Abstract
Background
Metered dose inhalers (MDIs) are important devices for delivering inhaled medications; however, they have an outsized carbon footprint due to their propellant gas. Many short-acting beta-agonist inhalers contain HFA-134a which has a global warming potential >1000 fold higher than carbon dioxide. We aimed to determine the practices around MDI use and disposal within Australia’s major lung function testing laboratories and identify the actions that most influence the carbon footprint of bronchodilator responsiveness (BDR) testing.
Methods
Australia’s 45 accredited lung function laboratories were invited to participate in an online survey asking about their volume of BDR testing, as well as practices around MDI use such as the number of actuations per BDR test, reuse of MDIs between patients and disposal method. We calculated MDI-associated carbon dioxide equivalent (CO2e) emissions by combining previously published estimates.
Results
39 laboratories completed the survey. Most laboratories used 4 actuations of salbutamol per BDR test for both adults (27/34, 79.4%) and children (17/20, 85%), but this ranged from 2 to 12. Only three (7.7%) laboratories did not routinely reuse MDIs between patients; however, they all sent their used MDIs for high-temperature incineration. Based on different combinations of observed MDI practices in Australia, we identified a potential sixfold difference in CO2e per 100 BDR tests, from as low as 23.3 kg CO2e up to 166 kg CO2e.
Conclusions
We identified three key practices to reduce the carbon footprint of BDR testing: disposing of MDIs via high-temperature incineration, reducing the number of actuations per BDR test and reusing MDIs between patients.
Keywords: Respiratory Function Test, Inhaler devices
WHAT IS ALREADY KNOWN ON THIS TOPIC
Metered dose inhalers (MDI) are known to have an outsized carbon footprint due to their propellant gas. While lung function testing laboratories are hot spots of MDI use, there have been no previous attempts to quantify their MDI-associated carbon footprint or to determine the most effective strategies to minimise their environmental impact.
WHAT THIS STUDY ADDS
This prospective survey of Australian lung function laboratories highlighted a variety of practices around the use, reuse and disposal of MDIs. Among observed practices, there was up to a 6-fold difference in carbon footprint, this increased to a 100-fold difference when the worst possible combination of practices was considered.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
To minimise their MDI-associated carbon footprint most effectively, lung function testing laboratories can reuse MDIs between patients, use fewer actuations of inhaled therapy and dispose of used MDIs via high-temperature incineration.
Introduction
Climate change represents one of the most serious threats to human health.1 The healthcare system is a significant contributor to climate change, responsible for 4%–5% of global greenhouse gas emissions.2 3 Metered dose inhalers (MDIs) have a disproportionate impact on healthcare’s carbon footprint due to the propellant gases they contain—commonly used propellants such as HFA-134a have a global warming potential more than 1000-fold higher than carbon dioxide.4 Propellant gases can be destroyed during the disposal of MDIs; however, this relies on these devices undergoing high-temperature incineration (ie, >1000°C). In the UK, it is estimated that MDIs alone are responsible for 3% of the entire National Health Service carbon footprint.5 Reducing the climate impacts of inhaler therapy, without compromising patient safety or leading to significant increases in cost, is currently a focus of peak respiratory bodies worldwide.6 7 Additionally, strategies to reduce the carbon footprint of healthcare often have financial cobenefits.8
Lung function testing is an important means of diagnosing and monitoring lung disease. Bronchodilator responsiveness (BDR) testing involves the measurement of spirometry before and after the administration of a short-acting beta-agonist (SABA) bronchodilator and assessing change in forced expiratory volume in 1 s and forced vital capacity against defined criteria.9 10 Spirometry guidelines recommend use of a standardised bronchodilator protocol for BDR testing.11 12 The default administration method for most laboratories is use of an MDI and spacer. We identified MDI use within lung function laboratories as a potentially significant and modifiable source of MDI-associated emissions, and a topic that had never previously been studied. We aimed to estimate the volume and determine the pattern of MDI use within Australia’s major lung function testing laboratories, as well as describe the practices that most influence the MDI-associated carbon footprint of BDR testing.
