Highlights
-
•
Beneficial properties of Polygonaceae in animal nutrition and health.
-
•
Relevant information about 11 species of Polygonaceae was found.
-
•
Potentiality of Polygonaceae in nutrition, disease prevention, and ecology.
Keywords: Buckwheat, Knotweed, Dock, Forage, Bioactive phytochemicals, Animal health, Animal nutrition
Abstract
Herbs rich in secondary metabolites may possess beneficial properties in livestock nutrition and health. 49 Polygonaceae species of European mountain regions were included in a qualitative systematic review based on the methodological framework of the PRISMA statement. 174 relevant publications were identified. They comprised 231 in vitro and 163 in vivo experiments with cattle, sheep, goats, poultry, pigs, and rodents. For 16 Polygonaceae species no reports were found. Fagopyrum esculentum and Fagopyrum tataricum showed potential as anti-inflammatory, antioxidative and metabolic modifying herbs and feeds improving intake and nitrogen conversion in broiler as well as milk quality and ruminal biotransformation in dairy cows. Polygonum aviculare was promising as an antimicrobial and anti-inflammatory drug or feed, improving performance and affecting ruminal biotransformation in sheep, and Polygonum bistorta as an anti-inflammatory drug or feed, improving performance in broiler and mitigating methane emissions in ruminants. Rumex obtusifolius showed potential as an antibacterial drug or feed improving ruminal biotransformation and preventing bloating in cows, while Rumex acetosa and Rumex acetosella had antimicrobial and anti-inflammatory properties. Furthermore, Polygonum minus, Polygonum persicaria, Rumex crispus and Rumex patientia possess interesting anti-inflammatory and antimicrobial activities. In conclusion, some Polygonaceae species show relevant properties that might be useful to prevent and treat livestock diseases, combined with nutritional benefits in performance, product quality, lowering ruminal methane and ammonia formation and transferring omega-3 fatty-acids from feed to tissue. The potential of such multifunctional plants for a holistic integration of veterinary, nutritional and ecological perspectives under a one-health approach of livestock management is discussed.
1. Introduction
The Polygonaceae family includes about 1’200 species worldwide in approximately 50 genera (Lajter et al., 2013). Forty-seven species are part of Swiss native flora, which can be considered as representative for the European sub-alpine and alpine region. Some Polygonum and Rumex species frequently grow in grassland (Polygonum alpinum All., Polygonum bistorta L., Polygonum viviparum L., Rumex acetosa L., Rumex alpestris Jacq., Rumex alpinus L., Rumex crispus L., Rumex longifolius DC., Rumex obtusifolius L. and Rumex thyrsiflorus Fingerh.) (Flora, 2023). These species are foraged by ruminants or other livestock, either deliberately on pastures (Gorlier et al., 2012) or randomly in conserved feed. However, none of the Polygonaceae are established by purpose in agricultural grassland (UFA-Samen, 2024). Plant parts of wild or cultivated Polygonaceae species can be used as animal feed and human food (e.g. Rumex acetosa L., Rheum rhabarbarum L., and two Fagopyrum species providing grain (Christa & Soral-Smietana, 2008; Lajter et al., 2013; Leiber, 2016).
Furthermore, several Polygonaceae species are described in Swiss and European ethnoveterinary research and also in historical veterinary pharmacology textbooks for being used in European traditional veterinary medicine. For example, Rumex obtusifolius L. has a wide range of use in ethnoveterinary medicine, including treatment of diseases of the gastrointestinal tract and metabolism, skin, genitourinary system, udder, musculoskeletal system and respiratory system. (Disler et al., 2014; Stucki et al., 2019; Schlittenlacher et al., 2022). The family of Polygonaceae synthesize a broad variety of polyphenols and further bioactive compounds including flavonoids (e.g. rutin), gallotannins, naphthodianthones, anthraquinones (e.g. fagopyrin), stilbenoids and phenylpropanoids (Cherian et al., 2023; Huda et al., 2021), and various vitamins and minerals. In their natural composition, these plant secondary metabolites can have both pharmacological and nutritional effects. Concentration of such compounds is always highly depending on plant part (Leiber et al., 2012) and phenological stage (Kälber et al., 2014; Kreft et al., 2016). These compounds may have beneficial metabolic and health effects, in particular through antioxidative and antimicrobial properties (Nan et al., 2024; Zhang et al., 2015a). Furthermore, rutin bears effective protection of organs against various toxins (Rahmani et al., 2022).
There are numerous examples showing that bioactive plant compounds in livestock nutrition affect the nutritional quality of animal products (Leiber et al., 2005; Provenza et al., 2019; van Vliet et al., 2021). For instance, the level of natural antioxidants (such as rutin from buckwheat) in animal feed matters not only for animal health (Hassan et al., 2019), but also for increased vitamin E concentrations in livestock products (Leiber & Messikommer, 2011). Plant secondary metabolites in forage herbs modulate ruminal lipid metabolism by increasing the concentration of essential unsaturated fatty acids in ruminant products (Buccioni et al., 2012). Relevant to say, increased unsaturated fatty acids in animal tissues are first and foremost relevant for the physiology of the animal itself (Leiber et al., 2019). These examples imply that plant diversity and phytochemical richness in livestock feed has positive effects on animal welfare and health (Beck & Gregorini, 2020; Leiber et al., 2020b), as well as on human wellbeing via the increased nutritional quality of the products (Provenza et al., 2015). Furthermore, dietary herbs with relevant concentrations of secondary metabolites like tannins lower the ruminal production of methane (Hristov et al., 2022) and ammonia, and influence the nitrogen degradation (Molan et al., 2007) and efficiency in ruminants (Kapp-Bitter et al., 2023; Leiber et al., 2020a; Zhang et al., 2019). Feeding tannin-rich herbs as a potential anti-parasitic feed-additive has been shown to reduce methane and CO2 emissions in the mesocosm of the respective livestock farm (Verdú et at., 2020).
On the other hand, Polygonaceae also possess antinutritive properties as for example phytic acid and trypsin inhibitors, which may significantly inhibit mineral absorption, release and activity of trypsin, and cause tissue damages in the digestive tract (Berrens and Maranon, 1995; Nan et al., 2024; Zhang et al., 2015b). Protease inhibitors, however, also have antimicrobial, antifungal and antitumor effects (Chen & Ruan, 2016). Furthermore, the phototoxic fagopyrin from buckwheat and polygonum species may evoke photodermatitis and digestive disorders, which are scarcely described in scientific literature, though (Benkovic et al., 2014; Leiber, 2016). Concentration and severity of antinutritive factors and phototoxins also largely depend on plant part and developmental phase (Nan et al., 2024; Ozbolt et al., 2008; Zhang et al., 2015b).
In conclusion, there is obviously considerable overlap among nutritive, toxicological, and pharmaceutical properties of Polygonaceae species with respect to animals. This makes them interesting for a One-Health perspective on natural feed production, feeding practices and veterinary concepts regarding livestock. The One Health concept focuses on the transmission chains for pathogens, in particular zoonotic diseases (Sinclair, 2019; WHO, 2023), acknowledging that, besides sanitary and hygienic means, the general health of ecosystems, production systems and social communities have to be addressed in an interdisciplinary and holistic approach. In a perspective of preventive measures that foster human and animal wellbeing, resilience and robustness, a healthy and diverse nutrition plays a major role (Leiber et al., 2020b). With respect to Polygonaceae, there is evidence that they possess several of the above-mentioned nutritional (Islam et al., 2016; Leiber, 2016) and veterinary (Ayrle et al., 2016) properties. Thus, an analysis of parallel existing evidence for veterinary, nutritional, and environmental effects, narrowed down to this one botanical family, could provide an example for a holistic synopsis of nutritional and health aspects for livestock and humans in the agri-food chain.
Against this background, the aim of this systematic review was to compile, based on recent in vitro, in vivo and clinical research, an overview on how European alpine and sub-alpine native, introduced, or cultivated Polygonaceae species might be promising candidates (a) to increase feed diversity and richness in secondary metabolites, (b) improve livestock performance and quality of livestock products, and (c) to prevent and/or treat livestock diseases.
To the authors knowledge we conducted for the first time a review covering both the animal health and nutritional properties of plant species of the Polygonaceae family.
2. Material and methods
The recommendation of the PRISMA statement (Liberati et al., 2009) and the AMSTAR measurement tool (Shea et al., 2007) served as the basis for the design of the systematic review. The research question was designed following the PICOS scheme (Liberati et al., 2009):
Population were ruminants and other livestock species plus rodents, and the intervention was a treatment with raw material or extracts based on European alpine and sub-alpine Polygonaceae species. The comparator was either no treatment, a placebo, or a standard treatment. The outcome was the effect of the plant material or the extract of Polygonaceae species. The study design included in vitro (including rumen model), in vivo and clinical data (Additional file 1).
2.1. Relevance screening and selection of Polygonaceae species
To choose eligible Polygonaceae species, different initial sources were screened with regard to species frequently recommended for the treatment of livestock, and for their occurrence in the Swiss flora (Flora, 2023). The flora of Switzerland can be considered as representative for the European alpine Polygonaceae, given that it covers northern and southern alpine and sub-alpine slopes and all altitudes. As initial sources three historic veterinary pharmacology textbooks (Delafond, 1853; Fröhner, 1900; Röll, 1866), two publications focusing on European ethnoveterinary plant use (Mayer et al., 2014; Vogl et al., 2016), two publications on alpine forage plant species (Gorlier et al., 2012; Jayanegara et al., 2011), and a list of all plant species reported in recent ethnoveterinary studies in Switzerland (Bischoff et al., 2016; Disler et al., 2014; Mayer et al., 2017; Mertenat et al., 2020; Schmid et al., 2012; Stucki et al., 2019) were screened in order to identify relevant Polygonaceae species. Out of these seven sources the following information about all species of the Polygonaceae family were extracted: plant species, plant part, ATC vet Code and type of administration. All further 32 Swiss indigenous Polygonaceae species (including the 3 neophytic species of the Genus Reynoutria; Flora, 2023) were included in addition (Table 1).
Table 1.
Selection of relevant Polygonaceae species.
| Plant species | Initial sources |
ATC vet Code1 | EEV countries2 | Vet. Pharm. textbook3 | |
|---|---|---|---|---|---|
| EEV | SNF | ||||
| Fagopyrum esculentum Moench | x | x | QG (2) | A, CH | |
| Fagopyrum tataricum (L.) Gaertn. | x | ||||
| Fallopia aubertii (L. Henry) Holub | x | ||||
| Fallopia convolvulus (L.) A. Löve | x | ||||
| Fallopia dumetorum (L.) Holub | x | ||||
| Oxyria digyna (L.) Hill | x | ||||
| Polygonum alpinum All. | x | ||||
| Polygonum amphibium L. | x | ||||
| Polygonum arenastrum Boreau | x | ||||
| Polygonum aviculare L. | x | x | QA (1), QD (2), n.a (1) GS (1) | CH, G, E, I | |
| Polygonum bistorta L. | x | x | QA (4), n.a (2) | I | x (Delafond, 1853; Röll, 1866) |
| Polygonum capitatum D.Don | x | ||||
| Polygonum hydropiper L. | x | ||||
| Polygonum lapathifolium L. | x | ||||
| Polygonum minus Huds. | x | ||||
| Polygonum mite Schrank | x | ||||
| Polygonum nepalense Meisn. | x | ||||
| Polygonum orientale L. | x | ||||
| Polygonum perfoliatum L. | x | ||||
| Polygonum persicaria L. | x | x | QG (1) | E | |
| Polygonum polystachyum Meisn. | x | ||||
| Polygonum rurivagum Boreau | x | ||||
| Polygonum viviparum L. | x | ||||
| Reynoutria x bohemica Chrtek & Chrtkova | x | ||||
| Reynoutria japonica Houtt. | x | ||||
| Reynoutria sachalinensis (F. Schmidt) Nakai | x | ||||
| Rheum officinale Baill./palmatum L. | x | QR (1), QA (6) | HR | x (Fröhner, 1900) | |
| Rheum rhabarbarum (L.) | x | x | QA (5), QV (1) | x (Röll, 1866) | |
| Rumex x pratensis Mert. & W.W.J. Koch | x | ||||
| Rumex acetosa L. | x | x | Feed/food integrator (1), QA (1), QP (3), QR (1), others (3) | I, E | x (Delafond, 1853) |
| Rumex acetosella L. | x | x | QA (1) | HR | |
| Rumex alpestris Jacq. | x | x | |||
| Rumex alpinus L. | x | x | Feed/food integrator (3), QA (2), QD (6), QG52 (1), QM (1) | AL, I, CH | |
| Rumex aquaticus L. | x | ||||
| Rumex conglomeratus Murray | x | x | QA (1) | CH | |
| Rumex crispus L. | x | x | Feed/food integrator (1), QA (3), QD (1) | I | |
| Rumex hydrolapathum Huds. | x | ||||
| Rumex longifolius DC. | x | ||||
| Rumex maritimus L. | x | ||||
| Rumex nebroides Campd. | x | ||||
| Rumex nivalis Hegetschw. | x | ||||
| Rumex obtusifolius L. | x | x | Feed/food integrator (1), QA (16), QD (25), QG (1), QG52 (10), QM (14), QR (1), QV (4) | G, I, CH | x (Röll, 1866) |
| Rumex palustris Sm. | x | ||||
| Rumex patientia L. | x | ||||
| Rumex pulcher L. | x | x | Feed/food integrator (2), QP (1) | E | |
| Rumex sanguineus L. | x | x | Feed/food integrator (1) | I | |
| Rumex scutatus L. | x | ||||
| Rumex sp. | x | Feed/food integrator (3), QA (4), QG (1) | SRB, RO, I | ||
| Rumex thyrsiflorus Fingerh. | x | ||||
EEV: European ethnoveterinary research, SNF: Swiss native flora including 3 species of the neophytic genus Reynoutria.
ATC vet Code in European ethnoveterinary research and/or historical textbooks of veterinary pharmacology, QA: Alimentary tract and metabolism, QD: Dermatological, QG: Genitourinary system and sex hormones, QG52: Mastitis, QP: Antiparasitic products, insecticides and repellents, QR: Respiratory system, QV: Various, GS: General strengthening.
ethnoveterinary use in European countries, A: Austria (Vogl et al., 2016), CH: Switzerland (Bischoff et al., 2016; Disler et al., 2014; Mayer et al., 2017; Mertenat et al., 2020; Stucki et al., 2019; Schmid et al., 2012) , E: Spain (Bonet et al., 2007; Gonzalez et al., 2011), G: Germany (Bavaria; Schlittenlacher et al., 2022), I: Italy )Viegi et al., 2003; Manganelli et al. 2001), HR: Croatia (Pieroni et al., 2003; Vucevac-Bajt et al., 1994), AL: Albania (Pieroni et al., 2005), SRB: Serbia (Jari et al., 2007), RO: Romania (Papp et al., 2012)
historical textbooks of veterinary pharmacology (Delafond, 1853; Fröhner, 1900; Röll, 1866)
2.2. Selection of scientific references
2.2.1. Bibliographic search
Scientific information on these Polygonaceae species were searched in PubMed (PubMed.gov, 2015) and Web of Science (Web of Science, 2020). Both bibliographic sources were consulted in the time between 20.01.2021 and 27.01.2021 by one person. First, we created a search term which consisted of the Latin name, the English name and, if applicable, the pharmaceutical herbal drug name of the respective Polygonaceae species. We excluded information on use or effects in neoplastic diseases. This led to the following search term: (“Latin name” OR “English name” OR “herbal drug”) NOT (cancer* OR anticancer* OR anti-cancer* OR anti-tumor* OR antitumor* OR tumor*). The search term was used for each Polygonaceae species separately. The second part of the English name of some Polygonaceae species were knotweed, knotgrass and sorrel. Therefore, the search for these names was conducted in addition with the search term: (“knotweed” OR “knotgrass” OR “sorrel”) NOT (cancer* OR anticancer* OR anti-cancer* OR anti-tumor* OR antitumor* OR tumor*).
The year of publication was not narrowed down. In Web of Science searches the results were refined with the following research areas: Agricultural, Agriculture dairy animal science, Agriculture multidisciplinary, Agronomy, Allergy, Behavioural science, Biochemical research methods, Biochemistry molecular biology, Biology, Biotechnology applied microbiology, Cell biology, Chemistry analytical, Chemistry applied, Chemistry medicinal, Chemistry multidisciplinary, Chemistry organic, Chemistry physical, Dermatology, Engineering, Engineering chemical, Entomology, Food science technology, Green sustainable science technology, Immunology, Integrative complementary medicine, Microbiology, Multidisciplinary science, Mycology, Nutrition dietetics, Pharmacology and pharmacy, Physiology, Substance abuse, Surgery, Toxicology, Veterinary science, Virology, Zoology.
All detected publications were saved in one folder per species in an EndNote® 20 database.
2.2.2. Removing duplicates automatically and manually
The publications where the species could be clearly identified, were manually assigned from the folders knotweed, knotgrass and sorrel to the corresponding folders of Polygonaceae species. After that, duplicates were removed within the folder of each species. Duplicates which were not automatically detected were removed manually afterwards.
2.2.3. Refining with manual title and abstract check
Only peer-reviewed publications with an abstract written in English, German, Turkish, French or Spanish were considered for further evaluation. The manual title and abstract check was conducted separately for each Polygonaceae species by one person based on predefined detailed in- and exclusion criteria (chapter 2.2.4; Additional file 1). Only publications reporting in vivo and in vitro experiments were included. If the title or abstract was not sufficiently informative for decision making, the full text was also consulted (Additional file 1).
2.2.4. Definition of inclusion and exclusion criteria
The inclusion and exclusion criteria were pre-defined by three scientists. Included were publications that clearly contained a control group (placebo, untreated or positive control) , one of the Polygonaceae species and in vitro (including rumen model), ex vivo, or in vivo, nutritional, clinical or phytochemical data. A publication was excluded if it dealt with other plant species or with mixtures of different plant species, food quality, technology and packaging, plant genetics, cultivation or breeding of plants, weed control, plant pathology, fertilizers, plant protection systems or pesticides, ecology, geology, ethology, sociology, and ethnobotany (Additional file 1).
2.3. Definition and categorization of the publications (including one or more experiments)
After abstract check the publications were categorized into in vivo, in vitro, food processing, pharmacognosy, side effects/toxicology, various information and reviews (Table 2). For further assessment, the focus was only on in vivo and in vitro studies.
Table 2.
Details on the process of quantifying and categorizing scientific publications during the bibliographic search process.
| Basic information |
Further information |
Included information (Main issue of the publication) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant species | Remaining publications |
Review | Side effect/ Toxicology |
Various information | Pharma-cognostic | Food processing |
In vivo |
In vitro |
Total publications | |||||||
| after removal of duplicates | after title check | after abstract/ full text check | Livestock | Laboratory animals | Human | Livestock1 | Laboratory animals2 | Human3 | Others4 | |||||||
| Fagopyrum esculentum Moench | 4122 | 392 | 281 | 35 | 41 | 13 | 58 | 158 | 21 | 24 | 8 | 4 | 0 | 8 | 0 | 65 |
| Fagopyrum tataricum (L.) Gaertn. | 342 | 63 | 48 | 2 | 2 | 9 | 13 | 17 | 1 | 12 | 2 | 1 | 1 | 1 | 0 | 18 |
| Fallopia aubertii (L. Henry) Holub | 2 | 2 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fallopia convolvulus (L.) A. Löve | 365 | 5 | 3 | 0 | 2 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Fallopia dumetorum (L.) Holub | 311 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Oxyria digyna (L.) Hill | 25 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polygonum alpinum All. | 4 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polygonum amphibium L. | 36 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Polygonum arenastrum Boreau | 7 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Polygonum aviculare L. | 216 | 37 | 31 | 0 | 0 | 1 | 13 | 2 | 1 | 4 | 1 | 3 | 0 | 2 | 6 | 17 |
| Polygonum bistorta L. | 154 | 26 | 20 | 0 | 0 | 2 | 11 | 0 | 1 | 2 | 0 | 2 | 2 | 1 | 2 | 10 |
| Polygonum capitatum D.Don | 37 | 8 | 6 | 0 | 1 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Polygonum hydropiper L. | 171 | 43 | 28 | 0 | 9 | 3 | 16 | 1 | 0 | 2 | 0 | 2 | 2 | 0 | 5 | 11 |
| Polygonum lapathifolium L. | 47 | 4 | 4 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Polygonum minus Huds. | 172 | 21 | 18 | 0 | 1 | 1 | 9 | 3 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 6 |
| Polygonum mite Schrank | 3 | 3 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Polygonum nepalense Meisn. | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polygonum orientale L. | 64 | 8 | 7 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Polygonum perfoliatum L. | 61 | 9 | 7 | 0 | 0 | 2 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 |
| Polygonum persicaria L. | 88 | 6 | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Polygonum polystachyum Meisn. | 7 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polygonum rurivagum Boreau | 1485 | 4 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Polygonum viviparum L. | 40 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reynoutria x bohemica Chrtek & Chrtkova | 11 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Reynoutria japonica Houtt. | 151 | 17 | 11 | 0 | 0 | 3 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Reynoutria sachalinensis (F. Schmidt) Nakai | 75 | 9 | 4 | 0 | 2 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Rheum officinale Baill./palmatum L. | 619 | 69 | 27 | 3 | 4 | 16 | 9 | 1 | 1 | 4 | 1 | 3 | 3 | 1 | 4 | 17 |
| Rheum rhabarbarum (L.) | 45 | 19 | 12 | 1 | 1 | 3 | 7 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex x pratensis Mert. & W.W.J. Koch | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex acetosa L. | 193 | 18 | 16 | 0 | 1 | 0 | 3 | 6 | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 7 |
| Rumex acetosella L. | 98 | 12 | 10 | 0 | 2 | 0 | 3 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 3 |
| Rumex alpestris Jacq. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex alpinus L. | 17 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Rumex aquaticus L. | 17 | 13 | 1 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex conglomeratus Murray | 113 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex crispus L. | 187 | 24 | 16 | 0 | 4 | 2 | 5 | 4 | 0 | 1 | 0 | 0 | 1 | 1 | 4 | 7 |
| Rumex hydrolapathum Huds. | 13 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex longifolius DC. | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex maritimus L. | 11 | 2 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Rumex nebroides Campd. | 719 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex nivalis Hegetschw. | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex obtusifolius L. | 220 | 24 | 21 | 0 | 0 | 4 | 4 | 7 | 3 | 1 | 0 | 1 | 0 | 0 | 4 | 9 |
| Rumex palustris Sm. | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex patientia L. | 58 | 16 | 11 | 0 | 1 | 0 | 4 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | 6 |
| Rumex pulcher L. | 13 | 6 | 6 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex sanguineus L. | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex scutatus L. | 10 | 4 | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Rumex sp. | 164 | 14 | 13 | 0 | 1 | 0 | 5 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rumex thyrsiflorus Fingerh. | 9 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
Rumen model, cell lines.
