Abstract
Abstract
Objectives
The objectives of the study are to investigate infection risk in offspring born to women with systemic lupus erythematosus (SLE) compared with offspring born to women without SLE and examine the mediating role of preterm birth.
Design
This is a register-based cohort study.
Setting
Liveborn singletons born in Sweden, 2006–2021, were included in the study.
Participants
1248 infants born to mothers with SLE (≥2 International Classification of Diseases-coded visits in the National Patient Register (NPR) and Medical Birth Register, with ≥1 visit before pregnancy) and 34 886 infants born to women without SLE from the general population were included.
Primary and secondary outcome measures
The primary outcome was any visit for infection in the NPR or anti-infectives in the Prescribed Drug Register. The secondary outcome was hospitalised infection. Infection risks within 72 hours, within 1 month and within 1 year were estimated.
Results
SLE offspring had a higher risk of infection in the first 72 hours compared with non-SLE (2.1% vs 1.2%; risk ratios (RR) (95% CI) 1.62 (1.09 to 2.42)), the first month (5.2% vs 4.5%; RR 1.12 (0.88 to 1.43)) and first year of life (38.2% vs 37.2%; RR 1.09 (1.01 to 1.17)). The hospitalised infection risk for SLE offspring was similar to that of non-SLE (5.8% vs 5.5%, first year of life). The percentage of the total effect of maternal SLE on infant infection mediated through preterm birth was 86% for infection in the first 72 hours and 27% in the first year of life.
Conclusions
The risk of infection in SLE offspring is most increased in the first 3 days after birth, and a proportion of this association can be explained by preterm birth. To prevent early neonatal infections, maternal SLE could be considered as a risk factor before allowing early discharge from postnatal care.
Keywords: EPIDEMIOLOGY, RHEUMATOLOGY, Pregnancy, Child
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The use of prospectively collected, contemporary, population-based data from the entire Swedish population minimised selection bias and increased power.
We were able to link infants to mothers with and without systemic lupus erythematosus (SLE) and follow them for 1 year after birth by using each individual’s unique personal identification number.
Mothers with SLE might seek and/or receive more healthcare than mothers without SLE which would result in an overestimate of the association between maternal SLE and infant infection.
The national registers used in this study do not capture information on lupus disease activity, clinical phenotype or severity which might modify the risk of infant infection.
Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic inflammatory autoimmune disease that predominantly affects women of childbearing age. Women with SLE have a 20–30% risk of preterm delivery which is two to three times higher than the risk among women without SLE.1,5 The downstream effect of maternal SLE on infants is not as clear. A previous study from Sweden reported that 21% of infants born to women with SLE had an infection during their first year of life compared with 14% of infants born to mothers without SLE.4 As the nature of this study was strictly descriptive, it remains unclear if the association between maternal SLE exposure during pregnancy and infections in infants is still present after considering confounding factors and the mediating effect of preterm birth.
Preterm birth (birth before 37 weeks of gestation) is likely an important mediator in the association between maternal SLE and infant infections. Being born preterm is a risk factor for infections, especially early-onset infections of which clinical manifestations usually appear within the first 72 hours.6 7 This is partly due to maternal infection (one cause of preterm birth) and immature organs (eg, lungs and skin). Also, preterm infants often require prolonged intravenous access, endotracheal intubation or other invasive procedures that provide a portal of entry or impair barrier and clearance mechanisms, placing them at increased risk for infections.6 7 Infants born preterm have on average 1.5 times the total number of infections in the first year of life compared with infants born full term.8
The current study investigates the association between maternal SLE and the risk of infection in infants during the first year of life and how much of the association can be explained by preterm birth. We used nationwide population-based registers in Sweden to compare infection risk in infants born to women with SLE to infants born to women without SLE.
Methods
Study setting and sources
In Sweden, access to healthcare is universal and residents have a unique personal identification number that allows for the linkage of their records in registers. Maternal health during pregnancy, delivery and neonatal outcomes of over 98% of deliveries in Sweden are registered in the Medical Birth Register (MBR) starting in 1973. Until July 2008, births≥28 gestational weeks and live births were included. From July 2008, the MBR also included stillbirths≥22 gestational weeks. Information on sex, year of birth, county of residence and immigration, from 1968 onward, was captured in the Total Population Register (TPR). The date of death is collected in the Cause of Death Register. Hospitalisation data with national coverage from 1987 onward and non-primary care specialised outpatient visits since 2001 are captured in the National Patient Register (NPR). Primary and secondary diagnoses are listed for each visit using International Classification of Diseases (ICD) codes. Since July 2005, dispensed prescriptions of medications in the Swedish population are captured in the Prescribed Drug Register (PDR) using Anatomical Therapeutic Chemical (ATC) codes.
