utilization and homeostasis. Studies have demonstrated that 5-ALA supplementation can improve glucose tolerance and insulin sensitivity in various animal models and human subjects. The data provides in vivo evidence indicating that 5-ALA deficiency diminishes mitochondrial function and leads to impaired glucose tolerance and insulin resistance (IR) in an age-dependent manner.96 Muscle mitochondrial activation in mice treated with 5-ALA is suggested to be beneficial for treating sarcopenia and glucose intolerance.97 In addition, supplementation with 5-ALA induces beneficial alterations in lipid metabolism, leading to decreased levels of circulating triglycerides and enhancements in lipid profiles.98
Recent studies have demonstrated that oral administration of 5-ALA may offer protection against mild hyperglycemia and serve as a preventive measure against T2DM.14,15,16,17,18,19 These investigations strongly suggest an association between 5-ALA or heme and glucose metabolism in vivo. In a study conducted on Zucker diabetic fatty rats, it was observed that the oral administration of 5-ALA in combination with SFC for 6 weeks led to a reduction in plasma glucose and hemoglobin A1c (HbA1c) levels, without impacting plasma insulin levels.99 Additionally, the 5-ALA/SFC treatment significantly improved glucose tolerance. Notably, the administration of 5-ALA/SFC induced the HO-1 expression in white adipose tissue and liver, and the levels of induced HO-1 expression correlated with the glucose-lowering effects of 5-ALA/SFC. These findings suggest that 5-ALA combined with ferrous iron effectively reduces hyperglycemia T2DM without affecting plasma insulin levels. The induction of HO-1 may play a role in the mechanisms underlying the glucose-lowering effect of 5-ALA/SFC.
Furthermore, heme has been shown to regulate hepatic glucose production by modulating the expression of key enzymes involved in gluconeogenesis and glycogenolysis. Animal studies have demonstrated that both 5-ALA and heme have the potential to decrease hepatic glucose output and enhance liver insulin sensitivity, thereby playing a role in maintaining overall glucose homeostasis. In hepatic cells, the transcriptional repressor Rev-erbα detects heme via its heme-binding domain, essential for its repressor function.100 This detection mechanism plays a crucial role in regulating glucose homeostasis by inhibiting glucose production and the expression of gluconeogenic genes such as glucose 6-phosphatase and phosphoenolpyruvate carboxykinase. Moreover, Rev-erbα controls circadian rhythms by regulating the Bmal1 gene and modulates energy metabolism by supplying heme for mitochondrial respiration, among other functions.101
There is growing evidence indicating that the combination of 5-ALA and SFC may have positive effects on the function and survival of pancreatic β-cells by enhancing mitochondrial function within these cells. Indeed, numerous studies have demonstrated the advantageous impact of 5-ALA/SFC on glycemic control among individuals with prediabetes and T2DM.14,15,16,17,18,19
The research conducted on obese Wistar rats fed a high-fat diet revealed that administering 5-ALA/SFC in different dosages daily for 6 months effectively reduced plasma glucose levels.102 The study showed a significant reduction in the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) index in the groups treated with 5-ALA/SFC, suggesting its effectiveness in improving IR. However, it also suggested that 5-ALA/SFC might have no impact on pancreatic β cells. Despite the enhancement of appetite by 5-ALA/SFC, reductions in both body weight and visceral fat were noted. The authors suppose that the promotion of appetite might rely on the regulation of two appetite-regulating factors, namely amylin and peptide YY. Additionally, adiponectin, monocyte chemotactic protein-1, and TNF-α may contribute to glucose and lipid metabolism. It is important to note that 5-ALA/SFC likely regulated the expression of cytochrome c oxidase subunit IV (COXIV), uncoupling protein 1 and 2 (UCP1 and UCP2), glucose transporter 2 (GLUT2), and sterol regulatory element-binding protein 1c (SREBP-1c) in relevant tissues. This regulation helps maintain homeostasis in mitochondrial metabolism, decrease accumulation of fat in the liver, reduce visceral fat deposition, and further enhance glucose and fat metabolism. These findings suggest that 5-ALA/SFC could ameliorate hyperglycemia and dyslipidemia, decrease fat accumulation and body weight by promoting mitochondrial activity, and modulating genes related to lipid and glucose metabolism.
5-ALA administration induced exogenous HO-1 production at plaque sites in low-density lipoprotein (LDL) receptor-deficient mice, leading to improved lipid profiles (including reduced oxidized LDL) and attenuated atherosclerotic plaque progression in vivo.21 Another investigation affirms that 5-ALA/SFC effectively decreased body weight, fat mass, hepatic lipid accumulation, and enhanced blood glucose levels and oral glucose tolerance test outcomes in diabetic mice subjected to a high-fat diet for 9 weeks.20 Furthermore, these substances inhibited the augmented glomerular tuft area, correlated with elevated HO-1 protein expression. Researchers concluded that 5-ALA/SFC shows promise in addressing conditions associated with obesity or T2DM, such as diabetic nephropathy and nonalcoholic fatty liver disease. It was found that 5-ALA activates the AMP-activated protein kinase (AMPK) signaling pathway, leading to increased lipolysis and fatty acid β-oxidation.103 Treatment of human hepatocarcinoma (HepG2) cells with 5-ALA resulted in elevated expression of lipolysis-related genes, including PGC-1α. These findings suggest that 5-ALA may have potential as a novel treatment for nonalcoholic fatty liver disease by restoring AMPK phosphorylation and acetyl-CoA levels, thereby enhancing the expression of PGC1α and carnitine palmitoyltransferase 1α.
These findings underscore the promise of 5-ALA as a therapeutic agent for preventing and managing T2DM and other metabolic disorders characterized by impaired carbohydrate and lipid metabolism.
Therapeutic applications of 5-aminolevulinic acid and its prospects as a treatment for metabolic disorders
The medical applications of 5-ALA extend beyond its primary role in heme synthesis, encompassing diverse therapeutic potentials. One notable application of 5-ALA is in PDT, utilized for treating various cancers, skin conditions, and cosmetic imperfections.4,5,104,105 In PDT, exogenously administered 5-ALA is preferentially taken up by tumor cells and metabolized to protoporphyrin IX, which upon activation by light leads to the generation of cytotoxic ROS, resulting in tumor cell death. Recently, the potential application of 5-ALA as a photosensitizer in photodynamic therapy for the comprehensive treatment of chronic wounds in patients with ischemic and mixed forms of diabetic foot syndrome has been identified.10 Combined light exposure using the photosensitizer not only stimulates the ROS formation but also enhances the effect of phagocytic cells. This enhancement is evident in the activation of neutrophil chemotaxis, adhesion, and endocytosis. The PDT utilizing 5-ALA as a photosensitizer augments the molecular mechanisms involved in intercellular interaction at all stages of primary immune system activation.
