ABSTRACT
Background and Aims
The current study aimed to compare the effects of 12 weeks of moderate versus high‐intensity water aerobics on muscle strength, body composition, lipid profile, blood pressure, and quality of life in both adults and older adults.
Methods
Twenty‐one women (65.19 ± 9.37 years) were randomly allocated to moderate (MIG; n = 11) or high‐intensity groups (HIG; n = 10). Both groups attended 45‐min sessions twice a week for 12 weeks. Assessments at baseline and posttraining included muscle endurance, explosive strength, body mass, body mass index, fat mass, fat‐free mass, triglycerides, total cholesterol, blood pressure, resting heart rate, general quality of life, and general health, and the physical, psychological, social relationships, and environmental domains of quality of life.
Results
HIG experienced greater reductions in cholesterol (η p 2 = 0.28) and fat mass (η p 2 = 0.35), and an increase in fat‐free mass (η p 2 = 0.35), compared to the MIG. The HIG also showed greater improvements in triglycerides (η p 2 = 0.24) and physical quality of life (η p 2 = 0.19) than MIG. No significant group × time interactions were found in the other variables. Nevertheless, muscular endurance and psychological quality of life were improved in both groups (p < 0.05, ES ≥ 0.57). The HIG was the only group to experience a reduction in diastolic blood pressure (p = 0.04, ES = −0.71), while the MIG showed no significant change. Explosive strength did not change in either group.
Conclusion
The study suggests that high‐intensity water aerobics provide additional benefits over moderate intensity for body composition, blood lipids, diastolic blood pressure, and physical quality of life in adults and older adults. However, both intensities effectively improved muscular endurance and psychological quality of life. Future studies should include a larger number of participants and groups (e.g., control group), longer interventions, and controlled dietary intake. Nonetheless, the current results demonstrate that exercise intensity is an important variable for optimal water aerobics outcomes.
Keywords: aquatic exercise, health‐related, intensity, physical fitness, quality of life
1. Introduction
Health and sports professionals have increasingly recommended engaging in aquatic exercise [1, 2]. Among aquatic activities, water aerobics has been particularly recognized by the scientific community for its ability to improve cardiorespiratory capacity [3, 4, 5], muscle strength [2, 5, 6, 7] and anthropometric parameters [2, 6, 8]. The specific properties of water, such as buoyancy, drag forces, and lack of hypogravity [2, 9] contribute to the improvements in health and physical fitness that make aquatic activities highly recommended for older adults and individuals recovering from injury [10, 11]. Recent evidence suggests that aquatic programs can promote improvements in balance, quality of life, strength, pain, and gait in individuals with chronic diseases (e.g., fibromyalgia, bone diseases, some heart diseases, diabetes, multiple sclerosis, and Parkinson's), although adaptations are specific to each disease [12]. Furthermore, aquatic exercise has also been shown to improve strength, balance, and cardiorespiratory fitness in healthy populations [12].
Most studies conducted on adults and older adults have primarily focused on the effects of water aerobics on muscle strength and body composition [2, 5, 7, 8, 13, 14]. This interest is likely due to the increased risk of sarcopenia among individuals aged 50–80 years [15, 16], as well as the fact that water aerobics is an activity widely practiced by people with obesity and/or locomotor problems [1]. However, despite its popularity, there is a lack of scientific evidence supporting its effects on lipid profile, blood pressure, and quality of life [2, 8, 17]. Water aerobics classes can play a crucial role in improving various health parameters, as they are closely linked to cardiovascular diseases [18, 19]. Furthermore, as individuals age, their quality of life tends to decline [20], making water aerobics classes all the more valuable.
While the results of water aerobics programs are often positive, the impact of various training variables has been inadequately studied. In terms of training volume, that is, the time dedicated to water aerobics sessions, research suggests that durations between 45 and 60 min per class, conducted 2–3 times per week, tend to yield favorable results, particularly for training periods lasting 12–24 weeks [2, 6, 7, 8, 10, 14, 21]. However, many programs fail to monitor and/or report crucial information about exercise intensity and its effects [12]. The intensity at which an exercise is performed is a vital aspect of designing and controlling any exercise program [22]. Additionally, aquatic exercise requires higher effort intensity but elicits a lower perception of effort compared to similar land‐based activities [13]. This can facilitate performing aquatic exercise at high intensities, although limited information exists on its effects in water aerobics. Furthermore, no studies have analyzed the effects of performing water aerobics at moderate versus high intensities.
To comprehend the optimal intensity of water aerobics for attaining optimal health and fitness enhancements, it is essential to assess the effects of water aerobics based on the intensity utilized. Given that water aerobics is widely practiced by adults and older individuals, it is crucial to investigate the ideal intensity for this particular population. To the best of our knowledge, no study has examined this in adults and older adults. For this reason, we aimed to analyze the impact of 12 weeks of moderate and high‐intensity water aerobics on muscle strength, body composition, lipid profile, blood pressure, and quality of life in adults and older adults. We hypothesized that all variables would improve following training, regardless of intensity. Furthermore, we hypothesized that there would be greater improvements across all parameters with high‐intensity training compared to moderate intensity.
2. Materials and Methods
2.1. Study Design
This was a pragmatic randomized controlled trial (pRCT) (Registration: NCT06509217), in adults and older adults, with balanced randomization (1:1) to each group (moderate or high intensity), conducted in Portugal. This was a double‐blinded study to analyze the effects of 12 weeks of moderate and high‐intensity water aerobics on muscle strength, body composition, lipid profile, blood pressure, and quality of life in adults and older adults.
2.2. Participants
The participants were recruited from the same residential area and municipal swimming pool in Tramagal, Abrantes, Portugal. The participants from various classes received both oral and written instructions regarding the study's objectives. Those who agreed to take part in the study signed the informed consent form and were randomly assigned to each group. Although no dietary intake was assessed, the participants were asked not to alter their eating habits. The study protocol was approved by the Ethics Committee of the University of Beira Interior, Portugal (CE‐UBI‐Pj‐2019‐051). To be eligible for the study, individuals had to be 18 years old or older and attend two classes per week. The following criteria were used to exclude participants: taking part in another physical exercise program, being recently hospitalized, having severe motor or cognitive problems, or having any medical restrictions on physical exercise. While being a man was not considered an exclusion criterion, only women completed the study.
2.3. Intervention
Participants in the study were randomly assigned to either the moderate‐intensity group (MIG) or the high‐intensity group (HIG). The water aerobics classes for both groups lasted approximately 45 min and were held twice a week for a period of 12 weeks. The MIG classes were conducted at moderate intensities, ranging from 60% to 70% of the maximum heart rate (HRmax) predicted by age (HRmax = 207–0.7 × age) [23]. Each session comprised a 5‐min warm‐up (88.14 ± 7.98 bpm), a 35‐min aerobic workout (113.70 ± 6.23 bpm), and a 5‐min cool‐down (93.45 ± 7.86 bpm). The HIG classes were similar to the MIG classes but were conducted at high intensities, ranging from 80% to 90% of the HRmax predicted by age. The HIG recorded the following heart rate values: warm‐up (96.35 ± 9.35 bpm), workout (124.73 ± 7.37 bpm), and cool‐down (101.95 ± 7.38 bpm). In both groups, the intensity of the classes was manipulated through the musical cadence [24, 25], and the heart rate was monitored once every 2 weeks for all participants. The warm‐up and cool‐down included exercises, such as walking forward and backward, performing lateral movements, and stretching all major muscle groups. The workout comprised a variety of exercises, including runs, steps sideways, swings, kicks, jumps, squats, hip flexion, extension, abduction, and adduction, both with and without equipment (e.g., dumbbells, water noodles, etc.). Table 1 provides the main exercises of routine workouts of the water aerobics programs [26, 27, 28]. The water aerobics classes were held at a temperature of about 29.0℃ and a depth of 1.50 m. The instructor of the water aerobics classes was informed about the study, and no external pressure was exerted to influence the instructor's activities. The instructor conducted exercises typically performed before the study began but with different intensities in each group.
