Abstract
Objectives
Post-stroke emotionalism (PSE) is a common, under-researched neurologic symptom of stroke, characterised by frequent crying episodes not under usual social control. Currently, there are no data on carer strain in the context of emotionalism after stroke. We aimed to explore the degree of carer strain in carers of individuals with diagnosed PSE compared with carers of individuals with stroke but no PSE to examine whether carer strain varies with particular characteristics of the cared for individual (patient age, sex, social deprivation, stroke type, functional status, mood status) and to quantify the impact of PSE on carer strain, after accounting for other factors.
Design
Cross-sectional observation study.
Setting
Nine secondary care stroke units in Scotland, UK.
Participants
102 informants of people with stroke.
Primary and secondary outcome measures
The Modified Carer Strain Index was completed at 6 months post-stroke as part of the Testing Emotionalism After Recent Stroke (TEARS) longitudinal cohort study between 1 October 2015 and 30 September 2018. Stroke survivor diagnostic status was determined using TEARS-Diagnostic Interview based on published, widely accepted diagnostic criteria of emotionalism.
Results
There was little evidence of association between carer strain and sex, age, deprivation level or stroke type of the cared for individual. There was strong evidence that carer strain was associated with both increased functional dependence post-stroke (−0.30 to −0.02, p=0.026) and presence of PSE (0.16 to 1.73, p=0.019).
Conclusions
Even after accounting for increased functional dependence, our study data indicates that caring in a PSE context may significantly increase carer strain, comparable to a six-point reduction on the Barthel Index.
Trial registration number
NRS Stroke Research Network ID 18980.
Keywords: Stroke, MENTAL HEALTH, Caregiver Burden
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is the first analysis of carer strain associated with emotionalism following a stroke.
However, the data were derived using only one measure at one time point when the caregiver was present and willing to participate.
Research into larger cohorts of stroke survivors with emotionalism and their carers is needed.
Background
Post-stroke emotionalism (PSE) is a widely acknowledged, clinically prevalent neurological stroke sequela characterised by frequent, sudden onset crying episodes (occasionally laughter), not under usual social control and which represent a change from pre-stroke functioning.1,3 At least one in five stroke survivors at 6 months suffer PSE4 5 yet compared with other common neurological stroke conditions, for example, neglect or aphasia, PSE remains under-researched.6 Emotionalism is not stroke specific and can arise following a range of neurological diseases impacting brain areas and pathways functionally linked to emotion expression and regulation.7 In the stroke context, PSE is associated with younger age, cognitive impairment, larger lesions and strokes disrupting serotonergic and/or bulbar brain networks.5 7 PSE can be upsetting and confusing for patients and families, and potentially misunderstood for depression by clinicians because of the central crying component. There may be co-presentation with clinical depression, adding to the risk of misdiagnosis. Standardised assessments are available.8 9 If ignored or missed, PSE can disrupt stroke rehabilitation as patients disengage or avoid therapies for fear of crying, distress or embarrassment, in turn eroding functional and quality of life outcomes.10
Caregiver burden is a widely recognised phenomenon in the stroke context, prevalent in 25%–54% of cases, and associated with a range of stroke survivor characteristics, including more severe illness and behavioural changes due to mood and/or cognition impairments.11 12 Surprisingly, however, despite the prevalence and nature of PSE and its psychological associations established by quantitative and recent qualitative research,13,16 little is specifically known regarding the impact of caring for people living with PSE.
Colamonico and colleagues progressed an online survey which included carers of people with and without emotionalism following a range of precipitating neurological diseases, including stroke.10 Three self-report measures were completed by the carers: the Center for Epidemiologic Studies Depression Scale 10-item (CESD-10),17 the Screen for Caregiver Burden (SCB)18 and the Work Productivity and Activity Impairment questionnaire (WPAI).19 20 Interestingly, those caring for people with emotionalism showed equivalent levels of depression to carers of people without emotionalism. Whereas among the emotionalism carers, there were more frequent burdensome events, higher distress levels, greater disruption to work productivity and work impairment, including missed time at work, and more caregiver burden overall.10
These are important data. They suggest increased carer burden and occupational dysfunction linked specifically to emotionalism, over and above the impact of caring in the context of the index neurological disease without concomitant emotionalism.
