Key Points
Question
Is the timing of phenotype-desirable antimicrobial therapy (PDAT; ie, a β-lactam antibiotic with the narrowest spectrum of activity to effectively treat the pathogen’s phenotype) associated with clinical outcomes among patients hospitalized with Enterobacterales bloodstream infections?
Findings
In this cohort study with 8193 adult patients, those receiving early PDAT had a 52.5% probability of a more desirable clinical outcome than patients receiving delayed PDAT. The observation persisted in the adjusted analysis, as patients receiving early PDAT had a 52.0% probability for a better clinical outcome than patients receiving delayed PDAT.
Meaning
In this study, receiving early PDAT was associated with favorable 30-day clinical outcomes among patients hospitalized with Enterobacterales bloodstream infections.
This cohort study evaluates whether the timing of phenotype-desirable antimicrobial therapy (PDAT; ie, receipt of a β-lactam antibiotic with the narrowest spectrum of activity to effectively treat the pathogen’s phenotype) is associated with clinical outcomes among hospitalized patients.
Abstract
Importance
Initiating effective therapy early is associated with improved survival among patients hospitalized with gram-negative bloodstream infections; furthermore, providing early phenotype-desirable antimicrobial therapy (PDAT; defined as receipt of a β-lactam antibiotic with the narrowest spectrum of activity to effectively treat the pathogen’s phenotype) is crucial for antimicrobial stewardship. However, the timing of targeted therapy among patients hospitalized with gram-negative bloodstream infections is not well understood.
Objective
To compare the clinical outcomes between patients who were hospitalized with Enterobacterales bloodstream infections receiving early vs delayed PDAT.
Design, Setting, and Participants
This retrospective cohort study used a large, geographically diverse, hospital-based US database (PINC AI Healthcare Database). Participants were adult (aged ≥18 years) patients with an inpatient admission between January 1, 2017, and June 30, 2022, with at least 1 blood culture isolate positive for Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, or Proteus mirabilis and receiving PDAT on blood culture collection days 0 to 4.
Exposure
Early vs delayed PDAT, with early PDAT defined as receipt of PDAT on blood culture collection days 0 to 2.
Main Outcomes and Measures
The main outcome was desirability of outcome ranking, in which patients were assigned a mutually exclusive rank 1 through 5. Rank 1 indicated the most desirable outcome (alive with no events), whereas rank 5 indicated the least desirable outcome and included all patients who died within 30 days of blood culture collection.
Results
Among 8193 eligible patients (mean [SD] age, 69.0 [16.4] years; 4758 [58.1%] female; 1200 [14.6%] African American or Black, 729 [8.9%] Hispanic, and 5778 [70.5%] White) from 252 hospitals, 5033 (61.4%) received early PDAT. Patients receiving early PDAT were similar in age (mean [SD], 68.2 [16.9] vs 70.3 [15.6] years) but more likely to have a lower median (IQR) Charlson-Deyo comorbidity score (2 [1-5] vs 3 [1-5]) compared with patients receiving delayed PDAT. After adjusting for comorbidities and severity of illness, patients receiving early PDAT were 20% less likely to be readmitted within 30 days compared with those receiving delayed PDAT (odds ratio, 0.80; 95% CI, 0.69-0.92; P < .001). A higher percentage of patients receiving early PDAT had a desirability of outcome ranking of 1 compared with patients receiving delayed PDAT (56.3% vs 52.2%, P < .001). Those receiving early PDAT had a 52.5% probability (95% CI, 51.3%-53.7%) of a more desirable outcome than those receiving delayed PDAT, a finding that persisted in the adjusted analysis (probability, 52.0%; 95% CI, 50.9%-53.2%).
Conclusions and Relevance
Receiving early PDAT was associated with favorable 30-day clinical outcomes among patients hospitalized with Enterobacterales blood stream infections. Early PDAT may be important not only for antimicrobial stewardship but also for improving patient outcomes.
Introduction
Conventional antimicrobial susceptibility testing (AST) methods may take 2 to 4 days from blood culture collection (BCC) to results.1 Because of delays in organism identification and AST results, there are subsequent delays in optimizing antibiotic therapy that can negatively impact outcomes in patients with bloodstream infections (BSIs).2,3,4 Among patients hospitalized with BSI in the United States receiving systemic antibiotics, approximately 1 in every 5 received discordant empirical agents.5 Empirical treatment is prone to introducing either ineffective or unnecessarily broad-spectrum antibiotic therapy, both with their unique downstream consequences.2,6,7 Discordant antibiotic therapy, particularly undertreatment, is more likely to occur among patients infected with antibiotic-resistant pathogens and result in overall poor patient outcomes, such as increased risk of mortality.5,6 On the other hand, broad-spectrum antibiotic therapy may also increase the risk of adverse patient events and contribute to antibiotic resistance, and in some populations, overtreatment is associated with increased mortality.6,7,8 Earlier administration of phenotype-desirable antimicrobial therapy (PDAT; the agent with the narrowest spectrum of activity that effectively treats the causative pathogen) and earlier deescalation of broad-spectrum agents can potentially lead to better outcomes, fewer antibiotic-associated adverse events, and reduced emergence of antibiotic resistance.9,10,11
Although clinicians strive to provide timely PDAT further research is warranted to understand the impact of the timing of PDAT on clinical outcomes. In addition, traditional noninferiority approaches in assessing clinical outcomes do not account for both benefits and harms.12 The desirability of outcome ranking (DOOR) analysis allows for a holistic patient assessment by combining the benefits and risks of the proposed therapy into a single outcome measure.12,13 This study aimed to compare the clinical outcomes between patients receiving early vs delayed PDAT among patients hospitalized with Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, or Proteus mirabilis BSIs using the DOOR analysis.
Methods
Data Source and Study Design
We performed a retrospective observational cohort study using the Premier PINC AI Healthcare Database (PHD).14 The PHD is an all-payer hospital administrative database for geographically diverse inpatient and outpatient visits from more than 1300 hospitals.14,15 The standard hospital discharge files include demographic characteristics, disease states, and a time-stamped log of billed items (eg, procedures, medications, laboratory services, and diagnostic services) at the patient level as well as geographic location, rural/urban populations served, teaching status, and bed capacity at the hospital-level. A subset of hospitals (approximately 25%) submit microbiology laboratory data to the PHD. All data are statistically deidentified and compliant with the Health Insurance Portability and Accountability Act. Based on US Title 45 Code of Federal Regulations, Part 46, the study was exempt from institutional review board approval. We did not pursue informed consent from study participants due to the nature of deidentified data. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)16 reporting guideline.