Additionally, 5-ALA has shown promise in the management of neurological disorders, such as Alzheimer’s disease, through its neuroprotective and anti-inflammatory effects.106,107 Findings suggest that 5-ALA and SFC may be promising therapeutic agents for combating SARS-CoV-2 infection by suppressing viral growth and mitigating the risk of post-COVID conditions.108,109,110 Moreover, emerging research suggests potential therapeutic avenues for 5-ALA in addressing prediabetes and T2DM,18,20,76,111,112,113 obesity,20 and cardiovascular diseases.22,114,115,116,117
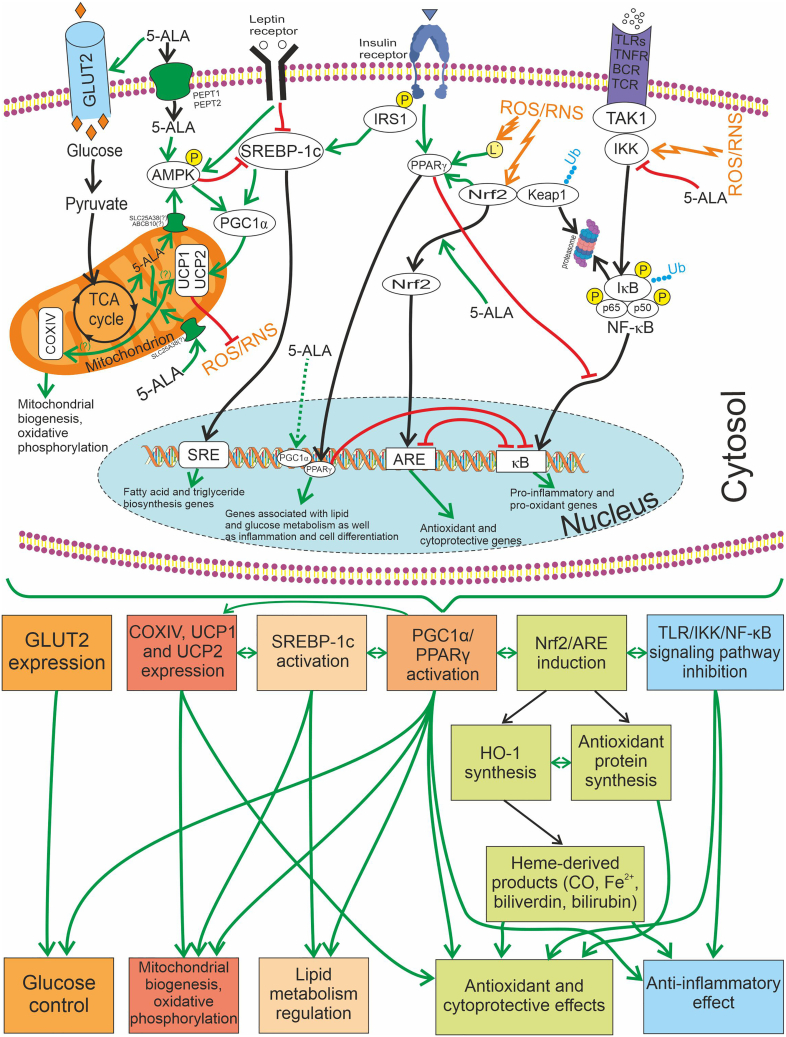
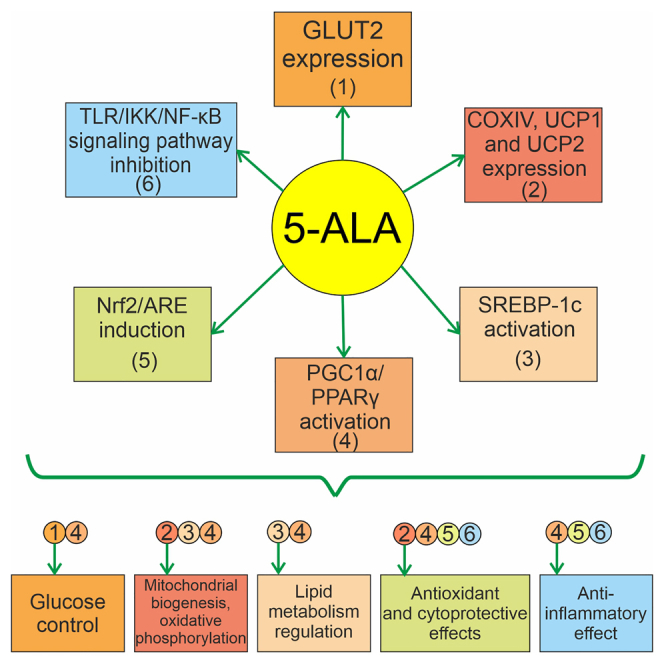
Current research supports the involvement of SIR, mitochondrial dysfunction, the production of damaging ROS/RNS, as well as decreased insulin secretion and increased IR in metabolic syndrome118,119,120,121,122 and T2DM.123,124 Examining the mechanism of action of 5-ALA, an amino acid synthesized in the mitochondria, and a novel supplement containing 5-ALA that has recently appeared on the market, research demonstrates its impact on diminishing pro-inflammatory and pro-oxidant TLR/NF-κB-dependent signaling pathways. Moreover, it activates anti-inflammatory signaling systems such as Nrf2-antioxidant responsive element, and induces HO-1, which breaks down heme into CO, biliverdin-IXα, and bilirubin-IXα, known to provide cytoprotective effects. The molecular mechanisms underlying the physiological and pharmacological effects of 5-ALA in SIR and metabolic disorders are schematically depicted in Figure 2.
Figure 2.
The molecular mechanisms underlying the physiological and pharmacological effects of 5-ALA (in the presence of Fe2+)
The green line indicates positive regulation, the red line indicates inhibition, the dashed line represents indirect effects. 5-ALA reveals an anti-inflammatory effect by inhibiting the NF-κB signaling pathway through IKK and activating PPARγ signaling (via PGC1α). Its impact on pro-oxidant cascades is mitigated by its ability to induce the Nrf2/ARE signaling pathway and PPARγ. Enhanced Nrf2-dependent synthesis of HO-1 leads to the production of heme-derived reaction products (CO, ferrous iron, biliverdin, and bilirubin), which may contribute to cytoprotection through antioxidant and immunomodulatory effects. Additionally, 5-ALA, whether imported into the mitochondrion exogenously (possibly via transport proteins like SLC25A38) or endogenously produced, regulates the expression of mitochondrial proteins (COXIV, UCP1, and UCP2), promoting mitochondrial biogenesis and activating oxidative phosphorylation. The increase in SREBP-1c expression driven by 5-ALA enhances the biosynthesis of fatty acids and triglycerides; however, it is subject to inhibitory control by another target of 5-ALA, AMPK. Furthermore, AMPK activates UCP1 and UCP2 via PGC1α. The decrease in mitochondrial membrane potential (ΔΨ) due to UCP2 may reduce the potential for ROS/RNS formation in mitochondria: this occurs by decreasing the efficiency of electron transport, which reduces the likelihood of free radical formation. By increasing the expression of GLUT1 and GLUT2, 5-ALA enhances the cellular uptake of glucose. Once inside the cell, glucose can undergo glycolysis, a metabolic process that generates ATP. This promotes glucose uptake and utilization, supporting the energy requirements of the cell, thus ensuring proper cellular function and metabolism. Abbreviations: ABCB10, ATP-binding cassette subfamily B member 10; 5-ALA, 5-aminolevulinic acid; AMPK, adenosine monophosphate-activated protein kinase; ARE, antioxidant response element; BCR, B cell receptor; COXIV, cytochrome c oxidase subunit IV; GLUT1, glucose transporter 1; GLUT2, glucose transporter 2; HO-1, heme oxygenase-1; IκB, NF-κB inhibitory protein; IKK, IκB kinase; IRS-1, insulin receptor substrate 1; Keap1, Kelch-like ECH-associated protein 1; L˙, lipid radical; NF-κB, nuclear factor κB; Nrf2, nuclear factor erythroid 2-related factor 2; P, phosphorylation; PEPT1, peptide transporter 1; PEPT2, peptide transporter 2; PGC1α, peroxisome proliferator-activated receptor γ coactivator 1α; PPARγ, peroxisome proliferator-activated receptor γ; ROS, reactive oxygen species; RNS, reactive nitrogen species; SLC25A38, solute carrier family 25 member 38; SRE, sterol regulatory element; SREBP-1c, sterol regulatory element-binding protein 1c; TAK1, transforming growth factor-β-activated kinase; TCA, tricarboxylic acid; TCR, T cell receptor; TLRs, Toll-like receptors; TNFR, tumor necrosis factor receptor; Ub, ubiquitination; UCP1, uncoupling protein 1; UCP 2, uncoupling protein 2.