Notably, however, carers in the Colamonico survey were from a mixed neurological disease sample. Under one-sixth of the carer samples were stroke carers (n=59), and data specific to caring in the context of stroke emotionalism are not separately reported.10 Thus, the specific psychological impact of caring for people with emotionalism after a stroke is not known, and we could find no other studies which have explored this topic directly. This is an important gap. Stroke is distinct aetiologically from other neurological diseases causing emotionalism (eg, multiple sclerosis, Parkinson’s disease) and with separate clinical care pathways. It is thus crucial not only to understand the distinctive nature of emotionalism following stroke but also the distinct nature of carer strain linked to emotionalism following stroke.
Our primary aim was to address this knowledge gap, via an analysis of Modified Caregiver Strain Index (MCSI)21 data, collected from carers of individuals with or without diagnosed PSE 6 months post index stroke derived from the TEARS (Testing Emotionalism After Recent Stroke; NRS Stroke Research Network ID 18980) longitudinal cohort study.5
Aims
As the TEARS data set contains MCSI scores from carers of individuals with stroke and PSE but also stroke and no PSE, the study had three aims:
To explore the degree of carer strain in carers of individuals with diagnosed PSE and to compare this to the degree of carer strain in carers of individuals with stroke but no diagnosis of PSE.
To examine whether carer strain varies with particular characteristics of the cared for individual: patient age, sex, social deprivation, stroke type, functional status and mood status (depression, anxiety).
To quantify the impact of PSE on carer strain, after accounting for other factors.
Methods
Sample size
The TEARS cohort study recruited n=277 participants. Of these, n=102 had informants at 6-month follow-up who reported carer strain data. Of these, 66 were shown not to have PSE at 6 months, 16 to have PSE at 6 months and 20 not to have PSE status determined. To compare a normally distributed outcome measure between the 66 non-PSE and 16 PSE carers, there would be 81% power to detect a standardised measure of 0.8 and 94% power to detect a standardised measure of 1.0. These differences can be compared with the range of MSCI which is 0–26. Thus, there was sufficient power to detect clinical differences in the continuous outcome, provided that it was sufficiently close to normal in distribution.
Participants
Participants were recruited into TEARS from nine Scottish hospital acute stroke units within 2 weeks of sustaining stroke (https://www.stroke.org.uk/research/understanding-difficulty-controlling-emotions-after-stroke; full protocol available from NB) between 1 October 2015 and 30 September 2018. For each participant, an informant (spouse or closest relative) was recruited.
All stroke participants were male or non-pregnant female, ≥18 years of age, with a clinical diagnosis of first ever or repeat ischaemic or haemorrhagic stroke. We excluded on the basis of subarachnoid haemorrhage, other extra-axial bleeds, transient ischaemic attack, severe concurrent medical conditions, severe distressing behaviours precluding participation, aphasia (score <25 on Frenchay Aphasia Screening Test),22 life expectancy ≤3 months and/or lack of spoken English. Our clinical research file and site staff training required that the MCSI was completed for all patients ‘by interviewing the nearest relative/carer’. Furthermore, the MCSI instructional set referred to the respondent as ‘caregiver’. We did not gather data on informant respondent characteristics, other than confirming next of kin or equivalent to the stroke participant, nor the relationship between the participant and informant or whether the relative/carer interviewed was definitely the primary caregiver.
Measures and procedure
TEARS had a priori ethical approval from the Scotland A Research Ethics Committee (IRAS Reference 157483). All participants gave written informed consent, including those informant participants whose data are reported here.
Findings are based on data (all measures) gathered face-to-face at the 6-month assessment point by pre-trained stroke research nurses based on the stroke units.
Carer strain was determined using the MCSI.21 The scale was developed for use with family carers based on the original Caregiver Strain Index version.23 MCSI is self-report and contains 13 items including prompting examples across a range of pertinent strain domains (physical, personal, psychological, financial, social) with item responses captured as follows: ‘Yes, on a regular basis’ (scored 2), ‘Yes, sometimes’ (scored 1) or ‘No’ (scored 0). This gives a possible total score ranging from 0 (no carer strain) to 26 (highest carer strain).
MCSI has acceptable internal (alpha=0.90) and test-retest reliability (alpha=0.86) based on the Thornton and Travis sample21 and has been used previously to screen carer burden in the context of neurological disease,24 25 including stroke.26
Diagnosis of PSE status was reached using Testing Emotionalism After Recent Stroke-Diagnostic Interview (TEARS-IV) 5. TEARS-IV is a detailed, semistructured diagnostic interview comprising three sections addressing post-stroke crying (screen questions, case characteristics, frequency and impact), post-stroke laughter (screen questions, case characteristics) and diagnostic summary. Final TEARS-IV diagnosis is reached based on published, widely accepted PSE diagnostic criteria1 13 14 27: (1) increased tearfulness, (2) crying comes on suddenly, with no warning, (3) crying not under usual social control and (4) crying episodes occur at least once weekly.