Study population
Infants born to mothers with SLE (exposed) and without SLE from the general population (unexposed) were identified by linking the NPR to the MBR. Women with ≥1 ICD-coded visits for SLE (ICD-10: M32, excluding M32.0 drug-induced lupus) in the inpatient or outpatient records of the NPR were matched to 10 randomly sampled comparators from the general population without SLE, identified in the TPR, on year of birth, sex, calendar time and residential location. We further restricted the women with SLE to have ≥2 visits listing SLE, at least one of which was required to be given at a department or specialist that diagnoses, treats or manages SLE (rheumatology, dermatology, nephrology, internal medicine and/or paediatrics). This definition is estimated to have a positive predictive value of 80% in women,9 but its accuracy has not been evaluated for identifying pregnant women with prevalent SLE.
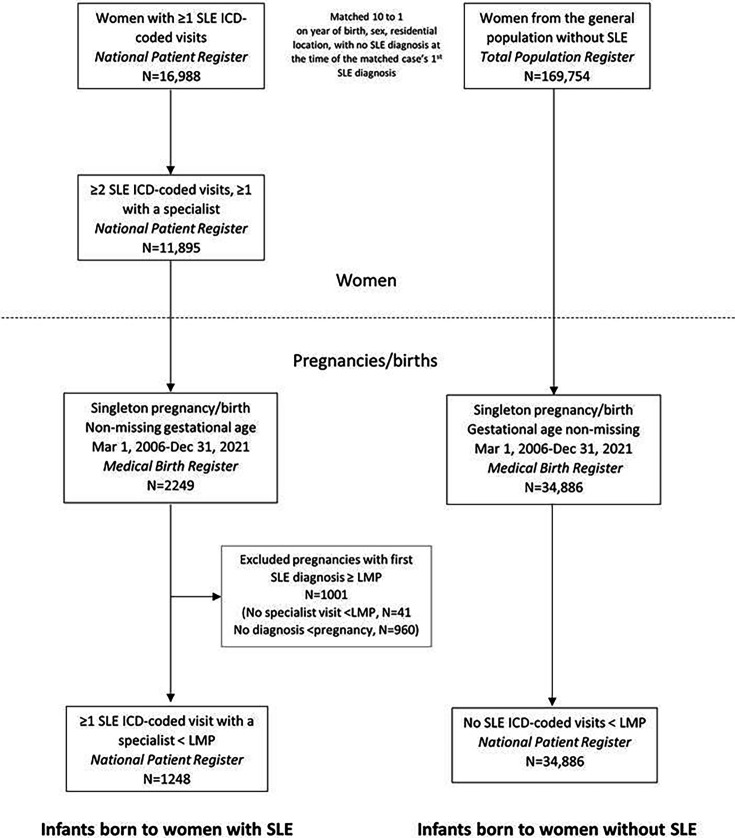
From this population of women with SLE and the general population comparators without SLE, we identified those who gave birth to a liveborn singleton registered in the MBR between March 2006 (as the PDR started in July 2005) and December 2021 without missing data on gestational age. A flow chart of the study population selection is depicted in figure 1.
Figure 1. Flow chart of study population selection. ICD, International Classification of Diseases; LMP, last menstrual period; SLE, systemic lupus erythematosus.
Maternal SLE at delivery
The infants were considered to be born to a mother with SLE if the mother had at least one SLE discharge code before pregnancy at a specialist clinic (rheumatology, dermatology, nephrology, internal medicine and/or paediatrics). The first observed SLE discharge code was used as a proxy for the diagnosis date as it is the first observed diagnosis in our data, which does not include primary care. However, SLE diagnosis typically is given by specialists; therefore, this is a reasonable proxy. Infants born to women with only one visit for SLE before delivery or with no visits with a specialist before pregnancy were excluded to minimise misclassification of maternal SLE. There were no general population comparators with an SLE discharge code before pregnancy. The study period was from the infant’s date of birth for 1 year, death or emigration, whichever came first.
