Summary
Background
The interplay between diet and gut microbiome substantially influences host metabolism, but uncertainties remain regarding their relationships tailored for each subject given the huge inter-individual variability. Here we aim to investigate diet-gut microbiome interaction at single-subject resolution and explore its effects on blood glucose homeostasis.
Methods
We conducted a series of nutritional n-of-1 trials (NCT04125602), in which 30 participants were assigned high-carbohydrate (HC) and low-carbohydrate (LC) diets in a randomized sequence across 3 pair of cross-over periods lasting 72 days. We used shotgun metagenomic sequencing and continuous glucose monitoring systems to profile the gut microbiome and blood glucose, respectively. An independent cohort of 1219 participants with available metagenomics data are included as a validation cohort.
Findings
We demonstrated that the gut microbiome exhibited both intra-individually dynamic and inter-individually personalized signatures during the interventions. At the single-subject resolution, we observed person-specific response patterns of gut microbiota to interventional diets. Furthermore, we discovered a personal gut microbial signature represented by a carb-sensitivity score, which was closely correlated with glycemic phenotypes during the HC intervention, but not LC intervention. We validate the role of this score in the validation cohort and find that it reflects host glycemic sensitivity to the personal gut microbiota profile when sensing the dietary carbohydrate inputs.
Interpretation
Our finding suggests that the HC diet modulates gut microbiota in a person-specific manner and facilitates the connection between gut microbiota and glycemic sensitivity. This study represents a new paradigm for investigating the diet–microbiome interaction in the context of precision nutrition.
Funding
This work was supported by the National Key R&D Program of China, National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation of China.
Keywords: Precision nutrition, High-carbohydrate diet, Gut microbiome, n-of-1 trial
Research in context.
Evidence before this study
The debate continues over the optimal dietary macronutrient distribution for metabolic health. Growing evidence has revealed that the interplay between diet and gut microbiome substantially influences host metabolism, but uncertainties remain regarding their relationships tailored for each subject given the huge inter-individual variability. Therefore, microbiome-based personalized approaches are warranted when contemplating nutritional interventions to improve host metabolism.
Added value of this study
Taking advantage of the unique design of nutritional n-of-1 trials, our study suggests that the gut microbiome exhibits both intra-individual dynamics and inter-individually conserved personalities during the dietary interventions. Furthermore, at the single-subject resolution, person-specific response patterns of gut microbiota to both high-carbohydrate and low-carbohydrate diets exists. Finally, this study identifies a personal as well as conserved gut microbial signature which reflects host glycemic sensitivity to the personal gut microbiota profile when sensing the dietary carbohydrate inputs.
Implications of all the available evidence
Our findings suggest that n-of-1 trial may be a suitable approach to investigating the diet–microbiome interaction at single-subject resolution. The generalizable microbiota-based carb-sensitivity score we have developed may be informative for dietary modifications tailored to each individual.
Introduction
The specific metabolic consequences of consuming different energy-yielding macronutrients have long been controversial.1 The benefits of a high-carbohydrate low-fat (HC) diet on metabolic health are indicated by several dietary guidelines, which are derived from the observational evidence and the knowledge that fats are more calorically dense than carbohydrate.2, 3, 4, 5 On the other hand, theoretical formulations implicate dysmetabolic consequences of the HC diet on postprandial glucose profiles and insulin-adipocyte physiology.6,7 Currently, the debate continues over whether HC or low-carbohydrate (LC) diet is better for optimizing metabolic health, without sufficient evidence to favor one over another.
The gut microbiota has been considered a “virtual organ” for the host and plays a critical role in maintaining host health.8,9 Diet has a substantial impact on the gut microbiota. At the same time the microbiota per se can also determine the physiological consequence of the diet, which together stimulate a widely recognized field of microbiome-based personalized nutrition research.10, 11, 12, 13, 14 However, there is still a knowledge gap regarding the role of personalized gut microbiota profiles in the effect of dietary macronutrients on host metabolism, which may help interpret the mixed results from previous studies comparing the effects of HC and LC diet on metabolic homeostasis.15, 16, 17, 18
Given the complex nature of the gut microbiota variability across individuals, we propose a new type of study design: nutritional n-of-1 trial, which potentially enables individual- and group-level data analysis for either metabolic data or even gut microbiota data.19, 20, 21 The results from this study design could provide a new angle to understand the diet-gut microbiome interplay at single-subject resolution for human health. This may also help address the debate behind HC and LC diets, and their interaction with the gut microbiome, for metabolic health.
Here, based on a series of nutritional n-of-1 trials, we observed striking person-specific gut microbiota response patterns across participants during the intervention. At the group level, we identified a substantial interplay between the gut microbiota and HC diet to modulate the glycemic phenotypes. The glycemic sensitivity to HC depends on a specific personal gut microbiota signature, based on which we created a carb-sensitivity score. In an independent cohort involving 1219 participants, we validated the associations between the carb-sensitivity score and host blood glycemic phenotypes among participants with HC diet habits. The microbiota-based carb-sensitivity score we have developed could help tailor personalized dietary macronutrient intake advice to optimize metabolic health.
Methods
Study population
The WE-MACNUTR was an integration of a serials of n-of-1 trials, which was conducted between October 20, 2019 and December 31, 2019.17,22 This study contained 3 cycles (with 3 pair of cross-over periods) of whole feeding primarily aiming to investigate individual postprandial glycemic as well as gut microbial responses to HC and LC diets. The study consisted of three successive 12-day dietary intervention pairs including a 6-day washout period between each 6-day intervention. The diets were isocaloric with fat and carbohydrate contents as their primary distinguishing features. The HC diet consist of 65–75% total energy intake (E) from carbohydrate and LC diet consist of 15–25%E from carbohydrate. Protein provided 15% energy in all dietary interventions. To ensure maximum control over dietary factors other than the macronutrients distribution, we designed identical food recipes for both HC and LC diets. Specifically, the food items were identical between the two groups, with macronutrient distributions achieved mainly through modifications of staple foods (such as rice, steamed bread, or fried potatoes) and cooking oils. Participants were instructed to consume only the provided foods or beverages and no relevant adverse events were recorded. A total of 30 participants were enrolled and 28 participants completed at least 5 intervention periods. The mean adherence rate of the 28 participants was 98%, with the rate of a single person defined as the number of meals a person consumed divided by the total number of the meals offered in the study.17 In the present analysis, 28 participants (male: 9, female: 19; aged 22–34 years) with 317 repeatedly collected stool samples and more than 110,000 glucose measurements were included.
Guangzhou Nutrition and Health Study (GNHS) cohort included 4048 participants aged 45–70 years at recruitment between 2008 and 2013, and participants were followed up about every 3 years.23 The study was registered on clinicaltrials.gov (NCT03179657). In the present study, we included 1219 participants who had available metagenomic sequencing data for fecal samples collected between 2013 and 2021. Of these participants, 505 participants had metagenomic sequencing data for repeatedly collected samples at a 3-year interval and 609 participants further provided both frequency questionnaire (FFQ) data and clinical information on fasting blood glucose and glycosylated hemoglobin concentration (HbA1c). Among the 609 participants, targeted metabolomics profiling of serum was performed for 539 of them. The blood samples for metabolome measurement and the fecal samples for the short-gun metagenomics sequencing were collected at the same time point during follow-up.
Detailed information on Data generation and preprocessing was summarized in the Supplementary methods.
Statistical analysis
Assessment of the dynamics of gut microbial diversity
For the dynamic analysis of gut microbial alpha diversity before and after each intervention period, we combine the interventional effects on the alpha diversity across different cycles. Specifically, the intra-subject variation stems from the 3 repeated measurements taken before each identical intervention (concurrently, this time point occurs after the identical washout period) across 3 cycles. We then computed the mean value of the alpha diversity measurements before and after the interventions, respectively, for each participant. Consequently, we obtained four average alpha diversity values for each participant, corresponding to the time points before and after the HC/LC interventions. Thereafter, we applied paired t-test to examine the statistical significance of the intervention effects on the alpha diversity at group level.
We applied the vegdist function from the R package vegan to calculate the Bray–Curtis distance based on gut microbial composition to assess intra-individual and inter-individual dissimilarities, respectively. The intra-individual distance was based on paired data, with the baseline gut microbiota data serving as the reference. With regard to the divergence between individuals, the inter-individual distance was evaluated at various time points, separately. At each time point, the inter-individual divergence within a sample set was quantified as the average Bray–Curtis distance of each sample from the group mean. Consequently, every participant possessed a dissimilarity value (calculated by their deviation from the group mean) for each time point. As each participant provided only one sample at each time point, therefore there was no intra-subject variations at each time point.
