Summary
Background
Surgical interventions for spontaneous supratentorial intracerebral haemorrhage (ICH) include conventional craniotomy (CC), decompressive craniectomy (DC), and minimally invasive surgery (MIS), with the latter encompassing endoscopic surgery (ES) and minimally invasive puncture surgery (MIPS). However, the superiority of surgery over conservative medical treatment (CMT) and the comparative benefits of different surgical procedures remain unclear. We aimed to evaluate the efficacy and safety of various surgical interventions for treating ICH.
Methods
In this systematic review and network meta-analysis, we searched PubMed, Cochrane Central Register of Controlled Trials, Embase, and ClinicalTrials.gov from inception to June 16, 2024. Eligible studies were randomised controlled trials (RCTs) comparing surgery (i.e., CC, ES, MIPS, or DC) with CMT or comparing different types of surgeries in patients with spontaneous supratentorial ICH. Paired reviewers independently screened citations, assessed the risk of bias of included trials, and extracted data. Primary outcomes were good functional outcome and mortality at 6 months. Secondary outcomes were good functional outcome and mortality at different follow-up times, complications (rebleeding, brain infection, pulmonary infection), and hematoma evacuation rate. The frequentist pairwise and network meta-analysis (NMA) were performed. The GRADE approach was used to evaluate the certainty of evidence. This study is registered with PROSPERO, CRD42024518961.
Findings
Of the 8573 total records identified by our searches, 31 studies (6448 patients) were eligible for the systematic review and network analysis. Compared with CMT, moderate certainty evidence showed that surgery improved good functional outcome (risk ratio [RR] 1.31, 95% CI 1.13–1.52; risk difference [RD] 9.1%, 95% CI 3.8 to 15.3; I2 = 36%) and reduced mortality (RR 0.82, 95% CI 0.71–0.95; RD −5.1%, 95% CI −8.2 to −1.4; I2 = 14%). Moderate certainty evidence from NMA suggested that compared with CMT, both ES (RR 1.51, 95% CI 1.18–1.93; RD 9.4%, 95% CI 3.3–17.1) and MIPS (RR 1.48, 95% CI 1.24–1.76; RD 15.7%, 95% CI 7.9–24.9) improved good functional outcome at 6 months, and both ES (RR 0.66, 95% CI 0.52–0.85; RD −17.0%, 95% CI −24.0 to −7.5) and CC (RR 0.75, 95% CI 0.60–0.94; RD −6.3%, 95% CI −10.1 to −1.5) reduced mortality at 6 months, whereas MIPS and DC showed a trend, although not statistically significant, towards a reduction in mortality. ES and MIPS also reduced pulmonary infection risk (ES RR 0.39, 95% CI 0.23–0.69; MIPS RR 0.35, 95% CI 0.20–0.60; RD −5.3%, 95% CI −6.6 to −3.3). ES showed higher hematoma evacuation than CC (MD: 7.03, 95% CI: 3.42–10.65; I2 = 94%). No difference in rebleeding or brain infection was found between CC and MIS.
Interpretation
Current moderate certainty evidence suggested that surgical intervention of spontaneous supratentorial ICH, may be associated with improved functional outcomes and a reduced risk of death at 6 months. The advantages of surgical haematoma removal are particularly pronounced when MIS including ES and MIPS are employed. ES could improve functional outcomes, reduce the risk of mortality and pulmonary infection, and have a high hematoma evacuation rate, suggesting that it might be an optimal surgical treatment.
Funding
National Natural Science Foundation of China, National Science Fund for Distinguished Young Scholars, Fundamental Research Funds for the Central Public Welfare Research Institutes, and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University.
Keywords: Intracerebral haemorrhage, Surgical intervention, Systematic review, Network meta-analysis
Research in context.
Evidence before this study
In the preliminary search of PubMed, Embase, Cochrane Central Register of Controlled Trials and ClinicalTrials.gov, we scoped the existing evidence on the surgical interventions for spontaneous supratentorial intracerebral haemorrhage from inception through December 2023, with a restriction to English-language publications. Our search terms included “intracranial haemorrhage OR cerebral haemorrhage” AND “surgery OR craniotomy OR endoscopy OR minimally invasive surgical procedures OR decompressive craniectomy”. We updated the search on June 2024. We identified a number of studies on surgical interventions for spontaneous supratentorial intracerebral haemorrhage. A systematic review and network meta-analysis published in 2020 on the topic included 20 randomised clinical trials. It included three types of surgical interventions (craniotomy, endoscopy, and minimally invasive surgical procedures), and integrated data from different follow-up time points.
Added value of this study
This study used a comprehensive search including both database specific subject headings and elaborated key words, an additional surgical intervention (decompressive craniectomy), and added recently published peer-reviewed publications from 2020 to 2024. This updated systematic review included 31 randomised trials, of which five were not included in the most recent systematic review on this topic in 2020, and six were published after the 2019 search, resulting in 11 additional included trials. We found that surgical intervention could improve functional and survival outcomes at 6 months in patients with spontaneous supratentorial intracerebral haemorrhage, with greater functional improvements for patients undergoing surgery within 24 h. We also observed that endoscopic surgery and minimally invasive puncture surgery could improve functional outcomes, while endoscopic surgery and craniotomy could improve survival benefits. Endoscopic surgery and minimally invasive puncture surgery also reduced pulmonary infection risk. In addition, endoscopic surgery has a high hematoma evacuation.
Implications of all the available evidence
Findings from this study demonstrate that for patients with spontaneous supratentorial intracerebral haemorrhage, surgical interventions, especially endoscopic surgery and minimally invasive procedure surgery, could be considered in clinical practice, and craniotomy may be used as a life-saving measure. While surgical intervention is most beneficial when performed within the first 24 h post-onset, it retains clinical significance even when performed up to 72 h after the onset of symptoms. Nonetheless, these findings still need to be verified by future well-designed and rigorously conducted clinical trials. Additionally, individual meta-analyses should elucidate the impact of various factors, such as the location of the hematoma, its volume, and the level of patient consciousness, on patient outcomes and the selection of surgical strategies.
Introduction
Intracerebral haemorrhage (ICH) represents a significant global health concern, resulting in high rates of morbidity and mortality.1,2 The 30-day mortality rate can reach 40%,3 with survivors suffering from functional and cognitive impairments.4,5 The primary treatment for ICH is conservative medical treatment (CMT), which, however, has limited efficacy.6,7 Surgical intervention has garnered attention for its potential to reduce hematoma volume, alleviate mass effects, and potentially improve patient prognosis.8 Despite these potential benefits, the efficacy of surgical treatment of ICH remains controversial. While the American Stroke Association (ASA) and European Stroke Organization (ESO) guidelines recommend surgery as a lifesaving strategy in certain contexts, its impact on improving functional outcomes remains uncertain.6,7
Surgical interventions for spontaneous supratentorial ICH include decompressive craniectomy (DC), conventional craniotomy (CC), and minimally invasive surgery (MIS), with the latter encompassing endoscopic surgery (ES) and minimally invasive puncture surgery (MIPS). In contrast to previous RCTs that failed to show the functional benefits of surgical interventions like CC and MIS,9, 10, 11, 12 the recent ENRICH (Early Minimally-Invasive Removal of Intracerebral Haemorrhage) trial represents a pivotal advancement, demonstrating that minimally invasive surgery within 24 h of ICH onset improves functional recovery at 6 months.13 Additionally, a recent trial conducted the first comparison of DC with CMT,14 sparking interest in evaluating the efficacy and safety of different surgical procedures with CMT or in comparison to each other.