Infections in infants
Any infant infection was identified using both primary and secondary ICD-coded visits in the inpatient and outpatient records of the NPR and dispensed prescriptions of anti-infectives in the PDR (the majority of which were antibiotics). We also examined hospitalised infections separately, defined as a hospitalisation listing infection as the primary diagnosis in the inpatient records of the NPR. The first infection during follow-up was categorised into upper respiratory, lower respiratory, gastrointestinal and other infections. For a list of ICD codes for infections and ATC codes for dispensed prescriptions of anti-infectives, see online supplemental table 1, adapted from Sørup et al10 Miller et al11 and Bröms et al.12 For any infection and hospitalised infection, we identified those occurring in the first 3 days (early-onset), within 1 month and within 1 year of birth. We examined infections in the first 3 days because it has a different pathogenesis than infections later in life and is associated with serious complications that can be life-threatening.
Preterm birth and other covariates
Preterm birth (birth before 37 weeks of gestation) and very preterm birth (birth before 32 weeks of gestation) were identified in the MBR. Additional data from the MBR included the infant’s sex, infant’s date of birth, parity (first or subsequent birth), gestational age in weeks, maternal age at delivery and self-reported maternal first trimester smoking (yes/no/missing). Maternal height and weight collected at the first prenatal visit were used to calculate the body mass index (BMI) as weight divided by height squared (kg/m2). Maternal infection during pregnancy was collected from the NPR and defined as an inpatient or outpatient visit listing an ICD code for infection.
Statistical analysis
Continuous variables were described using means and SD, and categorical variables were described with frequencies and column percentages. We calculated risk ratios (RR) and corresponding 95% CIs using modified Poisson regression models to estimate the risk of infant infection comparing infants born to mothers with SLE with infants born to general population comparators.13 Models were adjusted for maternal age at delivery (continuous), maternal first-trimester smoking (yes/no/missing) and calendar year (continuous). RRs for any infant infection in three time windows were estimated: (1) within the first 72 hours, (2) the first month and (3) the first year of life, overall and by preterm birth. RRs were also estimated for hospitalised infection in the three time windows. In a sensitivity analysis, we reran all models among only first births. It has been shown that outcomes from the first pregnancy might be less favourable than subsequent pregnancies.14
We conducted a mediation analysis using a casual inference counterfactual approach to examine how much of the association between maternal SLE and any infection in infants could be explained by preterm birth.15 Mediation analysis can be used to assess factors that are caused by the exposure (maternal SLE) and cause the outcome (infant infection) to better understand the relationship between exposure and outcome and to ultimately identify factors which can be intervened upon. Causal mediation analysis accommodates interaction between the exposure and the mediator. We investigated how much of the association between maternal SLE and infant infection operates through the mediating effect of preterm birth. Total effects were separated into natural direct effects and natural indirect effects through preterm birth. Results were reported on the OR scale which estimates the RR and is comparable with the RR. Based on knowledge from the literature, causal mediation models were adjusted for maternal age at delivery (continuous), maternal first-trimester smoking (yes/no/missing) and calendar year (continuous) to account for exposure–mediator, mediator–outcome and exposure–outcome confounding.16 We included an interaction between maternal SLE and preterm birth in the model. Because the maternal infection is associated with both preterm birth and infant infection, we also performed a sensitivity analysis excluding mothers with an infection during pregnancy in a sensitivity analysis. All data management and analyses were performed using SAS, V.9.4.
Results
Baseline characteristics of mothers and infants
We included 1248 infants born to mothers with SLE and 34 886 infants born to general population comparators (table 1). The proportion of births that were first born was higher among SLE-exposed infants compared with those infants born to mothers without SLE (44.7% vs 40.9%), and SLE-exposed infants were almost three times more likely to be born preterm (13.5% vs 4.6%). Mothers with SLE were less likely to smoke during the first trimester (4.2%) than general population comparators (5.1%). On average, maternal age at delivery and maternal BMI were comparable between mothers with SLE and the general population comparators.