Table 1.
The main exercises performed in the workout routine of the moderate‐intensity group and high‐intensity group through the 12 weeks.
| Workout | Duration (min) | Exercises | Brief description |
|---|---|---|---|
| Warm‐up | 5 | Walking forward and backward, performing lateral movements, stretching all major muscle groups | |
| Main training program | 35 | Stationary running and walking | Right hip and knee flexion to 90°, followed by its extension* , # |
| Frontal kicks | Right hip flexes to 45°, followed by the knee extension and the ankle plantar flexing. Then, the right hip extends* , # | ||
| Alternate forward kicks | Simultaneously with the forward leg kick, the opposite arm is pushed out sideways and vice versa. The kicking foot must be raised to hip height. | ||
| Alternate sideways kicks | At the same time as the leg kicking sideways, the opposite arm is pushed forward, and the other arm is pushed out sideways. The kicking foot should be raised to hip height. | ||
| Squat jumps | Knee flexion of 90°, torso straight, and feet shoulder‐width apart. When jumping, legs should be fully extended. Upon landing, both feet should come together in an upright position with fully extended knees# | ||
| Jumping jacks | In the first jump, abduction of both arms and legs at the same time. Then, in the second jump, adduction of the arms and legs to the initial position | ||
| Cross country skiing | Right hip flexion to 60°, knee semi‐flexed, and ankle dorsiflexed. Then, during a jump, extend the right hip and knee while maintaining the ankle dorsiflexed. Simultaneously, the left lower limb performs the opposite movement# | ||
| Cool down | 5 | Walking forward and backward, performing lateral movements, stretching all major muscle groups | |
Alternating with the left upper/lower limb.
Simultaneously performing movements with the upper limbs.
2.4. Outcome Measures
The primary objectives of this study were to evaluate changes in muscle strength, body composition, lipid profile, and blood pressure, with the secondary objectives being focused on quality of life. To achieve this, the variables were assessed in the week before the start of the program (Pre) and in the week following its conclusion (Post). Assessments were conducted by the same team of experienced evaluators in a room suitable for evaluations, located within the municipal swimming pool facility. The two assessment days were separated by a 72‐h interval. On the first day, body composition, blood pressure, and explosive strength were evaluated, while on the second day, 72 h later, lipid profile, muscle endurance, and quality of life were assessed. The order of assessments was maintained both during the pre and postevaluations. To ensure the best possible results, all participants were verbally encouraged to perform to the best of their abilities on all assessments.
2.5. Muscle Strength
The explosive strength of the lower and upper limbs was assessed using the countermovement jump (CMJ) and medicine ball throw (MBT), respectively. For the CMJ, participants commenced the test on an Optojump platform (Optojump, Microgate, Bolzano, Italy) with feet positioned shoulder‐width apart and hands resting on the waist. Three jumps with countermovement were performed, with participants instructed to jump as high as possible, with 2 min of rest between repetitions [29]. The CMJ demonstrated an average intraclass correlation coefficient (ICC) of 0.98 (95% CI: 0.96–0.99) and a coefficient of variation (CV) of 5.84%.
In the 3‐kilogram MBT, participants were instructed to sit in a chair against the wall, resting their backs on the chair and supporting their feet on the floor. Each participant was then encouraged to throw the 3‐kilogram medicine ball (Vinex model VMB‐003R, Bhalla International, Delhi, India) as far as possible. To obtain the values, a tape measure was used. Each individual performed three throws, with a 3‐min rest between each attempt [30]. For both CMJ and MBT, the mean values were considered. The mean ICC for MBT performance was 0.96 (95% CI: 0.91–0.98), and the CV values were 1.67%.
The chair stand test and arm curl test were performed to assess the lower and upper limb endurance strength, respectively [31]. The chair stand test entailed the participant sitting in the center of the chair with a straight back and feet shoulder‐width apart and in full contact with the floor. The upper limbs were crossed at the wrist level and positioned against the chest. Upon receiving the starting signal, the participant rose to maximum extension and returned to the initial sitting position, striving to complete the maximum number of repetitions within a 30‐second time frame.
In the arm curl test, the participant was seated in a chair with the back straight and fully leaning against the chair, with their feet flat on the floor. A 2 kg dumbbell was held in their dominant hand, and the test commenced with the forearm in a lower position, near the chair, perpendicular to the floor. Upon receiving the start signal, the participant performed a complete flexion of the forearm and then returned to the initial position of forearm extension. The evaluator encouraged the participant to perform as many repetitions as possible within a time limit of 30 s, counting each correct flexion performed [31].
2.6. Body Composition
The body composition parameters evaluated were body mass, body mass index (BMI), fat mass percentage, and fat‐free mass. The BMI was calculated by dividing the body mass value by the height squared. For this, each participant's height was measured using a precision stadiometer with a scale of 0.001 m (Seca 213, Hamburg, Germany). A bioimpedance balance was used to obtain body mass, body fat, and fat‐free mass (Tanita, BC418 MA, Tokyo, Japan). For the correct extraction of these tests, participants were barefoot and dressed in as little clothing as possible [32].
2.7. Lipid Profile
Using lancets and specific strips, blood samples were collected from the participant's fingertip to assess triglycerides and total cholesterol (Accutrend Plus, La Roche, Germany). Blood samples were collected before exercise (after a 15‐min rest period) and at least 2 h after the last meal. The assessment was carried out in the morning and the recommended meal, when taken, was recommended to be light. Each participant was advised to repeat the same meal and time procedures in both evaluations (pre‐ and post). The 2‐h fasting period was selected to balance the need for accurate lipid profile measurements with the practical considerations of participants' comfort and study logistics, particularly for older adults. While extended fasting periods (typically 8 h) are standard in some clinical lipid assessments, research suggests that shorter fasting periods (2–4 h) provide reliable data and are clinically relevant for assessing cardiovascular risk [33, 34, 35]. We focused on observing changes in lipid profiles over time rather than establishing absolute baseline levels. Therefore, we considered a 2‐h fasting period appropriate. Shorter fasting periods improve participant compliance and comfort, especially in older adults, and minimize disruptions to their daily routine. This pragmatic approach is aligned with our study design and ensures reliable lipid measurements while maintaining high participant engagement.
2.8. Blood Pressure Measurements
Blood pressure was assessed using an automatic blood pressure monitor (OMRON M4‐1, Hoofddorp, Netherlands) after the participant was seated and resting for at least 20 min [36]. This test assessed systolic blood pressure, diastolic blood pressure, and heart rate at rest.
2.9. Quality of Life
The WHOQOL‐BREF (Portuguese version) questionnaire with 26 items was used to assess the participants' quality of life [37]. Of the 26 items, 2 are related to the general quality of life and general health, and the remaining 24 items assess the perception of quality of life in 4 domains: physical, psychological, social relationships, and environment. Following the scoring guidelines, the domain scores were transformed into a linear scale between 0 and 100 [37]. The higher the final score, the higher the participant's quality of life in each domain.
2.10. Sample Size
An a priori power analysis was conducted using G*Power (v3.1.9.8, University of Kiel, Germany) [38] for sample size estimation and considering data from Neiva et al. [2]. The effect size was determined as medium, using Cohen [39] criteria (converted to an effect size f of 0.31), and the correlation among repeated measurements to be more than 0.60. With a significance criterion of α = 0.05 and power = 0.80, the minimum sample size needed for each group with this effect size is n = 10 (ratio 1:1), considering a 2‐tailed analysis of variance with repeated measurements (two groups, pre and posttest assessments). To allow for potential dropouts (considering the drop‐out rate of 20%) we decided to select at least four participants to the sample before starting. The obtained final sample size for analysis of n = 21 (10 in the HIG and 11 in the MIG) is more than adequate to test the study hypothesis.