Several factors, such as the surgical time window,15 hematoma size,16,17 and postoperative complications,18 crucially impact surgical outcomes and prognosis. Delayed surgery, averaging 27–58 h post-ICH onset, has shown limited functional improvement in previous trials.9, 10, 11, 12 Hematoma expansion occurring in 20% of patients within 3 h can worsen outcomes significantly, with a 3 mL increase tripling death and disability risks.17,19,20 Therefore, addressing the importance of a comprehensive understanding of the surgical time window is crucial.15 Larger hematomas pose higher risks due to mass effect and intracranial pressure,16,21 and the efficacy of surgery for different sizes remains debated. Additionally, research on complications like postoperative bleeding and infection is scarce but essential.
Recently, several pivotal RCTs on the efficacy and safety of different surgical interventions have been published,13,14,22, 23, 24, 25 providing an important opportunity to determine the impact of surgical interventions in patients with ICH. Therefore, we performed a systematic review and network meta-analysis of RCTs to assess the effect of surgical interventions on ICH and identify the optimal surgical treatment.
Methods
Search strategy and study selection
This was a prospectively registered systematic review and network meta-analysis (PROSPERO CRD42024518961) that followed the reporting guideline of PRISMA for systematic reviews.26 Changes from protocol are presented in supplement materials (Appendix 1). Two reviewers (JH and YM) independently screened titles/abstracts and full texts for eligibility, assessed the risk of bias, and collected data from each eligible study using a standardized template. Any disagreements were resolved by discussion, if needed, by consulting a third reviewer (LL).
Eligible studies were English-language RCTs comparing surgery (i.e., CC, ES, MIPS, or DC) with CMT or comparing different types of surgeries in patients with spontaneous supratentorial ICH. The included studies had to have a minimum follow-up period of three months and report at least one outcome of interest (see below). The primary outcomes were: (1) good functional outcome at 6 months; (2) death at 6 months. The secondary outcomes were: (1) good functional outcome at 3 months, 12 months, and the end of follow-up; (2) death at 7 days, 1 month, 3 months, 12 months, and end of follow-up; (3) rebleeding; (4) pulmonary infection; (5) brain infection; (6) hematoma evacuation rate.
PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched to identify relevant RCTs from inception to June 2024. ClinicalTrials.gov was also searched to identify potentially eligible RCTs (Appendix 2). The reference lists of the included articles and relevant reviews were checked to ensure all relevant articles were included in the final analysis.
Data analysis
The following information was collected from each eligible RCT: 1) Study characteristics: year of publication, the first author, sample size, duration of follow-up, region, centre; 2) Interventions and controls: type of surgical intervention, type of control group, specific treatment received by patients in the intervention and control groups, time from symptom onset to surgery (surgical time window); 3) Patient's characteristics: age, sex (male proportion), ICH location, and ICH volume; 4) Outcomes: number of events, patients included for analyses in each group, mean and standard deviation of results for continuous outcomes.
We defined “good functional outcome” in the study based on the criteria commonly used in previous research.27,28 The following benchmarks was considered to determine a good functional outcome: a modified Rankin Scale (mRS) score of 0–2, a Glasgow Outcome Scale (GOS) score of 4–5, an extended Glasgow Outcome Scale score (eGOS) score of 5–8, or a Barthel Index (BI) score of ≥60. Additionally, any alternative or self-defined criteria used in the RCTs were included if they aligned with the overall criterion of “no severe disability”. For outcomes with multiple follow-up time points documented in the eligible studies, data were extracted for each specified follow-up time point.
Statistical analyses were performed with R version 4.3.2. We synthesized dichotomous outcomes as relative risks (RRs) and risk differences (RDs) accompanied by their 95% confidence intervals (CIs), whereas for continuous outcomes we used mean differences (MD). We used the following formula: RD = baseline risk∗(RR−1),29 baseline risk was considered the rate of occurrence of the event of interest in control group. To assess the presence of statistical heterogeneity among the included studies, we employed both the Q statistic and the I2 statistic. Considering the inevitable heterogeneity across studies, we employed random effects models for data pooling. For instances where ten or more studies were compared, publication bias was evaluated by funnel plot inspection and Egger's test.
Conventional pairwise meta-analysis was employed to compare the role of all surgical types with conventional medical treatment (meta package, version 6.5-0). A frequentist network meta-analysis (NMA) was then performed to compare different surgical approaches (netmeta package, version 2.8-2). The assumption of transitivity was assessed to ensure that the included studies comparing different surgical treatments shared the necessary similarity to generate credible indirect evidence. This involved examining the distribution of variables—including age, sex (male proportion), ICH volume, and sample size—that could influence outcomes across treatment comparisons. The node-splitting method was used to examine the consistency assumption between direct and indirect evidence. Potential inconsistency within the network was quantified by calculating the ratio of direct to indirect estimates, accompanied by their respective 95% CIs, and by determining the P value for inconsistency.
We conducted two prespecified subgroup hypotheses and assessed the credibility of any apparent subgroup effects (i.e., the P-value of interaction test ≤0.05) with the Instrument for the Credibility of Effect Modification Analyses (ICEMAN criteria)30: time window between symptom onset and surgery (<24 h vs. <72 h; larger effect in trials involving patients undergoing surgery within 24 h), and haemorrhage volume at baseline (<50 mL vs. ≥50 mL; larger effect in trials involving patients with haemorrhage volume <50 mL). As the data available at 6 months were limited and insufficient to support subgroup analyses, we used the data at the end of the follow-up for our analyses. Given the heterogeneity of different scales used in the studies, we conducted three sensitivity analyses for the good functional outcome by only including studies that reported mRS 0 to 2, mRS 0 to 3 and mRS 0 to 4. We also conducted four sensitivity analyses using odds ratio (OR) and excluding studies with high risk of bias. To investigate the impact of small studies effect, we omitted those having sample size less than 50. Additionally, to assess temporal differences, we excluded studies published more than 20 years ago.
We assessed the risk of bias of included studies using the revised Cochrane risk of bias tool for RCTs (RoB 2.0),31 which includes randomization process, deviations from the intended interventions, missing outcome data, measurement of outcome, and selection of the reported result. Each domain was answered with ‘low risk of bias’, ‘some concerns’, or ‘high risk of bias’. A trial was considered to be at high risk of bias overall if any domain was at high risk of bias. Then a web application was used to generate risk of bias assessment figures.32
The certainty of the evidence was rated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE).33,34 RCTs begin as high-quality evidence and can be rated down by risk of bias, imprecision, inconsistency, indirectness, and publication bias. We adopted the minimally contextualized framework to rate the imprecision and draw conclusions from an NMA.35
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The data were available to all authors on request. The corresponding author had final responsibility for the decision to submit for publication.
Results
Of 8573 potentially relevant records identified, 31 trials involving 6448 patients with a mean age of 60 years met our inclusion criteria (Fig. 1). Additionally, 12 ongoing trials were identified (eTable 1). Three of the 31 trials were three-arm RCTs25,36,37 and the remaining 28 were two-arm RCTs.9, 10, 11, 12, 13, 14,22, 23, 24,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 The sample size of the included trials ranged from 10 to 1033. Six were multinational,9, 10, 11, 12, 13, 14 eight were multicentral within a single region,24,25,37,41,42,46, 47, 48 and the remaining 17 were limited to a single institutional setting.22,23,36,38, 39, 40,43, 44, 45,49, 50, 51, 52, 53, 54, 55, 56 Nine studies focused on deep ICH,14,23,36,42,45, 46, 47,53,56 one study10 focused on lobar ICH, and the remaining 21 studies included both lobar and deep ICH.9,11, 12, 13,22,24,25,37, 38, 39, 40, 41,43,45,48, 49, 50, 51, 52,54,55 27 studies reported baseline haemorrhage volume,9, 10, 11, 12, 13, 14,22, 23, 24, 25,36,37,39, 40, 41, 42, 43, 44, 45, 46, 47,49,50,52,53,55,56 with mean ICH volumes ranged from 23 mL to 65 mL. Of the 24 studies reported maximum time window from ICH onset to surgery,9, 10, 11, 12, 13, 14,22,23,25,36, 37, 38, 39, 40, 41, 42, 43,45, 46, 47,49,50,52,55 all patients underwent surgery within 72 h of ICH onset, whereas 29 (7.5%) of patients in the Kim45 study underwent surgery within 7 days (Table 1).