Table 1. Baseline characteristics of 1248 infants born to women with systemic lupus erythematosus (SLE) and 34 886 infants born to general population comparators.
| Infants born to mothers with SLE(n=1248) | Infants born to general population comparators(n=34 886) | |
| Female offspring, n (%) | 640 (51.3) | 16 926 (48.5) |
| Year of birth, n (%) | ||
| 2006–2011 | 370 (29.7) | 15 381 (44.1) |
| 2012–2017 | 400 (32.0) | 10 911 (31.3) |
| 2018–2021 | 478 (38.4) | 8594 (24.6) |
| First birth, n (%) | 558 (44.7) | 14 280 (40.9) |
| Gestational age, mean±SD completed weeks | 38.7 (2.5) | 39.8 (1.8) |
| Term birth, n (%) | 1080 (86.4) | 33 291 (95.4) |
| Preterm birth, n (%) | 168 (13.5) | 1595 (4.6) |
| 32–37 weeks of gestation | 136 (10.9) | 1354 (3.9) |
| <32 weeks of gestation | 32 (2.6) | 241 (0.7) |
| Maternal age at delivery, years, mean±SD | 32.3 (4.6) | 32.0 (5.0) |
| Maternal age at delivery in categories, years, n (%) | ||
| <35 | 882 (70.7) | 25 161 (72.1) |
| ≥35 | 366 (29.3) | 9725 (27.9) |
| Maternal infection during pregnancy, n (%) | 117 (9.4) | 1358 (3.9) |
| Maternal body mass index, mean±SD* | 24.4 (4.3) | 24.9 (4.7) |
| Missing data on body mass index, n (%) | 100 (8.0) | 2223 (6.4) |
| Maternal smoking during the first trimester, n (%)* | 53 (4.2) | 1763 (5.1) |
| Missing data on first-trimester smoking, n (%) | 71 (5.7) | 1767 (5.1) |
Mean or percentage excludes missing values.
Risk of infant infections
6 (2.1%) infants born to mothers with SLE and 414 (1.2%) infants born to mothers without SLE were diagnosed with any infection in the first 72 hours of life (table 2). The most common first registered infection in the first year of life was upper respiratory infection (SLE: 13.9% and non-SLE: 12.9%; online supplemental table 2).
Table 2. Risk ratios for any infection in the first year of life comparing infants born to mothers with systemic lupus erythematosus (SLE) to infants born to mothers from the general population, overall and by preterm birth.
| Infants born to mothers with SLE, no. cases/total (%) | Infants born to general population comparators, no. cases/total (%) | Unadjusted risk ratio(95% CI) | Adjusted risk ratio(95% CI)* | |
| Any infection in the first 3 days | ||||
| Overall | 26/1248 (2.1) | 414/34 886 (1.2) | 1.75 (1.19 to 2.60) | 1.63 (1.09 to 2.42) |
| Term birth | 11/1083 (1.0) | 313/33 291 (0.9) | 1.08 (0.60 to 1.97) | 0.98 (0.53 to 1.80) |
| Preterm birth (32–37 weeks) | 4/136 (2.9) | 38/1354 (2.8) | 1.05 (0.38 to 2.89) | 1.01 (0.36 to 2.79) |
| Very preterm birth (<32 weeks) | 11/32 (34.4) | 63/241 (26.1) | 1.32 (0.78 to 2.22) | 1.32 (0.77 to 2.25) |
| Any infection in the first month of life | ||||
| Overall | 65/1248 (5.2) | 1586/34 886 (4.5) | 1.15 (0.90 to 1.46) | 1.12 (0.88 to 1.43) |
| Term birth | 44/1080 (4.1) | 1436/33 291 (4.3) | 0.94 (0.70 to 1.27) | 0.92 (0.68 to 1.23) |
| Preterm birth (32–37 weeks) | 10/136 (7.4) | 80/1354 (5.9) | 1.24 (0.66 to 2.34) | 1.18 (0.62 to 2.26) |
| Very preterm birth (<32 weeks) | 11/32 (34.4) | 70/241 (29.1) | 1.18 (0.71 to 1.99) | 1.19 (0.71 to 2.02) |
| Any infection in the first year of life | ||||
| Overall | 478/1248 (38.3) | 12 985/34 886 (37.2) | 1.03 (0.96 to 1.11) | 1.09 (1.01 to 1.17) |
| Term birth | 400/1080 (37.0) | 12 270/33 291 (36.9) | 1.00 (0.93 to 1.09) | 1.07 (0.99 to 1.16) |
| Preterm birth (32–37 weeks) | 59/136 (42.4) | 569/1354 (42.0) | 1.03 (0.84 to 1.26) | 1.06 (0.86 to 1.29) |
| Very preterm birth (<32 weeks) | 19/32 (59.4) | 146/241 (60.6) | 0.98 (0.72 to 1.33) | 0.99 (0.73 to 1.35) |
Any infection is defined as a visit listing an ICDInternational Classification of Diseases code for infection as a primary or secondary diagnosis in the inpatient or outpatient components of the National Patient Register or a dispensed anti-infective listed in the Prescribed Drug Register.