To analyze the divergence between specific time points (i.e., baseline compared to subsequent time points) at the group level, we employed a paired t-test among those participants who have repeat measurements at both time points (∗, p < 0.05; ∗∗∗, p < 0.001; NS, non-significant). The PCoA analysis for the stool samples collected after each intervention or wash-out also based on the species-level Bray–Curtis distance.
Identification of specific species to the interventional diets
We performed this visual analysis only for those who completed all 3 cycles of HC or LC dietary interventions. For each participant, we calculate the fold-change of each species after each dietary intervention. If the fold-changes of a species were consistently larger than 2 or smaller than 0.5 across the 3 cycles, then this species was defined as a potential responsive species to the interventional diet for this participant. Crucially, we maintained a consistent threshold for both HC and LC interventions, making the results comparable between the diets. As a result, each participant could have a pattern of gut microbial responses to the intervention diet, which included positively responsive species, negatively responsive species as well as non-responsive species. For a specific species, it could also respond consistently or distinctively to the same diet across participants. We consider this part of description on fold changes as visual analysis, other than rigorous statistical approaches.
Principal component analysis to reduce dimensions of the gut microbiota data
We propose a concept that there might be gut microbial features that were associated with host glycemic response (monitored by the CGM) to the dietary interventions in the WE-MACNUTR study. Therefore, we aimed to identify the potential gut microbial features. Before further analysis, we individually averaged each sequential pair of gut microbiota data before and after HC or LC diet intervention to represent the gut microbiota composition accompanying the corresponding period, during which the continuous blood glucose was monitored by the CGM.
Due to the high dimensionality of gut microbiota data, we initially conducted a principal component analysis (PCA) to reduce the dataset to a lower-dimensional space, while preserving most of the information from the original data. As the top PCs capture a substantial proportion of data variance and represent specific gut microbial features, we used the PCs as the proxies of main gut microbial features to test their associations with host glycemic responses. To confirm that the concept is valid, we performed a centered log-ratio (CLR) transformation prior to the principal component analysis to examine the robustness of the results. The CLR transformation for the compositional data was performed within each sample independently. Specifically, for each taxon within a sample, the log-ratio of the counts for this taxon divided by the geometric mean of the counts of all taxa within the sample were calculated. We used multiplicative imputation to handle zero values before CLR transformation by applying the cmultRepl function from the zCompositions package in R.24 We applied the PCA function from the R package FactoMineR and factoextra vegan to perform this principal component analysis.
CGM-derived glycemic features
Based on the continuous glucose monitoring measurements, the reliability of which had been widely recognized in the field,25, 26, 27 we calculated three postprandial glycemic indexes, including MPG, MAGE and TAR, to reflect host glycemic sensitivity to the interventional diets. MPG is the peak value of CGM within 3 h after the first bite of a meal or the maximum CGM value between two meals when the interval is less than 3 h; MAGE is obtained by measuring the arithmetic mean of the differences between consecutive peaks and nadirs provided that the differences are greater than 1 SD around the mean glucose values; TAR is defined as the percentage of time spent when the blood glucose concentration was above the range of 3.9–7.8 mmol/L. Previously, a Bayesian model was used to predict responders with the posterior probability of any 1 of the 3 outcomes reaching a clinically meaningful difference.17 In the present analysis, during each period of HC or HC dietary intervention period, we averaged the repeatedly measured MPG, MAGE and TAR as outcomes for the downstream analysis.
Linear mixed effect regression analysis
The top PCs explaining more than 5% of the total compositional variance were fitted as exposure variables representing gut microbial features in the regression analysis. During each period of HC or LC dietary intervention period, we averaged the repeatedly measured MPG, MAGE and TAR as outcome variables. As both the exposure and outcome variables were repeatedly measured during the study, there are both within- and between-subject variations. We therefore fitted a linear mixed effect model with subject ID as the random variable to address the within-subject variations and test for associations of each PC with MPG, MAGE and TAR, respectively. Potential confounding variables including age, sex, BMI, and physical activity were also included in the regression model. The prototype equation of the linear mixed model is: dependent variable ∼ (intercept) + independent variable + age + sex + BMI+ physical activity+ (1| subject), with the subject ID as the random variable.
External validations of dietary carbohydrate-dependent host–microbe interaction using machine-learning model
Among 609 participants who have complete metagenome sequencing data, food frequency questionnaire data and blood glucose homeostasis data, we defined a total of 353 cases of pre-Diabetes or type 2 diabetes. To validate that the relationship between gut microbiota and host blood glucose homeostasis may depend on dietary carbohydrate intakes, we first classified 305 participants into HC dietary habits group if the ratio of habitual dietary carbohydrate to fat was higher than the median value. Accordingly, we classified 304 participants into the other group with the ratio of habitual dietary carbohydrate to fat lower than the median value. Then we modeled the prediction algorithms in the two subgroups independently. Specifically, we used gradient boosting decision trees from the Light Gradient Boosting Machine (LightGBM) to predict the risk of pre-diabetes or type 2 diabetes in the HC dietary habits group, LC dietary habits group, and the combined group, respectively. The species-level taxonomic abundance was used as the predictive features. A ten-fold cross-validation predictive implementation was used to generate risk of pre-diabetes or type 2 diabetes for each participant. The performance of the model was quantified using the AUC of the receiver operating characteristic for classification. R package pROC was used for receiver operating characteristic curve analyses and DeLong’s test method was used to test the difference between the classifier’s predictive performance.28
External validations of dietary carbohydrate-dependent host–microbe interaction using linear regression model
First of all, we extracted the leading taxa whose relative abundance contributed most to the identified PC3 in the n-of-1 trials, assuming these leading taxa in together may indicate the gut microbial pattern featured by the identified PC. In the external validation cohort, we then mimic this gut microbial pattern using the total relative abundance of these leading taxa. To make this score as simplified or generalizable as possible, we take the sum of the relative abundance of top 10 species contributing to the identified PC3 as the score (termed carb-sensitivity score). The linear regression was used to assess the associations of the carb-sensitivity score (standardized using z-score method) with glycemic traits in the external independent cohort, adjusted for age, sex, BMI, energy intake from protein, physical activity, smoking and drinking status. This analysis was separately performed in the two subgroups stratified by the habitual dietary carbohydrate status. We transformed the values of fasting glucose to normal distribution using negative inverse square before regression analysis due to the skewed distribution of raw data. In this model, the dependent variables are fasting glucose or HbA1c, while the independent variable is the carb-sensitivity score. The prototype equation of the linear model is: dependent variable ∼ (intercept) + independent variable + age + sex + BMI + physical activity + dietary_protein_proportion + smoking_status + alcohol_drinking.
Distance matrix-based variance estimation
We applied the permutational multivariate analysis of variance (PERMANOVA) based on the Bray–Curtis distance matrices to estimate contributions of the carb-sensitivity score-based grouping (top quartile vs. bottom quartile) to inter-individual variations of the gut microbial composition as well as functionality. This PERMANOVA analysis was performed among 1219 participants whose gut metagenomics data as well as information on potential confounding factors were available, and the adonis function from vegan (version 2.5.5) with 999 times permutation were used.
Correlations between the carb-sensitivity score and gut microbial functionality
To assess associations between the carb-sensitivity score and the relative abundance of predicted KEGG orthologs, we performed Spearman correlation analysis separately in the two sub-groups stratified by the habitual dietary carbohydrate status. The corrected p value was calculated using the Bonferroni procedure.
Mediation analysis
Firstly, we screened the potential mediators from CAZymes those were significantly associated with host fasting blood glucose or HbA1c using linear regression analysis with covariables including age, sex, BMI, dietary protein intake, smoking and drinking status. Thereafter, we performed mediation analysis to evaluate whether the screened 16 CAZymes could potentially mediate the association of carb-sensitivity score with host fasting blood glucose or HbA1c. In the mediation analysis, the covariates included age, sex, BMI, dietary protein intake, smoking and drinking status. The mediation analysis was performed using the R-mediation package with same parameter settings (boot = “TRUE”, boot.ci.type = “perc”, conf.level = 0.95, sims = 5000).29 The total effect was obtained through the sum of a direct effect and a mediated (indirect) effect. The FDR (based on the Benjamini–Hochberg procedure) was calculated to correct the multiple test p values for mediation effect.
