Abstract
Macrophages play an important role in the development of vascular diseases, with their homeostasis closely linked to metabolic reprogramming. This study aims to explore the role of circular RNA-mediated epigenetic remodeling in maintaining macrophage homeostasis during diabetes-induced microvascular dysfunction. We identified a circular RNA, circRNA-sperm antigen with calponin homology and coiled-coil domains 1 (cSPECC1), which is significantly up-regulated in diabetic retinas and in macrophages under diabetic stress. cSPECC1 knockdown in macrophages attenuates M1 macrophage polarization and disrupts macrophage-endothelial crosstalk in vitro. cSPECC1 knockdown in macrophages mitigates diabetes-induced retinal inflammation and ameliorates retinal vascular dysfunction. Mechanistically, cSPECC1 regulates GPX2 expression by recruiting eIF4A3, enhancing GPX2 mRNA stability and altering arachidonic acid metabolism. The metabolic intermediate 12-HETE has emerged as a key mediator, regulating both macrophage homeostasis and the crosstalk between macrophages and endothelial cells. Exogenous 12-HETE supplementation interrupts the anti-angiogenic effects of cSPECC1 knockdown. Collectively, circSPECC1 emerges as a novel regulator of macrophage-mediated vascular integrity and inflammation. Targeting the metabolic reprogramming of macrophages presents a promising therapeutic strategy for mitigating diabetes-induced vascular dysfunction.
Keywords: Vascular dysfunction, Macrophage homeostasis, Circular RNAs, Diabetic retinopathy, Metabolic reprogramming
Graphical abstract
Highlights
-
•
A significant up-regulation of cSPECC1 expression in diabetic retinas and in macrophages responding to diabetic stress.
-
•
CSPECC1 knockdown in macrophages attenuates M1 macrophage polarization and disrupts macrophage-endothelial crosstalk.
-
•
CSPECC1 knockdown attenuates macrophage-mediated retinal inflammation and diabetic vascular function.
-
•
CSPECC1 orchestrates macrophage metabolic reprogramming by stabilizing GPX2 mRNA.
-
•
12-HETE is a critical mediator in macrophage homeostasis and its interplay with endothelial cells, and Exogenous 12-HETE supplementation interrupts the anti-angiogenic effects of cSPECC1 knockdown.
1. Introduction
The vascular system constitutes the largest network in human body, which can deliver nutrients, immune cells, and other molecules to all organs. However, risk factors such as hyperglycemia, hypertension, and hyperlipidemia can lead to vascular injuries, resulting in conditions such as hypoperfusion, vessel leakage, and abnormal angiogenesis [1]. Vascular dysfunction occurs in the pathogenesis of several human diseases, such as malignancies, ischemic conditions, inflammatory disorders, and sight-threatening ocular diseases [2,3]. Currently, a major focus in the therapy of vascular diseases, especially in ocular dieases, centers on preventing re-endothelialization and angiogenesis [4]. Several pro-angiogenic factors, including VEGF and FGF, have been identified and approved as the therapeutic targets for vascular diseases [5]. Anti-VEGF agents exhibit significant therapeutic efficacy; however, their use faces with several challenges. VEGF blockade, while effectively inhibiting pathological angiogenesis, may also cause the potential injuries to healthy vessels. Prolonged anti-VEGF treatment can cause several adverse effects, such as hypertension, renal vascular dysfunction, and heart failure [6,7]. Thus, it is required to identify new signaling pathways involved in vascular dysfunction. Combining inhibitors that target these pathways with VEGF blockers may improve treatment outcomes for vascular diseases.
Vascular dysfunction encompasses structural and functional changes that occur in blood vessels [8]. Numerous epidemiological, clinical, and experimental studies have revealed a tight link between vascular dysfunction and inflammation [9,10]. The pathogenesis of vascular diseases involves the interplays among local microenvironment, inflammatory mediators, and infiltrating immune cells [11]. These inflammation-related cells include macrophages, lymphocytes, neutrophils, and natural killer cells [12,13]. In addition to their role in regulating inflammation and orchestrating immune responses, these cells can participate in the pathogenesis of vascular diseases [14,15].
Macrophages, as the specialized phagocytes, play a crucial role in maintaining body equilibrium by eliminating apoptotic cells, cellular debris, and degraded matrix, or by releasing immunomodulatory cytokines [16]. Macrophage homeostasis is critical for function maintenance of several biological processes, such as tissue homeostasis, infection, tissue repair, inflammatory response, and metabolic process [17]. In various (patho-) physiological conditions, vascular dysfunction and inflammation often coexist [18]. During vascular dysfunction, the equilibrium of macrophage homeostasis is disrupted, causing the secretion of pro-angiogenic stimuli by macrophages [19,20]. The pro-angiogenic signals released by macrophages induce abnormal endothelial cell activation, leading to vessel leakage and angiogenesis [21,22]. Abnormal crosstalk between macrophages and endothelial cells is implicated in the progression of vascular dysfunction. Thus, investigating interventions to modulate this interaction may provide new strategies for treating vascular diseases.
The retinal vasculature is an ideal model for studying vascular function because it can be directly and non-invasively observed. Vascular dysfunction is evident in various retinal disorders, including diabetic retinopathy (DR) [23]. DR stands as the major cause of legal blindness among adults aged 20–74 years. Vision loss in DR occurs due to retinal detachment, vitreous hemorrhage, macular edema, and capillary non-perfusion [24,25]. The pathogenesis of DR is tightly associated with chronic low-grade inflammation, up-regulated inflammatory factors, and activated inflammation-related cells [26,27]. Under hyperglycemic condition, macrophage homeostasis is disturbed, which can mediate inflammatory response and abnormal endothelial activation in DR [26]. Nevertheless, the exact mechanisms governing macrophage biology and the intricacies of macrophage-endothelial crosstalk remain incompletely understood.
Circular RNAs (circRNAs) are a class of stable, conserved, and covalently closed-loop RNAs [28]. Mounting evidence suggests that circRNAs participate in metabolic diseases, cardiovascular diseases, and cancers by acting as miRNA sponges or interacting with RNA binding proteins [29]. These molecules are implicated in various processes, such as cell apoptosis, cell cycle regulation, cell proliferation, and cell metabolism [30]. Moreover, circRNAs have the abilities to regulate gene expression at multiple molecular levels with diverse target regulatory patterns [31]. Herein, we investigated the role of circular RNA-mediated epigenetic remodeling in macrophage homeostasis during retinal vascular dysfunction. Knockdown of circRNA-sperm antigen with calponin homology and coiled-coil domains 1 (cSPECC1) in macrophages proves effective in reducing diabetes-induced retinal inflammation and ameliorating retinal vascular dysfunction by reshaping arachidonic acid metabolism. Restoring macrophage homeostasis and modulating macrophage-endothelial cell crosstalk through epigenetic remodeling represent promising therapeutic strategies for vascular diseases.
2. Results
2.1. Identification of cSPECC1 as a high glucose-regulated circRNA in macrophages
To validate circular RNA feature of cSPECC1 in macrophages, Sanger sequencing, Actinomycin D, and RNase R treatment were performed. Sanger sequencing revealed that cSPECC1 sequence obtained from Sanger sequencing was consistent with cSPECC1 sequence reported in circBase (Fig. 1A). RNase R treatment could digest most of linear SPECC1 mRNA but did not degrade cSPECC1 (Fig. 1B). Actinomycin D treatment revealed that cSPECC1 in macrophages was more stable than its linear transcript, SPECC1 mRNA (Fig. 1C). Cellular localization analysis through RNA fluorescence in situ hybridization (FISH) assays indicated that cSPECC1 was predominantly expressed in the cytoplasm of macrophages (Fig. 1D).
Fig. 1.
