Abstract
Background
Spinal cord infarction (SCI) is associated with poor clinical outcome. Intravenous thrombolysis (IVT) is a well-established treatment for cerebral ischaemic stroke. However, its efficacy in SCI is unknown.
Objective
We present a case of acute spinal cord ischaemia with significant improvement following thrombolysis and review the current literature to explore the safety and feasibility of this treatment.
Methods
We reviewed the literature for cases of SCI that were treated with IVT. We reviewed their medical history, clinical presentation and the reported outcome.
Results
Other than our case, our review includes 19 cases of SCI treated with IVT. Their mean age was 62.87±15.27 and 36% of them were women. Most of the cases were spontaneous and treated within 240 min of onset. Favourable outcome was achieved in 89% of cases, including the few cases treated within extended time window. No clinical worsening due to haemorrhage was reported in either case.
Conclusions
IVT may be considered in certain settings as treatment for SCI following the appropriate work-up. Favourable outcome was achieved in most cases and no case experienced clinical worsening due to post-thrombolysis haemorrhage. Safety and efficacy of this approach need further investigation.
Keywords: STROKE, CEREBROVASCULAR, MYELOPATHY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Intravenous thrombolysis is an established treatment for cerebral ischaemia, yet there are currently no guidelines for the acute treatment of spinal cord ischaemia.
WHAT THIS STUDY ADDS
This study reviews the cases of spinal cord ischaemia treated with thrombolysis, showing improvement in most cases and no clinical worsening due to haemorrhage in any.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Given the limited data, this study further explores this treatment option.
Introduction
Spinal cord infarctions (SCIs) are generally considered rare, yet associated with high morbidity.1 Their clinical presentation may vary from unilateral weakness to complete para- or tetraplegia.2
The diagnosis of SCI can be challenging. In contrast to cerebral stroke, SCI may manifest gradually and be associated with acute pain. SCIs are especially challenging to diagnose without an inciting event, such as a preceding surgical procedure.1 Radiologically, SCIs are also harder to distinguish than cerebral strokes. Specific imaging features include focal diffusion restriction of the spinal cord. Other less specific, but supportive features, are T2-hyperintensity patterns, including longitudinally extensive ‘pencil-like’ signals on sagittal views and ‘owl eyes’/’snake-eye’ pattern on axial views. Gadolinium enhancement is frequently presented subacutely.3
There are currently no standardised guidelines regarding the acute treatment of spinal ischaemia due to limited data. Although systemic intravenous thrombolysis (IVT) is a well-established and effective treatment for acute cerebral ischaemia, little is known about its use in spinal stroke.4
We report a case of a female patient diagnosed with spinal cord ischaemia in whom treatment with systemic IVT led to significant improvement. We also reviewed all reported cases from the literature of SCI treated with IVT to assess its safety, feasibility and effectiveness.
Case report
A 72-year-old woman presented with acute-onset back pain that had developed 3 hours earlier, followed by right hemiparesis and hypoesthesia. She did not report a history of headache, trauma, fever or substance abuse. Her medical history was positive for hypertension, hyperlipidaemia and chronic right carotid artery occlusion, treated with Clopidogrel. Her surgical history included right total knee replacement.
On neurological examination, she was fully alert. Her right upper limb had a flaccid tone with distal 2/5 more than proximal 4/5 weakness. Her left lower limb had proximal 2/5 weakness more than distal 4/5. Deep tendon reflexes +1 on the right compared with +2 on the left. Sensory examination showed decreased pinprick sensation on her right side compared with her left side with normal position sense.
Initial stroke protocol imaging did not show the acute signs of stroke or haemorrhage and an aorta protocol CT angiogram was normal. Her National Institutes of Health Stroke Scale (NIHSS) score was 5. With informed consent, the patient was treated with IVT 4 hours after symptom onset with the standard dosage of 0.9 mg/kg over 60 min, including an initial bolus of 10%.
By the first day of hospitalisation, the patient reported subjective improvement in her right limb weakness but developed new left-sided sensory deficits with bilateral extensor plantar reflexes. A follow-up head CT performed 24 hours post-thrombolysis revealed two minor bilateral subarachnoid haemorrhages.