Fig. 1.
Flowchart of study selection.
Table 1.
Characteristics of included studies.
| Study | Sample size, n | Region | Center | Intervention | Age, mean | Male, n (%) | ICH location | ICH volume, mean mL | Time to surgery, hrs | Follow up, mons |
|---|---|---|---|---|---|---|---|---|---|---|
| Auer 198938 | 100 | Austria | Single | ES/CMT | NA | 61 (61) | Lobar and Deep | NA | <48 | 6 |
| Juvela 198939 | 52 | Finland | Single | CC/CMT | 52 | 30 (58) | Lobar and Deep | 61 | <48 | 12 |
| Morgenstern 199840 | 34 | USA | Single | CC/CMT | 54a | 22 (65) | Lobar and Deep | 46b | <12 | 6 |
| Zuccarello 199937 | 20 | USA | Multi | CC/MIPS/CMT | 62 | 11 (55) | Lobar and Deep | 42 | <24 | 3 |
| Teernstra 200341 | 70 | Netherlands | Multi | MIPS/CMT | 68 | 40 (57) | Lobar and Deep | 59 | <72 | 6 |
| Hattori 200442 | 242 | Japan | Multi | MIPS/CMT | 61 | 148 (61) | Deep | 44 | <24 | 12 |
| Mendelow 20059 | 1033 | Multinational | Multi | Surgery (75% CC)/CMT | 62a | 591 (57) | Lobar and Deep | 38b | <72 | 6 |
| Pantazis 200643 | 108 | Greece | Single | CC/CMT | 61 | 60 (56) | Lobar and Deep | 56 | <8 | 12 |
| Cho 200636 | 90 | China | Single | CC/MIPS/ES | 56 | 60 (67) | Deep | 43 | <24 | 3 |
| Miller 200844 | 10 | USA | Single | ES/CMT | 59 | 9 (90) | Lobar and Deep | 48 | NA | 3 |
| Kim 200945 | 387 | Korea | Single | MIPS/CMT | 66 | 289 (75) | Deep | 23 | <168 | 6 |
| Wang 200946 | 377 | China | Multi | MIPS/CMT | 57 | 236 (63) | Deep | 33 | <72 | 3 |
| Sun 201047 | 304 | China | Multi | CC/MIPS | 56 | 196 (64) | Deep | 52 | <72 | 3 |
| Zhou 201148 | 168 | China | Multi | CC/MIPS | 58 | 109 (65) | Lobar and Deep | NA | NA | 12 |
| Mendelow 201310 | 601 | Multinational | Multi | Surgery (98% CC)/CMT | 64 | 340 (57) | Lobar | 41 | <72 | 6 |
| Zhang 201449 | 51 | China | Single | CC/ES | 61 | 38 (75) | Lobar and Deep | 60 | <24 | 6 |
| Hanley 201611 | 96 | Multinational | Multi | MIPS/CMT | 61 | 63 (66) | Lobar and Deep | 46 | <72 | 12 |
| Vespa 201650 | 56 | USA | Single | ES/CMT | 61a | 37 (66) | Lobar and Deep | 40 | <72 | 12 |
| Feng 201651 | 184 | China | Single | CC/ES | 68 | 114 (62) | Lobar and Deep | NA | NA | 6 |
| Bhaskar 201752 | 61 | India | Single | CC/CMT | 55 | 37 (61) | Lobar and Deep | 65 | <72 | 6 |
| Ge 201853 | 196 | China | Single | CC/MIPS | 59 | 118 (60) | Deep | 44 | NA | 3 |
| Rasras 201856 | 30 | Iran | Single | DC/CC | 59 | 43 (13) | Deep | 47 | NA | 6 |
| Hanley 201912 | 506 | Multinational | Multi | MIPS/CMT | 62a | 305 (60) | Lobar and Deep | 42b | <72 | 12 |
| Gui 201954 | 126 | China | Single | CC/ES | 53 | 75 (60) | Lobar and Deep | NA | NA | 3 |
| Luan 201955 | 80 | China | Single | CC/MIPS | 57 | 43 (54) | Lobar and Deep | 43 | <72 | 3 |
| Deng 202224 | 78 | China | Multi | MIPS/CMT | 62 | 48 (62) | Lobar and Deep | 35 | NA | 6 |
| Noiphithak 202322 | 188 | Thailand | Single | CC/ES | 51a | 130 (69) | Lobar and Deep | 50 | <12 | 6 |
| Lv 202323 | 128 | China | Single | CC/ES | 56 | 85 (66) | Deep | 30 | <24 | 6 |
| Pradilla 202413 | 150 | Multinational | Multi | MIPS/CMT | 63 | 150 (50) | Lobar and Deep | 55b | <24 | 6 |
| Beck 202414 | 201 | Multinational | Multi | DC/CMT | 61a | 134 (68) | Deep | 57b | <72 | 12 |
| Xu 202425 | 721 | China | Multi | CC/MIPS/ES | 57 | 497 (69) | Lobar and Deep | 49 | <36 | 6 |
ICH, intracerebral hemorrhage; hrs, hours; mons, months; ES, endoscopic surgery; CC, conventional craniotomy; MIPS, minimally invasive puncture surgery; DC, decompressive craniectomy; CMT, conservative medical treatment; NA, not available.
Median age.
Median ICH volume.
Detailed procedures of surgical intervention were provided in all studies except the one by Juvela et al.,39 and we summarized the details of surgeries and CMT for each study in the Appendix (eTable 2). CC were performed in 19 studies,9,10,22,23,25,36,37,39,40,43,47, 48, 49,51, 52, 53, 54, 55, 56 ES in ten studies,22,23,25,36,38,44,49, 50, 51,54 MIPS in 15 studies,11, 12, 13,24,25,36,37,41,42,45, 46, 47, 48,53,55 and DC in two studies.14,56 The procedure of CC was similar among studies, and typically involves opening the skull, removing the blood clot, and controlling bleeding. Similarly, the procedure for DC was consistent across the studies, involving the removal of a portion of the skull to facilitate brain tissue expansion, with hematoma excision typically not being part of this procedure. The ES procedure exhibited a notable degree of uniformity across the studies, with the procedure typically included minimal incisions, burr hole, insertion of sheath, endoscope-guided hematoma removal and coagulation. Cather placement in the cavity after colt removal was conducted in some of the trials.36,38,44,51,54 In the MIPS for ICH, there were variations among studies in terms of the surgical tools used and the thrombolytic agents administered. Some studies employed needles, while others utilized soft catheters. Additionally, the types of thrombolytic drugs and their methods of administration, such as dosages and times, varied. CMT was based on guideline-directed standard therapy or what was referred to as the best treatment in the studies, encompassed a range of basic treatments to maintain vital signs, including maintenance of blood pressure, fluid management, intracranial pressure monitoring, thromboprophylaxis, airway management, and nutritional support, etc.