Identification of cSPECC1 as a high glucose-regulated circRNA in macrophages
A, Sanger sequencing was conducted to detect cSPECC1 expression in macrophages. The result of Sanger sequencing (bottom) was consistent with the sequence of cSPECC1 reported in circBase (top). B, Total RNAs were digested with RNase R (3U/μg) at 37 °C for 30 min followed by qRT-PCR assay of cSPECC1 expression. SPECC1 mRNA was detected as the RNase R-sensitive control (n = 5, Student t-test). ∗P < 0.05 versus Mock group. C, qRT-PCR assays were conducted to detect the amount of cSPECC1 and SPECC1 mRNA in THP-1-derived macrophages following treatment with Actinomycin D (5 μM, n = 5, repeated-measures ANOVA with Bonferroni test). D, RNA-FISH assays conducted to detect the cellular distribution of cSPECC1 in THP-1-derived macrophages. Nuclei were stained with DAPI. Scale bar, 20 μm. 18S rRNA and U6 was detected as the cytoplasmic and nucleus control (n = 4). E, THP-1-derived macrophages were incubated with normal glucose (NG, 5.55 mM), osmotic control (OS, 5.55 mM glucose plus 24.45 mM mannitol), or high glucose (HG, 30 mM) for 24 h qRT-PCR assays were conducted to detect cSPECC1 expression (n = 5, ∗P < 0.05 versus Ctrl group, One-way ANOVA with Bonferroni test). F, qRT-PCRs were conducted to detect cSPECC1 expression in HRMECs, pericytes, Müller cells, RPEs, and THP-1-derived macrophages without (Ctrl) or with high glucose (HG, 30 mM) for 24 h (n = 4, ∗P < 0.05 versus Ctrl group, Mann-Whitney U test). G, qRT-PCRs were conducted to detect cSPECC1 expression in STZ-induced diabetic retinas and non-diabetic controls (Ctrl) at 1-month, 3-month, or 6-month following diabetes induction (n = 5, ∗P < 0.05 versus Ctrl group, Student t-test).
Next, we explored the expression pattern of cSPECC1 in macrophages following high glucose stress. THP-1-derived macrophages were incubated with normal glucose (NG, 5.55 mM), osmotic control (OS, 5.55 mM glucose plus 24.45 mM mannitol), or high glucose (HG, 30 mM) for 24 h. qRT-PCR assays revealed that the levels of cSPECC1 expression were obviously up-regulated in high glucose-treated group compared that in NG group or OS group (Fig. 1E). We also compared cSPECC1 expression pattern in other retinal cells between normal glucose condition and high glucose condition. The greatest change of cSPECC1 expression was observed in THP-1-derived macrophages following high glucose stress (Fig. 1F). We also isolated bone marrow-derived monocytes (BMDMs) (Figs. S1A and S1B) and exposed BMDMs to high glucose stress. The results showed that high glucose treatment led to increased levels of cSPECC1 expression (Fig. S1C). Furthermore, we assessed the expression pattern of cSPECC1 in diabetic animals. The levels of cSPECC1 expression were markedly up-regulated in diabetic retinas compared to non-diabetic controls at 1-month, 3-month, and 6-month intervals following diabetes induction (Fig. 1G). Collectively, these findings demonstrate that cSPECC1 is a potential regulator of macrophage homeostasis following high glucose stress in vitro and in vivo.
2.2. cSPECC1 regulates macrophage homeostasis in vitro
Macrophages can be categorized into two activation states: a pro-inflammatory phenotype (M1) and an anti-inflammatory phenotype (M2). It is well-established that diabetes is associated with chronic inflammation, and elevated glucose levels can disrupt macrophage homeostasis [32]. To investigate the effects of high glucose on macrophage homeostasis, PMA-differentiated THP-1 macrophages were incubated with normal glucose (5.55 mM) or high glucose (30 mM) for 12 h or 24 h. To evaluate macrophage activation under high glucose, we detected macrophage polarization-associated markers by western blots, flow cytometry analysis, and qRT-PCRs. Western blots revealed that the pro-inflammatory phenotype of macrophages was confirmed by increased levels of M1-like markers (iNOS and CD80) and decreased levels of M2-type markers (Arg-1 and CD206) following high glucose stress (Fig. S2A). Flow cytometry analysis and qRT-PCR assays revealed that high glucose treatment increased the ratio of CD68+/CD80+ cells and enhanced the expression levels of M1-type associated cytokines (IL-1β, IL-6, and TNF-α) (Figs. S2B and 2C).
To investigate the role of cSPECC1 in macrophage homeostasis in vitro, the levels of cSPECC1 expression were reduced in THP-1-derived macrophages by targeting the back-splicing site of cSPECC1 (Fig. S3). Flow cytometry analysis demonstrated that cSPECC1 knockdown significantly decreased the ratio of M1 macrophages induced by high glucose (Fig. 2A). qRT-PCR and ELISA assays were conducted to detect the expression levels of inflammation-associated cytokines in macrophages. cSPECC1 knockdown in macrophages reduced the expression levels of M1-associated ctokines (IL-1β, IL-6, and TNF-α) (Fig. 2B and C). Immunofluorescence staining and western blots further confirmed that cSPECC1 knockdown inhibited macrophage differentiation to M1 phenotype induced by high glucose (Fig. 2D and E), suggesting that cSPECC1 plays a crucial role in the activation of M1 macrophages.
Fig. 2.
cSPECC1 regulates macrophage homeostasis in vitro
THP-1-derived macrophages were transfected with scrambled (Scr) siRNA or cSPECC1 siRNA (si-cSPECC1), or left untreated (Ctrl) for 12 h, and then exposed without or with high glucose (HG, 30 mM) for 24 h. The expression of M1-like macrophage marker (CD68+/CD80+) was detected by flow cytometry (A, n = 5). The cytokine expression of M1-like macrophage (IL-1β, IL-6, and TNF-α) was detected by qRT-PCR assays (B, n = 5). The cytokine expression of M1-like macrophage (IL-1β, IL-6, and TNF-α) was detected by ELISA assays (C, n = 5). The expression of M1-like macrophage markers (iNOS and CD80) was detected by Western blot. β-actin as the internal reference (D, n = 5). The expression of M1-like macrophage marker (F4/80+/iNOS+) was detected by immunofluorescent staining. Scale bar, 20 μm (E, n = 5). ∗P < 0.05 versus Ctrl group, #P < 0.05 HG group versus HG group + si-cSPECC1 group; One-way ANOVA with Bonferroni test.
2.3. cSPECC1 regulates macrophage-endothelial cell crosstalk in vitro
The accumulation of high glucose and metabolites can trigger the activation of resident macrophages and endothelial cells. The communication between macrophages and endothelial cells leads to the production of various cytokines and chemokines, which play a role in regulating diabetes-induced retinal vascular dysfunction [33]. We thus employed cell co-culture system to explore the role of cSPECC1 in macrophage-endothelial cell crosstalk (Fig. 3A). Compared with the control group, co-culture with cSPECC1-knockdown macrophages led to a marked reduction of the proliferation, migration and tube-formation ability of HRMECs (Fig. 3B–D). We further investigated the effects of gain function of cSPECC1 in macrophages on endothelial angiogenic effects. cSPECC1 overexpression led to increased levels of cSPECC1 expression in macrophages (Fig. S4A). Functional experiments revealed that cSPECC1 overexpression in macrophages enhanced the proliferation, migration, and tube formation ability of endothelial cells (Figs. S4B–D). Collectively, these findings suggest that cSPECC1 regulates macrophage-endothelial cell crosstalk in vitro.
Fig. 3.
cSPECC1 regulates macrophage-endothelial cell crosstalk in vitro
A, A schematic diagram showing the experimental procedure of macrophage-endothelial cell co-culture. B-D, THP-1-derived macrophages were transfected with scrambled (Scr) siRNA or cSPECC1 siRNA (si-cSPECC1), or left untreated (Ctrl) and co-cultured with ECs in high glucose (HG, 30 mM) for 24 h. Cell proliferation was detected by EdU assays. Proliferating cells were calculated as the ratio of EdU-positive and DAPI-positive cells and are shown relative to the control. Scale bar, 20 μm (B, n = 5). Cell migration was detected by transwell assays. The number of migrated cells was estimated by ImageJ software using the auto-count function. Data are expressed as fold change with the controls. Scale bar, 20 μm (C, n = 5). Tube formation activity was detected by Matrigel assays. Scale bar, 100 μm (D, n = 5). ∗P < 0.05 versus Ctrl group; One-way ANOVA with Bonferroni test.