Associations of the carb-sensitivity score with serum metabolites
Spearman correlation was applied to assess the correlation between the carb-sensitivity score and serum metabolites stratified by the habitual dietary carbohydrate status. The FDR was calculated to correct the multiple test p values using the Benjamini–Hochberg procedure.
All statistical analyses were performed using R software (version: 4.1.1) or STATA (version 15.0) unless otherwise specified.
Ethics
Ethical approval for the study was obtained from the Research Ethics Committee of Westlake University and the Ethics Committee of the School of Public Health at Sun Yat-sen University. All participants provided written informed consent. The trial was registered on ClinicalTrials.gov (registration number: NCT04125602) and run in accordance with the Declaration of Helsinki and Good Clinical Practice.
Role of funders
The funders were not involved in any part of the study design, data collection, data analyses, interpretation, or writing of report.
Results
The discovery dataset was collected from 28 healthy adults who completed the WE-MACNUTR whole feeding n-of-1 trials.17,22 These consisted of gut microbiome profiling and postprandial glycemic responses (MPG, maximum postprandial glucose; MAGE, mean amplitude of glycemic excursion; TAR, time above the range) to sequential dietary challenges. The study design was reported previously and described in detail in the Fig. 1, and the descriptive characteristics of study subjects are presented in Supplementary Table S1. Findings were validated in 1219 middle-aged participants who had available metagenomics data, food frequency questionnaire information as well as glycemic phenotypes in the Guangzhou Nutrition and Health Study. The descriptive characteristics of participants (n = 1219) are presented in Supplementary Table S2.
Fig. 1.
Overview of study cohorts and analysis workflow. The top two boxes is specific to the workflows in the discovery cohort. The flowchart in the first box outlines the study design of n-of-1 trials. The workflow in the second box illustrates the bioinformatics and statistical analyses of the project. From left to right, these analyses encompass taxonomic profiling and dynamics of gut microbiota, individual-level and population-level responsive gut microbial features, dimension reduction of gut microbial features and their associations with host glycemic sensitivity to high-carbohydrate diets. The bottom two boxes are specific to the workflows in the validation cohort. The flowchart in the third box indicates the sample size of our validation dataset and the specific number of participants with available metagenomics, dietary, blood glucose, and serum metabolomics data. The workflow in the bottom box demonstrates the validation analyses. From left to right, these analyses include FFQ-based dietary macronutrient proportion analysis, species-based carb-sensitivity score construction, gut microbial function annotation, validation of the carb-sensitivity score, functional associations and potential mechanisms.
Gut microbiota is intra-individually dynamic and inter-individually personalized in response to sequential dietary challenges
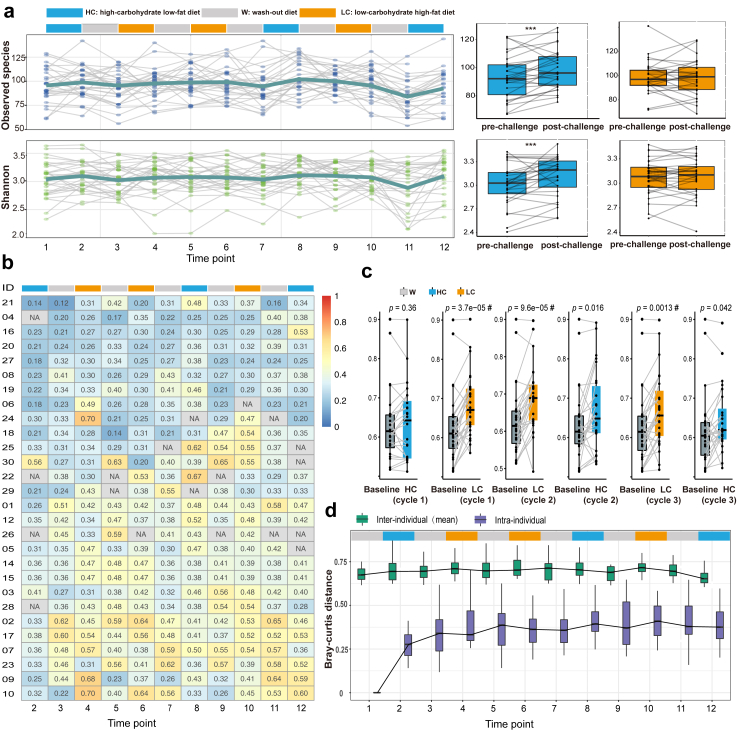
We first depicted the dynamics of the gut microbiota alpha diversity metrics, including the Shannon index, observed species, and Simpson index. These diversity metrics fluctuated strikingly in response to the sequential test-meal challenges at the individual level, and the dynamic patterns were personalized across participants (Fig. 2a and Supplementary Fig. S1a). At the group level, HC diet exhibited much more consistent effects on the alpha diversity across participants than the LC diet (Fig. 2a and Supplementary Fig. S1b and c). Further beta diversity analysis based on Bray–Curtis dissimilarity also confirmed that the intra-individual dynamics of gut microbiota composition were personalized and quite diverse across participants (Fig. 2b), and the HC diet induced much less inter-individual divergence in the gut microbial composition than the LC diet (Fig. 2c).
Fig. 2.
Individual- and group-level gut microbiota dynamics in response to HC and LC interventions. (a) Dynamics of the gut microbial alpha diversity (top, Shannon index; bottom, Observed species) in response to 3 cycles of randomly sequential HC and LC interventions. Each gray line represents the longitudinal dynamics within a participant, and the dark-green line represent the average dynamics among the 28 participants. The boxplots on the right side represent population level average effects of interventional diets on the Shannon index (top) and Observed species (bottom), stratified by macronutrient distributions. The 3-time repeatedly measured alpha diversity data before and after each HC or LC intervention were averaged respectively before the statistical test. The p value from the paired t-test (two sided, n = 28) is shown (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). The boxes represent the interquartile range (IQR), from the first to third quartiles, and the inside line represents the median. The upper and lower whiskers extend to the largest and smallest value no further than 1.5 × IQR, respectively. (b) The longitudinal dynamics of intra-individual species-level Bray–Curtis dissimilarity in gut microbial composition for each participant with their baseline data as reference. The number on the heatmap indicates the specific Bray–Curtis distance which is used to quantify the compositional dissimilarity. (c) The inter-individual divergence of the species-level gut microbial composition at each time point of sample collection. To analyze the divergence between specific time points (i.e., baseline compared to subsequent time points) at the group level, we employed a paired t-test among those participants who have repeat measurements at both time points (the sample sizes for the consecutive paired t-tests were 24, 28, 26, 26, 26, 24) # indicates the Bonferroni corrected p value < 0.05; the adjusted p value was obtained by multiplying the raw p value by the number of independent tests (n = 6). The boxes represent the IQR, from the first to third quartiles, and the inside line represents the median. The whiskers denote the lowest and highest values within 1.5 × IQR from the first and third quartiles. (d) The comparison of inter-individual Bray–Curtis distance of species composition at each time point across participants, as well as the intra-individual Bray–Curtis distance within each participant comparing with their own baseline gut microbiota composition. The mean of pair-wise inter-individual Bray–Curtis distances for each participant compared with other participants, as well as the intra-individual Bray–Curtis distance from the baseline for each participant were presented in the plot. The sample sizes for the consecutive paired t-tests were 28, 24, 28, 28, 26, 27, 27, 26, 27, 26, 26, 24. The boxes represent the IQR and the inside line represents the median. The whiskers denote the lowest and highest values within 1.5 × IQR from the first and third quartiles.
Although gut microbiota was intra-individually dynamic in response to dietary challenges, we found significantly less variation within individuals compared to the variation observed between individuals in terms of the gut microbial composition during the intervention (Fig. 2d). This result suggests that an individual’s overall microbial composition after rigorous feeding intervention is still more similar to their own baseline composition than to those of other participants, which support the relatively conserved and personalized nature of the gut microbiota within a person.
Then we profiled the dynamics of gut microbiota at the phylum level for each participant independently. Both the abundance and dynamic patterns of Bacteroidetes and Firmicutes were strikingly different across these participants. This result suggests an individualized relationship between the interventional diets and gut microbiota. Moreover, Bacteroidetes and Firmicutes showed strictly opposite responses to the dietary challenges among all participants, indicating potential fierce niche competition of these dominant phyla (Supplementary Fig. S2).