The effectiveness of 5-ALA/SFC application in prediabetes and T2DM, including those treated with oral hypoglycemic agents, is confirmed by clinical and experimental studies (Table 1).
Table 1.
Efficacy of 5-ALA/SFC in treatment of metabolic disorders and its mechanisms
| Metabolic disorder | Mechanism of action of 5-ALA | Reference |
|---|---|---|
| T2DM conditions in Otsuka Long-Evans Tokushima Fatty rats | 5-ALA ameliorates diabetic abnormalities in rats by reducing visceral fat mass (in the retroperitoneal region) through a decrease in adipocyte mitochondrial content | Sato et al.111 |
| Lipid accumulation in hepatocytes and hyperglycemia during obesity and T2DM | 5-ALA administration with ferrous citrate increases aerobic glycolysis, lipolysis and leads to higher expression of HO-1 and UCP1 | Kamiya et al.20 |
| Muscle and mitochondrial abnormalities during T2DM | 5-ALA administration with ferrous citrate changes skeletal muscle transcriptome leading to increased expression of glucose uptake and mitochondrial oxidative phosphorylation-related genes | Kitamura et al.112 |
| Decreased glucose tolerance during high-fat diet | 5-ALA administration with ferrous citrate increases GLUT1 translocation in the plasma membrane without affecting GLUT1 expression | Kuroda et al.113 |
| Increased fasting blood glucose level and decreased glucose tolerance in the patients with mitochondrial diabetes mellitus | 5-ALA administration with ferrous citrate reduces blood glucose levels and improves glucose tolerance by enhancing mitochondrial function | Nakamura et al.18 |
| Increased intensity of anaerobic glycolysis during breast cancer | 5-ALA administration leads to increased heme synthesis, which destabilizes Bach1 and decreases anaerobic glycolysis in favor of TCA cycle activation through AMPK | Kaur et al.125 |
| Endoplasmic reticulum stress caused by excessive intake of palmitic acid | 5-ALA induces HO-1 expression and decreases Bach1 expression | Hamada et al.126 |
Clinical studies have demonstrated that the use of 5-ALA offers the benefit of minimal adverse effects, as its safety and tolerance have been established in clinical trials.14,15,16,17,18,19,127,128 Additionally, it is expected to be more cost-effective compared to certain oral hypoglycemic agents.16,17 However, further research is required to confirm the efficacy of 5-ALA in diabetes treatment, particularly in terms of reducing HbA1c levels. Concerning safety, 5-ALA has been avoided in patients with porphyria due to the pathophysiology of the disease. Generally, studies involving 5-ALA have consistently reported low levels of adverse events, with some studies even indicating no adverse events at all. Reported events such as common cold symptoms, menstrual pain, diarrhea, and headache were typically mild, with no serious adverse events documented.76
Researchers have also shown significant enhancements in self-perception of effort expenditure, reduced feelings of loneliness, and improved coping abilities among middle-aged and older adults with prediabetes after a 12-week regimen of 5-ALA supplementation.129 These improvements in mood and coping skills may help individuals overcome emotional barriers that hinder the adoption of healthy lifestyle practices, thereby aiding in the prevention of diabetes onset. These findings are consistent with the results of a randomized, double-blind, placebo-controlled, parallel study demonstrating oral 5-ALA reduces weakness and negative mood in individuals experiencing persistent physical fatigue.130
Conclusions
Beyond its primary function in heme synthesis, 5-ALA demonstrates a range of physiological effects, serving as a signaling molecule that influences cellular processes including oxidative stress response, mitochondrial function, and gene expression. Moreover, recent research highlights its involvement in cellular metabolism, indicating potential implications for energy regulation and the management of metabolic disorders.
The ability of 5-ALA to influence immune response and inflammation, oxidative/nitrosative stress, antioxidant system, mitochondrial functions, as well as carbohydrate and lipid metabolism is mediated by molecular mechanisms associated with the suppression of the TLR/IKK/NF-κB signaling pathway, activation of the Nrf2/HO-1 system leading to the formation of heme-derived reaction products (CO, ferrous iron, biliverdin, and bilirubin), which may contribute to HO-1-dependent cytoprotection through antioxidant and immunomodulatory effects. Additionally, it regulates the expression of PGC1α, COXIV, UCP1, UCP2, GLUT1, GLUT2, and SREBP-1c in relevant tissues.
The majority of studies investigating the physiological or pharmacological effects of 5-ALA have been conducted on farm animals. The findings obtained from these studies require further preclinical and clinical validation. An important aspect of further research is to clarify the significance of combining 5-ALA with ferrous iron preparations.
Randomized controlled trials have confirmed the effects of 5-ALA on glucose control in both prediabetic and diabetic patients, noting its safety and tolerability, as well as the safety of its combined use with oral hypoglycemic agents. Only minor side effects have been reported. However, the impact of 5-ALA on markers of systemic inflammation, oxidative and nitrosative stress, and dyslipidemia was not evaluated in these studies.
At the same time, preparations of 5-ALA may potentially be effective not only in the treatment of prediabetes and T2DM, but also in other conditions associated with systemic inflammation, oxidative or nitrosative stress, mitochondrial dysfunction, as well as disorders of carbohydrate and lipid metabolism.
New strategies for preventing and treating these diseases may depend on the promising development of formulations containing 5-ALA, followed by subsequent preclinical and clinical trials.
Declaration of interests
Authors declare no competing interests.
References
- 1.Jiang M., Hong K., Mao Y., Ma H., Chen T., Wang Z. Natural 5-Aminolevulinic Acid: Sources, Biosynthesis, Detection and Applications. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.841443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang Z., Zhang J., Zhou J., Qi Q., Du G., Chen J. Recent advances in microbial production of δ-aminolevulinic acid and vitamin B12. Biotechnol. Adv. 2012;30:1533–1542. doi: 10.1016/j.biotechadv.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang X., Tian Y., Liao X., Tang Y., Ni Q., Sun J., Zhao Y., Zhang J., Teng Z., Lu G. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid Interface Sci. 2020;565:483–493. doi: 10.1016/j.jcis.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Sansaloni-Pastor S., Lange N. Unleashing the potential of 5-Aminolevulinic acid: Unveiling a promising target for cancer diagnosis and treatment beyond photodynamic therapy. J. Photochem. Photobiol., B. 2023;247 doi: 10.1016/j.jphotobiol.2023.112771. [DOI] [PubMed] [Google Scholar]
- 5.Simmons B.J., Griffith R.D., Falto-Aizpurua L.A., Nouri K. An update on photodynamic therapies in the treatment of onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2015;29:1275–1279. doi: 10.1111/jdv.12950. [DOI] [PubMed] [Google Scholar]