2.11. Randomization
The study design was a double‐blind pRCT with participants being blinded to allocation to each experimental group. The study participants were allocated using a simple randomization method. A computer‐generated list of random numbers was used to assign the participants to two study groups in a 1:1 ratio. Each number corresponded to a sealed opaque envelope containing details about the study group, ensuring that the randomization procedure was concealed. The sequential numbers remained undisclosed until the interventions were assigned. The randomization was conducted by three authors, with the researcher responsible for assigning participants to specific groups not informed in advance about the treatment regimen allocated to each participant. Due to the nature of this study, the main researcher and instructor, who were involved in both the testing and training at different intensities, were unable to be blinded. However, the research team involved in the evaluation (pre and posttest assessments) were also blinded.
2.12. Statistical Analysis
We used the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, version 28.0, IBM Corp., Armonk, NY, USA) to perform the statistical analysis. Standard statistical procedures were selected to determine means, standard deviations, and 95% confidence limits. To test normality, the Shapiro‐Wilks test (n < 30) was used, followed by the Levene test to confirm the homogeneity of variances. The reliability of measurements was assessed using ICC and CV across the three attempts. The ICC, calculated using a bidirectional mixed random effects model with absolute agreement, evaluates the consistency of the measurements, while the CV provides an additional measure of reliability. To compare pre‐training variables between groups, the independent sample t‐test was used, allowing to determine any significant differences at baseline between groups. A two‐way mixed design analysis of variance with one factor with repeated measures (pretest and posttest) was used to examine how different interventions affect outcomes over time, assessing both the within‐subject effects (changes over time) and between‐subject effects (differences between groups). To further explore changes within each group from pretest to posttest, paired samples t‐tests were performed. This test was chosen because it accounts for the paired nature of pre and postintervention data within the same participants, allowing us to directly assess whether the intervention produced significant improvements in the measured outcomes. Additionally, the measurements met the assumptions of the test, ensuring its suitability and robustness for detecting significant changes within each group. We also calculated the effect size to estimate the variance between groups, using the partial eta squared (ηp 2) and Cohen's d z (ES) was calculated for within‐subject comparisons [39, 40]. ES values were considered small (0.20–0.49), medium (0.50–0.79), and large (≥ 0.80), and the ηp 2 values were classified as small (0.01–0.08), moderate (0.09–0.24) and large (≥ 0.25). A 2‐sided p ≤ 0.05 was considered statistically significant.
3. Results
The recruitment took place in September 2022 and the follow‐up occurred between October and December 2022. A total of 29 individuals were assessed for eligibility. Of these, 3 refused to participate in the study, and 2 did not meet the inclusion criteria. Twenty‐two women and two men were considered eligible and were enrolled in the study. Of these, 12 were randomly allocated to the MIG and 12 to the HIG. However, 1 participant of the HIG lost to follow‐up after completing the pretest due to being hospitalized by illness, while 2 other people, one from each group discontinued intervention due to injury. Thus, 21 women completed the study and were used in the analysis, with 10 included in the HIG and 11 in the MIG. Figure 1 shows the participant flow diagram throughout the study.
Figure 1.
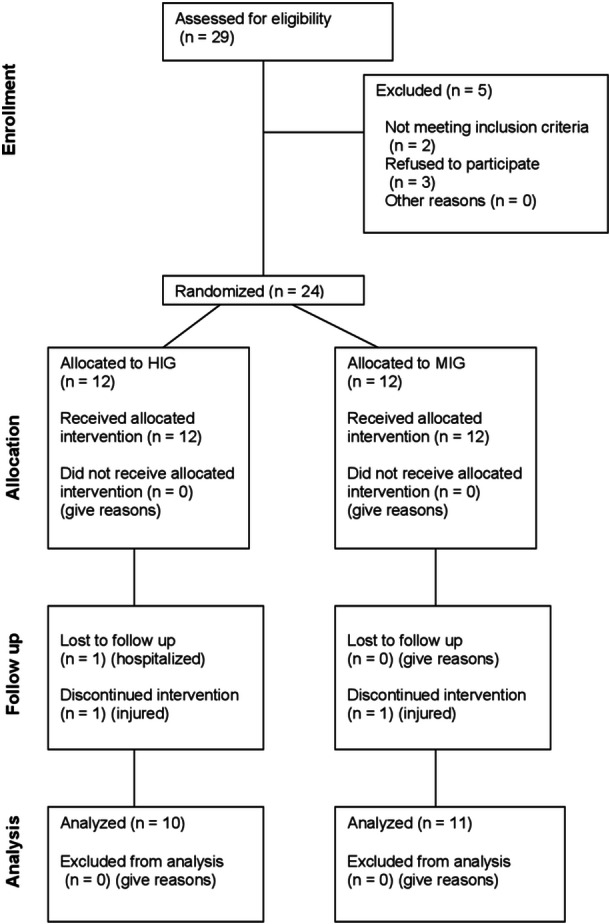
Flow diagram of the progress in a pragmatic randomized controlled trial to assess the effect of 12 weeks of moderate and high‐intensity water aerobics on muscle strength, body composition, lipid profile, blood pressure, and quality of life in adults and older adults.
Table 2 lists the characteristics of the study participants. There were no significant between‐group differences existed at baseline for any of the measures (all p > 0.05).
Table 2.
Characteristics of the study participants of the MIG and HIG in baseline (mean ± SD).
| Variable | MIG (n = 11) | HIG (n = 10) |
|---|---|---|
| Age (years) | 63.18 ± 8.64 | 67.40 ± 10.09 |
| Heigh (m) | 1.58 ± 0.07 | 1.56 ± 0.06 |
| Body mass (kg) | 69.65 ± 10.44 | 75.94 ± 17.66 |
| BMI (kg/m2) | 28.16 ± 4.76 | 31.39 ± 7.63 |
| Fat mass (%) | 38.99 ± 5.68 | 41.53 ± 6.34 |
| Fat‐free mass (kg) | 39.81 ± 3.15 | 41.30 ± 5.21 |
Abbreviations: BMI, Body Mass Index; HIG, high intensity group; MIG, moderate intensity group; SD, standard deviation.
3.1. Primary Outcomes
Table 3 displays the variables evaluated before and after training in the MIG and HIG groups. Concerning the muscle strength outcomes, no significant group × time interactions were identified for lower and upper body explosive strength assessed through CMJ (F = 0.32, p = 0.58, ηp 2 = 0.02) and MBT (F = 0.42, p = 0.53, ηp 2 = 0.02), as well as for lower and upper body muscular endurance measured using chair stand test (F = 0.91, p = 0.35, ηp 2 = 0.05) and arm curl test (F = 0.50, p = 0.49, ηp 2 = 0.03). However, a significant time effect was observed for upper and lower muscular endurance in both groups, as their performance improved on the chair stand and arm curl tests. It is worth noting that no main effects were observed for upper and lower explosive strength.
Table 3.