Next, we independently characterized the specific species responsive to the interventional diets for each participant. We identified a total of 67 and 83 species responsive to the HC and LC diet, respectively, in at least one participant (Fig. 3a and b and Supplementary Tables S3 and S4). Specifically, 70 species responded uniquely to one of the dietary interventions, and 16 species responded oppositely to HC or LC at the same time (Fig. 3c). The response directions of the identified species were generally consistent across their hosts, which include 123 common responses across participants (Fig. 3a and b). Of note, we also observed person-specific response patterns to both interventional diets, with as many as 22 species showing opposite responses to the LC diet and only 4 species to the HC diet in at least two participants (Fig. 3a and b). Among the 4 species responded inconsistently to the HC diet, Clostridium citroniae was the most robust biomarker for the prediction of incident type 2 diabetes,30 while Bifidobacterium longum had shown beneficial effect on host metabolic homeostasis from preclinical animal studies to human intervention studies.31, 32, 33, 34 These findings suggest considerable inter-individual variation in the response of clinically important bacterial taxa to the same intervention, which calls for personalized approaches when contemplating nutritional interventions to shape the gut microbiota. Moreover, a previous study had demonstrated that the initial or baseline profiling of gut microbiota determined the inter-individual responses of bacterial taxa to nutritional interventions,35 which indicated the importance of the conserved and personalized part of the gut microbiota.
Fig. 3.
Response patterns of gut microbiota to dietary interventions at single-subject resolution. Responsive species to HC diets (a) or LC diets (b) for each participant (Red, positive response; Light blue, negative response). For each participant, the fold-change of each species after each dietary intervention was calculated. If the fold-change of a species was consistently larger than 2 (positive response) or smaller than 0.5 (negative response) across the 3 cycles, then this species was defined as a responsive species to the interventional diet for this participant. (c) Venn plot indicating the common or specific species responsive to HC or LC diets.
Personal gut microbial features modulate host glycemic sensitivity under an HC dietary background
The inter-individual variability in postprandial glycemic responses following the sequential standardized test-meal challenge had been characterized in our previous report.17 Here, we further investigated the potential role of the gut microbiota in modulating individualized host glycemic sensitivity during the HC and LC intervention. Firstly, to reduce the dimensions of the gut microbial composition data, we performed a principal component analysis (PCA) to extract the main components that substantially explained total compositional variance (see the Supplementary methods). As a result, each of the top 3 principal components (PCs) explained more than 5% of the total compositional variance (Supplementary Fig. S3a and b). The values were comparable across the top 3 PCs. However, the intra-individual fluctuations (represented as SDs of time-series values along each PC within each participant) were much more dynamic for PC2 and relatively stable for PC1 and PC3 (Supplementary Fig. S3c and d). Further correlation analysis indicated that the top 3 PCs showed a distinct pattern of correlations with the alpha diversity (Supplementary Fig. S3e). These results together imply that the PC1 and PC3, compared to PC2, capture more conserved nature of gut microbiota composition in response to the dietary challenge.
Based on more than 110,000 glucose measurements collected by the continuous glucose monitoring systems throughout the study, we calculated three postprandial glycemic indexes, including maximum postprandial glucose (MPG), mean amplitude of glycemic excursions (MAGE), and time above the range (TAR) to reflect host glycemic sensitivity to the interventional HC or LC diet (see the Supplementary methods). During the period of HC intervention, the PC3 showed significantly positive associations with MPG (p < 0.01), MAGE (p < 0.001) and TAR (p < 0.05) (Fig. 4a). By contrast, during the period of LC intervention, none of the top 3 PCs were found significantly associated with the MPG, MAGE or TAR (Fig. 4a).
Fig. 4.
Associations of the dimensionality-reduced gut microbial features with postprandial glycemic responses and configurations of the features. (a) Forest plots presenting associations of the dimensionality-reduced gut microbial features (top 3 principal components) with the maximum postprandial glucose (MPG), the time above range (TAR) and the mean amplitude of glycemic excursions (MAGE), stratified by the interventional diets (HC vs. LC). The linear mixed model was used to assess the associations of each top 3 PC (z-score standardized) with MPG, MAGE and TAR, respectively, adjusted for age, sex, BMI and physical activity. Error bars are beta coefficients with 95% confidence intervals. 28 participants with repeat measures were included in this analysis, and # indicates the Bonferroni corrected p value < 0.05; The adjusted p value was obtained by multiplying the raw p value by the number of independent tests (3 outcomes∗2 models). (b) Taxonomic tree based on the collection of 26 leading species that contributed substantially (top 10) to the PC1, PC2 or PC3. The nodes from the inner to the outer circles represented the bacterial taxa from the phylum to the species level, with the nodes at genus and species level colored according to their assignment to families. The notes of bacterial families corresponding to the color of nodes are located at the upper right corner of the graph. The blue rectangles, green triangles and red rectangles on the outermost ring denote the leading contributors to PC1, PC2 and PC3 respectively. (c) Venn plot indicating common and specific of leading contributors to PC1, PC2 and PC3.
In conjunction with the understanding that gut microbiota is relevant for food metabolism and glycemic control,36 our findings imply that we have identified a predominant gut microbial pattern that may predict an individual's glycemic sensitivity under an HC dietary background. Given that distinct microbial profiles can serve as a fingerprint for the host,12 our results may partially explain the individualized postprandial glycemic responses (PPGRs) observed after consuming identical HC meals.
Dietary carbohydrate determines the predictive capacity of gut microbiota for disorders of glucose metabolism in an independent cohort
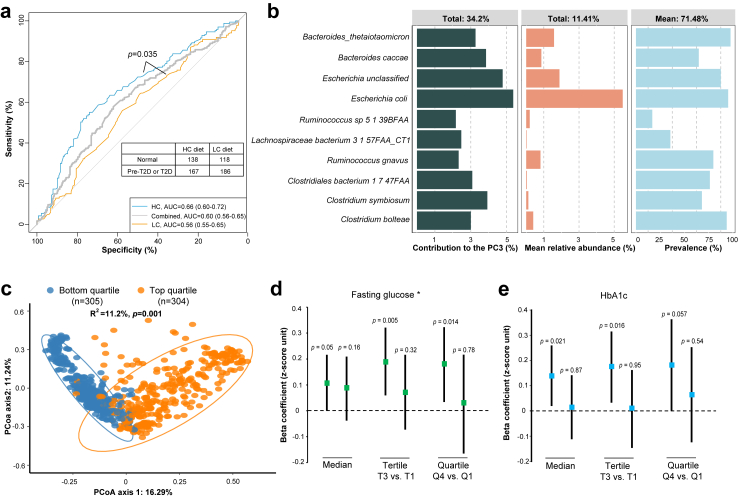
To validate that the associations of gut microbiota with host blood glucose homeostasis depend on the dietary carbohydrate intakes, we examined the predictive capacity of gut microbiota for disorders of glucose metabolism in the GNHS cohort (Supplementary methods and Tables S2 and S5). The overall gut microbial features showed a relatively high predictive capacity for pre-diabetes or diabetes, only among the habitual HC group (AUC, 0.66) but not the habitual LC group (AUC, 0.56, Fig. 5a). Moreover, the difference in the predication performance reached statistical significance (p = 0.035), confirming a critical role of dietary carbohydrate inputs in driving the associations between gut microbiota and host blood glucose homeostasis.
Fig. 5.