- 6.Millesi M., Kiesel B., Mischkulnig M., Martínez-Moreno M., Wöhrer A., Wolfsberger S., Knosp E., Widhalm G. Analysis of the surgical benefits of 5-ALA-induced fluorescence in intracranial meningiomas: experience in 204 meningiomas. J. Neurosurg. 2016;125:1408–1419. doi: 10.3171/2015.12.JNS151513. [DOI] [PubMed] [Google Scholar]
- 7.Della Pepa G.M., Ius T., La Rocca G., Gaudino S., Isola M., Pignotti F., Rapisarda A., Mazzucchi E., Giordano C., Dragonetti V., et al. 5-Aminolevulinic Acid and Contrast-Enhanced Ultrasound: The Combination of the Two Techniques to Optimize the Extent of Resection in Glioblastoma Surgery. Neurosurgery. 2020;86:E529–E540. doi: 10.1093/neuros/nyaa037. [DOI] [PubMed] [Google Scholar]
- 8.Suero Molina E., Kaneko S., Black D., Stummer W. 5-Aminolevulinic Acid-Induced Porphyrin Contents in Various Brain Tumors: Implications Regarding Imaging Device Design and Their Validation. Neurosurgery. 2021;89:1132–1140. doi: 10.1093/neuros/nyab361. [DOI] [PubMed] [Google Scholar]
- 9.Ubbink R., Prens E.P., Mik E.G. Quantitative intracellular oxygen availability before and after 5-aminolevulinic acid skin photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021;36 doi: 10.1016/j.pdpdt.2021.102599. [DOI] [PubMed] [Google Scholar]
- 10.Ivanova Y., Gramatiuk S., Prasol V., Kryvoruchko I., Miasoiedov K., Mitchell C., Hartl G., Sargsyan K. The use of 5-aminolevulinic acid gel in the treatment of diabetic foot syndrome. Probl Endocrin Pathol. 2022;79:74–84. doi: 10.21856/j-PEP.2022.4.10. [DOI] [Google Scholar]
- 11.Suzuki K., Yamamoto J., Toh K., Miyaoka R. 5-aminiolevulinic acid induces a radiodynamic effect with enhanced delayed reactive oxygen species production under hypoxic conditions in lymphoma cells: An in vitro study. Exp. Ther. Med. 2023;26:360. doi: 10.3892/etm.2023.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishio Y., Fujino M., Zhao M., Ishii T., Ishizuka M., Ito H., Takahashi K., Abe F., Nakajima M., Tanaka T., et al. 5-Aminolevulinic acid combined with ferrous iron enhances the expression of heme oxygenase-1. Int. Immunopharmacol. 2014;19:300–307. doi: 10.1016/j.intimp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Fujino M., Nishio Y., Ito H., Tanaka T., Li X.K. 5-Aminolevulinic acid regulates the inflammatory response and alloimmune reaction. Int. Immunopharmacol. 2016;37:71–78. doi: 10.1016/j.intimp.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez B.L., Curb J.D., Davis J., Shintani T., Perez M.H., Apau-Ludlum N., Johnson C., Harrigan R.C. Use of the dietary supplement 5-aminiolevulinic acid (5-ALA) and its relationship with glucose levels and hemoglobin A1C among individuals with prediabetes. Clin. Transl. Sci. 2012;5:314–320. doi: 10.1111/j.1752-8062.2012.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashikawa F., Noda M., Awaya T., Tanaka T., Sugiyama M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition. 2013;29:1030–1036. doi: 10.1016/j.nut.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita N., Watanabe A., Kondo H., Kawata S., Tanaka T., Nakajima M. Safety test of a supplement, 5-aminolevulinic acid phosphate with sodium ferrous citrate, in diabetic patients treated with oral hypoglycemic agents. Functional Foods in Health and Disease. 2014;4:415–428. doi: 10.31989/ffhd.v4i9.151. [DOI] [Google Scholar]
- 17.Al-Saber F., Aldosari W., Alselaiti M., Khalfan H., Kaladari A., Khan G., Harb G., Rehani R., Kudo S., Koda A., et al. The Safety and Tolerability of 5-Aminolevulinic Acid Phosphate with Sodium Ferrous Citrate in Patients with Type 2 Diabetes Mellitus in Bahrain. J. Diabetes Res. 2016;2016 doi: 10.1155/2016/8294805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura Y., Haraguchi A., Shigeno R., Ito A., Horie I., Kawakami A., Abiru N. A single-arm, open-label, intervention study to investigate the improvement of glucose tolerance after administration of 5-aminolevulinic acid (5-ALA) in patients with mitochondrial diabetes mellitus. Medicine. 2021;100 doi: 10.1097/MD.0000000000025100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura Y., Haraguchi A., Horie I., Kawakami A., Abiru N. Pilot Trial on the Effect of 5-Aminolevulinic Acid on Glucose Tolerance in Patients with Maternally Inherited Diabetes and Deafness. Diabetes Ther. 2023;14:447–459. doi: 10.1007/s13300-022-01335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamiya A., Hara T., Tsuda M., Tsuru E., Kuroda Y., Ota U., Karashima T., Fukuhara H., Inoue K., Ishizuka M., et al. 5-Aminolevulinic acid with ferrous iron improves early renal damage and hepatic steatosis in high fat diet-induced obese mice. J. Clin. Biochem. Nutr. 2019;64:59–65. doi: 10.3164/jcbn.18-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagisawa K., Ayaori M., Ikewaki K., Nakajima M., Morimoto Y. 5-Aminolevulinic Acid Attenuates Atherosclerotic Plaque Progression in Low-Density Lipoprotein Receptor-Deficient Mice by Heme Oxygenase-1 Induction. Circ. Rep. 2019;2:60–68. doi: 10.1253/circrep.CR-19-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orekhov A.N., Nikiforov N.G., Sobenin I.A., Sinyov V., Marey M., Sukhanova I., Manukhova L., Vyssokikh M. Subcellular anti-atherosclerotic therapy: 5-aminolevulinic acid as a potential drug affecting dysfunctional mitochondria. Atherosclerosis. 2020;315:E132. doi: 10.1016/j.atherosclerosis.2020.10.408. [DOI] [Google Scholar]
- 23.Sato K., Matsushita K., Takahashi K., Aoki M., Fuziwara J., Miyanari S., Kamada T. Dietary supplementation with 5-aminolevulinic acid modulates growth performance and inflammatory responses in broiler chickens. Poult. Sci. 2012;91:1582–1589. doi: 10.3382/ps.2010-01201. [DOI] [PubMed] [Google Scholar]
- 24.Hendawy A.O., Shirai M., Takeya H., Sugimura S., Miyanari S., Taniguchi S., Sato K. Effects of 5-aminolevulinic acid supplementation on milk production, iron status, and immune response of dairy cows. J. Dairy Sci. 2019;102:11009–11015. doi: 10.3168/jds.2018-15982. [DOI] [PubMed] [Google Scholar]
- 25.Hendawy A.O., Khattab M.S., Sugimura S., Sato K. Effects of 5-Aminolevulinic Acid as a Supplement on Animal Performance, Iron Status, and Immune Response in Farm Animals: A Review. Animals. 2020;10:1352. doi: 10.3390/ani10081352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Direkbusarakom S., Kinoshita H., Pooljun C., Saeng-ngern S., Wuthisuthimethavee S. 5-aminolevulinic Acid’s Effects on Immune-related Gene Expression and Acute Hepatopancreatic Necrosis Disease (AHPND) Protection in Pacific White Shrimp Litopenaeus vannamei. Fish Pathol. 2021;55:125–131. doi: 10.3147/jsfp.55.125. [DOI] [Google Scholar]
- 27.Chen J., Wang H., Wu Z., Gu H., Li C., Wang S., Liu G. Effects of 5-aminolevulinic acid on the inflammatory responses and antioxidative capacity in broiler chickens challenged with lipopolysaccharide. Animal. 2022;16 doi: 10.1016/j.animal.2022.100575. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki K., Watanabe M., Tanaka T., Tanaka T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002;58:23–29. doi: 10.1007/s00253-001-0858-7. [DOI] [PubMed] [Google Scholar]
