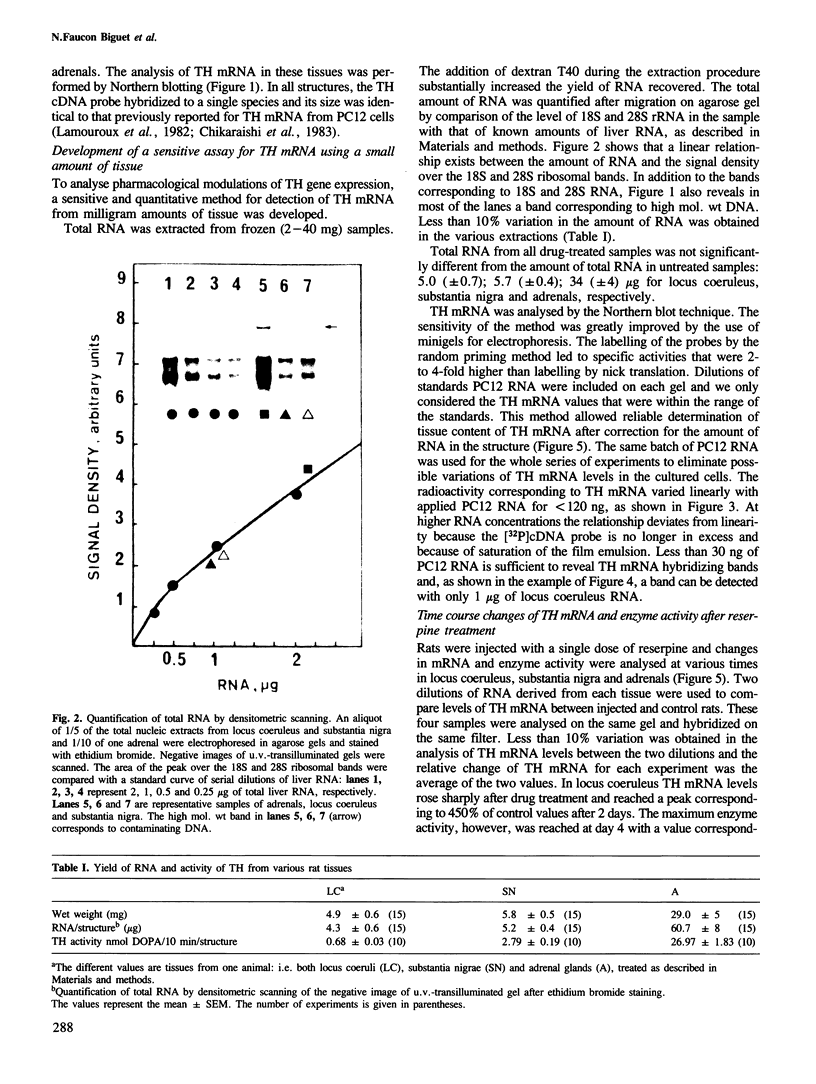
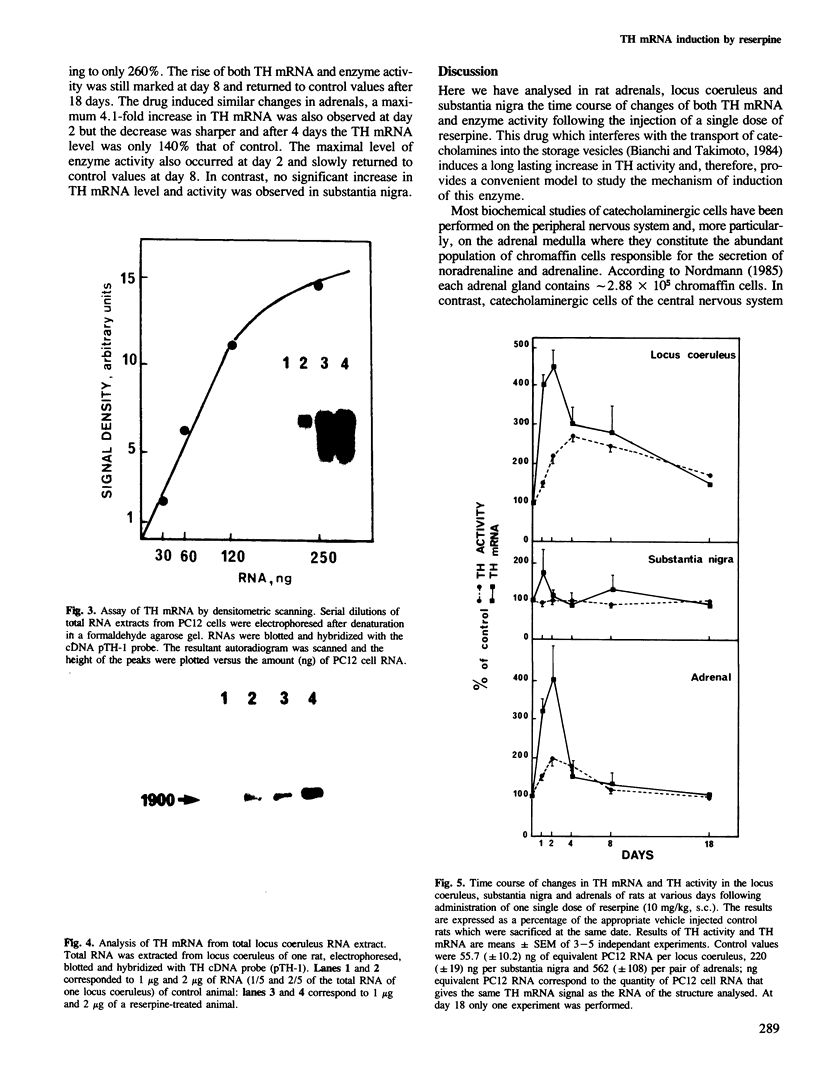
Abstract
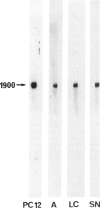
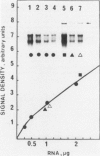
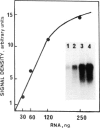
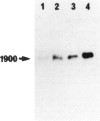
A single injection of reserpine causes a long lasting enhancement of the activity of tyrosine hydroxylase (TH), the enzyme catalyzing the rate-limiting step in the biosynthesis of catecholamines. A sensitive method has been developed to assay both TH mRNA level and enzyme activity in tissue from a single rat. The time course of the induction was analysed in adrenals, locus coeruleus and substantia nigra. In both locus coeruleus and adrenals reserpine caused respectively 4.2- and 4.5-fold increase of TH mRNA which was maximal 2 days after drug injection. This increase is about twice that of the enzyme activity. No change was observed in substantia nigra. The effect lasted longer in locus coeruleus than in adrenal. In the latter, TH mRNA had almost returned to initial values at day 4 whereas at this time it is 3-fold higher in locus coeruleus and still significant at day 18. This result suggests that induction of TH results from an enhanced transcription of the TH gene. The time course difference between locus coeruleus and adrenals is most likely to result from a difference in the stability of TH mRNA in the two structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi B. R., Takimoto G. S. Lability in storage of 3H-dopamine and 3H-norepinephrine in crude synaptosome (P2) and vesicle-associated fractions of rat brain. Life Sci. 1984 Feb 13;34(7):607–615. doi: 10.1016/0024-3205(84)90223-6. [DOI] [PubMed] [Google Scholar]
- Black I. B., Chikaraishi D. M., Lewis E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985 Jul 22;339(1):151–153. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- Buda M., Roussel B., Renaud B., Pujol J. F. Increase in tyrosine hydroxylase activity in the locus coeruleus of the rat brain after contralateral lesioning. Brain Res. 1975 Aug 15;93(3):564–569. doi: 10.1016/0006-8993(75)90200-0. [DOI] [PubMed] [Google Scholar]