Muscle strength, anthropometric, lipid profile, blood pressure, and quality of life values of the Moderate Intensity Group and High Intensity Group in pre and posttraining.
| Moderate Intensity Group (MIG) (n = 11) | High Intensity Group (HIG) (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change (± 95% CI) | p value | Pre | Post | Change (± 95% CI) | p value | |
| Strength | ||||||||
| CMJ [cm] | 6.56 ± 3.49 | 7.22 ± 3.42 | 0.66 (± 0.98) | 0.16 | 5.34 ± 3.57 | 5.67 ± 3.53 | 0.34 (± 0.79) | 0.36 |
| MBT [m] | 2.29 ± 0.32 | 2.34 0 ± 0.27 | 0.05 (± 0.12) | 0.35 | 2.12 ± 0.42 | 2.24 ± 0.60 | 0.12 (± 0.22) | 0.24 |
| Chair stand test [reps] | 14.00 ± 2.93 | 15.55 ± 3.88 | 1.55 (± 1.33) | 0.03* | 15.80 ± 5.94 | 18.30 ± 5.96 | 2.50 (± 1.85) | 0.01* |
| Arm Curl [reps] | 19.18 ± 2.99 | 21.09 ± 4.23 | 1.91 (± 1.82) | 0.04* | 22.10 ± 7.46 | 25.1 ± 5.96 | 3.00 (± 2.74) | 0.04* |
| Anthropometric | ||||||||
| Body mass [kg] | 69.65 ± 10.44 | 69.78 ± 10.54 | 0.13 (± 0.68) | 0.68 | 75.94 ± 17.66 | 76.14 ± 17.06 | 0.20 (± 0.78) | 0.58 |
| BMI [kg/m2] | 28.16 ± 4.76 | 28.21 ± 4.85 | 0.05 (± 0.23) | 0.66 | 31.39 ± 7.63 | 31.44 ± 7.33 | 0.05 (± 0.29) | 0.71 |
| Fat mass [%] | 38.99 ± 5.68 | 40.15 ± 4.88 | 1.16 (± 1.15) | 0.05* | 41.53 ± 6.34 | 40.31 ± 6.71 | −1.22 (± 1.21) | 0.05* |
| Fat‐free mass [kg] | 39.81 ± 3.15 | 39.19 ± 3.70 | −0.63 (± 0.57) | 0.04* | 41.30 ± 5.21 | 42.24 ± 5.50 | 0.94 (± 0.86) | 0.04* |
| Lipid profile | ||||||||
| Triglycerides [mg/dl] | 204.36 ± 59.53 | 216.82 ± 58.21 | 12.45 (± 21.16) | 0.22 | 187.70 ± 60.83 | 162.30 ± 46.83 | −25.40 (± 23.93) | 0.04* |
| Total cholesterol [mg/dl] | 201.27 ± 30.34 | 215.27 ± 33.88 | 14.00 (± 17.73) | 0.11 | 210.90 ± 41.77 | 190.30 ± 53.30 | −20.60 (± 18.85) | 0.04* |
| Blood pressure | ||||||||
| Systolic BP [cmHg] | 12.98 ± 2.04 | 12.76 ± 1.87 | −0.22 (± 0.82) | 0.56 | 13.81 ± 2.41 | 13.35 ± 2.23 | −0.46 (± 0.77) | 0.21 |
| Diastolic BP [cmHg] | 7.60 ± 1.23 | 7.33 ± 0.92 | −0.27 (± 0.38) | 0.14 | 8.19 ± 1.37 | 7.74 ± 1.51 | −0.45 (± 0.43) | 0.04* |
| Resting heart rate [bpm] | 72.64 ± 8.05 | 76.45 ± 8.66 | 3.82 (± 5.09) | 0.13 | 72.80 ± 11.35 | 74.00 ± 8.89 | 1.20 (± 4.34) | 0.55 |
| Quality of life | ||||||||
| QoL‐general | 62.50 ± 13.69 | 64.77 ± 12.27 | 2.27 (± 3.40) | 0.17 | 55.00 ± 19.72 | 60.00 ± 19.36 | 5.00 (± 7.54) | 0.17 |
| QoL‐physical | 68.18 ± 15.69 | 67.53 ± 17.01 | −0.65 (± 5.86) | 0.81 | 50.36 ± 15.47 | 57.14 ± 16.75 | 6.79 (± 5.04) | 0.01* |
| QoL‐psychological | 69.32 ± 15.17 | 74.24 ± 12.33 | 4.92 (± 3.93) | 0.02* | 58.33 ± 16.32 | 65.00 ± 13.78 | 6.67 (± 5.48) | 0.02* |
| QoL‐SR | 71.21 ± 14.12 | 70.45 ± 15.53 | −0.76 (± 8.84) | 0.85 | 68.33 ± 16.10 | 70.83 ± 14.30 | 2.50 (± 6.91) | 0.43 |
| QoL‐environment | 64.77 ± 13.04 | 66.19 ± 12.17 | 1.42 (± 3.02) | 0.32 | 54.06 ± 10.83 | 59.06 ± 10.77 | 5.00 (± 6.76) | 0.13 |
Abbreviations: BMI, Body Mass Index; BP, blood pressure; CI, confidence interval; CMJ, countermovement jump; MBT, medicine ball throw; QoL, quality of life; SR, social relationships.
p < 0.05.
Significant group × time interactions with large effect sizes were observed for fat mass (F = 10.36, p = 0.01, ηp 2 = 0.35) and fat‐free mass (F = 9.99, p = 0.01, ηp 2 = 0.35), favoring the HIG. Consequently, a significant time effect was verified in these variables, with fat mass decreased, and fat‐free mass increased in the HIG, while the opposite occurred in MIG. In contrast, no interactions or main effects were found for body mass (F = 0.03, p = 0.88, ηp 2 ≤ 0.01) and BMI (F ≤ 0.01, p = 0.98, ηp 2 ≤ 0.01).
The lipid profile exhibited significant group × time interactions with large effects for total cholesterol (F = 7.38, p = 0.01, ηp 2 = 0.28) and moderate effects for triglycerides (F = 6.03, p = 0.02, ηp 2 = 0.24), with the HIG showing greater improvements. A significant group effect was also found in total cholesterol and triglycerides, reducing with the HIG, while no main effects existed in the MIG. Additionally, no interactions existed for diastolic blood pressure (F = 0.48, p = 0.50, ηp 2 = 0.03), but a significant group effect was observed, improving in the HIG, without main effects on MIG. No interactions or main effects were verified for systolic blood pressure (F = 0.23, p = 0.64, ηp 2 = 0.01), and resting heart rate (F = 0.75, p = 0.40, ηp 2 = 0.04).
The results indicate that the HIG exhibited greater effects in comparison to the MIG. The chair stand test demonstrated positive large effects in the HIG (ES = 0.92 [mean value]), while the arm curl (ES = 0.75), fat‐free mass (ES = 0.75), fat mass (ES = −0.69), total cholesterol (ES = −0.62), triglycerides (ES = −0.62), and diastolic blood pressure (ES = −0.71) displayed medium effects. In contrast, the MIG showed medium negative changes in fat‐free mass (ES = −0.57) and fat mass (ES = 0.66). As depicted in Figure 2, the mean ES values and 95% confidence interval confirm these findings.
Figure 2.
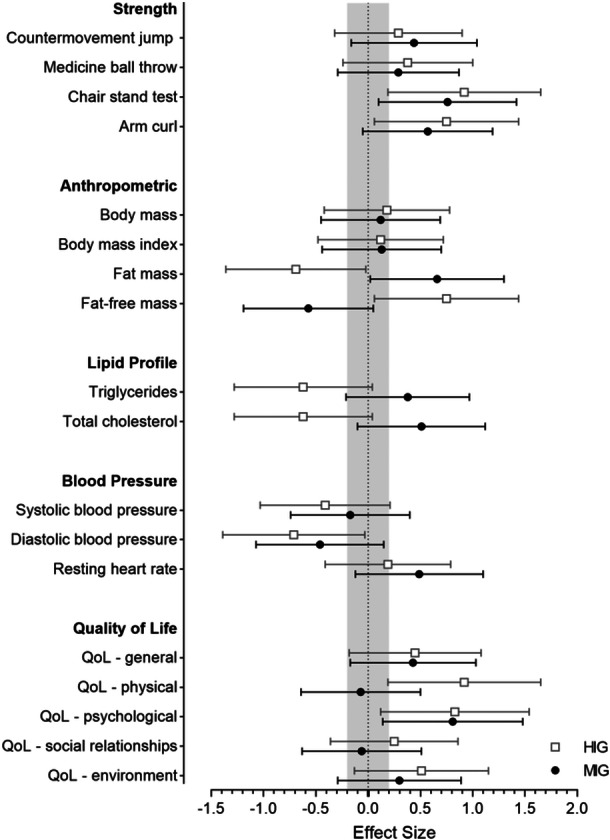
Standardized differences (effect size) between post and pre values of muscle strength, body composition, lipid profile, blood pressure, and quality of life (QoL) variables in the high‐intensity (HIG) and moderate‐intensity (MIG) water aerobics program. The trivial effect size interval is gray‐colored. Error bars indicate 95% confidence interval.