Study Population
Adults (aged ≥18 years) who had an inpatient visit between January 1, 2017, and June 30, 2022; had at least 1 blood culture isolate belonging to E coli, K oxytoca, K pneumoniae, or P mirabilis; and received effective β-lactam or select oral antibiotic therapy within 2 days of BCC (eFigure 1 in Supplement 1) were included in the study. Patients were excluded if they (1) had polymicrobial infection, defined as positive cultures belonging to more than 1 pathogen from blood or any other site within 30 days before and 5 days after the index BCC date; (2) did not have AST results available by BCC day 7; (3) had insufficient AST results to define susceptibility profile; (4) did not receive systemic β-lactam or select oral antibiotic treatment for at least 3 consecutive days on or after BCC day 0; (5) had an intra-abdominal infection (ie, principal or secondary diagnosis of peritonitis, cholangitis, diverticulitis, cholecystitis, pancreatitis, appendicitis, pseudocyst, or intra-abdominal abscess); (6) were transferred from another acute-care facility; (7) died, were discharged, or were transferred to another hospital within 2 days of BCC day 0; and 8) were from hospitals without continuous data submission during the 3 months before and 30 days after the visit. If a patient had multiple hospitalizations meeting the selection criteria, the earliest admission meeting all inclusion criteria was considered the index hospitalization.
Definitions of Early vs Delayed PDAT
PDAT was defined as a β-lactam antibiotic with the narrowest spectrum of activity to effectively treat the causative pathogen, as denoted in the modified Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR-MAT) framework (eFigure 4 in Supplement 1). β-Lactam antibiotics were cross-classified by spectrum of activity for 4 possible resistance phenotypes using the DOOR-MAT framework, adapted from approach 1 (naive) in the paper by Perez et al17 and described in detail elsewhere.7,18 Briefly, DOOR-MAT ranks antimicrobials based on desirability in the context of antimicrobial spectrum, the isolate’s resistance profile, and antimicrobial stewardship goals. Only patients receiving PDAT within 4 days of BCC were included in the analysis (eFigure 1 in Supplement 1). Among these patients, early PDAT was defined as PDAT within 2 days of BCC (day 0 to 2) and delayed PDAT otherwise (BCC day 3 to 4).
Ordinal Outcomes for DOOR
Patients were assigned a mutually exclusive rank 1 through 5 (Box). Rank 1 indicated the most desirable outcome and included anyone who was alive and did not experience any of the undesirable events within 30 days of the reference date (either BCC day 0 or discharge date), whereas rank 5 indicated the least desirable outcome and included all patients who died within 30 days of BCC. In-hospital mortality and the 8 undesirable events were used for ranking as described in the Box.
Box. Ordinal Outcomes for DOORs.
DOOR Rankings
1, Alive with no events
2, Alive with 1 event
3, Alive with 2 events
4, Alive with ≥3 events
5, In-hospital mortality within BCC day 30
Eventsa
Alive in hospital on BCC day 30 or discharged not to home by BCC day 30
Readmission within 30 d from discharge date
Transfer to intensive care unit within BCC day 30
Acquisition of multidrug-resistant organisms within BCC day 30
HO-CDI within BCC day 30
New indication for invasive mechanical ventilator use within BCC day 30
New indication for kidney replacement therapy within BCC day 30
New indication for vasopressor use within BCC day 30
These DOOR events were based on a previously validated study with a similar scope and framework by Tamma et al.19 We made the following modifications due to the limitations using an administrative database. First, because clinical response could not be directly assessed, transfer to intensive care unit and new indications for invasive mechanical ventilator, kidney replacement therapy, and vasopressor use within 30 days of BCC were used as proxies of clinical response. New indications were defined as receiving invasive mechanical ventilation, kidney replacement therapy, or vasopressor between index BCC day 2 through 30, only among patients who did not receive invasive mechanical ventilation, kidney replacement therapy, and vasopressor between admission date and index BCC day 2. Kidney replacement therapy was only assessed among patients without prior evidence of kidney replacement therapy, chronic kidney disease, or acute kidney injury (AKI; ie, if AKI was present on admission, dialysis-related procedures were recorded within 90 days on or prior to index BCC day 0, or chronic kidney disease–related diagnosis is recorded 180 days on or prior to index BCC day 0). Second, because the event rate was very low for relapse of BSI, we used acquisition of multidrug-resistant organisms and hospital-onset Clostridioides difficile infection (CDI) within 30 days of BCC as markers of adverse antibiotic events. Acquisition of multidrug-resistant organisms was defined when Staphylococcus aureus, Enterococci, Enterobacterales, Acinetobacter, or Pseudomonas aeruginosa was detected in culture results of any specimen and had corresponding antimicrobial susceptibility testing results as defined by the US Centers of Disease Control and Prevention20 between index BCC days 3 and 30. Hospital-onset CDI was defined using principal or secondary ICD-10-CM discharge diagnosis code of CDI with “present on admission” flag not equal to yes during index visit or a positive C difficile culture or molecular testing result plus CDI treatment (receiving oral vancomycin, fidaxomicin, or metronidazole) between index BCC days 3 through 30, or principal or secondary ICD-10-CM discharge diagnosis code CDI during any subsequent inpatient or outpatient visit to the same hospital within 30 days.
Patient, Hospital, and Visit Characteristics and Health Care Cost
Baseline patient characteristics, including age, sex, race (African American or Black, Asian, White, or other [American Indian or Alaska Native, Pacific Islander, and multiracial]), and ethnicity (Hispanic, non-Hispanic, and unknown), and hospital characteristics, including geographical region, hospital size, urbanicity of served population, and teaching status, were provided by the hospitals. Charlson-Deyo comorbidities and AKI were identified during the index visit and any visit to the same hospital within 6 months prior to the index visit using International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) and ICD-10, Procedural Coding System (ICD-10-PCS) codes (eTables 1 and 2 in Supplement 1). The Charlson-Deyo Comorbidity Index (CCI) score was calculated using a previously validated method.21,22
Administration of antibiotic agents was identified using hospital chargemaster descriptions and recorded in reference to BCC date (BCC day 0 to 4). If a patient received more than 1 applicable agent on the same day, all were captured (ie, the number of effective antibiotics could be greater than the number of effectively treated patients). Length of hospital stay (LOS) was reported by the hospitals. Index hospitalization cost included the sum of all costs incurred by the hospital (ie, room and board, pharmacy, laboratory, imaging, and central supply) during index hospitalization, and 30-day follow-up costs included the sum of all costs incurred by the hospital during any subsequent inpatient or outpatient visits within 30 days from the discharge date of index hospitalization. All costs were adjusted to 2022 US dollars based on the Consumer Price Index for inpatient services.23
Statistical Analysis
Descriptive statistics were used to present baseline patient and hospital characteristics of patients with gram-negative BSIs and their outcomes. Continuous variables were reported as mean (SD) or median (IQR), and categorical variables were reported as counts and percentages. For statistical difference between 2 groups, the Student t test or Mann-Whitney test was used for continuous variables, as indicated, and the Pearson χ2 test or Fisher exact test for categorical variables. Statistical significance was defined as P < .05.