We classified stroke using the Oxford Classification System28 and used the Hospital Anxiety and Depression Scale (HADS)29 to measure anxiety and depression, Barthel Activities of Daily Living Index30 for functional outcome and computed social deprivation level using the Scottish Index of Multiple Deprivation rank provided by the Scottish Government. This is based on 6976 data zones. A rank of 1 corresponds to the most deprived area or data zone and 6976 the least deprived.31
Patient and public involvement
We originally targeted PSE for clinical observation research based on a national stroke research setting exercise involving Scottish stroke patients, carers and health professionals undertaken by James Lind Alliance.32 This identified ‘What are the best ways to help people come to terms with the long-term consequences of stroke?’ as the second top research priority. We did not involve patients in the design of the study, nor in the recruitment to, and conduct of, the study. The final TEARS-Q and TEARS-IV outcome measures were endorsed by a person with personal experience of stroke. Interested study participants could contact the study team to receive a summary of the study results posted to them.
Statistical analysis
All inferential analyses were conducted using R software V.4.2.3.33 We deployed initial cross tabulation and follow-up statistics characterised the subsample from whom carer strain data were collected. Cross tabulation was used to explore the association of carer strain (MCSI) with stroke classification recorded at baseline and PSE status (yes/no on TEARS-IV), anxiety (HADS-A), depression (HADS-D) and Barthel Activities of Daily Living Index measured at 6-month follow-up when MCSI was also measured.
On initial inspection, the MCSI carer strain data had a skewed distribution. To improve variance stabilisation, we used the square root of MCSI, hereafter sqrt.MCSI, for modelling.
Univariate analyses were undertaken, regressing sqrt.MCSI on each covariate separately to identify covariates with the strongest association with sqrt.MCSI. Then, a suitable regression model was fitted. In particular, we focused our interest on PSE which was considered to lead to greater anxiety and depression. Thus, the model for sqrt.MCSI was regressed on PSE status and other covariates but not HADS-A nor HADS-D. The final model was selected to be a parsimonious fit for which all regression coefficients were statistically significant at the 5% level. We note that this is an exploratory approach and the parsimonious model fit avoids any nuisance variables.
Results
Participants
Characteristics of the final sample are shown in table 1.
Table 1. Participant characteristics, cross-classified by collection of caregiver strain on MCSI.
| Variable | Levels | No MCSI data collected (%) | MCSI data collected (%) | P value |
| N of participants | Total | 175 | 102 | |
| Centre | A | 51 (82.3) | 11 (17.7) | <0.001 |
| B | 5 (100) | 0 (0) | ||
| C | 20 (83.3) | 4 (16.7) | ||
| D | 51 (53.1) | 45 (46.9) | ||
| E | 9 (75.0) | 3 (25.0) | ||
| F | 2 (50.0) | 2 (50.0) | ||
| G | 15 (41.7) | 21(58.3) | ||
| H | 11 (52.4) | 10 (47.6) | ||
| I | 11 (64.7) | 6 (35.3) | ||
| Sex (%) | Female | 80 (65.6) | 42 (34.4) | 0.543 |
| Male | 95 (61.3) | 60 (38.7) | ||
| Age at stroke | Mean (SD) | 66.6 (14.8) | 65.7 (14.1) | 0.618 |
| SIMD | Mean (SD) | 2600 (2020) | 2966 (2032) | 0.152 |
| Anxiety (HADS-A) | Mean (SD) | 5.6 (4.5) | 5.6 (4.8) | 0.979 |
| Depression (HADS-D) | Mean (SD) | 4.4 (4.1) | 4.6 (3.8) | 0.772 |
| Stroke classification | PACS | 66 (65.3) | 35 (34.7) | 0.854 |
| LACS | 51 (60.7) | 33 (39.3) | ||
| POCS | 39 (66.1) | 20 (33.9) | ||
| TACS | 12 (54.5) | 10 (45.5) | ||
| Not recorded | 7 (63.6) | 4 (36.4) | ||
| Emotionalism 6 months | PSE | 16 (50.0) | 16 (50.0) | <0.001 |
| No PSE | 61 (48.0) | 66 (52.0) | ||
| Not recorded | 98 (83.1) | 20 (16.9) |
HADS-A, Hospital Anxiety and Depression Scale, Anxiety subscale29; HADS-D, Hospital Anxiety and Depression Scale, Depression subscale29LACS, Lacunar Stroke; PACS, Partial Anterior Circulation Stroke; POCS, Posterior Circulation Stroke; SIMD, Scottish Index of Multiple Deprivation31; TACS, Total Anterior Circulation Stroke28
As is evident, there were no significant differences between participants whose carers completed MCSI compared with those who did not, on variables of sex, age, social deprivation, anxiety, depression and stroke classification. There was MCSI variation by TEARS recruiting centre, likely reflective of differences in unit resourcing rather than participant differences. Furthermore, when PSE status was not recorded, MCSI data was also not recorded, largely explained by participants (and therefore carers) not attending for 6-month data collection.