Methods
Definitions of terms
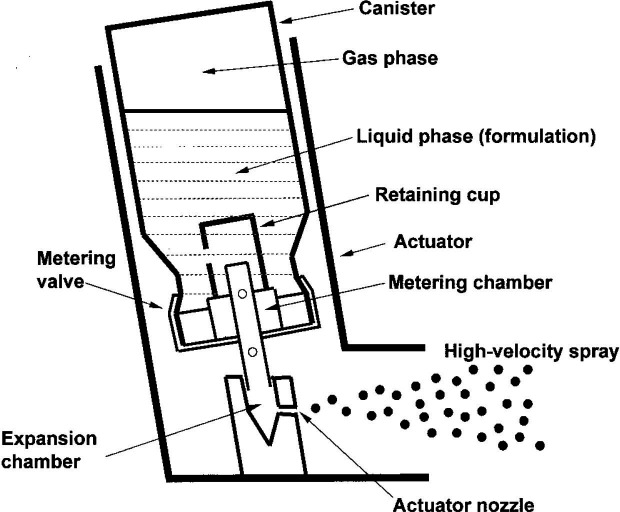
For the purposes of this paper, when discussing MDI components, ‘canister’ refers to the metal cylinder containing the active drug and propellant, while ‘actuator’ refers to the plastic outer shell that houses the canister and directs the aerosol spray (figure 1).13
Figure 1. Schematic of a typical metered dose inhaler. Reproduced with permission from Newman et al.13.
Study design, setting and participants
All Thoracic Society of Australia and New Zealand (TSANZ) accredited lung function laboratories in Australia were invited to participate in an online survey.14 Invitations were sent via email to a senior respiratory scientist at each lung function laboratory in September 2023, with a reminder email sent a fortnight later.
Survey content
The survey consisted of four main sections (online supplemental material). The first section recorded general laboratory information, including the location, the population serviced and the volume of lung function testing in 2022. The second section focused on the indications for and frequency of BDR testing. The third section focused on the volume and type of MDI routinely used by the laboratory in 2022. The final section focused on the reuse of MDIs (including if and how components were cleaned between patients), and disposal practices for canisters.
Carbon footprint calculations
We set out to provide MDI-associated carbon footprint calculations according to different combinations of laboratory practices. Based on our knowledge of Australian lung function laboratories, we anticipated most centres would be using a 200-dose GSK-licensed HFA-134a salbutamol MDI for their BDR testing, for which a formal carbon footprint assessment is publicly available.15
For our analysis, we assessed the carbon footprint based on the following assumptions:
Manufacture: 1.0 kg carbon dioxide equivalent (CO2e) per MDI.15
Patient use: 26.6 kg CO2e per 210 actuations,15 assuming 5% overfill.16
Disposal (propellant): for disposal via high-temperature incineration, we used a destruction removal efficiency (DRE) of 99.99%, meaning that only 0.01% of residual HFA-134a would remain intact. Due to the lack of published DRE data for high-temperature incineration and hydrofluorocarbon gases, we approached international experts belonging to a Montreal Protocol Technical Options Committee who provided this estimate based on preliminary data about the incineration process from a major healthcare waste disposal company (M. Loftus, personal correspondence). If disposal did not involve high-temperature incineration, we assumed all residual HFA-134a would reach the atmosphere intact. We assumed ‘empty’ MDI canisters still had 10 actuations’ worth of propellant remaining, based on our 5% overfill assumption.
Disposal (other): for other emissions associated with high-temperature incineration, we adopted calculations from Rizan et al,17 substituting the 2023 Australian emission factor for electricity generation (0.68 kg CO2e per kWh).18 Our estimate was, therefore, 1013 kg CO2e per tonne of waste (880 from direct emissions, 133 from indirect emissions). The weight of a full MDI canister was estimated to be 28.6 g, and an empty canister 14.8 g based on Di Paolo et al.16 We did not calculate the carbon footprint of the process of landfill disposal itself, this minor additional contribution would be dwarfed by the impact of the residual propellant released.
The carbon footprint of MDI distribution was not included as it was anticipated to be very low15 and would also vary between centres.
The financial cost of each MDI to a lung function laboratory was assumed to be $A6.50, based on pharmacy records at four participating hospitals from three different Australian states.
Statistical analysis
Continuous values were reported as medians with IQR. To facilitate comparisons between laboratories performing different volumes of BDR testing, we calculated carbon footprints in kg CO2e per 100 BDR tests.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Laboratory demographics and volume of testing
45 laboratories were invited to participate in the survey, 39 completed the survey and were included in our analysis.