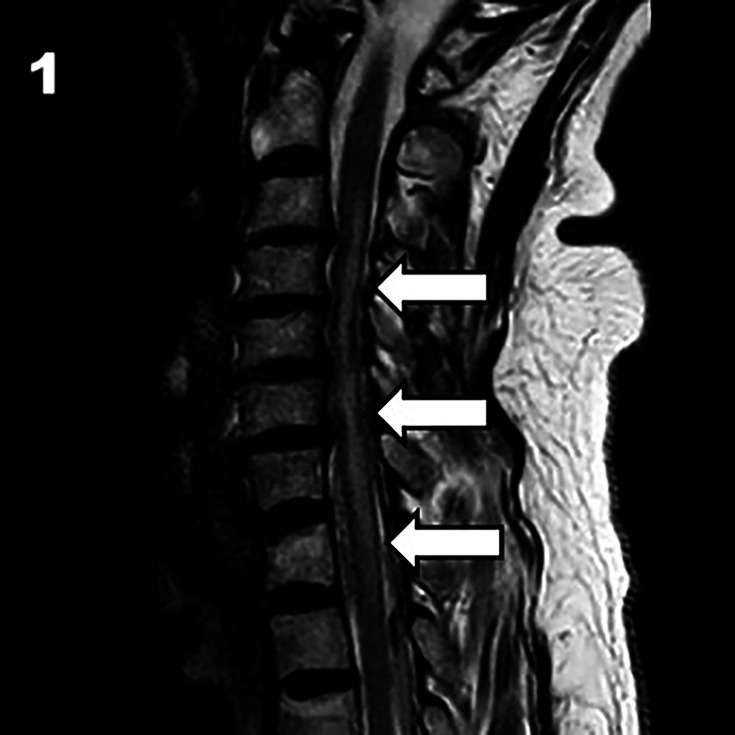
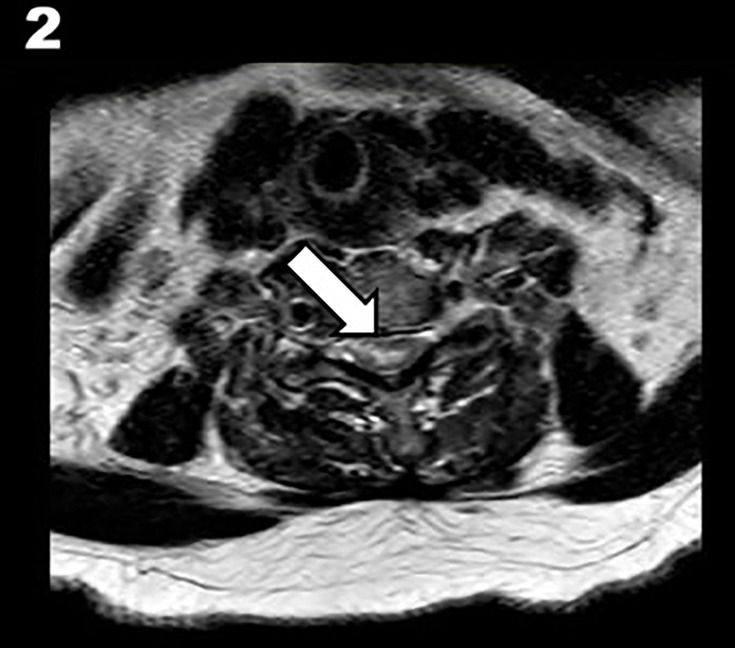
Subsequent spinal MRI demonstrated an abnormal, elongated T2 signal from C3 to T2 level, predominantly affecting grey matter with gadolinium enhancement at C5–6 levels (see figures1 2). These findings were consistent with acute spinal stroke. Minor discopathies were also noted on MRI. The patient was treated with dexamethasone and a buprenorphine patch with improvement. The patient has a few vascular risk factors, and even though a degenerative disc disease was noted on imaging, we believe the aetiology of the SCI to be atherosclerotic.
Figure 1. Sagittal T2-weighted MRI of the cervical spine showing high-signal intensity extending from C3 to T2 (white arrows).
Figure 2. Axial T2-weighted MRI of the spine at C5–6 level showing high-signal intensity in the spinal cord (white arrow).
