ABSTRACT
Background and Aims
Sub‐Saharan Africa drives global HIV‐related mortality, and patients continuously present with advanced HIV disease (AHD) at diagnosis. We describe prevalence, predictors, and treatment outcomes in HIV clients with AHD.
Methods
We systematically reviewed PUBMED, SCOPUS, Web of Science, JSTOR, and CINAHL for relevant studies conducted in Sub‐Saharan Africa from 2010 to 2022. We used a narrative synthesis to describe included studies and a random effect meta‐analysis to determine AHD pooled prevalence. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist guided the reporting, while the Joanna Briggs Institute's quality assessment checklist assessed the quality of included articles. Cochrane's Q and the I 2 tests assessed heterogeneity between included studies.
Results
We included 24 studies with a sample size of 322,676. Prevalence of AHD ranged from 11.0% to 89.7% with an overall pooled prevalence of 58.7% (95% CI: 51.3%, 66.0%): 66.1% (95% CI: 58.8%, 73.4%) between 2010 and 2016, and 51.2% (95% CI: 37.7%, 64.6%) from 2017 to 2022. Predictors of AHD include male sex, older age (≥ 35), widowed or divorced, unemployment, gap in care of ≥ 12 months before antiretroviral therapy (ART) initiation, no history of HIV testing, and seeking care from a traditional healer before presenting for HIV care. Loss to follow‐up ranged from 6.7%–58.3%, while the proportion of death ranged from 1.8%–13.1%. Predictors of death were being male, advanced age (≥ 50 years), advanced clinical stages, late ART initiation, higher mean log viral load, CD4+ cell count < 50 cells/mm3 and severe anaemia.
Conclusions
The high baseline prevalence of AHD suggests the need for targeted, people‐centred HIV testing in Sub‐Saharan Africa. Country HIV programs should accelerate the implementation of comprehensive HIV services that identify clients at risk of AHD for early enrolment with systems for monitoring the WHO care package for preventing, diagnosing, and treating AHD and associated comorbid conditions.
PROSPERO number: 2022 CRD42022336487.
Keywords: advanced HIV Disease, HIV testing, mortality, opportunistic infections, people living with HIV, prevalence
1. Introduction
Sub‐Saharan African countries, with nearly two‐thirds of the global HIV burden, have made remarkable progress in responding to the HIV pandemic. In 2023, antiretroviral therapy (ART) coverage for the estimated 25.1 million people living with HIV (PLHIV) has reached 78%, while HIV‐related mortality and incidence decreased by 57% and 56% respectively, compared to 2010 [1]. Reassuring, five countries in sub‐Saharan Africa (SSA) had achieved the Joint United Nations Program on HIV/AIDS (UNAIDS) targets of 95% of all PLHIV identified, of which 95% are on ART and 95% are virally suppressed by 2023 [2].
Despite progress, HIV remains one of the leading causes of death in the region, with over 25% of deaths attributable to HIV in the five highest‐burden countries [3]. After the implementation of the 2016 World Health Organization (WHO) “test and treat” guidelines, which advocate for ART initiation regardless of the CD4 cell count, the CD4 count at HIV diagnosis has increased significantly, and the proportion of clients commencing ART with AHD has also declined [4]. Clients presenting with advanced HIV disease (AHD), defined as those with CD4 measurements < 200 cells/µL, are at higher risk of death, hospital admission and other unfavourable outcomes [5, 6, 7]. Despite widespread access to HIV testing and treatment, 20% of newly enrolled patients with a baseline CD4 test present with AHD, with CD4 cell count measurements below 200 cells/µL [8, 9].
In the context of near‐universal treatment coverage, HIV‐associated morbidity and mortality increasingly occur among ART‐experienced patients interrupting or failing treatment rather than in the relatively smaller pool of ART naive patients [10]. Hence, identifying clients with AHD at HIV diagnosis and ensuring they are managed appropriately is vital to achieving the UNAIDS 2030 targets of ending AIDS as a public health threat [11, 12]. Understanding factors for delay in health‐seeking and presentation with advanced HIV or AIDS‐defining illness is essential as HIV clients presenting with AHD are severely immune suppressed with opportunistic infections such as disseminated tuberculosis, cryptococcal meningitis, and cancers [13]. With the implementation of “test and start” guidelines, the de‐emphasis of a requirement of baseline CD4 testing for treatment initiation has resulted in lower rates of CD4 test coverage, which may impede the effective identification of clients with AHD for effective management [14].
In 2013, the “Reduction of EArly mortaLITY in HIV‐infected Adults and Children Starting Antiretroviral Therapy (REALITY)” study showed that among HIV‐infected clients with advanced immunosuppression, enhanced antimicrobial prophylaxis combined with ART resulted in reduced rates of death at both 24 and 48 weeks without compromising viral suppression or increasing toxic effects [15]. To this end, WHO recommended a package for AHD to improve survival and treatment outcomes, including Rapid ART initiation, screening and treatment of opportunistic infections, and treatment support [16]. Within the package, clients with CD4 < 100 cells/µL should also be screened for serum cryptococcal antigen to identify those who could benefit from preemptive fluconazole treatment before the onset of cryptococcal meningitis.
The prevalence of AHD at ART enrolment over time in most countries declined with greater ART availability. However, by 2015, when the WHO recommended “test and start” guidelines and strategies to facilitate earlier HIV testing and treatment were published, approximately a third of new ART clients still initiated ART late in African countries, including Mozambique, Namibia, Nigeria, Swaziland, Uganda, and Zimbabwe as well as Vietnam, and Haiti [17]. These strategies contributed to the reduction in HIV incidence. Still, due to late presentation to or disengagement from ART, AHD remains common in SSA. Therefore, there is a need to understand the predictors of AHD and design interventions to address the barriers to early and rapid ART initiation.
Reliable data on the prevalence, predictors, and outcomes of care for PLHIV who present with AHD in SSA is limited. This could be attributed to the different contexts in HIV programming across countries and frequent changes in treatment guidelines to accommodate new treatment algorithms. To address this, we aimed to describe the prevalence, predictors, and treatment outcomes in HIV clients with AHD and to identify opportunities for improving care outcomes for clients with AHD in SSA using a systematic literature review of studies from 2010 to 2022.
2. Methods
2.1. Database Search, Search Strategy, and Terms
This review and the original protocol were registered with PROSPERO (Registration Number: PROSPERO 2022 CRD42022336487) and are reported based on the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) [18]. We searched PUBMED, SCOPUS, Web of Science, JSTOR, and CINAHL with a manual internet search for studies conducted in Africa from 2010 to 2022. We searched for studies that described at least one of the following: the prevalence, predictors, or outcome of care for PLHIV enrolling in treatment. We first searched for keywords and MeSH terms from the title of the review in PUBMED, SCOPUS and Web of Science. Next, we searched the databases for articles using the different keywords and MeSH terms identified in the first step. We conducted database searches from September 7 to September 9, 2022, and search terms used for PUBMED include “((“Prevalence”” [MeSH Terms] OR ““Prevalence”” [Text Word] OR ““epidemiolog” *” [Text Word]) AND “(“Health Belief Model”” [MeSH Terms] OR ““predict” *” [Text Word] OR” “factor” *” [Text Word]) AND “(“Treatment Outcome”” [MeSH Terms] OR ““Outcomes”” [Text Word]) AND “(“advanced”” [Text Word] OR” “late present” *” [Text Word]) AND “(“HIV Infections”” [MeSH Terms] OR ““HIV disease*” [Text Word])) AND (2010:2022[pdat]). The search terms for other databases are presented in Supporting Information S1: File 1. Finally, we conducted a manual internet search and references of select articles identified from Step 2 above for additional articles for inclusion.
2.2. Study Selection
Study selection involved title/abstract screening, and full‐text reviews were conducted independently by three different reviewers before final inclusion for data extraction. All extracted articles were initially entered into EndNote (Clarivate Analytics, Philadelphia, USA) for deduplication and afterwards uploaded into Rayyan software (https://www.rayyan.ai/) for title abstract screening using prespecified eligibility criteria [19]. Rayyan software enables double‐blinded independent review of articles (title/abstract and full text) to limit bias in selected articles. Three reviewers (S.A., V.W., T.M.) independently conducted the title/abstract screening and conflicts were resolved by two other reviewers (S.H., S.O.). The full text of articles from the title/abstract screening considered for full‐text review was retrieved, uploaded into Rayyan and matched to their respective titles. Two reviewers conducted the full‐text review, and a third (A.M.) resolved conflicts. Articles not meeting the inclusion criteria at both stages and those without the full text were excluded from the review. We could not retrieve the full text for three articles, and the corresponding authors have yet to respond to emails requesting the articles.
2.3. Eligibility Criteria
Articles included in the review were original studies (case‐control, cohort studies, cross‐sectional studies, and clinical trials) conducted in SSA (African countries south of the Sahara desert) and published from 2010 to 2022. Additional inclusion criteria were: (a) study participants should be aged ≥ 15 years with a positive HIV status regardless of clinical status, (b) studies should specifically indicate participants were enrolled as they were commencing ART or entering care, and (c) studies should have data on baseline CD4 and/or WHO staging or predictors of AHD. We excluded articles conducted outside Africa, those outside the specified study period, other study designs (case reports, letters to the editor, review papers, and opinion papers), and those including pregnant women and children as study participants. Articles that included population‐level analysis, regardless of when the included participants commenced ART or entered, were excluded.