Cell lines.
Bacterial, viral, fungal cultures.
Trials investigating effects of biomass or preparations from Polygonaceae species on diseases, digestion, performance, or other parameters in living animals and humans were categorized as “in vivo”. Investigations using pathogens, cellular or ex vivo models (e.g. rumen fermentation models) were categorized as “in vitro”. Subgroups were formed for in vivo (including (a) livestock, (b) laboratory animals and (c) human) and in vitro (including (a) livestock directed research like rumen model or livestock-derived cell lines, (b) laboratory animal-derived cell lines, (c) human cell lines, and (d) further research mainly utlizing bacterial, fungal or viral cultures).
The finally included publications were categorized by one person into two main groups: “in vivo” and “in vitro” publications. In vivo publications were papers with in vivo data even if they included in addition in vitro data, while in vitro publications contained solely in vitro data. Given that publications often reported testing of several extracts of a given plant and, also both in vivo and in vitro data, we defined the “experiment” as key “unit” in our review, subdivided into in vivo and in vitro experiments.
In vivo experiments: Each trial within different or the same publication documenting two or more groups of animals kept under the same conditions during the same time period, receiving raw material or an extract of one plant part (or of the whole plant) of a Polygonaceae species (publication x trial x plant species x plant part x extracting agent) compared to a control group.
Thus, a publication including two trials with Fagopyrum esculentum Moench, one in turkeys and one in chicken was counted as 2 experiments. A publication reporting a trial with 10 groups of animals receiving different plant species, two of them from the Polygonaceae family (e.g. Fagopyrum esculentum Moench or Fagopyrum tataricum (L.) Gaertn.) was also counted as 2 experiments. A publication reporting a trial with three different extracts of Rumex maritimus L. was counted as 3 experiments (three lines in Additional file 2).
In vitro experiment: Each in vitro experiment in a publication in which a specific extract of a particular plant part (or a whole plant) of a plant species was tested for one or more effects (publication x plant species x plant part x extracting agent). Testing of an acqueous extract of Rumex crispus L. for antibacterial and antifungal effects by agar dilution was counted as 1 experiment (one line in Additional file 3), while the testing of four different extracts of Polygonum aviculare L., was counted as 4 experiments (four lines in Additional file 3).
2.4. Assessment of the experiments
Information were systematically recorded from in vivo and in vitro experiments. For in vivo experiments information about Polygonaceae species, plant part, extracting agent, animal species, total number of animals, control groups, experimental groups, topic, effects and physiological effect on the rumen were assessed (Additional file 2). For in vitro experiments the information on Polygonaceae species, plant part, extracting agent, topic (including type of method), effects and physiological effect on rumen were assessed (Additional file 3). For each investigated effect the outcomes were categorized as follows: Positive (p) meaning there was a significant positive effect; (no) meaning that no significant effect was observed; negative (n) meaning that significant negative effect was seen; question mark (?) meaning that the effect was inconsistent (Table 3). For example in in vivo experiments regarding performance a statistical significant higher daily weight gain, compared to an untreated control group, was counted as (p), while a lower daily weight gain was counted as (n) and no statistical difference as (no).
Table 3.
Scoring system used in the systematic literature research for each parameter measured in each experiment.
| Effects* | Experiments that compared a medicinal plant-based treatment only with an antiparasitic, antibacterial or another treatment as control. | Experiments that compared a medicinal plant-based treatment at least with a negative control group (placebo-treatment or no treatment), sometimes, in addition, with an antiparasitic, antibacterial or another treatment as control. | |
|---|---|---|---|
| p | Positive effect (in case of several dosages of one plant material at least one dosage showed a positive effect, other dosages showed no effect). | The medicinal plant-based treatment showed a significant positive effect compared to any control group or no difference compared to the antiparasitic / antibacterial treatment | Medicinal plant-based treatment showed a significant positive effect compared to the negative control. |
| no | No significant effect compared to the negative control group | There is no significant difference compared to the negative control group or a significant negative difference compared to the antiparasitic / antibacterial treatment control | Medicinal plant-based treatment showed no significant difference to the negative control. |
| n | Negative effect (in case of several dosages of one plant material at least one dosage was negative, other dosages showed no effect) | Medicinal plant-based treatment showed a significant negative effect compared to the negative control. | |
| na | No data available | ||
| ? | In case of experiments with several dosages of one plant material: if at least one dosage showed a positive and another dosage a negative effect compared to negative control group (inconsistent effect). | ||
as this study was not designed as a meta-analysis but more as a qualitative systematic review a detailed prove of the statistical methods was not conducted. Only the results that the authors presented as statistical significant were considered.
3. Results and discussion
3.1. Procedure of this systematic literature review
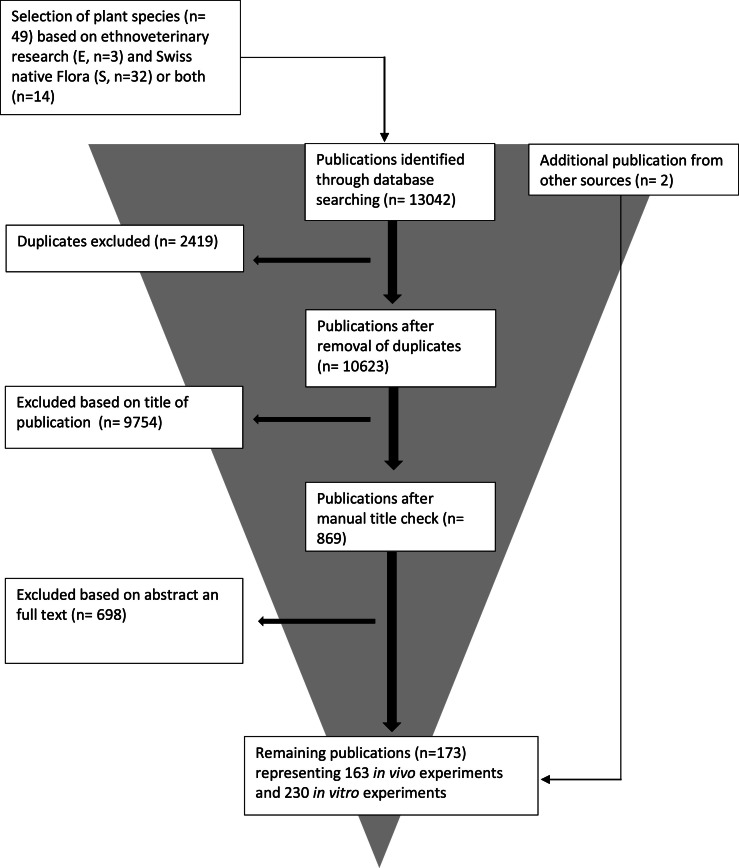
The screening of ethnoveterinary research and Swiss native flora (initial sources) led to a total of 49 Polygonaceae species (Table 2). In the subsequent bibliographic search, 10’623 publications (after removal of duplicates) were retrieved. The following manual title and abstract check reduced the number to 869 potentially interesting publications. The detailed screening of these publications finally led to 173 publications (Fig. 1) representing 393 experiments (163 in vivo and 230 in vitro). A total of 10’450 publications were excluded because they did not match the predefined criteria. The main reason for exclusion was that the title and content of the abstract did not match with the focus of the review. Other reasons were lack of an abstract, or unavailability of publications online as full text. As our systematic review was not designed as a meta-analysis but more as a qualitative review a detailed prove of the statistical methods was not conducted. This might be a certain weakness. However, we only included studies with defined and clearly described control groups that conducted and provided a statistic.
Fig. 1.
Selection process for publications included in the literature analysis.
3.1.1. Frequencies of published evidence for Polygonaceae species
We found a large amount of evidence-based information on Polygonaceae, represented by 10’623 publications focusing on the selected 49 Polygonaceae species. However, in comparison with 30 plant species that are well known for their therapeutic use (Ayrle et al., 2016), we found on average about 3 times less publications per Polygonaceae species reporting experimental based knowledge about veterinary or nutritional use in farm animals.
In our review approximately four fifths of the publications were dealing with food processing, pharmacognosy, side effects/toxicology, or other data not of within the scope of this review, or they were reviews by themselves. Information from publications dealing solely with side effects and toxicology (75 mainly about Fagopyrum esculentum Moench) were taken into account in the discussion of the respective Polygonaceae species. For some species a high number of publications was identified in the initial search (more than 113 publications per species), but only approx. 10 % (e.g. for Fagopyrum esculentum Moench, Fallopia convolvulus (L.) A. Löve, Polygonum minus Huds., Polygonum rurivagum Boreau, Reynoutria japonica Houtt., Rheum officinale/palmatum L., Rumex acetosa L., Rumex conglomeratus MURRAY, Rumex crispus L., Rumex obtusifolius L.) or even none (e.g. for Fallopia dumetorum (L.) Holub, Rumex nebroides Campd.) of these publications remained after title and/or abstract check.
More than 10 publications with in vivo or in vitro data were found for 7 Polygonaceae species, namely Fagopyrum esculentum Moench (65), Fagopyrum tataricum (L.) Gaertn. (18), Polygonum aviculare L. (17), Polygonum bistorta L. (10), Polygonum hydropiper L. (11), and Rheum officinale/palmatum L. (17). Less than 10 publications reporting in vivo or in vitro data were found for 26 Polygonaceae species, and for 16 species no publications with in vivo or in vitro data were identified. The number of publications containing in vivo information was comparable to that of papers describing only in vitro data.
3.2. Frequencies of published evidence
The 173 publications referred to 163 in vivo experiments (Additional file 2) and 230 in vitro experiments (Additional file 3).
3.2.1. Veterinary / medical effects
Antibacterial effects of Polygonaceae species were investigated most frequently (148 times), followed by anti-inflammatory (75 times), antifungal (66 times), antihyperlipidemic (35 times) and antioxidant (32 times) properties (Table 4). Hepatoprotective (12 times), antiviral (10 times), antinociceptive/analgesic (10 times), antidiarrheal (9 times) and prebiotic (5 times) effects were less frequently reported for the 51 Polygonaceae species analyzed in this review.
Table 4.
In vivo and in vitro effects reported for Polygonaceae species.
| Plant species | Plant parts | Number of publications | Type of experiments | Antioxidative | Antiviral | Antibacterial | Prebiotic | Antifungal | Hepatoprotective | Anti-inflammatory | Antinociceptive/ analgesic | Antidiarrheal | Antihyperlipidemic | Livestock related effects | Other effects | Total effects of individual species x plant part |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n | p/no/n/? | pos | none | neg | ? | ||||||
| Fagopyrum esculentum Moench | Whole plant | 11 | In vitro | 1/1/-/- | 1 | 1 | ||||||||||||||
| 42 | Ruminant | 3/7/-/- | 3 | 7 | ||||||||||||||||
| 13 | LA | -/2/-/- | 2 | |||||||||||||||||
| Grain/Seed | 74 | In vitro | 1/-/- | 1/-/- | 2/2/-/- | A | 4 | 2 | 1 | |||||||||||
| 15 | Ruminant | -/2/-/- | 2 | |||||||||||||||||
| 86 | LA | 2/2/- | 1/-/- | -/1/- | 6/2/- | 4/13//2/- | 13 | 18 | 2 | |||||||||||
| 67 | OA | 1/-/- | 5/5/1/- | B | 6 | 5 | 1 | |||||||||||||
| Hull/Bran | 18 | In vitro | 1/-/- | 1 | ||||||||||||||||
| 39 | LA | 1/-/- | 1/-/- | -/3/-/- | 2 | 3 | ||||||||||||||
| 410 | OA | 1/-/- | 1/1/- | -/2/- | -/1/- | 3/5/-/- | 5 | 9 | ||||||||||||
| n.a | 411 | In vitro | 1/-/- | 1/-/- | C | 2 | ||||||||||||||
| 312 | Ruminant | 2/2/-/- | 2 | 2 | ||||||||||||||||
| 713 | LA | 1/-/- | 4/1/- | -/9/3/- | 5 | 10 | 3 | |||||||||||||
| 914 | OA | 1/-/- | 1/2/- | 1/3/- | 2/1/-/- | 5 | 6 | |||||||||||||
| Sprout | 215 | In vitro | 2/-/- | 2 | ||||||||||||||||
| 216 | LA | 1/-/- | 1/-/- | -/1/2/- | 2 | 1 | 2 | |||||||||||||
| Leaves/Flower | 317 | LA | 3/-/- | 2/-/- | -/3/3/- | 5 | 3 | 3 | ||||||||||||
| 118 | OA | D | ||||||||||||||||||
| Herb | 119 | OA | E | |||||||||||||||||
| Straw | 220 | Ruminant | 5/1/-/- | 5 | 1 | |||||||||||||||
| Total effects | 13/2/- | - | 1/1/- | 2/2/- | - | - | 2/3/- | - | - | 18/7/- | 24/57/11/- | 60 | 72 | 12 | - | |||||
| Fagopyrum tataricum (L.) Gaertn. | Grain/Seed | 221 | In vitro | 4/-/- | 4 | |||||||||||||||
| 422 | LA | 5/-/- | 3/-/- | 3/-/- | 2/-/- | 1/1/1/- | 14 | 1 | 1 | |||||||||||
| n.a | 123 | In vitro | 1/-/- | 1/-/- | 2 | |||||||||||||||
| 224 | OA | 2/-/- | -/2/- | 2 | 2 | |||||||||||||||
| 525 | LA | 1/-/- | -/1/- | 3/-/- | 2/-/- | -/5/-/- | 6 | 6 | ||||||||||||
| Sprout | 126 | In vitro | 1/-/- | 1 | ||||||||||||||||
| Aerial parts | 127 | LA | 2/-/- | 2/-/- | 4 | |||||||||||||||
| Bran | 128 | Ruminant | 1/-/- | 3/-/-/- | 4 | |||||||||||||||
| 229 | LA | 1/-/- | -/1/- | 1/1/- | 2/1/-/- | 4 | 3 | |||||||||||||
| Total effects | 12/-/- | - | - | - | - | 6/-/- | 12/1/- | - | - | 5/3/- | 6/7/1/- | 41 | 12 | 1 | - | |||||
| Polygonum aviculare L. | Aerial part | 430 | In vitro | 3/-/- | 4/-/-/- | F | 7 | |||||||||||||
| 331 | LA | 1/-/- | -/1/- | -/1/1/- | 1 | 2 | 1 | |||||||||||||
| Leaves/Flower | 232 | In vitro | 4/1/- | 4/-/- | 8 | 1 | ||||||||||||||
| 133 | LA | G | ||||||||||||||||||
| Herbs | 234 | In vitro | -/1/- | 1/-/- | 1 | 1 | ||||||||||||||
| n.a | 435 | In vitro | 2/-/- | 2/-/- | 1/-/- | 5 | ||||||||||||||
| 136 | LA | 1/-/- | 1/-/- | 1/-/-/- | 3 | |||||||||||||||
| Stem | 137 | In vitro | 4/-/- | 4/-/- | 8 | |||||||||||||||
| Whole plant | 138 | In vitro | 1/-/- | 1 | ||||||||||||||||
| 139 | OA | -/2/-/- | H | 2 | ||||||||||||||||
| Root | 140 | OA | 1/-/- | 1 | ||||||||||||||||
| Total effects | 1/-/- | -/1/- | 14/-/- | - | 10/-/- | 1/-/- | 4/-/- | - | - | -/1/- | 5/3/1/- | 35 | 6 | 1 | - | |||||
| Polygonum bistorta L. | Aerial parts | 341 | In vitro | 4/-/-/- | I | 4 | ||||||||||||||
| Fruits | 142 | In vitro | 1/-/- | 1/-/- | 2 | |||||||||||||||
| Herbs | 143 | In vitro | -/1/- | 1 | ||||||||||||||||
| N.a | 144 | In vitro | 1/-/- | 1 | ||||||||||||||||
| 145 | OA | 1/1/-/- | H | 1 | 1 | |||||||||||||||
| Rhizome/Root/Tuber | 446 | In vitro | 1/-/- | 2/-/- | 2/2/- | 1/-/- | J/K | 6 | 2 | |||||||||||
| 247 | LA | 1/-/- | 1/-/- | 1/-/- | 3 | |||||||||||||||
| Total effects | 1/-/- | 2/1/- | 2/-/- | - | - | - | 4/2/- | - | 3/-/- | - | 5/1/-/- | 17 | 4 | - | - | |||||
| Polygonum hydropiper L. | Aerial part | 348 | In vitro | 6/-/- | 6/-/- | 4/-/-/- | 16 | |||||||||||||
| Herbs | 249 | In vitro | -/1/- | 4/-/- | 4 | 1 | ||||||||||||||
| Leaves | 250 | In vitro | 2/-/- | 1/-/- | 3 | |||||||||||||||
| n.a | 151 | In vitro | 1/-/- | 1 | ||||||||||||||||
| Stem/Stalks | 152 | In vitro | 2/-/- | 2 | ||||||||||||||||
| 153 | LA | 1/-/- | 1/-/- | 2 | ||||||||||||||||
| Whole plant | 154 | In vitro | 1/-/- | 1 | ||||||||||||||||
| 155 | LA | 3/-/- | 3 | |||||||||||||||||
| Total effects | 1/-/- | -/1/- | 11/-/- | - | 11/-/- | - | 2/-/- | 3/-/- | - | - | 4/-/-/- | 32 | 1 | - | - | |||||
| Polygonum minus Huds. | Aerial part | 156 | In vitro | 1/-/- | 1 | |||||||||||||||
| 157 | LA | 1/-/- | 1 | |||||||||||||||||
| Leaves | 358 | In vitro | 1/-/- | 2/2/- | L | 3 | 2 | |||||||||||||
| 259 | LA | 1/-/- | 2/-/- | M | 3 | |||||||||||||||
| Total effects | 1/-/- | - | 1/-/- | - | 2/2/- | - | 4/-/- | - | - | - | - | 8 | 2 | - | - | |||||
| Polygonum persicaria L. | Aerial parts | 260 | In vitro | 1/-/- | 4/1/- | 5 | 1 | |||||||||||||
| Herbs | 161 | In vitro | -/1/- | 1 | ||||||||||||||||
| Total effects | - | -/1/- | 1/-/- | - | 4/1/- | - | - | - | - | - | - | 5 | 2 | - | - | |||||
| Rheum officinale/ palmatum L. | n.a | 262 | In vitro | 1/-/- | 1/-/- | N | 2 | |||||||||||||
| 363 | LA | 1/-/- | 2/-/- | O | 3 | |||||||||||||||
| Rhizome/Root | 1064 | In vitro | 1/-/- | 1/-/- | 3/-/- | -/-/1 | 1/-/- | -/2/- | 2/-/-/- | P | 8 | 2 | 1 | |||||||
| 165 | LA | -/1/- | 1 | |||||||||||||||||
| 266 | OA | -/2/-/- | 2 | |||||||||||||||||
| Total effects | 1/-/- | 1/-/- | 4/-/- | -/-/1 | 1/-/- | 1/1/- | 3/2/- | - | - | - | 2/2/-/- | 13 | 5 | 1 | - | |||||
| Rumex acetosa L. | Aerial parts | 367 | In vitro | 1/-/- | 2/-/- | 3 | ||||||||||||||
| Herbs | 168 | In vitro | 1/2/- | 1 | 2 | |||||||||||||||
| Root | 169 | In vitro | 3/-/- | 3 | ||||||||||||||||
| Whole plant | 270 | In vitro | 2/-/- | Q | 2 | |||||||||||||||
| 171 | OA | |||||||||||||||||||
| 272 | LA | 2/-/- | R | 2 | ||||||||||||||||
| Leaves | 173 | LA | -/-/-/1 | 1 | ||||||||||||||||
| Total effects | - | - | 5/2/- | - | 2/-/- | - | 4/-/- | - | - | - | -/-/-/1 | 11 | 2 | - | 1 | |||||
| Rumex acetosella L. | n.a | 174 | In vitro | 2/-/- | 2/-/- | 4 | ||||||||||||||
| Whole plant | 175 | In vitro | -/3/- | 3 | ||||||||||||||||
| Root | 176 | LA | 1/-/- | /-/- | 1/-/-/- | 3 | ||||||||||||||
| Total effects | - | - | 2/3/- | - | 2/-/- | 1/-/- | - | - | - | 1/-/- | 1/-/-/- | 7 | 3 | - | - | |||||
| Rumex crispus L. | Aerial parts | 277 | In vitro | 4/1/- | -/3/- | 4 | 4 | |||||||||||||
| 178 | LA | -/2/- | -/2/- | 4 | ||||||||||||||||
| Herbs | 179 | In vitro | 1/2/- | 1 | 2 | |||||||||||||||
| Leaves | 380 | In vitro | 6/4/- | 4/3/- | 10 | 7 | ||||||||||||||
| n.a | 181 | In vitro | 1/-/- | 1 | ||||||||||||||||
| Root | 382 | In vitro | 9/1/- | 5/2/- | 14 | 3 | ||||||||||||||
| Seed | 183 | In vitro | 1/2/- | -/3/- | 1 | 5 | ||||||||||||||
| Total effects | - | - | 21/10/- | - | 9/11/- | - | 1/2/- | -/2/- | - | - | - | 31 | 25 | - | - | |||||
| Rumex obtusifolius L. | Herbs | 284 | In vitro | 1/2/- | -/-/1/- | 1 | 2 | 1 | ||||||||||||
| Leaves | 185 | In vitro | 1/-/- | 1 | ||||||||||||||||
| n.a | 286 | Ruminants | ||||||||||||||||||
| Root | 287 | In vitro | 1/2/- | 1/-/- | 2 | 2 | ||||||||||||||
| 188 | LA | 1/-/- | 1 | |||||||||||||||||
| Seed | 289 | In vitro | 3/-/- | S | 3 | |||||||||||||||
| Whole plant | 390 | Ruminants | 1/-/-/- | 1 | ||||||||||||||||
| Total effects | - | - | 5/4/- | - | 1/-/- | - | 2/-/- | - | - | - | 1/-/-/1 | 9 | 4 | - | - | |||||
| Rumex patientia L. | Leaves/Flower | 391 | In vitro | 2/5/- | 1/-/- | 3 | 5 | |||||||||||||
| Root | 292 | In vitro | 4/-/- | 4 | ||||||||||||||||
| 293 | LA | 8/1/- | 8 | 1 | ||||||||||||||||
| Stem | 194 | In vitro | 1/-/- | 1/-/- | 2 | |||||||||||||||
| Fruits | 195 | LA | 1/-/- | 1 | ||||||||||||||||
| Total effects | - | - | 7/5/- | - | 2/-/- | - | 9/1/- | - | - | - | - | 18 | 6 | - | - | |||||
| Other plant species (n = 36) | 2596 | All groups | - | 1/2/- | 29/19/- | - | 6/2/- | 1/-/- | 14/3/- | 5/-/- | 4/2/- | - | 3/-/-/- | 63 | 28 | - | - | |||
| Total effects of all Polygonaceae species | 30/2/- | 4/6/- | 103/45/- | 2/2/1 | 50/16/- | 10/2/- | 61/14/- | 8/2/- | 7/2/- | 24/11/- | 51/70/14/1 | 356 | 172 | 15 | 1 | |||||
p (positive) = a positive effect, no (none) = no effect, n (negative) = a negative effect, question mark (?) = inconsistent effect; In vivo: Ruminant, labor animals (LA), other animals (OA); detailed information about the scoring procedure could be found in Table 3.
