Abstract
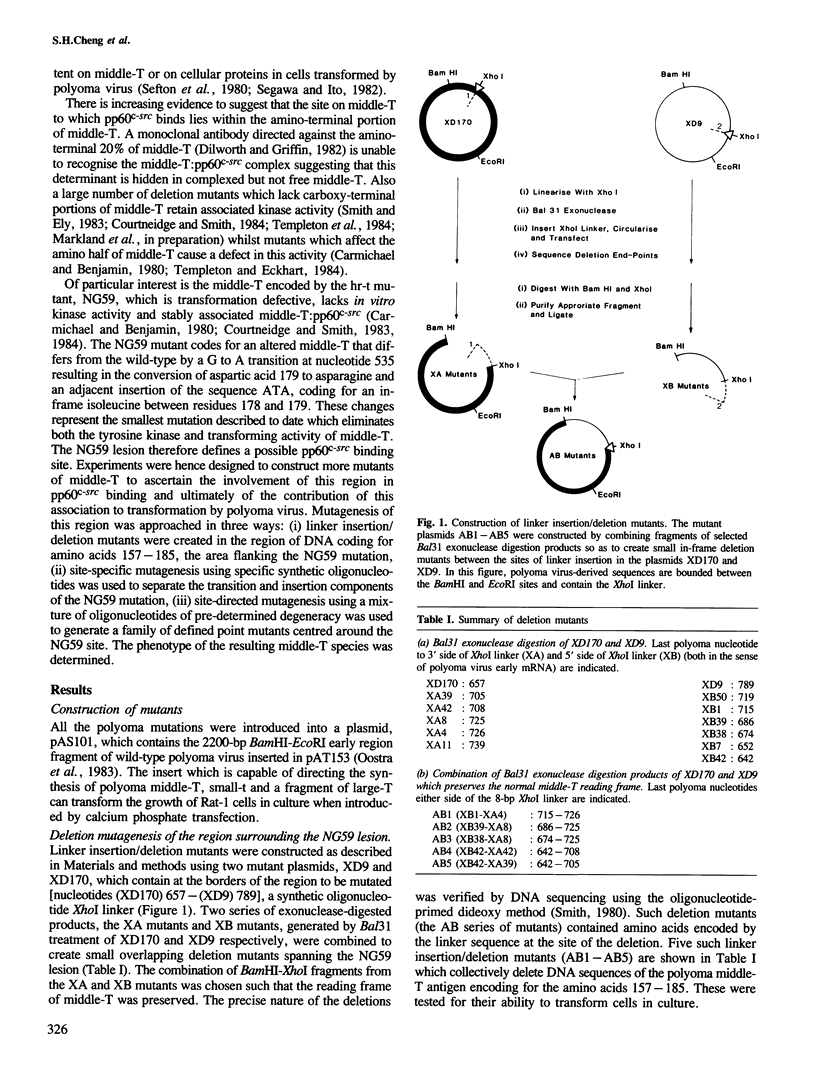
The transforming activity of polyoma virus middle-T antigen is believed to be dependent on its ability to form a complex with the cellular tyrosine protein kinase, pp60c-src. This hypothesis is based on observations of mutants of middle-T which demonstrated a correlation between these two activities. To investigate further the significance of pp60c-src association in transformation by middle-T, a series of deletion and point mutants were constructed around the NG59 lesion since this region has been implicated in pp60c-src binding. Analysis of the middle-T variants revealed a complete correlation between the presence of associated activated pp60c-src and the ability to transform. Further, this ability of pp60c-src to associate with middle-T may depend on the presence of a beta-turn between amino acids 177 and 180. The results indicate the NG59 phenotype results from the introduction of an isoleucine residue between amino acids 177 and 178 rather than the transition mutation at 179. The mutant MG1 is a single point mutation (at residue 180) and represents the smallest change in the middle-T which abolishes both the transformating and kinase activity of middle-T. Taken together, the data suggest the region surrounding the NG59 lesion is involved in the formation of an active complex between middle-T and pp60c-src and strongly suggest that this association is an absolute requirement for polyoma virus-induced transformation.