- Coyle J. T. Tyrosine hydroxylase in rat brain--cofactor requirements, regional and subcellular distribution. Biochem Pharmacol. 1972 Jul 15;21(14):1935–1944. doi: 10.1016/0006-2952(72)90006-8. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Affolter H. U., Herbert E., Hotchkiss A. J. Alternative modes of enkephalin biosynthesis regulation by reserpine and cyclic AMP in cultured chromaffin cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3949–3953. doi: 10.1073/pnas.81.13.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux A., Blanot F., Biguet N. F., Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):617–621. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Costa E. Commentary: Trans-synaptic regulation of typrosine 3-mono-oxygenase biosynthesis in rat adrenal medulla. Biochem Pharmacol. 1977 May 1;26(9):817–823. doi: 10.1016/0006-2952(77)90393-8. [DOI] [PubMed] [Google Scholar]
- Hefti F., Gnahn H., Schwab M. E., Thoenen H. Induction of tyrosine hydroxylase by nerve growth factor and by elevated K+ concentrations in cultures of dissociated sympathetic neurons. J Neurosci. 1982 Nov;2(11):1554–1566. doi: 10.1523/JNEUROSCI.02-11-01554.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joh T. H., Baetge E. E., Ross M. E., Reis D. J. Evidence for the existence of homologous gene coding regions for the catecholamine biosynthetic enzymes. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):327–335. doi: 10.1101/sqb.1983.048.01.036. [DOI] [PubMed] [Google Scholar]
- Joh T. H., Geghman C., Reis D. Immunochemical demonstration of increased accumulation of tyrosine hydroxylase protein in sympathetic ganglia and adrenal medulla elicited by reserpine. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2767–2771. doi: 10.1073/pnas.70.10.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Changeux J. P. Activity regulates the levels of acetylcholine receptor alpha-subunit mRNA in cultured chicken myotubes. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4558–4562. doi: 10.1073/pnas.82.13.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouroux A., Faucon Biguet N., Samolyk D., Privat A., Salomon J. C., Pujol J. F., Mallet J. Identification of cDNA clones coding for rat tyrosine hydroxylase antigen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3881–3885. doi: 10.1073/pnas.79.12.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J., Faucon Biguet N., Buda M., Lamouroux A., Samolyk D. Detection and regulation of the tyrosine hydroxylase mRNA levels in rat adrenal medulla and brain tissues. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):305–308. doi: 10.1101/sqb.1983.048.01.033. [DOI] [PubMed] [Google Scholar]