As 175 participants did not return a measure of carer strain and only 102 did, we did not consider imputation methods for subsequent analyses. Instead, we continued noting that, based on participants’ characteristics, there was little evidence of any association with the presence or absence of carer strain measurements, and thus, no selection bias was suspected.
Carer strain
Scores on MCSI range from 0 to 26 and were distributed as per online supplemental figure 1. As is evident, the distribution was heavily skewed. For better modelling, we therefore computed sqrt.MCSI, plotted in online supplemental figure 2. Prior to transformation, the MCSI distribution had a median of 4.0 and a mean of 6.0. Following transformation, the distribution had a median of 2.0 and mean of 2.0.
Association of MCSI total score with appropriate putative predictors
Next, we examined the association of participant sex, age, social deprivation, anxiety, depression, Barthel Index, stroke classification, carer strain and sqrt.MCSI by PSE status at 6 months.
As is evident in table 2, anxiety was higher in patients known to have PSE, but not statistically significant in this sample. The Barthel Index was lower in patients known to have PSE, suggesting that emotionalism associates with greater functional dependence. The sqrt.MCSI stabilised the variance (and therefore the SD), with carer strain higher for those with known PSE.
Table 2. Participant characteristics, cross-classified by PSE status at 6 months.
| PSE | No PSE | Not recorded | P value | |
| N of participants | 16 | 66 | 20 | |
| Sex=male (%) | 10 (62.5) | 37 (56.1) | 13 (65.0) | 0.736 |
| Age at stroke (mean, SD) | 56.75 (14.91) | 66.05 (13.06) | 71.55 (13.98) | 0.006 |
| SIMD (mean, SD) | 2000.31 (1841.62) | 3341.26 (2093.65) | 2472.84 (1637.68) | 0.029 |
| Anxiety HADS-A (mean, SD) | 7.33 (5.77) | 5.46 (4.47) | 3.62 (4.47) | 0.183 |
| Depression HADS-D (mean, SD) | 4.40 (4.50) | 4.64 (3.63) | 4.62 (3.70) | 0.975 |
| Barthel ADL (mean, SD) | 17.88 (3.69) | 19.23 (1.58) | 14.74 (6.05) | <0.001 |
| Stroke classification (%) | 0.069 | |||
| PACS | 4 (25.0) | 23 (34.8) | 8 (40.0) | |
| LACS | 6 (37.5) | 23 (34.8) | 4 (20.0) | |
| POCS | 4 (25.0) | 14 (21.2) | 2 (10.0) | |
| TACS | 1 (6.2) | 3 (4.5) | 6 (30.0) | |
| Not recorded | 1 (6.2) | 3 (4.5) | 0 (0.0) | |
| Carer strain MCSI | 9.75 (8.52) | 4.23 (4.89) | 8.75 (6.48) | <0.001 |
| sqrt.MCSI | 2.73 (1.56) | 1.61 (1.29) | 2.60 (1.44) | 0.001 |
ADL, Barthel Activities of Daily Living Index30; HADS-A, Hospital Anxiety and Depression Scale, Anxiety subscale29; HADS-D, Hospital Anxiety and Depression Scale, Depression subscale29; LACS, Lacunar Stroke; MCSI, Modified Carer Strain Index21PACS, Partial Anterior Circulation Stroke; POCS, Posterior Circulation Stroke; SIMD, Scottish Index of Multiple Deprivation31; TACS, Total Anterior Circulation Stroke28
To establish the associations between carer strain and patient characteristics, sqrt.MCSI was therefore regressed on age, sex, deprivation (as measured by Scottish Index of Multiple Deprivation (SIMD) rank), Barthel Index, stroke class (Oxford classification measured at baseline) and PSE status at 6 months. Covariates that did not achieve statistical significance (by Wald test) were dropped to obtain a parsimonious final model. HADS anxiety and HADS depression were not included in this modelling since they were considered to arise from PSE and functional status (as measured by the Barthel Index).