Models are adjusted for maternal age at delivery (continuous), maternal first-trimester smoking (yes/no/missing), and calendar year (continuous).
The risk of infection in the first 72 hours associated with maternal SLE was 63% higher than infants born to mothers without SLE (adjusted RR 1.63 (95% CI 1.09 to 2.42)). The RR for any infection in the first month of life was 1.12 (95% CI 0.88, 1.43), and results did not differ greatly when stratified by preterm birth (table 2). When examining up to 1 year after birth, the risk of any infection was significantly higher in infants born to mothers with SLE (38.3%) than in infants born to mothers without SLE (37.2%), with a corresponding adjusted RR of 1.09 (95% CI 1.01 to 1.17).
In the first 72 hours, there was a higher percentage of infants born very preterm with an infection to mothers with SLE (11/32, 34.4%) than to mothers without SLE (63/241, 26.1%), although the corresponding RR was not significantly higher (1.32 (95% CI 0.77 to 2.25); table 2). Results among term and preterm births were similar to the overall estimates for the other time windows.
73 (5.8%) infants born to mothers with SLE and 1923 (5.5%) infants born to mothers without SLE were hospitalised for infections in the first year of life, and 6 (0.5%) and 162 (0.5%) of those, respectively, occurred in the first 3 days (table 3). Overall, the number of hospitalised infections was too small to stratify by preterm birth.
Table 3. Risk ratios for hospitalised infection in the first year of life comparing infants born to mothers with systemic lupus erythematosus (SLE) to infants born to mothers from the general population, overall and by preterm birth.
| Infants born to mothers with SLE, no. cases/total (%) | Infants born to general population comparators, no. cases/total (%) | Unadjusted risk ratio(95% CI) | Adjusted risk ratio(95% CI)* | |
| Hospitalised infection in the first 3 days | ||||
| Overall | 6/1248 (0.5) | 162/34 886 (0.5) | 1.04 (0.46 to 2.33) | 0.97 (0.43 to 2.23) |
| Term birth | 5/1080 (0.5) | 159/33 291 (0.5) | 0.97 (0.40 to 2.36) | 0.91 (0.37 to 2.22) |
| Preterm birth (32–37 weeks) | 1/136 (0.7) | 3/1354 (0.2) | NE | NE |
| Very preterm birth (<32 weeks) | 0/32 (0.0) | 0/241 (0.0) | NE | NE |
| Hospitalised infection in the first month of life | ||||
| Overall | 22/1248 (1.8) | 485/34 886 (1.4) | 1.27 (0.83 to 1.94) | 1.25 (0.81 to 1.91) |
| Term birth | 19/1080 (1.8) | 473/33 291 (1.4) | 1.24 (0.79 to 1.95) | 1.21 (0.77 to 1.92) |
| Preterm birth (32–37 weeks) | 3/136 (2.2) | 12/1354 (0.9) | NE | NE |
| Very preterm birth (<32 weeks) | 0/32 (0.0) | 0/241 (0.0) | NE | NE |
| Hospitalised infection in the first year of life | ||||
| Overall | 73/1248 (5.8) | 1923/34 886 (5.5) | 1.06 (0.85 to 1.33) | 1.13 (0.90 to 1.42) |
| Term birth | 59/1080 (5.5) | 1776/33 291 (5.3) | 1.02 (0.80 to 1.32) | 1.10 (0.85 to 1.41) |
| Preterm birth (32–37 weeks) | 11/136 (8.1) | 110/1354 (8.1) | 1.00 (0.55 to 1.80) | 1.03 (0.56 to 1.88) |
| Very preterm birth (<32 weeks) | 3/32 (9.4) | 37/241 (15.4) | 0.61 (0.20 to 1.87) | 0.64 (0.20 to 1.97) |
HospitalizedHospitalised infections are defined as International Classification of Diseases-coded visits listing infection infections are an ICD-coded visits lisiting infection as the primary diagnosis in the inpatient component of the National Patient Register.