Adjustment using inverse probability weighting (IPW) based on propensity scores was performed because we observed that patients receiving early PDAT were more likely to have medical conditions independently associated with less desirable outcomes compared with patients receiving delayed PDAT. Age category, sex, race, ethnicity, year of admission, admission from intermediate or long-term care facility, phenotype pattern, onset of BSI (community vs hospital), COVID-19 diagnosis, CCI score category, AKI present on admission, vasopressor use within 2 days of BCC, invasive mechanical ventilator use within 2 days of BCC, and hospital characteristics (ie, hospital size, teaching status, urbanicity of served population, and geographic region) were selected as covariates in calculating propensity scores. Race and ethnicity were included because patient demographic characteristics can influence treatment and outcome. Absolute standardized mean differences in covariates between early and delayed PDAT groups after IPW were all less than 0.05 (eFigure 2 in Supplement 1).
The primary outcome was IPW-adjusted DOOR proportions, and the probability that a randomly selected patient with early PDAT had a more desirable DOOR (vs patient with delayed PDAT) was determined using a previously validated method and tool.12,19,24 A probability of 50% implied no difference between DOOR distribution of the 2 groups, whereas a probability greater than 50% with a 95% CI that excludes 50% implied patients with early PDAT have more desirable outcomes than patients with delayed PDAT. Bootstrapping with 1000 replicates with replacement was used to calculate the 95% CI. All analyses and figures were performed and generated using R version 3.6.3 or higher (R Foundation for Statistical Computing).
Results
We identified 86 177 hospitalized adult patients with blood culture isolates belonging to the gram-negative pathogens of interest. Among these patients, 8193 patients (9.5%; mean [SD] age, 69.0 [16.4] years; 4758 [58.1%] female; 1200 [14.6%] African American or Black, 729 [8.9%] Hispanic, and 5778 [70.5%] White) from 252 hospitals met all study selection criteria and received PDAT (eFigure 1 in Supplement 1). Among patients receiving PDAT, 5033 (61.4%) received early PDAT. Overall, the mean (SD) time to the first AST result was 72.0 (26.7) hours. There was only a 2-hour difference in time to results between patients in the early vs delayed PDAT groups (mean [SD], 71.2 [27.7] hours vs 73.2 [24.9] hours).
Patients receiving early PDAT, compared with those receiving delayed PDAT, were more likely to be Hispanic (509 [10.1%] vs 220 [7.0%]), younger (mean [SD] age, 68.2 [16.9] vs 70.3 [15.6] years), and less likely to have Medicare (3425 [68.1%] vs 2321 [73.4%] (Table 1). They were also more likely to visit a small hospital (1-299 beds: 2026 [40.3%] vs 1188 [37.6%]), a rural hospital (852 [16.9%] vs 414 [13.1%]), and hospitals in the West (347 [6.9%] vs 136 [4.3%]). Patients receiving early PDAT had a lower median (IQR) CCI score than patients receiving delayed PDAT (2.0 [1.0-5.0] vs 3.0 [1.0-5.0]).
Table 1. Demographic, Hospital, and Visit and Clinical Characteristics of Patients Hospitalized With Gram-Negative Bloodstream Infection, Stratified by the Timing of PDAT.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Overall (n = 8193) | PDAT | ||
| Early (n = 5033) | Delayed (n = 3160) | ||
| Patient demographic characteristics | |||
| Age category, y | |||
| 18-39 | 535 (6.5) | 366 (7.3) | 169 (5.3) |
| 40-59 | 1404 (17.1) | 919 (18.3) | 485 (15.3) |
| 60-74 | 2835 (34.6) | 1726 (34.3) | 1109 (35.1) |
| ≥75 | 3419 (41.7) | 2022 (40.2) | 1397 (44.2) |
| Age, continuous, y | |||
| Mean (SD) | 69.0 (16.4) | 68.2 (16.9) | 70.3 (15.6) |
| Median (IQR) | 71.0 (60.0-81.0) | 71.0 (59.0-81.0) | 72.0 (62.0-82.0) |
| Sex | |||
| Male | 3435 (41.9) | 2079 (41.3) | 1356 (42.9) |
| Female | 4758 (58.1) | 2954 (58.7) | 1804 (57.1) |
| Race | |||
| African American or Black | 1200 (14.6) | 677 (13.5) | 523 (16.6) |
| Asian | 396 (4.8) | 269 (5.3) | 127 (4.0) |
| White | 5778 (70.5) | 3549 (70.5) | 2229 (70.5) |
| Othera | 703 (8.6) | 460 (9.1) | 243 (7.7) |
| Unknown | 116 (1.4) | 78 (1.5) | 38 (1.2) |
| Ethnicity | |||
| Hispanic | 729 (8.9) | 509 (10.1) | 220 (7.0) |
| Non-Hispanic | 6393 (78.0) | 3854 (76.6) | 2539 (80.3) |
| Unknown | 1071 (13.1) | 670 (13.3) | 401 (12.7) |
| Insurance type | |||
| Medicare | 5746 (70.1) | 3425 (68.1) | 2321 (73.4) |
| Medicaid | 870 (10.6) | 571 (11.3) | 299 (9.5) |
| Commercial insurance | 1140 (13.9) | 746 (14.8) | 394 (12.5) |
| Uninsured | 273 (3.3) | 187 (3.7) | 86 (2.7) |
| Other or unknown | 164 (2.0) | 104 (2.1) | 60 (1.9) |
| Hospital characteristics | |||
| Hospital size | |||