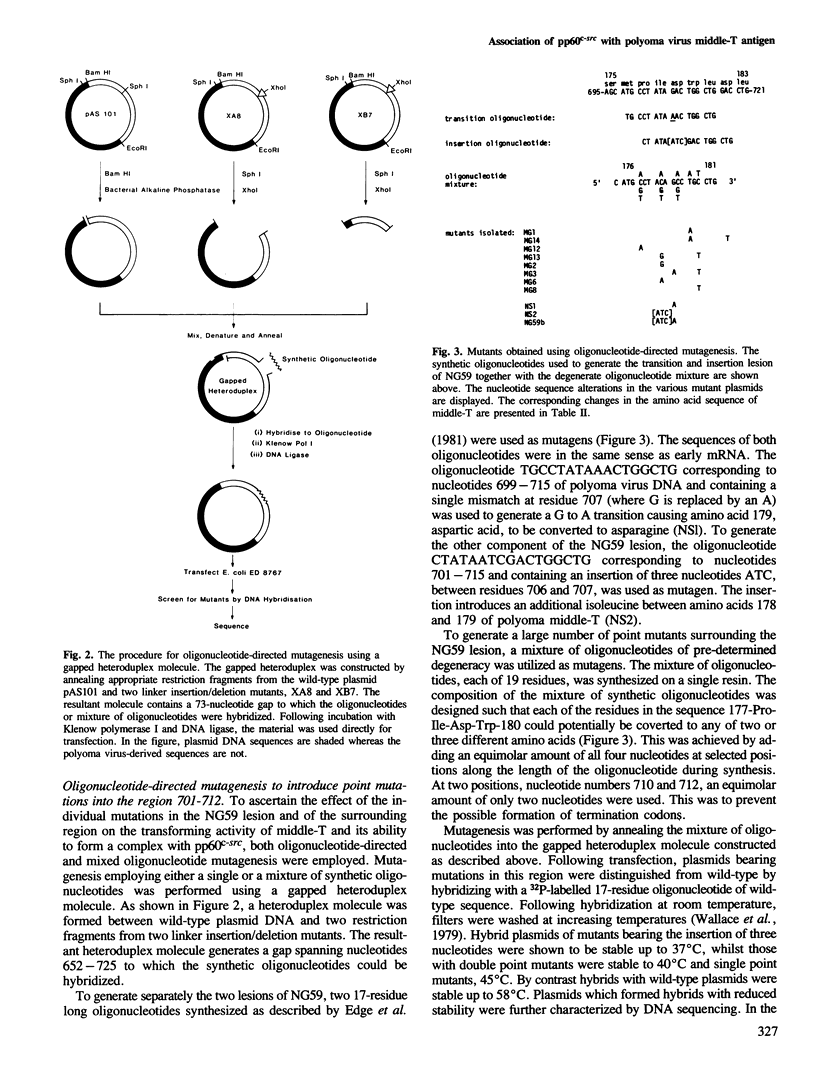
Full text
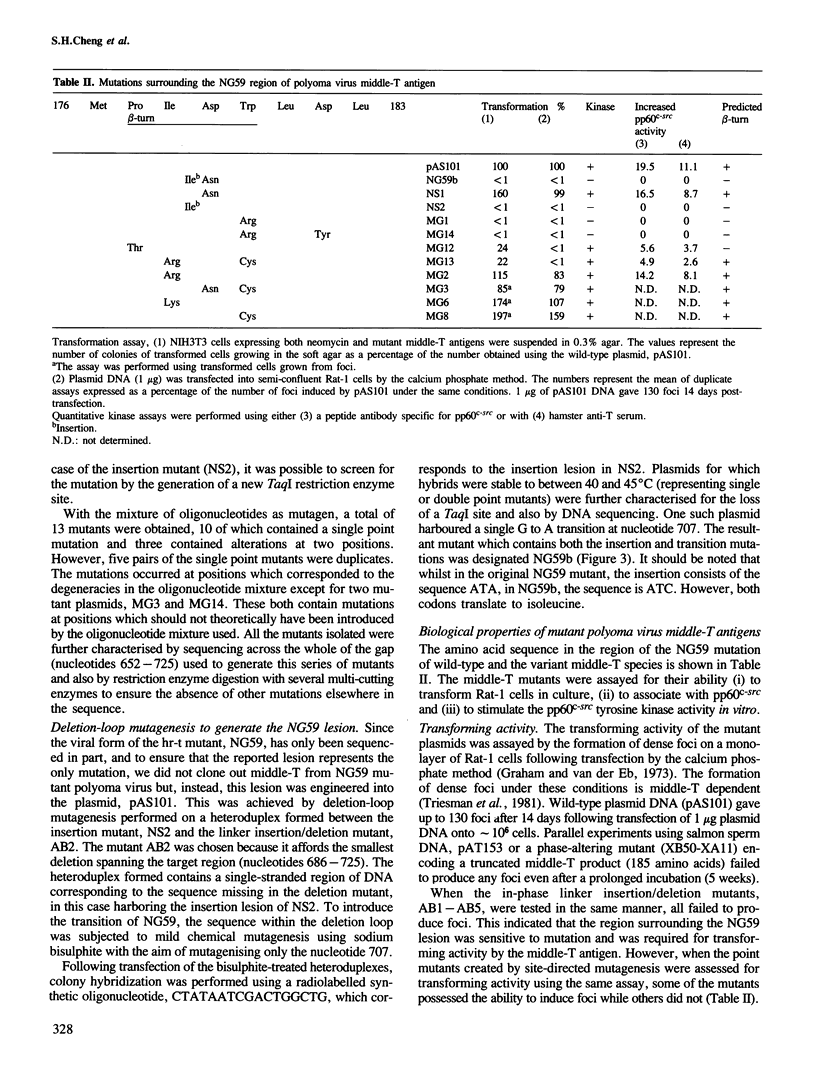
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
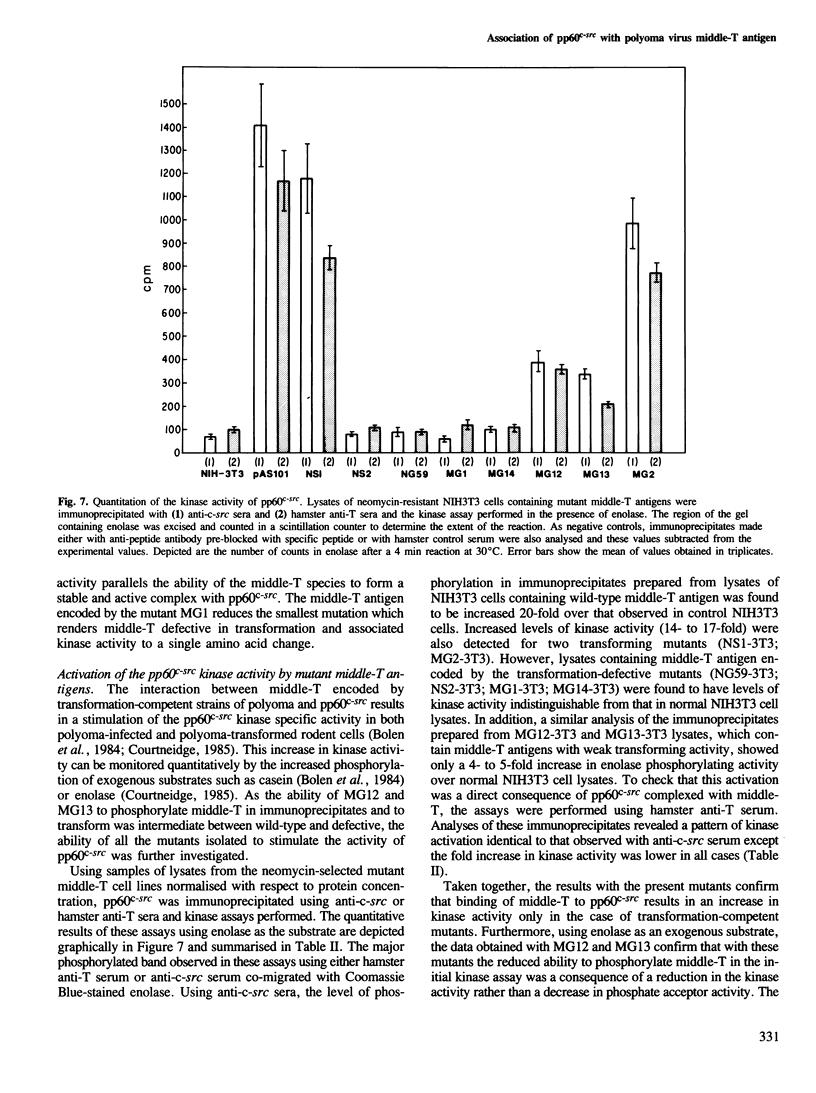
- Bolen J. B., Israel M. A. Middle tumor antigen of polyomavirus transformation-defective mutant NG59 is associated with pp60c-src. J Virol. 1985 Jan;53(1):114–119. doi: 10.1128/jvi.53.1.114-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Benjamin T. L. Identification of DNA sequence changes leading to loss of transforming ability in polyoma virus. J Biol Chem. 1980 Jan 10;255(1):230–235. [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J. L., Lipscomb W. N., Schellman C. G. The reverse turn as a polypeptide conformation in globular proteins. Proc Natl Acad Sci U S A. 1973 Feb;70(2):538–542. doi: 10.1073/pnas.70.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V., Bastien C., Scherneck S., Feunteun J. A new member of the polyomavirus family: the hamster papovavirus. Complete nucleotide sequence and transformation properties. EMBO J. 1985 May;4(5):1279–1286. doi: 10.1002/j.1460-2075.1985.tb03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth S. M., Griffin B. E. Monoclonal antibodies against polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1059–1063. doi: 10.1073/pnas.79.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Edge M. D., Green A. R., Heathcliffe G. R., Meacock P. A., Schuch W., Scanlon D. B., Atkinson T. C., Newton C. R., Markham A. F. Total synthesis of a human leukocyte interferon gene. Nature. 