- 29.Peoc'h K., Nicolas G., Schmitt C., Mirmiran A., Daher R., Lefebvre T., Gouya L., Karim Z., Puy H. Regulation and tissue-specific expression of δ-aminolevulinic acid synthases in non-syndromic sideroblastic anemias and porphyrias. Mol Genet Metab. 2019;128:190–197. doi: 10.1016/j.ymgme.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Tian Q., Li T., Hou W., Zheng J., Schrum L.W., Bonkovsky H.L. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011;286:26424–26430. doi: 10.1074/jbc.M110.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Handschin C., Lin J., Rhee J., Peyer A.K., Chin S., Wu P.H., Meyer U.A., Spiegelman B.M. Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1alpha. Cell. 2005;122:505–515. doi: 10.1016/j.cell.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 32.Chen J., Wang Y., Guo X., Rao D., Zhou W., Zheng P., Sun J., Ma Y. Efficient bioproduction of 5-aminolevulinic acid, a promising biostimulant and nutrient, from renewable bioresources by engineered Corynebacterium glutamicum. Biotechnol. Biofuels. 2020;13:41. doi: 10.1186/s13068-020-01685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakizaki S., Yamamoto Y., Ueda A., Moore R., Sueyoshi T., Negishi M. Phenobarbital induction of drug/steroid-metabolizing enzymes and nuclear receptor CAR. Biochim. Biophys. Acta. 2003;1619:239–242. doi: 10.1016/s0304-4165(02)00482-8. [DOI] [PubMed] [Google Scholar]
- 34.Martini C.N., Romero D.G., Yanes L.L., Vila M.d.C. Induction of 5-aminolevulinate synthase by activators of steroid biosynthesis. Life Sci. 2007;81:19–25. doi: 10.1016/j.lfs.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F.L., Shen G.M., Liu X.L., Wang F., Zhao H.L., Yu J., Zhang J.W. Hypoxic induction of human erythroid-specific δ-aminolevulinate synthase mediated by hypoxia-inducible factor 1. Biochemistry. 2011;50:1194–1202. doi: 10.1021/bi101585c. [DOI] [PubMed] [Google Scholar]
- 36.Schranzhofer M., Schifrer M., Cabrera J.A., Kopp S., Chiba P., Beug H., Müllner E.W. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 37.Doyle F., Tenenbaum S.A. Trans-regulation of RNA-binding protein motifs by microRNA. Front. Genet. 2014;5:79. doi: 10.3389/fgene.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yien Y.Y., Perfetto M. Regulation of Heme Synthesis by Mitochondrial Homeostasis Proteins. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.895521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu-Farha M., Niles J., Willmore W.G. Erythroid-specific 5-aminolevulinate synthase protein is stabilized by low oxygen and proteasomal inhibition. Biochem. Cell. Biol. 2005;83:620–630. doi: 10.1139/o05-045. [DOI] [PubMed] [Google Scholar]
- 40.Dailey T.A., Woodruff J.H., Dailey H.A. Examination of mitochondrial protein targeting of haem synthetic enzymes: in vivo identification of three functional haem-responsive motifs in 5-aminolaevulinate synthase. Biochem. J. 2005;386:381–386. doi: 10.1042/BJ20040570. Erratum in: Biochem J. 2005 Nov 1;391(Pt 3):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medlock A.E., Dailey H.A. New Avenues of Heme Synthesis Regulation. Int. J. Mol. Sci. 2022;23:7467. doi: 10.3390/ijms23137467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labib P.L., Yaghini E., Davidson B.R., MacRobert A.J., Pereira S.P. 5-Aminolevulinic acid for fluorescence-guided surgery in pancreatic cancer: Cellular transport and fluorescence quantification studies. Transl. Oncol. 2021;14 doi: 10.1016/j.tranon.2020.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan Z., Duan J., Luo P., Shao L., Chen Q., Tan X., Zhang L., Xu X. SLC25A38 as a novel biomarker for metastasis and clinical outcome in uveal melanoma. Cell Death Dis. 2022;13:330. doi: 10.1038/s41419-022-04718-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayeva M., Khechaduri A., Wu R., Burke M.A., Wasserstrom J.A., Singh N., Liesa M., Shirihai O.S., Langer N.B., Paw B.H., Ardehali H. ATP-binding cassette B10 regulates early steps of heme synthesis. Circ. Res. 2013;113:279–287. doi: 10.1161/CIRCRESAHA.113.301552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seguin A., Takahashi-Makise N., Yien Y.Y., Huston N.C., Whitman J.C., Musso G., Wallace J.A., Bradley T., Bergonia H.A., Kafina M.D., et al. Reductions in the mitochondrial ABC transporter Abcb10 affect the transcriptional profile of heme biosynthesis genes. J. Biol. Chem. 2017;292:16284–16299. doi: 10.1074/jbc.M117.797415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez L., Batlle A., Di Venosa G., MacRobert A.J., Battah S., Daniel H., Casas A. Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PEPT2 transporters as a tool to improve photodynamic therapy of tumours. Int. J. Biochem. Cell Biol. 2006;38:1530–1539. doi: 10.1016/j.biocel.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Hu Y., Shen H., Keep R.F., Smith D.E. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J. Neurochem. 2007;103:2058–2065. doi: 10.1111/j.1471-4159.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- 48.Xie Y., Hu Y., Smith D.E. The proton-coupled oligopeptide transporter 1 plays a major role in the intestinal permeability and absorption of 5-aminolevulinic acid. Br. J. Pharmacol. 2016;173:167–176. doi: 10.1111/bph.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Killer M., Wald J., Pieprzyk J., Marlovits T.C., Löw C. Structural snapshots of human PepT1 and PepT2 reveal mechanistic insights into substrate and drug transport across epithelial membranes. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abk3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalmasso G., Charrier-Hisamuddin L., Nguyen H.T.T., Yan Y., Sitaraman S., Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovacs-Nolan J., Zhang H., Ibuki M., Nakamori T., Yoshiura K., Turner P.V., Matsui T., Mine Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta. 2012;1820:1753–1763. doi: 10.1016/j.bbagen.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Tsiftsoglou A.S., Tsamadou A.I., Papadopoulou L.C. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol. Ther. 2006;111:327–345. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Chiabrando D., Marro S., Mercurio S., Giorgi C., Petrillo S., Vinchi F., Fiorito V., Fagoonee S., Camporeale A., Turco E., et al. The mitochondrial heme exporter FLVCR1b mediates erythroid differentiation. J. Clin. Invest. 2012;122:4569–4579. doi: 10.1172/JCI62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryter S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants. 2022;11:555. doi: 10.3390/antiox11030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu B., Wu Y., Tang W. Heme Catabolic Pathway in Inflammation and Immune Disorders. Front. Pharmacol. 2019;10:825. doi: 10.3389/fphar.2019.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijayan V., Wagener F.A.D.T.G., Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Liu R., Yang J., Li Y., Xie J., Wang J. Heme oxygenase-1: The roles of both good and evil in neurodegenerative diseases. J. Neurochem. 2023;167:347–361. doi: 10.1111/jnc.15969. [DOI] [PubMed] [Google Scholar]