Of the 39 laboratories, 36 (92.3%) were in metropolitan locations and three (7.7%) in regional centres. 19 laboratories (48.7%) exclusively tested adults, 5 (12.8%) exclusively tested children and the remaining 15 (38.5%) tested both, although these latter laboratories were skewed towards adult patients, with a median of 92% (IQR 90%–95%) of tests performed on adults.
The median number of lung function tests performed in the preceding calendar year (2022) was 3670 (IQR 2350–4842; range 1074–19,500). 38 laboratories reported on the proportion of lung function tests accompanied by BDR testing, with a median of 57.5% (IQR 40%–85%, range 8%–99%). Fewer laboratories (n=20) reported on the proportion of their BDR tests demonstrating significant change, with a median of 16.5% (IQR 10%–30%, range 3%–50%).
Metered dose inhalers
All laboratories used one of three different salbutamol MDIs, 100 μg per actuation, containing HFA-134a: Ventolin (n=24, 61.5%), Zempreon (n=10, 25.6%) or Asmol (n=5, 12.8%). The most common number of actuations of MDI used for BDR testing was 4, either when testing adults (27/34, 79.4%) or children (17/20, 85%). All other laboratories used 2 or 3 actuations for adults or 6 for children, except for 1 site that gave 12 actuations to all patients over 6 years of age. Most sites reused MDI canisters between patients, but three laboratories (7.7%) reported using a completely new MDI for every patient.
The median number of MDI canisters used by a laboratory in 2022 was 50 (IQR 40–74) but ranged widely from 15 to 5100. The two highest values (5100 and 396) were from single-use laboratories. To correct for laboratories’ volume of testing, data on both MDI use (n=37) and BDR test numbers (n=38) were required; both data points were available from 36 (92.3%) laboratories. Corrected for volume of testing, the median number of MDI canisters per 100 BDR tests was 2.22 (IQR 2.00–2.88, range 0.94–100). The laboratories with the three highest values (100, 99.9 and 98.5 canisters per 100 BDR tests) did not reuse MDIs between patients, and the fourth highest value (17.1) belonged to the laboratory performing 12 actuations per BDR test.
Regarding disposal of MDI canisters, only four laboratories (4/35, 11.4%) placed MDIs into a pharmaceutical waste stream to undergo high-temperature incineration—this included all three laboratories that did not reuse MDIs. 30/35 (85.7%) laboratories disposed of canisters into general or clinical waste streams (ultimately ending up in landfill, without destruction of residual propellant), while one final laboratory reported all used MDIs were collected by a clinician with an interest in propellant recycling.
Carbon footprint calculations
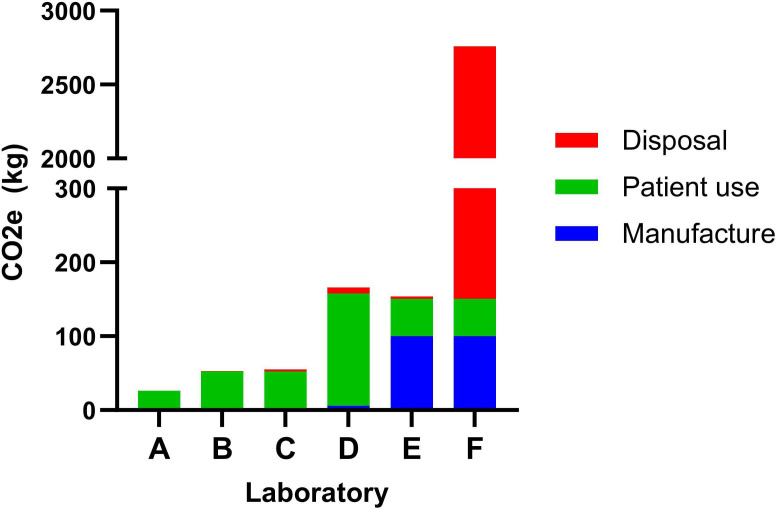
We calculated the MDI-associated carbon footprint of six hypothetical scenarios based on different combinations of laboratory practices (table 1 and figure 2). These are related to (1) the number of actuations of salbutamol per BDR test, (2) whether MDIs were reused between patients and (3) the method of MDI disposal. Unless otherwise specified, all CO2e and cost estimates are ‘per 100 BDR tests.’