Other effects A: Cytotoxic effect, B: Modulatory effect of satiety hormones (DM2), C/H: Cytoprotective effect; modulatory effect on microorganisms, D: Stimulated growth of E. Ramulus, E: Supportive treatment of CVI, F: Antiobesity effect, G: Effective for kidney stones, I/K/L/Q: No toxic effect, J: Antispasmodic effect, M: Laxative effect, N: No wound healing, O: Smooth muscle reactivity, P: Spasmogenic effect, R: Antibiotic modulatory effect
Other plant species contain all plants with less than three publications: Fallopia aubertii (L.Henry) Holub, Fallopia convolvulus (L.) A. Löve (seed), Fallopia dumetorum (L.) Holub, Oxyria digyna (L.) Hill, Polygonum alpinum All., Polygonum amphibium L. (herb, whole plant), Polygonum arenastrum Boreau (leaves, stem), Polygonum capitatum D.Don (whole plant), Polygonum lapathifolium L. (herb, root/rhizome), Polygonum mite Schrank (herb), Polygonum nepalense Meisn., Polygonum orientale L. (aerial parts), Polygonum perfoliatum L. (herb, whole plant), Polygonum polystachyum Meisn., Polygonum rurivagum Boreau, Polygonum viviparum L., Reynoutria x bohemica Chrtek & Chrtkova (rhizome), Reynoutria japonica Houtt (rhizome)., Reynoutria sachalinensis (F. Schmidt) Nakai (rhizome), Rheum rhabarbarum L. (root), Rumex x pratensis Mert. & W.W.J. Koch, Rumex alpestris Jacq., Rumex alpinus L. (aerial part, flower, leaves, root), Rumex aquaticus L. (herb, root), Rumex conglomeratus Murray (herbs), Rumex hydrolapathum Huds. (leaves, root), Rumex longifolius DC., Rumex maritimus L. (root), Rumex nebroides Campd., Rumex nivalis Hegetschw., Rumex palustris Sm., Rumex pulcher (L.), Rumex sanguineus L. (whole plant), Rumex scutatus L. (whole plant), Rumex sp., Rumex thasiflorus Fingerh. (herb, root)
(Amelchanka et al., 2010; Hur et al., 2011; Metzger et al., 2007; Leiber et al., 2012; Paśko et al., 2019; Sinz et al., 2019; Swiatecka et al., 2013)
(Choi et al., 2007; Lee et al., 2014; Zduńczyk et al., 2006; Kim et al., 2012, 2012; Fotschki et al., 2020; Préstamo et al., 2003; Tomotake et al., 2006)
(Han et al., 2019; Huang et al., 2020; Kayashita et al., 1995, 1996, 1999; Li et al., 2020; Tomotake et al., 2001)
(Bijlani et al., 1985; Dong et al., 2019; Fang et al., 2007; Imaki et al., 1990; Jacob et al., 2008; Jacob & Carter 2008; Mišan et al., 2017; Wieslander et al., 2011, 2007)
(Bae et al., 1998; Zhang et al., 2011; García-González et al., 2008a, b; Yu et al., 2005; Goto et al., 1996; Blaszczyk et al., 2000; Kosikowska et al., 2010; Nanasombat et al., 2018; Lin et al., 2009)
(Harrold et al., 1980; Smolarz & Skwarek, 1999; Özbay & Alim, 2009; Ceylan et al., 2019; Liao et al., 2011; Keleş et al., 2001; Smolarz & Skwarek, 1999; Gou et al., 2017; Saleem et al., 2018; Liu et al., 2012; Nawrot-Hadzik et al., 2019; Rehman et al., 2019; Ozturk & Ozturk, 2007; Orbán-Gyapai et al., 2017; Rouf et al., 2003)
As to single species, effects of Fagopyrum esculentum Moench were investigated the most (144 times, with 131 in vivo and 13 in vitro experiments). The second most frequently studied species was Rumex crispus L. (56 times, with 52 in vivo and 4 in vitro experiments) followed by Fagopyrum tataricum (L.) Gaertn. (54 times, including 47 in vivo and 7 in vitro experiments), Polygonum aviculare L. (42 times; 10 in vivo and 32 in vitro experiments) and Polygonum hydropiper L. (33 times, with 5 in vivo and 28 in vitro experiments).
Most frequently, antibacterial effects were investigated for Rumex crispus L. (31 times) and Polygonum aviculare L. (15 times), anti-inflammatory effects for Fagopyrum tataricum (L.) Gaertn. (13 times) and Rumex patientia L. (10 times), antifungal effects for Rumex crispus L. (20 times) and Polygonum hydropiper L. (11 times), antihyperlipidemic effects for Fagopyrum esculentum Moench (25 times) and Fagopyrum tataricum (L.) Gaertn. (8 times), antioxidative effects for Fagopyrum esculentum Moench (15 times) and Fagopyrum tatatricum (L.) Gaertn. (12 times).
3.2.2. Effects potentially relevant for livestock nutrition
Most frequently, effects on growth performance were studied (49 times), followed by effects on feed intake (39 times) and feed conversion rate (17 times; Table 5, Table 6). Effects on methane reduction (12 times), ammonia reduction (9 times), meat quality (6 times) and milk quality (4 times) were studied less frequently. With regard to specific species again most studies were dealing with Fagopyrum esculentum Moench (93 times containing 87 in vivo and 6 in vitro effects). A lower number of studies was dealing with Fagopyrum tataricum (L.) Gaertn. (14 times containing solely in vivo), Polygonum aviculare L. (9 times containing 5 in vivo and 4 in vitro) and Polygonum bistorta L. (6 times containing 2 in vivo and 4 in vitro). These four Polygonaceae species were the most intensively researched species with regard to feeding effects in livestock.
Table 5.
Potentially livestock-relevant effects of 11 Polygonaceae species.
| Plant species | Plant parts | Number of publications | Type of experiments | Ammonia reduction | Methane reduction | Milk quality | Meat quality | Improved performance | Improved feed intake | Improved conversion rate | Total effects of individual species x plant part |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p/no/n | p/no/n | p/no/n | p/no/n | p/no/n/? | p/no/n | p/no/n | p | no | n | ? | ||||
| Fagopyrum esculentum Moench | Whole plant | 21 | In vitro (rumen content of cow) | 1/1/- | 1/1/- | 2 | 2 | |||||||
| 42 | Cows | 1/-/- | 2/2/- | -/2/-/- | 1/3/- | 4 | 7 | |||||||
| 13 | Mice | -/1/-/- | -/1/- | 2 | ||||||||||
| Grain/Seed | 34 | In vitro (rumen content of cow) | 2/1/- | 1/1/1 | 3 | 2 | 1 | |||||||
| 15 | Cows | -/1/- | -/1/- | 2 | ||||||||||
| 76 | Mice, rats | 1/6/2/- | 1/6/- | 2/1/- | 4 | 13 | 2 | |||||||
| 57 | Broilers | 1/2/-/- | 2/1/- | 1/1/1 | 4 | 4 | 1 | |||||||
| Laying hen | -/1/-/- | 1/-/- | -/1/- | 1 | 2 | |||||||||
| Hull/Bran | 28 | Mice, rats | -/2/-/- | -/1/- | 3 | |||||||||
| 49 | Pigs | -/1/- | 1 | |||||||||||
| Laying hen | 1/-/-/- | 1/-/- | -/1/- | 2 | 1 | |||||||||
| Piglets | -/1/- | 1/-/-/- | -/1/- | 1 | 3 | |||||||||
| n.a | 310 | Goat | ||||||||||||
| Lambs | 1/-/- | -/1/-/- | 1/-/- | -/1/- | 2 | 2 | ||||||||
| 511 | Mice, rats | -/4/2/- | -/4/1 | -/1/- | 9 | 3 | ||||||||
| 412 | Barrow | |||||||||||||
| Broiler | -/1/-/- | 1/-/- | 1/-/- | 2 | 1 | |||||||||
| Chicken | ||||||||||||||
| Turkey | ||||||||||||||
| Sprout | 213 | Mice, rats | -/-/2/- | -/1/- | 1 | 2 | ||||||||
| Leaves/Flower | 214 | Rats | -/2/2/- | -/1/1 | -/2/- | 3 | 3 | |||||||
| Straw | 215 | Lambs | 1/-/- | 1/1/-/- | 2/-/- | 1/-/- | 5 | 1 | ||||||
| Total effects | 2/1/- | - | 2/2/- | 2/3/- | 5/20/8/- | 9/20/2 | 4/9/1 | 24 | 57 | 12 | - | |||
| Fagopyrum tataricum (L.) Gaertn. | Grain/Seed | 216 | Mice, Rats | 1/-/1/- | -/1/- | 1 | 1 | 1 | ||||||
| n.a | 317 | Mice, rats | -/3/-/- | -/2/- | 5 | |||||||||
| Bran | 118 | Lambs | 1/-/- | 1/-/-/- | 1/-/- | 3 | ||||||||
| 219 | Rats | 1/1/-/- | 1/-/- | 2 | 1 | |||||||||
| Total effects | - | - | - | 1/-/- | 3/4/1/- | 2/3/- | - | 6 | 7 | 1 | - | |||
| Polygonum aviculare L. | Aerial part | 220 | In vitro (rumen content of sheep) | 2/-/- | 2/-/- | 4 | ||||||||
| 121 | Mice | -/-/1/- | -/1/- | 1 | 1 | |||||||||
| n.a | 122 | Mice | 1/-/-/- | 1 | ||||||||||
| Whole plant | 123 | Broiler | -/1/-/- | -/1/- | 2 | |||||||||
| Total effects | 2/-/- | 2/-/- | - | - | 1/1/1/- | -/1/- | -/1/- | 5 | 3 | 1 | - | |||
| Polygonum bistorta L. | Aerial part | 224 | In vitro (rumen content of sheep) | 2/-/- | 2/-/- | 4 | ||||||||
| n.a | 125 | Broiler | 1/-/-/- | -/1/- | 1 | 1 | ||||||||
| Total effects | 2/-/- | 2/-/- | - | - | 1/-/-/- | - | -/1/- | 5 | 1 | - | - | |||
| Polygonum hydropiper L. | Aerial part | 226 | In vitro (rumen content of sheep) | 2/-/- | 2/-/- | 4 | ||||||||
| Total effects | 2/-/- | 2/-/- | - | - | - | - | - | 4 | - | - | - | |||
| Rheum officinale/ palmatum L. | Rhizome/root | 227 | In vitro (rumen content of sheep) | 2/-/- | 2 | |||||||||
| 128 | Piglets | -/1/- | 2 | |||||||||||
| Total effects | - | 2/-/- | - | - | -/-/-/- | -/1/- | - | 2 | 2 | - | - | |||
| Rumex acetosa L. | Whole plant | 129 | Chicks | |||||||||||
| Leaves | 130 | Rats | -/-/-/1 | 1 | ||||||||||
| Total effects | - | - | - | - | -/-/-/1 | - | - | - | - | - | 1 | |||
| Rumex acetosella L. | Root | 131 | Rats | 1/-/-/- | 1 | |||||||||
| Total effects | - | - | - | - | 1/-/-/- | - | - | 1 | - | - | - | |||
| Rumex obtusifolius L. | herbs | 132 | In vitro (rumen content of steer) | 1/-/- | 1 | |||||||||
| n.a | 233 | Lambs | ||||||||||||
| Sheep | ||||||||||||||
| Whole plant | 134 | Lambs | ||||||||||||
| Sheep | ||||||||||||||
| Total effects | - | 1/-/- | - | - | - | - | - | 1 | - | - | - | |||
| Fallopia convolvulus (L.) A. Löve | Seed | 135 | Rats | 1/-/-/- | 1/-/- | 1/-/- | 3 | |||||||
| Total effects | - | - | - | - | 1/-/-/- | 1/-/- | 1/-/- | 3 | - | - | - | |||
| ∑ total effects | 8/1/- | 9/2/1 | 2/2/- | 3/-/- | 12/26/10/1 | 12/25/2 | 5/11/1 | 58 | 72 | 14 | 1 | |||
P (positive) = is a positive effect, no (none) = is no effect, n (negative) = a negative effect, question mark (?) = inconsistent effect; detailed information about the scoring procedure could be found in Table 3.
(Choi et al., 2007; Zduńczyk et al., 2006; Kim et al., 2012, 2012; Fotschki et al., 2020; Préstamo et al., 2003; Tomotake et al., 2006)
Table 6.
Further information about potentially livestock-relevant effects of 8 Polygonaceae species.