2.4. cSPECC1 regulates diabetes-induced macrophage activation in vivo
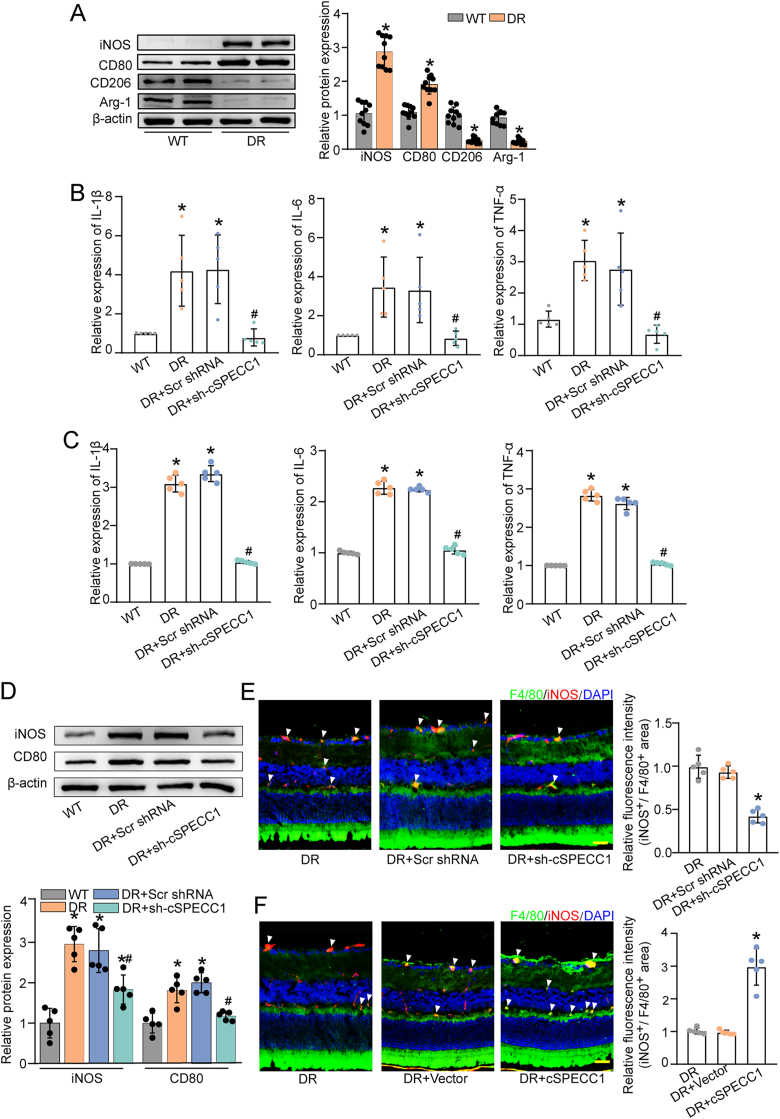
Long-term diabetes can destroy retinal vascular system, which is tightly associated with chronic inflammation. Macrophages play essential roles at all stages of diabetic vascular complications [34]. Compared with the non-diabetic controls, western blots revealed that diabetes induction enhanced the expression levels of M1-type macrophages associated markers (iNOS and CD80) and reduced the expression levels of M2-type macrophages-associated markers (Arg-1 and CD206) (Fig. 4A), suggesting that high glucose stress can lead to the differentiation of macrophages towards an M1 phenotype.
Fig. 4.
cSPECC1 regulates diabetes-induced macrophage homeostasis in vivo
A, The retinas of diabetic mice (DR) and wild-type mice (WT) were collected. Western blots were conducted to detect the specific markers of different macrophage phenotypes in the retinas (n = 5). β-actin was detected as the internal control. ∗P < 0.05 versus WT group. B-E, Diabetic C57BL/6J mice received intravitreous injections of scrambled (Scr) shRNA (DR + Scr shRNA), cSPECC1 shRNA (DR + sh-cSPECC1), or left untreated (DR). Non-diabetic C57BL/6J mice (WT) were served as the controls. The expression levels of cytokines in M1-like macrophages (IL-1β, IL-6, and TNF-α) were detected by qRT-PCRs (B, n = 5) and ELISA assays (C, n = 5). The expression of M1-like macrophage markers (iNOS and CD80) was detected by western blots. β-actin was detected as the internal control (D, n = 5). ∗P < 0.05 versus WT group, #P < 0.05 DR + Scr shRNA group versus DR + cSPECC1 shRNA group; Kruskal-Wallis test followed by Bonferroni test. The expression of M1-like macrophage marker (F4/80+/iNOS+) was detected by immunofluorescent staining of retinal frozen sections. Scale bar, 50 μm; ∗P < 0.05 versus DR group; Kruskal-Wallis test followed by Bonferroni test (E, n = 5). Diabetic C57BL/6J mice received intravitreous injections of Scr vector (DR + Vector), cSPECC1 overexpression vector (DR + cSPECC1), or left untreated (DR). The expression of M1-like macrophage marker (F4/80+/iNOS+) was detected by immunofluorescent staining of retinal frozen sections. Scale bar, 50 μm (F, n = 5). ∗P < 0.05 versus DR group; Kruskal-Wallis test with Bonferroni test.
Diabetic murine model was built by intraperitoneal injections of STZ to assess the functional significance of cSPECC1 in macrophage activation in vivo. The mice received an intravitreous injection of cSPECC1 shRNA to reduce cSPECC1 level (Fig. S5). Three months following diabetes induction, qRT-PCR and ELISA assays revealed that diabetes led to obvious activation of macrophages as shown by increased number of M1-type macrophages, whereas cSPECC1 knockdown blocked the activation of macrophages as shown by reduced levels of M1-type associated cytokines (IL-1β, IL-6, and TNF-α) (Fig. 4B and C). Western blot analysis revealed that cSPECC1 knockdown led to reduced levels of iNOS and CD80, displaying the inhibitory effects of cSPECC1 knockdown on macrophage activation (Fig. 4D). In addition, immunofluorescence staining was performed to detect the change of macrophage homeostasis. F4/80+/iNOS+ cells were identified as M1 macrophage phenotype. Long-term induction of diabetes increased the number of F4/80+/iNOS+ cells. cSPECC1 knockdown reduced the number of F4/80+/iNOS+ cells (Fig. 4E). By contrast, cSPECC1 overexpression had the opposite effect (Fig. 4F), suggesting that cSPECC1 regulates diabetes-induced macrophage activation in vivo.
2.5. cSPECC1 regulates retinal vascular inflammation and vascular integrity in vivo
Diabetes mellitus is a chronic systemic disease that is becoming increasingly prevalent worldwide. A crucial pathological aspect of diabetes involves the interplay and exchange of information among multiple cell types. Following diabetic injury, leukocyte infiltration is a hallmark of retinal vascular inflammation [35]. FITC-ConA assays revealed that, compared with the non-diabetic retinas, long-term diabetes led to increased number of infiltrating leukocytes. Knockdown of cSPECC1 significantly decreased the number of infiltrating leukocytes (Fig. 5A).
Fig. 5.
cSPECC1 regulates retinal vascular inflammation and vascular integrity in vivo
A-C, Diabetic C57BL/6J mice received an intravitreous injection of scrambled (Scr) shRNA (DR + Scr shRNA), cSPECC1 shRNA (DR + sh-cSPECC1), or left untreated (DR). Non-diabetic C57BL/6J mice (WT) were taken as the control. The infiltration of leukocytes in retinal vessels was detected by fluorescein-isothiocyanate (FITC)-coupled concanavalin A (FITC-conA) lectin perfusion assays. Adherent leukocytes (red arrows) were stained with FITC-conA (20 μg/mL) and observed under a fluorescence microscope. Scale bar, 50 μm (A, n = 5). The mice were perfused with Evans blue dye (45 mg/kg) for 2 h. The fluorescence signal of flat-mounted retina was detected using a fluorescence microscope. A representative image is shown. Scale bar, 200 μm (B, n = 5). Retinal trypsin digestion was conducted to detect acellular capillaries. Acellular capillaries were quantified in 30 random fields per retina and averaged. Yellow arrows indicated acellular capillaries. Scale bar, 10 μm (C, n = 5). ∗P < 0.05 versus WT group, #P < 0.05 DR + Scr shRNA group versus DR + cSPECC1 shRNA group; Kruskal-Wallis test followed by Bonferroni test. D, Retinal vaso-obliteration (VO) and neovascularization of cSPECC1 knockdown mice and the matched control mice at P17 were shown by isolectin B4 (IB4) staining. Avascular area was highlighted by white staining. Pathological angiogenic area (at P17) was highlighted by yellow staining. Low panels showed the high magnification of neovascular tufts. Avascular area and angiogenic area were statistically analyzed. Scale bar, 200 μm (n = 5). ∗P < 0.05 versus OIR group; Kruskal-Wallis test with Bonferroni test.
We next explored the involvement of cSPECC1 in diabetes-induced retinal vascular dysfunction in vivo. Evans blue assays were conducted to assess retinal vascular permeability. In the initial month following diabetes induction, neither hyperglycemia nor cSPECC1 knockdown demonstrated a significant impact on vascular leakage (Fig. S6). Three months after diabetes induction, hyperglycemia significantly increased the degree of retinal vascular leakage. By contrast, cSPECC1 knockdown exhibited the opposite effect, reducing retinal vascular leakage (Fig. 5B). Retinal vascular alterations were also assessed using PAS staining. Compared with DR group, cSPECC1 knockdown decreased the number of acellular capillaries in diabetic retinas (Fig. 5C). Furthermore, OIR model was employed to investigate the effects of cSPECC1 knockdown on retinal function. At the peak of retinal angiogenesis on postnatal day 17, IB4 staining was conducted to label avascular regions and neovascular regions. cSPECC1 knockdown was found to reduce the area of non-perfusion regions and angiogenic regions (Fig. 5D).