- 58.Ito H., Nishio Y., Hara T., Sugihara H., Tanaka T., Li X.K. Oral administration of 5-aminolevulinic acid induces heme oxygenase-1 expression in peripheral blood mononuclear cells of healthy human subjects in combination with ferrous iron. Eur. J. Pharmacol. 2018;833:25–33. doi: 10.1016/j.ejphar.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Medina M.V., Sapochnik D., Garcia Solá M., Coso O. Regulation of the Expression of Heme Oxygenase-1: Signal Transduction, Gene Promoter Activation, and Beyond. Antioxid. Redox Signal. 2020;32:1033–1044. doi: 10.1089/ars.2019.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu Z., Pei G., Wang P., Yang J., Zhu F., Guo Y., Wang M., Yao Y., Zeng R., Liao W., Xu G. Biliverdin Reductase A (BVRA) Mediates Macrophage Expression of Interleukin-10 in Injured Kidney. Int. J. Mol. Sci. 2015;16:22621–22635. doi: 10.3390/ijms160922621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee Y., Kim H., Kang S., Lee J., Park J., Jon S. Bilirubin Nanoparticles as a Nanomedicine for Anti-inflammation Therapy. Angew. Chem. Int. Ed. Engl. 2016;55:7460–7463. doi: 10.1002/anie.201602525. [DOI] [PubMed] [Google Scholar]
- 62.Chiang S.K., Chen S.E., Chang L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018;20:39. doi: 10.3390/ijms20010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang D.D., Liang Y.F., Qi J., Kang K.B., Yu X.J., Gao H.L., Liu K.L., Chen Y.M., Shi X.L., Xin G.R., et al. Carbon Monoxide Attenuates High Salt-Induced Hypertension While Reducing Pro-inflammatory Cytokines and Oxidative Stress in the Paraventricular Nucleus. Cardiovasc. Toxicol. 2019;19:451–464. doi: 10.1007/s12012-019-09517-w. [DOI] [PubMed] [Google Scholar]
- 64.Layer G., Reichelt J., Jahn D., Heinz D.W. Structure and function of enzymes in heme biosynthesis. Protein Sci. 2010;19:1137–1161. doi: 10.1002/pro.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miura M., Ito K., Hayashi M., Nakajima M., Tanaka T., Ogura S.i. The Effect of 5-Aminolevulinic Acid on Cytochrome P450-Mediated Prodrug Activation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsiftsoglou S.A. Heme Interactions as Regulators of the Alternative Pathway Complement Responses and Implications for Heme-Associated Pathologies. Curr. Issues Mol. Biol. 2023;45:5198–5214. doi: 10.3390/cimb45060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaketani K., Nakajima M. Case reports: safety, tolerability and efficacy of 5-aminolevulinic acid phosphate, an inducer of heme oxygenase 1, in combination with sodium ferrous citrate for the treatment of COVID-19 patients. The Open COVID Journal. 2021;1:52–61. doi: 10.2174/2666958702101010052. [DOI] [Google Scholar]
- 68.Darwish A., Almadani A., Alsalman J., Atkin S., Murad M., Nakajima M., Rehani R., Ebeling A., Stocker M., Stummer W., Berenzen N. Safety and immune-supportive potential of the food supplement 5-aminolevulinic acid phosphate for patients with COVID-19: An open-label, non-randomized pilot study. Bioact. Compd. Health Dis. 2024;7:17–35. doi: 10.31989/bchd.v7i1.1290. [DOI] [Google Scholar]
- 69.Wang J.P., Jung J.H., Kim I.H. Effects of dietary supplementation with delta-aminolevulinic acid on growth performance, hematological status, and immune responses of weanling pigs. Livest. Sci. 2011;140:131–135. doi: 10.1016/j.livsci.2011.02.017. [DOI] [Google Scholar]
- 70.Wang J.P., Kim I.H. Effects of iron injection at birth on neonatal iron status in young pigs from first-parity sows fed delta-aminolevulinic acid. Anim. Feed Sci. Technol. 2012;178:151–157. doi: 10.1016/j.anifeedsci.2012.08.011. [DOI] [Google Scholar]
- 71.Chen Y.J., Kim I.H., Cho J.H., Min B.J., Yoo J.S., Wang Q. Effect of δ-aminolevulinic acid on growth performance, nutrient digestibility, blood parameters, and the immune response of weanling pigs challenged with Escherichia coli lipopolysaccharide. Livest. Sci. 2008;114:108–116. doi: 10.1016/j.livsci.2007.04.015. [DOI] [Google Scholar]
- 72.Mateo R.D., Morrow J.L., Dailey J.W., Ji F., Kim S.W. Use of δ-Aminolevulinic Acid in Swine Diet: Effect on Growth Performance, Behavioral Characteristics, and Hematological/Immune Status in Nursery Pigs. Asian-Australas. J. Anim. Sci. 2005;19:97–101. doi: 10.5713/ajas.2006.97. [DOI] [Google Scholar]
- 73.Chen Y., Kim I., Cho J., Yoo J., Kim H., Shin S. Utilization of δ-aminolevulinic acid for livestock: Blood characteristics and immune organ weight in broilers. J. Anim. Feed Sci. 2008;17:215–223. doi: 10.22358/jafs/66601/2008. [DOI] [Google Scholar]
- 74.Wang J.P., Yan L., Lee J.H., Zhou T.X., Kim I.H. Effects of dietary delta-aminolevulinic acid and vitamin C on growth performance, immune organ weight, and iron status in broiler chicks. Livest. Sci. 2011;135:148–152. doi: 10.1016/j.livsci.2010.06.161. [DOI] [Google Scholar]
- 75.Liu C., Fujino M., Zhu S., Isaka Y., Ito H., Takahashi K., Nakajima M., Tanaka T., Zhu P., Li X.K. 5-ALA/SFC enhances HO-1 expression through the MAPK/Nrf2 antioxidant pathway and attenuates murine tubular epithelial cell apoptosis. FEBS Open Bio. 2019;9:1928–1938. doi: 10.1002/2211-5463.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rehani P.R., Iftikhar H., Nakajima M., Tanaka T., Jabbar Z., Rehani R.N. Safety and Mode of Action of Diabetes Medications in comparison with 5-Aminolevulinic Acid (5-ALA) J. Diabetes Res. 2019;2019 doi: 10.1155/2019/4267357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin G., Guo N., Liu Y., Zhang L., Chen L., Dong T., Liu W., Zhang X., Jiang Y., Lv G., et al. 5-aminolevulinate and CHIL3/CHI3L1 treatment amid ischemia aids liver metabolism and reduces ischemia-reperfusion injury. Theranostics. 2023;13:4802–4820. doi: 10.7150/thno.83163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L., Zhang L., Jin G., Liu Y., Guo N., Sun H., Jiang Y., Zhang X., He G., Lv G., et al. Synergy of 5-aminolevulinate supplement and CX3CR1 suppression promotes liver regeneration via elevated IGF-1 signaling. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112984. [DOI] [PubMed] [Google Scholar]
- 79.Li S., Takahara T., Li X.K., Fujino M., Sugiyama T., Tsukada K., Liu C., Kakuta Y., Nonomura N., Ito H., et al. 5-Aminolevulinic acid combined with ferrous iron ameliorate ischemia-reperfusion injury in the mouse fatty liver model. Biochem. Biophys. Res. Commun. 2016;470:900–906. doi: 10.1016/j.bbrc.2016.01.136. [DOI] [PubMed] [Google Scholar]