Table 1. Six hypothetical laboratories and their metered dose inhaler-associated carbon footprint and medication cost, per 100 BDR tests.
| A | B | C | D | E | F | |
| Laboratory practice | ||||||
| Actuations per BDR test | 2 | 4 | 4 | 12 | 4 | 4 |
| Reuse of MDIs | Yes | Yes | Yes | Yes | No | No |
| Disposal | Incineration | Incineration | Landfill | Landfill | Incineration | Landfill |
| Consumption (per 100 BDR tests) | ||||||
| MDIs | 1 | 2 | 2 | 6 | 100 | 100 |
| Actuations | 200 | 400 | 400 | 1200 | 400 | 400 |
| Medication cost (per 100 BDR tests) | ||||||
| $A | $A6.5 | $A13 | $A13 | $A39 | $A650 | $A650 |
| Carbon footprint in kg CO2e (per 100 BDR tests) | ||||||
| Manufacture | 1 | 2 | 2 | 6 | 100 | 100 |
| Patient use | 25.3 | 50.7 | 50.7 | 152 | 50.7 | 50.7 |
| Disposal (propellant) | <0.001 | <0.001 | 2.66 | 7.98 | 0.26 | 2607 |
| Disposal (other) | 0.015 | 0.030 | 0 | 0 | 2.9 | 0 |
| Total kg, CO2e | 26.3 | 52.7 | 55.4 | 166 | 154 | 2758 |
The colours in the above table indicate the carbon footprint associated with that particular laboratory practice. Actions with the lowest carbon footprint have green shading, actions with the highest carbon footprint have red shading, while orange indicates an intermediate carbon footprint.
$A, Australian dollars; BDR, bronchodilator responsiveness; CO2e, carbon dioxide equivalent; MDI, metered dose inhaler
Figure 2. Carbon footprint of different combinations of laboratory practices, per 100 bronchodilator responsiveness tests.
The lowest carbon footprint scenario involved using two actuations of salbutamol per BDR test, reusing MDI canisters between patients, and disposing of MDIs via high-temperature incineration (example A, 26.3 kg CO2e). Among surveyed laboratories, the most frequently observed combination of practices was four actuations per BDR test, reuse of canisters and disposal via landfill (example C, 55.4 kg CO2e).
The major contributor to overall CO2e for most laboratories was the ‘patient use’ component, relating to the number of actuations given per BDR test. For a typical laboratory (example C), switching from 4 actuations to 12 (example D) led to a tripling of the overall carbon footprint. The carbon savings from MDI reuse could be cancelled out by such a high number of actuations—example D’s carbon footprint of 166 kg CO2e was higher than a laboratory with a single-use MDI policy that only administered 4 actuations per test and incinerated used canisters (example E, 154 kg CO2e).
The MDI disposal method can potentially have a very large impact on a laboratory’s carbon footprint, but its influence is relative to the volume of residual propellant in discarded canisters. For a typical laboratory reusing MDIs, disposal via general waste (example C) versus incineration (example B, 52.7 kg CO2e) only increases its carbon footprint by 2.7 kg CO2e or 5.1%. Whereas for laboratories with a single-use policy, discarding near-full canisters, disposal via general waste (example F, 2758 kg CO2e) versus incineration (example E) increases carbon footprint by over 2600 kg CO2e—a more than 15-fold rise. For an average Australian laboratory conducting 2000 BDR tests per year, this would equate to an increase in its annual carbon footprint of 55 200 kg CO2e—a similar impact to driving around Australia in a petrol car over 20 times.19