2.4. Data Extraction
A standardised data extraction form was developed in an Excel spreadsheet (Supporting Information S2: File 2). The reviewers validated this extraction form to address all vital information relevant to the review questions. Two reviewers (V.W., T.M.) independently extracted data from the selected articles. Studies selected for data extraction were assigned similar but different codes before extraction to enable comparison at the end of data extraction, e.g., codes 1A and 1B were assigned to the same article by the two reviewers. At the end of data extraction, the two spreadsheets were reviewed, and conflicts were resolved before merging to form the final spreadsheet for analysis.
2.5. Quality Assessment of Included Studies
Two reviewers (VW and TM) independently conducted the quality assessment of the studies included in this review using the Joana Briggs Institute's critical appraisal tool for case series [20] (Supporting Information S1: File 3). We used the tool for case series since, in all our included studies, the participants all had the same clinical condition (HIV cases), and the outcomes considered were those related to HIV [21]. The appraisal tool is a 10‐item checklist, and articles were graded as good (score of 8–10), fair (score of 5–7) and poor (score of 1–4). Conflicts in assigned scores were resolved by discussion with a third reviewer (AM).
2.6. Summary Measures and Statistical Analyses
We used a narrative synthesis approach to describe the methodology and quality of included studies. The study population's three outcomes—the prevalence, predictors, and treatment outcomes—were described separately. As the prevalence summarises the proportion of AHD in the study population, we first summarised the prevalence from the individual study. Then, we conducted a meta‐analysis to estimate the pooled prevalence from all the studies using the Stata command metaprop and the random‐effects model. We also assessed pooled prevalence by the African region (West, East, or Southern African Region) the study was conducted in, the year or timeline the study was published divided into two categories (2010–2016 and 2017–2022) and assessed the effect of female sex and study sample size on AHD prevalence. To assess between‐study heterogeneity for the pooled prevalence, we estimated I 2 statistics expressed as a percentage and Cochrane's Q statistic (p < 0.05) [22]. We assessed the risk of bias across studies using funnel plots and the Eggers test.
For the studies that described the predictors of AHD, we identified and described the different predictors and also used the forest plot to describe adjusted odd ratios of all the predictors of AHD. The risk ratio reported in one study was recomputed to derive the odds ratio for consistency [23]. Subgroup analysis was conducted to describe the predictors based on the participant's age, sex, marital status, and some clinical parameters.
3. Results
We extracted 967 articles from the different databases and included 626 articles for the title/abstract screening after excluding duplicates (Figure 1). After title/abstract screening, 151 articles met the inclusion criteria for the full‐text review, including 16 additional articles identified through a manual internet search. Following the full‐text review, 24 articles [17, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45] which met the inclusion criteria were selected for synthesis. One hundred and twenty‐seven articles were excluded.
Figure 1.
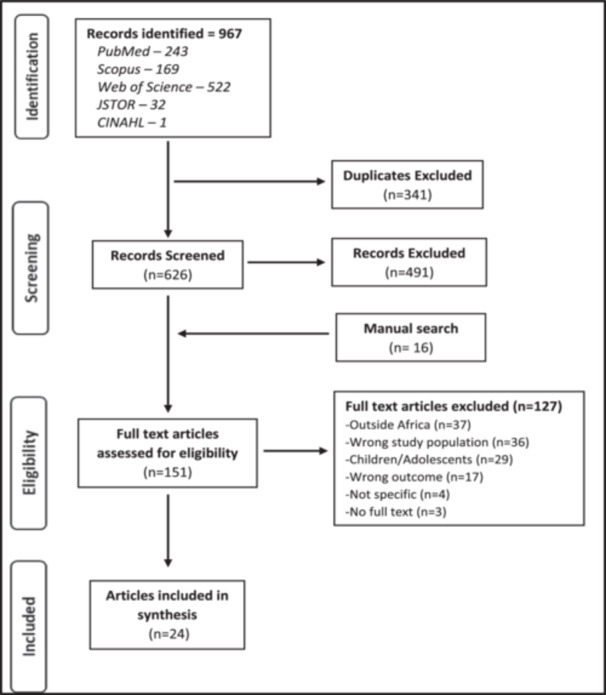
PRISMA flow chart for included studies.
3.1. Description of Included Studies
Table 1 describes the studies included in this review. They were from 16 African countries, including Botswana, which had four studies; South Africa and Mozambique, which had three studies each; Tanzania, Ethiopia, Senegal, and Rwanda, which had two studies each; and Uganda, Malawi, Cote d' Ivoire, Lesotho, Nigeria, Eswatini, Guinea Bissau, and Cameron, which each had one study.
Table 1.
Description of studies included in the review.
| First Authors Surname, Year, Country | Year patients were seen | Aim of the study | Study design | Study population | Sample size (N) | Female (%) | Age (median/IQR) |
|---|---|---|---|---|---|---|---|
| May et al. 2010 [25]; Cote d' Ivoire, South Africa, Malawi | 2004–2007 | To estimate the probability of death in patients commencing antiretroviral therapy in sub‐Saharan Africa. | Cross‐sectional | Adults starting ART. ≥ 15 years | 11,153 | 68 | 34 (29, 41) |
| Auld et al. 2011 [17]; Mozambique | 2004–2007 | To investigate mortality rates, attrition (death, loss to follow‐up, or treatment cessation), immunologic treatment failure, regimen‐switch, and determinants of selected outcomes in patients commencing ART treatment. | Retrospective cohort | Adults initiating ART ≥ 15 years | 2596 | 62 | 34 (28, 42) |
| Mills et al. 2011 [26]; Uganda | 2004–2010 | To determine if men have different treatment outcomes compared to women. | Prospective cohort | Adults initiating ART. ≥ 15 years | 22,315 | 69 | |
| Wandeler et al. 2012 [27]; Zimbabwe, Mozambique, Lesotho | 2005–2010 | To describe risk factors for no follow‐up, LTFU and mortality in patients commencing ART treatment. | Prospective cohort | New patients starting ART. ≥ 15 years. Mainly rural—90% of farmers | 7725 | 65 | 38 (32, 46) |
| Cornell et al. 2012 [28]; South Africa | 2002–2009 | To examine the magnitude and risk factors for gender differences in mortality for ART clients. | Prospective cohort | ART‐naive adults starting ART. ≥ 15 years | 46,201 | 65 | 35 (30, 42) |
| Chalamilla et al. 2012 [29]; Tanzania | 2004–2008 | To describe mortality and immunological outcomes and response to ART treatment, and determine the predictors of mortality in newly enrolled ART clients. | Prospective cohort | New HIV clients starting ART. ≥ 15 years | 12,842 | 66 | 36 (31‐43) |
| Fatti et al. 2014 [30]; South Africa | 2004–2010 | To compare baseline features, clinical, virologic and immunologic outcomes of older versus young adults commencing ART. | Retrospective cohort | ART‐naive clients starting ART. ≥ 25 years | 90,071 | 66 | 59 (57, 62) 35 (31, 41) |
| Aliyu et al. 2014 [31]; Nigeria | 2009–2011 | To describe the predictors of delayed ART initiation and trends in mortality and retention in a cohort of patients initiating ART. | Retrospective cohort | ART‐naive clients starting ART. ≥ 15 years | 869 | 64 | 34 (28, 40) |
| Melaku et al. 2015 [32]; Ethiopia | 2006–2011 | To describe trends in characteristics and outcomes in adults starting ART treatment and outcomes after enrolment in care and ART initiation. | Retrospective cohort | ART‐naive adults starting ART. ≥ 15 years | 53,300 | 59 | |
| Mhozya et al. 2015 [33]; Tanzania | 2013 | To determine the proportion of HIV patients presenting with WHO clinical stages 3 and 4 diseases and the level of immunity at the time of enrolment. | Cross‐sectional | ART‐naive adults starting ART. ≥ 15 years | 366 | 64.2 | 36 (30, 43) |
| Mutimura et al. 2015 [34]; Rwanda | 2003–2012 | Describe barriers and enablers to timely ART initiation and compare ART enrolment before and after guideline changes. | Cross‐sectional | ART‐naive adults starting ART. ≥ 15 years | 4486 | 60 | 35 (29, 42) |
| Hønge et al. 2016 [35]; Guinea‐Bissau | 2004–2014 | To describe patients presenting late for ART care and those with AHD, identify risk factors and evaluate patient outcomes at an HIV clinic in Guinea‐Bissau. | Retrospective cohort | ART patients newly enrolled into care. ≥ 15 years | 3720 | 67 | 36 (29, 45) |
| Naidoo et al. 2017 [36]; South Africa | 2004–2013 | To describe the users of the current HIV treatment services. | Retrospective cohort | ART‐naive adults starting ART. ≥ 15 years | 4043 | 59 | 32 (28, 36) |