In contrast, cSPECC1 overexpression aggravated retinal inflammation, as evidenced by an increased number of infiltrating leukocytes (Fig. S7A). cSPECC1 overexpression aggravated hyperglycemia-induced retinal vascular injury, as evidenced by increased extravasation of Evans blue dye, a higher number of acellular capillaries, and enlarged avascular and neovascular areas (Figs. S7B–D). These results indicate that cSPECC1 plays a crucial role in diabetes-induced retinal vascular dysfunction by regulating macrophage homeostasis.
2.6. Conditional knockdown of cSPECC1 in macrophages alleviates retinal vascular dysfunction in vivo
Subsequently, we investigated the effects of conditional knockdown of cSPECC1 in macrophages on retinal vascular dysfunction in vivo. LysM is specifically expressed in myelomonocytic cells, encompassing monocytes, macrophages, and granulocytes in mice. We employed Cre dependent adeno-associated virus (AAV)-mediated gene knockdown strategy to reduce cSPECC1 expression in macrophages. The expression levels of cSPECC1 in macrophages but not endothelial cells were significantly down-regulated in Cre-dependent cSPECC1 shRNA-injected group (Fig. S8). FITC-labelled ConA staining showed a marked reduction in the number of adherent intravascular leukocytes in Cre-dependent shRNA-injected group compared with the non-DR-Cre group or DR-Cre group (Fig. 6A). Furthermore, we investigated the effects of macrophage-conditional knockdown of cSPECC1 on retinal vascular function in vivo. Evans blue assays demonstrated a significant reduction in vascular leakage in cSPECC1-knockdown group (Fig. 6B). Trypsin digest and PAS staining assays showed that conditional knockdown of cSPECC1 in macrophages could reduce the number of acellular capillaries (Fig. 6C).
Fig. 6.
Conditional knockdown of cSPECC1 in macrophages alleviates retinal vascular dysfunction in vivo
A, The infiltration of leukocytes in retinal vessels was detected by FITC-coupled ConA perfusion in non-diabetic C57BL/6J Cre mice (Cre), diabetic Cre mice (Cre-DR) with or without intravitreous injection of Scr shRNA or cSPECC1 shRNA. Scale bar, 50 μm (n = 5). B, The mice were perfused with Evans blue dye (45 mg/kg) for 2 h. The fluorescence signal of flat-mounted retina was detected using a fluorescence microscope. A representative image was shown. Scale bar, 200 μm (n = 5). C, Retinal trypsin digestion was conducted to detect the number of acellular capillaries. Acellular capillaries were quantified in 30 random fields per retina and averaged. Yellow arrows indicated acellular capillaries (n = 5). Scale bar, 10 μm ∗P < 0.05 versus Cre group; #P < 0.05 Cre-DR + Scr shRNA group versus Cre-DR + sh-cSPECC1 group. D, Retinal vaso-obliteration (VO) and neovascularization was detected by isolectin B4 (IB4) staining. Avascular area was highlighted using white staining. Pathologic angiogenic area (at P17) was highlighted using yellow staining. Low panels showed high magnification of neovascular tufts. Avascular area and pathologic angiogenic area were statistically analyzed. Scale bar, 200 μm (n = 5). ∗P < 0.05 versus Cre group; #P < 0.05 Cre-DR + Scr shRNA group versus Cre-DR + sh-cSPECC1 group; Kruskal-Wallis test with Bonferroni test.
OIR model was employed to investigate the effects of conditional knockdown of cSPECC1 on macrophage homeostasis during vascular dysfunction. IB4 staining assays revealed that conditional knockdown of cSPECC1 in macrophages significantly decreased the area of avascular regions and neovascular regions and reduced the number of neovascular tufts (Fig. 6D).
2.7. cSPECC1 regulates macrophage homeostasis through arachidonic acid pathway
To elucidate the mechanism by which cSPECC1 regulated macrophage homeostasis, RNA-Seq was conducted to identify the potential targets of cSPECC1. The results revealed that 47 genes were differentially expressed between these two groups, including 13 up-regulated genes and 34 down-regulated genes (Fig. 7A). KEGG enrichment analysis revealed that several signaling pathways were involved in macrophage homeostasis, including arachidonic acid (AA) signaling, Ras signaling, MAPK signaling, and PI3K-Akt signaling. Remarkably, AA signaling pathway was identified as the top-ranked signaling pathway affected by cSPECC1 knockdown (Fig. 7B and C). Based on gene enrichment analysis, we obtained the potential genes involved in AA signaling, which were PLAAT3 and glutathione peroxidase 2 (GPX2). qRT-PCR assays revealed that cSPECC1 knockdown led to reduced levels of GPX2 expression under normal glucose and high glucose condition (Fig. 7D). PLAAT3 gene was ruled out for further analysis due to its low expression level (data not shown). RIP assays revealed that the amount of GPX2 protein in the immunoprecipitates pulled down by cSPECC1 was not significantly different from that in the immunoprecipitates pulled down by IgG, indicating that there was no direct interaction between GPX2 and cSPECC1 (Fig. 7E).
Fig. 7.
cSPECC1 regulates macrophage homeostasis through arachidonic acid pathway
A, Screening for the potential genes affected by cSPECC1 knockdown (red spots, up-regulated genes; blue spots, down-regulated genes). B and C, KEGG pathway analysis was conducted to predict the signaling pathways associated with cSPECC1 knockdown. D, THP-1-derived macrophages were transfected without or with cSPECC1 siRNA for 12 h, and then exposed to normal glucose (NG, 5.55 mM) or high glucose (HG, 30 mM) for 24 h. The cells without cSPECC1 siRNA transfection in normal glucose (NG) was taken as the control group. The expression levels of GPX2 were detected by qRT-PCR assays (n = 5, ∗P < 0.05, Student t-test). E, RNA immunoprecipitation (RIP) was conducted using GPX2 antibody or IgG negative control. RIP-derived RNAs were measured by qRT-PCR assays and cSPECC1 level was expressed as a percentage of the input. ns, no significant (n = 4). F, The expression levels of arachidonic acid and 12-HETE were determined by metabolic analysis in high glucose-treated (30 mM) group (HG) and cSPECC1 siRNA-transfected group with high glucose (30 mM) treatment (HG + si-cSPECC1). ∗P < 0.05 versus HG group. G, The macrophages were transfected cSPECC1 siRNA or left untreated, and cultured in normal glucose (NG, 5.55 mM) or high glucose (HG, 30 mM) for 24 h. The levels of 12-LOX expression were detected by qRT-PCR assays (n = 5, ∗P < 0.05, Student t-test). H, THP-1-derived macrophages were treated as shown and then co-cultured with ECs for another 24 h. The proliferation ability of ECs was analyzed by EdU assays. Scale bar, 20 μm. The migration ability of ECs was analyzed by transwell assays. Scale bar, 20 μm. The tube formation ability of ECs was analyzed by matrigel assays. Scale bar, 100 μm (n = 5, ∗P < 0.05 versus Ctrl group, #P < 0.05 versus si-cSPECC1 group).
To clarify the role of GPX2 in cSPECC1-mediated macrophage homeostasis, we conducted flow cytometry assays and immunofluorescence staining assays to detect macrophage homeostasis. cSPECC1 overexpression increased the degree of M1 macrophage polarization. By contrast, reduction of GPX2 expression could interrupt cSPECC1 overexpression-induced M1 macrophage polarization (Fig. S9), suggesting that GPX2 was a downstream target of cSPECC1.
To further investigate whether cSPECC1 regulates macrophage homeostasis through AA pathway, we detected the metabolites from macrophages using targeted AA metabolomics analysis. The results indicated a significant decrease in the levels of AA and its subsequent metabolites in cSPECC1-knockdown group compared with the control group (Figure S10 and Fig. 7F). Among these altered metabolites, 12-HETE drew our interests due to its greatest reduction following cSPECC1 knockdown.
12-HETE is a stable hydroxyl fatty acid derived from AA through metabolism by 12-LOX, which serves as the primary enzyme responsible for generating 12-HETE. High glucose enhanced the levels of 12-LOX, while cSPECC1 knockdown reduced the levels of 12-LOX under both normal glucose and high glucose condition (Fig. 7G). Previous studies have reported that the level of 12-LOX is regulated via a NOX1-dependent superoxide production mechanism. GPX2 acts as an upstream regulator of NOX1 expression [36,37]. High glucose enhanced the level of 12-LOX, while GPX2 silencing led to reduced level of 12-LOX (Fig. S11), suggesting that GPX2 acts as an upstream regulator of 12-LOX expression.