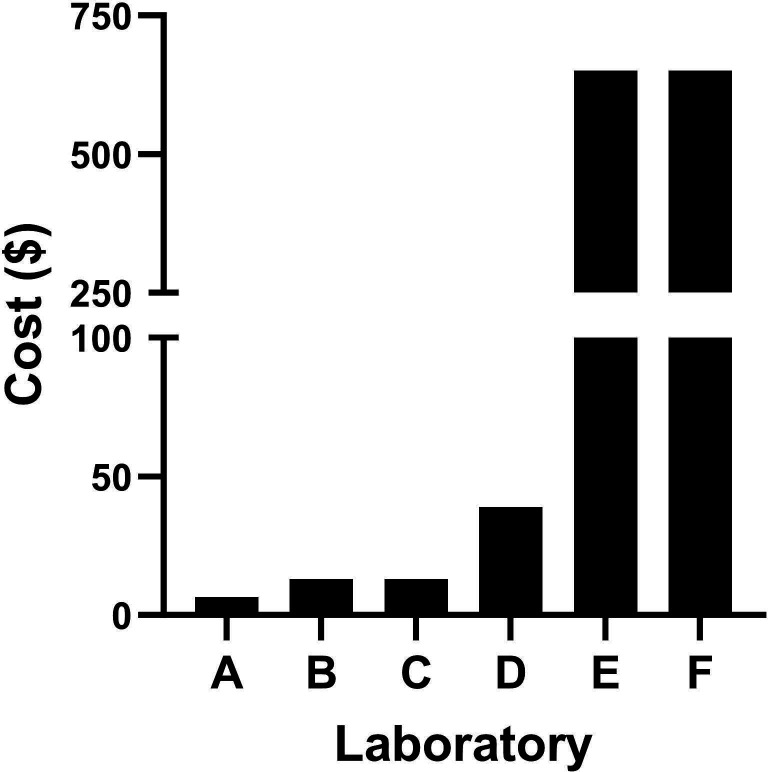
Unsurprisingly, there were financial savings for laboratories reusing their MDI canisters (figure 3). For an average laboratory conducting 2000 BDR tests per year and administering four actuations of salbutamol, a single-use versus multiple-use policy (laboratory B vs laboratory E) could result in annual medication cost savings of over $A12 000 ($A13 000 vs $A260).
Figure 3. Medication cost of different combinations of laboratory practices, per 100 bronchodilator responsiveness tests. CO2e, carbon dioxide equivalent.
Infection prevention
Of the 36 laboratories reusing MDIs between patients, 29 (80.6%) reused the entire device, whereas 7 (19.4%) reused the canister alone and discarded the actuator between patients.
Laboratories took a range of approaches to cleaning the MDI canister. For laboratories that reused canisters, 25 (69.4%) wiped down the canister with some form of disinfectant wipe, 6 (16.7%) performed no cleaning and 5 (13.9%) did not describe their practice in sufficient detail to be categorised. There was more variation in how laboratories cleaned the actuator. Seven laboratories purchased single-use actuators (not reused between patients). Of the remaining 29 laboratories, 11 (37.9%) soaked their actuators in disinfectant within their laboratory, 9 (31.0%) used some form of disinfectant wipe, 5 (17.2%) used thermal disinfection, 2 (6.9%) reported performing no cleaning between patients and two gave responses that lacked sufficient detail for categorisation.
Five of the laboratories that reused at least one component of the MDI (5/36, 14.3%) reported that they excluded one or more patient groups from reused MDIs. Indications for excluding patients included cystic fibrosis (n=2), previous lung transplant (n=2) or a known history of multidrug resistant bacteria in their sputum (n=2).
Discussion
We surveyed TSANZ-accredited lung functional laboratories in Australia to explore common practices regarding BDR testing and the use of MDIs, as part of the first-ever study of the carbon footprint of lung function laboratory practices. Among commonly observed practices (examples A–E), there was up to a sixfold difference in MDI-associated carbon footprints. The three primary determinants of a laboratory’s carbon footprint were whether MDIs were reused, the number of salbutamol actuations given and the method of MDI disposal. All laboratories except three reused MDI canisters, a practice that was also associated with significant financial savings. Reassuringly, all laboratories that discarded near-full MDI canisters after every patient did so via high-temperature incineration. This ensured that residual propellant was destroyed, avoiding a near 20-fold increase in their own carbon footprints and a scenario (example F) 100-fold worse than the lowest footprint example.