External validations for the dietary carbohydrate-dependent associations between the gut microbiota and host blood glucose homeostasis in an independent cohort. (a) ROC curves illustrating the predictive utility of gut microbiota to distinguish the type 2 diabetes or pre-type 2 diabetes from the remaining participants of the cohort. Red curve, predictive performance among participants with HC dietary habits; Green curve, predictive performance among participants without HC dietary habits; Grey curve, predictive performance among available participants regardless of their habitual macronutrient distributions. This analysis is performed among 609 participants who have complete metagenome sequencing data, food frequency questionnaire data and blood glucose homeostasis data. (b) The left bar plot indicates effect size of contributions to PC3 of the top 10 species used for carb-sensitivity score construction, and middle and right bar plots show the relative abundance and prevalence of the top 10 species, respectively, in the independent cohort involving 1219 participants with available metagenome sequencing data. (c) The association of the carb-sensitivity score (based on the identified 10 contributors to the PC3 in the nutritional n-of-1 trials) with the overall microbiota composition, among participants (n = 1219) with available metagenome sequencing data, was evaluated using principal coordinates analysis (PCoA) of Bray–Curtis distance at species level. Each dot represented an individual. Permutational ANOVA (PERMANOVA, 999 permutations) was used to estimate the compositional variation comparing individuals whose carb-sensitivity score ranked in the top quartile (n = 304) with those in the bottom quartile (n = 305). (d) Forest plots showing associations of the carb-sensitivity score with fasting glucose among participants with or without HC dietary habits defined with various thresholds (median, tertile or quartile). (e) Forest plots showing associations of the carb-sensitivity score with HbA1c among participants with or without HC dietary habits defined with various thresholds (median, tertile or quartile). For (d) and (e), the linear regression was used to assess the associations of the carb-sensitivity score (standardized using z-score method) with glycemic traits, adjusted for age, sex, BMI, energy intake from protein, physical activity, smoking and drinking status. This analysis is performed among 609 participants who have complete metagenome sequencing data, food frequency questionnaire data and blood glucose homeostasis data. Error bars are beta coefficients with 95% confidence intervals. ∗, the values of fasting glucose were transformed to normal distribution using negative inverse square.
Configuration of the gut microbiota-based carb-sensitivity score for glycemic control
To characterize specific gut microbes which may predict the glycemic response to dietary carbohydrate, we extracted the leading 10 species contributing to the above-identified PC3. As a comparison, we also extracted the leading 10 species for PC1 and PC2, respectively. There were low overlaps of the leading species among the 3 PCs (Fig. 4b and c), and the contributions, relative abundance as well as prevalence of the leading 10 species in the independent cohort were illustrated in Fig. 5b. The 10 species include 6 species assigned to phylum Firmicutes, 2 to Bacteroidetes and Proteobacteria, respectively. Two species (Escherichia coli and Escherichia unclassified) belonging to the Enterobacteriaceae family are the most abundant, with a mean relative abundance of 5.48% and 1.89%, respectively. The secondly abundant species include two species (Bacteroides caccae and Bacteroides thetaiotaomicron) belonging to the Bacteroidaceae family and one species (Ruminococcus gnavus) assigned to the Lachnospiraceae family, and the mean relative abundance of these species ranges from 0.81% to 1.58%. The remaining species are less abundant, with a mean relative abundance lower than 0.5%.
We constructed an abundance-based gut microbial score in the GNHS cohort based on the 10 leading species to represent the functional role of PC3 microbiota, named carb-sensitivity score (Supplementary methods). We first presented the long-term stability of gut microbiota as well as the carb-sensitivity score among 505 participants who repeatedly provided stool samples with a 3-year interval. An individual’s overall gut microbial composition was more similar to their own composition about 3 years later than to those of other people (p < 0.001, Supplementary Fig. S4a) and a significant correlation was observed between the carb-sensitivity score at baseline and follow-up (r = 0.34, p < 0.001, Supplementary Fig. S4b), which further confirmed the conserved and personalized nature of the gut microbiota.
Then we sought to replicate the interplay between the score and dietary carbohydrates on the glycemic phenotypes (Fig. 1). As negative controls, we also constructed gut microbial scores for PC1- and PC2-captured microbial features, termed PC1- and PC2-score, respectively. To examine whether the 10-species-based score could reflect whole compositional patterns of the gut microbiota, we separated the 1219 GNHS participants into 4 groups by quartiles of the carb-sensitivity score and found there were significant differences regarding the gut microbiota composition comparing participants in the top quartile to those in the bottom quartile (PERMANOVA; R2 = 11.2%, p < 0.001; Fig. 5c).
Based on the FFQ data, participants were classified into the HC group if they maintained a HC dietary habit. Independent replication results suggested significant associations of the gut microbiota-based carb-sensitivity score with fasting glucose (normalization-transformed, β = 0.107, p = 0.05) and HbA1c (β = 0.094, p = 0.02) among participants with HC dietary habits, but not among those without HC dietary habits (Fig. 5d and e). Furthermore, the sensitivity analyses of setting various thresholds to distinguish individuals with habitual HC diets showed consistent results (Fig. 5d and e).
The carb-sensitivity score reflects the metabolic potential of the gut microbiota in sensing dietary carbohydrate input
To better understand to what extent the generalizable carb-sensitivity score may reflect the gut microbiota functional potential, we examined the explained variance of gut microbiota functional capacity by quartiles of the score. Comparing the participants in the top vs. bottom quartile of the score, the grouping explained 19.37% of the total compositional variation for KOs (PERMANOVA, p = 0.001, Fig. 6a).
Fig. 6.
Associations of the generalizable carb-sensitivity score with the gut microbiota functional potential and host serum metabolome. (a) The association of the generalizable carb-sensitivity score with the gut microbiota functional potential among participants (n = 1219) with available metagenome sequencing data was evaluated using principal coordinates analysis (PCoA) of KO. Each dot represented an individual. Permutational ANOVA (PERMANOVA, 999 permutations) was used to estimate the compositional variation in KO patterns comparing individuals whose carbohydrate score ranked in the top quartile (n = 304) with those in the bottom quartile (n = 305). (b) Correlation coefficients of functional KOs with the carb-sensitivity score and negative controls (PC1-score and PC2-score). 1219 participants were included in this analysis. The boxes represent the IQR, from the first to third quartiles, and the inside line represents the median. The whiskers denote the lowest and highest values within 1.5 × IQR from the first and third quartiles. Outliers are plotted individually and the violin plots indicate the distribution of the correlation coefficients. (c) Correlation between carb-sensitivity score and the summed relative abundance of KOs mapped to carbohydrate metabolism. Each dot represents one participant, and the red dashed line shows the linear regression line. The association strength was assessed using Spearman correlation (two sided; the correlation coefficient and p values are reported). (d) Analysis of the association between carb-sensitivity score and HbA1c as mediated by CAZYmes. Asterisks indicate statistically significant regression coefficients between carb-sensitivity score and CAZYmes or between CAZYmes and HbA1c (∗∗FDR < 0.05, ∗∗∗FDR < 0.01). The symbol # indicate significant mediation effect of CAZymes on the associations between carb-sensitivity score and HbA1c, with corrected q value < 0.05. (e) Heatmap of statistically significant correlations (Spearman) between the generalizable carb-sensitivity score and individual serum metabolite in two subgroups stratified by the habitual dietary macronutrient distribution. This heatmap only included metabolites that survived the FDR thresholds of 0.01 in at least one of the subgroups, and the colored entries represent the correlation coefficients. Asterisks indicate a statistically significant correlation coefficients (∗FDR < 0.1, ∗∗FDR < 0.05, ∗∗∗FDR < 0.01) and the six blocks on the right represent the classes (e.g., carbohydrates, bile acids and organic acids) corresponding to each metabolite on the left. This analysis was limited in 539 participants who have complete metagenome sequencing data, food frequency questionnaire data and serum metabolomics data.
We further performed a correlation analysis between the constructed scores and each of the KOs. The median value of the correlation coefficients for the carb-sensitivity score was 0.29. In contrast, the PC1 score and PC2 score had median correlation coefficients of 0.12 and 0.14, respectively (Fig. 6b). These results suggest that the carb-sensitivity score is more effective at capturing intensive gut microbiota functional patterns compared to other negative control scores (p < 0.001). We then focused on the function of carbohydrate metabolism and found that a higher carb-sensitivity score was associated with a higher relative abundance of KOs mapped to carbohydrate metabolism (p < 0.0001, Fig. 6c). Therefore, the carb-sensitivity score might be a biomarker of gut microbial metabolic potential after sensing the dietary carbohydrate input.
Microbial carbohydrate-active enzymes mediate the associations between carb-sensitivity score and host blood glucose homeostasis
As the carb-sensitivity score was highly correlated with the functional potential of carbohydrate metabolism, we further examined whether the carbohydrate-active enzymes (CAZymes) encoded in gut metagenomes had a mediation effect on the associations between carb-sensitivity score and host blood glycemic phenotypes. Among the subgroup of participants who kept an HC dietary habit, we identified a total of 16 CAZymes that were significantly associated with host HbA1c after adjusting for potential confounders (false discover rate (FDR) < 0.05, Supplementary Table S6). By contrast, we did not find any fasting glucose or HbA1c-associated CAZymes among the other subgroup without an HC dietary habit (Supplementary Table S7).