The risk of bias was assessed separately for good functional outcome, mortality and other secondary outcomes. Among the 29 RCTs reporting good functional outcome, seven studies (24.1%) had a high risk of bias due to randomization process37,44 or deviations from intended interventions39,46,47,52,55 and seven studies9, 10, 11, 12, 13, 14, 25 (24.1%) had a low risk of bias (eFig. 1). Among the 31 RCTs reporting mortality, two studies37,44 (6.5%) had a high risk of bias due to the randomization process and seven studies9, 10, 11, 12, 13, 14, 25 (22.6%) had a low risk of bias (eFig. 2). Among the 20 RCTs reporting other secondary outcomes, one study (5.0%) had a high risk of bias due to randomization process,44 and six studies10, 11, 12, 13, 14, 25 (30.0%) had a low risk of bias (eFig. 3).
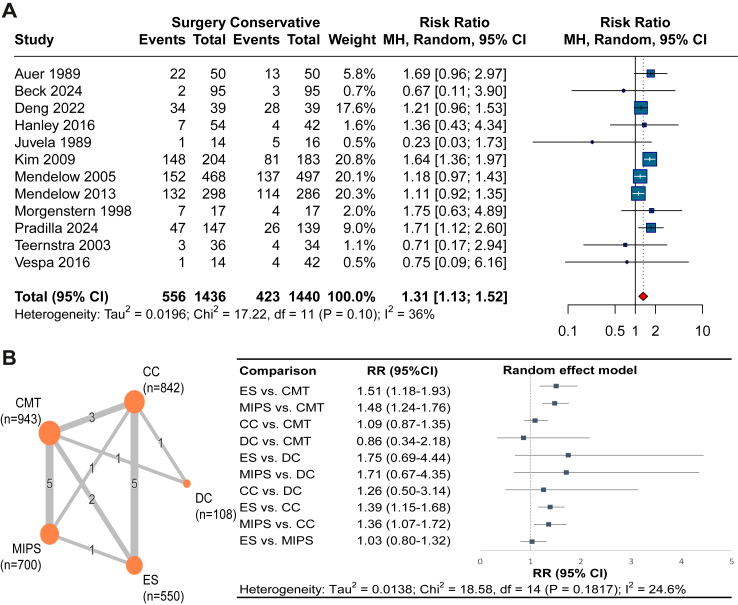
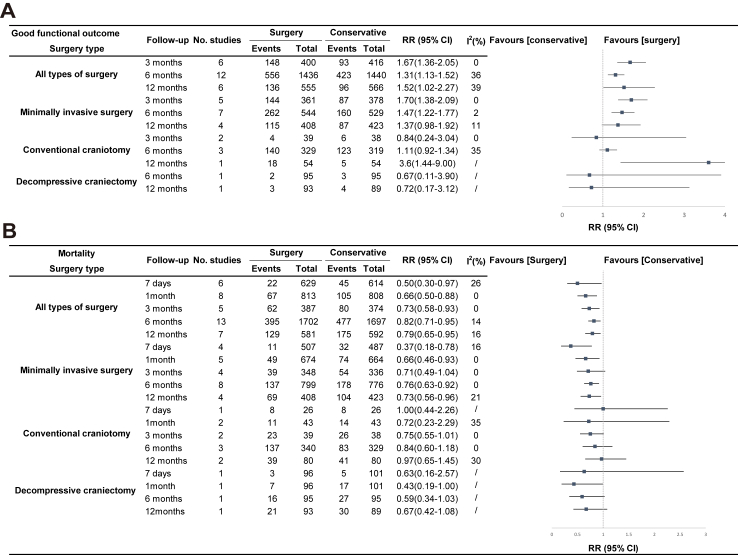
Moderate certainty evidence from 12 RCTs (2876 patients)9, 10, 11, 12, 13, 14,22, 23, 24,37, 38, 39,41, 42, 43,45, 46, 47, 48, 49, 50, 51, 52,54, 55, 56 showed that compared to CMT, surgery improved the proportion of ICH patients who achieved good functional outcome at 6 months (RR 1.31, 95% CI 1.13–1.52; RD 9.1%, 95% CI 3.1–15.3; I2 = 36%; Fig. 2 and Table 2). We found no evidence of publication bias (Egger's test P = 0.54; eFig. 4). MIS including ES and MIPS increased the chance of a good functional outcome at 3 months, 6 months, and 12 months (Fig. 3).
Fig. 2.
Forest plot of good functional outcome at 6 months, (A) all type of surgeries vs. conservative medical treatment, (B) network meta-analysis of different types of surgeries. ES, endoscopic surgery; CC, conventional craniotomy; MIPS, minimally invasive puncture surgery; DC, decompressive craniectomy; CMT, conservative medical treatment.
Table 2.
GRADE evidence profile of surgical interventions for spontaneous supratentorial intracerebral haemorrhage.
| Certainty assessment |
Summary of findings |
Certainty of evidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Study event rates |
Relative effect (RR (95% CI)) | Absolute effects estimates |
|||
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | ||||||||
| Good functional outcome at 6 months; surgery vs. CMT | |||||||||||
| 2916 (12) | Serious limitationsa | No serious limitations | No serious limitationsa | No serious limitations | Undetected | 556/1476 (37.7%) | 423/1440 (29.4%) | RR 1.31 (1.13–1.52) | 514/1000 | 423/1000 | ⊕⊕⊕Ο Moderate |
| Difference: 91 more per 1000 (from 38 more to 153 more) | |||||||||||
| Mortality at 6 months; surgery vs. CMT | |||||||||||
| 3399 (13) | Serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | 395/1702 (23.2%) | 477/1697 (28.1%) | RR 0.82 (0.71–0.95) | 230/1000 | 281/1000 | ⊕⊕⊕Ο Moderate |
| Difference: 51 fewer per 1000 (from 82 fewer to 14 fewer) | |||||||||||
| Pulmonary infection; surgery vs. CMT | |||||||||||
| 1250 (5) | Serious limitationsa | No serious limitations | No serious limitations | Serious limitationsc | Undetected | 65/638 (10.2%) | 87/612 (14.2%) | RR 0.69 (0.43–1.11) | 98/1000 | 142/1000 | ⊕⊕ΟΟ Low |
| Difference: 44 fewer per 1000 (from 81 fewer to 16 more) | |||||||||||
| Rebleeding; MIS vs. CC | |||||||||||
| 1798 (8) | No serious limitations | No serious limitations | No serious limitations | very serious limitationsd | Undetected | 82/1046 (7.8%) | 87/752 (11.6%) | RR 0.75 (0.49–1.16) | 87/1000 | 116/1000 | ⊕⊕ΟΟ Low |
| Difference: 29 fewer per 1000 (from 59 fewer to 19 more) | |||||||||||
| Brain infection; MIS vs. CC | |||||||||||
| 1026 (4) | No serious limitations | No serious limitations | No serious limitations | very serious limitationse | Undetected | 30/627 (4.8%) | 23/399 (5.8%) | RR 0.80 (0.43–1.51) | 46/1000 | 58/1000 | ⊕⊕ΟΟ Low |
| Difference: 12 fewer per 1000 (from 33 fewer to 29 more) | |||||||||||
| Evacuation rate; ES vs. CC | |||||||||||
| 797 (6) | Serious limitationsa | No serious limitations | No serious limitations | No serious limitations | Undetected | No application | No application | MD 7.03 (3.42–10.65) | No application | No application | ⊕⊕⊕Ο Moderate |
| Difference: 7.03 mL more (from 3.42 more to 10.65 more) | |||||||||||
| Evacuation rate; MIPS vs. CC | |||||||||||
| 797 (6) | Serious limitationsa | Serious limitationsb | No serious limitations | very serious limitationsf | Undetected | No application | No application | MD −13.13 (−38.81 to 12.54) | No application | No application | ⊕ΟΟΟ Very low |
| Difference: 13.13 mL fewer (from 38.81 fewer to 12.54 more) | |||||||||||
Absolute risk difference was estimated based on the calculated risk ratio and the overall event rate across the control groups for each outcome.