Due to the minor haemorrhage detected on CT, the patient was initiated on single antiplatelet therapy (Aspirin) and Atorvastatin. Throughout her hospitalisation, she underwent physiotherapy and occupational therapy with gradual functional improvement. On her discharge to a rehabilitation centre, she demonstrated minor improvement in her lower right limb. At the 3-month follow-up, she could walk independently with a cane displaying only minor weakness on her right lower limb and minimal overall disability.
Methods
We reviewed all reported cases of SCI in the literature treated with IVT. We searched PubMed combining the keywords “thrombolysis” and/or “tpa” and/or “alteplase” with “spinal cord ischemia” and/or “spinal stroke” and/or “spinal artery syndrome” and/or “ischemic myelopathy” and/or “spinal cord infarction”. The first author reviewed all identified articles and extracted clinical and other relevant data from the selected articles.
Results
Our search initially identified 21 relevant reports; however, after review, only 11 articles presented the cases of SCI treated with IVT. An additional 4 articles were identified through references in the reviewed papers, making the total sum of 19 cases of SCI treated with IVT.24,17 The mean age of SCI cases was 62.87±15.27 and 36% of them were women (7 out of 19). MRI confirmed the diagnosis in 15 out of 19 cases212,17 (table 1).
Table 1. Patient’s characteristics.
| Sex/Age | Risk factors | Clinical deficit | Spontaneous or periprocedural | NIHSS admission | Time to IVT (min) | mRS at day 7 | mRS at 3 months | |
| Jankovic et al2 | F, 57 | Hypercholesterolemia | Left leg sensorimotor deficit | Spontaneous | 4 | 135 | 3 | 3 |
| Jankovic et al2 | M, 83 | Smoking, hypercholesterolemia | Paraplegia | Spontaneous | 10 | 240 | 4 | 5 |
| Jankovicet al 2 | F, 82 | Smoking, T2DM, hypertension | Left motor-sensory hemisyndrome | Spontaneous | 9 | 245 | 3 | 3 |
| Jankovic et al2 | F, 74 | Smoking, hypercholesterolemia | Loss of right arm dexterity, unstable gait | Spontaneous | 11 | 190 | 1 | 1 |
| Restrepo10 | M, 71 | ‘severe systemic atherosclerosis’ | Paraparesis | Periprocedural, diagnostic aortography | 6 | 110 | 0 (day 3) | 0 |
| Muller et al11 | M, 68 | Smoking, hypertension | Left>right hemiparesis | Spontaneous | 15 | 270 | 4 | 3 |
| Lee et al12 | M, 58 | Hypertension, hepatocellular carcinoma (vertebral artery coil embolisation of C2 metastasis) | Left numbness, right hemiparesis, C4 sensory level | Periprocedural | 9 | 90 | 2 | 1 (3 weeks) |
| Koch et al13 | M, 81 | Hypertension | Paraparesis, sensory-level T12 | Spontaneous | 7 | 220 | 4 | 3 |
| *Etgen and Höcherl14 (early repeated IVT) | F, 72 | None | Right hemiparesis and trunk ataxia | Spontaneous | 4 | 30 | 0 | NA |
| Right motor-sensory hemisyndrome | Spontaneous | 7 | (less than) 180 | 4 (day 2) | NA | |||
| Dorodnicov et al15 | M, 55 | Obesity, hypertension, dyslipidaemia | Tetraparesis, dizziness | Spontaneous | 16 | 240 | 4 | NA (mild improvement) |
| Wiszniewska and Harat16 | M, 61 | None | Right hemiparesis | Spontaneous | 8 | 90 | 0 (12 hours) | 1 (16 hours after recurrence of ischaemia) |
| Chandak et al17 | M, 50s | Hypertension | Paraparesis, difficulty passing urine | Spontaneous | 9 | 428 | NA | 3 |
| Fock and Seitz4 | F, 17 | None | Paraplegia | Spontaneous | NA | 270 | 0 | 0 |
| Almutlaq et al5 | F, 81 | Hypertension, dyslipidaemia | Paraplegia | Spontaneous | NA | 285 | 2 (several days) | 1 (1 month) |
| Lawson et al6 | M, 58 | Smoker, previous strokes, hypertension | Acute right-sided headache and pain in neck, arm and leg proceeding to hemiparesis | Spontaneous | 11 | NA | 2 (probably) | 2 |
| Oliveira and Sousa7 | M, 45 | Smoker (after weight lifting) | Paraparesis | Spontaneous | 4 | 330 | 4 | 1 (11 months) |
| Xiao and Huang8 | M,61 | Smoker | Hemiparesis | Spontaneous | 4 | 120 | 4 | 3 |
| Pikija et al9 | F, 57 | NA | ‘ASA syndrome’ | NA | NA | Within 270 min | 5 unknown time | |
| Pikija et al9 | M, 57 | NA | ‘Anterior and posterior cervical syndrome’ | NA | NA | Within 270 min | 3 unknown time |
In literature cases, NIHSS and mRS were estimated from the case descriptions if not stated in the article.