We further investigated whether 12-HETE was a pivotal mediator of macrophage-endothelial cell crosstalk. Cell co-culture assays revealed that cSPECC1 knockdown in macrophages led to reduced anti-angiogenic effects on endothelial cells, as evidenced by decreased proliferative ability, tube formation, and migration ability. Administration of 12-HETE could reverse the anti-angiogenic effects mediated by cSPECC1 knockdown in macrophages, thereby increasing the proliferative ability and enhancing tube formation and migration ability in endothelial cells (Fig. 7H). Collectively, these data indicate that cSPECC1 regulates macrophage homeostasis through AA pathway.
2.8. cSPECC1 regulates GPX2 expression by recruiting eIF4A3 to enhance GPX2 stability
We next investigated the potential mechanism about cSPECC1-mediated GPX2 expression. We used the CircInteractome database to predict the potential RNA-binding proteins that could interact with cSPECC1. AGO2, eIF4A3, FUS, and U2AF65 were predicted as the potential regulators of cSPECC1. We further conducted RNA pull-down assays using the biotinylated probes targeting cSPECC1 back-spliced sequence to verify cSPECC1-protein interaction. Silver staining assay showed that cSPECC1 was potentially interacted with the protein bands observed at 40–55 kDa (Fig. 8A). Mass spectrometry analysis combined with the CircInteractome database revealed that eIF4A3 was a candidate interactor of cSPECC1 (Fig. 8B). RNA immunoprecipitation assays demonstrated that cSPECC1 showed significant enrichment in the immunoprecipitate pulled down by eIF4A3 antibody, whereas it was not notably present in the IgG-pulled immunoprecipitate (Fig. 8C). Immunofluorescence staining and FISH assays further verified the co-localization between cSPECC1 and eIF4A3 in macrophages (Fig. 8D)
Fig. 8.
cSPECC1 regulates GPX2 expression by recruiting eIF4A3 to enhance GPX2 stability
A, Following the treatment with PMA (100 ng/mL, 48 h) to differentiate to macrophages, cell lysates were incubated with biotin-labelled cSPECC1 probes (100 μM). Silver-stained sodium dodecyl sulfate polyacrylamide gel of the proteins immunoprecipitated by cSPECC1 and its antisense sequence. eIF4A3 expression was detected by western blots (n = 4, ∗P < 0.05, Mann-Whitney U test). B, Schematic representation of screening for cSPECC1-binding targets. eIF4A3 belonged to the union set of CircInteractome predictive RNAs and pull-down RNAs. C, Total cellular fractions were isolated from macrophages and immunoprecipitated using eIF4A3 or IgG antibody. The amount of cSPECC1 in the immunoprecipitate was detected by qRT-PCR assays (n = 4, ∗P < 0.05, Mann-Whitney U test). D, Immunofluorescence staining and FISH assays were conducted to detect the co-localization between cSPECC1 and eIF4A3 in macrophages. Scale bar, 20 μm (n = 4). E, Immunostaining assays were conducted to detect the localization of eIF4A3 protein transfected with cSPECC1 siRNA or Scr siRNA following high glucose stress (HG, 30 mM) for 24 h. Scale bar, 20 μm (n = 4). F, The macrophages were transfected with scramble (Scr) siRNA or cSPECC1 siRNA for 24 h. Cytoplasmic and nucleus levels of eIF4A3 expression were detected by western blots. β-actin was detected as the cytoplasmic control. PCNA was detected as the nucleus control (n = 4). G, eIF4A3 expression was detected by western blots in macrophages transfected with Scr siRNA or cSPECC1 siRNA for 24 h (n = 4). H, Left: Identification of the proteins pulled down by GPX2 probe (100 μM) in the protein extracts of macrophages. The arrow indicates the band containing eIF4A3. Right: Immunoblot analysis of eIF4A3 expression following pull-down assays by GPX2 probe (n = 5, ∗P < 0.05 versus GPX2 anti-sense group). I, RIP assays were conducted to verify the interaction between GPX2 and eIF4A3 (n = 4, ∗P < 0.05 versus IgG group, Mann-Whitney U test). J, THP-1-derived macrophages were transfected with scramble (Scr) siRNA or eIF4A3 siRNA for 12 h, and then exposed to normal glucose (Ctrl, 5.55 mM) or high glucose (HG, 30 mM) for 24 h qRT-PCR assays were conducted to detect the relative levels of GPX2 (n = 5, Student t-test). K, The stability of GPX2 mRNA in macrophages transfected with eIF4A3 siRNA or left untreated (Ctrl) was detected by qRT-PCRs after the administration of Actinomycin D (5 μM, n = 5, repeated-measures ANOVA with Bonferroni test, ∗P < 0.05 versus Ctrl group). L, THP-1-derived macrophages were treated as shown and GPX2 mRNA stability was detected by qRT-PCRs following Actinomycin D treatment at the indicated time points (0 h, 6 h, 12 h and 18 h, n = 5, Repeated-measures ANOVA with Bonferroni test, ∗P < 0.05 si-cSPECC1+eIF4A3 group versus si-cSPECC1 group).
eIF4A3 is involved in several post-transcriptional processes, such as RNA splicing, nonsense-mediated mRNA decay, translation, and localization. We next investigated subcellular localization of eIF4A3 following cSPECC1 knockdown. Immunostaining assays and western blots revealed that cSPECC1 knockdown caused the translocation of eIF4A3 from the cytoplasm to the nucleus (Fig. 8E and F). However, cSPECC1 knockdown did not affect the total amount of eIF4A3 at the protein level (Fig. 8G).
We next investigated the interaction between eIF4A3 and GPX2. eIF4A3 could be pulled down by GPX2 probe (Fig. 8H). RNA immunoprecipitation (RIP) assays revealed that GPX2 was highly enriched in the immunoprecipitants pulled down by eIF4A3 compared with IgG (Fig. 8I). qRT-PCR assays showed that eIF4A3 knockdown led to reduced levels of GPX2 expression (Fig. 8J). Since eIF4A3 can interact with circRNAs to regulate the stability of downstream genes, we used Actinomycin D assays to evaluate the stability of GPX2 mRNA in THP-1-derived macrophages. The results demonstrated that eIF4A3 knockdown reduced the stability of GPX2 mRNA (Fig. 8K), mimicking the effects of cSPECC1 knockdown on GPX2 mRNA stability. Conversely, eIF4A3 overexpression mitigated the effects of cSPECC1 knockdown on GPX2 stability and increased the stability of GPX2 mRNA (Fig. 8L). Collectively, these data suggest that cSPECC1 regulates GPX2 expression by recruiting eIF4A3 to enhance GPX2 stability.
3. Discussion
Accumulating evidence suggests that macrophage homeostasis is crucial in the context of diabetic complications. In this study, we elucidate the role of circRNA-mediated epigenetic remodeling in maintaining macrophage homeostasis. We identify a macrophage-enriched circRNA, cSPECC1, and demonstrate that its overexpression increases susceptibility to diabetes-induced retinal vascular dysfunction by modulating macrophage homeostasis. Mechanistically, increased cSPECC1 expression contributes to macrophage activation by recruiting eIF4A3 to enhance GPX2 stability and boost the production of 12-HETE. Excessive production of 12-HETE from macrophages exacerbates local retinal inflammation and activates endothelial cells. Collectively, our findings reveal a critical mechanism involving cSPECC1 in macrophage homeostasis and highlight it as a potential therapeutic target for diabetic complications.
Macrophages are highly plastic and heterogeneous regulators of inflammation [38]. Previous studies have highlighted the association between chronic hyperglycemia and macrophage differentiation into a pro-inflammatory phenotype [39,40]. This process involves an increased secretion of pro-inflammatory chemokines, which accelerates the progression of diabetic complications. Our findings demonstrate that high glucose conditions promote the polarization of macrophages toward a pro-inflammatory phenotype, aligning with previous studies [39,40]. We aimed to identify the key regulator of macrophage function under high glucose stress. Our results indicate that cSPECC1 is predominantly expressed in macrophages, and its knockdown inhibits macrophage activation under high glucose conditions, suggesting that cSPECC1 is essential for maintaining macrophage homeostasis in diabetic environments.