| Gunda et al. 2017 [37]; Tanzania | 2004–2008 | To determine the proportion and risk factors of poor immune recovery in adult HIV patients. | Retrospective cohort | ART‐naive adults starting ART. ≥ 18 years | 734 | 67 | 39 (21, 60) |
| Gesesew et al. 2018 [38]; Ethiopia | 2003–2015 | To determine the prevalence, trends, outcomes, and risk factors for late presentation to HIV care. | Retrospective cohort | HIV‐infectedadult patients aged ≥ 15 years enrolled in ART care | 4900 | 59.8 | 30 (15, 81) |
| Luma et al. 2018 [39]; Cameroon | 1996–2014 | To describe late presentation for HIV care, associated factors and outcomes of patient follow‐up. | Retrospective cohort | Adults newly enrolled in ART care. ≥ 18 years | 1886 | 45 | Mean 40 (10) |
| Benzekri et al1; 2019 [40]; Senegal | 2017–2018 | To determine the frequency and predictors of traditional healer use, and to determine if traditional healer use is associated with HIV outcomes among people living with HIV. | Prospective study | Adults living with HIV commencing treatment. | 157 | 70 | 37 (30, 45) |
| Lifson et al. 2019 [23]; Botswana | 2015–2017 | To describe baseline clinical characteristics of adults presenting late to HIV care with advanced HIV disease. | Cross‐sectional | Adults newly enrolled in care. ≥ 18 years | 1799 | 59 | 34 |
| Mupfumi et al. 2019 [41]; Botswana | 2011–2015 | To determine the incidence of TB in the national database of HIV clients receiving care | Retrospective cohort | Adults newly enrolled in care. ≥ 18 years | 45,729 | 65 | 37 (31, 45) |
| Benzekri et al2; 2019 [42]; Senegal | 2017–2018 | To determine the prevalence and predictors of advanced disease among individuals initiating ART and to evaluate adherence to the WHO guidelines for managing advanced HIV disease. | Cross‐sectional | People living with HIV (PLHIV). ≥ 18 years (Included 6 participants < 18) | 198 | 70 | 35 |
| Lebelonyane et al. 2020 [24]; Botswana | 2018 | To determine the proportion of HIV‐positive individuals presenting for HIV care with AHD, describe factors associated with AHD, and assess clinical outcomes using baseline CD4 count. | Cross‐sectional | Clients newly linked to ART 16–64‐year‐old, new and old HIV+ cases not on ART | 2499 | 62.9 | 36 (29, 44) |
| Kerschberger et al. 2020 [43]; Eswatini | 2014–2016 | To assess different associations with treatment outcomes under Treat‐All and the concurrent national standard of care (SOC) at the time and to compare programmatic outcomes between the two interventions. | Prospective cohort | HIV‐positive patients initiated on 1st line ART. ≥ 16‐year‐old | 3170 | 71.3 | 31 (25, 38) |
| Magro et al. 2021 [44]; Mozambique | 2017 | To describe factors associated with early loss to follow‐up, retention in care and ART adherence during the first year of follow‐up. | Cross‐sectional | New ART patients starting treatment. ≥ 14 years | 960 | 73 | 36 (28, 75) |
| Musengimana et al. 2022 [45]; Rwanda | 2016–2018 | To determine the prevalence of AHD in patients presenting for care and compare clinical outcomes between patients. | Retrospective cohort | Patients newly enrolled in care aged ≥ 15 years | 957 | 66 |
Abbreviation: ART, antiretroviral therapy.
The studies aimed at describing characteristics of PLHIV commencing ART, late presentation to care, and treatment outcomes. Two of the studies were multi‐country studies conducted in three countries. About half of the included studies used a retrospective cohort design (N = 11), with the rest using either a cross‐sectional study design (N = 7) or a prospective cohort design (N = 6). The study population consisted of PLHIV who were newly enrolled on HIV care or were ART naive, mostly aged ≥ 15 years, regardless of sex. One of the studies [27] focused on PLHIV from a rural location. The sample size of included studies ranged from 157 to 90,071 with a combined total sample size of 322,676 PLHIV. Except for one study [39], ≥ 60% of study participants across the different studies were females, and the median age of study participants was 35 years. Most studies included in the review had a satisfactory quality assessment, with 19 studies having a good score across all areas assessed and five studies with a fair score in one or two assessed components. No study had a poor‐quality score, and no study was excluded based on the quality assessment. The performance of each study based on the different assessment parameters is available in Supporting Information S1: File 4.
3.2. Prevalence of AHD (by Sex, Age)
The prevalence of AHD at baseline in the included studies ranged from 11% to 89.7%. It was primarily determined using the cut‐off baseline CD4 count < 200/mm3 or the presence of WHO stage 3 or 4 diseases at baseline (Table 2). The trend of AHD prevalence declined overall when the studies were ranked by year from 2010 to 2022 (Figure 2). The proportion of participants from the included studies with a baseline CD4 < 200/mm3 ranged from 17.2% to 88%, while the proportion with WHO Stage 3 or 4 diseases ranged from 11% to 85%; the most common Stage 3 or 4 conditions was tuberculosis.
Table 2.
Prevalence of AHD in included studies.
| First authors surname, year, country | Number with CD4 | Number with CD4 < 200 | % CD4 < 200 | % WHO Stage 3/4 | Stage 3/4 defining disease | Duration of follow‐up (months) | AHD Prevalence |
|---|---|---|---|---|---|---|---|
| May et al. 2010 [25]; Cote d' Ivoire, South Africa, Malawi | 11,153 | 9471 | 84.9% | 85.0% | Severe anaemia 37% | 12 | 85% |
| Auld et al. 2011 [17]; Mozambique | 2596 | 1500 | 57.8% | 53.0% | TB 11% | Median 1.3 years (0.7, 2.2) | 57% |
| Mills et al. 2011 [26]; Uganda | 18,498 | 9804 | 53.0% | 42.0% | TB 5% | Median months 32 (20, 47) | 53% |
| Wandeler et al. 2012 [27]; Zimbabwe, Mozambique, Lesotho | — | — | 70.4% | — | 36 | 70.4% | |
| Cornell et al. 2012 [28]; South Africa | 39,990 | 35,191 | 88.0% | 79.0% | 36 | 88.0% | |
| Chalamilla et al. 2012 [29]; Tanzania | 8655 | 6855 | 78.9% | 79.2% | TB 13.4% | 36 | 79.2% |
| Fatti et al. 2014 [30]; South Africa | 90,071 | 63,410 | 70.0% | 47.0% | — | 60 | 70.0% |
| Aliyu et al. 2014 [31]; Nigeria | 790 | 529 | 61.0% | 67.0% | — | 67.0% | |
| Melaku et al. 2015 [32]; Ethiopia | 44,132 | 28,686 | 59.0% | 65.0% | — | 36 | 65.0% |
| Mhozya et al. 2015 [33]; Tanzania | 243 | 153 | 41.8% | 62.6% | — | — | 63.0% |
| Mutimura et al. 2015 [34]; Rwanda | 4486 | 158 | 21.0% | 27.0% | — | — | 46.0% |
| Hønge et al. 2016 [35]; Guinea‐Bissau | 3720 | 1810 | 49.0% | — | — | 120 | 48.0% |
| Naidoo et al. 2017 [36]; South Africa | 4043 | 2978 | 74.0% | 11.0% | TB 4% | 72 | 74.0% |
| Gunda et al. 2017 [37]; Tanzania | 723 | 545 | 75.4% | 57.4% | 48 | 75.4% | |
| Gesesew et al. 2018 [38]; Ethiopia | 4900 | 3607 | 73.6% | 54.3% | TB 28% | 49 | 36.5% |
| Luma et al. 2018 [39]; Cameroon | 1324 | 1188 | 63.7% | 58.2% | TB 21% | 89.7% | |
| Benzekri et al1; 2019 [40]; Senegal | 128 | 83 | 52.8% | 65.0% | — | 12 | 65.0% |
| Lifson et al. 2019 [23]; Botswana | 1559 | 554 | 39.0% | 41.0% | — | 60.0% | |
| Mupfumi et al. 2019 [41]; Botswana | 20,509 | 7166 | 35.0% | — | TB 2% | 20 (6, 37) | 35.0% |
| Benzekri et al. 2019 [42]; Senegal | 144 | 102 | 55.0% | 53.0% | — | 71.0% | |
| Lebelonyane et al. 2020 [24]; Botswana | 2499 | 430 | 17.2% | — | — | 12 | 17.2% |
| Kerschberger et al. 2020 [43]; Eswatini | 2782 | 943 | 29.7% | 13.8% | TB 7% | 36 | 33.9% |
| Magro et al. 2021 [44]; Mozambique | 893 | 416 | 46.6% | 14.8% | — | — | 46.6% |
| Musengimana et al. 2022 [45]; Rwanda | 555 | 61 | 58.0% | — | — | 18 | 11.0% |
Figure 2.
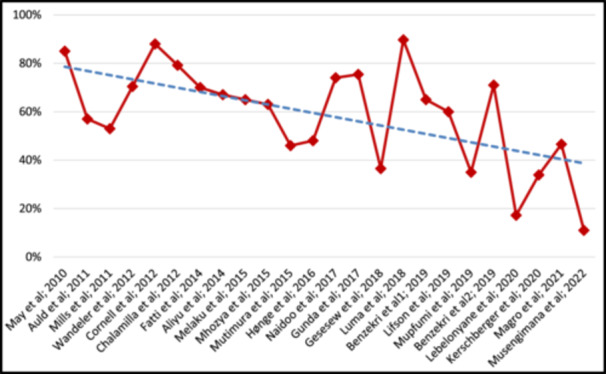
Prevalence of AHD ranked by year of study publication (2010 to 2022).