Models are adjusted for maternal age at delivery (continuous), maternal first-trimester smoking (yes/no/missing), and calendar year (continuous).
NE, not estimatable due to low number of events
All the results remained similar to the main results in a sensitivity analysis including only first births, although with limited power for some subgroups (online supplemental table 3).
The mediating role of preterm birth
Considering all births, 85% (95% CI 27% to 144%) of the association between maternal SLE and any infection in the first 3 days of life was mediated by preterm birth (table 4). The proportion mediated through preterm birth for the first year of life was 28% (95% CI −10% to 66%). Looking at first births only, the proportion mediated was 59% (95% CI 19% to 98%) for any infection in the first 3 days and 77% (95% CI −117% to 272%) in the first year (online supplemental table 4). A small proportion of mothers had a visit in inpatient or outpatient care listing an ICD code for infection during pregnancy (9.4% SLE, 3.9% general population), and excluding these pregnancies did not considerably change the estimates in mediation analyses (online supplemental table 4).
Table 4. Estimates of the mediating effect of preterm birth (<37 weeks gestation) in the association between maternal systemic lupus erythematosus and infant infections within the first 3 days and the first year of life. Direct, indirect and total effects are estimated with ORs and 95% CIs (OR 95% CI).
| Mediator: preterm birth vs full term | Direct effectOR (95% CI) | Indirect effect through preterm birthOR (95% CI) | Total effectOR 95% CI | % mediated through preterm birth (95% CI) |
| Outcome | ||||
| Any infection in the first 3 days of life | 1.10 (0.60 to 1.61) | 1.55 (1.18 to 1.93) | 1.71 (1.00 to 2.43) | 85 (27 to 144) |
| Any infection in the first year of life | 1.10 (0.97 to 1.23) | 1.03 (0.99 to 1.07) | 1.13 (1.00 to 1.27) | 28 (−10 to 66) |
OR odds ratio; confidence interval. Models are adjusted for maternal age at delivery (continuous), maternal first-trimester smoking (yes/no/missing), and calendar year (continuous).
Discussion
In this population-based study in Sweden, infants born to mothers with SLE had a 63% increased risk of any infection in the first 3 days of life compared with infants born to general population comparators. In the first year of life, the risk of any infection in infants born to mothers with SLE was 9% higher than in infants who were not exposed to maternal SLE. Being born preterm accounted for a proportion of the association between maternal SLE and any infection in the first 3 days of life. We did not find a significant association between maternal SLE and hospitalised infection at any time point in the first year of life.
Information on the risk of infections in infants exposed to maternal SLE during pregnancy is limited. In a previous descriptive study of SLE pregnancies in Sweden using the same data sources but with data only through 2012, the 1-year infection risk was significantly higher in infants born to women with SLE compared with infants born to general population comparators.4 In contrast, we used a broader definition of infections with more ICD-10 codes and anti-infective medications for systemic use and births through 2021. Ignacio and colleagues reported risks of infant infections associated with exposure to maternal SLE, with the risk of any infection in the first 30 days of life of 3.9% in SLE-exposed infants and 2.3% in unexposed infants born to general population comparators.17 In comparison, we observed a slightly higher risk of infection in both SLE (5.2%) and general population (4.5%) for the same time period.
Women with SLE in our study were three times more likely to deliver preterm than their general population comparators, which has been reported by others.4 5 18 Mediation analysis showed that preterm birth explained a proportion of the association between infection in the first 3 days of life and maternal SLE. Maternal infection is a risk factor for preterm birth and also for neonatal infection, and the SLE mothers had a higher prevalence of infection during pregnancy compared with the general population.7 However, when pregnancies with a maternal infection-related hospitalisation or outpatient specialist visit were excluded, estimates remained similar to the main analysis. We cannot exclude the possibility that there is residual confounding related to maternal infection or other unmeasured confounders, and therefore, these results should be interpreted cautiously. We are most likely not capturing all maternal infections by using ICD-coded visits. Efforts to decrease infant infection should focus on preventing preterm delivery when possible. Maternal SLE should be considered a risk factor for early neonatal infections and could be used to assess risk when considering early hospital discharge.