| Plant species | Plant parts | Number of publications | Type of experiments | Further information |
|---|---|---|---|---|
| Fagopyrum esculentum Moench | Whole plant | 21 | In vitro (rumen content of cow) | Buckwheat forage and silage had no significant effect on in vitro ruminal degradability and short chain fatty acid concentration. Buckwheat forage enhanced estimated microbial Nitrogen growth efficiency. Buckwheat forage reduced the number of bacteria in the incubated fluid, while Buckwheat silage reduced that of holotrich protozoa significantly compared to control. |
| 42 | Cows | Transfer rate of a-linoleic acid from feed to milk was significantly higher than ryegrass, but there was no effect on alpha-tocopherol in milk. Ruminal Nitrogen balance was significantly lower, fecal Nitrogen loss and Nitrogen utilization for milk were significantly higher than ryegrass. | ||
| Grain/Seed | 34 | In vitro (rumen content of cow) | There was no effect on in vitro ruminal degradability and short chain fatty acid concentration. pH was significantly higher than wheat. Nitrogen supply, apparent degraded Nitrogen, degraded but not recovered Nitrogen, non-ammonia Nitrogen decreased significantly compared to wheat. Holotrich protozoa counts were significantly higher and total gas was significantly lower than wheat; There was no significant effect on total gas production and microbial count. Short chain fatty acid, Propionate, acetate concentration increased significantly compared to acacia. | |
| 15 | Cows | There was no significant effect on milk quality and feed intake compared to the control diet. | ||
| 57 | Broilers | Ileal digestibility of Phytate phosphorus was significantly higher than control diet (0.15 % lower non-Phytate). Phytase activity was significantly higher, but decreased in digestive tract parts. Phytase activity was significantly lower in ileum. Bone quality (Tibia, Femur) and Phosphorus and Nitrogen retention were significantly higher, Total Phosphorus excretion was significantly lower than control diet (0.16 % lower non-Phytate). | ||
| Laying hen | Egg production rate, egg quality and Phosphorus and Nitrogen retention were significantly higher than control diet (0.16 % lower non-Phytate). There was no significant effect on egg quality. | |||
| Hull/Bran | 49 | Pigs | There was no significant effect on meat oxidative stability compared to the control diet (some contain additionally oat or vitamin E). | |
| Laying hen | Egg production rate was significantly higher than control diet. There was no effect on egg quality. Buckwheat bran was preferred to soybean in the free-choice feeding test. | |||
| Piglets | Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), alkaline phosphatase (ALP), Calcium (CA) levels were significantly lower in Probiotics + Buckwheat than only probiotics. There was no significant probiotic effect. Intestinal morphology, total number and density of goblet cells had no significant changes. Ki-67+ cells were significantly higher in colon. CD3+ cells were significantly lower in Jejunum and higher in Colon. | |||
| n.a | 310 | Goat | Phosphorus and Crude protein degradability of Buckwheat was comparable with other cereals. Starch degradability was slower than the other cereals. | |
| Lambs | There was no significant effect on Carcass parameters. Yellowness of M. longissimus dorsi of lambs was significantly lower, but the Total phenolic content was significantly higher than maize. | |||
| 412 | barrow | Apparent total tract digestibility of Organic matter was significantly higher and Neutral detergent fiber/Acid detergent fiber were significantly lower than control diet (Soybean). There was no significant effect on Energy balance and energy content. There was no significant effect on true and apparent Phosphorus digestibility. | ||
| chicken | True metabolizable energy was lower compared to control (fasted): The content was higher compared to NRC. | |||
| turkey | True metabolizable energy was lower compared to control (fasted): The content was higher compared to NRC. | |||
| Straw | 215 | Lambs | Dry matter digestibility and Nitrogen retention were higher, while Nitrogen excretion was lower than control diet. Nutrient digestibility was significantly higher than control diet. The ruminal microbial diversity was declined and the microbiome tends to be simplified and some maleficent bacteria abundance was increased. | |
| Fagopyrum tataricum (L.) Gaertn. | Bran | 118 | Lambs | There was no significant effect on serum parameters. Kidney, rumen and omasum weights were significantly lower, Reticulum and small intestine weights were significantly higher than basal diet. |
| Polygonum aviculare L. | Aerial part | 220 | In vitro (rumen content of sheep) | 30 % less methane production and 80 % less ammonia production than ryegrass. True organic matter digestibility, crude protein, gas production, volatile fatty acid were significantly lower than ryegrass. |
| Whole plant | 123 | Broiler | There was no significant effect on bloody diarrhea, survival, lesion score. Oocyst excretion was lower than model group (infected). There was no significant anticoccidial effect. | |
| Polygonum bistorta L. | Aerial part | 224 | In vitro (rumen content of sheep) | 30 % less methane production and 80 % less ammonia production than ryegrass. True organic matter digestibility, gas production, volatile fatty acid were significantly lower than ryegrass. Crude protein was significantly higher than ryegrass. |
| n.a | 125 | Broiler | Serum Glucose and total protein were significantly higher, Urea nitrogen was significantly lower than model group (infected). Carcass fat weight and grid reference tissue depth increased while drip loss decreased. There was an anticoccidial activity. | |
| Polygonum hydropiper L. | Aerial part | 226 | In vitro (rumen content of sheep) | 30 % less methane production and 80 % less ammonia production than ryegrass. True organic matter digestibility, crude protein, gas production, volatile fatty acid were significantly lower than ryegrass. |
| Rheum officinale/ palmatum L. | Rhizome/root | 227 | In vitro (rumen content of sheep) | Dry matter digestibility, neutral detergent fiber digestibility, total gas production, total volatile fatty acid production decreased significantly compared to control; Dry matter digestibility, cell wall digestibility, total gas production, total volatile fatty acid production decreased significantly compared to control. |
| 128 | Piglets | Fecal N loss and energy digestibility and metabolizability were significantly higher, fecal energy loss was significantly lower than basal diet. There was no significant effect on nitrogen and energy balance. Dry matter content of the feces was decreased linearly to the increasing amount of rhubarb. Higher dose of rhubarb (1 %) caused a laxative effect. | ||
| Rumex acetosa L. | Whole plant | 129 | Chicks | Coppersulphate, brassica compestris and cisplatin induced emesis were significantly decreased compared to negative control. |
| Rumex obtusifolius L. | n.a | 233 | Lambs | Mineral content of rumen and feces and mineral availability changed. The dock particles in the rumen typically had a low ratio of length to width and it seemed that dock particles did not need to be reduced in size as much as ryegrass particles before passing out of the rumen. Fibrosity index, time spent ruminating and time spent eating was lower than ryegrass. |
| Sheep | Mineral content in rumen changed. Fibrosity index was lower than ryegrass. | |||
| Whole plant | 134 | Lambs | Feed intake and Body weight gain were lower compared to ryegrass. Organic matter digestibility was lower and dry matter digestibility was higher than ryegrass. Dry weight in rumen was lower and fecal loss of rumen content was higher than ryegrass. | |
| Sheep | Dry matter digestibility was higher and organic matter digestibility was lower than ryegrass. |
Specifically, effects on growth performance were investigated most frequently (Fagopyrum esculentum Moench (33 times), and Fagopyrum tataricum (L.) Gaertn. (8 times)), followed by effects on feed intake (Fagopyrum esculentum Moench (31 times), and Fagopyrum tataricum (L.) Gaertn. (5 times)), and effects on feed conversion rate (Fagopyrum esculentum Moench (14 times)), effects on milk and meat quality (Fagopyrum esculentum Moench (9 times)), and effects on ammonia and methane reduction (Fagopyrum esculentum Moench (6 times)).
3.3. Outcome of published evidence regarding veterinary, pharmacological and/or biological effects
For 33 of the investigated 49 Polygonaceae species, publications reporting on pharmacological and/or biological effects were found. No studies on immunomodulatory, antitussive and astringent effects were identified, and only sparse information on antiviral, antiparasitic, prebiotic, probiotic, modulatory, antidiarrheal, hepatoprotective and antinociceptive/ analgesic effects was found. Studies were predominantly dealing with antibacterial, anti-inflammatory, antifungal, antioxidative and antihyperlipidemic properties (Table 4). The investigated Polygonaceae species showed some in vitro antibacterial activity against numerous gram positive (Streptococcus pyogenes, Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus saprophyticus, Bacillus subtilis) and gram negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aerigunosa, Neisseria gonorrhoeae, Salmonella typhi, Salmonella paratyphi, Salmonella enteriditis, Shigella flexneri, Achinetobacter baumanii) (Additional file 3). Since E.coli-induced digestive disorders are frequently observed in the postweaning period of piglets, Polygonaceae species might be useful for this treatment (Madec et al., 1998).
Numerous in vitro experiments indicated antifungal properties of the Polygonaceae species, with activity against a wide spectrum of fungi (Aspergillus flavus, Aspergillus fumigatus, Aspergillus niger, Cryptococcus neoformans, Candida albicans, Candida krusei, Microsporum gypseum, Tychophyton rubrum, Trychophyton mentagophytes, Saccharomyces cerevisiae, Alternia solani, Harpophora maydis; Additional file 3). Given that livestock diseases like cattle ringworm may be caused by T. mentagophytes (Rook & Frain-Bell, 1954), some Polygonaceae species may be useful as a supportive treatment.
Many in vitro and in vivo experiments were dealing with anti-inflammatory activity of Polygonaceae species. On a mechanistic level a decrease of proinflammatory cytokines, such as tumor necrosis factor-a (TNF-a), interleukins (IL) and lipopolysaccharides (LPS), or the inhibition of cyclooxygenases were reported. Activity against ethanol-induced gastric ulcers and inhibition of carrageenan- and xylene-induced edema were described. The anti-inflammatory activity may be particularly useful for diseases in young animals, such as respiratory or gastrointestinal diseases (Ayrle et al., 2016). Polygonaceae species are rich in phenolic compounds and therefore act as antioxidants. Beside the well known direct radical scavenging activity of polyphenols, the experimental shown antioxidative activity is also based on increasing levels of antioxidant enzyme activities, such as superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT), which are important for detoxifying of free radicals.
Fagopyrum species are interesting with regard to their anti-inflammatory, antioxidative and antihyperlipidemic activities. The genera “Polygonum” and “Rumex” show mainly antibacterial, antifungal and anti-inflammatory activities. In addition, antioxidative properties are shown by Polygonum species and antihyperlipidemic properties in Rumex species. Few experiments were conducted with the genus “Rheum”, which showed antioxidative, antibacterial, antifungal and anti-inflammatory activities.
Thus, Polygonum and Rumex species may be useful in the case of bacterial and fungal infections. Species from almost all genera exhibited some anti-inflammatory activity and may thus be useful to mitigate inflammatory ailments. On the other hand, only Fagopyrum species showed a pronounced cholesterol-lowering activity.
Some Polygonaceae species (17 species, e.g. Polygonum aviculare L., Rumex crispus L. and Rumex obtusifolius L.) seem to play an important role in European ethnoveterinary medicine and are commonly used for the treatment of disorders of the gastrointestinal tract and metabolism, and skin diseases (Table 1). However, for 32 species no ethnoveterinary use has been documented yet.
Data only from in vitro experiments or in vivo experiments with laboratory animals have been reported for Polygonum minus Huds., Polygonum persicaria L., Rumex crispus L. and Rumex patientia L., but without a link to livestock relevant diseases (Table 4). Extracts of Polygonum minus Huds. showed anti-inflammatory properties. The plant contains phenolic compounds (Qader et al., 2012). No ethnoveterinary uses have been reported for Polygonum minus Huds. Extracts of Polygonum persicaria L. showed antifungal properties in vitro against a wide range of fungi (Additional file 3). The plant contains compounds like sesquiterpenes and flavonoids (Derita & Zacchino, 2011). The ethnoveterinary use of Polygonum persicaria L. in case of genitourinary diseases has so far not been corroborated by in vitro and/or in vivo studies (Mayer et al., 2014). Extracts of Rumex crispus L. showed activity against a wide range of gram positive and negative bacteria, and fungi (Additional file 3), supporting the ethnoveterinary use for the treatment of skin and gastrointestinal diseases (Mayer et al., 2014). Extracts of Rumex patientia L. showed antibacterial and anti-inflammatory activities (Additional file 3). Also, such extracts were effective in vivo against gastric ulcers (Süleyman et al., 2004) and inhibited carrageenan-induced edema (Süleyman et al., 1999). There are no reported ethnoveterinary uses for Rumex patientia L. Both. Rumex crispus L. and Rumex patientia L. contain phenolic compounds, such as anthraquinones, flavonoids and stilbenes (Orbán-Gyapai et al., 2017).
3.4. Specific livestock nutrition-related properties of Polygonaceae species
Plants relevant for various livestock animals are predominantly the cultivated Polygonaceae species, in particular buckwheat which is used as poultry feed (Jacob & Carter, 2008; Leiber, 2016) but also considered as feed for pigs (Dong et al., 2019; Farrell, 1978) and a concentrate or forage for ruminants (Leiber, 2016; Mu et al., 2019; Omokanye et al., 2021). Rhubarb (mainly roots or rhizomes) is considered as an alternatives to antibiotic growth promoters in pig diets (Straub et al., 2005). Of the wild Polygonaceae abundant in Europe, mainly Polygonum species occurring in natural pastures are mentioned in the literature as feed selected by grazing ruminants (Leps et al., 1995; Krahulec et al., 2001; Gorlier et al., 2012; Kazmin et al., 2016). However, also Rumex species, which are almost exclusively considered as weeds, are eaten by ruminants (Hejcman et al., 2014; Sormunen-Cristian et al., 2012; Waghorn & Jones, 1989; Zaller, 2006). For buckwheat, interesting effects on rumen fermentation are reported, which affect fatty acid composition of the products (Kälber et al., 2011), as well as methane formation (Leiber et al., 2012) and ammonia levels (Kälber et al., 2012). Rumen-modulating effects were also shown for rhubarb roots (García-González et al., 2008b; Kim et al., 2016). Rumex obtusifolius may have effects against bloating (Waghorn & Jones, 1989) and affects ruminal protein degradation and growth of proteolytic bacteria (Molan et al., 2007).
For 11 of the investigated 49 Polygonaceae species, 66 publications on effects potentially relevant for animals nutrition and performance were found (Table 5, Table 6). Growth performance, milk yield, egg yield, feed intake and feed conversion rate were investigated in cows, mice, rats, chicken, pigs, goats, lambs, barrows, broiler and turkey. The majority of these publications showed no effects on feed intake, growth performance and feed conversion rate. Of the few effects reported, positive effects predominated over negative properties. Thus, some Polygonaceae species may improve the above parameters to some extent. The effects on the quality of milk, meat and eggs were either positive or absent.
The plant most frequently studied in controlled feeding experiments was buckwheat. A total of 12 ruminant experiments with Fagopyrum esculentum, and one with Fagopyrum tataricum have been reported. Buckwheat was the only species for which negative effects on feed intake and performance were never reported (except for rodents). Some positive effects were found, such as an improved ruminal or overall N conversion, lower methanogenesis, and positive effects on milk or meat quality. Three studies (four experiments) considering roots of Rheum spp. as a feedstuff for farm animals were reported, showing in vitro mitigation of ruminal methanogenesis but no effects on performance of piglets in vivo. We found four experiments with Rumex obtusifolius in which lowering of methane production and inhibition of proteolytic bacteria was shown in vitro, and prevention of bloating in vivo. Polygonum bistorta is the most abundant species of Polygonaceae in pastures, and is clearly selected by ruminants (Leps et al., 1995; Krahulec et al., 2001; Gorlier et al., 2012). Two in vitro experiments with this species revealed mitigation of ruminal methane and ammonia formation.
Feeding buckwheat, rhubarb or Polygonaceae has modulating effects on the rumen processes. This is evident from in vivo and in vitro experiments where either methane and ammonia production or fatty acid profiles were taken into account.
3.5. Polygonaceae species most relevant for veterinary treatment and nutrition of farm animals
3.5.1. Fagopyrum esculentum Moench and Fagopyrum tataricum (L.) Gaertn.
In experiments with both Fagopyrum species, sprouts, leaves, stems and flowers, but mainly the seeds, hull and bran and whole plants were investigated. For both species in vivo and in vitro experiments showed their antioxidative activity. In addition, Fagopyrum esculentum Moench (grains and seeds, hulls and brans, sprouts, leaves and flowers) showed antihyperlipidemic, and the grains and seed of Fagopyrum tataricum (L.) Gaertn anti-inflammatory activity (Table 4). Flavonoids and other phenolics are known to possess antioxidant properties (Đurendić-Brenesel et al., 2013; Flis et al., 2010; Jin et al., 2020), while flavonoids of Fagopyrum esculentum Moench may be responsible for the antihyperlipidemic activity. Buckwheat seeds could contribute to the improvement of the lipid profile in serum and liver (Choi et al., 2007). Seeds of Fagopyrum tataricum (L.) Gaertn. are rich in rutin which may explain, in part, their anti-inflammatory activity (Choi et al., 2015). Gastrointestinal diseases with inflammatory manifestation are a major challenge in piglets (Ayrle et al., 2016), and Fagopyrum tataricum (L.) Gaertn. may be useful in the treatment of postweaning diarrhea. Antioxidant activity of buckwheat hulls and brans were reported in pigs (Flis et al., 2010). Administration of buckwheat hulls and brans lowered the Enterobacteriaceae count in piglets and improved growth performance in the adaptive phase of postweaning (Gāliņa & Valdovska, 2017). One of the most important diseases of dairy cows are disturbance of fat and liver metabolism in early lactation (Durrer et al., 2020). The antihyperlipidemic activities of Fagopyrum esculentum Moench might be of interest in this context. However, phototoxic fagopyrins in Fagopyrum species may limit feedable amounts (Benković et al., 2014). Although Fagopyrum esculentum Moench is reported in ethnoveterinary sources for its fertility (Vogl et al., 2016) purposes, no in vivo or in vitro data on this use could be found in the recent literature.
The above-mentioned phenolic compounds (mainly Rutin; Leiber et al., 2012) are essentially involved in the modulating effects of buckwheat seeds and whole plant on rumen processes. It was shown that dietary buckwheat can lower methane formation. Also, ammonia production was lowered, as shown by in vivo and in vitro studies (Table 5; Sinz et al., 2019). Thus could imply a lesser burden to the liver of ruminants (Parker et al., 1995), lower metabolic urea levels, and a better utilization of dietary nitrogen (Kälber et al., 2012; Kapp-Bitter et al., 2023) resulting in lower urinary nitrogen emissions. A higher transfer rate of omega-3 fatty acids from feed to milk in cows supplemented with buckwheat (Kälber et al., 2011), are possibly due to a protective effect in the rumen of buckwheat polyphenols on these fatty acids (Buccioni et al., 2012). This is beneficial for the animal itself (Leiber et al., 2019) as well as for the consumer (Sinclair et al., 2002).
Numerous studies investigated the basic dietary properties of Fagopyrum esculentum Moench in cows, mice, rats, broilers, laying hens, pigs, piglets, goat, lambs, barrows, chicken and turkeys. In the majority of experiments no effects were observed on growth performance, feed intake and feed conversion rate. Nevertheless, some experiments show significant effects, with either improvement or impairment of intake and performance (Table 5). For all investigated farm animals, the feed properties led to an improvement, while impairment was predominantly seen in laboratory animals. Compared to leaves, flowers and sprouts of Fagopyrum esculentum Moench, grains and seeds, hull and brans and straw showed improved dietary properties. Seeds, hulls and brans of buckwheat increased the egg production rate in laying hens, as well as intake and nitrogen efficiency of broilers, and meat quality in lambs and milk quality in cows (Table 5). Whether the negative effects seen in some studies are linked to the concentration of phototoxic fagopyrins in the feeds (highest concentrations in flowers, leaves, and lowest in seeds) needs further investigation.
Less information on feed properties in rats, mice and lambs is available for Fagopyrum tataricum. Growth performance and feed intake were improved in lambs, but impaired in most laboratory animals.
In conclusion, Fagopyrum esculentum Moench and Fagopyrum tataricum (L.) Gaertn. exhibit a range of interesting antioxidative, antihyperlipidemic and anti-inflammatory properties and are valuable candidates for future studies in inflammatory gastrointestinal and metabolic diseases in livestock.
Fagopyrum esculentum may be beneficial as a feed with positive effects on the animals’ constitution (less ruminal ammonia formation, improved provision with functional fatty acids, antioxidative potential), the environment (less emissions of nitrogen and methane), and human health (beneficial fatty acid profiles of the product). Taken together, feeding of farm animals with suitable buckwheat products may contribute to a one-health approach from the nutritional side.
3.5.2. Polygonum aviculare L.
Experiments with Polygonum aviculare L. were using the aerial parts (leaves, flowers, herbs, stem, whole plant) and roots. In vitro antibacterial and antifungal activities, and in vitro and in vivo anti-inflammatory properties have been reported for this species (Table 4). In vitro antibacterial activity against gram positive and gram negative bacteria (Additional file 3), including E.coli and some Salmonella species, P. aviculare might be useful in the treatment of young livestock diseases (Ayrle et al., 2016). However, the impact of P. aviculare on the composition of the rumen microflora remains to be studied. The plant showed in vitro antifungal against various Aspergillus sp. (Additional file 3). Secondary metabolites contained in the plants, such as tannins and flavonoid, are known to possess antibacterial and antifungal properties (Salama & Marraiki, 2010). Extracts exhibited cyclooxygenases (Tunón et al., 1995) and lowered the concentrations of pro-inflammatory cytokines (Park et al., 2018). Wound healing properties were recently reported (Seo et al., 2016), which corroborates the ethnoveterinary use in skin diseases (Mayer et al., 2014). On the other hand, P. aviculare showed no antiparasitic activity against oocysts of Eimeria tenella in broilers (Youn & Noh, 2001). No toxic properties of Polygonum aviculare L. were reported.
Polygonum aviculare L. lowered ammonia and methane production in rumen of sheep (Table 5). This may be due to the high concentration of polyphenols which tend to interfere with enteric methane production and proteolysis in the rumen (Macheboeuf et al., 2014; Niderkorn & Macheboeuf, 2014; ). Polygonum aviculare L. seems to be highly fermentable with a low methane production and may be nutritionally and environmentally beneficial when consumed by ruminants. Less information is available about feed properties in broilers and mice. For the investigated livestock, the growth performance, feed intake, and feed conversion rate was not affected, but there were positive and negative effects on growth performance of laboratory animals.
To conclude, Polygonum aviculare L. shows interesting antibacterial, antifungal and anti-inflammatory activities. Further studies are therefore warranted for the potential treatment of microbial infections and inflammatory gastrointestinal diseases in livestock. The reduction of methane and ammonia levels in sheep is also of interest in a health and environment perspective. There is no information about effects on livestock product quality.
3.5.3. Polygonum bistorta L.
In experiments with Polygonum bistorta L. the aerial parts (fruits, herb), roots and rhizomes were used. In vitro and in vivo experiments showed anti-inflammatory and antidiarrheal activities (Table 4). Polygonum bistorta L. contains tannins and other phenolic compounds. The polyphenols may contribute to the anti-inflammatory properties (Khushtar et al., 2018) and antidiarrheal activity (Yu et al., 2005). Polygonum bistorta L. reportedly has antiparasitic activity against coccidial oocysts of Eimeria spp. in broilers (Khan et al., 2008).
Some in vivo investigations were conducted in the context of gastrointestinal diseases. Antidiarrheal activity in mice and on the colonic mucosa of rats was reported (Ali et al., 2015; Yu et al., 2015). Interestingly, the beneficial effect on experimental gastric ulcers in rats (Khushtar et al., 2018) supports the ethnoveterinary use of P. bistorta (Delafond, 1853; Mayer et al., 2014; Röll, 1866). No toxicological findings have been reported for Polygonum bistorta L.
The aerial parts of Polygonum bistorta L. show an in vitro ammonia and methane-lowering effect in rumen of sheep (Table 5). This may be due again to the high concentrations of polyphenols which tend to interfere with enteric methane production and proteolysis in the rumen (Macheboeuf et al., 2014; Niderkorn & Macheboeuf, 2014). Polygonum bistorta L. was shown to improve growth performance, but without effect on feed conversion rate in broilers.