1981 Aug 20;292(5825):756–762. doi: 10.1038/292756a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Oostra B. A., Ely B. K., Smith A. E. Deletion loop mutagenesis: a novel method for the construction of point mutations using deletion mutants. Nucleic Acids Res. 1982 Sep 11;10(17):5161–5171. doi: 10.1093/nar/10.17.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Smith A. E. In vitro mutagenesis of a putative DNA binding domain of SV40 large-T. Virology. 1984 Nov;139(1):109–137. doi: 10.1016/0042-6822(84)90334-9. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Oostra B. A., Harvey R., Ely B. K., Markham A. F., Smith A. E. Transforming activity of polyoma virus middle-T antigen probed by site-directed mutagenesis. Nature. 1983 Aug 4;304(5925):456–459. doi: 10.1038/304456a0. [DOI] [PubMed] [Google Scholar]
- Raptis L., Lamfrom H., Benjamin T. L. Regulation of cellular phenotype and expression of polyomavirus middle T antigen in rat fibroblasts. Mol Cell Biol. 1985 Sep;5(9):2476–2486. doi: 10.1128/mcb.5.9.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Lodge J., Kaplan D., Roberts T. M. Expression of polyoma early gene products in E. coli. Nucleic Acids Res. 1985 Jan 25;13(2):501–519. doi: 10.1093/nar/13.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Fried M., Ito Y., Spurr N., Smith R. Is polyoma virus middle T antigen a protein kinase? Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):141–147. doi: 10.1101/sqb.1980.044.01.016. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Griffin B., Fried M. Protein kinase activity associated with polyoma virus middle T antigen in vitro. Cell. 1979 Dec;18(4):915–924. doi: 10.1016/0092-8674(79)90204-6. [DOI] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Templeton D., Eckhart W. N-terminal amino acid sequences of the polyoma middle-size T antigen are important for protein kinase activity and cell transformation. Mol Cell Biol. 1984 May;4(5):817–821. doi: 10.1128/mcb.4.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D., Voronova A., Eckhart W. Construction and expression of a recombinant DNA gene encoding a polyomavirus middle-size tumor antigen with the carboxyl terminus of the vesicular stomatitis virus glycoprotein G. Mol Cell Biol. 1984 Feb;4(2):282–289. doi: 10.1128/mcb.4.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R., Novak U., Favaloro J., Kamen R. Transformation of rat cells by an altered polyoma virus genome expressing only the middle-T protein. Nature. 1981 Aug 13;292(5824):595–600. doi: 10.1038/292595a0. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemoto W., Jarvis-Morar M., Brugge J. S., Bolen J. B., Israel M. A. Tyrosine phosphorylation within the amino-terminal domain of pp60c-src molecules associated with polyoma virus middle-sized tumor antigen. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4568–4572. doi: 10.1073/pnas.82.14.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]