Presentation with CD4 < 200/mm3 or WHO Stage 3 or 4 diseases were more common amongst males compared to females [23, 24, 28, 30, 44], and males and other clients with baseline CD4 < 200/mm3 had higher attrition rates during follow‐up [24]. Similarly, clients who presented late with lower baseline CD4 < 200/mm3 had a lower median CD4 at 12 months than those who presented early [39].
3.2.1. Pooled Prevalence of ADH
The pooled summary of AHD prevalence from the different studies is presented in Figure 3. The prevalence ranged from 10.9% (95% CI: 8.5%, 13.9%) to 89.7% (95% CI: 88.0%, 91.3%), with an overall pooled prevalence of 58.7% (95% CI: 51.3%, 66.0%). The estimated I 2 was 99.95%, p < 0.01. A pooled prevalence of studies by major African regions (West, East and Southern Africa) was estimated and is described in Figure 4a. The pooled prevalence was 57.8% (95% CI: 45.4%, 70.2%), 54.4% (95% CI: 44.5%, 64.2%) and 68.2% (95% CI: 46.9%, 89.5%) in the Southern, East and West African region respectively. When assessed by timeline (Figure 4b ) , for the period 2010–2016, the pooled prevalence was 66.1% (95% CI: 58.8%, 73.4%) and 51.2% (95% CI: 37.7%, 64.6%) from 2017 to 2022. The heterogeneity test for between‐study effect showed the different studies were heterogenous (Q statistic = 15.55; p < 0.01), while between‐group differences indicated the pooled prevalence by region and timeline was homogenous (Q statistic = 1.36; p = 0.507 and Q statistic = 3.67; p = 0.06, respectively). An assessment of the effect of the female sex on AHD prevalence showed a negative correlation. Still, it was not statistically significant (p = 0.2850), while the study sample size had a positive correlation with AHD prevalence, though it was not also statistically significant (p = 0.287) (Supporting Information S1: File 5a and 5b). A visual assessment of the funnel plot for small study effects showed well‐dispersed estimates without any clear aggregation area around the pooled estimate (Figure 5). An evaluation of the funnel plot with the Eggers test showed no evidence of a small study effect, p = 0.51.
Figure 3.
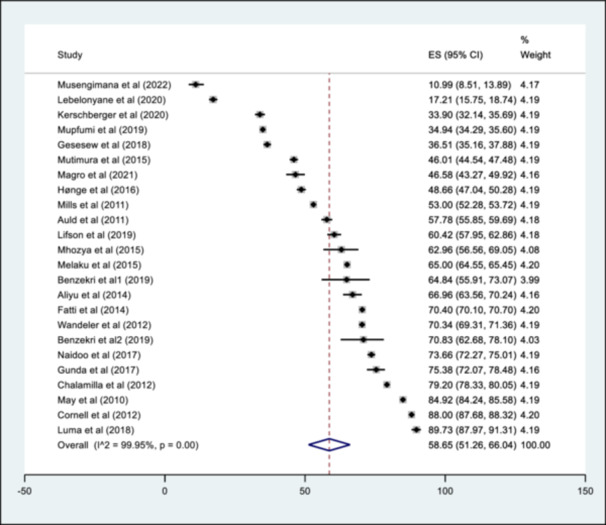
Forest plot showing the pooled summary of AHD Prevalence.
Figure 4.
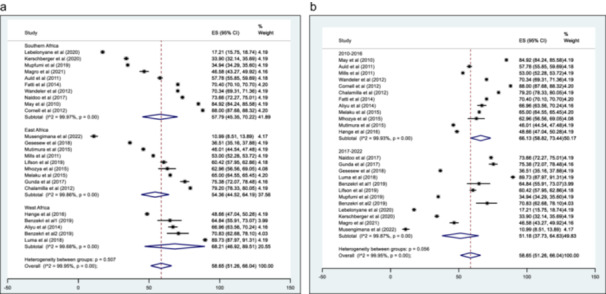
a: Pooled prevalence of AHD by African region (Southern, East and West Africa) b: Pooled prevalence of AHD by Timeline (2010–2016 and 2017–2022).
Figure 5.
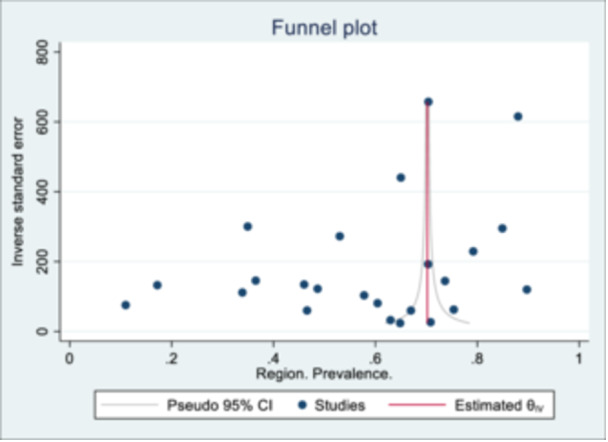
Funnel plot to assess for small study effects (Eggers test: p = 0.510).
3.3. Predictors of AHD
Of the 24 studies included in this review, eight provided information on the predictors of AHD [23, 24, 34, 35, 38, 39, 40, 45] (Table 3). Overall, the male sex compared to females [23, 24, 34, 35]; older age (≥ 35 years) compared to younger age [23, 24, 35, 38, 40]; single, widowed, or divorced compared to living with a partner [24, 35]; unemployment [23]; gap in care of ≥ 12 months before ART initiation [34]; TB co‐infection [38]; no history of HIV testing, mobile HIV testing and a new HIV diagnosis [24, 38]; and seeking care from a traditional healer before presenting to HIV care [42] had increased odds for AHD while having a partner and living with a partner [34, 39]; younger adults and being a student [23, 38] and PMTCT/Blood donation [39] had reduced odds for AHD. Seven studies with effect measures (adjusted odd ratio) are summarised in a forest plot (Figure 6). A complete meta‐analysis was not feasible as few studies showed a small study effect characterised by an asymmetrical funnel plot and significant Eggers test (Supporting Information S1: File 6).
Table 3.
Predictors of AHD from the different studies.
| First authors surname, year, country | Predictor 1 (OR/RR/HR with 95% CI) | Predictor 2 (OR/RR/HR with 95% CI) | Predictor 3 (OR/RR/HR with 95% CI) |
|---|---|---|---|
| Mutimura et al. 2015 [34]; Rwanda | Male sex (aOR = 1.66, 95% CI: 1.34–2.05). | Older age: (aOR 36–45 vs. < 25 = 1.68, 95% CI: 1.11–2.56, and aOR 46–55 vs. < 25 = 2.26, 95% CI: 1.20–4.30) | Gap in care of 12 or more months before ART initiation (aOR = 5.23, 95% CI: 1.23–21.13). |
| Living with a partner versus married=0.75, 95% CI: 0.58–0.98 | |||
| Unknown eligibility versus eligible for ART aOR = 0.16, 95% CI: 0.04–0.63. | |||
| Hønge et al. 2016 [35]; Guinea‐Bissau | Male gender (aOR 1.49), single versus married (aOR 1.30), | age 30–49 years (aOR 1.66), age = 50 years (aOR 1.48), | Fula ethnicity (aOR 1.47) Mandinga ethnicity (aOR 2.04). |
| Gesesew et al. 2018 [38]; Ethiopia | Older adults aged between 25–< 50 years (aOR = 0.4, 95% CI: 0.3–0.6) and 50+ years (aOR = 0.4, 95% CI: 0.2–0.6 | Females were 20% high likely (aOR = 1.2, 95% CI: 1.03–1.5) to present late for HIV care than their comparator. | No previous history of HIV testing (aOR = 1.2, 95% CI: 1.1–1.4) was a risk factor for LP |
| TB/HIV co‐infection (aOR = 1.6, 95% CI: 1.09–2.1 | HIV care enrolment period in 2012 and after (aOR = 0.8, 95% CI: 0.7–0.9) was a protective factor for LP | ||
| Luma et al. 2018 [39]; Cameroon | PMTCT/blood donation (OR = 0.16, 95% CI 0.10–0.29), | Students (Unemployed) had lower odds of presenting late compared to people who had employment (OR = 0.50, 95%CI = 0.26–0.98). | Calendar time OR = 1.64, 95% CI = 1.08–2.48 for ≥ 2010 versus < 2005) increased the odds of late presentation. |
| Having a positive partner (OR = 0.16, 95% CI = 0.10–0.26), and routine screening (OR = 0.13, 95% CI = 0.10–0.19) reduced the odds of presenting late compared to clinical suspicion. | |||
| Lifson et al. 2019 [23]; Botswana | Males were associated with advanced HIV disease (adjusted RR = 1.20, 95% CI, 1.10–1.31, p < 0.001) | Unemployment (adjusted RR = 1.28, 95% CI, 1.15–1.42, p < 0.001) | ≤ 25 years of age were less likely to have advanced HIV disease (adjusted RR = 0.83, 95% CI, 0.74–0.94, p = 0.001) |
| Benzekri et al.2 2019 [42]; Senegal | Age ≥ 35 (OR 5.80, 95% CI 2.35–14.30) | Having sought care from a traditional healer before presentation at a health facility (OR 3.86, 95% CI 1.17–12.78) | |
| Lebelonyane et al. 2020 [24]; Botswana | Males [23.7 vs. 13.4%, adjusted odds ratio (aOR) 1.9, 95% CI 1.5–2.3]. | Increasing age [9.1% of those < 25 years, 15.0% of those aged 25–34 years, and 20.4% of those> 35 years; aOR relative to the < 25 years category of 1.5 (95% CI 1.0–2.4) and 2.2 (95% CI 1.4–3.2) respectively. | A new (rather than previously known) HIV diagnosis (aOR 1.3, 95% CI 1.0–1.6); |
| HIV testing through mobile rather than home‐based contact (aOR 1.3, 95% CI 1.0–1.6); | |||
| Widowed or divorced (aOR 2.6, 95% CI 1.6–4.3) | |||
| Musengimana et al. 2022 [45]; Rwanda | Older age was significantly associated with advanced HIV disease, with 39.0% of advanced HIV patients being ≥ 45 years compared to 19.0% of non‐advanced. | Be identified through inpatient settings rather than through voluntary or prenatal testing | Compared to non‐AHD patients, AHD patients were more likely to be underweight (BMI < 18.5, 20.9%. vs 9.6%) and to be severely underweight (BMI < 16, 8.6% vs. 1.5%). |
Abbreviations: HR, hazzard ratio; OR, odds ratio; RR, risk ratio.