The results of modelling are provided in table 3 with univariable results included for reference, since these were used to suppress terms with little evidence of association to sqrt.MCSI (p values >0.1). As is evident, the final model had two covariates: Barthel Index and PSE status. These were the root sources of the associations within the data. Participants with a lower Barthel had higher associated values of sqrt.MCSI (carer strain). The size of the effect was such that a six unit decrease in Barthel Index, say from 20 to 14, was associated with a unit (1.0) increase in sqrt.MCSI.
Table 3. Table of regression coefficients, with sqrt.MCSI as a dependent variable.
| Coefficient (univariable) | Coefficient (multivariable) | ||
| Sex | Female | – | – |
| Male | 0.09 (−0.49 to 0.67, p=0.756) | – | |
| Age at stroke | −0.00 (−0.03 to 0.02, p=0.630) | – | |
| Social deprivation | −0.17 (−0.30 to −0.03, p=0.19) | – | |
| Barthel ADL | −0.14 (−0.21 to −0.06, p<0.001) | −0.16 (−0.30 to −0.02, p=0.026) | |
| Stroke Classification | PACS | – | – |
| LACS | 0.09 (−0.60 to 0.77, p=0.806) | – | |
| POCS | 0.43 (−0.37 to 1.22, p=0.291) | – | |
| TACS | 1.14 (0.12 to 2.16, p=0.029) | – | |
| Unknown | −0.13 (−1.63 to 1.37, p=0.868) | – | |
| PSE status 6 months | No PSE | – | |
| PSE | 1.13 (0.38 to 1.87, p=0.004) | 0.95 (0.16 to 1.73, p=0.019) |
Barthel ADLBarthel Activities of Daily Living Index30
Moreover, PSE was associated with a 0.91 point increase in sqrt.MCSI. While it is difficult to practically assess patients on a sqrt.MCSI scale, the effect size of PSE on MCSI would be similar to a six point difference on the Barthel Index.
Table 2 shows the unadjusted average carer strain of those with PSE to be 9.75 compared with an unadjusted average of 4.23 in those without PSE. Further modelling was undertaken to explore the nonlinear effects of social deprivation, age and Barthel Index. These are not reported since the effects were acceptable as linear.
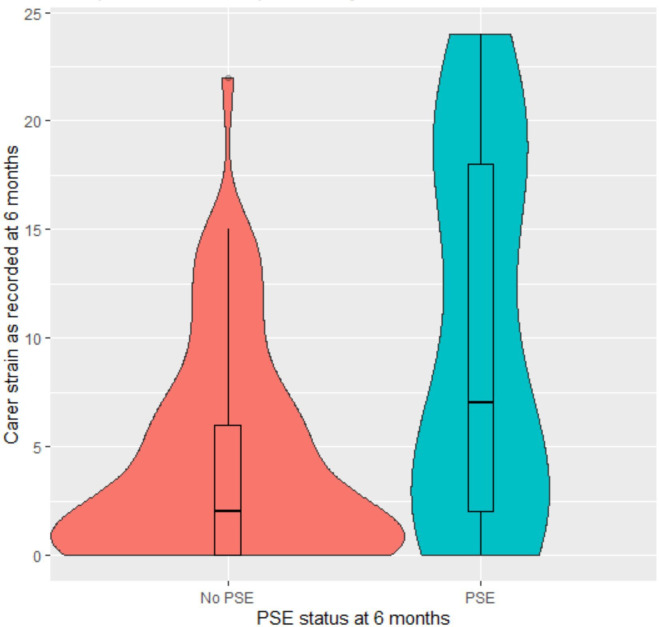
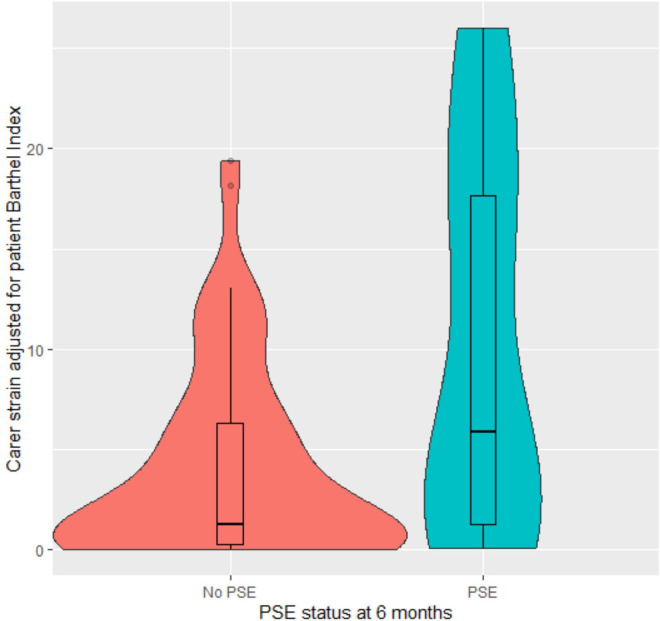
Finally, we produced a violin plot with boxplot within to reflect more details of the distribution of carer strain (figure 1), including carer strain adjusted from the final model for the association with Barthel (figure 2). As is evident, the distribution of MCSI data among carers of people with PSE had a higher median, greater IQR with more frequent low scores, which held for the distribution adjusted for Barthel.