The current study has several strengths. We used a register-based linkage of prospectively collected, population-based data of the entire Swedish population and their infants including the most up-to-date data with follow-up until 31 December 2022. We used a register-based definition of SLE which has good validity.9 Also, the results of this study can be generalisable to populations with universal access to healthcare.
We realise that our study also has several limitations. We do not have information on breastfeeding, which is associated with a lower risk of respiratory infections with fever, middle ear infection and infective gastroenteritis in infants.19 Women with SLE may breastfeed their infants less, especially those born preterm, than general population comparators. While most SLE medications are likely safe in breastfeeding, worries about medication use is an important reason for women with SLE not to breastfeed.20 21 Also, there is evidence that infants exposed to maternal SLE born preterm are less likely to be breastfed than babies born at term.20 21 Mothers with SLE might seek and/or receive more healthcare than mothers without SLE which would result in more registered outpatient infections and more prescriptions of anti-infectives. By using ICD and ATC codes to identify infant infections, the exact cause of the infection is not clear as we did not have access to laboratory results and not all infants may have received a laboratory test for the type of infection. The national registers do not capture information on disease activity, clinical phenotype or severity and thus were not accounted for in this study. Lupus disease activity is an important risk factor for pregnancy complications including preterm delivery.22 Lupus disease activity and phenotype are strongly related to medication use, and all of these factors could affect infant infection. We do not have information on rituximab use during pregnancy, which depletes B cells in the mother and baby and affects infant infection risk. However, during the study’s time period, rituximab was not recommended for use during pregnancy, except in extremely rare cases, according to treatment guidelines by the Swedish Society of Rheumatology. Therefore, we do not believe that rituximab has greatly affected our results. Future studies should investigate the relationship between SLE-related characteristics and infant infection with more clinically detailed data, with a focus on preterm infants who carry the majority of the risk. Some analyses were limited in power, resulting in wide confidence intervals, and the proportion-mediated estimates are unstable when sample sizes are small.23 Although we adjusted for several confounders of the exposure–mediator and mediator–outcome relationships, there is likely to be residual confounding.
In conclusion, the risk of infection in infants born to mothers with SLE is slightly increased during the first year of life. The relative risk is highest during the first days after birth, and some of the increased risk was accounted for by preterm birth. The role of maternal infection, immune dysfunction and/or inflammation should be clarified in future studies. Our findings underscore the importance of preventing preterm delivery whenever possible, but preterm delivery may not be avoidable in some circumstances, and it is sometimes necessary for the health of the mother and infant. Therefore, to prevent early neonatal infections, maternal SLE could be considered as a risk factor before allowing early discharge from postnatal care. Awareness about the higher infection risk in the first weeks of life, avoiding crowds and people with infections and vigilance about infection symptoms should perhaps be recommended to mothers with SLE.
supplementary material
Footnotes
Funding: This study was funded by the Ingegerd Johansson Donation (Swedish Society of Medicine SLS-714651).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-090555).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The individual-level data used in this study cannot be publicly made available due to legal restrictions. Please send any requests for the study data to the corresponding author.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: Ethical approval was granted by the Ethics Review Authority in Sweden (DNR 2021–01148).
Contributor Information
Sofie A M Gernaat, Email: S.A.M.Gernaat-2@umcutrecht.nl.
Julia F Simard, Email: jsimard@stanford.edu.
Maria Altman, Email: maria.altman@ki.se.
Elisabet Svenungsson, Email: Elisabet.Svenungsson@ki.se.
Elizabeth V Arkema, Email: Elizabeth.arkema@ki.se.
Data availability statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available.