Figure 6.
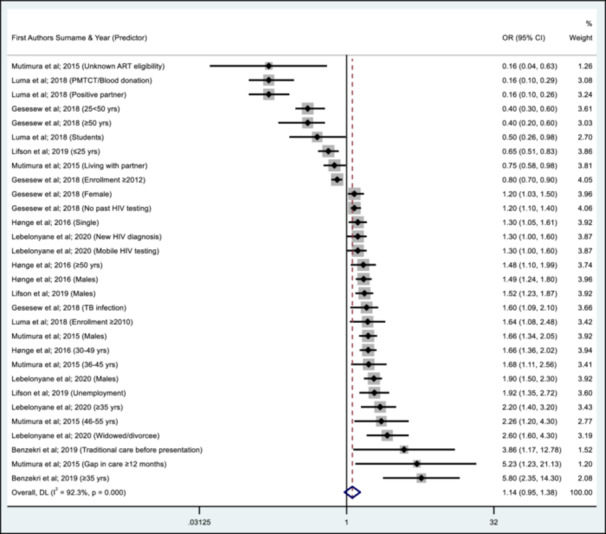
Summary of predictors of AHD from the different studies.
3.4. Treatment Outcomes
3.4.1. Retention in Care and Viral Load Suppression
The presentation of findings on final treatment outcomes for all clients in the different articles varied (Table 4). Nine studies provided information on the proportion of clients still in care (retention) at the end of the follow‐up period or the termination of the study. Retention in care ranged from 23% for a study conducted in Guinea‐Bissau [35] to 97% for a study conducted in Botswana [24]. Besides a South African study with a retention of 92% [36], the reported retention in the other studies ranged between 65%–80% [17, 27, 28, 32, 38]. Six studies had data on viral load suppression, with the highest suppression of 94% each for studies conducted in Botswana and South Africa [36, 41], followed by 87% for another study conducted in Botswana [24]. The other reported viral load suppression rates ranged from 68% to 85% [30, 31, 45]. Three studies reported treatment failure of 2.2%, 18.7%, and 19.7%, respectively [17, 43, 45].
Table 4.
Treatment outcomes by author, year and country study was conducted.
| First authors surname, year, country | In‐care % | Suppressed VL % | Unsuppressed VL % | Treatment failure | LTFU % | Transferred‐Out % | Died % |
|---|---|---|---|---|---|---|---|
| May et al. 2010 [25]; Cote d' Ivoire, South Africa, Malawi | 79.6% | — | — | — | 7.4% | 5% | 8.2% |
| Auld et al. 2011 [17]; Mozambique | 71.5% | — | — | 18.7 | 21.7% | — | 6.3% |
| Mills et al. 2011 [26]; Uganda | — | — | — | — | M‐7.5% | — | 8.7% |
| F‐ 5.9% | |||||||
| Wandeler et al. 2012 [27]; Zimbabwe, Mozambique, Lesotho | 77.1% | — | — | — | 22.9% | — | 9.6% |
| Cornell et al. 2012 [28]; South Africa | 64.7% | — | — | — | 7.9% | 10.4% | 8.5% |
| Chalamilla et al. 2012 [29]; Tanzania | — | — | — | — | — | 13.1% | |
| Fatti et al. 2014 [30]; South Africa | — | 85% | — | — | 12.8% | — | 5.9% |
| Aliyu et al. 2014 [31]; Nigeria | — | 68% | — | — | 29% | — | 3% |
| Melaku et al. 2015 [32]; Ethiopia | 70% | — | — | — | 23% | — | 9% |
| Hønge et al. 2016 [35]; Guinea‐Bissau | 23% | — | — | — | 58.3% | 4.8% | 8.7% |
| Naidoo et al. 2017 [36]; South Africa | 92% | 94% | — | — | 10% | — | 10% |
| Gunda et al. 2017 [37]; Tanzania | — | — | — | — | — | — | — |
| Gesesew et al. 2018 [38]; Ethiopia | 72.3% | — | — | — | — | — | 3.8% |
| Luma et al. 2018 [39]; Cameroon | — | — | — | — | — | — | 11.6% |
| Mupfumi et al. 2019 [41]; Botswana | — | 94% | 6% | — | — | — | |
| Lebelonyane et al. 2020 [24]; Botswana | 97% | 91% (86.6% if CD4 < 200) | 9% | — | — | — | 2.3% (4.9% if CD4 < 200) |
| Kerschberger et al. 2020 [43]; Eswatini | — | — | — | 2.2% | 24% | 3.1% | 1.8% |
| Musengimana et al. 2022 [45]; Rwanda | — | 77% | 23% | 19.7% | 16.5% | 2.8% |
Abbreviations: LTFU, loss to follow‐up; VL, viral load.
3.4.2. Loss to Follow‐Up, Transfer Out and Death
Twelve studies reported the loss to follow‐up (LTFU) rate. The lowest was 6.7% from a Ugandan study [26], while the highest was 58.3% from a study conducted in Guinea‐Bissau [35]. Three studies had an LTFU rate of < 10% [25, 26, 28] and 10%–20% [30, 36, 45], while five studies had an LTFU rate of 20%–30% [17, 27, 31, 32, 43]. Males, compared to females, weight < 45 kg, WHO stage IV, lower median CD+ count, late ART initiation and lower levels of education increased the likelihood of LTFU while pregnancy and breastfeeding and older adults were less likely to be LTFU [15, 23, 25, 27, 41].
Four studies reported the proportion of clients transferred out to be 3.1%, 4.8%, 5%, and 10.4%, respectively [25, 28, 35, 43]. Sixteen studies reported death outcomes for their clients. The lowest occurrence of death was from a study conducted in Eswatini at 1.8% [43], while the highest was 13.1% from a study conducted in Tanzania [29]. Overall, five studies recorded death rates below 5% [24, 31, 38, 43, 45]; eight studies had death rates of 5%–10% [17, 25, 26, 27, 28, 30, 32, 35], while three studies had death rates ≥ 10% [29, 36, 39].
Death as an outcome was associated with the males compared to females, advanced age (≥ 50 years), advanced clinical stages (WHO clinical Stages 3–4), late ART initiation, higher mean log viral load, CD4+ cell count < 50 cells/mm3, and severe anaemia with haemoglobin < 8.5 g/dL [17, 25, 27, 28, 29, 36, 45]. During follow‐up, two studies reported survival in males was lower than in females [26, 36], and most deaths occurred within the first 3 months of initiating treatment [26, 35, 36].
3.4.3. Opportunistic Infections (OI) and Who Stages III/IV Conditions
Eleven studies reported the occurrence of opportunistic infections (OIs), and the commonest was tuberculosis (2%– 28%), followed by severe anaemia at 37% [17, 25, 26, 29, 36, 38, 39, 40, 41, 42, 43]. Opportunistic infections were more likely to occur in males and those with lower CD4 and higher viral loads at baseline than in females and those with a higher CD4 and lower baseline viral load [26, 38, 39, 41]. Other reported OIs include bacterial pneumonia, herpes zoster, skin Kaposi sarcoma, candidiasis, cryptococcal meningoencephalitis, cerebral toxoplasmosis, tuberculous meningitis, HIV encephalitis, pulmonary aspergillosis, pulmonary Kaposi sarcoma, microsporidia, and cryptosporidiosis. The OIs were more common in clients who presented with CD4 < 200/mm3 than those with CD4 > 200/mm3 at 3, 6, and 12 months. In addition to these OIs, other reported clinical symptoms included weight loss, chronic fatigue, chronic fevers, chronic pain, chronic cough, and chronic diarrhoea. Males were more likely to report these symptoms than females (p = 0.008) [23, 40].