Figure 1. Violin plot with nested boxplot of unadjusted carer strain.
Figure 2. Violin plot with nested boxplot of adjusted carer strain.
Discussion
To the best of our knowledge, this is the first study to examine carer strain in the context of emotionalism after stroke. We deployed a widely used, validated measure of carer strain and compared the psychological impact of caring for people with stroke and emotionalism to the impact of caring for people with stroke but no emotionalism. We also considered whether carer strain varied with the particular characteristics of the cared for individual, including age, sex, stroke type, deprivation, mood and functional status.
Several key findings emerged. First, we did not observe any significant association between carer strain measured on MCSI and sex, age, deprivation level or stroke type of the cared for individual. By contrast, we observed a clear and substantial association between carer strain and functional dependence on the Barthel Index. Specifically, greater functional dependence is associated with greater carer strain. Moreover, we also observed a significant association between carer strain and PSE status, strain being greater among carers of individuals with diagnosed PSE, compared with carers of individuals with stroke but no PSE. Consistent with these effects, when we regressed carer strain on age, sex, deprivation, Barthel Index, stroke type and PSE status at 6 months, the final model had two covariates: Barthel Index and PSE status. Again, higher carer strain was associated with greater functional dependence and diagnosed PSE.
Overall then, our analyses suggest a substantial association between functional dependence and carer strain but also between PSE status and carer strain, remaining after accounting for the impact of functional dependence measured by the Barthel Index. Consistent with the effects Calamonico and colleagues observed across the neurological disorders, our analyses indicate that caring in a PSE context significantly increases carer strain, equivalent to a six-point difference on the Barthel scale.
This is a unique study of carer strain in stroke patients assessing the impact of PSE on carers and the first to directly address this topic. We analysed MCSI data collected at 6 months post index event rather than acutely. Our assessments were conducted face to face with participants and their caregivers rather than online. Colamonico and colleagues took an online survey approach, using the self-report Centre for Neurologic Studies-Lability Scale9 to denote emotionalism status of the cared for individuals. By contrast, we diagnosed PSE status using a semistructured diagnostic interview, constructed based on House diagnostic criteria of PSE,1 delivered face-to-face to people with stroke and their caregivers by stroke research nurses, pre-trained by the senior author, an emotionalism expert.
A number of study limitations must be highlighted. Although larger than the cohort of stroke caregivers surveyed by Colamonico, our sample size of 102 caregivers is still relatively small making the study underpowered with data obtained from an observational study. Moreover, only 82 were associated to stroke survivors with known PSE status and with MCSI data returned, and only 16 were carers of people with PSE. There are also likely to be some unmeasured differences between those who did, and those who did not, complete the follow-up MCSI survey. Replication will be required using a larger cohort of stroke survivors with PSE and their carers, although our sample is similarly modest to that of Colamonico and colleagues (59 stroke caregivers) who observed a similar effect.