The subsequent mediation analyses among the HC subgroup indicated potential mediation effects for 4 out of the 16 CAZymes regarding the associations between carb-sensitivity score and HbA1c (Fig. 6d, Supplementary Fig. S5), including 1 glycosyl transferases (GT19), 1 glycoside hydrolases (GH5), 1 polysaccharide lyases (PL4) and 1 carbohydrate esterase (CE4). We summarized the potential substrates of these CAZymes in the Supplementary Table S10.
The links between carb-sensitivity score and host serum metabolome are dependent on dietary carbohydrate intakes
To further validate if the dietary carbohydrate-dependent gut–host interaction was reflected in the serum metabolome as functional readouts, we examined the associations between the carb-sensitivity score and 199 serum metabolites. Among the subgroup of participants who kept an HC dietary habit (n = 270), we found 5 and 13 carb-sensitivity score-related metabolites which survived the FDR thresholds of 0.05 and 0.1, respectively (Fig. 6e, Supplementary Table S8). Among the identified serum metabolites, 2-hydroxybutyric acid, glycoursodeoxycholic acid (GUDCA) and indoleacetic acid were positively associated with the carb-sensitivity score. The remaining metabolites, including 3-indolepropionic acid, hydrocinnamic acid, hippuric acid, glycodeoxycholicacid (GDCA) and deoxycholicacid (DCA), were negatively associated with the score. By contrast, we did not find any significant serum metabolites (all at FDR > 0.1, Supplementary Table S9) among the other dietary subgroup (n = 269). These results suggest that dietary carbohydrate inputs were essential for linking the score-captured gut microbiome pattern and the host serum metabolomics profile.
Moreover, most of the identified score-related metabolites have been well studied and correlated to glycemic traits. For example, both 2-Hydroxybutyric acid and GUDCA were positively associated with fasting blood glucose and HbA1c in prior studies, including a mendelian randomization study suggesting a causal effect of 2-Hydroxybutyric acid on glycemic traits.37,38 On the other hand, 3-indolepropionic acid, hydrocinnamic acid (as a major component of cinnamon extract), hippuric acid, DCA and GDCA had been related to beneficial effects on glycemic traits and shown great potential as candidates for the treatment of disorders associated with glucose homeostasis.39, 40, 41, 42, 43, 44
Discussion
Using a series of nutritional n-of-1 trials, we have identified both common and person-specific gut microbial species responsive to HC and LC diets and demonstrated intra-individually dynamic and inter-individually personalized signatures of the gut microbiome in response to the standardized meals. Our study proposed an externally validated gut microbial feature, named carb-sensitivity score, reflecting an individual’s glycemic sensitivity to carbohydrate intakes. This feature may characterize a gut microbiota pattern that is highly capable of carbohydrate metabolism.
The gut microbiome is generally considered a dynamic ecosystem that can be substantially shaped by the dietary inputs. However, most prior studies focused on population averages and high inter-person variability in response to diet demands developing more personalized approaches.45,46 The present study, taking advantage of the unique design of nutritional n-of-1 trials, demonstrated both common and personalized responses of gut microbiota to identical feeding meals and identified specific responsive species to both HC and LC diets for each participant. Despite the dynamic characteristics, the gut microbiome is also considered a personal signature predictive of host metabolic response to food categories or patterns.11,12,27 Our study proposes that although the gut microbiota is intra-individually dynamic in response to dietary challenges, the dynamics are very conserved compared to the inter-individual dissimilarities at the population level. Thus, the inter-individual variation and conserved nature of the gut microbial ecosystem make the gut microbiome a potential personal signature that influences the diet-health connection.
Our study, aggregating repeated measurements of both gut microbiome and high-resolution PPGRs, identified a relatively conserved gut microbial feature that was significantly associated with PPGRs to the HC dietary challenges. Although this association did not occur with the LC diets, the statistical interaction between the microbial feature and interventional diets did not reach significance, possibly due to the limited sample size. Therefore, caution should be taken when interpreting the findings. Nevertheless, we successfully validated that this microbial feature could indicate the host’s glycemic sensitivity to a HC diet in an independent cohort.
Moreover, the effectiveness of this feature in indicating sensitivity to HC diets might be not contingent upon the consumption of any specific type of carbohydrate, as we did not limit the characterization of HC dietary habits to particular food items within our validation cohort. To the best of our knowledge, this is the first reported nutritional n-of-1 study that provides a microbiota-based index to help quantify a personal glycemic sensitivity to the HC diet. These findings reaffirm that not everyone responds equally to the HC diet and highlight a concept of gut microbiome-modulated glycemic sensitivity to a HC diet. Our findings support the application of the n-of-1 trial, integrated with gut microbiome data, to design precision nutritional strategies to promote health in clinical practice.47
Most of the 10 species used to quantify host glycemic sensitivity to HC diet, including species Bacteroides_thetaiotaomicron, Clostridium bolteae, Clostridiales bacterium_1_7_47FAA, and Ruminococcus_gnavus, have been reported to have big influences on host health or are closely related with the fermentation of carbohydrate. Bacteroides_thetaiotaomicron serves as a classical example showing that microbial contributions to carbohydrate metabolism are responsible for the conversion of many dietary substances into molecules that can be absorbed and utilized by the host.48,49 Animal studies also reported that Bacteroides_thetaiotaomicron could produce changes in the expression of a number of host genes involved in the processing and absorption of carbohydrate, as well as the breakdown and absorption of dietary lipids.48,50 C. bolteae is one of the most robust species showing predictive capability of incident type 2 diabetes, and both Clostridium_bolteae and Clostridiales_bacterium_1_7_47FAA are involved in carbohydrate metabolism and glycan biosynthesis.30,51 Another well-studied species is Ruminococcus_gnavus which has been reported to be positively correlated with fasting blood glucose,52 and this is in line with our study.
We speculated that the identified taxonomy-based feature or the 10-species-based carb-sensitivity score is more likely to be a biomarker of a specific gut microbiome pattern. As expected, the 10-species-based score could properly separate participants of the independent cohort into subgroups with a distinct pattern of gut microbial composition. Thus, what is more important might be the myriad of functions this specific pattern performs. Functional metagenomic profiling of specific gut microbiome patterns could help advance the understanding of roles of the gut microbiome in the diet-health sequence, which enables us to comprehend further how diet impacts health explicitly at the individual level. As this study focused on the diet–microbiota interplays as moderators of human metabolism, more attention was paid to the gut microbiome metabolic potential. The high-score-represented gut microbiome pattern was characterized by the higher metabolic capability of carbohydrates. This functional profile of the gut microbiome pattern matched well with that the score was associated with host blood glucose homeostasis when sensing the carbohydrate inputs.
Further exploration of the CAZymes encoded in gut metagenomes identified potential mediators between the carb-sensitivity score and host HbA1c among participants sensing HC inputs. Among these potential mediators, glycoside hydrolases are the largest and best-studied class of CAZymes responsible for carbohydrate degradation.53 Interestingly, Bacteroides_thetaiotaomicron, one of the most important components of the carb-sensitivity score, was reported dedicating remarkable proportions of their genomes to glycoside hydrolases and polysaccharide lyases.54,55 These findings highlight that the functional capacity of the score-represented gut microbiota ecosystem supports its influence on carbohydrate degradation, and that the release of these functional potentials depends on dietary carbohydrate intakes. Taking the starch utilization system of Bacteroides_thetaiotaomicron as an example, prior studies have shown that the bacteria expend energy to express genes involved in nutrients metabolism only when the particular nutrient is available in the gut ecosystem.56 Specifically, the genome of Bacteroides_thetaiotaomicron contained genes involved in starch metabolism and a transcriptional factor that responded to the presence of glucose oligomers by increasing the transcription of metabolic genes.57,58 On the other hand, Bacteroides_thetaiotaomicron was recently reported to decrease the active glucagon like peptide-1 and disrupts host glucose metabolism through expressing dipeptidyl peptidase 4 (DPP4).59 Thus, the Bacteroides_thetaiotaomicron would endow the host with a high sensitivity to glucose oligomers and impaired glucose tolerance.