In summary, Polygonum bistorta L. shows interesting anti-inflammatory and antidiarrheal activities and may thus be useful in the treatment of inflammatory gastrointestinal diseases. A lower ruminal methane and ammonia formation implies both potential benefits for animal health and the environment. Polygonum bistorta L. is abundant in alpine pastures and readily consumed by cattle, leading to improved fatty acid profiles in milk from grazing cows (Gorlier at al., 2012).
3.5.4. Rumex obtusifolius L.
Experiments with Rumex obtusifolius L. were done with herbs, leaves, seeds, whole plant and roots. In vitro data showed antibacterial activities (Table 4) against gram negative and gram positive bacteria (Additional file 3). Saponins and other isoprenoids, tannins, flavonoids and coumarins have been reported for the species. The high concentration of saponins and polyphenols may be responsible for the antibacterial activity (Ginovyan et al., 2020; Ginovyan & Trchounian, 2019). Due to its in vitro antibacterial against E. coli, R. obtusifolius might be interesting in the treatment neonatal diarrhea in piglets and calves (Ayrle et al., 2016). Rumex obtusifolius L. is an undesirable weed in fields, but has been frequently reported in ethnoveterinary and historical sources to be used to treat disorders of the gastrointestinal tract, skin, genitourinary system, udder, and the musculoskeletal and respiratory systems (Mayer et al., 2014; Röll, 1866). However, no in vivo data are available that would support these traditional uses. No toxicological activities have been reported for Rumex obtusifolius L.
Rumex obtusifolius L. lowered methane production in the rumen of steers (Table 5). The high content in condensed tannins is known to reduce methanogenesis and thus may have contributed to the lower methane output (Purcell et al., 2012). Also, the reduced methane output could be due to the fact that the plant does not need to be fully digested before leaving the rumen. A short ruminating time may reduce methane formation (Wilman et al., 1997).
To conclude, Rumex obtusifolius L. shows interesting antibacterial activities and may be useful in the treatment of infectious diseases. Little information about feed properties is available to date, but the prevention of bloating in ruminants (Waghorn & Jones, 1989) is a relevant factor in cattle nutrition and health. The methane-lowering effect may be environmentally beneficial.
3.5.5. Rumex acetosa L. and Rumex acetosella L.
In experiments with these two Rumex species the aerial parts (herbs, leaves, whole plants) and roots were investigated. Rumex acetosa L. and R. acetosella both showed in vitro antibacterial activity against gram negative and gram positive bacteria, and antifungal properties (Additional file 3). In addition, Rumex acetosa L. showed in vitro and in vivo anti-inflammatory activity (Table 4).
Both Rumex species contain saponins, tannins, flavonoids, anthranoids and stilbenes. The antibacterial activity could be due, at least in part, to the polyphenols and saponins. Calves may be infected with Salmonella within hours after birth, which is a common cause of neonatal morbidity and mortality (Mohler et al., 2009). As Rumex acetosella L. is in vitro antibacterial against S. enteritidis, it might be considered as a supporting treatment option. Emodin may display anti-ulcerogenic and anti-inflammatory activities (Bae et al., 2012). These findings fit well with the ethnoveterinary use of Rumex acetosa L. in gastrointestinal diseases (Delafond, 1853; Mayer et al., 2014). However, investigations on beneficial effects of R. acetosa in parasitic and respiratory diseases are lacking, as well as studies with Rumex acetosella in the context of gastrointestinal diseases. For both Rumex species the influence on growth performance was investigated in rats. Administration of Rumex acetosella showed positive effects, whereas the effects with Rumex acetosa was inconsistent. No toxicological findings have been reported for both species.
To conclude, R. acetosa and R. acetosella possess potentially beneficial properties for livestock, such as antibacterial, antifungal and anti-inflammatory activities. No information is currently available about livestock performance, product quality and ruminal biotransformation.
4. Conclusion
In the European alpine regions, some 50 species of the Polygonaceae family occur, most of them wild and very few cultivated. The spectrum of phytochemicals in the family renders these plants interesting as options for the treatment and nutrition of farm animals. Our qualitative systematic review showed that the majority of published data was dealing with pharmacological activities, while fewer publications were addressing specific veterinary issues related to animal nutrition and disesae prevention. For two cultivated (Fagopyrum spp.) and five wild (two Polygonum spp. and three Rumex spp.) species desirable effects for veterinary treatment and animal nutrition were reported. Beneficial effects of plants may be multifacetted. For example, antibacterial properties may be beneficial in the prevention of gastrointestinal infection in young animals on the one hand, and increase nutrient conversion in ruminants on the other. Possible, but not yet evaluated preventive properties against gastrointestinal bacterial infections in piglets (Fagopyrum spp.) or parasite infections in chicken (Polygonum bistorta) could be provided by the plants when used as a feed or feed additive. In ruminants, proven modulating effects on the digestive process by phytochemicals from Polygonaceae may positively impact animal health and resilience, the environment and climate, as well as the health of human consumers of the animal products. This perspective on promising yet under-utilized plants as feed and preventive treatment implies that a One Health approach can be developed and applied also at the level of agricultural animal systems.
Ethical statement
Due to the fact that the submitted manuscript is a review article, an ethical statement seems to be not applicable for the authors.
Additional files
Additional file 1: Protocol of systematic review
Additional file 2:In vivo experiments
Additional file 3:In vitro experiments
CRediT authorship contribution statement
Zafide Türk: Writing – original draft, Visualization, Investigation, Formal analysis, Data curation. Florian Leiber: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Theresa Schlittenlacher: Writing – review & editing, Visualization, Resources, Project administration. Matthias Hamburger: Writing – review & editing, Supervision, Methodology, Conceptualization. Michael Walkenhorst: Writing – review & editing, Writing – original draft, Supervision, Project administration, Data curation, Conceptualization.
Declaration of competing interest
The authors declare to have no conflict of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors. Many thanks from the authors go to Bernadette Waldmeier for her technical support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2024.100416.
Appendix. Supplementary materials
References
- Akgunlu S., Sekeroglu N., Koca-Caliskan U., Ozkutlu F., Ozcelik B., Kulak M., Gezici S. Research on selected wild edible vegetables: Mineral content and antimicrobial potentials. Annals of Phytomedicine. 2016;5(2):50–57. doi: 10.21276/ap.2016.5.2.6. [DOI] [Google Scholar]
- Ali M.Z., Janbaz K.H., Mehmood M.H., Gilani A.H. Antidiarrheal and antispasmodic activities of Polygonum bistorta rhizomes are mediated predominantly through K+ channels activation. Bangladesh Journal of Pharmacology. 2015;10(3):627–634. doi: 10.3329/bjp.v10i3.23714. [DOI] [Google Scholar]
- Alkushi A.G. Protective effect of sorrel extract on adult rats treated by carbon tetrachloride. Pharmacognosy Research. 2017;9(2):200–207. doi: 10.4103/0974-8490.204653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelchanka S., Kreuzer M., Leiber F. Utility of buckwheat (Fagopyrum esculentum Moench) as feed: Effects of forage and grain on in vitro ruminal fermentation and performance of dairy cows. Animal Feed Science and Technology. 2010;155(2-4):111–121. doi: 10.1016/j.anifeedsci.2009.10.007. [DOI] [Google Scholar]
- Ayaz M., Junaid M., Ullah F., Sadiq A., Ovais M., Ahmad W., Zeb A. Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Complementary and Alternative Medicine. 2016;16(1):1–14. doi: 10.1186/s12906-016-1491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrle H., Mevissen M., Kaske M., Nathues H., Gruetzner N., Melzig M., Walkenhorst M. Medicinal plants–prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Veterinary Research. 2016;12(1):1–31. doi: 10.1186/s12917-016-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae E.-A., Han M.J., Kim N.-J., Kim D.-H. Anti-helicobacter pylori activity of hearbal medicines. Biological & Pharmaceutical Bulletin. 1998;21(9):990–992. doi: 10.1248/bpb.21.990. [DOI] [PubMed] [Google Scholar]
- Bae J.-Y., Lee Y.S., Han S.Y., Jeong E.J., Lee M.K., Kong J.Y., Lee D.H., Cho K.J., Lee H.-S., Ahn M.-J. A comparison between water and ethanol extracts of rumex acetosa for protective effects on gastric ulcers in mice. Biomolecules & Therapeutics. 2012;20(4):425–430. doi: 10.4062/biomolther.2012.20.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M.R., Gregorini P. How dietary diversity enhances hedonic and eudaimonic well-being in grazing ruminants. Frontiers in Veterinary Science. 2020;7:191. doi: 10.3389/fvets.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begné M.G., Yslas N., Reyes E., Quiroz V., Santana J., Jimenez G. Clinical effect of a Mexican sanguinaria exftract (Polygonum aviculare L.) on gingivitis. Journal of Ethnopharmacology. 2001;74(1):45–51. doi: 10.1016/s0378-8741(00)00338-x. [DOI] [PubMed] [Google Scholar]
- Benković E.T., Žigon D., Friedrich M., Plavec J., Kreft S. Isolation, analysis and structures of phototoxic fagopyrins from buckwheat. Food Chemistry. 2014;143:432–439. doi: 10.1016/j.foodchem.2013.07.118. [DOI] [PubMed] [Google Scholar]
- Benvenuti M.N., Giuliotti L., Pasqua C., Gatta D., Bagliacca M. Buckwheat bran (Fagopyrum esculentum) as partial replacement of corn and soybean meal in the laying hen diet. Italian Journal of Animal Science. 2012;11(1):e2. doi: 10.4081/ijas.2012.e2. [DOI] [Google Scholar]
- Berrens L., Maranon F. IgE-binding trypsin inhibitors in plant pollen extracts. Experientia. 1995;51:953–955. doi: 10.1007/BF01921746. [DOI] [PubMed] [Google Scholar]
- Bijlani R., Sud S., Sahi A., Gandhi B., Tandon B. Effect of sieved buckwheat (Fagopyrum esculentum) flour supplementation on lipid profile and glucose tolerance. Indian Journal of Physiology and Pharmacology. 1985;29(2):69–74. [PubMed] [Google Scholar]
- Bineshian F., Bakhshandeh N., Nazari H. Anti-Candida and antioxidant activities of hydroalcohlic extract of Rumex obtusifolius leaves. Pakistan Journal of Pharmaceutical Sciences. 2019;32(3) [PubMed] [Google Scholar]
- Bischoff T., Vogl C.R., Ivemeyer S., Klarer F., Meier B., Hamburger M., Walkenhorst M. Plant and natural product based homemade remedies manufactured and used by farmers of six central Swiss cantons to treat livestock. Livestock Science. 2016;189:110–125. doi: 10.1016/j.livsci.2016.05.003. [DOI] [Google Scholar]
- Blaszczyk T., Krzyzanowska J., Lamer-Zarawska E. Screening for antimycotic properties of 56 traditional Chinese drugs. Phytotherapy Research. 2000;14(3):210–212. doi: 10.1002/(sici)1099-1573(200005)14:3%3C210::aid-ptr591%3E3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bonet M.A., Vallès J. Ethnobotany of montseny biosphere reserve (Catalonia, Iberian peninsula): plants used in veterinary medicine. Journal of Ethnopharmacology. 2007;110:130–147. doi: 10.1016/j.jep.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Buccioni A., Decandia M., Minieri S., Molle G., Cabiddu A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Animal Feed Science and Technology. 2012;174:1–25. doi: 10.1016/j.anifeedsci.2012.02.009. [DOI] [Google Scholar]
- Ceylan S., Cetin S., Camadan Y., Saral O., Ozsen O., Tutus A. Antibacterial and antioxidant activities of traditional medicinal plants from the Erzurum region of Turkey. Irish Journal of Medical Science (1971-) 2019;188(4):1303–1309. doi: 10.1007/s11845-019-01993-x. [DOI] [PubMed] [Google Scholar]
- Chen H., Ruan J. Molecular breeding and nutritional aspects of buckwheat. Elsevier Academic Press; 2016. Protease inhibitors in buckwheat; pp. 355–363. [DOI] [Google Scholar]
- Cherian S., Hacisayidli K.M., Kurian R., Mathews A. Therapeutically important bioactive compounds of the genus Polygonum L. and their possible interventions in clinical medicine. Journal of Pharmacy and Pharmacology. 2023;75:301–327. doi: 10.1093/jpp/rgac105. [DOI] [PubMed] [Google Scholar]
- Choi I., Seog H., Park Y., Kim Y., Choi H. Suppressive effects of germinated buckwheat on development of fatty liver in mice fed with high-fat diet. Phytomedicine. 2007;14(7-8):563–567. doi: 10.1016/j.phymed.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Choi S.Y., Choi J.Y., Lee J.M., Lee S., Cho E.J. Tartary buckwheat on nitric oxide-induced inflammation in RAW264. 7 macrophage cells. Food & Function. 2015;6(8):2664–2670. doi: 10.1039/C5FO00639B. [DOI] [PubMed] [Google Scholar]
- Choi A.S., Bae I.Y., Lee H.G. Predicting buckwheat flavonoids bioavailability in different food matrices under in vitro simulated human digestion. Cereal Chemistry. 2017;94(2):310–314. doi: 10.1094/CCHEM-03-16-0054-R. [DOI] [Google Scholar]
- Chowdhury R., Koh K. Growth performance, bone quality, and phosphorus availability in broilers given phosphorus-deficient diets containing buckwheat (Fagopyrum esculentum) Journal of Poultry Science. 2018;55(4):249–256. doi: 10.2141/jpsa.0170178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Koh K. Phytase activity in the digesta from different parts of the digestive tract and ileal digestibility of nutrients in broilers fed with buckwheat diets. Journal of Poultry Science. 2018;55(4):274–279. doi: 10.2141/jpsa.0170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R., Koh K. Phosphorus availability in laying hens given non-phytate phosphorus deficient diets containing buckwheat. Journal of Poultry Science. 2019;56(1):58–64. doi: 10.2141/jpsa.0170178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christa K., Soral-Smietana M. Buckwheat grains and buckwheat products - nutritional and prophylactic value of their components – a review. Czech Journal of Food Science. 2008;26:153–162. doi: 10.17221/1602-CJFS. [DOI] [Google Scholar]
- Coman M.M., Verdenelli M.C., Cecchini C., Silvi S., Vasile A., Bahrim G.E., Orpianesi C., Cresci A. Effect of buckwheat flour and oat bran on growth and cell viability of the probiotic strains Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and their combination SYNBIO®, in synbiotic fermented milk. International Journal of Food Microbiology. 2013;167(2):261–268. doi: 10.1016/j.ijfoodmicro.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Coruh I., Gormez A., Ercisli S., Sengul M. Total phenolic content, antioxidant, and antibacterial activity of Rumex crispus grown wild in Turkey. Pharmaceutical Biology. 2008;46(9):634–638. doi: 10.1080/13880200802182240. [DOI] [Google Scholar]
- Cui Y., Zhao Z., Liu Z., Liu J., Piao C., Liu D. Purification and identification of buckwheat hull flavonoids and its comparative evaluation on antioxidant and cytoprotective activity in vitro. Food Science and Nutrition. 2020;8(7):3882–3892. doi: 10.1002/fsn3.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delafond, O. (1853). Allgemeines Lehrbuch der Phytotherapie.
- Derita M., Zacchino S. Validation of the ethnopharmacological use of polygonum persicaria for its antifungal properties. Natural Product Communications. 2011;6(7) doi: 10.1177/1934578X1100600702. [DOI] [PubMed] [Google Scholar]
- Derrick R., Moseley G., Wilman D. Intake, by sheep, and digestibility of chickweed, dandelion, dock, ribwort and spurrey, compared with perennial ryegrass. Journal of Agricultural Science. 1993;120(1):51–61. doi: 10.1017/S0021859600073585. [DOI] [Google Scholar]
- Disler M., Ivemeyer S., Hamburger M., Vogl C.R., Tesic A., Klarer F., Meier B., Walkenhorst M. Ethnoveterinary herbal remedies used by farmers in four north-eastern Swiss cantons (St. Gallen, Thurgau, Appenzell Innerrhoden and Appenzell Ausserrhoden) Journal of Ethnobiology and Ethnomedicine. 2014;10(1):1–23. doi: 10.1186/1746-4269-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Wang Q., Chen J., Liu L., Zhang S. Apparent total tract digestibility of nutrients and the digestible and metabolizable energy values of five unconventional feedstuffs fed to growing pigs. Journal of Applied Animal Research. 2019;47(1):273–279. doi: 10.1080/09712119.2019.1625778. [DOI] [Google Scholar]
- Ðurendić-Brenesel M., Popović T., Pilija V., Arsić A., Milić M., Kojić D., Jojić N., Milić N. Hypolipidemic and antioxidant effects of buckwheat leaf and flower mixture in hyperlipidemic rats. Bosnian Journal of Basic Medical Sciences. 2013;13(2):100–108. doi: 10.17305/bjbms.2013.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrer M., Mevissen M., Holinger M., Hamburger M., Graf-Schiller S., Mayer P., Potterat O., Bruckmaier R., Walkenhorst M. Effects of a multicomponent herbal extract on the course of subclinical ketosis in dairy cows–a blinded placebo-controlled field-study. Planta Medica. 2020;86(18):1375–1388. doi: 10.1055/a-1260-3148. [DOI] [PubMed] [Google Scholar]
- Fang R., Li T., Yin F., Yin Y., Kong X., Wang K., Yuan Z., Wu G., He J., Deng Z. The additivity of true or apparent phosphorus digestibility values in some feed ingredients for growing pigs. Asian-Australasian Journal of Animal Science. 2007;20(7):1092–1099. doi: 10.5713/ajas.2007.1092. [DOI] [Google Scholar]
- Farrell D.J. A nutritional evaluation of buckwheat (Fagopyrum esculentum) Animal Feed Science and Technology. 1978;3:95–108. doi: 10.1016/0377-8401(78)90038-x. [DOI] [Google Scholar]
- Flis M., Sobotka W., Antoszkiewicz Z., Lipiński K., Zduńczyk Z. The effect of grain polyphenols and the addition of vitamin E to diets enriched with α-linolenic acid on the antioxidant status of pigs. Journal of Animal and Feed Sciences. 2010;19(4):539–553. doi: 10.22358/jafs/66319/2010. [DOI] [Google Scholar]
- Flora . Info Flora; 2023. Das nationale daten- und informationszentrum der schweizer flora.https://www.infoflora.ch/de/ Retrieved from. Accessed May 10, 2023. [Google Scholar]
- Fotschki B., Juśkiewicz J., Jurgoński A., Amarowicz R., Opyd P., Bez J., Muranyi I., Lykke Petersen I., Laparra Llopis M. Protein-rich flours from quinoa and buckwheat favourably affect the growth parameters, intestinal microbial activity and plasma lipid profile of rats. Nutrients. 2020;12(9):2781. doi: 10.3390/nu12092781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhner, E. (1900). Lehrbuch der allgemeinen Therapie für Thierärzte.