| 1-299 beds | 3214 (39.2) | 2026 (40.3) | 1188 (37.6) |
| 300-499 beds | 2029 (24.8) | 1204 (23.9) | 825 (26.1) |
| ≥500 beds | 2950 (36.0) | 1803 (35.8) | 1147 (36.3) |
| Teaching status | |||
| Teaching hospital | 3690 (45.0) | 2264 (45.0) | 1426 (45.1) |
| Nonteaching hospital | 4503 (55.0) | 2769 (55.0) | 1734 (54.9) |
| Population served | |||
| Rural | 1266 (15.5) | 852 (16.9) | 414 (13.1) |
| Urban | 6927 (84.5) | 4181 (83.1) | 2746 (86.9) |
| Geographic location | |||
| Midwest | 1146 (14.0) | 699 (13.9) | 447 (14.1) |
| Northeast | 1791 (21.9) | 1021 (20.3) | 770 (24.4) |
| South | 4773 (58.3) | 2966 (58.9) | 1807 (57.2) |
| West | 483 (5.9) | 347 (6.9) | 136 (4.3) |
| Visit and clinical characteristics | |||
| Charlson-Deyo comorbidities | |||
| Myocardial infarction | 1176 (14.4) | 723 (14.4) | 453 (14.3) |
| Congestive heart failure | 2100 (25.6) | 1221 (24.3) | 879 (27.8) |
| Peripheral vascular disease | 859 (10.5) | 466 (9.3) | 393 (12.4) |
| Cerebrovascular disease | 1023 (12.5) | 594 (11.8) | 429 (13.6) |
| Dementia | 1491 (18.2) | 878 (17.4) | 613 (19.4) |
| Chronic pulmonary disease | 2161 (26.4) | 1323 (26.3) | 838 (26.5) |
| Rheumatic disease | 352 (4.3) | 217 (4.3) | 135 (4.3) |
| Peptic ulcer disease | 185 (2.3) | 98 (1.9) | 87 (2.8) |
| Mild liver disease | 186 (2.3) | 117 (2.3) | 69 (2.2) |
| Diabetes | 3697 (45.1) | 2233 (44.4) | 1464 (46.3) |
| Hemiplegia or paraplegia | 213 (2.6) | 119 (2.4) | 94 (3.0) |
| Kidney disease | 2627 (32.1) | 1577 (31.3) | 1050 (33.2) |
| Moderate or severe liver disease | 211 (2.6) | 121 (2.4) | 90 (2.8) |
| Any malignant neoplasm | 1288 (15.7) | 736 (14.6) | 552 (17.5) |
| Metastatic solid tumor | 444 (5.4) | 230 (4.6) | 214 (6.8) |
| HIV | 36 (0.4) | 23 (0.5) | 13 (0.4) |
| CCI score category | |||
| 0 | 1204 (14.7) | 820 (16.3) | 384 (12.2) |
| 1-4 | 4658 (56.9) | 2861 (56.8) | 1797 (56.9) |
| 5 | 2331 (28.5) | 1352 (26.9) | 979 (31.0) |
| CCI score, continuous | |||
| Mean (SD) | 3.32 (2.83) | 3.16 (2.77) | 3.56 (2.91) |
| Median (IQR) | 3.00 (1.00-5.00) | 2.00 (1.00-5.00) | 3.00 (1.00-5.00) |
| Phenotype patternsb | |||
| S-S-S-S | 3811 (46.5) | 1582 (31.4) | 2229 (70.5) |
| R-S-S-S | 2323 (28.4) | 1838 (36.5) | 485 (15.3) |
| R-R-S-S | 2055 (25.1) | 1610 (32.0) | 445 (14.1) |
| R-R-R-S | 4 (<0.1) | 3 (0.1) | 1 (<0.1) |
| Types of infection organism | |||
| Escherichia coli | 6395 (78.1) | 4071 (80.9) | 2324 (73.5) |
| Klebsiella oxytoca | 104 (1.3) | 69 (1.4) | 35 (1.1) |
| Klebsiella pneumoniae | 1124 (13.7) | 619 (12.3) | 505 (16.0) |
| Proteus mirabilis | 570 (7.0) | 274 (5.4) | 296 (9.4) |
| Acute kidney injury, present on admission | 4064 (49.6) | 2384 (47.4) | 1680 (53.2) |
| Any IMV use during index hospitalization | 408 (5.0) | 256 (5.1) | 152 (4.8) |
| IMV use within 2 d of BCC | 288 (3.5) | 189 (3.8) | 99 (3.1) |
| Vasopressor use within 2 d of BCC | 1165 (14.2) | 678 (13.5) | 487 (15.4) |
| First PDAT antibiotic | |||
| Narrow-spectrum penicillins (eg, ampicillin, amoxicillin) | 1362 (16.6) | 629 (12.5) | 733 (23.2) |
| Narrow-spectrum cephalosporins (eg, cefazolin, cephalexin) | 2603 (31.8) | 1056 (21.0) | 1547 (49.0) |
| Intermediate I (eg, ceftriaxone, cefotaxime) | 2323 (28.4) | 1838 (36.5) | 485 (15.3) |
| Intermediate II (eg, cefepime, piperacillin-tazobactam) | 0 | 0 | 0 |
| Broad (eg, ertapenem, doripenem) | 2052 (25.0) | 1609 (32.0) | 443 (14.0) |
| Broadest (eg, ceftazidime-avibactam) | 4 (<0.1) | 3 (0.1) | 1 (<0.1) |
| Time from BCC to first PDAT antibiotic, d | |||
| Mean (SD) | 1.81 (1.38) | 0.88 (0.87) | 3.29 (0.45) |
| Median (IQR) | 2.00 (0.00-3.00) | 1.00 (0.00-2.00) | 3.00 (3.00-4.00) |
Abbreviations: BCC, blood culture collection; CCI, Charlson-Deyo Comorbidity Index; IMV, invasive mechanical ventilator; PDAT, phenotype-desirable antimicrobial therapy; R-S-S-S, resistant to narrow-spectrum penicillins or cephalosporins but susceptible to immediate I or oral third-generation cephalosporins, intermediate II, broad, and broadest antibiotics; R-R-S-S, resistant to narrow-spectrum penicillins or cephalosporins and immediate I or oral third-generation cephalosporins but susceptible to intermediate II, broad, and broadest antibiotics; R-R-R-S, resistant to narrow-spectrum penicillins or cephalosporins, immediate I or oral third-generation cephalosporins, intermediate II, and broad antibiotics but susceptible to broadest antibiotics; S-S-S-S, susceptible to narrow-spectrum penicillins or cephalosporins, immediate I or oral third-generation cephalosporins, intermediate II, broad, and broadest antibiotics.
Other race includes American Indian or Alaska Native and Pacific Islander individuals as well as individuals with multiple races.
Phenotype patterns are illustrated in eFigure 4 in Supplement 1.