Gut microbiota-related metabolites are important intermediates in the cross-talk between the gut microbiota and the host. Prior studies had suggested that 2-hydroxybutyric acid was gut-derived and could be regulated by altering the gut microbiota.60 Moreover, both observational and Mendelian randomization studies supported a causal effect of 2-hydroxybutyric acid on glycemic traits.37,61,62 Our study yielded consistent results that the carb-sensitivity score was positively associated with the serum 2-hydroxybutyric acid among participants having HC habits. At the same time, the carb-sensitivity score was negatively associated with the serum level of beneficial metabolites (e.g., 3-indolepropionic acid, hippuric acid and GDCA) among participants who kept HC habits, further indicating the dependence of gut–host interaction on dietary inputs. Of note, these findings are only correlations which may help in understanding the reason why the score is predictive of host response to HC diets. However, further research is warranted to clarify the underlying mechanisms through reliable functional interpretations.
This study possesses several limitations. Firstly, the generalizability of the findings regarding the diet-gut microbiome interaction at the individual level is highly restricted, given that the data collection, analysis, and interpretation were performed independently for each participant. Nonetheless, the group-level analysis may yield insights that can be more widely applied. Secondly, we employed principal components to capture high-dimensional gut microbiome features. This strategy, however, may be subject to a loss of precision, despite the external validation conducted. Thirdly, the design of the validation analysis was not based on an interventional trial but rather on an observational cohort study. In this study, information on dietary intakes was collected using a food frequency questionnaire, which may have limitations in terms of accurately capturing dietary intakes and introducing possible measurement errors. Moreover, only participants with available data were included in the analyses, which may introduce potential bias in our findings and interpretations.
Conclusions
In conclusion, we discover a species-based microbiota score which reflects personalized glycemic sensitivity to HC diets. This finding suggests that there is no “one-size-fits-all” solution to the debate regarding HC and LC diets, and the optimal dietary choice may partially depend on the unique composition of an individual's gut microbiota. This study points to a new direction of precision nutrition research, wherein nutritional n-of-1 trials and gut microbiome analysis are integrated to discover personalized dietary guidelines.
Contributors
Conceptualization: J.-S.Z. and Y.F.; Methodology: Y.F., W.G.; Participant recruitment and study visits: H.-L.Z., Y.T., X.L., M.S. Z.J. and L.-B. Z.; Statistical analysis: Y.F., W.G. and H.Z.; Visualization: Y.F., W.G., and H.Z.; Resources: J.-S.Z., Y.-m. C.; Writing (original draft): Y.F.; Writing (review and editing): J.-S.Z, Y.-m. C., and J.M.O.; Accessed and verified the underlying data: J.-S.Z. and Y.F.; Supervision: J.-S.Z, Y.-m. C.; Project administration: Y.F., Y.T., J.T. and H.-L. Z.; Funding acquisition: J.-S.Z., Y.-m. C. and Y.F.; Y.F., W.G., and H.-L. Z. contributed equally to this work. All authors revised and approved the final version of the manuscript for publication.
Data sharing statement
The raw data of metagenomic sequencing in this study have been deposited in the Genome Sequence Archive (GSA) (https://ngdc.cncb.ac.cn/gsa/) at accession number CRA010236 and CRA008796. The MAGs used in this study have been deposited in our website (https://zheng.lab.westlake.edu.cn/Resource.htm). The serum metabolomics data have been deposited in the Metabolomics Workbench (https://www.metabolomicsworkbench.org/) at study ID ST001669. UCSC hg19 is available from https://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_19/GRCh37.p13.genome.fa.gz. All data supporting the findings of this study are available within the paper and its Supplementary materials.
Declaration of interests
The authors declare no conflict of interest.
Acknowledgements
Funding: This study was funded by the National Key R&D Program of China (2022YFA1303900), National Natural Science Foundation of China (82103826, 82073529, 92374112, 82073546, U21A20427), “Pioneer” and “Leading goose” R&D Program of Zhejiang (2024SSYS0032, 2022C03102), Zhejiang Provincial Natural Science Foundation of China (LQ21H260002), the Research Program (No. 202208012) of Westlake Laboratory of Life Sciences and Biomedicine.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105483.
Contributor Information
Yu-ming Chen, Email: chenyum@mail.sysu.edu.cn.
Ju-Sheng Zheng, Email: zhengjusheng@westlake.edu.cn.
Appendix ASupplementary data
References
- 1.San-Cristobal R., Navas-Carretero S., Martínez-González M.Á., Ordovas J.M., Martínez J.A. Contribution of macronutrients to obesity: implications for precision nutrition. Nat Rev Endocrinol. 2020;16:305–320. doi: 10.1038/s41574-020-0346-8. [DOI] [PubMed] [Google Scholar]
- 2.Wolever T.M.S. Carbohydrate and the regulation of blood glucose and metabolism. Nutr Rev. 2003;61:S40–S48. doi: 10.1301/nr.2003.may.S40-S48. [DOI] [PubMed] [Google Scholar]
- 3.Seid H., Rosenbaum M. Low carbohydrate and low-fat diets: what we don’t know and why we should know it. Nutrients. 2019;11:2749. doi: 10.3390/nu11112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cybulska B., Kłosiewicz-Latoszek L. Landmark studies in coronary heart disease epidemiology. The framingham heart study after 70 years and the seven countries study after 60 years. Kardiol Pol. 2019;77:173–180. doi: 10.5603/KP.a2019.0017. [DOI] [PubMed] [Google Scholar]
- 5.Dietary fat and its relation to heart attacks and strokes. Report by the central committee for medical and community Program of the American heart association. JAMA. 1961;175:389–391. [PubMed] [Google Scholar]
- 6.Ludwig D.S., Aronne L.J., Astrup A., et al. The carbohydrate-insulin model: a physiological perspective on the obesity pandemic. Am J Clin Nutr. 2021;114:1873–1885. doi: 10.1093/ajcn/nqab270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig D.S. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 8.Evans J.M., Morris L.S., Marchesi J.R. The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol. 2013;218:R37–R47. doi: 10.1530/JOE-13-0131. [DOI] [PubMed] [Google Scholar]
- 9.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 10.Mills S., Stanton C., Lane J., Smith G., Ross R. Precision nutrition and the microbiome, Part I: current state of the science. Nutrients. 2019;11:923. doi: 10.3390/nu11040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeevi D., Korem T., Zmora N., et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. doi: 10.1016/j.cell.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Chen L., Wang D., Garmaeva S., et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell. 2021;184:2302–2315.e12. doi: 10.1016/j.cell.2021.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Wang D.D., Nguyen L.H., Li Y., et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med. 2021;27:333–343. doi: 10.1038/s41591-020-01223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 15.Lammert O., Grunnet N., Faber P., et al. Effects of isoenergetic overfeeding of either carbohydrate or fat in young men. Br J Nutr. 2000;84:233–245. [PubMed] [Google Scholar]
- 16.Horton T.J., Drougas H., Brachey A., Reed G.W., Peters J.C., Hill J.O. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62:19–29. doi: 10.1093/ajcn/62.1.19. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y., Fu Y., Tian Y., et al. Individual postprandial glycemic responses to diet in n-of-1 trials: westlake N-of-1 trials for macronutrient intake (WE-MACNUTR) J Nutr. 2021;151:3158–3167. doi: 10.1093/jn/nxab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanamori K., Ihana-Sugiyama N., Yamamoto-Honda R., et al. Postprandial glucose surges after extremely low carbohydrate diet in healthy adults. Tohoku J Exp Med. 2017;243:35–39. doi: 10.1620/tjem.243.35. [DOI] [PubMed] [Google Scholar]
- 19.Potter T., Vieira R., De Roos B. Perspective: application of N-of-1 methods in personalized nutrition research. Adv Nutr. 2021;12:579–589. doi: 10.1093/advances/nmaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston B.C., Mills E. N-of-1 randomized controlled trials: an opportunity for complementary and alternative medicine evaluation. J Alternative Compl Med. 2004;10:979–984. doi: 10.1089/acm.2004.10.979. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Wilt G.J., Stunnenberg M.B.C. 2011. Combining N-of-1 trials to estimate population clinical effectiveness of drugs using Bayesian hierarchical modeling; the case of Mexiletine for patients with Non-Dystrophic Myotonia. [Google Scholar]