- García-González R., López S., Fernández M., Bodas R., González J.S. Screening the activity of plants and spices for decreasing ruminal methane production in vitro. Animal Feed Science and Technology. 2008;147(1-3):36–52. doi: 10.1016/j.anifeedsci.2007.09.008. [DOI] [Google Scholar]
- García-González R., López S., Fernández M., González J.S. Dose–response effects of Rheum officinale root and Frangula alnus bark on ruminal methane production in vitro. Animal Feed Science and Technology. 2008;145(1-4):319–334. doi: 10.1016/j.anifeedsci.2007.05.040. [DOI] [Google Scholar]
- George A., Chinnappan S., Chintamaneni M., Kotak V., Choudhary Y., Kueper T., Radhakrishnan A.K. Anti-inflammatory effects of Polygonum minus (Huds) extract (Lineminus™) in in-vitro enzyme assays and carrageenan induced paw edema. BMC Complementary and Alternative Medicine. 2014;14(1):1–7. doi: 10.1186/1472-6882-14-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginovyan M., Trchounian A. Novel approach to combat antibiotic resistance: Evaluation of some Armenian herb crude extracts for their antibiotic modulatory and antiviral properties. Journal of Applied Microbiology. 2019;127(2):472–480. doi: 10.1111/jam.14335. [DOI] [PubMed] [Google Scholar]
- Ginovyan M., Ayvazyan A., Nikoyan A., Tumanyan L., Trchounian A. Phytochemical screening and detection of antibacterial components from crude extracts of some Armenian herbs using TLC-bioautographic technique. Current Microbiology. 2020;77(7):1223–1232. doi: 10.1007/s00284-020-01929-0. [DOI] [PubMed] [Google Scholar]
- González J.A., García-Barriuso M., Gordaliza M., Amich F. Traditional plant-based remedies to control insect vectors of disease in the Arribes del Duero (western Spain): an ethnobotanical study. Journal of Ethnopharmacolology. 2011;138:595–601. doi: 10.1016/j.jep.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Gorlier A., Lonati M., Renna M., Lussiana C., Lombardi G., Battaglini L.M. Changes in pasture and cow milk compositions during a summer transhumance in the western Italian Alps. Journal of Applied Botany and Food Quality. 2012;85:216–223. doi: 10.5073/jabfq.2325.85.2.216. [DOI] [Google Scholar]
- Goto W., Kusumoto I.T., Kadota S., Namba T., Kurokawa M., Shiraki K. Suppression of hepatitis B virus surface antigen secretion by traditional plant medicines. Phytotherapy Research. 1996;10(6):504–507. doi: 10.1002/(sici)1099-1573(199609)10:6%3C504::aid-ptr894%3E3.3.co;2-v. [DOI] [Google Scholar]
- Gou J., Zou Y., Ahn J. Enhancement of antioxidant and antimicrobial activities of Dianthus superbus, Polygonum aviculare, Sophora flavescens, and Lygodium japonicum by pressure-assisted water extraction. Food Science and Biotechnology. 2011;20(1):283–287. doi: 10.1007/s10068-011-0040-7. [DOI] [Google Scholar]
- Gou K.-J., Zeng R., Dong Y., Hu Q.-Q., Hu H.-W.-Y., Maffucci K.G., Dou Q.-L., Yang Q.-B., Qin X.-H., Qu Y. Anti-inflammatory and analgesic effects of Polygonum orientale L. extracts. Frontiers in Pharmacology. 2017;8:562. doi: 10.3389/fphar.2017.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürbüz I., Özkan A.M., Yesilada E., Kutsal O. Anti-ulcerogenic activity of some plants used in folk medicine of Pinarbasi (Kayseri, Turkey) Journal of Ethnopharmacology. 2005;101(1):313–318. doi: 10.1016/j.jep.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Gāliņa D., Valdovska A. Effect of probiotics and herbals on health and shedding of resistant Escherichia coli in piglets. Research for Rural Development. 2017;1 doi: 10.22616/RRD.23.2017.037. [DOI] [Google Scholar]
- Gāliņa D., Ansonska L., Valdovska A. Effect of probiotics and herbal products on intestinal histomorphological and immunological development in piglets. Veterinary Medicine International. 2020;2020 doi: 10.1155/2020/3461768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Han F., Wang Y., Fan L., Song G., Chen X., Jiang P., Miao H., Han Y. Digestible indispensable amino acid scores of nine cooked cereal grains. British Journal of Nutrition. 2019;121(1):30–41. doi: 10.1017/s0007114518003033. [DOI] [PubMed] [Google Scholar]
- Harrold R., Craig D., Nalewaja J., North B. Nutritive value of green or yellow foxtail, wild oats, wild buckwheat or redroot pigweed seed as determined with the rat. Journal of Animal Science. 1980;51(1):127–131. doi: 10.2527/jas1980.511127x. [DOI] [PubMed] [Google Scholar]
- Hassan F.A.M., Roushdy E.M., Kishawy A.T.Y., Zaglool A.W., Tukur H.A., Saadeldin I.M. Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of Rutin. Animals. 2019;9:7. doi: 10.3390/ani9010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejcman M., Strnad L., Hejcmanova P., Pavlu V. Biological control of Rumex obtusifolius and Rumex crispus by goat grazing. Weed Biology and Management. 2014;14:115–120. doi: 10.1111/wbm.12038. [DOI] [Google Scholar]
- Hosaka T., Sasaga S., Yamasaka Y., Nii Y., Edazawa K., Tsutsumi R., Shuto E., Okahisa N., Iwata S., Tomotake H. Treatment with buckwheat bran extract prevents the elevation of serum triglyceride levels and fatty liver in KK-Ay mice. Journal of Medical Investigation. 2014;61(3.4):345–352. doi: 10.2152/jmi.61.345. [DOI] [PubMed] [Google Scholar]
- Hristov A.N., Melgar A., Wasson D., Arndt C. Symposium review: Effective nutritional strategies to mitigate enteric methane in dairy cattle. Journal of Dairy Science. 2022;10:8543–8557. doi: 10.3168/jds.2021-21398. [DOI] [PubMed] [Google Scholar]
- Huang Z.-R., Deng J.-C., Li Q.-Y., Cao Y.-J., Lin Y.-C., Bai W.-D., Liu B., Rao P.-F., Ni L., Lv X.-C. Protective mechanism of common buckwheat (fagopyrum esculentum moench.) against nonalcoholic fatty liver disease associated with dyslipidemia in mice fed a high-fat and high-cholesterol diet. Journal of Agricultural and Food Chemistry. 2020;68(24):6530–6543. doi: 10.1021/acs.jafc.9b08211. [DOI] [PubMed] [Google Scholar]
- Huda M.N., Lu S., Jahan T., Ding M.Q., Jha R., Zhang K.X., Zhang W., Georgiev M.I., Park S.U., Zhou M.L. Treasure from garden: Bioactive compounds of buckwheat. Food Chemistry. 2021;335 doi: 10.1016/j.foodchem.2020.127653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur S.-J., Park S.-J., Jeong C.-H. Effect of buckwheat extract on the antioxidant activity of lipid in mouse brain and its structural change during in vitro human digestion. Journal of Agricultural and Food Chemistry. 2011;59(19):10699–10704. doi: 10.1021/jf202279r. [DOI] [PubMed] [Google Scholar]
- Hussain F., Ahmad B., Hameed I., Dastagir G., Sanaullah P., Azam S. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of Polygonaceae. African Journal of Biotechnology. 2010;9(31):5032–5036. [Google Scholar]
- Hussain M., Raza S.M., Janbaz K.H. Pharmacologically mechanistic basis for the traditional uses of Rumex acetosa in gut motility disorders and emesis. Bangladesh Journal of Pharmacology. 2015;10(3):548–554. doi: 10.3329/bjp.v10i3.23406. [DOI] [Google Scholar]
- Idris O.A., Wintola O.A., Afolayan A.J. Evaluation of the bioactivities of Rumex crispus L. leaves and root extracts using toxicity, antimicrobial, and antiparasitic assays. Evidence-Based Complementary and Alternative Medicine. 2019;2019 doi: 10.1155/2019/6825297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihme N., Kiesewetter H., Jung F.a., Hoffmann K., Birk A., Müller A., Grützner K. Leg oedema protection from a buckwheat herb tea in patients with chronic venous insufficiency: A single-centre, randomised, double-blind, placebo-controlled clinical trial. European Journal of Clinical Pharmacology. 1996;50(6):443–447. doi: 10.1007/s002280050138. [DOI] [PubMed] [Google Scholar]
- Imaki M., Miyoshi T., Fujii M., Sei M., Tada T., Nakamura T., Tanada S. Study on digestibility and energy availability of daily food intake in Japanese (Part 3. Cereals) Nippon Eiseigaku Zasshi. 1990;45(2):635–641. doi: 10.1265/jjh.45.635. [DOI] [PubMed] [Google Scholar]
- Islam M.S., Siddiqui M.N., Sayed M.A., Tahjib-Ul-Arif M., Islam M.A., Hossain M.A. Dietary effects of buckwheat (Fagopyrum esculentum) and black cumin (Nigella sativa) seed on growth performance, serum lipid profile and intestinal microflora of broiler chicks. South African Journal of Animal Science. 2016;46:103–111. doi: 10.4314/sajas.v46i1.13. [DOI] [Google Scholar]
- Jacob J., Carter C. Inclusion of buckwheat in organic broiler diets. Journal of Applied Poultry Research. 2008;17(4):522–528. doi: 10.3382/japr.2008-00004. [DOI] [Google Scholar]
- Jacob J., Noll S., Brannon J. Comparison of metabolic energy content of organic cereal grains for chickens and turkeys. Journal of Applied Poultry Research. 2008;17(4):540–544. doi: 10.3382/japr.2008-00026. [DOI] [Google Scholar]
- Jamal P., Karim I.A., Abdullah E., Raus R.A., Hashim Y.Z. Phytochemical screening for antibacterial activity of potential Malaysian medicinal plants. African Journal of Biotechnology. 2011;10(81):18795–18799. doi: 10.5897/ajb11.2755. [DOI] [Google Scholar]
- Jari S., Popovi Z., Maukanovi -Joci M., Djurdjevi L., Mijatovi M., Karadži B., Mitrovi M., Pavlovi P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia) Journla of Ethnopharmacology. 2007;111:160–175. doi: 10.1016/j.jep.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Jayanegara A., Marquardt S., Kreuzer M., Leiber F. Nutrient and energy content, in vitro ruminal fermentation characteristics and methanogenic potential of alpine forage plant species during early summer. Journal of the Science of Food and Agriculture. 2011;91(10):1863–1870. doi: 10.1002/jsfa.4398. [DOI] [PubMed] [Google Scholar]
- Jeppesen A.S., Soelberg J., Jäger A.K. Antibacterial and COX-1 inhibitory effect of medicinal plants from the Pamir mountains. Afghanistan Plants. 2012;1(2):74–81. doi: 10.3390/plants1020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Deng Y., Yang A., Lu Z., Chen Y., Liu X., Han L., Zou C. Rhubarb enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification. Frontiers in Pharmacology. 2020;11:1092. doi: 10.3389/fphar.2020.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H.-R., Lee S., Choi S.-J. Pharmacokinetics and protective effects of Tartary buckwheat flour extracts against ethanol-induced liver injury in rats. Antioxidants. 2020;9(10):913. doi: 10.3390/antiox9100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johann S., Silva D.L., Martins C.V., Zani C.L., Pizzolatti M.G., Resende M.A. Inhibitory effect of extracts from Brazilian medicinal plants on the adhesion of Candida albicans to buccal epithelial cells. World Journal of Microbiology and Biotechnology. 2008;24(11):2459–2464. doi: 10.1007/s11274-008-9768-5. [DOI] [Google Scholar]
- Johann S., Cisalpino P.S., Watanabe G.A., Cota B.B., de Siqueira E.P., Pizzolatti M.G., Zani C.L., de Resende M.A. Antifungal activity of extracts of some plants used in Brazilian traditional medicine against the pathogenic fungus Paracoccidioides brasiliensis. Pharmaceutical Biology. 2010;48(4):388–396. doi: 10.3109/13880200903150385. [DOI] [PubMed] [Google Scholar]
- Kapp-Bitter A.N., Berard J., Amelchanka S.L., Baki C., Kunz C., Steiner A.K., Kreuzer M., Leiber F. Effects of dietary Sanguisorba minor, Plantago lanceolata, and Lotus corniculatus on urinary N excretion of dairy cows. Animal Production Science. 2023;63:1494–1504. doi: 10.1071/AN22300. [DOI] [Google Scholar]
- Kapp-Bitter A.N., Berard J., Amelchanka S.L., Baki C., Kunz C., Steiner A.K.…Leiber F. Effects of dietary Sanguisorba minor, Plantago lanceolata, and Lotus corniculatus on urinary N excretion of dairy cows. Animal Production Science. 2023;63:1494–1504. doi: 10.1071/AN22300. [DOI] [Google Scholar]
- Kayashita J., Shimaoka I., Nakajyoh M. Hypocholesterolemic effect of buckwheat protein extract in rats fed cholesterol enriched diets. Nutrition Research. 1995;15(5):691–698. doi: 10.1016/0271-5317(95)00036-i. [DOI] [Google Scholar]
- Kayashita J., Shimaoka I., Nakajoh M., Kato N. Feeding of buckwheat protein extract reduces hepatic triglyceride concentration, adipose tissue weight, and hepatic lipogenesis in rats. Journal of Nutritional Biochemistry. 1996;7(10):555–559. doi: 10.1016/s0955-2863(96)00110-6. [DOI] [Google Scholar]
- Kayashita J., Shimaoka I., Nakajoh M., Kondoh M., Hayashi K., Kato N. Muscle hypertrophy in rats fed on a buckwheat protein extract. Bioscience Biotechnology and Biochemistry, 1999;63(7):1242–1245. doi: 10.1271/BBB.63.1242. [DOI] [PubMed] [Google Scholar]
- Kazmin V.D., Abaturov B.D., Demina O.N., Kolesnikov M.P. Fodder resources and feeding of semifree bison (bison bison) on a steppe pasture in the Western Manych valley. Biology Bulletin. 2016;43:926–935. doi: 10.1134/S1062359016080112. [DOI] [Google Scholar]
- Keles G., Kocaman V., Ustundag A.O., Zungur A., Ozdogan M. Growth rate, carcass characteristics and meat quality of growing lambs fed buckwheat or maize silage. Asian-Australasian Journal of Animal Science. 2018;31(4):522–528. doi: 10.5713/ajas.17.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleş O., Bakirel T., Şener S., Aydın H., Alpınar K. Antiinflammatory and antipyretic activity of polygonum lapathifolium in rats. Turkish Journal of Veterinary & Animal Sciences. 2001;25(4):623–628. [Google Scholar]
- Khan M., Younas M., Khan I., Abbas R., Ali M. Comparative efficacy of some herbal and homeopathic preparations against coccidiosis in broilers. International Journal of Agriculture and Biology. 2008;10(3):358–360. [Google Scholar]
- Khushtar M., Ahmad A., Rahman M.A. Gastroprotective effect of hydro-alcoholic extract of polygonum bistorta lin root in indomethacin-induced gastric ulcers in sprague dawley rats. Indian Journal of Pharmaceutical Education and Research. 2018;52(4):618–625. doi: 10.5530/ijper.52.4.72. [DOI] [Google Scholar]
- Kim J.Y., Son B.K., Lee S.S. Effects of adlay, buckwheat, and barley on transit time and the antioxidative system in obesity induced rats. Nutrition Research and Practice, 2012;6(3):208–212. doi: 10.4162/nrp.2012.6.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Shin H., Lee S. Cardioprotective effects of diet with different grains on lipid profiles and antioxidative system in obesity-induced rats. International Journal for Vitamin and Nutrition Research. 2012;82(2):85–93. doi: 10.1024/0300-9831/a000097. [DOI] [PubMed] [Google Scholar]
- Kim H.K., Arokiyraj S., Lee J., Oh Y.K., Chung H.Y., Jin G.D., Kim E.B., Kim E.K., Lee Y., Baik M. Effect of rhubarb (Rheum spp.) root on in vitro and in vivo ruminal methane production and a bacterial community analysis based on 16S rRNA sequence. Animal Production Science. 2016;56:402–408. doi: 10.1071/AN15585. [DOI] [Google Scholar]
- Kim S.-Y., Lee M.-S., Chang E., Jung S., Ko H., Lee E., Lee S., Kim C.-T., Kim I.-H., Kim Y. Tartary buckwheat extract attenuated the obesity-induced inflammation and increased muscle PGC-1a/SIRT1 expression in high fat diet-induced obese rats. Nutrients. 2019;11(3):654. doi: 10.3390/nu11030654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosikowska U., Smolarz H., Malm A. Antimicrobial activity and total content of polyphenols of Rheum L. species growing in Poland. Open Live Sciences. 2010;5(6):814–820. doi: 10.2478/s11535-010-0067-4. [DOI] [Google Scholar]
- Kozan E., Küpeli E., Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. Journal of Ethnopharmacology. 2006;108(2):211–216. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Krahulec F., Skálová H., Herben T., Hadincová V., Wildová R., Pecháčková S. Vegetation changes following sheep grazing in abandoned mountain meadows. Applied Vegetation Science. 2001;4(1):97–102. doi: 10.1111/j.1654-109X.2001.tb00239.x. [DOI] [Google Scholar]
- Kreft I., Wieslander G., Vombergar B. Molecular breeding and nutritional aspects of buckwheat. Elsevier Academic Press; 2016. Bioactive flavonoids in buckwheat grain and green parts; pp. 161–167. [DOI] [Google Scholar]
- Kälber T., Meier J.S., Kreuzer M., Leiber F. Flowering catch crops used as forage plants for dairy cows: Influence on fatty acids and tocopherols in milk. Journal of Dairy Science. 2011;94(3):1477–1489. doi: 10.3168/jds.2010-3708. [DOI] [PubMed] [Google Scholar]
- Kälber T., Kreuzer M., Leiber F. Silages containing buckwheat and chicory: quality, digestibility and nitrogen utilisation by lactating cows. Archives of Animal Nutrition. 2012;66(1):50–65. doi: 10.1080/1745039x.2011.630213. [DOI] [PubMed] [Google Scholar]
- Kälber T., Kreuzer M., Leiber F. Effect of feeding buckwheat and chicory silages on fatty acid profile and cheese-making properties of milk from dairy cows. Journal of Dairy Research. 2013;80(1):81–88. doi: 10.1017/s0022029912000647. [DOI] [PubMed] [Google Scholar]
- Kälber T., Kreuzer M., Leiber F. Milk fatty acid composition of dairy cows fed green whole-plant buckwheat, phacelia or chicory in their vegetative and reproductive stage. Animal Feed Science and Technology. 2014;193:71–83. doi: 10.1016/j.anifeedsci.2014.04.007. [DOI] [Google Scholar]
- Ladeji O., Okoye Z.S.C., Waidu Z. Effect of supplementation of laboratory chow with leaf of Rumex acetosa (sorrel) on body weight and serum levels of amino acids and minerals in rat. Food Chemistry. 1997;59(1):15–17. doi: 10.1016/0308-8146(95)00233-2. [DOI] [Google Scholar]
- Lajter I., Zupkó I., Molnár J., Jakab G., Balogh L., Vasas A., Hohmann J. Antiproliferative activity of polygonaceae species from the carpathian basin against human cancer cell lines. Phytotherapy Research. 2013;27(1):77–85. doi: 10.1002/ptr.4690. [DOI] [PubMed] [Google Scholar]
- Lee T., Vairappan C. Antioxidant, antibacterial and cytotoxic activities of essential oils and ethanol extracts of selected South East Asian herbs. Journal of Medicinal Plants Research. 2011;5 [Google Scholar]
- Lee J.-S., Bok S.-H., Jeon S.-M., Kim H.-J., Do K.-M., Park Y.-B., Choi M.-S. Antihyperlipidemic effects of buckwheat leaf and flower in rats fed a high-fat diet. Food Chemistry. 2010;119(1):235–240. doi: 10.1016/j.foodchem.2009.06.014. [DOI] [Google Scholar]
- Lee C.-C., Shen S.-R., Lai Y.-J., Wu S.-C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food & Function. 2013;4(5):794–802. doi: 10.1039/c3fo30389f. [DOI] [PubMed] [Google Scholar]
- Lee Y.-J., Kim K.-J., Park K.-J., Yoon B.-R., Lim J.-H., Lee O.-H. Buckwheat (Fagopyrum esculentum M.) sprout treated with methyl jasmonate (MeJA) improved anti-adipogenic activity associated with the oxidative stress system in 3T3-L1 adipocytes. International Journal of Molecular Sciences. 2013;14(1):1428–1442. doi: 10.3390/ijms14011428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-J., Lee S.Y., Kim Y.-C., Choi I., Hur S.J. Influence of buckwheat extract on various dietary lipid-induced oxidative status of the mice brain. Current Topics in Nutraceutical Research, R. 2014;12:155. + [Google Scholar]