4. Discussion
We aimed to describe the prevalence, predictors, and treatment outcomes in HIV clients with AHD and to identify opportunities for improving care outcomes for clients with AHD in SSA in the era of accessible case identification services, immediate ART for all, and enhanced guidelines for managing AHD. We included 24 articles from 16 SSA countries in the final review. The prevalence of AHD from the studies ranged from 10.9% (95% CI: 8.5%, 13.9%) to 89.7% (95% CI: 88.0%, 91.3%) and the overall pooled prevalence was 58.7% (95% CI: 51.3%, 66.0%). The pooled prevalence in Southern, East and West African regions was 57.8% (95% CI: 45.4%, 70.2%), 54.4% (95% CI: 44.5%, 64.2%) and 68.2% (95% CI: 46.9%, 89.5%) respectively. Based on the timeline, the pooled prevalence was 66.1% (95% CI: 58.8, 73.4) for the years 2010–2016, decreasing to 51.2% (95% CI: 37.7%, 64.6%) for the years 2017–2022.
The male sex compared to females; older age compared to younger age; single, widowed or divorced; Unemployment; gap in care of ≥ 12 months before ART initiation; TB co‐infection; no history of HIV testing; mobile HIV testing; a new HIV diagnosis; and seeking care from a traditional healer before presenting to HIV care were identified as positive predictors of AHD at presentation while having a partner and being a young adult reduced the odds of AHD. The proportion of clients retained in care varied from 23% to 97%, while six studies reported a 68%–94% viral load suppression rate. The LTFU rate ranged from 6.7% to 58.3%, with significant predictive factors being male sex, presenting with WHO stage IV disease and a lower level of education. Death in the study sample population varied from 1.8% to 13.1% and was associated with male sex, advanced age at presentation, WHO stage III‐IV, high viral load and severe anaemia. Opportunistic infections were more likely to occur in males with lower baseline CD4 count and high viral load, the most common being tuberculosis.
The pooled prevalence of AHD reported in this review (58.7%) indicates approximately one in two new clients present with AHD. This prevalence is high, given the increased access to antiretrovirals. It compares with data from a multi‐country study on the prevalence of AHD where Mozambique, Namibia, Eswatini and Nigeria had a prevalence of 58%, 58%, 54%, and 60%, respectively in 2010 [17]. A South African study using laboratory data of PLHIV reported a decline in the proportion of clients entering care with AHD from 46.8% in 2004 to 35.6% in 2011, reducing slightly to 32.9% in 2016 [46]. Although we observed a decline in prevalence after commencing “test and treat” compared to the prior period, the observed prevalence is still high. It indicates a need to scale up screening of clients at risk for early diagnosis and treatment to avert possible mortality from Stage 3 and 4 diseases [16, 47]. The pooled prevalence was higher in West Africa than in other regions (East and Southern). This is counter‐intuitive as countries in Southern Africa characteristically report the highest prevalence of HIV [48, 49]. The matured HIV programs in Eastern and Southern Africa, which result in increased access to HIV testing and treatment compared to West African countries, may account for these slight differences. As expected, the prevalence of AHD characterised by different Stage 3 & 4 conditions was highest amongst males, as reported in South Africa, Botswana and Mozambique [23, 24, 28, 30, 44].
The male sex as a predictor of AHD is consistent with previous studies conducted in Guinea‐Bissau, Botswana, Rwanda and South Africa [23, 24, 34, 35]. This finding is attributed to gender norms, the limited opportunities men have to visit health facilities compared to most females who, at a certain point, have to attend ante‐natal services, and the fact most males are often employed and also have a higher likelihood of being involved in migrant work which limits their access to health services [50, 51, 52]. These structural and cultural barriers discourage health‐seeking behaviour and contribute to men's lower participation in HIV testing. Flexibility in operating hours of HIV services, multiple convenient locations, mobile testing and male‐dedicated sections at health facilities are cited as facilitating factors enhancing male participation in HIV testing [53]. Older age (≥ 35 years) as a risk factor for AHD could be attributed to waning immunity, employment, non‐adherence to treatment and age‐associated comorbidities, which increase susceptibility to complications [54, 55]. A gap in care and accessing alternative care as predictors of AHD indicates limited access and delays to effective treatment, which results in lower immunity and increased viraemia, further predisposing clients to stages 3 and 4 conditions. This necessitates recognising client health education and alternative medicine practitioners as key components of the HIV care cascade [56, 57, 58, 59, 60]. The positive impact of having a partner emphasises the importance of partner disclosure, which increases psychosocial support for clients accessing care and limits poor adherence, which may result in treatment failure and AHD [61].
Client retention in care, viral suppression rate, loss to follow‐up, transfer out, and death of HIV clients varied by study. These variations depend on several factors, including but not limited to the set‐up of the different HIV programs, location, adoption of other international treatment guidelines, various socioeconomic and psychosocial factors, and population group. A higher loss to follow‐up rate and death in males confirms existing evidence. It could be attributed to increased exposure to risks by males, migratory work, and limited access to health services, resulting in late ART initiation and poor survival compared to females [50, 51, 52]. The most common OI remained tuberculosis, although other OIs were reported and more common in males. These OIs usually define the clinical stage of clients, are sometimes difficult to diagnose depending on presentation and may result in increased hospitalisation, morbidity and death with delayed diagnosis and treatment [13, 16, 62]. There are opportunities for using human‐centred design (HCD) to improve the implementation of the WHO AHD package. HCD is about understanding human needs and how design can respond to these needs by applying the three core characteristics of HCD: understanding people, stakeholder engagement throughout the HCD process and a systems approach towards the development of new products, services, and strategies. Through HCD, individual settings will engage, segment and respond to predictors of AHD, quality of life among patients with AHD, and care outcomes [63, 64].
This systematic review presents findings on the prevalence, predictors, and treatment outcomes in clients with AHD in SSA. There is no previous review on this topic, and this review fills that gap. Findings can guide country programs as they plan for the ageing population of PLHIV served by their programs. The review was exhaustive, including five major databases and a manual Internet search, thus ensuring that relevant articles were not excluded. Finally, the review adopted a rigorous article screening and selection approach using an article screening software with a double‐blinding option to limit bias in article selection, confirmed with a standardised quality assessment for included articles.
This review has some limitations. First, the included studies were from different settings with different approaches to HIV care and treatment and focused on diverse population groups. Second, the included studies are mostly observational studies using programmatic data with limited probability sampling, reducing the quality of the evidence. Third, studies differed in their approach to recruiting clients for inclusion. For instance, some studies only assessed AHD clients, making it impossible to determine the prevalence of AHD.
In contrast, others included all clients receiving care, making it impossible to determine those with AHD at first presentation. Fourth, we may have missed studies that were not included in any database, but we tried overcoming this by conducting a manual internet search. Finally, our findings may have limited external validity as the studies did not represent all the countries in SSA. To address this, we grouped countries to indicate AHD prevalence by African region. Despite these limitations, our review provides valuable findings to guide country programs in planning care for PLHIV.
With the high AHD prevalence in all the SSA regions and our finding that clients with limited HIV testing had an increased risk of AHD, we recommend increased access to unrestricted HIV testing through any of the available testing modalities and rapid initiation of antiretroviral therapy, which enables screening for different risk factors, OIs and immune responses. To address challenges with males accessing services, country programs should engage with male PLHIV groups and address the structural (such as mobility of men) and cultural (such as gender norms and health‐seeking behaviour) factors that contribute to men's lower participation in HIV testing to identify innovative models of service delivery targeting different male sub‐population groups. Client information and health education on risks and available services can ensure clients do not drop out of care when encountering treatment‐related challenges. Given the critical roles of traditional medicine practitioners and traditional birth attendants within the communities where clients reside, country programs should have fora for frequent engagement and training of these alternative practitioners on where their care intersects with orthodox medical practice, referrals and use of aseptic techniques when attending to clients [65].
5. Conclusion
Our review indicates the prevalence of AHD in people enrolling on HIV care is still high despite the availability of different modalities of HIV testing and effective ART options. The commonest predictors of AHD were males, older age, and those with limited access to HIV testing. The high mortality in this group indicates an accelerated need for targeted HIV services delivery to increase access to HIV testing and comprehensive care for those in the high‐risk group. Further studies to understand regional‐level variations and predictors of AHD are required to guide HIV Programs in planning context‐specific interventions for clients. Additionally, country HIV programs should ensure the adoption and implementation of the WHO AHD care package with measures for its routine monitoring and evaluation to ensure that provided care is optimal and continuously meets clients' needs.
Author Contributions
S.H., S.A., and S.O. conceptualised the review. S.H., S.A., V.W., T.M., A.M., and S.O. conducted article screening, while V.W, T.M., S.A., and A.M. extracted data from the selected articles. V.W. and T.M. analysed the data, and S.H., S.A., S.O., and V.W. wrote the first draft of the manuscript. S.H., S.A., and S.O. revised the first draft, and all authors reviewed and approved the final draft.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Samson Haumba affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Supporting information.