A higher percentage of patients receiving early PDAT compared with those receiving late PDAT had E coli infection (4071 [80.9%] vs 2324 [73.5%]) and had more resistant phenotypes (R-R-S-S or R-R-R-S, 1613 [32.1%] vs 446 [14.1%]). The most common first effective antibiotic(s) were intermediate I (56.7%), followed by intermediate II (53.8%), broad (42.1%), and broadest (27.8%) among patients receiving early PDAT (eFigure 3 in Supplement 1). For patients receiving delayed PDAT, the most common first effective antibiotics were intermediate II (71.8%), followed by intermediate I (59.3%), narrow-spectrum cephalosporins (49.0%), and narrow-spectrum penicillins (23.2%).
Early PDAT and Individual Clinical Outcomes
In-hospital mortality within 30 days of BCC was similar between patients receiving early PDAT delayed PDAT (323 [6.4%] vs 216 [6.8%]; P = .45) (Table 2). However, lower proportions of patients receiving early PDAT were either still hospitalized or discharged to a place other than home by BCC day 30 (1438 [30.5%] vs 1004 [34.1%]; P = .001) and had a readmission within 30 days of discharge (578 [12.3%] vs 435 [14.8%]; P = .002) compared with patients receiving delayed PDAT. After IPW adjustment, patients receiving early PDAT were 20% less likely to be readmitted to the same hospital within 30 days compared with patients receiving delayed PDAT (odds ratio, 0.80; 95% CI, 0.69-0.92).
Table 2. Clinical Outcomes, Health Care Resource Use, and Health Care Cost of Patients Hospitalized With Gram-Negative Bloodstream Infection, Stratified by the Timing of PDAT.
| Outcome | Patients, No. (%) | P value | ||
|---|---|---|---|---|
| Overall (N = 8193) | PDAT status | |||
| Early (n = 5033) | Delayed (n = 3160) | |||
| Individual outcomes | ||||
| 30-d In-hospital mortality | 539 (6.6) | 323 (6.4) | 216 (6.8) | .45 |
| 60-d In-hospital mortality | 656 (8.0) | 381 (7.6) | 275 (8.7) | .07 |
| Alive in hospital or discharged not to home | 2442 (31.9) | 1438 (30.5) | 1004 (34.1) | .001 |
| 30-d Readmission | 1013 (13.2) | 578 (12.3) | 435 (14.8) | .002 |
| Transfer to intensive care unit | 1230 (56.1) | 718 (56.4) | 512 (55.7) | .71 |
| Acquisition of MDRO | ||||
| Overall | 240 (2.9) | 168 (3.3) | 72 (2.3) | .006 |
| MRSA | 46 (0.6) | 29 (0.6) | 17 (0.5) | .82 |
| VRE | 43 (0.5) | 30 (0.6) | 13 (0.4) | .26 |
| CRE | 10 (0.1) | 3 (0.1) | 7 (0.2) | .04 |
| ESBL-producing Enterobacterales | 156 (1.9) | 114 (2.3) | 42 (1.3) | .003 |
| CRAsp | 2 (<0.1) | 2 (<0.1) | 0 | .26 |
| MDR-PSA | 0 | 0 | 0 | |
| 30-d HO-CDI | 127 (1.6) | 70 (1.4) | 57 (1.8) | .14 |
| 60-d HO-CDI | 165 (2.0) | 96 (1.9) | 69 (2.2) | .39 |
| New indication for invasive mechanical ventilator use | 113 (1.4) | 63 (1.3) | 50 (1.6) | .22 |
| New indication for kidney replacement therapy | 42 (1.1) | 26 (1.1) | 16 (1.2) | .68 |
| New indication for vasopressor use | 138 (2.0) | 76 (1.7) | 62 (2.3) | .09 |
| Index visit LOS, d | ||||
| Mean (SD) | 7.33 (7.17) | 7.06 (6.99) | 7.77 (7.42) | .001 |
| Median (IQR) | 5.00 (4.00-8.00) | 5.00 (4.00-8.00) | 6.00 (4.00-9.00) | |
| LOS after index blood culture collection, d | ||||
| Mean (SD) | 6.92 (5.88) | 6.62 (5.43) | 7.40 (6.52) | .001 |
| Median (IQR) | 5.00 (4.00-8.00) | 5.00 (4.00-7.00) | 6.00 (4.00-8.00) | |
| Total index hospitalization cost, 2022 US $ | ||||
| Mean (SD) | 18 677 (27 972) | 18 125 (25 535) | 19 554 (31 447) | .03 |
| Median (IQR) | 12 317 (8338-19 906) | 11 895 (8043-19 316) | 13 041 (8838-20 947) | |
| Total index hospitalization and 30-d follow-up cost, 2022 US $ | ||||
| Mean (SD) | 22 103 (32 147) | 21 193 (29 671) | 23 549 (35 691) | .002 |
| Median (IQR) | 13 778 (8995-23 927) | 13 256 (8671-22 940) | 14 860 (9577-25 247) | |
Abbreviations: CRAsp, carbapenem-resistant Acinetobacter; CRE, carbapenem-resistant Enterobacterales; ESBL, extended-spectrum β-lactamases; HO-CDI, hospital-onset Clostridioides difficile infection; LOS, length of stay; MDRO, multidrug-resistant organism; MDR-PSA, multidrug-resistant Pseudomonas aeruginosa; MRSA, methicillin-resistant Staphylococcus aureus; PDAT, phenotype-desirable antibiotic therapy; VRE, vancomycin-resistant Enterococci.
Patients receiving PDAT had a shorter mean (SD) LOS after BCC than those receiving delayed PDAT (6.6 [5.4] vs 7.4 [6.5] days, P = .001). The median (IQR) cost of index hospitalization and 30-day follow-up was higher among patients receiving delayed PDAT compared with those patients receiving early PDAT ($14 860 [$9577-$25 247] vs $13 256 [$8671-$22 940]; P = .001).
DOOR Analysis
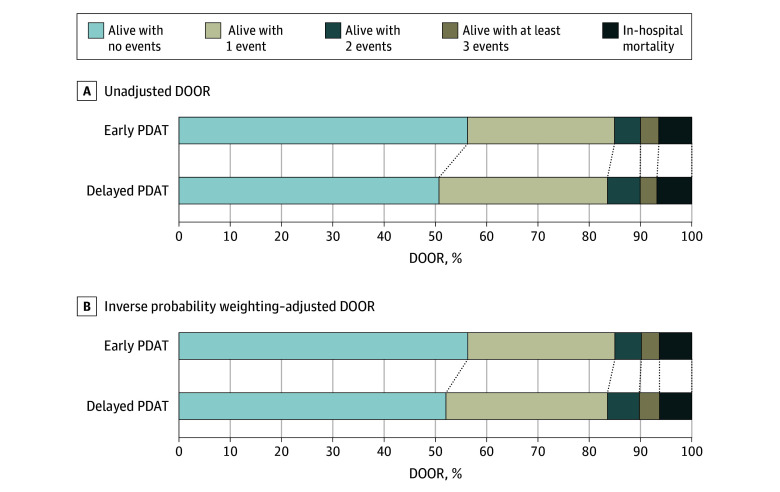
A higher percentage of patients receiving early PDAT had a DOOR of 1 (alive with no events) compared with patients receiving delayed PDAT (56.3% vs 50.8%; P = .001) (Figure 1A). After IPW adjustment, 52.2% of patients receiving delayed PDAT had a DOOR of 1 (Figure 1B).