- Leiber F., Messikommer R. Proceedings of the society of nutrition physiology. Vol. 20. 2011. Buckwheat grain as a feed component in layer diets: effects on egg quality; p. 97. [Google Scholar]
- Leiber F., Kreuzer M., Nigg D., Wettstein H.R., Scheeder M.R.L. A study on the causes for the elevated n-3 fatty acids in cows’ milk of alpine origin. Lipids. 2005;40:191–202. doi: 10.1007/s11745-005-1375-3. [DOI] [PubMed] [Google Scholar]
- Leiber F., Messikommer R., Wenk C. Buckwheat: A feed for broiler chicken? Agrarforschung. 2009;16:448–453. [Google Scholar]
- Leiber F., Kunz C., Kreuzer M. Influence of different morphological parts of buckwheat (Fagopyrum esculentum) and its major secondary metabolite Rutin on rumen fermentation in vitro. Czech Journal of Animal Science. 2012;57:10–18. doi: 10.17221/5479-CJAS. [DOI] [Google Scholar]
- Leiber F., Willems H., Werne S., Ammer S., Kreuzer M. Effects of vegetation type and breed on n-3 and n-6 fatty acid proportions in heart, lung and brain phospholipids of lambs. Small Ruminant Research. 2019;171:99–107. doi: 10.1016/j.smallrumres.2018.12.003. [DOI] [Google Scholar]
- Leiber F., Arnold N., Heckendorn F., Werne S. Assessing effects of tannin-rich sainfoin supplements for grazing dairy goats on feed protein efficiency. Journal of Dairy Research. 2020;87:397–399. doi: 10.1017/S0022029920000965. [DOI] [PubMed] [Google Scholar]
- Leiber F., Walkenhorst M., Holinger M. The relevance of feed diversity and choice in nutrition of ruminant livestock. Landbauforschung. 2020;70:35–38. doi: 10.3220/LBF1592393539000. [DOI] [Google Scholar]
- Leiber F. Molecular breeding and nutritional aspects of buckwheat. Elsevier Academic Press; 2016. Buckwheat in the nutrition of livestock and poultry; pp. 229–238. [DOI] [Google Scholar]
- Lepš J., Michálek J., Kulíšek P., Uhlík P. Use of paired plots and multivariate analysis for the determination of goat grazing preference. Journal of Vegetation Science. 1995;6(1):37–42. doi: 10.2307/3236254. [DOI] [Google Scholar]
- Li H., Jiang B., Zhou Y. Effects of rumen-protected methionine supplementation on growth performance, nitrogen balance, carcass characteristics, and meat quality of lambs fed diets containing buckwheat straw. Canadian Journal of Animal Science. 2020;100(2):337–345. doi: 10.1139/cjas-2018-0180. [DOI] [Google Scholar]
- Li Y., Li W., Wang X., Ding C., Liu J., Li Y., Li W., Sun Y. High-salt diet-induced gastritis in C57BL/6 mice is associated with microbial dysbiosis and alleviated by a buckwheat diet. Molecular Nutrition & Food Research. 2020;64(8) doi: 10.1002/mnfr.201900965. [DOI] [PubMed] [Google Scholar]
- Liao S.-G., Zhang L.-J., Sun F., Zhang J.-J., Chen A.Y., Lan Y.-Y., Li Y.-J., Wang A.-M., He X., Xiong Y., Dong L., Chen X.-J., Li Y.-T., Zuo L., Wang Y.-L. Antibacterial and anti-inflammatory effects of extracts and fractions from Polygonum capitatum. Journal of Ethnopharmacology. 2011;134(3):1006–1009. doi: 10.1016/j.jep.2011.01.050. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lin Y.-L., Wu C.-F., Huang Y.-T. Effects of rhubarb on migration of rat hepatic stellate cells. Journal of Gastroenterology and Hepatology. 2009;24(3):453–461. doi: 10.1111/j.1440-1746.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- Liu C.-L., Chen Y.-S., Yang J.-H., Chiang B.-H. Antioxidant activity of tartary ( Fagopyrum tataricum (L.) Gaertn.) and Common ( Fagopyrum esculentum Moench) buckwheat sprouts. Journal of Agricultural and Food Chemistry. 2008;56(1):173–178. doi: 10.1021/jf072347s. [DOI] [PubMed] [Google Scholar]
- Liu Q., Luyten W., Pellens K., Wang Y., Wang W., Thevissen K., Liang Q., Cammue B.P.A., Schoofs L., Luo G. Antifungal activity in plants from Chinese traditional and folk medicine. Journal of Ethnopharmacology. 2012;143(3):772–778. doi: 10.1016/j.jep.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Liu Y., Nielsen M., Staerk D., Jäger A.K. High-resolution bacterial growth inhibition profiling combined with HPLC–HRMS–SPE–NMR for identification of antibacterial constituents in Chinese plants used to treat snakebites. Journal of Ethnopharmacology. 2014;155(2):1276–1283. doi: 10.1016/j.jep.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Luo X., Xue L., Xu H., Zhao Q.-Y., Wang Q., She Y.-S., Zang D.-A., Shen J., Peng Y.-B., Zhao P., Yu M.-F., Chen W., Ma L.-Q., Chen S., Chen S., Fu X., Hu S., Nie X., Shen C.…Liu Q.-H. Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle. Science Reports-UK. 2018;8(1):3114. doi: 10.1038/s41598-018-20409-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macheboeuf D., Coudert L., Bergeault R., Lalière G., Niderkorn V. Screening of plants from diversified natural grasslands for their potential to combine high digestibility, and low methane and ammonia production. Animal. 2014;8(11):1797–1806. doi: 10.1017/S1751731114001785. [DOI] [PubMed] [Google Scholar]
- Mackeen M.M., Ali A.M., El-Sharkawy S.H., Manap M.Y., Salleh K.M., Lajis N.H., Kawazu K. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (Ulam) International Journal of Pharmacognosy. 1997;35(3):174–178. doi: 10.1076/phbi.35.3.174.13294. [DOI] [Google Scholar]
- Madec F., Bridoux N., Bounaix S., Jestin A. Measurement of digestive disorders in the piglet at weaning and related risk factors. Preventive Veterinary Medicine. 1998;35(1):53–72. doi: 10.1016/s0167-5877(97)00057-3. [DOI] [PubMed] [Google Scholar]
- Manganelli R.E., Camangi F., Tomei P.E. Curing animals with plants: traditional usage in Tuscany (Italy) Journal of Ethnopharmacology. 2001;78:171–191. doi: 10.1016/s0378-8741(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Mayer M., Vogl C.R., Amorena M., Hamburger M., Walkenhorst M. Treatment of organic livestock with medicinal plants: A systematic review of European ethnoveterinary research. Journal of Complementary Medicine Research. 2014;21(6):375–386. doi: 10.1159/000370216. [DOI] [PubMed] [Google Scholar]
- Mayer M., Zbinden M., Vogl C.R., Ivemeyer S., Meier B., Amorena M., Maeschli A., Hamburger M., Walkenhorst M. Swiss ethnoveterinary knowledge on medicinal plants – a within-country comparison of Italian speaking regions with north-western German speaking regions. Journal of Ethnobiology and Ethnomedicine. 2017;13(1):1. doi: 10.1186/s13002-016-0106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertenat D., Cero M.D., Vogl C.R., Ivemeyer S., Meier B., Maeschli A., Hamburger M., Walkenhorst M. Ethnoveterinary knowledge of farmers in bilingual regions of Switzerland – is there potential to extend veterinary options to reduce antimicrobial use? Journal of Ethnopharmacology. 2020;246 doi: 10.1016/j.jep.2019.112184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger B.T., Barnes D.M., Reed J.D. Insoluble fraction of buckwheat (fagopyrum esculentum moench) protein possessing cholesterol-binding properties that reduce micelle cholesterol solubility and uptake by caco-2 cells. Journal of Agricultural and Food Chemistry. 2007;55(15):6032–6038. doi: 10.1021/jf0709496. [DOI] [PubMed] [Google Scholar]
- Milan P., Nejad D.M., Ghanbari A.A., Soleimani Rad J., Tayefi Nasrabadi H., Habibi Roudkenar M., Mohammadi Roushandeh A., Goldust M. Effects of polygonum aviculare herbal extract on sperm parameters after EMF exposure in mouse. Pakistan Journal of Biological Science. 2011;14:720–724. doi: 10.3923/pjbs.2011.720.724. [DOI] [PubMed] [Google Scholar]
- Mišan A., Petelin A., Stubelj M., Mandić A., Šimurina O., Pojić M., Milovanović I., Jakus T., Filipčev B., Jenko Pražnikar Z. Buckwheat – enriched instant porridge improves lipid profile and reduces inflammation in participants with mild to moderate hypercholesterolemia. Journal of Functional Foods. 2017;36:186–194. doi: 10.1016/j.jff.2017.06.056. [DOI] [Google Scholar]
- Mohler V.L., Izzo M.M., House J.K. Salmonella in calves. Veterinary Clinics of North America, 2009;25(1):37–54. doi: 10.1016/j.cvfa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Molan A.L., Attwood G.T., McNabb W.C. The impact of condensed tannins from dock (Rumex obtusifolius) on the growth of rumen proteolytic bacteria in vitro. Journal of Animal and Feed Sciences. 2007;16(Suppl. 2):118–123. doi: 10.22358/jafs/74467/2007. [DOI] [Google Scholar]
- Mu C.T., Ding N., Hao X.Y., Zhao Y.B., Wang P.J., Zhao J.X., Ren Y.S., Zhang C.X., Zhang W.J., Xiang B.W., Zhang J.X. Effects of different proportion of buckwheat straw and corn straw on performance, rumen fermentation and rumen microbiota composition of fattening lambs. Small Ruminant Research. 2019;181:21–28. doi: 10.1016/j.smallrumres.2019.09.006. [DOI] [Google Scholar]
- Mukoda T., Sun B., Ishiguro A. Antioxidant activities of buckwheat hull extract toward various oxidative stress in vitro and in vivo. Biological & Pharmaceutical Bulletin. 2001;24(3):209–213. doi: 10.1248/bpb.24.209. [DOI] [PubMed] [Google Scholar]
- Nan G., Zhao H., Wu Q., Liu L., Guan Z., Li C., Wu H., Xiang D., Wu Q. Comparative study of the effects of Tartary buckwheat seed and sprout consumption on the physiological indices and gut microbiota OFC 57BL/6J mice. Food Science and Human Wellness. 2024;13:791–800. doi: 10.26599/FSHW.2022.9250067. [DOI] [Google Scholar]
- Nanasombat S., Kuncharoen N., Ritcharoon B., Sukcharoen P. Antibacterial activity of Thai medicinal plant extracts against oral and gastrointestinal pathogenic bacteria and prebiotic effect on the growth of lactobacillus acidophilus. Chiang Mai Journal of Science, 2018;45:33–44. [Google Scholar]
- Nasir A., Khan M., Rehman Z., Khalil A.A.K., Farman S., Begum N., Irfan M., Sajjad W., Parveen Z. Evaluation of alpha-amylase inhibitory, antioxidant, and antimicrobial potential and phytochemical contents of polygonum hydropiper L. Plants. 2020;9(7):852. doi: 10.3390/plants9070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot-Hadzik I., Hadzik J., Fleischer M., Choromańska A., Sterczała B., Kubasiewicz-Ross P., Saczko J., Gałczyńska-Rusin M., Gedrange T., Matkowski A. Chemical composition of east asian invasive knotweeds, their cytotoxicity and antimicrobial efficacy against cariogenic pathogens: An in-vitro study. Medical Science Monitor. 2019;25:3279–3287. doi: 10.12659/msm.913855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderkorn V., Macheboeuf D. Identification of bioactive grassland plants for reducing enteric methane production and rumen proteolysis using an in vitro screening assay. Animal Production Science. 2014;54(10):1805–1809. doi: 10.1071/an14168. [DOI] [Google Scholar]
- Omokanye A., Hernandez G., Lardner H.A., Al-Maqtari B., Gill K.S., Lee A. Alternative forage feeds for beef cattle in Northwestern Alberta, Canada: Forage yield and nutritive value of forage brassicas and forbs. Journal of Applied Animal Research. 2021;49:203–210. doi: 10.1080/09712119.2021.1933990. [DOI] [Google Scholar]
- Orbán-Gyapai O., Liktor-Busa E., Kúsz N., Stefkó D., Urbán E., Hohmann J., Vasas A. Antibacterial screening of Rumex species native to the Carpathian Basin and bioactivity-guided isolation of compounds from Rumex aquaticus. Fitoterapia. 2017;118:101–106. doi: 10.1016/j.fitote.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Ozturk S., Ozturk A. Antibacterial activity of aqueous and methanol extracts of rumex alpinus. and rumex caucasicus. Pharmaceutical Biology. 2007;45(2):83–87. doi: 10.1080/13880200601105285. [DOI] [Google Scholar]
- Ožbolt L., Kreft S., Kreft I., Germ M., Stibilj V. Distribution of selenium and phenolics in buckwheat plants grown from seeds soaked in Se solution and under different levels of UV-B radiation. Food Chemistry. 2008;110:691–696. doi: 10.1016/j.foodchem.2008.02.073. [DOI] [Google Scholar]
- Özbay H., Alim A. Antimicrobial activity of some water plants from the Northeastern Anatolian region of Turkey. Molecules. 2009;14(1):321–328. doi: 10.3390/molecules14010321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Zhao X., Kim S.-H., Kang S.-A., Kim Y.-G., Park K.-Y. Anti-inflammatory effects of Beopje curly dock (Rumex crispus L.) in LPS-induced RAW 264.7 cells and its active compounds. Journal of Food Biochemistry. 2020;44(7):e13291. doi: 10.1111/jfbc.13291. [DOI] [PubMed] [Google Scholar]
- Papp N., Birkás-Frendl K., Farkas A., Pieroni A. An ethnobotanical study on home gardens in a Transylvanian Hungarian Csángó village (Romania) Genetic Resources and Crop Evolution. 2012;60:1423–1432. doi: 10.1007/s10722-012-9930-7. [DOI] [Google Scholar]
- Park S.H., Jang S., Son E., Lee S.W., Park S.D., Sung Y.-Y., Kim H.K. Polygonum aviculare L. extract reduces fatigue by inhibiting neuroinflammation in restraint-stressed mice. Phytomedicine. 2018;42:180–189. doi: 10.1016/j.phymed.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Parker, D.S., Lomax, M.A., Seal, C.J., Wilton, J.C. (1995). Metabolic implications of ammonia production in the ruminant. The Proceedings of the Nutrition Society 54, 549–563. doi: 10.1079/pns19950023. [DOI] [PubMed]
- Pawłowska K.A., Hałasa R., Dudek M.K., Majdan M., Jankowska K., Granica S. Antibacterial and anti-inflammatory activity of bistort (Bistorta officinalis) aqueous extract and its major components. Justification of the usage of the medicinal plant material as a traditional topical agent. Journal of Ethnopharmacology. 2020;260 doi: 10.1016/j.jep.2020.113077. [DOI] [PubMed] [Google Scholar]
- Paśko P., Tyszka-Czochara M., Namieśnik J., Jastrzębski Z., Leontowicz H., Drzewiecki J., Martinez-Ayala A.L., Nemirovski A., Barasch D., Gorinstein S. Cytotoxic, antioxidant and binding properties of polyphenols from the selected gluten-free pseudocereals and their by-products: In vitro model. Journal of Cereal Science. 2019;87:325–333. doi: 10.1016/j.jcs.2019.04.009. [DOI] [Google Scholar]
- Peng L., Zhang Q., Zhang Y., Yao Z., Song P., Wei L., Zhao G., Yan Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Science and Nutrition. 2020;8(1):199–213. doi: 10.1002/fsn3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieroni A., Giusti M.E., Münz H., Lenzarini C., Turkovi G., Turkovi A. Ethnobotanical knowledge of the Istro-Romanians of Žejane in Croatia. Fitoterapia. 2003;74:710–719. doi: 10.1016/j.fitote.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Pieroni A., Dibra B., Grishaj G., Grishaj I., Maçai S.G. Traditional phytotherapy of the Albanians of Lepushe, Northern Albanian Alps. Fitoterapia. 2005;76:379–399. doi: 10.1016/j.fitote.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Pirvu L., Sha`at F., Miclea L., Miclea, Savopol T., Neagu G., Denisa I., Udeanu D., Moisescu G. Polygonum bistorta L. Herba et flores. Polyphenols profile, antioxidant properties and cytotoxic effect on murine fibroblast cell line NIH3T3. Farmacia. 2017;65 [Google Scholar]
- Proestos C., Boziaris I., Kapsokefalou M., Komaitis M. Natural antioxidant constituents from selected aromatic plants and their antimicrobial activity against selected pathogenic microorganisms. Food Technology and Biotechnology. 2008;46:151–156. [Google Scholar]
- Provenza F.D., Meuter M., Gregorini P. Our landscapes, our livestock, ourselves: Restoring broken linkages among plants, herbivores, and humans with diets that nourish and satiate. Appetite. 2015;95:500–519. doi: 10.1016/j.appet.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Provenza F.D., Kronberg S.L., Gregorini P. Is grassfed meat and dairy better for human and environmental health? Frontiers in Nutrition. 2019;6(26) doi: 10.3389/fnut.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Préstamo G., Pedrazuela A., Peñas E., Lasunción M.A., Arroyo G. Role of buckwheat diet on rats as prebiotic and healthy food. Nutrition Research. 2003;23(6):803–814. doi: 10.1016/S0271-5317(03)00074-5. [DOI] [Google Scholar]
- PubMed.gov. (2015). "PubMed." Retrieved from https://pubmed.ncbi.nlm.nih.gov/. Accessed January 27, 2021.
- Pulkrabek M., Rhee Y., Gibbs P., Hall C. Flaxseed- and buckwheat-supplemented diets altered enterobacteriaceae diversity and prevalence in the cecum and feces of obese mice. Journal of Dietary Supplements. 2017;14(6):667–678. doi: 10.1080/19390211.2017.1305477. [DOI] [PubMed] [Google Scholar]
- Purcell P.J., Boland T.M., O'Brien M., O'Kiely P. In vitro rumen methane output of forb species sampled in spring and summer. Agricultural Food Science. 2012;21(2):83–90. doi: 10.23986/afsci.4811. [DOI] [Google Scholar]
- Qader S.W., Abdulla M.A., Chua L.S., Najim N., Zain M.M., Hamdan S. Antioxidant, total phenolic content and cytotoxicity evaluation of selected Malaysian plants. Molecules. 2011;16(4):3433–3443. doi: 10.3390/molecules16043433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qader S.W., Abdulla M.A., Chua L.S., Sirat H.M., Hamdan S. Pharmacological mechanisms underlying gastroprotective activities of the fractions obtained from polygonum minus in sprague dawley rats. International Journal of Molecular Sciences. 2012;13(2):1481–1496. doi: 10.3390/ijms13021481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman E., Goni S.A., Rahman M.T., Ahmed M. Antinociceptive activity of polygonum hydropiper. Fitoterapia. 2002;73(7):704–706. doi: 10.1016/s0367-326x(02)00239-3. [DOI] [PubMed] [Google Scholar]
- Rahmani S., Naraki K., Roohbakhsh A., Hayes A.W., Karimi G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Science & Nutrition. 2022;11:39–56. doi: 10.1002/fsn3.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P.G., Leary P.D., Gregoire B.R., Finley J.W., Lindlauf J.E., Johnson L.K. Selenium bioavailability from buckwheat bran in rats fed a modified AIN-93G torula yeast–based diet. Journal of Nutrition. 2005;135(11):2627–2633. doi: 10.1093/jn/135.11.2627. [DOI] [PubMed] [Google Scholar]
- Rehman A., Hesheng H., Rahman M., Jingyi X., Shuang L., Yannan G., Qamar S.H. Evaluation of efficacy of compound Chinese medicinal herbs against mycoplasma synoviae using different lab tests in mouse and chicken. International Journal of Agriculture and Biology. 2019 doi: 10.17957/IJAB/15.1111. [DOI] [Google Scholar]
- Rook A.J., Frain-Bell W. Cattle ringworm. BMJ-British Medical Journal. 1954;2(4898):1198–1200. doi: 10.1136/bmj.2.4901.1420-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouf A.S.S., Islam M.S., Rahman M.T. Evaluation of antidiarrhoeal activity Rumex maritimus root. Journal of Ethnopharmacology. 2003;84(2):307–310. doi: 10.1016/S0378-8741(02)00326-4. [DOI] [PubMed] [Google Scholar]
- Röll, M.F. (1866). Lehrbuch der Arzneimittellehre für Tierärzte.