- 80.Otaka Y., Kanai K., Mori A., Okada D., Nagai N., Yamashita Y., Ichikawa Y., Tajima K. 5-ALA/SFC Ameliorates Endotoxin-Induced Ocular Inflammation in Rats by Inhibiting the NF-κB Signaling Pathway and Activating the HO-1/Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2023;24:8653. doi: 10.3390/ijms24108653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Otaka Y., Kanai K., Okada D., Nagai N., Yamashita Y., Ichikawa Y., Tajima K. Effects of Oral 5-Aminolevulinic Acid on Lipopolysaccharide-Induced Ocular Inflammation in Rats. Vet. Sci. 2023;10:207. doi: 10.3390/vetsci10030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang S.N., Chu G.M., Song Y.M., Jin S.K., Hwang I.H., Kim I.S. The effects of replacement of antibiotics with by-products of oriental medicinal plants on growth performance and meat qualities in fattening pigs. Anim. Sci. J. 2012;83:245–251. doi: 10.1111/j.1740-0929.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 83.Saga N., Hu A., Yamaguchi T., Naraoka Y., Kobayashi H. The Impact of 5-Aminolevulinic Acid Supplementation on Redox Balance and Aerobic Capacity. Int. J. Mol. Sci. 2024;25:988. doi: 10.3390/ijms25020988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Masuki S., Morita A., Kamijo Y.i., Ikegawa S., Kataoka Y., Ogawa Y., Sumiyoshi E., Takahashi K., Tanaka T., Nakajima M., Nose H. Impact of 5-aminolevulinic acid with iron supplementation on exercise efficiency and home-based walking training achievement in older women. J. Appl. Physiol. 2016;120:87–96. doi: 10.1152/japplphysiol.00582.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki H., Masuki S., Morikawa A., Ogawa Y., Kamijo Y.I., Takahashi K., Nakajima M., Nose H. Effects of 5-aminolevulinic acid supplementation on home-based walking training achievement in middle-aged depressive women: randomized, double-blind, crossover pilot study. Sci. Rep. 2018;8:7151. doi: 10.1038/s41598-018-25452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bechara E.J.H., Dutra F., Cardoso V.E.S., Sartori A., Olympio K.P.K., Penatti C.A.A., Adhikari A., Assunção N.A. The dual face of endogenous alpha-aminoketones: pro-oxidizing metabolic weapons. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;146:88–110. doi: 10.1016/j.cbpc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Frenkel Y., Cherno V., Kostenko V. Nrf2 Induction Alleviates Metabolic Disorder and Systemic Inflammatory Response in Rats under a Round-the-Clock Lighting and High-Carbohydrate–Lipid Diet. Romanian Journal of Diabetes Nutrition and Metabolic Diseases. 2022;29:194–201. doi: 10.46389/rjd-2022-1092. [DOI] [Google Scholar]
- 88.Kozaeva R., Klymenko M.O., Katrushov O.V., Kostenko V.O. Bioflavonoids as agents for correcting nitro-oxidative stress and salivary gland functions in rats exposed to alcohol during modeled lipopolysaccharide-induced systemic inflammatory response. Wiad. Lek. 2022;75:685–690. doi: 10.36740/WLek202203121. [DOI] [PubMed] [Google Scholar]
- 89.Frenkel’ Y., Cherno V.S., Kostenko V.O. Effect of NF-κB and Nrf2 Transcription Factor Modulators on Indicators of Oxidative–Nitrosative Stress in Skeletal Muscles of Rats under Chronic Hypomelatoninemia and Carbohydrate-Lipid Diet. Fiziol. Zh. 2023;69:11–18. doi: 10.15407/fz69.02.011. [DOI] [Google Scholar]
- 90.Kostenko V., Akimov O., Gutnik O., Kostenko H., Kostenko V., Romantseva T., Morhun Y., Nazarenko S., Taran O. Modulation of redox-sensitive transcription factors with polyphenols as pathogenetically grounded approach in therapy of systemic inflammatory response. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gutnik O.M., Nazarenko S.M., Kostenko V.O. Effect of quercetin on oxidative and nitrosative stress in kidney tissues of rats during acute desynchronosis and lipopolysaccharide-induced inflammation. Fiziol. Zh. 2024;70:33–41. doi: 10.15407/fz70.04.033. [DOI] [Google Scholar]
- 92.Opryshko V., Prokhach A., Akimov O., Riabushko M., Kostenko H., Kostenko V., Mishchenko A., Solovyova N., Kostenko V. Desmodium styracifolium: Botanical and ethnopharmacological insights, phytochemical investigations, and prospects in pharmacology and pharmacotherapy. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e25058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez-Guzman O., Willoughby M.M., Saini A., Dietz J.V., Bohovych I., Medlock A.E., Khalimonchuk O., Reddi A.R. Mitochondrial-nuclear heme trafficking in budding yeast is regulated by GTPases that control mitochondrial dynamics and ER contact sites. J. Cell Sci. 2020;133 doi: 10.1242/jcs.237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nozawa N., Noguchi M., Shinno K., Saito T., Asada A., Ishii T., Takahashi K., Ishizuka M., Ando K. 5-Aminolevulinic acid bypasses mitochondrial complex I deficiency and corrects physiological dysfunctions in Drosophila. Hum. Mol. Genet. 2023;32:2611–2622. doi: 10.1093/hmg/ddad092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimura M., Nozawa N., Ogawa-Tominaga M., Fushimi T., Tajika M., Ichimoto K., Matsunaga A., Tsuruoka T., Kishita Y., Ishii T., et al. Effects of 5-aminolevulinic acid and sodium ferrous citrate on fibroblasts from individuals with mitochondrial diseases. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-46772-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saitoh S., Okano S., Nohara H., Nakano H., Shirasawa N., Naito A., Yamamoto M., Kelly V.P., Takahashi K., Tanaka T., et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fujii C., Miyashita K., Mitsuishi M., Sato M., Fujii K., Inoue H., Hagiwara A., Endo S., Uto A., Ryuzaki M., et al. Treatment of sarcopenia and glucose intolerance through mitochondrial activation by 5-aminolevulinic acid. Sci. Rep. 2017;7:4013. doi: 10.1038/s41598-017-03917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sakai A., Iwatani N., Harada K. Improvement Effect of 5-Aminolevulinic Acid on Hyperlipidemia in Miniature Schnauzer Dogs: An Open Study in 5 Cases of One Pedigree. Yonago Acta Med. 2020;63:234–238. doi: 10.33160/yam.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hara T., Koda A., Nozawa N., Ota U., Kondo H., Nakagawa H., Kamiya A., Miyashita K., Itoh H., Nakajima M., Tanaka T. Combination of 5-aminolevulinic acid and ferrous ion reduces plasma glucose and hemoglobin A1c levels in Zucker diabetic fatty rats. FEBS Open Bio. 2016;6:515–528. doi: 10.1002/2211-5463.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raghuram S., Stayrook K.R., Huang P., Rogers P.M., Nosie A.K., McClure D.B., Burris L.L., Khorasanizadeh S., Burris T.P., Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat. Struct. Mol. Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin L., Wu N., Lazar M.A. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal. 2010;8 doi: 10.1621/nrs.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu Z., Zhou F., Chen H., Hara T., Nakajima M., Zheng C., Tanaka T., Lu D. The chronic effects of 5-aminolevulinic acid plus sodium ferrous citrate on pre-diabetic Wistar rats. Int. J. Clin. Exp. Med. 2019;12:8227–8238. [Google Scholar]
- 103.Yu H., Zhang M., Ma Y., Lu J., Pan J., Pan P., Chen H., Jia W. 5-ALA ameliorates hepatic steatosis through AMPK signaling pathway. J. Mol. Endocrinol. 2017;59:121–128. doi: 10.1530/JME-16-0260. [DOI] [PubMed] [Google Scholar]
- 104.Pignatelli P., Umme S., D’Antonio D.L., Piattelli A., Curia M.C. Reactive Oxygen Species Produced by 5-Aminolevulinic Acid Photodynamic Therapy in the Treatment of Cancer. Int. J. Mol. Sci. 2023;24:8964. doi: 10.3390/ijms24108964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naraoka Y., Hu A., Yamaguchi T., Saga N., Kobayashi H. 5-Aminolevulinic Acid Improves Skin Hydration and Reduces Wrinkles. Health. 2020;12:709–716. doi: 10.4236/health.2020.127052. [DOI] [Google Scholar]
- 106.Omori C., Motodate R., Shiraki Y., Chiba K., Sobu Y., Kimura A., Nakaya T., Kondo H., Kurumiya S., Tanaka T., et al. Facilitation of brain mitochondrial activity by 5-aminolevulinic acid in a mouse model of Alzheimer's disease. Nutr. Neurosci. 2017;20:538–546. doi: 10.1080/1028415X.2016.1199114. [DOI] [PubMed] [Google Scholar]