Figure 1. Unadjusted Desirability of Outcome Ranking (DOOR) Proportions at Day 30 and Inverse Probability Weighted–Adjusted DOOR Proportions at Day 30, by Timing of Phenotype-Desirable Antimicrobial Treatment (PDAT).
In the unadjusted DOOR analysis, patients receiving early PDAT had a 52.5% probability (95% CI, 51.3%-53.7%) for a more desirable clinical outcome than patients receiving delayed PDAT. The observation persisted in the IPW-adjusted DOOR analysis, as patients receiving early PDAT had a 52.0% probability (95% CI, 50.9%-53.2%) for a better clinical outcome than patients receiving delayed PDAT (Figure 2).
Figure 2. Forest Plot Demonstrating the Inverse Probability Weighted–Adjusted Desirability of Outcome Ranking (DOOR) Analysis for Each Component.
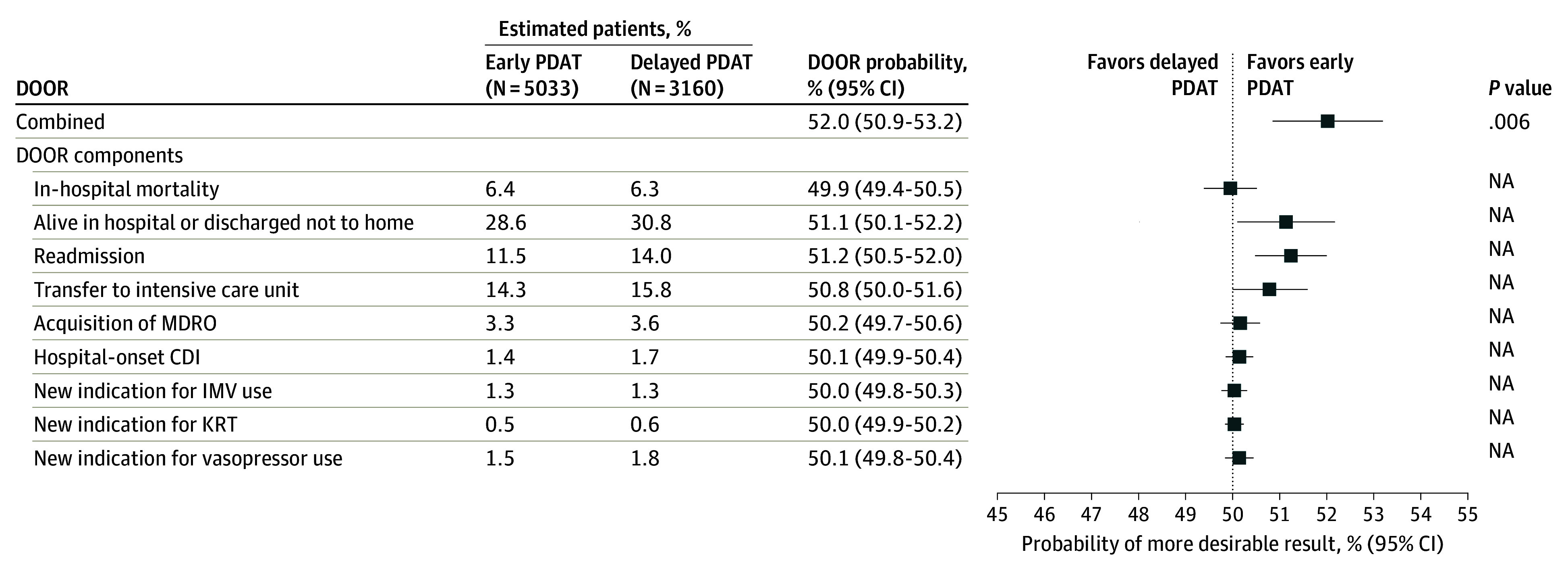
CDI indicates Clostridioides difficile infection; IMV, invasive mechanical ventilation; KRT, kidney replacement therapy; MDRO, multidrug-resistant organism; NA, not applicable; and PDAT, phenotype-desirable antimicrobial treatment.
Discussion
We used one of the largest hospital-based administrative databases in the United States to examine the differences in clinical outcomes among patients with gram-negative BSI receiving early and delayed PDAT. In addition, we implemented a holistic approach that accounts for both benefits and harms (DOOR analysis) in assessing clinical outcomes. Our findings showed that the receipt of early PDAT was associated with favorable 30-day clinical outcomes among patients hospitalized with Enterobacterales BSIs.
Many studies have demonstrated the harm associated with delayed appropriate antibiotic therapy in gram-negative BSIs, but few studies have evaluated the possible harm associated with delayed targeted antibiotic therapy (ie, PDAT).5,6,11,25 Seddon et al26 observed that more than 48 hours of antipseudomonal β-lactams (ABPL) resulted in a higher incidence of CDI compared with 48 hours or less of APBL for the treatment of Enterobacterales BSI in a propensity score matched analysis of 808 patients. Our study observed a lower rate of CDI overall (1.6%), without any significant difference between early and delayed PDAT. Teshome et al27 found that each day of broad-spectrum therapy increased the risk of infection with a multidrug-resistant organism (MDRO) in a critically ill cohort study, suggesting excessive unnecessary antibiotic exposure can contribute to antibiotic resistance development.
A randomized clinical trial (RAPIDS-GN) comparing clinical impact of rapid identification and phenotypic AST in gram-negative BSI demonstrated that gram-negative antibiotic modifications were made 24 hours faster for patients who received rapid testing methods than those who were randomized to receive conventional testing methods.28 The study was performed in areas with low prevalence of antibiotic resistance and was not sufficiently powered to detect differences in clinical outcomes. A secondary analysis of the RAPIDS-GN trial by Banerjee et al29 reported that faster and more frequent appropriate antibiotic therapy via rapid AST methods benefited the management of antibiotic-resistant E coli, Klebsiella, and Proteus bacteremia. Additionally, the proportion of patients with early PDAT (39% vs 23%) was higher with rapid testing than conventional methods for patients with susceptible pathogens.