One of the primary factors influencing MDI-associated carbon footprint was whether MDIs were used just once or multiple times. A potential concern with MDI reuse is the risk of transmitting infections. Although almost all surveyed laboratories reused MDI canisters between patients, and the majority reused actuators after some form of cleaning, there is relatively little published data to support one practice over another. In some jurisdictions, ‘common canister’ protocols have been developed for inpatient settings,20 yet these have undergone little evaluation. The microbiology data to support the common canister approach is primarily based on small published studies or conference abstracts—these only found either no growth of pathogens21 22 or very low rates of growth of predominantly skin flora,23 especially if cleaning processes were not followed.24
A second practice with a major impact on MDI-associated emissions is the number of bronchodilator actuations per BDR test, with our surveyed laboratories administering between 2 and 12. Current European Respiratory Society and American Thoracic Society (ERS/ATS) spirometry guidelines propose administering four actuations of bronchodilator11; however, this is arbitrary and based on an example provided in the original 2005 guidelines that has since been widely adopted.12 The original recommendation was not supported by specific evidence beyond a desire to ensure a patient’s response was high on the SABA dose-response curve. We note that early salbutamol studies demonstrated rapid onset of bronchodilator effect with doses equivalent to two to three actuations,25 26 and the most recent ERS/ATS standard on interpretive strategies for lung function testing acknowledged that the relative merits of different BDR dosing protocols are unclear.9 Over 80% of the laboratories we surveyed used four actuations for BDR testing, far more uniform than a similar assessment in 2000 when less than half of all laboratories routinely used four actuations.27 A reduction to two or three actuations per BDR test could lead to a 50% or 25% reduction in ‘patient use’ emissions, respectively—more modest than the reduction achieved by shifting to reuse of MDIs, but a possible next step for laboratories already reusing their inhalers. The environmental benefits of this approach would need to be carefully weighed against a potential reduction in the diagnostic yield of the BDR test, to ensure that the test remains clinically useful. Further research is needed to establish whether reducing actuations is appropriate, and for which patient groups.
Our research highlights the importance of correct MDI disposal for restricting damaging emissions. High-temperature incineration is required to destroy residual hydrofluorocarbon gas inside canisters and prevent it from exerting an outsized environmental impact due to its very high global warming potential. While the advent of dose counters can help prevent MDIs from being prematurely discarded before they are ‘empty’, all MDIs are overfilled to facilitate uniform drug delivery so still contain residual propellant when the dose counter reaches zero. Despite most laboratories being colocated within tertiary or secondary hospitals that would have established pathways for handling medication waste, only a minority (under 15%) disposed of MDIs into a pharmaceutical waste stream for incineration. Addressing MDI disposal practices within the controlled environment of lung function laboratories may be relatively easy, through targeted education for respiratory scientists, however, MDI disposal should also be a focus in the community where the majority of MDI use occurs. Multiple small surveys in the UK demonstrated that >90% of patients disposed of their inhalers in household waste.28 29 Ideally a recycling programme would be in place to reclaim unused propellant, but no such programme currently exists in Australia. A pilot study in the UK demonstrated the feasibility of an inhaler postal recovery scheme, saving 119.3 tonnes CO2e from 20 049 inhalers across just 12 months.30
In the community setting, dry powder inhalers (DPIs) are viewed as a key lower-carbon alternative to MDIs. However, in the lung function laboratory setting, it may not be practical to switch to DPIs. First, in the Australian context, there is not currently a salbutamol-only DPI option. Second, DPIs are in direct contact with patients (unlike MDIs which are separated from the patient by a spacer), meaning they would have to be discarded after every use. So, while the ‘per actuation’ carbon footprint would be lower, this would introduce additional carbon footprint associated with the manufacture and distribution of so many extra DPI devices, as well as a higher financial cost for laboratories to purchase single-use inhalers. In contexts where salbutamol-only DPIs are commonly used, allowing or encouraging patients to bring their own devices for BDR testing could be a feasible strategy to reduce the carbon footprint of lung function via DPIs.