ES, endoscopic surgery; CC, conventional craniotomy; MIPS, minimally invasive puncture surgery; CMT, conservative medical treatment; MIS, minimally invasive surgery.
Rated down 1 level due to risk of bias.
Rated down 1 level due to moderate heterogeneity.
The 95% CI includes both important benefit (−8.1%) and important harm (1.6%).
The 95% CI includes both important benefit (−5.9%) and important harm (1.9%).
The 95% CI includes both important benefit (−3.3%) and important harm (2.9%).
The 95% CI includes both important benefit (12.54 mL) and important harm (38.81 mL).
Fig. 3.
Forest plot of surgery vs. conservative medical treatment at different follow-up times, (A) good functional outcome, (B) mortality.
Our NMA found moderate certainty evidence that compared to CMT, both ES (RR 1.51, 95% CI 1.18–1.93; RD 9.4%, 95% CI 3.3–17.1) and MIPS (RR 1.48, 95% CI 1.24–1.76; RD 15.7%, 95% CI 7.9–24.9) were more effective in improving good functional outcome whereas no significant difference was found for CC (RR 1.09, 95% CI 0.87–1.35; RD 3.5%, 95% CI −5.0 to 13.5) or DC (RR 0.86, 95% CI 0.34–2.18; RD −0.4%, 95% CI −2.1 to 3.7) at 6 months. Besides, there is no significant different between ES and MIPS (RR 1.03, 95% CI 0.80–1.32; RD 1.0%, 95% CI −6.5 to 10.5) at 6 months (Fig. 2 and eTables 4 and 5).
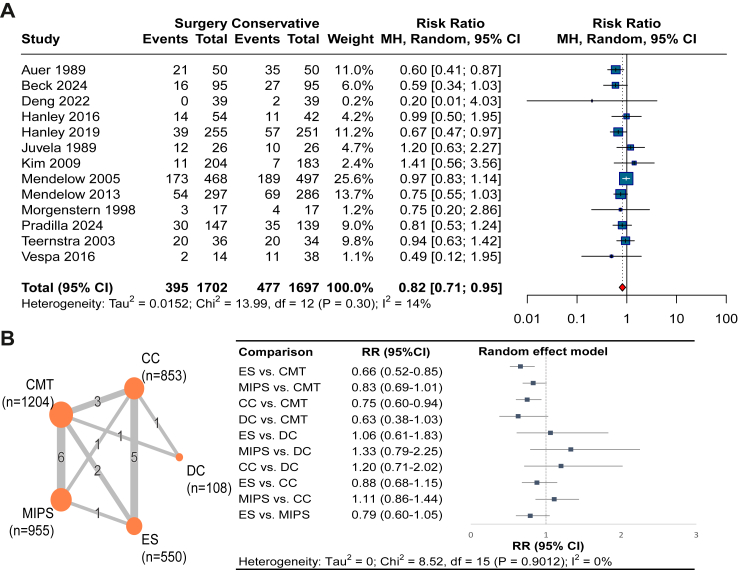
Moderate certainty evidence from 13 RCTs (3399 patients)9, 10, 11, 12, 13, 14,24,38, 39, 40, 41,45,50 suggested that compared with CMT, surgical treatment reduced the risk of death at 6 months (RR 0.82, 95% CI 0.71–0.95; RD −5.1%, 95% CI −8.2 to −1.4; I2 = 14%; Fig. 4 and Table 2). We found no evidence of publication bias (Egger's test P = 0.26; eFig. 12). The pooled results of mortality assessed at 7 days, 1 month, 3 months, 6 months, and 12 months showed similar results (Fig. 3).
Fig. 4.
Forest plot of mortality at 6 months, (A) all type of surgeries vs. conservative medical treatment, (B) network meta-analysis of different types of surgeries. ES, endoscopic surgery; CC, conventional craniotomy; MIPS, minimally invasive puncture surgery; DC, decompressive craniectomy; CMT, conservative medical treatment.
The NMA found moderate certainty evidence that compared to CMT, ES (RR 0.66, 95% CI 0.52–0.85; RD −17.0%, 95% CI −24.0 to −7.5) and CC (RR 0.75, 95% CI 0.60–0.94; RD −6.3%, 95% CI −10.1 to −1.5) reduced the risk of mortality at 6 months. Low certainty evidence suggested that MIPS (RR 0.83, 95% CI 0.69–1.01; RD −3.3%, 95% CI −5.9 to 0.2) and DC (RR 0.63, 95% CI 0.38–1.03; RD −10.5%, 95% CI −17.6 to 0.9) may show no significant difference in mortality compared with CMT (Fig. 4 and eTables 6 and 7).
Studies that compared the rebleeding rate after surgery were eligible for this analysis. Low certainty evidence from eight RCTs (1798 patients)22,25,36,47, 48, 49,53,55 suggested insignificant difference in the rebleeding rate between MIS and CC (7.8% vs. 11.6%; RR 0.75, 95% CI 0.49–1.16; RD −2.9%, 95% CI −5.9 to 1.9; I2 = 36%; eFig. 17 and Table 2).
The NMA found low certainty evidence that there was no significant difference between ES and MIPS (RR 0.71, 95% CI 0.33–1.53; RD −1.6%, 95% CI −3.7 to 2.9; eFig. 18 and eTables 8 and 9).
Low certainty evidence from four RCTs (1026 patients)23,25,49,54 suggested that there was no significant difference between MIS and CC in brain infection (4.8% vs. 5.8%; RR 0.80, 95% CI 0.43–1.51; RD −1.2%, 95% CI −3.3 to 2.9; I2 = 0%; eFig. 19 and Table 2).
Low certainty evidence from five RCTs (1250 patients)10,11,13,14,24 suggested that compared with CMT, surgery failed to reduce pulmonary infection (10.2% vs. 14.2%; RR 0.69, 95% CI 0.43–1.11; RD −4.4%, 95% CI −8.1 to 1.6; I2 = 33%; eFig. 20 and Table 2).
The NMA found moderate certainty evidence that both ES (RR 0.39, 95% CI 0.23–0.69) and MIPS (RR 0.35, 95% CI 0.20–0.60; RD −5.3%, 95% CI −6.6 to −3.3) were reduced risk of pulmonary infection compared to CMT, while CC (RR 0.74, 95% CI 0.46–1.18; RD −3.6%, 95% CI −7.4 to 2.5) and DC (RR 0.93, 95% CI 0.55–1.58; RD −2.2%, 95% CI −14.0 to 18.0) did not show a significant difference. Moderate certainty evidence showed that compared to CC, both ES (RR 0.54, 95% CI 0.39–0.74; RD −12.6%, 95% CI −16.7 to −7.1) and MIPS (RR 0.48, 95% CI 0.33–0.68; RD −14.1%, 95% CI −18.1 to −8.7) decreased the risk of pulmonary infection (eFig. 21 and eTables 10 and 11).