3.2. Secondary Outcome
The results of the study revealed a significant interaction between group and time for physical quality of life, with a moderate effect size (F = 4.56, p = 0.05, ηp 2 = 0.19), indicating a preference for the HIG. No significant interactions group × time were observed in the other domains: general (F = 0.59, p = 0.45, ηp 2 = 0.03); psychological (F = 0.35, p = 0.56, ηp 2 = 0.02); social relationships (F = 0.41, p = 0.53, ηp 2 = 0.02); and environment (F = 1.27, p = 0.27, ηp 2 = 0.06). Additionally, a significant time effect was found in the psychological quality of life domain, suggesting improvement in both groups, in addition to a significant group effect observed in physical quality of life, favoring the HIG. However, no main effects were detected in the domains of general quality of life and general health, social relationships, and environment. The ES values observed in Figure 2 demonstrate favorable large effects on the physical quality of life in HIG (ES = 0.92), while both groups revealed positive large effects on the psychological quality of life (MIG, ES = 0.81; HIG, ES = 0.83).
4. Discussion
The primary purpose of this study was to evaluate the effects of engaging in water aerobics for 12 weeks at different intensities (moderate vs. high) on muscle strength, body composition, lipid profile, blood pressure, and quality of life in both adults and older adults. Our findings indicate that both exercise intensities led to significant and positive improvements in upper and lower muscle endurance and the psychological domain of quality of life. However, the HIG was found to yield superior benefits in several areas, including body composition (fat and fat‐free mass), lipid profile (triglycerides and cholesterol), and physical quality of life, when compared to MIG. Additionally, diastolic blood pressure was improved only after HIG. These results partially confirm our hypotheses that both interventions would yield positive adaptations and that higher exercise intensity would be associated with higher improvements. Although changes in several outcomes did not reach significance, most showed a trend toward improvement.
Our research findings align with previous evidence, demonstrating that engaging in water aerobics for 12 weeks can result in substantial gains in both upper and lower limb muscular endurance when performed at moderate or high intensity [5, 7, 14]. This improvement is likely due to the properties of the aquatic environment and the specificity of the exercise, which allow for a larger range of motion and greater resistance [41, 42], stimulating muscular endurance. This may have been intensified by the density of water, which provides 900 times greater resistance during movements than air [43]. Furthermore, the velocity at which the movements were performed was probably enough to maximize the strength needed to overcome the water's resistance [41] and to recruit motor units with higher excitability thresholds, contributing to improving strength indices [44].
Regarding explosive strength, it appears that a longer intervention duration may be necessary to produce meaningful improvements through water aerobics. In contrast to our findings, Neiva et al. [2] reported significant gains in upper limb explosive strength after 12 weeks of moderate‐intensity water aerobics (approximately 65% of HRmax). The discrepancy between our results and those of Neiva et al. [2] is likely due to the slightly longer session durations and possibly different exercise protocols employed in their study. In our study, we found some indications that upper limb explosive strength was marginally higher when performed at HIG, and the lower limbs were barely more enhanced with MIG. However, these changes were not statistically significant in either group. Overall, the intensity does not appear to be the primary variable influencing strength gains with water aerobics. This is consistent with the idea that water‐based exercise may not provide sufficient mechanical load to elicit significant increases in explosive strength, which typically requires greater external resistance or impact [10]. Factors such as exercise duration, the inclusion of specific strength‐training components, and the mechanical resistance offered by the water should be considered in future research to better understand how water aerobics can effectively improve explosive strength in adults and older adults.
The HIG was found to reduce fat mass and increase fat‐free mass, which aligns with previous research on similar aquatic programs and their effects on body composition [8, 42]. Notably, the study did not control the diet. Previous research suggests that a controlled diet, in addition to high‐intensity water aerobics, would further enhance body composition [13, 45]. However, in contrast, the MIG in the study increased fat mass and lost fat‐free mass during the intervention period. This suggests that water aerobics performed at 60‐70% HRmax can only enhance these variables with dietary control, as suggested by previous research [13]. In this intensity, it's possible that the execution velocity of exercises wasn't sufficient to significantly impact these variables [45]. The considerably lower baseline anthropometric values in the MIG may also have made it difficult to see improvements compared to the HIG. Regarding body mass and BMI, both groups were incapable of observing changes, corroborating the findings of Neiva et al. [2]. There were probably no changes because, in both groups, although fat mass decreased/increased, the opposite happened in fat‐free mass, approximately in the same proportion. These findings emphasize the importance of high‐intensity exercise when aiming to improve fat mass and fat‐free mass, in addition to the inefficacy of this training variable in body mass and BMI in adults and older adults. Thus, more research is needed to confirm the effects of water aerobics intensity and anthropometry.
The HIG led to improvements in the lipid profile, particularly total cholesterol and triglycerides. These findings are noteworthy, as elevated levels of these lipids increase the risk of cardiovascular disease [19]. On the other hand, MIG did not show changes in the lipid profile. Previous studies have reported no changes in cholesterol and triglycerides following a moderate‐intensity program with the same duration [2, 46]. These results suggest the potential value of higher intensities of water aerobics, as well as the uncertainty surrounding the effects of moderate intensities [47, 48]. Additionally, Viljoen and Christie [49]. found no changes in the lipid profile but did not achieve adequate reductions in body mass. In contrast, Kasprzak and Pilaczyńska‐Szcześniak [50] reported improved cholesterol levels, which were concurrent with improvements in body composition. In our study, the HIG showed significant improvements in both the lipid profile and body composition, while the MIG did not. Therefore, the effects of cholesterol and triglycerides on body composition, particularly reduced body fat, may be related to the intensity of the exercise [42]. HIG positively influenced body fat, which may explain the improved cholesterol and triglyceride levels observed in this group. These findings suggest that exercise intensity is crucial in improving cholesterol and triglyceride levels with water aerobics.
Blood pressure results were analyzed, and a significant group effect was detected, indicating that the HIG exhibited substantial improvements in diastolic blood pressure. This finding is consistent with previous studies that have reported similar reductions in both systolic and diastolic blood pressure in hypertensive individuals [51]. However, in our sample of non‐hypertensive individuals, only diastolic blood pressure decreased significantly (5.6%). Although some participants improved systolic blood pressure in HIG and MIG (evidenced by the absolute difference of approximately 3% and 1%, respectively), the mean difference did not reach statistically significant levels. The same happened with diastolic blood pressure in MIG, with a nonsignificant reduction of around 3%. It is noteworthy that even small reductions in both systolic and diastolic blood pressure are associated with decreased cardiovascular events [52]. Furthermore, previous studies have reported a higher likelihood of improving blood pressure in hypertensive individuals [53], highlighting the changes achieved in our study involving normotensive individuals. Probably the decrease in the activity of the sympathetic nervous system and the increase in the action of the parasympathetic nervous system contributed to this reduction [54]. Thus, the intensity of water aerobics seems to be important in the gains in diastolic blood pressure.
We were unable to demonstrate a difference between groups in resting heart rate. Contrary to the findings of Igarashi and Nogami [52], we anticipated a reduction in resting heart rate resulting from enhanced parasympathetic activity, a common response to warm water immersion [55]. However, this reduction typically occurs with water temperatures in the range of approximately 36°C, which differs from the temperature used in the present study (29°C). This disparity may explain the absence of a reduction in resting heart rate. Therefore, the intensity of water aerobics does not appear to have a significant impact on resting heart rate, with water temperature playing a more prominent role in this phenomenon.
The HIG demonstrated a substantial improvement in the physical domain of quality of life, while both groups experienced substantial improvements in the psychological domain. Furthermore, the HIG resulted in greater score improvements in the remaining quality‐of‐life domains, although no differences between groups. A study by Ayán et al. [56] found that high‐intensity aquatic exercise led to greater improvements in the quality of life for healthy women. Consequently, high‐intensity water aerobics may be a more effective means of improving quality of life. Additionally [17], suggested that 24 weeks of water aerobics improved quality of life, but no information was provided about the intensities used. However, the psychological domain did not show any significant changes. Although the literature is limited on this variable, the promising results warrant further investigation.