The measurements were carried out as part of the follow-up of the TEARS cohort study and thus represent observational data. Furthermore, the data were only collected at a 6-month follow-up session whenever the carer was present with the patient and willing to participate and we did not record carer characteristics or whether the relative/carer interviewed was the primary caregiver. We only examined respondent/non-respondent differences on certain key variables; and thus, although we observed no evidence of sampling bias, this could still have influenced the data. Our conclusions must also be limited as they may not apply to where the patients have more severe stroke (see table 1 for details). Consequently, they should be regarded as a convenience sample at one time point. Nonetheless, there is no suspicion of bias in the findings and participation of carers appeared to be representative of the observed characteristics of the patient population. Finally, while MCSI has acceptable psychometrics21 and previous use in stroke,26 the scale lacks established cut points. While this makes it hard to determine the clinical extent of carer strain seen in our data, the unadjusted average carer strain of those with PSE is 9.75, compared with 4.23 in those without PSE. The additional impact of caring in the PSE-specific context is thus clearly evident.
Clinical implications
While from a preliminary observational study and only at one time point, the data have important clinical and research implications. Clinically, additional psychological strain might be expected to arise in carers of individuals with PSE, something stroke clinicians and rehabilitation teams should hold in mind when PSE is detected. Targeted screening of carers of those with emotionalism using MCSI and other reliable measures could form an additional element of stroke care. Strain among carers of those with chronic PSE might be expected to escalate beyond 6 months, although larger scale longitudinal research beyond 6 months will be required to determine this. Although there has been recent qualitative research exploring the lived experience of emotionalism,15 16 research using qualitative approaches is also needed to improve our understanding of the lived experience of caring for someone with PSE, including what can help both the patient and the carer, psychologically. This important research could in turn inform badly needed work to adapt current evidence-based psychological interventions aimed at reducing carer strain in the broader stroke context and then test these for the PSE context.
supplementary material
Acknowledgements
We sincerely thank our stroke nurse, SSRN and NHS GGC R&D colleagues, our co-applicant colleagues who contributed to delivery of the wider TEARS study, our patient adviser colleague for their endorsement of the TEARS study measures and to all our study participants.
Footnotes
Funding: This work was supported by Stroke Association UK (grant number TSA 2013/03).
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-084079).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Consent obtained directly from patient(s).
Ethics approval: This study involves human participants and was approved by Scotland A Research Ethics Committee (IRAS Reference 157483). Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Niall Broomfield, Email: N.Broomfield@uea.ac.uk.
Matthew Walters, Email: matthew.walters@glasgow.ac.uk.
Robert M West, Email: r.m.west@leeds.ac.uk.
Data availability statement
Data are available upon reasonable request.
References
- 1.House AO, Dennis M, Molyneux A, et al. Emotionalism after stroke. BMJ . 1989;298:991–4. doi: 10.1136/bmj.298.6679.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Teng F, Chen Y, et al. Clinical Features and Related Factors of Poststroke Pathological Laughing and Crying: A Case-Control Study. J Stroke Cerebrovasc Dis. 2016;25:556–64. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Allida S, House A, Hackett ML. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database Syst Rev. 2022;11:CD003690. doi: 10.1002/14651858.CD003690.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillespie DC, Cadden AP, Lees R, et al. Prevalence of Pseudobulbar Affect following Stroke: A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Dis. 2016;25:688–94. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.038. [DOI] [PubMed] [Google Scholar]
- 5.Broomfield NM, West R, Barber M, et al. TEARS: a longitudinal investigation of the prevalence, psychological associations and trajectory of poststroke emotionalism. J Neurol Neurosurg Psychiatry. 2022;93:886–94. doi: 10.1136/jnnp-2022-329042. [DOI] [PubMed] [Google Scholar]