Previous studies have shown that, in addition to endothelial cells, inflammation and immune cells are closely associated with the pathogenesis of diabetic vascular complications. For example, pigment epithelium-derived factor (PEDF) inhibits macrophage polarization by regulating the activation of MAPK and Notch1 signaling. This significantly reduces M1 subtype-related iNOS and M2 subtype-related Arg-1 expression, suggesting that PEDF exerts a protective role against retinal neovascularization by regulating macrophage and endothelial cell crosstalk. Additionally, neutralization of interleukin-17A can alleviate ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization [41,42]. In this study, cell co-culture assays have unveiled the significance of cSPECC1 in mediating the crosstalk between macrophages and endothelial cells. cSPECC1 overexpression exacerbates retinal vascular dysfunction by inducing macrophage activation and intensifying crosstalk between macrophages and endothelial cells. Conversely, cSPECC1 knockdown exhibits a protective effect, reducing diabetes-induced vascular leakage and acellular vessel number by mitigating local inflammation. Given that macrophages play a pro-angiogenic role in diabetic vascular complications, inhibiting cSPECC1 expression in macrophages could serve as a powerful angiogenesis inhibitor by regulating the crosstalk between macrophages and endothelial cells.
Macrophages are involved in sensing, integrating, and responding to diabetic stress to maintain metabolic homeostasis by initiating an inflammation response [43]. The intricate crosstalk among signaling cascades, metabolic pathways, and metabolites alter transcription and epigenetic events [44]. Previous studies have shown that lipid metabolism and its products are key contributors to the inflammatory response. Omega-3-derived specialized pro-resolving mediators (SPMs) are essential for resolving acute inflammation. The initiation of the acute inflammatory response begins with changes in blood flow, stimulated by PGE2, PGI2, and LTB4, which are produced from AA pathway [45]. Activation of macrophages by lipopolysaccharide Kdo2-lipid A can induce great remodeling of subcellular lipidome. Altered lipid metabolism can potentially influence biological processes by acting as signaling molecules or as the components of cellular membranes [46]. We show that cSPECC1 governs macrophage homeostasis by targeting GPX2 within AA pathway. Notably, the involvement of GPX2 in AA metabolism has been identified in macrophage homeostasis [47]. GPX2 can regulate the synthesis of inflammatory lipid mediators, exerting anti-inflammatory effects by preventing abnormal production of PGE2 [37]. GPX2 is also implicated in the progression of malignant tumors, exhibiting parallel expression with various metabolic enzymes that regulate AA cascade. This consequently affects the production of pro-inflammatory cytokines [48]. We demonstrate that GPX2 knockdown reduces M1-type macrophage polarization by modulating AA pathway. This highlights the complex interplay between cSPECC1, GPX2, and the AA cascade, providing insights into potential therapeutic targets for modulating macrophage function in the context of diabetic stress and inflammation.
AA serves as a stimulant for Nox2-dependent NADPH oxidase. It is generated by DAG-lipase and phospholipase A2, and undergoes additional processing by enzymes including lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450. Apart from its direct activating role, the signaling metabolites of AA, such as 12-HETE and 15-HETE, exhibit the capability to trigger NADPH oxidase. We show that cSPECC1 knockdown reduces the levels of 12-HETE. The production of 12-HETE is mediated by 12-LOX, a process tightly associated with the levels of GPX2. Previous studies have demonstrated that 12-LOX and its main product, 12-HETE, activate retinal endothelial cells by inducing ER stress or engaging NADPH oxidase during retinal vascular dysfunction [49,50]. As a pro-inflammatory lipid metabolite, increased 12-HETE in endothelial cells and smooth muscle cells can cause elevated VEGF levels under high glucose condition [51]. We speculate that 12-HETE exerts a pro-inflammatory effect. Abnormal crosstalk between macrophages and endothelial cells subsequently contributes to retinal vascular dysfunction under diabetic conditions.
circRNAs play their roles by acting as the sponges for miRNAs or proteins, competing with linear mRNAs, or regulating the transcription of their parental genes. A recent study has reported that cSPECC1 binds with miR-145-5p to affect CDKN1A under oxidative stress in retinal pigment epithelial cells [52]. We unveil a distinct molecular mechanism of cSPECC1 in macrophages. Specifically, cSPECC1 interacts with eIF4A3 protein, an ATP-dependent RNA helicase and a member of eIF4A family. As a component of the exon junction complex, eIF4A3 is involved in various post-transcriptional processes, including splicing and nonsense-mediated mRNA decay. Additionally, eIF4A3 has been implicated in circRNA biogenesis by influencing its recruitment to target mRNA [53,54]. Previous studies have reported that circ_0020256 induces fibroblast activation to drive cholangiocarcinoma development via recruiting EIF4A3 to stabilize KLF4 mRNA. circMYH9 increases KPNA2 mRNA stability to promote hepatocellular carcinoma progression in an EIF4A3-dependent manner [55,56]. In this study, we identified eIF4A3 as a potential RNA-binding protein (RBP) for circSPECC1. The interaction between eIF4A3 and circSPECC1 has been confirmed through RNA-pulldown and RIP assays. Interestingly, knockdown of circSPECC1 caused eIF4A3 to translocate from the cytoplasm to the nucleus. These results suggest that circSPECC1 may act as a molecular sponge for eIF4A3, affecting its subcellular localization and availability, thereby modulating macrophage activation. In addition, bioinformatics analysis indicates that eIF4A3 may also act as an upstream regulator of circSPECC1. This bidirectional interaction highlights a complex regulatory relationship, where circSPECC1 not only affects eIF4A3 levels and function but may also be affected by eIF4A3. Further studies are warranted to investigate this interplay in greater depth, as the regulatory mechanisms involved are likely intricate and require additional experimental validation.
In conclusion, this study reveals a crucial mechanism involving circRNA-mediated epigenetic remodeling in macrophage homeostasis, inflammatory response, and vascular dysfunction. Specifically, cSPECC1 emerges as a novel regulator, affecting macrophage-mediated vascular integrity and inflammation through arachidonic acid pathway. This highlights the potential of targeting macrophage metabolic reprogramming as a promising strategy for addressing diabetes-induced vascular dysfunction.
4. Materials and methods
4.1. Cell culture
THP-1 cells were procured from the American Type Culture Collection (ATCC). They were maintained in RPMI-1640 medium (Sigma-Aldrich), supplemented with 10 % (v/v) fetal bovine serum (FBS, 11573397, Gibco, USA), and 1 % penicillin/streptomycin. To induce differentiation into macrophages, THP-1 monocytes were incubated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL, HY-18739, MCE, USA). The differentiation of macrophages was characterized by cell adhesion, increased granularity, a spread morphology and irregular nucleus shape. For the detection of macrophage polarization, F4/80 or CD68 were used as M0-like macrophage surface markers, iNOS and CD80 as M1-like macrophage surface markers, and CD206 and Arg-1 as M2-like macrophage surface markers. IL-1β, IL-6, and TNF-α were used as M1-like macrophage secretory markers. Human retinal microvascular endothelial cells (HRMECs) were obtained from Cell Systems Corporation (Kirkland, USA). They were cultured in endothelial basal medium (EBM‐2, CC‐3156, Lonza, USA) supplemented with 10 % FBS with 5 % CO2 and 95 % air at 37 °C. For all experiments, cells at passages 5–8 were used, ensuring consistency and reliability in the experimental procedures.
4.2. Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the ethics committee of the author's institute and adhered to the guidelines outlined in the Association for Research in Vision and Ophthalmology (ARVO) Statement.
4.3. Flow cytometry analysis
To determine M1/M2 proportion under diabetic condition, macrophages were collected and subjected for flow cytometry analysis [57,58]. The macrophages were stained with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD68 (333805, Biolegend, USA), Alexa Fluor 647 (APC)-conjugated anti-CD206 (321109, Biolegend, USA), and APC-conjugated anti-CD80 (305219, Biolegend, USA). Cells expressing both CD68 and CD80 were identified as M1-type macrophages, while cells expressing both CD68 and CD206 were identified as M2-type macrophages. For analysis of surface marker CD80, cell suspension was incubated with APC-CD80 antibody for 30 min at 4 °C. For CD68 and CD206 staining, macrophages were fixed and permeabilized using the fixation and permeabilization buffer. Then, cell suspension was labelled with FITC-CD68 antibody or APC-CD206 antibody. All antibodies were used at a final dilution of 1:50, and appropriate isotype control IgG was used. The macrophages were analyzed by a flow cytometry (FACSVerse, Becton Dickinson) and the data were analyzed using the FlowJo version 10.7.1 (Becton Dickinson Life Sciences). All experiments were repeated for 5 times. In each replicate, at least 25,000 macrophages were acquired.
4.4. RNA extraction and quantitative RT-PCR
Total RNAs were extracted from THP-1 cells or retinal tissues using TRIzol reagents (15596018, Invitrogen, USA) according to the manufacturer's instruction. For cDNA synthesis, total RNAs were quantified by a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA) and 300 ng of total RNAs were reversely transcribed into cDNAs using the PrimeScript RT Master Mix (RR037A, Takara Bio, Japan). qRT-PCRs were conducted using the SYBR Premix Ex TaqII (RR820A, Takara Bio, Japan) according to the manufacturer's protocol. qRT-PCRs were conducted as shown below: 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Relative gene expression was determined using 2−△△Ct method.