The potential limitations of our study must be acknowledged. Specifically, the lack of a control group in our study design presents a limitation. The inclusion of a control group would have allowed us to establish a baseline for comparison, providing a clearer understanding of the effects observed. Without a control group, it is difficult to completely rule out the possibility that some of the improvements observed in both groups could be partially attributed to external factors unrelated to the exercise intervention, such as lifestyle changes or other health behaviors. Future studies should consider including a control group to better isolate the effects of different exercise intensities and clarify the unique contribution of aquatic exercise to the observed health outcomes. Furthermore, the small sample size within each experimental group warrants caution in the interpretation and generalization of our findings. The limited number of participants increases the risk of type II errors and may compromise the detection of significant differences between groups. This limitation is particularly relevant when considering the generalizability of our findings to the broader population of older adults, including both men and women. A larger sample size would provide more statistical power and improve the robustness of the findings, enhancing their relevance to wider populations. Nonetheless, it is important to recognize that we are dealing with a long‐term follow‐up, which is often challenging in populations of the age group studied here. However, the extended follow‐up provides a comprehensive understanding of long‐term effects, even with a small sample size in each group. Future studies should include a larger sample size to clarify some of the findings. Although most water aerobics participants in Portugal are women, the absence of men in our study may limit the generalizability of the results.
The lack of diet monitoring could have influenced our findings, particularly regarding body composition and lipid profile outcomes. For example, participants with healthier diets might have experienced greater improvements in cholesterol and fat mass, while those with less healthy diets might have seen smaller benefits. Consequently, the variability in dietary habits might have contributed to the differences or lack of significant findings in some of the measured outcomes. However, it is important to note that the decision not to control diet was intentional and aligned with the pragmatic nature of our trial. Our primary aim was to assess the effects of different exercise intensities in a real‐world setting, where dietary habits typically vary among individuals. By not controlling for diet, we aimed to replicate a more naturalistic environment, reflecting how water aerobics might impact health outcomes in everyday life.
Additionally, we did not assess cardiorespiratory fitness, which would have been informative given the aerobic nature of the intervention. This limits our understanding of how different intensities of water aerobics might affect cardiovascular health in older adults. Nonetheless, we prioritized other relevant variables, such as muscle strength and power, due to the heightened risk of sarcopenia in older adults. Future studies should incorporate measures of cardiorespiratory fitness and balance, while also including both sexes, controlling for potential confounding factors, and considering the stratification of participants by baseline fitness or physical activity levels for a more comprehensive understanding of the impact of aquatic exercise. While this study provides valuable insights into the role of exercise intensity in aquatic aerobics for older adults, addressing these limitations in future research will strengthen the evidence base and enhance the applicability of the findings in real‐world settings.
In conclusion, HIG resulted in significant enhancements in lipid profile, physical quality of life, and body composition by reducing fat mass and increasing fat‐free mass, as compared to MIG. HIG also boosted upper and lower limb muscular endurance, and psychological quality of life, while reducing diastolic blood pressure. Conversely, while MIG did increase upper and lower limb endurance and psychological quality of life, it was insufficient to significantly improve body composition. Neither intensity had a meaningful effect on explosive strength. Therefore, while exercise intensity is indeed a critical training variable when designing and evaluating water aerobics programs, future studies with more diverse and larger populations, longer intervention periods, and controlled dietary intake are needed to validate and expand upon these results.
5. Perspective
The popularity of exercise in aquatic environments has increased exponentially over the last decade, with a growing number of participants. Health and sports professionals usually recommend aquatic programs such as water aerobics due to the benefits of the water environment, which can potentially improve health and physical fitness. However, some studies fail to report or monitor exercise intensity, leading to inconclusive results. Additionally, no studies have explored the differences between different intensities (i.e., moderate vs. high‐intensity) workouts in water aerobics. Results showed that both intensities of exercise improve muscular endurance and psychological quality of life. Nevertheless, higher‐intensity water aerobics can be more effective in improving body composition, lipid profile, physical quality of life, and diastolic blood pressure than moderate‐intensity exercise. Thus, the intensity of exercise is a significant factor in achieving optimal outcomes in water aerobics for adults and older adults. This study provides sports professionals with guidance on the appropriate intensity levels to achieve desired health and fitness goals. Future studies should investigate more variations in intensity (i.e., low vs. moderate vs. high intensity) and longer intervention periods to understand water aerobics trends and effects.
Author Contributions
Luís Brandão Faíl: conceptualization, data curation; formal analysis, investigation, methodology, project administration, writing–original draft. Daniel Almeida Marinho: conceptualization, formal analysis, funding acquisition, methodology, supervision, writing–review and editing. Elisa A Marques: conceptualization, investigation, methodology, supervision, writing–review and editing. Juliana Mendes Gonçalves: methodology, project administration, resources, software, writing–original draft. Maria Helena Gil: investigation, methodology, project administration, writing–original draft. Rogério Alves Pereira: investigation, methodology, project administration, software, writing–original draft. Mário Cardoso Marques: conceptualization, formal analysis, validation, visualization, writing–review and editing. Mikel Izquierdo: conceptualization, methodology, validation; visualization, writing–review and editing. Henrique Pereira Neiva: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing–review and editing.
Ethics Statement
The study protocol was approved by the Ethics Committee of the University of Beira Interior, Portugal (CE‐UBI‐Pj‐2019‐051).