- Mallet J., Faucon Biguet N., Buda M., Lamouroux A., Samolyk D. Detection and regulation of the tyrosine hydroxylase mRNA levels in rat adrenal medulla and brain tissues. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):305–308. doi: 10.1101/sqb.1983.048.01.033. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Translation of exogenous mRNAs in reticulocyte lysates. Methods Enzymol. 1983;101:606–615. doi: 10.1016/0076-6879(83)01041-1. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci. 1978;1:129–169. doi: 10.1146/annurev.ne.01.030178.001021. [DOI] [PubMed] [Google Scholar]
- Moore R. Y., Bloom F. E. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Mueller R. A., Thoenen H., Axelrod J. Inhibition of trans-synaptically increased tyrosine hydroxylase activity by cycloheximide and actinomycin D. Mol Pharmacol. 1969 Sep;5(5):463–469. [PubMed] [Google Scholar]
- Nordmann J. J. Combined stereological and biochemical analysis of storage and release of catecholamines in the adrenal medulla of the rat. J Neurochem. 1984 Feb;42(2):434–437. doi: 10.1111/j.1471-4159.1984.tb02696.x. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Joh T. H., Ross R. A. Effects of reserpine on activities and amounts of tyrosine hydroxylase and dopamine-beta-hydroxylase in catecholamine neuronal systems in rat brain. J Pharmacol Exp Ther. 1975 Jun;193(3):775–784. [PubMed] [Google Scholar]
- Reis D. J., Joh T. H., Ross R. A., Pickel V. M. Reserpine selectively increases tyrosine hydroxylase and dopamine-beta-hydroxylase enzyme protein in central noradrenergic neurons. Brain Res. 1974 Dec 6;81(2):380–386. doi: 10.1016/0006-8993(74)90956-1. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Induction of tyrosine hydroxylase in peripheral and central adrenergic neurones by cold-exposure of rats. Nature. 1970 Nov 28;228(5274):861–862. doi: 10.1038/228861a0. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Mueller R. A., Axelrod J. Trans-synaptic induction of adrenal tyrosine hydroxylase. J Pharmacol Exp Ther. 1969 Oct;169(2):249–254. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond R. E. Tyrosine hydroxylase activity in noradrenergic neurons of the locus coeruleus after reserpine administration: sequential increase in cell bodies and nerve terminals. J Neurochem. 1979 Jan;32(1):23–29. doi: 10.1111/j.1471-4159.1979.tb04505.x. [DOI] [PubMed] [Google Scholar]