ASAAnterior spinal arteryDWIdiffusion-weighted imagingIVTintravenous thrombolysismRSmodified Rankin scaleNAnot availableNIHSSNational Institutes of Health Stroke ScaleT2time-2 imagingT2DMtype 2 diabetes mellitus
Most cases were spontaneous, two cases were periprocedural10 12 and two were of unknown circumstances.9 The aetiology of the spinal stroke was atherosclerotic in most cases except one periprocedural, related to the embolisation of a C2 vertebral metastasis,12 three with undetermined aetiology9 14 and two from fibrocartilaginous embolism, one with degenerative cervical canal stenosis and mechanical compression of the anterior spinal artery16 and another with the presence of a thoracic disc herniation after physical exertion.7
Among the 19 cases, 10 underwent immediate MRI prior to IVT administration,2 4 5 11 13 16 17 whereas this was not performed in our case. Two cases did not provide this information.9 One case completed an MRI 3 days after the IVT due to a neurological deterioration, confirming a diagnosis of SCI and administered a second dose of thrombolysis afterwards. This was the only case with early repeated systemic thrombolysis treatment.14
12 out of 19 cases received alteplase at the standard dosage of 0.9 mg/kg over 60 min and an initial bolus of 10%.2 4 5 8 10 11 13 16 17 One case used tenecteplase at a dose of 0.25 mg/kg8 and another used intra-arterial alteplase as well as intravenous alteplase at a dosage of 2/3*0.9*bodyweight.12 Five cases did not provide this information.6 9 14 15Thrombolysis was administered within an ‘extended time window’ in 3 out of the 19 cases, ranging from 5 to 7 hours after onset.5 7 17
12 out of 19 cases ruled out an aortic dissection before IVT administration or had the diagnosis confirmed beforehand with a spinal MRI or angiography.24 5 7 10,13 17
17 out of 19 cases had favourable short-term and long-term outcomes, including the 3 cases treated within the extended time window24,17 as well as the cases of fibrocartilaginous aetiology.7 16 Favourable outcome was defined as an improvement in their clinical examination as described in the original report. None of the cases reviewed reported post-thrombolysis haemorrhage. Notably, even though our patient had minor subarachnoid haemorrhages, she had no clinical worsening and eventually improved significantly with rehabilitation.
Discussion
We report a patient presenting with acute hemiparesis due to a spinal stroke treated with IVT.
Acute SCI is a rare condition, typically associated with a poor prognosis.4 While bilateral presentations are usually more common, unilateral cases have been also reported.1 2 14 16 18 19 The absence of cranial nerve involvement and normal brain imaging can aid in making the diagnosis in such cases.17 Unilateral presentation may be either due to the occlusion of a unilateral sulcal artery or because incomplete collateralization with the posterior spinal artery maintains perfusion on one side of the cord.6
Sequela of SCI can be detrimental with only 47% of patients able to ambulate independently.1 Urinary symptoms and pain may persist for years.20 Given the limited data on treatment and the poor outcomes associated with SCI, IVT may be a valid option in certain settings.
In contrast to cerebral stroke, spinal strokes often manifest with preceding acute pain and can progress gradually. This can lead to potential misdiagnosis. Consequently, the time window for treatment with IVT might be missed, depriving patients of a potentially effective treatment as well as exposing them to unnecessary investigations and possibly unsuitable treatments.
Our review included 19 cases of patients with SCI treated with IVT, 17 of whom had an overall improvement. None of these cases had post-thrombolysis worsening due to haemorrhage. Despite our patient having minor haemorrhages, these did not result in clinical deterioration in the long term.