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Luís Brandão Faíl affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Acknowledgments
This work was supported by National Funds by FCT ‐ Foundation for Science and Technology under the following project UIDB/04045/2020 (https://doi.org/10.54499/UIDB/04045/2020).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions. All authors have read and approved the final version of the manuscript and Henrique Pereira Neiva had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Raffaelli C., Milanese C., Lanza M., and Zamparo P., “Water‐Based Training Enhances Both Physical Capacities and Body Composition in Healthy Young Adult Women,” Sport Sciences for Health 12, no. 2 (2016): 195–207, 10.1007/s11332-016-0275-z. [DOI] [Google Scholar]
- 2. Pereira Neiva H., Brandão Faíl L., Izquierdo M., Marques M. C., and Marinho D. A., “The Effect of 12 Weeks of Water‐Aerobics on Health Status and Physical Fitness: An Ecological Approach,” PLoS One 13, no. 5 (2018): e0198319, 10.1371/journal.pone.0198319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Broman G., Quintana M., Lindberg T., Jansson E., and Kaijser L., “High Intensity Deep Water Training Can Improve Aerobic Power in Elderly Women,” European Journal of Applied Physiology 98, no. 2 (2006): 117–123, 10.1007/s00421-006-0237-2. [DOI] [PubMed] [Google Scholar]
- 4. Nikolai A. L., Novotny B. A., Bohnen C. L., Schleis K. M., and Dalleck L. C., “Cardiovascular and Metabolic Responses to Water Aerobics Exercise in Middle‐Aged and Older Adults,” Journal of Physical Activity and Health 6, no. 3 (2009): 333–338, 10.1123/jpah.6.3.333. [DOI] [PubMed] [Google Scholar]
- 5. Hall López J. A., Ochoa Martínez P. Y., Alarcón Meza E. I., Moncada‐Jiménez J. A., Garcia Bertruy O., and Martin Dantas E. H., “Programa De Entrenamiento De Hidrogimnasia Sobre Las Capacidades Físicas De Adultas Mayores/Hydrogymnastics Training Program on Physical Fitness in Elderly Women,” Revista Internacional de Medicina y Ciencias de la Actividad Física y del Deporte 66, no. 66 (2017): 283–298, 10.15366/rimcafd2017.66.005. [DOI] [Google Scholar]
- 6. Tsourlou T., Benik A., Dipla K., Zafeiridis A., and Spiros K., “The Effects of a Twenty‐Four‐Week Aquatic Training Program on Muscular Strength Performance in Healthy Elderly Women,” Journal of Strength and Conditioning Research 38 (2020): 1422–1444. [DOI] [PubMed] [Google Scholar]
- 7. Moreira O. C., Lopes G. S., de Matos D. G., et al., “Impact of Two Hydrogymnastics Class Methodologies on the Functional Capacity and Flexibility of Elderly Women,” The Journal of Sports Medicine and Physical Fitness 59, no. 1 (2018): 126–131, 10.23736/S0022-4707.17.07872-0. [DOI] [PubMed] [Google Scholar]
- 8. Kantyka J., Herman D., Roczniok R., and Kuba L., “Effects of Aqua Aerobics on Body Composition, Body Mass, Lipid Profile, and Blood Count in Middle‐Aged Sedentary Women,” Human Movement 16, no. 1 (2015): 9–14, 10.1515/humo-2015-0020. [DOI] [Google Scholar]
- 9. Pöyhönen T., Sipilä S., Keskinen K. L., Hautala A., Savolainen J., and Mlki E., “Effects of Aquatic Resistance Training on Neuromuscular Performance in Healthy Women,” Medicine & Science in Sports & Exercise 34, no. 12 (2002): 2103–2109, 10.1097/00005768-200212000-00036. [DOI] [PubMed] [Google Scholar]
- 10. Colado J. C., Tella V., Triplett N. T., and González L. M., “Effects of a Short‐Term Aquatic Resistance Program on Strength and Body Composition in Fit Young Men,” Journal of Strength and Conditioning Research 23, no. 2 (2009): 549–559, 10.1519/JSC.0b013e31818eff5d. [DOI] [PubMed] [Google Scholar]
- 11. Benelli P., Ditroilo M., and De Vito G., “Physiological Responses to Fitness Activities‐A Comparison Between Land‐Based and Water Aerobics Exercise,” Journal of Strength and Conditioning Research 18, no. 4 (2004): 719–722, 10.1519/00124278-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 12. Faíl L. B., Marinho D. A., Marques E. A., et al., “Benefits of Aquatic Exercise in Adults With and Without Chronic Disease—A Systematic Review With Meta‐Analysis,” Scandinavian Journal of Medicine & Science in Sports 32, no. 3 (2022): 465–486, 10.1111/sms.14112. [DOI] [PubMed] [Google Scholar]
- 13. Jasiński R., Socha M., Sitko L., Kubicka K., Woźniewski M., and Sobiech K. A., “Effect of Nordic Walking and Water Aerobics Training on Body Composition and the Blood Flow in Lower Extremities in Elderly Women,” Journal of Human Kinetics 45, no. 1 (2015): 113–122, 10.1515/hukin-2015-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alves R. V., Mota J., Costa M. C., and Alves J. G. B., “Aptidão Física Relacionada À Saúde De Idosos: Influência Da Hidroginástica,” Revista Brasileira de Medicina do Esporte 10, no. 1 (2004): 31–37, 10.1590/s1517-86922004000100003. [DOI] [Google Scholar]
- 15. Beaudart C., Dawson A., Shaw S. C., et al., “Nutrition and Physical Activity in the Prevention and Treatment of Sarcopenia: Systematic Review,” Osteoporosis International 28 (2017): 1817–1833, 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buch A., Carmeli E., Boker L. K., et al., “Muscle Function and Fat Content in Relation to Sarcopenia, Obesity and Frailty of Old Age — An Overview,” Experimental Gerontology 76 (2016): 25–32, 10.1016/j.exger.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 17. Garrido N., Silva J. D. P., Novaes J. S., Cirilo‐Sousa M. S., and Neto G. R., “Effect of Water Aerobics on the Quality of Life, Satisfaction, and Perception of Body Image Among Elderly Women,” Journal of Exercise Physiology Online 19, no. 5 (2016): 30–37. [Google Scholar]
- 18. Lewington S., Clark R., Qizilbash N., Peto R., and Collins R., “Mortality: A Meta‐Analysis of Individual Data for One Million Adults in 61 Prospective Studies,” Lancet 360, no. 9349 (2002): 1903–1913. [DOI] [PubMed] [Google Scholar]
- 19. Magalhães M. E. C., “New Cholesterol Targets of SBC Guidelines on Dyslipidemia,” International Journal of Cardiovascular Sciences 30, no. 6 (2017): 466–468, 10.5935/2359-4802.20170090. [DOI] [Google Scholar]
- 20. Sewo Sampaio P. Y. and Ito E., “Activities With Higher Influence on Quality of Life in Older Adults in Japan,” Occupational Therapy International 20, no. 1 (2013): 1–10, 10.1002/oti.1333. [DOI] [PubMed] [Google Scholar]
- 21. Takeshima N., Rogers M. E., Watanabe E., et al., “Water‐Based Exercise Improves Health‐Related Aspects of Fitness in Older Women,” Medicine & Science in Sports & Exercise 34, no. 3 (2002): 544–551, 10.1097/00005768-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 22. Graef F. I. and Kruel L. F. M., “Freqüência Cardíaca E Percepção Subjetiva Do Esforço No Meio Aquático: Diferenças Em Relação Ao Meio Terrestre E Aplicações Na Prescrição Do Exercício ‐ Uma Revisão,” Revista Brasileira de Medicina do Esporte 12, no. 4 (2006): 221–228, 10.1590/s1517-86922006000400011. [DOI] [Google Scholar]
- 23. Gellish R. L., Goslin B. R., Olson R. E., McDonald A., Russi G. D., and Moudgil V. K., “Longitudinal Modeling of the Relationship Between Age and Maximal Heart Rate,” Medicine & Science in Sports & Exercise 39, no. 5 (2007): 822–829, 10.1097/mss.0b013e31803349c6. [DOI] [PubMed] [Google Scholar]
- 24. Barbosa T. M., Sousa V. F., Silva A. J., Reis V. M., Marinho D. A., and Bragada J. A., “Effects of Musical Cadence in the Acute Physiologic Adaptations to Head‐Out Aquatic Exercises,” Journal of Strength and Conditioning Research 24, no. 1 (2010): 244–250, 10.1519/JSC.0b013e3181b296fd. [DOI] [PubMed] [Google Scholar]
- 25. Costa M. J., Oliveira C., Teixeira G., Marinho D. A., Silva A. J., and Barbosa T. M., “The Influence of Musical Cadence Into Aquatic Jumping Jacks Kinematics,” Journal of Sports Science & Medicine 10, no. 4 (2011): 607–615. [PMC free article] [PubMed] [Google Scholar]
- 26. Alberton C., Tartaruga M., Pinto S., et al., “Vertical Ground Reaction Force During Water Exercises Performed at Different Intensities,” International Journal of Sports Medicine 34, no. 10 (2013): 881–887, 10.1055/s-0032-1331757. [DOI] [PubMed] [Google Scholar]
- 27. Pinto S., Cadore E., Alberton C., et al., “Cardiorespiratory and Neuromuscular Responses During Water Aerobics Exercise Performed With and Without Equipment,” International Journal of Sports Medicine 32, no. 12 (2011): 916–923, 10.1055/s-0031-1283176. [DOI] [PubMed] [Google Scholar]
- 28. Raffaelli C., Lanza M., Zanolla L., and Zamparo P., “Exercise Intensity of Head‐Out Water‐Based Activities (Water Fitness),” European Journal of Applied Physiology 109, no. 5 (2010): 829–838, 10.1007/s00421-010-1419-5. [DOI] [PubMed] [Google Scholar]
- 29. Ramírez‐Campillo R., Martínez C., De La Fuente C. I., et al., “High‐Speed Resistance Training in Older Women: The Role of Supervision,” Journal of Aging and Physical Activity 25, no. 1 (2017): 1–9, 10.1123/japa.2015-0122. [DOI] [PubMed] [Google Scholar]
- 30. Harris C., Wattles A. P., DeBeliso M., Sevene‐Adams P. G., Berning J. M., and Adams K. J., “The Seated Medicine Ball Throw as a Test of Upper Body Power in Older Adults,” Journal of Strength and Conditioning Research 25, no. 8 (2011): 2344–2348, 10.1519/JSC.0b013e3181ecd27b. [DOI] [PubMed] [Google Scholar]
- 31. Rikli R. E. and Jones C. J., “Functional Fitness Normative Scores for Community‐Residing Older Adults, Ages 60‐94,” Journal of Aging and Physical Activity 7, no. 2 (1999): 162–181, 10.1123/japa.7.2.162. [DOI] [Google Scholar]
- 32. Marfell‐Jones M., Stewart A. D., and Ridder J. H. International Standards for Anthropometric Assessment. (International Society for the Advancement of Kinanthropometry, ed.).; 2012.