References
- 1.Molokhia M, Maconochie N, Patrick AL, et al. Cross-sectional analysis of adverse outcomes in 1,029 pregnancies of Afro-Caribbean women in Trinidad with and without systemic lupus erythematosus. Arthritis Res Ther. 2007;9:R124. doi: 10.1186/ar2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan Yuen S, Krizova A, Ouimet JM, et al. Pregnancy outcome in systemic lupus erythematosus (SLE) is improving: Results from a case control study and literature review. Open Rheumatol J. 2008;2:89–98. doi: 10.2174/1874312900802010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnabe C, Faris PD, Quan H. Canadian pregnancy outcomes in rheumatoid arthritis and systemic lupus erythematosus. Int J Rheumatol. 2011;2011:345727. doi: 10.1155/2011/345727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkema EV, Palmsten K, Sjöwall C, et al. What to Expect When Expecting With Systemic Lupus Erythematosus (SLE): A Population‐Based Study of Maternal and Fetal Outcomes in SLE and Pre‐SLE. Arthritis Care & Research . 2016;68:988–94. doi: 10.1002/acr.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei S, Lai K, Yang Z, et al. Systemic lupus erythematosus and risk of preterm birth: a systematic review and meta-analysis of observational studies. Lupus (Los Angel) 2017;26:563–71. doi: 10.1177/0961203316686704. [DOI] [PubMed] [Google Scholar]
- 6.Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390:1770–80. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 7.Assuring Healthy O. In: Preterm Birth: Causes, Consequences, and Prevention. Washington (DC): National Academies Press (US) Copyright © 2007, National Academy of Sciences. Behrman RE, Butler AS, editors. 2007. The national academies collection: reports funded by national institutes of health. [PubMed] [Google Scholar]
- 8.Steiner L, Diesner SC, Voitl P. Risk of infection in the first year of life in preterm children: An Austrian observational study. PLoS One. 2019;14:e0224766. doi: 10.1371/journal.pone.0224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arkema EV, Jönsen A, Rönnblom L, et al. Case definitions in Swedish register data to identify systemic lupus erythematosus. BMJ Open. 2016;6:e007769. doi: 10.1136/bmjopen-2015-007769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sørup S, Benn CS, Poulsen A, et al. Simultaneous vaccination with MMR and DTaP-IPV-Hib and rate of hospital admissions with any infections: A nationwide register based cohort study. Vaccine (Auckl) 2016;34:6172–80. doi: 10.1016/j.vaccine.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JE, Hammond GC, Strunk T, et al. Association of gestational age and growth measures at birth with infection-related admissions to hospital throughout childhood: a population-based, data-linkage study from Western Australia. Lancet Infect Dis. 2016;16:952–61. doi: 10.1016/S1473-3099(16)00150-X. [DOI] [PubMed] [Google Scholar]
- 12.Bröms G, Kieler H, Ekbom A, et al. Paediatric infections in the first 3 years of life after maternal anti-TNF treatment during pregnancy. Aliment Pharmacol Ther. 2020;52:843–54. doi: 10.1111/apt.15971. [DOI] [PubMed] [Google Scholar]
- 13.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Díaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. doi: 10.1136/bmj.b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–50. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanderweele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York: Oxford University Press; 2015. [Google Scholar]
- 17.Bender Ignacio RA, Madison AT, Moshiri A, et al. A Population‐based Study of Perinatal Infection Risk in Women with and without Systemic Lupus Erythematosus and their Infants. Paediatric Perinatal Epid . 2018;32:81–9. doi: 10.1111/ppe.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simard JF, Chaichian Y, Rossides M, et al. Preterm Delivery Phenotypes in Systemic Lupus Erythematosus Pregnancies. Am J Perinatol. 2019;36:964–8. doi: 10.1055/s-0038-1675648. [DOI] [PubMed] [Google Scholar]
- 19.Frank NM, Lynch KF, Uusitalo U, et al. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019;19:339. doi: 10.1186/s12887-019-1693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikram N, Eudy A, Clowse MEB. Breastfeeding in women with rheumatic diseases. Lupus Sci Med . 2021;8:e000491. doi: 10.1136/lupus-2021-000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noviani M, Wasserman S, Clowse MEB. Breastfeeding in mothers with systemic lupus erythematosus. Lupus (Los Angel) 2016;25:973–9. doi: 10.1177/0961203316629555. [DOI] [PubMed] [Google Scholar]
- 22.Clowse MEB, Magder LS, Witter F, et al. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005;52:514–21. doi: 10.1002/art.20864. [DOI] [PubMed] [Google Scholar]
- 23.Mackinnon DP, Warsi G, Dwyer JH. A Simulation Study of Mediated Effect Measures. Multivariate Behav Res. 1995;30:41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]