Moderate certainty evidence from seven RCTs (1242 patients)22,23,25,36,49,51,54 suggested that the evacuation rate of ES was found to be higher than that of CC (MD 7.03, 95% CI 3.42–10.65; I2 = 94%; eFig. 22 and Table 2). Very low certainty evidence from two RCTs (542 patients)25,36 suggested that no difference between MIPS and CC (MD −13.13, 95% CI −38.81 to 12.54; I2 = 99%; eFig. 22 and Table 2).
Concerning the surgical timing, patients undergoing surgery within 24 h have a higher likelihood of a good functional outcome, when compared to those undergoing surgery within 72 h after ICH onset (test of interaction P = 0.03; within 24 h RR 1.70, 95% CI 1.35–2.14; within 72 h RR 1.23, 95% CI 1.04–1.45; Fig. 5). Applying ICEMAN criteria, we judged the credibility as moderate bordering on high (eTable 3). A large number of trials for between-trial comparisons, a priori specified direction of the effect, the implausibility of chance as an explanation, testing only a small number of effect modifiers, and the use of an appropriate random effect model in the analysis all support the credibility of the subgroup effect. The lack of the within-trial comparison decreases the credibility. The subgroup analyses provided no support for subgroup effects for good functional outcome based on haematoma volume (test of interaction P = 0.86), for mortality based on surgical time window (test of interaction P = 0.94), and for mortality based on haematoma volume (test of interaction P = 0.79).
Fig. 5.
Forest plot of subgroup analysis.
Sensitivity analyses showed that alterations in the definition of good functional outcome, the use of OR, the exclusion of studies with a high risk of bias, the exclusion of studies published more than two decades ago, and the exclusion of studies with fewer than 50 participants did not result in statistically significant alterations to the pooled effects for good functional outcomes (eFigs. 5–11) and mortality (eFigs. 13–16).
Discussion
Our systematic review and meta-analysis supports for the hypothesis that surgical intervention for evacuation of spontaneous supratentorial ICH is associated with improved functional outcomes and a reduced risk of death. The advantages of surgical haematoma removal are particularly pronounced when MIS including ES and MIPS are used and when the procedure is initiated within 24 h after symptom onset. We did not find subgroup effect of average baseline haemorrhage. In addition, MIS appears to mitigate the risk of pulmonary infections and does not elevate the likelihood of rebleeding. In particular, ES is associated with superior hematoma evacuation rates. Sensitivity analyses support the robustness of our results.
ICH is a neurological emergency, and 70% of patients are at risk of early neurological deterioration within the first 24 h.57 Surgery can prevent brain herniation, reduce intracranial pressure, and minimize the toxic effects of hematomas on surrounding brain tissue.8 Prompt hematoma evacuation prevents expansions and mitigates secondary brain injury, crucial for the patient's prognosis.15 ES is a minimally invasive procedure that facilitates hematoma aspiration under direct visual guidance, thereby minimizing disruption to surrounding brain tissue. Compared to other techniques, ES proves more effective in hematoma evacuation.8 A post hoc exploratory analysis of the MISTIE III trial revealed that achieving ≥70% hematoma reduction or reducing hematoma volume to ≤15 mL significantly enhances the likelihood of a favourable outcome.12 Furthermore, by reducing the incidence of pulmonary infections—which can worsen prognosis, prolong hospital stays, and increase mortality in ICH patients18—ES contributes to better overall patient outcomes. Importantly, ES does not elevate the risk of complications such as rebleeding and intracranial infection compared to other procedures.8
Six previous meta-analyses have explored the effect of surgery on patients with ICH.27,28,58, 59, 60, 61 Two previous meta-analyses compared surgery with CMT in ICH patients,27,58 which found that all types of surgery could improve functional outcomes and reduce mortality at the end of follow-up. Two previous meta-analyses compared MIS with CC and CMT,59,60 indicating that MIS could reduce the risk of disability and mortality at the end of follow-up. One previous NMA meta-analysis including 20 RCTs (3603 patients) compared CC, ES, MIPS, and CMT with each other,28 which found that both ES and MIPS could improve functional outcomes and reduce mortality at the end of follow-up. Four meta-analyses performed subgroup analyses to examine the impact of surgical time window on treatment effect with controversial results.27,59, 60, 61 One study found that the sooner the surgery the greater the benefit,27 while another study suggested that time to randomization of less than 8 h correlated with a decreased probability of a poor outcome.61 The remaining two studies showed that surgical intervention performed within either 24 or 72 h decreased poor outcomes with no specific subgroup effects.59,60
In comparison with the previous studies, our review has added substantial information. Firstly, we identified and incorporated several recently published high-quality RCTs and encompassed DC in addition to hematoma removal surgical procedures such as CC, ES, and MIPS, thereby allowing for a comprehensive assessment of the different surgical procedures. Secondly, following the Cochrane Handbook,62 we defined multiple outcomes based on different follow-up times and conducted separate analyses accordingly, to avoid selecting the longest follow-up time from each trial, which could mitigate potential heterogeneity between estimates and prevent biased conclusions. Thirdly, our study showed that CC reduced 6-month mortality, while MIPS didn't. This could be due to our larger sample size, which increased the statistical power to identify the effect of CC. Focusing on 6-month follow-up inadvertently reduced the number of studies of relevant studies for MIPS and limited its potential for indirect comparison. Fourthly, our prespecified subgroup analyses have provided additional evidence regarding the optimal surgical timing, highlighting the possibility that patients undergoing surgery within 24 h of ICH onset may benefit more from good functional outcome than those within 72 h. Fifthly, our study classified rebleeding as an outcome attributable to surgical procedures for hematoma removal and demonstrated no meaningful difference in the incidence of rebleeding among patients who underwent CC, ES, or MIPS.
This study has several strengths. Firstly, we have carefully integrated outcomes from various follow-up periods to comprehensively assess the short-term and long-term impacts of surgical intervention in patients with ICH, which allows for a more subtle understanding of how surgical treatments influence both the immediate and extended outcomes for these patients. Secondly, we have conducted preplanned subgroup analyses to explore the most two controversial and critical clinical factors of ICH surgery and assessed the credibility of subgroup effects by using ICEMAN criteria. Thirdly, we conducted a network meta-analysis to evaluate the comparative effectiveness of various surgical procedures. Fourthly, we used GRADE to grade the evidence, providing more information for clinical decision-making.
This study also has some limitations. Firstly, the review, although comprehensive, is not exempt from the challenges posed by the heterogeneity inherent in the body of research on ICH surgery. Variability in study design, demographics, interventions, and outcomes may impact the consistency and comparability of results, necessitating cautious interpretation of the pooled results, and therefore caution is warranted in concluding our findings. In particular, the definition of “good functional outcome” varied across studies. However, we conducted a sensitivity analysis including only studies reporting mRS 0 to 2, mRS 0 to 3 and 0 to 4 to assess the robustness of the results. Secondly, our study did not include non-English trials, which may inevitably lead to omits of some studies published in other languages, thus leading to language bias. Thirdly, the analysis did not account for the impact of ICH location on surgical outcomes, an important prognostic factor in ICH. Superficial bleeds are more accessible and may yield better surgical results, while deep bleeds are less suitable for surgery and carry a higher risk of tissue damage. Fourthly, according to the inclusion criteria, we did not include combined treatments for different surgical types. However, such studies also provide evidence for the effects of surgical intervention in ICH. For example, the CARICH study showed that clot removal within 72 h without DC reduced the rate of mRS 3–6 and mortality in patients with supratentorial ICH compared with clot removal with DC. The findings showed that DC with hematoma removal may not be beneficial. On the other hand, it also indirectly suggested that surgery to remove the haematoma (under intracranial pressure control) without decompression may improve prognosis.63
Our study indicates that for patients with spontaneous supratentorial ICH, surgical interventions, especially ES and MIPS, could be considered in clinical practice. CC may be used as a life-saving measure. While surgical intervention is most beneficial when performed within the first 24 h post-onset, it retains clinical significance even when performed up to 72 h after the onset of symptoms. Nonetheless, these findings still need to be verified by future carefully designed and rigorously conducted RCTs. Additionally, individual meta-analyses should elucidate the impact of various factors, such as the location of the hematoma, its volume, and the level of patient consciousness, on patient outcomes and the selection of surgical strategies. Current evidence favours surgical intervention within 72 h, with optimal results within the first 24 h, as early surgery may prevent hematoma expansion and mitigate secondary injury.15 Future research should further confirm the optimal timing of surgical intervention to determine whether earlier intervention results in outcomes, or whether a minimum surgical time window should be proposed to prevent complications such as rebleeding.