Spontaneous improvement of SCI is rare but well-documented. About 7% of patients may experience improvement within the first 24 hours.19 Therefore, it is possible that, in some of the cases reviewed, the outcomes were regardless of IVT. In our review, one case had improved completely following IVT during 24 hours but deteriorated at a later point.16 A few cases had improved in various degrees within the 24 hours following IVT, including our case.4,610 12 13 17 However, these cases improved even further with time. And so it is unlikely that the outcomes were not associated with IVT. One case was respiratory unstable and deteriorated hours after IVT11 and the rest did not provide clinical information about the patient during the 24 hours following IVT.27,9 14 15 Publication bias is a critical limitation when discussing case reports since positive outcomes are more likely to be published than negative or inconclusive outcomes. It is highly likely that there are cases of SCI treated with IVT that were not published due to poor or inconclusive results. This can skew the literature and lead to incomplete understanding of the effectiveness of IVT for SCI. Therefore, clinicians must be extremely cautious when implementing interventions based on such data.
Nevertheless, given the limited data, poor prognosis and lack of conclusive treatment guidelines for SCI, there is a growing argument in the literature advocating to present this treatment option to the patient within the first 4.5 hours of onset.1 4 The main challenge remains a timely diagnosis, especially since the main differential diagnoses are an absolute contraindication to IVT, such as dissections and vascular malformations.
Two of the cases reviewed were of fibrocartilaginous embolism and improved after IVT.7 16 Spinal stroke of fibrocartilaginous embolism is rare and caused by the migration of fibrocartilaginous material into the vessels.21 The history of physical exertion in such cases makes the diagnosis of spinal stroke even more challenging and so these cases might not be suitable for IVT because of the need for a timely diagnosis. Moreover, even though these two cases specifically improved following IVT, one had improved solely after the use of additional treatments, including steroids and neurosurgery,16 and causality definitely cannot be concluded from two cases. Since the embolism in these cases involves a different mechanism and not a thrombus, we believe that IVT may be less effective theoretically.
Early recognition of acute SCI may potentially mitigate its devastating consequences by administering prompt thrombolytic therapy. An urgent spinal MRI may expedite the process. However, because of the lack of clinical trials, IVT, if used, should be administered only after a thorough risk-benefit evaluation and with the patient’s informed consent.
Further studies are needed to assess the safety and efficacy of IVT for SCI, ideally through a randomised controlled trial. Conducting such a trial has many limitations. First, the scarcity of spinal stroke cases. Moreover, the need for a timely diagnosis for an urgent treatment, as delays could impact outcomes, and finally, ethical considerations due to investigating a novel treatment. However, we believe that a randomised controlled trial might be feasible with a multicentre collaboration in order to recruit sufficient participants; this approach also provides a more diverse patient population. Given the limited data and poor prognosis, such a trial could contribute immensely to the understanding and treatment of SCI.
In summary, our review revealed a majority of SCI cases treated with IVT with overall improvement, similar to our case. None of the cases experienced clinical worsening due to haemorrhage post-thrombolysis. Further studies are needed to assess the safety and efficacy of this approach.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; internally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Contributor Information
Haya Bishara, Email: haya.bishara@mail.huji.ac.il.