- 107.Takase N., Inden M., Sekine S.I., Ishii Y., Yonemitsu H., Iwashita W., Kurita H., Taketani Y., Hozumi I. Neuroprotective effect of 5-aminolevulinic acid against low inorganic phosphate in neuroblastoma SH-SY5Y cells. Sci. Rep. 2017;7:5768. doi: 10.1038/s41598-017-06406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakurai Y., Ngwe Tun M.M., Kurosaki Y., Sakura T., Inaoka D.K., Fujine K., Kita K., Morita K., Yasuda J. 5-amino levulinic acid inhibits SARS-CoV-2 infection in vitro. Biochem. Biophys. Res. Commun. 2021;545:203–207. doi: 10.1016/j.bbrc.2021.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ngwe Tun M.M., Sakura T., Sakurai Y., Kurosaki Y., Inaoka D.K., Shioda N., Yasuda J., Kita K., Morita K. Antiviral activity of 5-aminolevulinic acid against variants of severe acute respiratory syndrome coronavirus 2. Trop. Med. Health. 2022;50:6. doi: 10.1186/s41182-021-00397-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Imamura K., Sugiharaa H., Hirahata K. Phase 2 randomized clinical trial of 5-Aminolevulinic acid plus sodium citrate chloride vs placebo for Covid-19 infected patients recovered with sequelae. ALA-Porphyrin Science. 2021;10:15–22. [Google Scholar]
- 111.Sato T., Yasuzawa T., Uesaka A., Izumi Y., Kamiya A., Tsuchiya K., Kobayashi Y., Kuwahata M., Kido Y. Type 2 diabetic conditions in Otsuka Long-Evans Tokushima Fatty rats are ameliorated by 5-aminolevulinic acid. Nutr. Res. 2014;34:544–551. doi: 10.1016/j.nutres.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 112.Kitamura N., Zhang S., Morel J.D., Nagano U., Taworntawat T., Hosoda S., Nakamura A., Ogawa Y., Benegiamo G., Auwerx J., et al. Sodium ferrous citrate and 5-aminolevulinic acid improve type 2 diabetes by maintaining muscle and mitochondrial health. Obesity. 2023;31:1038–1049. doi: 10.1002/oby.23705. [DOI] [PubMed] [Google Scholar]
- 113.Kuroda Y., Kamiya A., Ishii T., Ishizuka M., Yamashita Y., Ashida H. 5-Aminolevulinic acid combined with ferrous iron improves glucose tolerance in high-fat diet-fed mice via upregulation of glucose transporter 1. Exp. Ther. Med. 2021;22:1454. doi: 10.3892/etm.2021.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao M., Zhu P., Fujino M., Nishio Y., Chen J., Ito H., Takahashi K., Nakajima M., Tanaka T., Zhao L., et al. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016;479:663–669. doi: 10.1016/j.bbrc.2016.09.156. [DOI] [PubMed] [Google Scholar]
- 115.Yang Y., Liu Y., Chen X., Gong J., Huang Z., Wang W., Shi Y., Wang Y., Yao J., Shen Z., et al. 5-Aminolevulinic Acid-Mediated Sonodynamic Therapy Alleviates Atherosclerosis via Enhancing Efferocytosis and Facilitating a Shift in the Th1/Th2 Balance Toward Th2 Polarization. Cell. Physiol. Biochem. 2018;47:83–96. doi: 10.1159/000489751. [DOI] [PubMed] [Google Scholar]
- 116.Cao Y., Yao J., Gao W., Cao Z., Diabakte K., Wang L., Sun X., Tian Y. Sonodynamic Therapy Promotes Efferocytosis via CD47 Down-Regulation in Advanced Atherosclerotic Plaque. Int. Heart J. 2022;63:131–140. doi: 10.1536/ihj.21-233. [DOI] [PubMed] [Google Scholar]
- 117.Shi Y., Hu F., Fu H., Li S., Lu C., Hu C. Low Dose of 5-Aminolevulinic Acid Hydrochloride Alleviates the Damage in Cardiomyocytes Induced by Lenvatinib via PI3K/AKT Signaling Pathway. Discov. Med. 2023;35:483–491. doi: 10.24976/Discov.Med.202335177.49. [DOI] [PubMed] [Google Scholar]
- 118.Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165838. [DOI] [PubMed] [Google Scholar]
- 119.Mohamed S.M., Shalaby M.A., El-Shiekh R.A., El-Banna H.A., Emam S.R., Bakr A.F. Metabolic syndrome: risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023;3 doi: 10.1016/j.focha.2023.100335. [DOI] [Google Scholar]
- 120.Frenkel’ Y., Zyuzin V.O., Cherno V.S., Kostenko V.O. Effect of epigallocatechin-3-gallate and quercetin on the production of reactive oxygen and nitrogen species in liver of rats exposed to round-the-clock light and kept on carbohydrate-lipid diet. Fiziol. Zh. 2022;68:20–27. doi: 10.15407/fz68.01.020. [DOI] [Google Scholar]
- 121.Frenkel Y., Cherno V., Kostenko H., Chopra H., Gautam R.K., Kostenko V. Dietary Supplementation with Resveratrol Attenuates Serum Melatonin Level, Pro-Inflammatory Response and Metabolic Disorder in Rats Fed High-Fructose High-Lipid Diet under Round-the-Clock Lighting. Pathophysiology. 2023;30:37–47. doi: 10.3390/pathophysiology30010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frenkel Y., Cherno V., Kostenko H., Kostenko V. Resveratrol attenuates the development of nitro-oxidative stress in the liver of rats under a round-the-clock lighting and high-carbohydrate-lipid diet. Romanian Journal of Diabetes, Nutrition and Metabolic Diseases. 2023;30:48–54. doi: 10.46389/rjd-2023-1217. [DOI] [Google Scholar]
- 123.Galicia-Garcia U., Benito-Vicente A., Jebari S., Larrea-Sebal A., Siddiqi H., Uribe K.B., Ostolaza H., Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020;21:6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Solis-Herrera C., Triplitt C., Cersosimo E. In: Endotext [Internet] Feingold K.R., Anawalt B., Blackman M.R., editors. MDText.com, Inc.; 2000. Pathogenesis of Type 2 Diabetes Mellitus; pp. 1–53.https://www.ncbi.nlm.nih.gov/books/NBK279115/ [Updated 2021 Sep 27] [Google Scholar]
- 125.Kaur P., Nagar S., Bhagwat M., Uddin M., Zhu Y., Vancurova I., Vancura A. Activated heme synthesis regulates glycolysis and oxidative metabolism in breast and ovarian cancer cells. PLoS One. 2021;16 doi: 10.1371/journal.pone.0260400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamada S., Mae Y., Takata T., Hanada H., Kubo M., Taniguchi S., Iyama T., Sugihara T., Isomoto H. Five-Aminolevulinic Acid (5-ALA) Induces Heme Oxygenase-1 and Ameliorates Palmitic Acid-Induced Endoplasmic Reticulum Stress in Renal Tubules. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241210151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanaka T., Tashiro M., Ota K., Fujita A., Sawai T., Kadota J., Fukuda Y., Sumiyoshi M., Ide S., Tachikawa N., et al. Safety and efficacy of 5-aminolevulinic acid phosphate/iron in mild-to-moderate coronavirus disease 2019: A randomized exploratory phase II trial. Medicine (Baltim.) 2023;102 doi: 10.1097/MD.0000000000034858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamura Y., Kaga H., Abe Y., Yoshii H., Seino H., Hiyoshi T., Kuribayashi N., Inoue I., Watada H. Efficacy and Safety of 5-Aminolevulinic Acid Combined with Iron on Skeletal Muscle Mass Index and Physical Performance of Patients with Sarcopenia: A Multicenter, Double-Blinded, Randomized-Controlled Trial (ALADDIN Study) Nutrients. 2023;15 doi: 10.3390/nu15132866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aquino R.K., Perez M., Sil P., Shintani T., Harrigan R., Rodriguez B. The Relationship of 5-Aminolevulinic Acid on Mood and Coping Ability in Prediabetic Middle Aged and Older Adults. Geriatrics. 2018;3:17. doi: 10.3390/geriatrics3020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Higashikawa F., Kanno K., Ogata A., Sugiyama M. Reduction of fatigue and anger-hostility by the oral administration of 5-aminolevulinic acid phosphate: a randomized, double-blind, placebo-controlled, parallel study. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72763-4. [DOI] [PMC free article] [PubMed] [Google Scholar]