- 6.Broomfield NM, Blake J, Gracey F, et al. Post-stroke emotionalism: Diagnosis, pathophysiology, and treatment. Int J Stroke. 2024;19:857–66. doi: 10.1177/17474930241242952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald S, Gracey F, Trigg E, et al. Predictors and correlates of emotionalism across acquired and progressive neurological conditions: A systematic review. Neuropsychol Rehabil. 2023;33:945–87. doi: 10.1080/09602011.2022.2052326. [DOI] [PubMed] [Google Scholar]
- 8.Broomfield NM, West R, House A, et al. Psychometric evaluation of a newly developed measure of emotionalism after stroke (TEARS-Q) Clin Rehabil. 2021;35:894–903. doi: 10.1177/0269215520981727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore SR, Gresham LS, Bromberg MB, et al. A self report measure of affective lability. Journal of Neurology, Neurosurgery & Psychiatry . 1997;63:89–93. doi: 10.1136/jnnp.63.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29:775–98. doi: 10.1007/s12325-012-0043-7. [DOI] [PubMed] [Google Scholar]
- 11.Rigby H, Gubitz G, Phillips S. A Systematic Review of Caregiver Burden following Stroke. Int J Stroke. 2009;4:285–92. doi: 10.1111/j.1747-4949.2009.00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Panzeri A, Rossi Ferrario S, Vidotto G. Interventions for Psychological Health of Stroke Caregivers: A Systematic Review. Front Psychol. 2019;10:1–16. doi: 10.3389/fpsyg.2019.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvert T, Knapp P, House AO. Psychological associations with emotionalism after stroke. J Neurol Neurosurg Psychiatry . 1998;65:928–9. doi: 10.1136/jnnp.65.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eccles S, House AO, Knapp P. Psychological adjustment and self reported coping in stroke survivors with and without emotionalism. Journal of Neurology, Neurosurgery & Psychiatry . 1999;67:125–6. doi: 10.1136/jnnp.67.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAleese N, Guzman A, O’Rourke SJ, et al. Post-stroke emotionalism: a qualitative investigation. Disabil Rehabil. 2019;28:1–9. doi: 10.1080/09638288.2019.1620876. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald S, Gracey F, Broomfield N. Post-stroke emotionalism (PSE): a qualitative longitudinal study exploring individuals’ experience with PSE. Disabil Rehabil. 2022;44:7891–903. doi: 10.1080/09638288.2021.2002439. [DOI] [PubMed] [Google Scholar]
- 17.Andresen EM, Malmgren JA, Carter WB, et al. Screening for Depression in Well Older Adults: Evaluation of a Short Form of the CES-D. Am J Prev Med. 1994;10:77–84. doi: 10.1016/S0749-3797(18)30622-6. [DOI] [PubMed] [Google Scholar]
- 18.Vitaliano PP, Russo J, Young HM, et al. The screen for caregiver burden. Gerontologist. 1991;31:76–83. doi: 10.1093/geront/31.1.76. [DOI] [PubMed] [Google Scholar]
- 19.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–65. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Reilly associates health outcomes research. http://www.reillyassociates.net n.d. Available.
- 21.Thornton M, Travis SS. Analysis of the Reliability of the Modified Caregiver Strain Index. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences . 2003;58:S127–32. doi: 10.1093/geronb/58.2.S127. [DOI] [PubMed] [Google Scholar]
- 22.Enderby P, Crow E. Frenchay Aphasia Screening Test: validity and comparability. Disabil Rehabil. 1996;18:238–40. doi: 10.3109/09638289609166307. [DOI] [PubMed] [Google Scholar]
- 23.Robinson BC. Validation of a Caregiver Strain Index. J Gerontol. 1983;38:344–8. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Kuzmik A, Boltz M, BeLue R, et al. The Modified Caregiver Strain Index in Black and White Dementia Caregivers at Hospital Discharge. Clin Gerontol. 2023;46:574–84. doi: 10.1080/07317115.2022.2106927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullen B, Pownall J, Cummings J, et al. Positive PsychoTherapy in ABI Rehab (PoPsTAR): A pilot randomised controlled trial. Neuropsychol Rehabil. 2018;28:17–33. doi: 10.1080/09602011.2015.1131722. [DOI] [PubMed] [Google Scholar]
- 26.Ogunlana MO, Dada OO, Oyewo OS, et al. Quality of life and burden of informal caregivers of stroke survivors. Hong Kong Physiother J. 2014;32:6–12. doi: 10.1016/j.hkpj.2013.11.003. [DOI] [Google Scholar]
- 27.Woodward TJ, Kim C, Calderon F, et al. Review of the diagnosis and management of pseudobulbar affect. US Pharm. 2017;42:31–5. [Google Scholar]
- 28.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 29.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 30.Wade DT, Collin C. The Barthel ADL Index: A standard measure of physical disability? Int Disabil Stud. 1988;10:64–7. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 31.Scottish government; 2012. [23-Sep-2020]. Scottish government index of multiple deprivation: SIMD.https://spatialdata.gov.scot/geonetwork/srv/eng/catalog.search Available. Accessed. [Google Scholar]
- 32.Pollock A, St George B, Fenton M, et al. Top 10 research priorities relating to life after stroke--consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9:313–20. doi: 10.1111/j.1747-4949.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2023. [Google Scholar]