Sivan Bloch, Email: Sivanbl2@clalit.org.il.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Zalewski NL, Rabinstein AA, Krecke KN, et al. Characteristics of Spontaneous Spinal Cord Infarction and Proposed Diagnostic Criteria. JAMA Neurol. 2019;76:56–63. doi: 10.1001/jamaneurol.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jankovic J, Rey Bataillard V, Mercier N, et al. Acute ischemic myelopathy treated with intravenous thrombolysis: Four new cases and literature review. Int J Stroke. 2019;14:893–7. doi: 10.1177/1747493019851289. [DOI] [PubMed] [Google Scholar]
- 3.English SW, Rabinstein AA, Flanagan EP, et al. Spinal cord transient ischemic attack. Neur Clin Pract. 2020;10:480–3. doi: 10.1212/CPJ.0000000000000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Focke JK, Seitz RJ. Reversal of Acute Spinal Cord Ischemia by Intravenous Thrombolysis. Neurol Clin Pract. 2021;11:e975–6. doi: 10.1212/CPJ.0000000000001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almutlaq A, Alkhalifa A, Bereznyakova O. Extended Window Thrombolysis in Acute Spinal Cord Ischemia - ERRATUM. Can J Neurol Sci. 2022;49:831. doi: 10.1017/cjn.2021.219. [DOI] [PubMed] [Google Scholar]
- 6.Lawson EC, Hu R, Silver M, et al. Administration of Recombinant Tissue Plasminogen Activator for Sulcal Artery Syndrome. Neurol Clin Pract . 2021;11:e901–3. doi: 10.1212/CPJ.0000000000000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira DS, Sousa L. Off-label tenecteplase use in spinal cord ischemia. Acta Neurol Belg. 2023;123:2409–12. doi: 10.1007/s13760-023-02228-8. [DOI] [PubMed] [Google Scholar]
- 8.Xiao M, Huang X. Anterior spinal artery syndrome, intravenous alteplase or not. Acta Neurol Belg. 2022;122:235–7. doi: 10.1007/s13760-021-01802-2. [DOI] [PubMed] [Google Scholar]
- 9.Pikija S, Kunz AB, Nardone R, et al. Spontaneous spinal cord infarction in Austria: a two-center comparative study. Ther Adv Neurol Disord. 2022;15:17562864221076321. doi: 10.1177/17562864221076321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Restrepo L. Acute spinal cord ischemia during aortography. Tex Heart Inst J. 2007;34:259. [PMC free article] [PubMed] [Google Scholar]
- 11.Müller KI, Steffensen LH, Johnsen SH. Thrombolysis in anterior spinal artery syndrome. BMJ Case Rep. 2012;2012:1–4. doi: 10.1136/bcr-2012-006862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K, Strozyk D, Rahman C, et al. Acute spinal cord ischemia: treatment with intravenous and intra-arterial thrombolysis, hyperbaric oxygen and hypothermia. Cerebrovasc Dis. 2010;29:95–8. doi: 10.1159/000259618. [DOI] [PubMed] [Google Scholar]
- 13.Koch M, Sepp D, Prothmann S, et al. Systemic thrombolysis in anterior spinal artery syndrome: what has to be considered? J Thromb Thrombolysis. 2016;41:511–3. doi: 10.1007/s11239-015-1281-8. [DOI] [PubMed] [Google Scholar]
- 14.Etgen T, Höcherl C. Repeated early thrombolysis in cervical spinal cord ischemia. J Thromb Thrombolysis. 2016;42:142–5. doi: 10.1007/s11239-015-1332-1. [DOI] [PubMed] [Google Scholar]
- 15.Dorodnicov E, Gelfand A, Milo R. Acute spinal cord ischemia treated with intravenous thrombolysis. J Neurol Sci. 2017;381:410. doi: 10.1016/j.jns.2017.08.3370. [DOI] [Google Scholar]
- 16.Wiszniewska M, Harat M. The positive effect of combined treatment with thrombolysis and neurosurgery for cervical myelopathy due to anterior spinal artery thrombosis. ppn . 2017;26:270–4. doi: 10.5114/ppn.2017.72439. [DOI] [Google Scholar]
- 17.Chandak SN, Chandak N, Kabra D, et al. Spinal Cord Infarction Thrombolysed at Seven Hours: A Case Report and Review of Literature. Cureus. 2024;16:e55983. doi: 10.7759/cureus.55983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheshire WP, Santos CC, Massey EW, et al. Spinal cord infarction: etiology and outcome. Neurology (ECronicon) 1996;47:321–30. doi: 10.1212/wnl.47.2.321. [DOI] [PubMed] [Google Scholar]
- 19.Nedeltchev K, Loher TJ, Stepper F, et al. Long-term outcome of acute spinal cord ischemia syndrome. Stroke. 2004;35:560–5. doi: 10.1161/01.STR.0000111598.78198.EC. [DOI] [PubMed] [Google Scholar]
- 20.Robertson CE, Brown RD, Wijdicks EFM, et al. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology (ECronicon) 2012;78:114–21. doi: 10.1212/WNL.0b013e31823efc93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagenkötter SS, Hammami F, Hagenkötter B. Fibrocartilaginous embolism after mountain cycling: a case report with clinical and radiological follow-up and almost complete recovery. BMJ Neurol Open. 2024;6:e000690. doi: 10.1136/bmjno-2024-000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.