In this meta-analysis of RCTs, current moderate certainty evidence suggested that surgery, compared to CMT, increased the likelihood of good functional outcome and reduced mortality at 6 months. A greater functional benefit was found for patients when surgery was performed within 24 h (moderate credibility). ES and MIPS both improved functional outcomes and reduced the risk of pulmonary infection, and ES and CC both reduced mortality at 6 months, ES also had a high haematoma evacuation rate. All these findings suggesting that ES might be an optimal surgical treatment, and well-designed RCTs are still needed to confirm the role of different surgeries in ICH patients.
Contributors
JH and MY contributed equally as co-first authors. JH and MY had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. XS, LL, and XH designed the study. JH and MY drafted the manuscript and conducted the statistical analysis. LL, XS, and XH gave administrative, technical, or material support and supervised the study. JH, MY, YM, FM, YL, XL, JL, HX, KZ, XH, LL, and XS critically revised the article. All authors analysed and interpreted the data. LM, CY and JX provided critical assessment of the neurosurgical procedures of different surgical types and contribute to the revision of the manuscript. JH, MY and LL have verified the underlying data. All authors critically reviewed or revised the manuscript and approved the final version of the manuscript.
Data sharing statement
No additional data available.
Declaration of interests
The authors declare that there is no conflict of interest.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No. 72204173 and 82274368), National Science Fund for Distinguished Young Scholars (Grant No. 82225049), Fundamental Research Funds for the Central Public Welfare Research Institutes (Grant No. 2020YJSZX-3) and 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant No. ZYGD23004).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102999.
Contributor Information
Xin Hu, Email: huxingxxy@gmail.com.
Ling Li, Email: liling@wchscu.cn.
Xin Sun, Email: sunxin@wchscu.cn.
Appendix A. Supplementary data
References
- 1.GBD 2019 Stroke Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 3.Poon M.T.C., Fonville A.F., Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 4.Li L., Poon M.T.C., Samarasekera N.E., et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral haemorrhage: pooled analyses of two population-based studies. Lancet Neurol. 2021;20:437–447. doi: 10.1016/S1474-4422(21)00075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Asch C.J., Luitse M.J., Rinkel G.J., Van Der Tweel I., Algra A., Klijn C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S.M., Ziai W.C., Cordonnier C., et al. 2022 Guideline for the management of patients with spontaneous intracerebral hemorrhage: a guideline from the American heart association/American stroke association. Stroke. 2022;53:e282–e361. doi: 10.1161/STR.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 7.Steiner T., Al-Shahi Salman R., Beer R., et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 8.de Oliveira Manoel A.L. Surgery for spontaneous intracerebral hemorrhage. Crit Care. 2020;24:45. doi: 10.1186/s13054-020-2749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet Lond Engl. 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 10.Mendelow A.D., Gregson B.A., Rowan E.N., Murray G.D., Gholkar A., Mitchell P.M. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet Lond Engl. 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanley D.F., Thompson R.E., Muschelli J., et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15:1228–1237. doi: 10.1016/S1474-4422(16)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley D.F., Thompson R.E., Rosenblum M., et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet Lond Engl. 2019;393:1021–1032. doi: 10.1016/S0140-6736(19)30195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pradilla G., Ratcliff J.J., Hall A.J., et al. Trial of early minimally invasive removal of intracerebral hemorrhage. N Engl J Med. 2024;390:1277–1289. doi: 10.1056/NEJMoa2308440. [DOI] [PubMed] [Google Scholar]
- 14.Beck J., Fung C., Strbian D., et al. Decompressive craniectomy plus best medical treatment versus best medical treatment alone for spontaneous severe deep supratentorial intracerebral haemorrhage: a randomised controlled clinical trial. Lancet Lond Engl. 2024;S0140-6736(24) doi: 10.1016/S0140-6736(24)00702-5. [DOI] [PubMed] [Google Scholar]
- 15.Kellner C.P., Schupper A.J., Mocco J. Surgical evacuation of intracerebral hemorrhage: the potential importance of timing. Stroke. 2021;52:3391–3398. doi: 10.1161/STROKEAHA.121.032238. [DOI] [PubMed] [Google Scholar]
- 16.LoPresti M.A., Bruce S.S., Camacho E., et al. Hematoma volume as the major determinant of outcomes after intracerebral hemorrhage. J Neurol Sci. 2014;345:3–7. doi: 10.1016/j.jns.2014.06.057. [DOI] [PubMed] [Google Scholar]
- 17.Morotti A., Boulouis G., Nawabi J., et al. Association between hematoma expansion severity and outcome and its interaction with baseline intracerebral hemorrhage volume. Neurology. 2023;101:e1606–e1613. doi: 10.1212/WNL.0000000000207728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faura J., Bustamante A., Miró-Mur F., Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. 2021;18:127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowlatshahi D., Demchuk A.M., Flaherty M.L., et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morotti A., Boulouis G., Dowlatshahi D., et al. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol. 2023;22:159–171. doi: 10.1016/S1474-4422(22)00338-6. [DOI] [PubMed] [Google Scholar]
- 21.Broderick J.P., Brott T.G., Duldner J.E., Tomsick T., Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 22.Noiphithak R., Yindeedej V., Ratanavinitkul W., Duangprasert G., Nimmannitya P., Yodwisithsak P. Treatment outcomes between endoscopic surgery and conventional craniotomy for spontaneous supratentorial intracerebral hemorrhage: a randomized controlled trial. Neurosurg Rev. 2023;46:136. doi: 10.1007/s10143-023-02035-y. [DOI] [PubMed] [Google Scholar]
- 23.Lv K., Wang Y., Chao H., Cao S., Cao W. Comparison of the efficacy of subosseous window neuro-endoscopy and minimally invasive craniotomy in the treatment of basal ganglia hypertensive intracerebral hemorrhage. J Craniofac Surg. 2023;34:e724–e728. doi: 10.1097/SCS.0000000000009461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deng C., Ji Y., Song W., Bi J. Clinical effect of minimally invasive aspiration and drainage of intracranial hematoma in the treatment of cerebral hemorrhage. Pak J Med Sci. 2022;38:95–99. doi: 10.12669/pjms.38.1.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Zhang H., Zhang J., et al. Minimally invasive surgeries for spontaneous hypertensive intracerebral hemorrhage (MISICH): a multicenter randomized controlled trial. BMC Med. 2024;22:244. doi: 10.1186/s12916-024-03468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 27.Sondag L., Schreuder F.H.B.M., Boogaarts H.D., et al. Neurosurgical intervention for supratentorial intracerebral hemorrhage. Ann Neurol. 2020;88:239–250. doi: 10.1002/ana.25732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo G., Pan C., Guo W., et al. Efficacy and safety of four interventions for spontaneous supratentorial intracerebral hemorrhage: a network meta-analysis. J Neurointerventional Surg. 2020;12:598–604. doi: 10.1136/neurintsurg-2019-015362. [DOI] [PubMed] [Google Scholar]