- Sadauskiene I., Liekis A., Bernotiene R., Sulinskiene J., Kasauskas A., Zekonis G. The effects of buckwheat leaf and flower extracts on antioxidant status in mouse organs. Oxidative Medicine and Cellular Longevity. 2018;2018 doi: 10.1155/2018/6712407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama H.M.H., Marraiki N. Antimicrobial activity and phytochemical analyses of Polygonum aviculare L. (Polygonaceae), naturally growing in Egypt. Saudi Journal of Biological Sciences. 2010;17(1):57–63. doi: 10.1016/j.sjbs.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M., Mushtaq M., Akhtar M.F., Saleem A., Zahid S., Akhtar B., Dar E., Ullah M., Badshah M. Validation of Hepatoprotective use of Polygonum Perfoliatum extract against Paracetamol induced toxicity in wistar rats. Acta Poloniae Pharmaceutica. 2018;76 doi: 10.32383/appdr/95224. [DOI] [Google Scholar]
- Saremi J., Kargar Jahromi H., Pourahmadi M. Effect of polygonum Aviculare L. on nephrolithiasis induced by ethylene glycol and ammonium chloride in rats. Urology Journal. 2018;15(3):79–82. doi: 10.22037/uj.v0i0.3815. [DOI] [PubMed] [Google Scholar]
- Sassi A.B., Harzallah-Skhiri F., Aouni M. Investigation of some medicinal plants from Tunisia for antimicrobial activities. Pharmaceutical Biology. 2007;45(5):421–428. doi: 10.1080/13880200701215406. [DOI] [Google Scholar]
- Sayed M., Islam T., Haque M.M., Shaha M.J.H., Ahmed R., Siddiqui M., Hossain M.A. Dietary effects of chitosan and buckwheat (Fagopyrum esculentum) on the performance and serum lipid profile of broiler chicks. South African Journal of Animal Science. 2015;45 doi: 10.4314/sajas.v45i4.9. [DOI] [Google Scholar]
- Schlittenlacher T., Knubben-Schweizer G., Dal Cero M., Vogl C.R., Maeschli A., Hamburger M., Walkenhorst M. What can we learn from past and recent Bavarian knowledge for the future development of European veterinary herbal medicine? An ethnoveterinary study. Journal of Ethnopharmacology. 2022 doi: 10.1016/j.jep.2021.114933. [DOI] [PubMed] [Google Scholar]
- Schmid K., Ivemeyer S., Vogl C., Klarer F., Meier B., Hamburger M., Walkenhorst M. Traditional use of herbal remedies in livestock by farmers in 3 Swiss cantons (Aargau, Zurich, Schaffhausen) Complementary Medicine Research. 2012;19(3):125–136. doi: 10.1159/000339336. [DOI] [PubMed] [Google Scholar]
- Schmuch J., Beckert S., Brandt S., Löhr G., Hermann F., Schmidt T.J., Beikler T., Hensel A. Extract from Rumex acetosa L. for prophylaxis of periodontitis: Inhibition of bacterial in vitro adhesion and of gingipains of porphyromonas gingivalis by epicatechin-3-O-(4β→8)-epicatechin-3-o-gallate (procyanidin-b2-di-gallate) PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S.H., Lee S.-H., Cha P.-H., Kim M.-Y., Min D.S., Choi K.-Y. Polygonum aviculare L. and its active compounds, quercitrin hydrate, caffeic acid, and rutin, activate the Wnt/β-catenin pathway and induce cutaneous wound healing. Phytotherapy Research. 2016;30(5):848–854. doi: 10.1002/ptr.5593. [DOI] [PubMed] [Google Scholar]
- Shea B.J., Grimshaw J.M., Wells G.A., Boers M., Andersson N., Hamel C., Porter A.C., Tugwell P., Moher D., Bouter L.M. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology. 2007;7(1):10. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmering R., Pforte H., Jacobasch G., Blaut M. The growth of the flavonoid-degrading intestinal bacterium, Eubacterium ramulus, is stimulated by dietary flavonoids in vivo. FEMS Microbiology Ecology. 2002;40(3):243–248. doi: 10.1016/S0168-6496(02)00238-6. [DOI] [PubMed] [Google Scholar]
- Sinclair A.J., Attar-Bashi N.M., Li D. What is the role of α-linolenic acid for mammals? Lipids. 2002;37(12):1113–1123. doi: 10.1007/s11745-002-1008-x. [DOI] [PubMed] [Google Scholar]
- Sinclair J.R. Importance of a one health approach in advancing global health security and the sustainable development goals. Revue Scientifique et Technique de l'Office International des Epizooties. 2019;38(1):145–154. doi: 10.20506/rst.38.1.2949. [DOI] [PubMed] [Google Scholar]
- Singh M., Purohit M. Anti-inflammatory activity of methanolic extract of roots of Rumex obtusifolius. International Journal of Pharmaceutics Science Research. 2018;9(8):3519–3522. [Google Scholar]
- Sinz S., Marquardt S., Soliva C.R., Braun U., Liesegang A., Kreuzer M. Phenolic plant extracts are additive in their effects against in vitro ruminal methane and ammonia formation. Asian-Australasian Journal of Animal Science. 2019;32(7):966–976. doi: 10.5713/ajas.18.0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin A.V., Macejková M., Tarko A., Fabova Z., Alrezaki A., Alwasel S., Harrath A.H. Effects of benzene on gilts ovarian cell functions alone and in combination with buckwheat, rooibos, and vitex. Environmental Science Pollution Research. 2021;28(3):3434–3444. doi: 10.1007/s11356-020-10739-7. [DOI] [PubMed] [Google Scholar]
- Smolarz H.D., Skwarek T. The investigations into the interferon-like activity of Polygonum L. genus. Acta Poloniae Pharmaceutica. 1999;56(6):459–462. [PubMed] [Google Scholar]
- Sormunen-Cristian R., Manninen M., Oksanen A. Mixed grazing by suckler cows, calves and lambs in a cultivated pasture. Livestock Science. 2012;145:258–265. doi: 10.1016/j.livsci.2012.02.014. [DOI] [Google Scholar]
- Straub R., Gebert S., Wenk C., Wanner M. Growth performance, energy, and nitrogen balance of weanling pigs fed a cereal-based diet supplemented with Chinese rhubarb. Livestock Production Science. 2005;92(3):261–269. doi: 10.1016/j.livprodsci.2004.09.005. [DOI] [Google Scholar]
- Stringer D.M., Taylor C.G., Appah P., Blewett H., Zahradka P. Consumption of buckwheat modulates the post-prandial response of selected gastrointestinal satiety hormones in individuals with type 2 diabetes mellitus. Metabolis. 2013;62(7):1021–1031. doi: 10.1016/j.metabol.2013.01.021. [DOI] [PubMed] [Google Scholar]
- Stucki K., Cero M.D., Vogl C.R., Ivemeyer S., Meier B., Maeschli A., Hamburger M., Walkenhorst M. Ethnoveterinary contemporary knowledge of farmers in pre-alpine and alpine regions of the Swiss cantons of Bern and Lucerne compared to ancient and recent literature – Is there a tradition? Journal of Ethnopharmacology. 2019;234:225–244. doi: 10.1016/j.jep.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Sung Y.-Y., Yoon T., Yang W.-K., Kim S.J., Kim D.-S., Kim H.K. The antiobesity effect of polygonum Aviculare L. ethanol extract in high-fat diet-induced obese mice. Evidence-Based Complementary and Alternative Medicine. 2013;2013 doi: 10.1155/2013/626397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatecka D., Markiewicz L.H., Wróblewska B. In vitro evaluation of the effect of the buckwheat protein hydrolysate on bacterial adhesion, physiology and cytokine secretion of Caco-2 cells. Central European Journal of Immunology. 2013;38(3):317–327. doi: 10.5114/ceji.2013.37753. [DOI] [Google Scholar]
- Süleyman H., Demirezer L.Ö., Kuruüzüm A., Banoğlu Z.N., Göçer F., Özbakir G., Gepdiremen A. Antiinflammatory effect of the aqueous extract from Rumex patientia L. roots. Journal of Ethnopharmacology. 1999;65(2):141–148. doi: 10.1016/s0378-8741(98)00175-5. [DOI] [PubMed] [Google Scholar]
- Süleyman H., Demirezer L.O., Kuruüzüm-Uz A. Effects of Rumex patientia root extract on indomethacine and ethanol induced gastric damage in rats. Pharmazie. 2004;59(2):147–149. [PubMed] [Google Scholar]
- Tomotake H., Shimaoka I., Kayashita J., Yokoyama F., Nakajoh M., Kato N. Stronger suppression of plasma cholesterol and enhancement of the fecal excretion of steroids by a buckwheat protein product than by a soy protein isolate in rats fed on a cholesterol-free diet. Bioscience Biotechnology and Biochemistry. 2001;65(6):1412–1414. doi: 10.1271/bbb.65.1412. [DOI] [PubMed] [Google Scholar]
- Tomotake H., Yamamoto N., Yanaka N., Ohinata H., Yamazaki R., Kayashita J., Kato N. High protein buckwheat flour suppresses hypercholesterolemia in rats and gallstone formation in mice by hypercholesterolemic diet and body fat in rats because of its low protein digestibility. Nutrition. 2006;22(2):166–173. doi: 10.1016/j.nut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Tsai J.-C., Tsai S., Chang W.-C. Effect of ethanol extracts of three Chinese medicinal plants with anti-diarrheal properties on ion transport of the rat intestinal epithelia. Journal of Pharmacological Sciences. 2004;94(1):60–66. doi: 10.1254/jphs.94.60. [DOI] [PubMed] [Google Scholar]
- Tunón H., Olavsdotter C., Bohlin L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. Journal of Ethnopharmacology. 1995;48(2):61–76. doi: 10.1016/0378-8741(95)01285-l. [DOI] [PubMed] [Google Scholar]
- UFA-Samen (2024). Field seed catalog 2024. Retrieved 15.11.2024 from https://landwirtschaft.ufasamen.ch/files/catalog/Feldsamenkatalog_DE.pdf.
- Ulukanli Z., Ulukanli S., Ozbay H., Ilcim A., Tuzcu M. Antimicrobial activities of some plants from the eastern Anatolia region of Turkey. Pharmaceutical Biology. 2005;43(4):334–339. doi: 10.1080/13880200590951757. [DOI] [PubMed] [Google Scholar]
- van Vliet S., Provenza F.D., Kronberg S.L. Health-promoting phytonutrients are higher in grass-fed meat and milk. Frontiers in Sustainable Food Systems. 2021;4(299) doi: 10.3389/fsufs.2020.555426. [DOI] [Google Scholar]
- Verdú J.R., Sánchez-Piñero F., Lobo J.M., Cortez V. Evaluating long-term ivermectin use and the role of dung beetles in reducing short-term CH4 and CO2 emissions from livestock faeces: A mesocosm design under Mediterranean conditions. Ecological Entomology. 2020;45(1):109–120. doi: 10.1111/een.12777. [DOI] [Google Scholar]
- Viegi L., Pieroni A., Guarrera P.M., Vangelisti R. A review of plants used in folk veterinary medicine in Italy as basis for a databank. Journal of Ethnopharmacology. 2003;89:221–244. doi: 10.1016/j.jep.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Vogl C.R., Vogl-Lukasser B., Walkenhorst M. Local knowledge held by farmers in Eastern Tyrol (Austria) about the use of plants to maintain and improve animal health and welfare. Journal of Ethnobiology and Ethnomedicine. 2016;12(1):40. doi: 10.1186/s13002-016-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucevac-Bajt V., Karlovi M. Traditional methods for the treatment of animal diseases in Croatia. OIE Revue Scientifique et Technique. 1994;1994,13:499–512. doi: 10.20506/rst.13.2.771. [DOI] [PubMed] [Google Scholar]
- WHO. One health, fact sheet. Retrieved from https://www.who.int/news-room/fact-sheets/detail/one-health. Accessed October 24, 2023.
- Waghorn G.C., Jones W.T. Bloat in cattle. Potential of dock (Rumex obtusifolius) as an antibloat agent for cattle. New Zealand Journal of Agricultural Research. 1989;32:227–235. doi: 10.1080/00288233.1989.10423458. [DOI] [Google Scholar]
- Wang M., Hu Y., Tan Z., Tang S., Sun Z., Han X. In situ ruminal phosphorus degradation of selected three classes of feedstuffs in goats. Livestock Science. 2008;117(2):233–237. doi: 10.1016/j.livsci.2007.12.016. [DOI] [Google Scholar]
- Wang M., Jiang J., Tan Z.L., Tang S.X., Sun Z.H., Han X.F. In situ ruminal crude protein and starch degradation of three classes of feedstuffs in goats. Journal of Applied Animal Research. 2009;36(1):23–28. doi: 10.1080/09712119.2009.9707023. [DOI] [Google Scholar]
- Wang M., Liu J.-R., Gao J.-M., Parry J.W., Wie Y.-M. Antioxidant activity of tartary buckwheat bran extract and its effect on the lipid profile of hyperlipidemic rats. Journal of Agricultural and Food Chemistry. 2009;57(11):5106–5112. doi: 10.1021/jf900194s. [DOI] [PubMed] [Google Scholar]
- Wang J.-B., Zhao H.-P., Zhao Y.-l., Jin C., Liu D.-J., Kong W.-J., Fang F., Zhang L., Wang H.-J., Xiao X.-H. Hepatotoxicity or Hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb rheum palmatum L. in treating rat liver injury. PLoS One. 2011;6(9):e24498. doi: 10.1371/journal.pone.0024498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasman S.Q., Abdulla M., Hamdan S., Amin Z., Ismail S. Cytoprotective activities of Polygonum minus aqueous leaf extract on ethanol-induced gastric ulcer in rats. Journal of Medicinal Plants Research. 2010;4 doi: 10.5897/jmpr09.412. [DOI] [Google Scholar]
- Watanabe M., Ayugase J. Effects of buckwheat sprouts on plasma and hepatic parameters in Type 2 diabetic db/db Mice. Journal of Food Science. 2010;75(9):H294–H299. doi: 10.1111/j.1750-3841.2010.01853.x. [DOI] [PubMed] [Google Scholar]
- WebOfScience. (2020). "Web of Science." Retrieved from https://apps.webofknowledge.com. Accessed January 27, 2021.
- Wei L., Luo Y., Zhang X., Liu Y., Gasser M., Tang F., Ouyang W.-W., Wei H., Lu S., Yang Z., Waaga-Gasser A.M., Deng C., Lin M. Topical therapy with rhubarb navel plasters in patients with chronic constipation: Results from a prospective randomized multicenter study. Journal of Ethnopharmacology. 2021;264 doi: 10.1016/j.jep.2020.113096. [DOI] [PubMed] [Google Scholar]
- Wieslander G., Fabjan N., Janson I.K.-C., Leanderson P., Norback D. Vol. 62. Wiley-Blackwell; NJ USA: 2007. Increased intake of the antioxiclant rutin in tartary buckwheat biscuits may reduce neutrophilic inflammation-an experimental study; pp. 442–443. (Allergy). 111 River St, Hoboken 07030-5774. [Google Scholar]
- Wieslander G., Fabjan N., Vogrincic M., Kreft I., Janson C., Spetz U., Vombergar B., Tagesson C., Leanderson P., Norbäck D. Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care centre staffs. Tohoku Journal of Experimental Medicine. 2011;225(2):123–130. doi: 10.1620/tjem.225.123. [DOI] [PubMed] [Google Scholar]
- Wilman D., Derrick R.W., Moseley G. Physical breakdown of chickweed, dandelion, dock, ribwort, spurrey and perennial ryegrass when eaten by sheep and when macerated. Journal of Agricultural Science. 1997;129(4):419–428. doi: 10.1017/S0021859697004759. [DOI] [Google Scholar]
- Wolter A., Hager A.-S., Zannini E., Arendt E.K. Influence of sourdough on in vitro starch digestibility and predicted glycemic indices of gluten-free breads. Food & Function. 2014;5(3):564–572. doi: 10.1039/c3fo60505a. [DOI] [PubMed] [Google Scholar]
- Yan F.W., Fang F., Jin H.M., Jin Y. Influence of tartary buckwheat extracts supplementation on oxidative stress induced by acute exhaustive exercise in rats. International Journal of Pharmacology. 2012;8(8):695–700. doi: 10.3923/ijp.2012.695.700. [DOI] [Google Scholar]
- Yang Y., Yu T., Jang H.-J., Byeon S.E., Song S.-Y., Lee B.-H., Rhee M.H., Kim T.W., Lee J., Hong S., Cho J.Y. In vitro and in vivo anti-inflammatory activities of polygonum hydropiper methanol extract. Journal of Ethnopharmacology. 2012;139(2):616–625. doi: 10.1016/j.jep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Yang H.J., Lim J.H., Park K.J., Kang S., Kim D.S., Park S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition. 2016;32(1):129–137. doi: 10.1016/j.nut.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Yang W.-T., Ke C.-Y., Wu W.-T., Harn H.-J., Tseng Y.-H., Lee R.-P. Effects of Angelica dahurica and Rheum officinale extracts on excisional wound healing in rats. Evidence-Based Complementary and Alternative Medicine. 2017 doi: 10.1155/2017/1583031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Luo C., Zhang X., Liu Y., Wang Z., Cacciamani P., Shi J., Cui Y., Wang C., Sinha B., Peng B., Tong G., Das G., Shah E., Gao Y., Li W., Tu Y., Qian D., Shah K.…Wang X. Tartary buckwheat extract alleviates alcohol-induced acute and chronic liver injuries through the inhibition of oxidative stress and mitochondrial cell death pathway. American Journal of Translational Research. 2020;12(1):70–89. [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Xu F., Ju X., Li Z., Wang L. Lipid-lowering effects and intestinal transport of polyphenol extract from digested buckwheat in Caco-2/HepG2 coculture models. Journal of Agricultural and Food Chemistry. 2020;68(14):4205–4214. doi: 10.1021/acs.jafc.0c00321. [DOI] [PubMed] [Google Scholar]
- Youn H.J., Noh J.W. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Veterinary Parasitology. 2001;96(4):257–263. doi: 10.1016/s0304-4017(01)00385-5. [DOI] [PubMed] [Google Scholar]
- Yu M., Luo Y.-L., Zheng J.-W., Ding Y.-H., Li W., Zheng T.-Z., Qu S.-Y. Effects of rhubarb on isolated gastric muscle strips of guinea pigs. World Journal of Gastroenterology. 2005;11(17):2670–2673. doi: 10.3748/wjg.v11.i17.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Jiang Y., Liu Y., Ma T., Yang H. Prevention of secretory diarrhea by ethanol extract of Bistortae rhizoma through inhibition of chloride channel. Bangladesh Journal of Pharmacology. 2015;10(3):543–547. doi: 10.3329/bjp.v10i3.23260. [DOI] [Google Scholar]
- Yıldırım A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of rumex crispus L. extracts. Journal of Agricultural and Food Chemistry. 2001;49(8):4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- Zaller J.G. Sheep grazing vs. cutting: Regeneration and soil nutrient exploitation of the grassland weed Rumex obtusifolius. Biocontrol. 2006;51:837–850. doi: 10.1007/s10526-005-5272-0. [DOI] [Google Scholar]
- Zduńczyk Z., Flis M., Zieliński H., Wróblewska M., Antoszkiewicz Z., Juśkiewicz J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats. Journal of Agricultural and Food Chemistry. 2006;54(12):4168–4175. doi: 10.1021/jf060224m. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ravipati A.S., Koyyalamudi S.R., Jeong S.C., Reddy N., Smith P.T., Bartlett J., Shanmugam K., Münch G., Wu M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. Journal of Agricultural and Food Chemistry. 2011;59(23):12361–12367. doi: 10.1021/jf203146e. [DOI] [PubMed] [Google Scholar]
- Zhang R.-Z., Qiu H., Wang N., Long F.-L., Mao D.-W. Effect of Rheum palmatum L. on NF-κB signaling pathway of mice with acute liver failure. Asian Pacific Journal of Tropical Medicine. 2015;8(10):841–847. doi: 10.1016/j.apjtm.2015.09.011. [DOI] [PubMed] [Google Scholar]
- Zhang G., Xu Z., Gao Y., Huang X., Zou Y., Yang T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. Journal of Food Science. 2015;80:1111–1119. doi: 10.1111/1750-3841.12830. [DOI] [PubMed] [Google Scholar]
- Zhang W., Pan Y., Qu S., Wang D., Cheng S., Liu X. Anti-inflammatory effects of an extract of polygonum hydropiper stalks on 2,4,6-trinitrobenzenesulphonic acid-induced intestinal inflammation in rats by inhibiting the NF-κB Pathway. Mediators of Inflammation. 2018 doi: 10.1155/2018/6029135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Guo Y., Zhao Q., Xue W., Li Y., Wu X., Huo S. Study on the bacteriostatic action of Chinese herbal medicine on avian Trichosporon. Poultry Science. 2020;99(9):4530–4538. doi: 10.1016/j.psj.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Xu X., Cao Z., Wang Y., Yang H., Azarfar A., Li S. Effect of different tannin sources on nutrient intake, digestibility, performance, nitrogen utilization, and blood parameters in dairy cows. Animals. 2019;9:507. doi: 10.3390/ani9080507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Li K., Su R., Liu W., Ren Y., Zhang C., Du M., Zhang J. Effect of dietary Tartary buckwheat extract supplementation on growth performance, meat quality and antioxidant activity in ewe lambs. Meat Science. 2017;134:79–85. doi: 10.1016/j.meatsci.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Zhou X.-L., Yan B.-B., Xiao Y., Zhou Y.-M., Liu T.-Y. Tartary buckwheat protein prevented dyslipidemia in high-fat diet-fed mice associated with gut microbiota changes. Food Chemistry, Toxicology. 2018;119:296–301. doi: 10.1016/j.fct.2018.02.052. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Jiang Q., Zhao S., Yan B., Zhou X. Impact of buckwheat fermented milk combined with high-fat diet on rats’ gut microbiota and short-chain fatty acids. Journal of Food Science. 2019;84(12):3833–3842. doi: 10.1111/1750-3841.14958. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhao S., Jiang Y., Wie Y., Zhou X. Regulatory function of buckwheat-resistant starch supplementation on lipid profile and gut microbiota in mice fed with a high-fat diet. Journal of Food Science. 2019;84(9):2674–2681. doi: 10.1111/1750-3841.14747. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wei Y., Yan B., Zhao S., Zhou X. Regulation of tartary buckwheat-resistant starch on intestinal microflora in mice fed with high-fat diet. Food Science and Nutrition. 2020;8(7):3243–3251. doi: 10.1002/fsn3.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.