We observed that the mean time to first AST results was approximately 3 days. Therefore, it is possible that many patients with delayed PDAT received treatment in response to AST. As described in our previous publication,18 there are potential patient factors (eg, evolution of clinical response or deterioration) and microbiology result factors (eg, notification of positive blood culture, gram stain results, molecular blood test results) that could have informed antibiotic decisions for patients receiving early PDAT.
These findings consistently point to the importance of early diagnostic testing and PDAT, especially for patients with a more resistant phenotype (ie, R-S-S-S, R-R-S-S, and R-R-R-S). Patients infected with a more resistant phenotype are less likely to receive appropriate empirical therapy and early availability of phenotypic susceptibility and/or detection of specific resistance mechanisms may improve outcomes. On the other hand, early availability of phenotypic AST may enable more timely antibiotic deescalation. The Infection Section of the European Society of Intensive Care Medicine and the European Society of Clinical Microbiology and Infectious Diseases consensus–recommended antibiotic deescalation is performed within 24 hours of definitive culture results and antibiogram availability in critically ill patients.30
In our study, while we did not observe a difference in mortality, patients receiving delayed PDAT were more likely to be still hospitalized or discharged to a place other than home (34.1% vs 30.5%) or have a readmission (14.8% vs 12.3%) within 30 days compared to patients receiving early PDAT. The overall LOS (mean, 7.4 vs 7.0 days) and LOS since BCC (mean, 6.5 vs 5.4 days) were both longer among patients receiving delayed PDAT than patients receiving early PDAT. Health care costs for both index hospitalization (mean, $31 447 vs $25 535) and index hospitalization plus 30-day follow-up (mean, $35 691 vs $29 671) were also higher for patients receiving delayed PDAT than for patients receiving early PDAT.
We extended our observation on individual clinical outcomes using DOOR to understand the totality of treatment outcomes. We observed that the overall desirability of the outcome in patients receiving early PDAT was preferable to that in patients receiving delayed PDAT in both unadjusted and IPW-adjusted DOOR analysis.
Limitations
This study has several limitations. First, this was a secondary data analysis using a hospital administrative database. Many clinical conditions, except those defined using microbiology data, were captured by ICD-10-CM or ICD-10-PCS codes, and potential coding errors may affect the accuracy of patient identification. Furthermore, the definition of present on admission was based on hospital reporting, which was not a requirement for reporting on some conditions. For microbiology data, measurement error was possible. We limited the time to first AST to 7 days and reported the more resistant phenotype when more than 1 result was available for microbiology data. Second, selection bias was possible, as the study cohort was selected from a subset of hospitals submitting microbiology data to PHD. Based on the overall hospital distribution in PHD,14 patients included in this study were more likely to be from large teaching hospitals in urban areas. However, the bias would have been nondifferential between the 2 comparison groups. Furthermore, as previously published,18 patient and hospital characteristics between patients receiving PDAT vs not receiving PDAT were similar. Third, the timestamp of the antibiotic administration was not available. Therefore, the timing of antibiotic administration in reference to BCC was based on the date (eg, BCC day 0-4), not time (eg, within 24 hours). Fourth, we were not able to assess the use and role of rapid diagnostic tests, including the impact these tests may have had on antibiotic therapy selection prior to the availability of AST results. Fifth, we excluded polymicrobial and intra-abdominal infections that often require broad-spectrum antibiotics, making the categorization of desirable treatment challenging. Sixth, we evaluated relapse of BSI but did not include it as a clinical outcome or part of the DOOR analysis due to the low number of observed repeat blood cultures in the dataset. Additionally, collider bias or unmeasured confounding was possible.
Conclusions
To our knowledge, this is first study to compare clinical outcomes between patients with E coli, K pneumoniae, K oxytoca, and P mirabilis BSIs receiving early PDAT vs delayed PDAT using DOOR analysis. Receiving early PDAT was associated with favorable 30-day clinical outcomes among patients hospitalized with Enterobacterales BSIs. Starting early PDAT may be important not only for antimicrobial stewardship but also for improving the clinical outcome of affected patients.
eTable 1. Code Types and Values for Clinical Conditions
eTable 2. Charlson-Deyo comorbidities and Related Diagnosis and Procedure Codes
eFigure 1. Flowchart of Patient Selection
eFigure 2. Standardized Mean Differences Before and After Inverse Probability Weighting Using Propensity Score
eFigure 3. First Effective Antibiotic(s) Received, Among Patients Receiving PDAT
eFigure 4. Grid Illustrating the Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR-MAT) for the Treatment of Bloodstream Infections Caused by Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, or Proteus mirabilis
Data Sharing Statement
Footnotes
Abbreviations: BCC, blood culture collection; DOOR, desirability of outcome rankings; HO-CDI, hospital-onset Clostridioides difficile infection.
Detailed definitions of events appear in the Methods section.