- 29.Newcombe R.G., Bender R. Implementing GRADE: calculating the risk difference from the baseline risk and the relative risk. BMJ Evid-Based Med. 2014;19:6–8. doi: 10.1136/eb-2013-101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schandelmaier S., Briel M., Varadhan R., et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ Can Med Assoc J. 2020;192:E901–E906. doi: 10.1503/cmaj.200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 32.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 33.What is GRADE? | BMJ Best Practice. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/
- 34.Izcovich A., Chu D.K., Mustafa R.A., Guyatt G., Brignardello-Petersen R. A guide and pragmatic considerations for applying GRADE to network meta-analysis. BMJ. 2023;381 doi: 10.1136/bmj-2022-074495. [DOI] [PubMed] [Google Scholar]
- 35.Brignardello-Petersen R., Florez I.D., Izcovich A., et al. GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ. 2020;371 doi: 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 36.Cho D.-Y., Chen C.-C., Chang C.-S., Lee W.-Y., Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006;65:547–555. doi: 10.1016/j.surneu.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Zuccarello M., Brott T., Derex L., et al. Early surgical treatment for supratentorial intracerebral hemorrhage: a randomized feasibility study. Stroke. 1999;30:1833–1839. doi: 10.1161/01.str.30.9.1833. [DOI] [PubMed] [Google Scholar]
- 38.Auer L.M., Deinsberger W., Niederkorn K., et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530–535. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 39.Juvela S., Heiskanen O., Poranen A., et al. The treatment of spontaneous intracerebral hemorrhage. A prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755–758. doi: 10.3171/jns.1989.70.5.0755. [DOI] [PubMed] [Google Scholar]
- 40.Morgenstern L.B., Frankowski R.F., Shedden P., Pasteur W., Grotta J.C. Surgical treatment for intracerebral hemorrhage (STICH): a single-center, randomized clinical trial. Neurology. 1998;51:1359–1363. doi: 10.1212/wnl.51.5.1359. [DOI] [PubMed] [Google Scholar]
- 41.Teernstra O.P.M., Evers SM.a.A., Lodder J., et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–974. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 42.Hattori N., Katayama Y., Maya Y., Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101:417–420. doi: 10.3171/jns.2004.101.3.0417. [DOI] [PubMed] [Google Scholar]
- 43.Pantazis G., Tsitsopoulos P., Mihas C., Katsiva V., Stavrianos V., Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: a prospective randomized study. Surg Neurol. 2006;66:492–501. doi: 10.1016/j.surneu.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 44.Miller C.M., Vespa P., Saver J.L., et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:441–446. doi: 10.1016/j.surneu.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Y.Z., Kim K.H. Even in patients with a small hemorrhagic volume, stereotactic-guided evacuation of spontaneous intracerebral hemorrhage improves functional outcome. J Korean Neurosurg Soc. 2009;46:109–115. doi: 10.3340/jkns.2009.46.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W.-Z., Jiang B., Liu H.-M., et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009;4:11–16. doi: 10.1111/j.1747-4949.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 47.Sun H., Liu H., Li D., Liu L., Yang J., Wang W. An effective treatment for cerebral hemorrhage: minimally invasive craniopuncture combined with urokinase infusion therapy. Neurol Res. 2010;32:371–377. doi: 10.1179/016164110X12670144526147. [DOI] [PubMed] [Google Scholar]
- 48.Zhou H., Zhang Y., Liu L., et al. A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011;11:76. doi: 10.1186/1471-2377-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H.-Z., Li Y.-P., Yan Z., et al. Endoscopic evacuation of basal ganglia hemorrhage via keyhole approach using an adjustable cannula in comparison with craniotomy. BioMed Res Int. 2014;2014 doi: 10.1155/2014/898762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vespa P., Hanley D., Betz J., et al. ICES (intraoperative stereotactic computed tomography-guided endoscopic surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke. 2016;47:2749–2755. doi: 10.1161/STROKEAHA.116.013837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y., He J., Liu B., Yang L., Wang Y. Endoscope-assisted keyhole technique for hypertensive cerebral hemorrhage in elderly patients: a randomized controlled study in 184 patients. Turk Neurosurg. 2016;26:84–89. doi: 10.5137/1019-5149.JTN.12669-14.0. [DOI] [PubMed] [Google Scholar]
- 52.Bhaskar M.K., Kumar R., Ojha B., et al. A randomized controlled study of operative versus nonoperative treatment for large spontaneous supratentorial intracerebral hemorrhage. Neurol India. 2017;65:752. doi: 10.4103/neuroindia.NI_151_16. [DOI] [PubMed] [Google Scholar]
- 53.Ge C., Zhao W., Guo H., et al. Comparison of the clinical efficacy of craniotomy and craniopuncture therapy for the early stage of moderate volume spontaneous intracerebral haemorrhage in basal ganglia: using the CTA spot sign as an entry criterion. Clin Neurol Neurosurg. 2018;169:41–48. doi: 10.1016/j.clineuro.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Gui C., Gao Y., Hu D., Yang X. Neuroendoscopic minimally invasive surgery and small bone window craniotomy hematoma clearance in the treatment of hypertensive cerebral hemorrhage. Pak J Med Sci. 2019;35:377–382. doi: 10.12669/pjms.35.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luan L., Li M., Sui H., Li G., Pan W. Efficacies of minimally invasive puncture and small bone window craniotomy for hypertensive intracerebral hemorrhage, evaluation of motor-evoked potentials and comparison of postoperative rehemorrhage between the two methods. Exp Ther Med. 2019;17:1256–1261. doi: 10.3892/etm.2018.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasras S., Safari H., Zeinali M., Jahangiri M. Decompressive hemicraniectomy without clot evacuation in supratentorial deep-seated intracerebral hemorrhage. Clin Neurol Neurosurg. 2018;174:1–6. doi: 10.1016/j.clineuro.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Lord A.S., Gilmore E., Choi H.A., Mayer S.A., VISTA-ICH Collaboration Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015;46:647–652. doi: 10.1161/STROKEAHA.114.007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prasad K., Mendelow A.D., Gregson B. Surgery for primary supratentorial intracerebral haemorrhage. Cochrane Database Syst Rev. 2008;CD000200 doi: 10.1002/14651858.CD000200.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X., Chen J., Li Q., et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. 2012;43:2923–2930. doi: 10.1161/STROKEAHA.112.667535. [DOI] [PubMed] [Google Scholar]
- 60.Scaggiante J., Zhang X., Mocco J., Kellner C.P. Minimally invasive surgery for intracerebral hemorrhage. Stroke. 2018;49:2612–2620. doi: 10.1161/STROKEAHA.118.020688. [DOI] [PubMed] [Google Scholar]
- 61.Gregson B.A., Broderick J.P., Auer L.M., et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage. Stroke. 2012;43:1496–1504. doi: 10.1161/STROKEAHA.111.640284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Higgins J.P., Li T., Deeks J.J. Cochrane Handbook for systematic reviews of interventions. John Wiley & Sons, Ltd; 2019. Choosing effect measures and computing estimates of effect; pp. 143–176. [Google Scholar]
- 63.Zhang C., Zhang S., Yin Y., et al. Clot removAl with or without decompRessive craniectomy under ICP monitoring for supratentorial IntraCerebral Hemorrhage (CARICH): a randomized controlled trial. Int J Surg Lond Engl. 2024;110:4804–4809. doi: 10.1097/JS9.0000000000001466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.