4.5. Enzyme‐linked immunosorbent (ELISA) assay
ELISA assay is a technique for cytokines measurement [59]. The concentrations of M1-type macrophage polarization associated cytokines (IL-1β, IL-6, and TNF-α) were determined by the ELISA kits. Briefly, 100 μL of the assay diluents was added to each well of 96-well microplates. Then, 100 μL of standard or samples was added to each well. They were mixed together and shaken for 2 h at room temperature. Following washing with the wash buffer three times, 200 μL of IL-1β/IL-6/TNF-α conjugate was added into each well for 2 h at room temperature on the shaker. Next, 200 μL of the substrate solution was added into each well for 30 min at room temperature. The enzyme-substrate reaction was stopped by adding stop solution and OD value at 450 nm was recorded by a microplate reader.
4.6. Western blot
Western blots were conducted to analyze macrophage polarization by detecting the expression of M1/M2 specific markers (M1-type, iNOS and CD80; M2-type, Arg-1 and CD206) [60]. THP-1 cells or retinal tissues were lysed in the RIPA buffer (P0013C, Beyotime, China) containing the protease inhibitor cocktails (539131, Sigma-Aldrich, USA). The lysates were collected and centrifuged at 12,000 g for 15 min. Protein concentrations were measured by a bicinchoninic acid assay (BCA) reagent kit (P0012S, Beyotime, China). Equal amounts of protein samples were electrophoresed on a 10 % polyacrylamide gel and transferred onto the polyvinylidene fluoride (PVDF) membranes (3010040001, Sigma-Aldrich, USA). After blocking with 5 % BSA for 1 h at room temperature, the membranes were probed with the primary antibodies overnight at 4 °C, followed by incubation with the secondary antibodies for 2 h at room temperature. After washing with PBST three times, the membranes were detected using a Pierce ECL Western Blotting Substrate kit (32209, Thermo Fisher Scientific, USA).
4.7. Immunofluorescence staining
Frozen sections were used to examine the expression of macrophage polarization markers by immunofluorescence staining. The eyeballs were enucleated and bisected at the equator. After fixation in 4 % PFA overnight, the samples were immersed in 30 % sucrose solution for 2 days at 4 °C and embedded in OCT compound (Sakura Finetechnical, Japan). The frozen sections (5-μm thickness) were fixed with 4 % PFA for 20 min and washed by PBS for 3 times. After blocking with 5 % BSA, the sections were incubated with the following primary antibodies overnight at 4 °C: rabbit polyclonal anti-iNOS (18985-1-AP, Proteintech, USA), or rat monoclonal anti-F4/80 (ab16911, Abcam, USA). Subsequently, the sections were re-warmed at room temperature for 1 h and washed by PBST for 3 times. Then, Alexa Fluor 594 or Alexa Fluor 488-labelled secondary antibodies (1:1000, Invitrogen, USA) were used to label M1-like and M2-like macrophages, respectively. The nuclei were visualized with 4, 6-diamidino-2-phenylindole (DAPI, 0.5 μg/mL, D1306, Molecular Probe, USA). The sections were observed and photographed under a fluorescence microscope.
4.8. STZ-induced diabetic mice
C57BL/6J mice (6–8 weeks old, male) were obtained from the Animal Core Facility of Nanjing Medical University (Nanjing, China), and LysM-2-Cre mice (6–8 weeks old, male) were obtained from the Animal Laboratory of Nanjing University. All mice were kept in a specific pathogen-free environment with ad libitum access to rodent chow and water, under a standard light/dark cycle. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 150 mg/mL, 572201, Sigma-Aldrich, USA) in a 10 mM citrate buffer (pH 4.5) vehicle. The control mice received an injection of citrate buffer alone. After being maintained on standard chow with unrestricted access to water for one week, the mice with fasting blood glucose levels equal to or exceeding 16.7 mmol/L were considered as diabetic. Blood glucose levels were measured via tail vein sampling using an OneTouch Ultra meter (LifeScan, USA).
4.9. Quantification of retinal leukocytosis
Under deep anesthesia, the chest cavity was opened to expose the heart and the right auricle was cut for drainage. Subsequently, a 27-G cannula was inserted into the left ventricle, and a 3-min perfusion with PBS was conducted to flush out erythrocytes and non-adherent leukocytes from retinal vessels. Following this, the mice underwent perfusion with FITC-conjugated concanavalin A lectin (20 μg/mL in PBS [pH 7.4], 5 mg/kg, 4 mL) to label adherent leukocytes and vascular endothelial cells. Any residual unbound lectin was removed through repeated PBS perfusion. After enucleation of the eyeballs, the retinas were dissected and flat-mounted for examination. Adherent leukocytes were visualized under a fluorescence microscope, allowing for detailed observation of their presence and distribution in retinal vasculature.
4.10. Retinal trypsin digestion assay
Retinal vessels were isolated by a trypsin digestion technique. Initially, the eyes were extracted and fixed in 4 % PFA for 24 h. Subsequently, the retinas were harvested and subjected to a 12-h washing process in distilled water. Trypsin digestion, comprising a 3 % trypsin solution in 0.1 M Tris buffer (pH 7.4), was used to separate retinal vessels. Retinal samples were gently agitated to liberate vessel network before being air-dried on glass slides. Retinal vessels were then stained with periodic acid Schiff and hematoxylin to delineate basement membranes and capillary nuclei. Enumeration of endothelial cells, pericytes, and migrated pericytes was performed across 10 randomly selected fields at 400× magnification per retina. Acellular capillaries were quantified using an ocular integration grid (Olympus, 400× magnification) and total count was normalized to mm2 of retinal area.
4.11. RNA pull-down assay
Biotinylated cSPECC1 and its anti-sense sequences were synthesized by RiboBio (Guangzhou, China). Cell lysates were incubated with a biotin-labelled cSPECC1 probe, facilitating the pull-down of biotin-coupled RNA complex. Finally, the interacting proteins were validated by western blots.
4.12. RNA immunoprecipitation (RIP) assay
RIP assays were performed using the Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) according to the manufacturer's instruction. The cells were lysed in the RIP lysis buffer supplemented with RNase inhibitors. Subsequently, the lysates were subjected to overnight incubation with magnetic beads conjugated with either eIF4A3 antibody, or IgG, while being rotated at 4 °C. RNAs extracted from the immunoprecipitated complexes were quantified using qRT-PCR assays.
4.13. Statistical analysis
All data are presented as mean ± SEM. Prior to statistical analysis, data normality was assessed using the Pearson normality test, and variance homogeneity was examined using Levene's test. For data that were normally distributed with equal variance, two-tailed Student's t-test or one-way, two-way, or repeated measures analysis of variance (ANOVA) followed by the post hoc Bonferroni test was used as appropriate. For data that were not normally distributed or had unequal variances, the nonparametric Mann-Whitney U test or Kruskal-Wallis test followed by Bonferroni post hoc test was employed for significance analysis.
CRediT authorship contribution statement
Qiu-Yang Zhang: Writing – review & editing, Writing – original draft, Methodology, Investigation. Hui-Ying Zhang: Writing – review & editing, Writing – original draft, Methodology, Investigation. Si-Guo Feng: Methodology, Investigation. Mu-Di Yao: Visualization, Data curation. Jing-Juan Ding: Methodology, Investigation. Xiu-Miao Li: Writing – review & editing. Rong Ye: Investigation. Qing Liu: Investigation. Jin Yao: Supervision, Project administration, Funding acquisition. Biao Yan: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was funded by the grants from National Natural Science Foundation of China (no.82171074 and 82225013 to Dr Yan; no. 82271107 to Dr Yao).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103449.
Contributor Information
Jin Yao, Email: jianyao1972@126.com.