- 22.Tian Y., Ma Y., Fu Y., Zheng J.S. Application of n-of-1 clinical trials in personalized nutrition research: a trial protocol for westlake n-of-1 trials for macronutrient intake (WE-MACNUTR) Curr Dev Nutr. 2020;4:1–8. doi: 10.1093/cdn/nzaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z.-Q., He L.-P., Liu Y.-H., Liu J., Su Y.-X., Chen Y.-M. Association between dietary intake of flavonoid and bone mineral density in middle aged and elderly Chinese women and men. Osteoporosis Int. 2014;25:2417–2425. doi: 10.1007/s00198-014-2763-9. [DOI] [PubMed] [Google Scholar]
- 24.Palarea-Albaladejo J., Martín-Fernández J.A. ZCompositions - R package for multivariate imputation of left-censored data under a compositional approach. Chemometr Intell Lab Syst. 2015;143:85–96. doi: 10.1016/j.chemolab.2015.02.019. [DOI] [Google Scholar]
- 25.Shilo S., Keshet A., Rossman H., et al. Continuous glucose monitoring and intrapersonal variability in fasting glucose. Nat Med. 2024;30:1424–1431. doi: 10.1038/s41591-024-02908-9. [DOI] [PubMed] [Google Scholar]
- 26.Keshet A., Shilo S., Godneva A., et al. CGMap: characterizing continuous glucose monitor data in thousands of non-diabetic individuals. Cell Metab. 2023;35:758–769.e3. doi: 10.1016/j.cmet.2023.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Berry S.E., Valdes A.M., Drew D.A., et al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–973. doi: 10.1038/s41591-020-0934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robin X., Turck N., Hainard A., et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valente M.J., Rijnhart J.J.M., Smyth H.L., Muniz F.B., MacKinnon D.P. Causal mediation programs in R, M plus , SAS, SPSS, and stata. Struct Equ Model. 2020;27:975–984. doi: 10.1080/10705511.2020.1777133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruuskanen M.O., Erawijantari P.P., Havulinna A.S., et al. Gut microbiome composition is predictive of incident type 2 diabetes in a population cohort of 5,572 Finnish adults. Diabetes Care. 2022;45:811–818. doi: 10.2337/dc21-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schellekens H., Torres-Fuentes C., van de Wouw M., et al. Bifidobacterium longum counters the effects of obesity: partial successful translation from rodent to human. EBioMedicine. 2021;63 doi: 10.1016/j.ebiom.2020.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben Othman M., Sakamoto K. Effect of inactivated Bifidobacterium longum intake on obese diabetes model mice (TSOD) Food Res Int. 2020;129 doi: 10.1016/j.foodres.2019.108792. [DOI] [PubMed] [Google Scholar]
- 33.Koutnikova H., Genser B., Monteiro-Sepulveda M., et al. Impact of bacterial probiotics on obesity, diabetes and non-alcoholic fatty liver disease related variables: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9:1–12. doi: 10.1136/bmjopen-2017-017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hao J., Zhang Y., Wu T., et al. The antidiabetic effects of Bifidobacterium longum subsp. longum BL21 through regulating gut microbiota structure in type 2 diabetic mice. Food Funct. 2022;13:9947–9958. doi: 10.1039/d2fo01109c. [DOI] [PubMed] [Google Scholar]
- 35.Chung W.S.F., Walker A.W., Bosscher D., et al. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 2020;20:1–14. doi: 10.1186/s12866-020-01968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D.D., Hu F.B. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:416–426. doi: 10.1016/S2213-8587(18)30037-8. [DOI] [PubMed] [Google Scholar]
- 37.Sun Y., Lu Y.-K., Gao H.-Y., Yan Y.-X. Effect of metabolite levels on type 2 diabetes mellitus and glycemic traits: a mendelian randomization study. J Clin Endocrinol Metab. 2021 doi: 10.1210/clinem/dgab581. [DOI] [PubMed] [Google Scholar]
- 38.Osuna-Prieto F.J., Rubio-Lopez J., Di X., et al. Plasma levels of bile acids are related to cardiometabolic risk factors in young adults. J Clin Endocrinol Metab. 2022;107:715–723. doi: 10.1210/clinem/dgab773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. The microbial metabolite indole-3-propionic acid improves glucose metabolism in rats, but does not affect behaviour. Arch Physiol Biochem. 2018;124:306–312. doi: 10.1080/13813455.2017.1398262. [DOI] [PubMed] [Google Scholar]
- 40.Li W., Liu R., Li X., et al. Saxagliptin alters bile acid profiles and yields metabolic benefits in drug-naïve overweight or obese type 2 diabetes patient. J Diabetes. 2019;11:982–992. doi: 10.1111/1753-0407.12956. [DOI] [PubMed] [Google Scholar]
- 41.de Mello V.D., Lankinen M.A., Lindström J., et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201700019. [DOI] [PubMed] [Google Scholar]
- 42.Kim W., Khil L.Y., Clark R., et al. Naphthalenemethyl ester derivative of dihydroxyhydrocinnamic acid, a component of cinnamon, increases glucose disposal by enhancing translocation of glucose transporter 4. Diabetologia. 2006;49:2437–2448. doi: 10.1007/s00125-006-0373-6. [DOI] [PubMed] [Google Scholar]
- 43.Khan A., Safdar M., Ali Khan M.M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 44.Mang B., Wolters M., Schmitt B., et al. Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 45.Beam A., Clinger E., Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13:2795. doi: 10.3390/nu13082795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merra G., Noce A., Marrone G., et al. Influence of mediterranean diet on human gut microbiota. Nutrients. 2020;13:7. doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J.S., Ordovás J.M. Precision nutrition for gut microbiome and diabetes research: application of nutritional n-of-1 clinical trials. J Diabetes. 2021;13:1059–1061. doi: 10.1111/1753-0407.13220. [DOI] [PubMed] [Google Scholar]
- 48.Zocco M.A., Ainora M.E., Gasbarrini G., Gasbarrini A. Bacteroides thetaiotaomicron in the gut: molecular aspects of their interaction. Dig Liver Dis. 2007;39:707–712. doi: 10.1016/j.dld.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Xu J., Bjursell M.K., Himrod J., et al. A genomic view of the human- Bacteroides thetaiotaomicron symbiosis. Science. 2003;299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 50.Bäckhed F., Ding H., Wang T., et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang R., Li F., Zhou Y., et al. Metagenome-wide association study of the alterations in the intestinal microbiome composition of ankylosing spondylitis patients and the effect of traditional and herbal treatment. J Med Microbiol. 2020;69:797–805. doi: 10.1099/jmm.0.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li G., Yin P., Chu S., et al. Correlation analysis between GDM and gut microbial composition in late pregnancy. J Diabetes Res. 2021;2021:1–17. doi: 10.1155/2021/8892849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wardman J.F., Bains R.K., Rahfeld P., Withers S.G. Carbohydrate-active enzymes (CAZymes) in the gut microbiome. Nat Rev Microbiol. 2022;20:542–556. doi: 10.1038/s41579-022-00712-1. [DOI] [PubMed] [Google Scholar]
- 54.Kaoutari A El, Armougom F., Gordon J.I., Raoult D., Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11:497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 55.Davies G.J., Gloster T.M., Henrissat B. Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr Opin Struct Biol. 2005;15:637–645. doi: 10.1016/j.sbi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Bjursell M.K., Martens E.C., Gordon J.I. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J Biol Chem. 2006;281:36269–36279. doi: 10.1074/jbc.M606509200. [DOI] [PubMed] [Google Scholar]
- 57.Reeves A.R., Wang G.R., Salyers A.A. Characterization of four outer membrane proteins that play a role in utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1997;179:643–649. doi: 10.1128/jb.179.3.643-649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Elia J.N., Salyers A.A. Effect of regulatory protein levels on utilization of starch by Bacteroides thetaiotaomicron. J Bacteriol. 1996;178:7180–7186. doi: 10.1128/jb.178.24.7180-7186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K., Zhang Z., Hang J., et al. Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target. Science. 2023;381 doi: 10.1126/science.add5787. [DOI] [PubMed] [Google Scholar]
- 60.Zheng N., Gu Y., Hong Y., et al. Vancomycin pretreatment attenuates acetaminophen-induced liver injury through 2-hydroxybutyric acid. J Pharm Anal. 2020;10:560–570. doi: 10.1016/j.jpha.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shantavasinkul P.C., Muehlbauer M.J., Bain J.R., et al. Improvement in insulin resistance after gastric bypass surgery is correlated with a decline in plasma 2-hydroxybutyric acid. Surg Obes Relat Dis. 2018;14:1126–1132. doi: 10.1016/j.soard.2018.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye D., Huang J., Wu J., et al. Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microb. 2023;15 doi: 10.1080/19490976.2022.2154552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.