References
- 1.Banerjee R, Humphries R. Rapid antimicrobial susceptibility testing methods for blood cultures and their clinical impact. Front Med (Lausanne). 2021;8:635831. doi: 10.3389/fmed.2021.635831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Timbrook TT, Morton JB, McConeghy KW, Caffrey AR, Mylonakis E, LaPlante KL. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis. 2017;64(1):15-23. doi: 10.1093/cid/ciw649 [DOI] [PubMed] [Google Scholar]
- 3.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time is of the essence: the impact of delayed antibiotic therapy on patient outcomes in hospital-onset enterococcal bloodstream infections. Clin Infect Dis. 2016;62(10):1242-1250. doi: 10.1093/cid/ciw110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lodise TP, Zhao Q, Fahrbach K, Gillard PJ, Martin A. A systematic review of the association between delayed appropriate therapy and mortality among patients hospitalized with infections due to Klebsiella pneumoniae or Escherichia coli: how long is too long? BMC Infect Dis. 2018;18(1):625. doi: 10.1186/s12879-018-3524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadri SS, Lai YL, Warner S, et al. ; forming the National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI) . Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021;21(2):241-251. doi: 10.1016/S1473-3099(20)30477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohnuma T, Chihara S, Costin B, et al. Association of appropriate empirical antimicrobial therapy with in-hospital mortality in patients with bloodstream infections in the US. JAMA Netw Open. 2023;6(1):e2249353. doi: 10.1001/jamanetworkopen.2022.49353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson BM, Jiang Y, Jump RLP, et al. Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR MAT): a framework for assessing antibiotic selection strategies in the presence of drug resistance. Clin Infect Dis. 2021;73(2):344-350. doi: 10.1093/cid/ciaa1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rhee C, Kadri SS, Dekker JP, et al. ; CDC Prevention Epicenters Program . Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. doi: 10.1001/jamanetworkopen.2020.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez MD, Chao T, Pettengill MA. Modern Blood Culture: Management Decisions and Method Options. Clin Lab Med. 2020;40(4):379-392. doi: 10.1016/j.cll.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Routsi C, Gkoufa A, Arvaniti K, et al. De-escalation of antimicrobial therapy in ICU settings with high prevalence of multidrug-resistant bacteria: a multicentre prospective observational cohort study in patients with sepsis or septic shock. J Antimicrob Chemother. 2020;75(12):3665-3674. doi: 10.1093/jac/dkaa375 [DOI] [PubMed] [Google Scholar]
- 11.Bonine NG, Berger A, Altincatal A, et al. Impact of delayed appropriate antibiotic therapy on patient outcomes by antibiotic resistance status from serious gram-negative bacterial infections. Am J Med Sci. 2019;357(2):103-110. doi: 10.1016/j.amjms.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 12.Howard-Anderson J, Dai W, Yahav D, et al. A desirability of outcome ranking analysis of a randomized clinical trial comparing seven versus fourteen days of antibiotics for uncomplicated gram-negative bloodstream infection. Open Forum Infect Dis. 2022;9(6):ofac140. doi: 10.1093/ofid/ofac140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis. 2015;61(5):800-806. doi: 10.1093/cid/civ495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.PINC AI Applied Sciences. Best in class: data that informs and performs. Accessed December 14, 2023. https://offers.pinc-ai.com/PINC-AI-Healthcare-Database-White-Paper-LP.html
- 15.Moon RC, Bleak TC, Rosenthal NA, et al. Epidemiology and economic burden of acute infectious gastroenteritis among adults treated in outpatient settings in US health systems. Am J Gastroenterol. 2023;118(6):1069-1079. doi: 10.14309/ajg.0000000000002186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 17.Perez F, Colindres RV, Wilson BM, et al. Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR MAT) reveals improvements in the treatment of bloodstream infections caused by Escherichia coli and Klebsiella pneumoniae in patients from the Veterans Health Administration. Clin Infect Dis. 2021;73(7):1231-1238. doi: 10.1093/cid/ciab384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon RM. MacVane SH, David J, Morton JB, Rosenthal N, Claeys KC. Incidence and variability in receipt of phenotype-desirable antimicrobial therapy for Enterobacterales bloodstream infections among hospitalized United States patients. Antimicrob Steward Healthc Epidemiol. 2024;4(1):e183. doi: 10.1017/ash.2024.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamma PD, Komarow L, Ge L, et al. ; Antibacterial Resistance Leadership Group . Clinical impact of ceftriaxone resistance in Escherichia coli bloodstream infections: a multicenter prospective cohort study. Open Forum Infect Dis. 2022;9(11):ofac572. doi: 10.1093/ofid/ofac572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.US Centers for Disease Control and Prevention . Antibiotic resistance threats in the United States, 2019. Accessed November 19, 2024. https://www.cdc.gov/antimicrobial-resistance/media/pdfs/2019-ar-threats-report-508.pdf
- 21.Rosenthal N, Cao Z, Chung J, et al. Updated coding algorithm for assessing Charlson Comorbidity Index using large hospital administrative data. Presented at: ISPOR 22nd Annual International Meeting; May 21, 2017; Boston, MA. [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 23.Bureau of Labor Statistics. Consumer Price Index. Accessed May 2, 2024. http://www.bls.gov/cpi/
- 24.Hamasaki T, Evans SR. The DOOR is open: a web-based application for analyzing the desirability of outcome ranking. Open Forum Infect Dis. 2023;10(Suppl 2):ofad500.439. doi: 10.1093/ofid/ofad500.439 [DOI] [Google Scholar]
- 25.Lodise TP, Kanakamedala H, Hsu WC, Cai B. Impact of incremental delays in appropriate therapy on the outcomes of hospitalized adult patients with gram-negative bloodstream infections: “every day matters”. Pharmacotherapy. 2020;40(9):889-901. doi: 10.1002/phar.2446 [DOI] [PubMed] [Google Scholar]
- 26.Seddon MM, Bookstaver PB, Justo JA, et al. Role of early de-escalation of antimicrobial therapy on risk of Clostridioides difficile infection following Enterobacteriaceae bloodstream infections. Clin Infect Dis. 2019;69(3):414-420. doi: 10.1093/cid/ciy863 [DOI] [PubMed] [Google Scholar]
- 27.Teshome BF, Vouri SM, Hampton N, Kollef MH, Micek ST. Duration of exposure to antipseudomonal β-lactam antibiotics in the critically ill and development of new resistance. Pharmacotherapy. 2019;39(3):261-270. doi: 10.1002/phar.2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banerjee R, Komarow L, Virk A, et al. Randomized trial evaluating clinical impact of Rapid Identification and Susceptibility Testing for Gram-Negative Bacteremia: RAPIDS-GN. Clin Infect Dis. 2021;73(1):e39-e46. doi: 10.1093/cid/ciaa528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banerjee R, Girl A, Komarow L, Souli M, Doernberg SB, Patel R. Impact of rapid antibiotic susceptibility testing for gram negative bacteremia varies by pathogen type and resistance: a secondary analysis of the RAPIDS-GN trial. Presented at: IDWeek 2023; October 11-15, 2023; Boston, MA. Session Poster #857 [DOI] [PubMed] [Google Scholar]
- 30.Tabah A, Bassetti M, Kollef MH, et al. Antimicrobial de-escalation in critically ill patients: a position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patients Study Group (ESGCIP). Intensive Care Med. 2020;46(2):245-265. doi: 10.1007/s00134-019-05866-w [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Code Types and Values for Clinical Conditions
eTable 2. Charlson-Deyo comorbidities and Related Diagnosis and Procedure Codes
eFigure 1. Flowchart of Patient Selection
eFigure 2. Standardized Mean Differences Before and After Inverse Probability Weighting Using Propensity Score
eFigure 3. First Effective Antibiotic(s) Received, Among Patients Receiving PDAT
eFigure 4. Grid Illustrating the Desirability of Outcome Ranking for the Management of Antimicrobial Therapy (DOOR-MAT) for the Treatment of Bloodstream Infections Caused by Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, or Proteus mirabilis
Data Sharing Statement