Acknowledgments
We thank Dr. Sharon Kibwana and Ms. Fezokuhle Khumalo for reviewing the final manuscript for grammatical errors and ensuring consistency. We also thank Ms. Jilly Motsa‐Dlamini for refining and formatting the graphical abstract. Finally, we acknowledge Georgetown Global Health Institute for providing a fellowship to one of the authors (S.A.). The authors received no specific funding for this work. However, one of the authors (S.A.) is a beneficiary of the Georgetown Global Health Institute student fellows' program, which provides an opportunity for talented undergraduate and graduate students to work with faculty on research projects, participate in global health‐related events, and network with one another and scholars in the global health field. The funding agency had no role in developing the study design, collection, analysis, interpretation of data, writing of the report, and the decision to submit the report for publication.
Data Availability Statement
Data for this review is available upon reasonable request to the corresponding author.
References
- 1. Joint United Nations AIDS Program , The Urgency of Now. AIDS at a Crossroads: 2024 Global AIDS Update [Internet] (Geneva: UNAIDS, 2024), https://www.unaids.org/sites/default/files/media_asset/2024-unaids-global-aids-update_en.pdf. [Google Scholar]
- 2. Joint United Nations AIDS Program , “The Path That Ends AIDS [Internet],” (2023), https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2023/july/unaids-global-aids-update.
- 3. Frank T. D., Carter A., Jahagirdar D., et al., GBD HIV Collaborators , “Global, Regional, and National Incidence, Prevalence, and Mortality of HIV, 1980–2017, and Forecasts to 2030, for 195 Countries and Territories: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017,” Lancet HIV 6, no. 12 (August 2019): e831–e859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaniewski E., Dao Ostinelli C. H., Chammartin F., et al., “Trends in CD4 and Viral Load Testing 2005 to 2018: Multi‐Cohort Study of People Living With HIV in Southern Africa,” Journal of the International AIDS Society 23, no. 7 (2020): e25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Post F. A., Szubert A. J., Prendergast A. J., et al., “Causes and Timing of Mortality and Morbidity Among Late Presenters Starting Antiretroviral Therapy in the Reality Trial,” Supplement, Clinical Infectious Diseases 66, no. S2 (March 2018): S132–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organisation , Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach [Internet] (Geneva: WHO, 2016), https://apps.who.int/iris/handle/10665/208825. [PubMed] [Google Scholar]
- 7. Moh R., Danel C., Messou E., et al., “Incidence and Determinants of Mortality and Morbidity Following Early Antiretroviral Therapy Initiation in HIV‐Infected Adults in West Africa,” AIDS 21, no. 18 (November 2007): 2483–2491. [DOI] [PubMed] [Google Scholar]
- 8. Leeme T., Mine M., Lechiile K., et al., “Utility of CD4 Count Measurement in the Era of Universal Antiretroviral Therapy: An Analysis of Routine Laboratory Data in Botswana,” HIV Medicine 22, no. 1 (2021): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lilian R. R., Rees K., Mabitsi M., McIntyre J. A., Struthers H. E., and Peters R. P. H., “Baseline CD4 and Mortality Trends in the South African Human Immunodeficiency Virus Programme: Analysis of Routine Data,” Southern African Journal of HIV Medicine 20, no. 1 (2019): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osler M., Hilderbrand K., Goemaere E., et al., “The Continuing Burden of Advanced HIV Disease Over 10 Years of Increasing Antiretroviral Therapy Coverage in South Africa,” supplement, Clinical Infectious Diseases 66, no. S2 (March 2018): S118–S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guaraldi G., Borghi V., Milic J., et al., “The Impact of COVID‐19 on UNAIDS 90–90–90 Targets: Calls for New HIV Care Models,” Open Forum Infectious Diseases 8, no. 7 (July 2021): ofab283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frescura L., Godfrey‐Faussett P., A. Feizzadeh A., et al, “Achieving the 95 95 95 Targets for All: A Pathway to Ending Aids,” PLoS One 17, no. 8 (2022): e0272405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker S. M., Cox E., Revill P., et al., “The Cost‐Effectiveness of Prophylaxis Strategies for Individuals With Advanced HIV Starting Treatment in Africa,” Journal of the International AIDS Society 23, no. 3 (March 2020): e25469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford N., Meintjes G., Vitoria M., Greene G., and Chiller T., “The Evolving Role of CD4 Cell Counts in HIV Care,” Current Opinion in HIV and AIDS 12, no. 2 (March 2017): 123–128. [DOI] [PubMed] [Google Scholar]
- 15. Hakim J., Musiime V., Szubert A. J., et al., “Enhanced Prophylaxis Plus Antiretroviral Therapy for Advanced HIV Infection in Africa,” New England Journal of Medicine 377, no. 3 (July 2017): 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organisation , Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy [Internet] (Geneva: WHO, 2017), https://www.who.int/publications-detail-redirect/9789241550062. [PubMed] [Google Scholar]
- 17. Auld A. F., Mbofana F., Shiraishi R. W., et al., “Four‐Year Treatment Outcomes of Adult Patients Enrolled in Mozambique's Rapidly Expanding Antiretroviral Therapy Program,” PLoS One 6, no. 4 (April 2011): e18453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page M. J., Moher D., Bossuyt P. M., et al., “PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews,” BMJ 372 (March 2021): n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ouzzani M., Hammady H., Fedorowicz Z., and Elmagarmid A., “Rayyan‐a web and Mobile App for Systematic Reviews,” Systematic Reviews 5, no. 1 (December 2016): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munn Z., Barker T. H., Moola S., et al., “Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool,” JBI Evidence Synthesis 18, no. 10 (October 2020): 2127–2133. [DOI] [PubMed] [Google Scholar]
- 21. Dekkers O. M., Egger M., Altman D. G., and Vandenbroucke J. P., “Distinguishing Case Series From Cohort Studies,” Annals of Internal Medicine 156, no. 1 pt. 1 (January 2012): 37–40. [DOI] [PubMed] [Google Scholar]
- 22. Higgins J. P. T., “Measuring Inconsistency in Meta‐Analyses,” BMJ 327, no. 7414 (September 2003): 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lifson A. R., Workneh S., Hailemichael A., et al., “Advanced HIV Disease Among Males and Females Initiating HIV Care in Rural Ethiopia,” Journal of the International Association of Providers of AIDS Care 18 (January 2019): 2325958219847199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lebelonyane R., Mills L. A., Mogorosi C., et al., “Advanced HIV Disease in the Botswana Combination Prevention Project: Prevalence, Risk Factors, and Outcomes,” AIDS 34, no. 15 (2020): 2223–2230. [DOI] [PubMed] [Google Scholar]
- 25. May M., Boulle A., Phiri S., et al., “Prognosis of Patients With HIV‐1 Infection Starting Antiretroviral Therapy in Sub‐Saharan Africa: A Collaborative Analysis of Scale‐Up Programmes,” Lancet 376, no. 9739 (August 2010): 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mills E. J., Bakanda C., Birungi J., et al., “Male Gender Predicts Mortality in a Large Cohort of Patients Receiving Antiretroviral Therapy in Uganda,” Journal of the International AIDS Society 14 (November 2011): 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wandeler G., Keiser O., Pfeiffer K., et al., “Outcomes of Antiretroviral Treatment Programs in Rural Southern Africa,” Journal of Acquired Immune Deficiency Syndromes 59, no. 2 (February 2012): e9–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cornell M., Schomaker M., Garone D. B., et al., “Gender Differences in Survival Among Adult Patients Starting Antiretroviral Therapy in South Africa: A Multicentre Cohort Study,” PLoS Medicine 9, no. 9 (2012): e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chalamilla G., Hawkins C., Okuma J., et al., “Mortality and Treatment Failure Among Hiv‐Infected Adults in Dar Es Salaam, Tanzania,” Journal of the International Association of Physicians in AIDS Care 11, no. 5 (September 2012): 296–304. [DOI] [PubMed] [Google Scholar]
- 30. Fatti G., Mothibi E., Meintjes G., and Grimwood A., “Antiretroviral Treatment Outcomes Amongst Older Adults in a Large Multicentre Cohort in South Africa,” PLoS One 9, no. 6 (2014): e100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aliyu M. H., Blevins M., Parrish D. D., et al., “Risk Factors for Delayed Initiation of Combination Antiretroviral Therapy in Rural North Central Nigeria,” Journal of Acquired Immune Deficiency Syndromes 65, no. 2 (February 2014): e41–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melaku Z., Lamb M. R., Wang C., et al., “Characteristics and Outcomes of Adult Ethiopian Patients Enrolled in HIV Care and Treatment: A Multi‐Clinic Observational Study,” BMC Public Health 15 (May 2015): 462, 10.1186/s12889-015-1776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mhozya H., Bintabara D., Kibusi S., Neilson E., and Mpondo B., “Late‐Stage Disease at Presentation to an HIV Clinic in Eastern Tanzania: A Retrospective Cross‐Sectional Study,” Malawi Medical Journal 27, no. 4 (December 2015): 125–127. [PMC free article] [PubMed] [Google Scholar]