- 33. Nordestgaard B. G., Langsted A., Mora S., et al., “Fasting is Not Routinely Required for Determination of a Lipid Profile: Clinical and Laboratory Implications Including Flagging at Desirable Concentration Cut‐Points ‐ A Joint Consensus Statement From the European Atherosclerosis Society and European Fede,” European Heart Journal 37, no. 25 (2016): 1944–1958, 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ridker P. M., “Fasting Versus Nonfasting Triglycerides and the Prediction of Cardiovascular Risk: Do We Need to Revisit the Oral Triglyceride Tolerance Test,” Clinical Chemistry 54, no. 1 (2008): 11–13, 10.1373/clinchem.2007.097907. [DOI] [PubMed] [Google Scholar]
- 35. Langsted A. and Nordestgaard B. G., “Nonfasting Versus Fasting Lipid Profile for Cardiovascular Risk Prediction,” Pathology 51, no. 2 (2019): 131–141, 10.1016/j.pathol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 36. American College of Sports Medicine , ACSM's Health‐Related Physical Fitness Assessment Manual, eds. Wolters Kluwer L. W. & W., 2013). 9th ed. [Google Scholar]
- 37. Serra A. V., Canavarro M. C., Simões M. R., et al., “Estudos Psicométricos Do Instrumento De Avaliação Da Qualidade De Vida Da Organização Mundial De Saúde (Whoqol‐Bref) Para Português De Portugal,” Psiquiatria Clínica 27 (2006): 41–49. [Google Scholar]
- 38. Kang H., “Sample Size Determination and Power Analysis Using the G*Power Software,” Journal of Educational Evaluation for Health Professions 18 (2021): 17, 10.3352/jeehp.2021.18.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen Cohen J., Statistical Power Analysis for the Behavioral Sciences Second Edition (Lawrence Erlbaum Associates, Publisher, 1998). Second edition. [Google Scholar]
- 40. Lakens D., “Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for T‐Tests and Anovas,” Frontiers in Psychology 4 (2013): 1–12, 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanitz A. C., Delevatti R. S., Reichert T., et al., “Effects of Two Deep Water Training Programs on Cardiorespiratory and Muscular Strength Responses in Older Adults,” Experimental Gerontology 64 (2015): 55–61, 10.1016/j.exger.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 42. Irandoust K. and Taheri M., “The Effects of Aquatic Exercise on Body Composition and Nonspecific Low Back Pain in Elderly Males,” Journal of Physical Therapy Science 27, no. 2 (2015): 433–435, 10.1589/jpts.27.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGinnis P. M., Biomechanics of Sport and Exercise. Third Edition. (Human Kinetics, ed.). Champaign; 2005.
- 44. Cadore E. L., Izquierdo M., Pinto S. S., et al., “Neuromuscular Adaptations to Concurrent Training in the Elderly: Effects of Intrasession Exercise Sequence,” Age 35, no. 3 (2013): 891–903, 10.1007/s11357-012-9405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Penaforte F. R. O., Calhau R., Mota G. R., and Chiarello P. G., “Impact of Short‐Term Water Exercise Programs on Weight, Body Composition, Metabolic Profile and Quality of Life of Obese Women,” Journal of Human Sport and Exercise 10, no. 4 (2015): 915–926, 10.14198/jhse.2015.104.07. [DOI] [Google Scholar]
- 46. Arca E. A., Martinelli B., Martin L. C., Waisberg C. B., and Franco R. J. S., “Aquatic Exercise Is as Effective as Dry Land Training to Blood Pressure Reduction in Postmenopausal Hypertensive Women,” Physiotherapy Research International 19, no. 2 (2014): 93–98, 10.1002/pri.1565. [DOI] [PubMed] [Google Scholar]
- 47. Sigal R. J., Kenny G. P., Boulé N. G., et al., “Effects of Aerobic Training, Resistance Training, or Both on Glycemic Control in Type 2 Diabetes: A Randomized Trial,” Annals of Internal Medicine 147, no. 6 (2007): 357–369, 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- 48. Kraus W. E., Houmard J. A., Duscha B. D., et al., “Effects of the Amount and Intensity of Exercise on Plasma Lipoproteins,” New England Journal of Medicine 347, no. 19 (2002): 1483–1492, 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 49. Viljoen J. and Christie C., “Resistance Training and Changes to Plasma Lipoproteins in Postmenopausal Women,” South African Journal of Sports Medicine 23, no. 2 (2011): 40, 10.17159/2078-516x/2011/v23i2a346. [DOI] [Google Scholar]
- 50. Kasprzak Z. and Pilaczyńska‐Szcześniak Ł., “Effects of Regular Physical Exercises in the Water on the Metabolic Profile of Women With Abdominal Obesity,” Journal of Human Kinetics 41, no. 1 (2014): 71–79, 10.2478/hukin-2014-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Terblanche E. and Millen A. M. E., “The Magnitude and Duration of Post‐Exercise Hypotension After Land and Water Exercises,” European Journal of Applied Physiology 112, no. 12 (2012): 4111–4118, 10.1007/s00421-012-2398-5. [DOI] [PubMed] [Google Scholar]
- 52. Igarashi Y. and Nogami Y., “The Effect of Regular Aquatic Exercise on Blood Pressure: A Meta‐Analysis of Randomized Controlled Trials,” European Journal of Preventive Cardiology 25, no. 2 (2018): 190–199, 10.1177/2047487317731164. [DOI] [PubMed] [Google Scholar]
- 53. Pescatello L. S., Franklin B. A., Fagard R., Farquhar W. B., Kelley G. A., and Ray C. A., “Exercise and Hypertension,” Medicine & Science in Sports & Exercise 36, no. 3 (2004): 533–553, 10.1249/01.MSS.0000115224.88514.3A. [DOI] [PubMed] [Google Scholar]
- 54. Nahimura K., Yianishi A., Komiyama M., et al., “Effects of Immersion in Different Water Temperature Before Exercise on Heart Rate, Cardiac Parasympathetic Nervous System and Rectal Temperature,” in The Book of Proceedings of the 1st International Scientific Conference of Aquatic Space Activities, eds. Nomura T. and Tskuba U. B. E. (University of Tskuba, 2008): 128–133, https://pub.uni-bielefeld.de/record/2688782. [Google Scholar]
- 55. Bergamin M., Ermolao A., Matten S., Sieverdes J. C., and Zaccaria M., “Metabolic and Cardiovascular Responses During Aquatic Exercise in Water at Different Temperatures in Older Adults,” Research Quarterly for Exercise and Sport 86, no. 2 (2015): 163–171, 10.1080/02701367.2014.981629. [DOI] [PubMed] [Google Scholar]
- 56. Ayán C., Carvalho P., Varela S., and Cancela J. M., “Effects of Water‐Based Exercise Training on the Cognitive Function and Quality of Life of Healthy Adult Women,” Journal of Physical Activity and Health 14, no. 11 (2017): 899–904, 10.1123/jpah.2017-0036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions. All authors have read and approved the final version of the manuscript and Henrique Pereira Neiva had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.