Biao Yan, Email: yanbiao@sjtu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Li X., Sun X., Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metabol. 2019;30(3):414–433. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Annex B.H., Cooke J.P. New directions in therapeutic angiogenesis and arteriogenesis in peripheral arterial disease. Circ. Res. 2021;128(12):1944–1957. doi: 10.1161/CIRCRESAHA.121.318266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenen M., et al. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021;128(7):951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumas S.J., et al. Phenotypic diversity and metabolic specialization of renal endothelial cells. Nat. Rev. Nephrol. 2021;17(7):441–464. doi: 10.1038/s41581-021-00411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonetti D.A., Silva P.S., Stitt A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021;17(4):195–206. doi: 10.1038/s41574-020-00451-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wubben T.J., Johnson M.W. Anti-vascular endothelial growth factor therapy for diabetic retinopathy: consequences of inadvertent treatment interruptions. Am. J. Ophthalmol. 2019;204:13–18. doi: 10.1016/j.ajo.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Mettu P.S., Allingham M.J., Cousins S.W. Incomplete response to anti-VEGF therapy in neovascular AMD: exploring disease mechanisms and therapeutic opportunities. Prog. Retin. Eye Res. 2021;82 doi: 10.1016/j.preteyeres.2020.100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eelen G., et al. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116(7):1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., et al. Improving immune-vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018;18(3):195–203. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan K.A., Kerbel R.S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 2018;15(5):310–324. doi: 10.1038/nrclinonc.2018.9. [DOI] [PubMed] [Google Scholar]
- 11.Jurisic V., et al. The concentration of TNF-alpha correlate with number of inflammatory cells and degree of vascularization in radicular cysts. Oral Dis. 2008;14(7):600–605. doi: 10.1111/j.1601-0825.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 12.Varricchi G., et al. Innate effector cells in angiogenesis and lymphangiogenesis. Curr. Opin. Immunol. 2018;53:152–160. doi: 10.1016/j.coi.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Biswas S.K., et al. Macrophage polarization and plasticity in health and disease. Immunol. Res. 2012;53(1–3):11–24. doi: 10.1007/s12026-012-8291-9. [DOI] [PubMed] [Google Scholar]
- 14.Sene A., Chin-Yee D., Apte R.S. Seeing through VEGF: innate and adaptive immunity in pathological angiogenesis in the eye, Trends. Mol. Med. 2015;21(1):43–51. doi: 10.1016/j.molmed.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapouri-Moghaddam A., et al. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K., et al. TREM2(hi) resident macrophages protect the septic heart by maintaining cardiomyocyte homeostasis. Nat. Metab. 2023;5(1):129–146. doi: 10.1038/s42255-022-00715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funes S.C., et al. Implications of macrophage polarization in autoimmunity. Immunology. 2018;154(2):186–195. doi: 10.1111/imm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalucka J., et al. Interaction of endothelial cells with macrophages-linking molecular and metabolic signaling. Pflugers. Arch. 2017;469(3–4):473–483. doi: 10.1007/s00424-017-1946-6. [DOI] [PubMed] [Google Scholar]
- 19.Fantin A., et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willenborg S., et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120(3):613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 21.Baer C., et al. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp. Cell Res. 2013;319(11):1626–1634. doi: 10.1016/j.yexcr.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore K.J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ola M.S., et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J. Diabetes. Complications. 2012;26(1):56–64. doi: 10.1016/j.jdiacomp.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang W., Lo A.C.Y. Diabetic retinopathy: pathophysiology and treatments. Int. J. Mol. Sci. 2018;19(6):1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang J., Kern T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W.W., Lin F., Fort P.E. The innate immune system in diabetic retinopathy. Prog. Retin. Eye Res. 2021;84 doi: 10.1016/j.preteyeres.2021.100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32(5):309–316. doi: 10.1016/j.tig.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020;21(8):475–490. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 30.Li Z., et al. The emerging landscape of circular RNAs in immunity: breakthroughs and challenges. Biomark. Res. 2020;8:25. doi: 10.1186/s40364-020-00204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao T., Pan Y.H., Xiong X.D. Circular RNA: an important player with multiple facets to regulate its parental gene expression. Mol. Ther. Nucleic Acids. 2020;23:369–376. doi: 10.1016/j.omtn.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strizova Z., et al. M1/M2 macrophages and their overlaps - myth or reality? Clin. Sci. (Lond). 2023;137(15):1067–1093. doi: 10.1042/CS20220531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu L., et al. Macrophage-to-endothelial cell crosstalk by the cholesterol metabolite 27HC promotes atherosclerosis in male mice. Nat. Commun. 2023;14(1):4101. doi: 10.1038/s41467-023-39586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee Y., et al. The role of atherogenic lipoproteins in diabetes: molecular aspects and clinical significance. J. Diabetes. Complications. 2023;37(8) doi: 10.1016/j.jdiacomp.2023.108517. [DOI] [PubMed] [Google Scholar]
- 35.Pezhman L., Tahrani A., Chimen M. Dysregulation of leukocyte trafficking in type 2 diabetes: mechanisms and potential therapeutic avenues. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.624184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Carvalho D.D., et al. Nox1 downstream of 12-lipoxygenase controls cell proliferation but not cell spreading of colon cancer cells. Int. J. Cancer. 2008;122(8):1757–1764. doi: 10.1002/ijc.23300. [DOI] [PubMed] [Google Scholar]
- 37.Koeberle S.C., et al. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-κB-driven inflammation through redox-active mechanisms. Redox Biol. 2020;28 doi: 10.1016/j.redox.2019.101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Castro I., et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunol. Lett. 2016;176:81–89. doi: 10.1016/j.imlet.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Y., et al. Mitochondrial ROS-induced lysosomal dysfunction impairs autophagic flux and contributes to M1 macrophage polarization in a diabetic condition. Clin. Sci. (Lond). 2019;133(15):1759–1777. doi: 10.1042/CS20190672. [DOI] [PubMed] [Google Scholar]
- 41.Gao S., et al. PEDF mediates pathological neovascularization by regulating macrophage recruitment and polarization in the mouse model of oxygen-induced retinopathy. Sci. Rep. 2017;7 doi: 10.1038/srep42846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y., et al. Interleukin-17A neutralization alleviated ocular neovascularization by promoting M2 and mitigating M1 macrophage polarization. Immunology. 2016;147(4):414–428. doi: 10.1111/imm.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odegaard J.I., Chawla A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saha S., Shalova I.N., Biswas S.K. Metabolic regulation of macrophage phenotype and function. Immunol. Rev. 2017;280(1):102–111. doi: 10.1111/imr.12603. [DOI] [PubMed] [Google Scholar]
- 45.Buckley C.D., Gilroy D.W., Serhan C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreyev A.Y., et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010;51(9):2785–2797. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez F.O., et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed K.M., et al. Glutathione peroxidase 2 is a metabolic driver of the tumor immune microenvironment and immune checkpoint inhibitor response. J. Immunother. Cancer. 2022;10(8) doi: 10.1136/jitc-2022-004752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elmasry K., et al. Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy. Diabetologia. 2018;61(5):1220–1232. doi: 10.1007/s00125-018-4560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibrahim A.S., et al. A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase. J. Lipid Res. 2015;56(3):599–611. doi: 10.1194/jlr.M056069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen S., Zou H. Key role of 12-lipoxygenase and its metabolite 12-hydroxyeicosatetraenoic acid (12-HETE) in diabetic retinopathy. Curr. Eye Res. 2022;47(3):329–335. doi: 10.1080/02713683.2021.1995003. [DOI] [PubMed] [Google Scholar]
- 52.Chen X., et al. m(6)A modification of circSPECC1 suppresses RPE oxidative damage and maintains retinal homeostasis. Cell Rep. 2022;41(7) doi: 10.1016/j.celrep.2022.111671. [DOI] [PubMed] [Google Scholar]
- 53.Wei Y., et al. EIF4A3-induced circular RNA ASAP1 promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1-2 signaling. Neuro Oncol. 2021;23(4):611–624. doi: 10.1093/neuonc/noaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang X., et al. EIF4A3-induced circARHGAP29 promotes aerobic glycolysis in Docetaxel-resistant prostate cancer through IGF2BP2/c-Myc/LDHA signaling. Cancer Res. 2022;82(5):831–845. doi: 10.1158/0008-5472.CAN-21-2988. [DOI] [PubMed] [Google Scholar]
- 55.Li Z., et al. Circ_0020256 induces fibroblast activation to drive cholangiocarcinoma development via recruitment of EIF4A3 protein to stabilize KLF4 mRNA. Cell. Death. Discov. 2023;9(1):161. doi: 10.1038/s41420-023-01439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia J., et al. CircMYH9 increases KPNA2 mRNA stability to promote hepatocellular carcinoma progression in an EIF4A3-dependent manner. Am. J. Cancer Res. 2022;12(9):4361–4372. [PMC free article] [PubMed] [Google Scholar]
- 57.Song H., et al. Circular RNA Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol. Ther. 2022;30(2):915–931. doi: 10.1016/j.ymthe.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imam S., et al. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J. Immunother. Cancer. 2019;7(1):3. doi: 10.1186/s40425-018-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurisic V. Multiomic analysis of cytokines in immuno-oncology. Expert Rev. Proteomics. 2020;17(9):663–674. doi: 10.1080/14789450.2020.1845654. [DOI] [PubMed] [Google Scholar]
- 60.Jurisic V., et al. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J. Membr. Biol. 2011;239(3):115–122. doi: 10.1007/s00232-010-9309-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.