- 34. Mutimura E., Addison D., Anastos K., et al., “Trends in and Correlates of CD4(+) Cell Count at Antiretroviral Therapy Initiation After Changes in National Art Guidelines in Rwanda,” AIDS 29, no. 1 (January 2015): 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hønge B. L., Jespersen S., Aunsborg J., et al., “High Prevalence and Excess Mortality of Late Presenters Among HIV‐1, HIV‐2 and HIV‐1/2 Dually Infected Patients in Guinea‐Bissau—A Cohort Study From West Africa,” Pan African Medical Journal 25 (2016): 40, https://www.scopus.com/inward/record.uri?eid=2-s2.0-84992108978&doi=10.11604%2fpamj.2016.25.40.8329&partnerID=40&md5=b76c8386d55474066da961b04e6b7d4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Naidoo K., Hassan‐Moosa R., Yende‐Zuma N., et al., “High Mortality Rates in Men Initiated on Antiretroviral Treatment in Kwazulu‐Natal, South Africa,” PLoS One 12, no. 9 (2017): 0184124, https://www.scopus.com/inward/record.uri?eid=2-s2.0-85029439886&doi=10.1371%2fjournal.pone.0184124&partnerID=40&md5=80b2712503b53d4af7efbaeee9559fde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gunda D. W., Godfrey K. G., Kilonzo S. B., and Mpondo B. C., “Cytopenias Among Art‐Naive Patients With Advanced HIV Disease on Enrolment to Care and Treatment Services at a Tertiary Hospital in Tanzania: A Crosssectional Study,” Malawi Medical Journal 29, no. 1 (May 2017): 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gesesew H. A., Ward P., Woldemichael K., and Mwanri L., “Late Presentation for HIV Care in Southwest Ethiopia in 2003–2015: Prevalence, Trend, Outcomes and Risk Factors,” BMC Infectious Diseases 18, no. 1 (2018): 59, https://www.scopus.com/inward/record.uri?eid=2-s2.0-85041054860&doi=10.1186%2fs12879-018-2971-6&partnerID=40&md5=d01c467992c5c150d4bd88b77c7f3c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luma H. N., Jua P., Donfack O. T., et al., “Late Presentation to HIV/AIDS Care at the Douala General Hospital, Cameroon: Its Associated Factors, and Consequences,” BMC Infectious Diseases 18, no. 1 (2018): 298, https://www.scopus.com/inward/record.uri?eid=2-s2.0-85049470599&doi=10.1186%2fs12879-018-3204-8&partnerID=40&md5=384172088c0a24865c7fce27659518a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benzekri N. A., Sambou J. F., Ndong S., et al., “Prevalence, Predictors, and Management of Advanced HIV Disease Among Individuals Initiating ART in Senegal, West Africa,” BMC Infectious Diseases 19, no. 1 (March 2019): 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mupfumi L., Moyo S., Shin S. S., et al., “High Incidence of Tuberculosis in the First Year of Antiretroviral Therapy in the Botswana National Antiretroviral Therapy Programme Between 2011 and 2015,” AIDS 33, no. 15 (December 2019): 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Benzekri N. A., Sambou J. F., Ndong S., et al., “Traditional Healers, HIV Outcomes, and Mortality Among People Living With HIV in Senegal, West Africa,” AIDS 33, no. 9 (2019): 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kerschberger B., Schomaker M., Jobanputra K., et al., “HIV Programmatic Outcomes Following Implementation of the ‘Treat‐All’ Policy in a Public Sector Setting in Eswatini: A Prospective Cohort Study,” Journal of the International AIDS Society 23, no. 3 (2020): 25458, https://www.scopus.com/inward/record.uri?eid=2-s2.0-85081043053&doi=10.1002%2fjia2.25458&partnerID=40&md5=7d36c5cfda7b6b2636b46dc3256239ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Magro P., Cerini C., da Gloria A., Tembe S., Castelli F., and Tomasoni L. R., “The Cascade of Care of HIV After One Year of Follow‐Up in a Cohort of HIV‐Positive Adult Patients in Three Health Settings of Morrumbene in Rural Mozambique,” Tropical Medicine & International Health 26, no. 11 (November 2021): 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Musengimana G., Umugisha J. P., Habinshuti P., et al., “Characteristics and Clinical Outcomes of Patients Presenting With Advanced HIV Disease in the “Treat All” Era: A Retrospective Cohort Study From Rural Rwanda,” BMC Infectious Diseases 22, no. 1 (August 2022): 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carmona S., Bor J., Nattey C., et al., “Persistent High Burden of Advanced HIV Disease Among Patients Seeking Care in South Africa's National HIV Program: Data From a Nationwide Laboratory Cohort,” supplement, Clinical Infectious Diseases 66, no. S2 (March 2018): S111–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. World Health Organization , Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach [Internet] (Geneva: WHO, 2021), https://www.ncbi.nlm.nih.gov/books/NBK572729/. [PubMed] [Google Scholar]
- 48. Dwyer‐Lindgren L., Cork M. A., Sligar A., et al., “Mapping HIV Prevalence in Sub‐Saharan Africa Between 2000 and 2017,” Nature 570, no. 7760 (June 2019): 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. World Health Organisation , The Global Health Observatory: Prevalence of HIV Among Adults Aged 15 to 49 (%) [Internet] (Geneva: WHO, 2023), https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-hiv-among-adults-aged-15-to-49-(-). [Google Scholar]
- 50. Fauk N. K., Sukmawati A. S., Berek P. A. L., et al., “Barriers to HIV Testing Among Male Clients of Female Sex Workers in Indonesia,” International Journal for Equity in Health 17, no. 1 (May 2018): 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sileo K. M., Reed E., Kizito W., et al., “Masculinity and Engagement in HIV Care Among Male Fisherfolk on HIV Treatment in Uganda,” Culture, Health & Sexuality 21, no. 7 (July 2019): 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lurie M. N., Williams B. G., Zuma K., et al., “The Impact of Migration on HIV‐1 Transmission in South Africa: A Study of Migrant and Nonmigrant Men and Their Partners,” Sexually Transmitted Diseases 30, no. 2 (February 2003): 149–156. [DOI] [PubMed] [Google Scholar]
- 53. Camlin C. S., Ssemmondo E., Chamie G., et al., “Men “Missing” From Population‐Based HIV Testing: Insights From Qualitative Research,” supplement, AIDS Care 28, no. S3 (June 2016): 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sadighi Akha A. A., “Aging and the Immune System: An Overview,” Journal of Immunological Methods 463 (December 2018): 21–26. [DOI] [PubMed] [Google Scholar]
- 55. Bloch M., John M., Smith D., Rasmussen T., and Wright E., “Managing HIV‐Associated Inflammation and Ageing in the Era of Modern ART,”supplement, HIV Medicine 21, no. S3 (2020): 2–16. [DOI] [PubMed] [Google Scholar]
- 56. Kunutsor S., Walley J., Muchuro S., et al., “Improving Adherence to Antiretroviral Therapy in Sub‐Saharan African HIV‐Positive Populations: An Enhanced Adherence Package,” AIDS Care 24, no. 10 (October 2012): 1308–1315. [DOI] [PubMed] [Google Scholar]
- 57. Wolf M. S., Davis T. C., Arozullah A., et al., “Relation Between Literacy and HIV Treatment Knowledge Among Patients on Haart Regimens,” AIDS Care 17, no. 7 (October 2005): 863–873. [DOI] [PubMed] [Google Scholar]
- 58. Medley A., Kennedy C., O'Reilly K., and Sweat M., “Effectiveness of Peer Education Interventions for HIV Prevention in Developing Countries: A Systematic Review and Meta‐Analysis,” AIDS Education and Prevention 21, no. 3 (June 2009): 181–206, 10.1521/aeap.2009.21.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nsagha D. S., Ayima C., Njamen T., and Assob J., “Review of the The Role of Traditional, Complementary/Alternative Medicine in Primary Healthcare, Adjunct to Universal Health Coverage in Cameroon: A Review of the Literature.” (April 2020): 37–47.
- 60. Peltzer K., Preez N. F., Ramlagan S., and Fomundam H., “Use of Traditional Complementary and Alternative Medicine for HIV Patients in Kwazulu‐Natal, South Africa,” BMC Public Health 8, no. 1 (July 2008): 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dessalegn N. G., Hailemichael R. G., Shewa‐Amare A., et al., “HIV Disclosure: HIV‐Positive Status Disclosure to Sexual Partners Among Individuals Receiving HIV Care in Addis Ababa, Ethiopia,” PLoS One 14, no. 2 (2019): e0211967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ford N., Shubber Z., Meintjes G., et al., “Causes of Hospital Admission Among People Living With HIV Worldwide: A Systematic Review and Meta‐Analysis,” The Lancet HIV 2, no. 10 (October 2015. Oct): e438–e444. [DOI] [PubMed] [Google Scholar]
- 63. Melles M., Albayrak A., and Goossens R., “Innovating Health Care: Key Characteristics of Human‐Centered Design,”supplement, International Journal for Quality in Health Care 33, no. S1 (January 2021): 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Erwin K. and Krishnan J. A., “Redesigning Healthcare to Fit With People,” BMJ 354 (August 2016): i4536. [DOI] [PubMed] [Google Scholar]
- 65. Homsy J., King R., Tenywa J., Kyeyune P., Opio A., and Balaba D., “Defining Minimum Standards of Practice for Incorporating African Traditional Medicine into HIV/Aids Prevention, Care, and Support: A Regional Initiative in Eastern and Southern Africa,” The Journal of Alternative and Complementary Medicine 10, no. 5 (October 2004): 905–910. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
Data for this review is available upon reasonable request to the corresponding author.