A strength of this study was its very high response rate (>85%), combined with a focus on TSANZ-accredited laboratories that are likely to have the highest testing volumes. Additionally, we have for the first time published an estimate of the DRE for high-temperature incineration of propellants within MDIs. There are some potential limitations to this research. First, we were unable to precisely quantify the degree of MDI overfill, despite contacting device manufacturers and multiple international experts. A higher overfill estimate would increase the calculated CO2e impact of MDIs being discarded into landfill, especially for laboratories reusing devices and discarding supposedly ‘empty’ canisters, increasing the importance of disposal method. If overfill was as high as 40%,31 the sole decision of disposal method (landfill vs incineration) would determine over one-third of laboratories’ carbon footprint when reusing devices. Second, our DRE estimate of 99.99% was based on incineration practices from one major Australian waste disposal company and may not be applicable globally. A lower DRE would increase the environmental impact of incinerated canisters; however, incineration would still lead to a lower overall CO2e than disposal via landfill. Third, we limited our carbon footprint calculations to the CO2e associated with MDIs alone, this was a pragmatic decision given their disproportionate environmental impact and the availability of published estimates. Other factors such as the CO2e contributions of spacers (which are typically single use) and patient travel were not considered. Lastly, we acknowledge that our survey may not be representative of private providers or smaller community-based providers, which additionally may have reduced access to pharmaceutical waste disposal.
In conclusion, in this first-ever study to assess the carbon footprint of BDR testing, our survey of major Australian lung function laboratories demonstrated a wide range of practices in how laboratories handle MDIs. We note the significant unnecessary variation in practice and feel there is a strong need to align procedures, to both maximise clinical value and ideally minimise unnecessary environmental impact. We have identified and quantified three key practices that can reduce laboratories’ MDI-associated carbon footprint, namely reusing MDI canisters between patients, using fewer actuations per BDR test and disposing of MDIs via high-temperature incineration. The method of disposal becomes especially important when discarded canisters are not empty. Ensuring correct disposal of MDIs should not only be a focus of lung function laboratories, but also wider community-based patient education efforts to help reduce MDIs’ outsized carbon footprint.
supplementary material
Acknowledgements
Mr Owen Eades (Monash University School of Public Health and Preventive Medicine) for assistance with survey design and management; the Board of the Australian and New Zealand Society of Respiratory Science (ANZSRS) for their support of the project and assistance distributing the survey; and Dr Helen Tope for assistance generating a destruction removal efficiency estimate for hydrofluorocarbon gases during high-temperature incineration. Dr Tope is employed by Planet Futures, a consulting business providing services to government, industry and other nongovernmental organisations on environmental issues. As an independent expert, she co-chairs the Medical and Chemicals Technical Options Committee, which provides technical and economic advice, including on inhalers and destruction technologies, to the Montreal Protocol.
The views expressed herein are those of the co-authors and do not represent those of the Medical and Chemicals Technical Options Committee.
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The data that support the findings of this study are available from the corresponding author on reasonable request.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval: Ethics approval was obtained from the Alfred Health Human Research Ethics Committee (Project number 495/23).
Contributor Information
Michael J Loftus, Email: michael.loftus@monash.edu.
Jayne Roberts, Email: roberts.jaynelouise@gmail.com.
Nicholas Romeo, Email: nicholas.romeo@nh.org.au.
Pam Matsas, Email: P.Matsas@alfred.org.au.
Karin Leder, Email: karin.leder@monash.edu.
Brigitte Borg, Email: b.borg@alfred.org.au.
Belinda R Miller, Email: b.miller@alfred.org.au.
Data availability statement
Data are available on reasonable request.
References
- 1.Costello A, Abbas M, Allen A, et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009;373:1693–733. doi: 10.1016/S0140-6736(09)60935-1. [DOI] [PubMed] [Google Scholar]
- 2.Lenzen M, Malik A, Li M, et al. The environmental footprint of health care: a global assessment. Lancet Planet Health. 2020;4:e271–9. doi: 10.1016/S2542-5196(20)30121-2. [DOI] [PubMed] [Google Scholar]
- 3.Pichler P-P, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004. doi: 10.1088/1748-9326/ab19e1. [DOI] [Google Scholar]
- 4.Pritchard JN. The Climate is Changing for Metered-Dose Inhalers and Action is Needed. Drug Des Devel Ther. 2020;14:3043–55. doi: 10.2147/DDDT.S262141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS England . NHS England; 2021. [2-Oct-2024]. Delivering a net zero national health service.https://www.england.nhs.uk/greenernhs/a-net-zero-nhs/ Available. Accessed. [Google Scholar]
- 6.Levy ML, Bateman ED, Allan K, et al. Global access and patient safety in the transition to environmentally friendly respiratory inhalers: the Global Initiative for Asthma perspective. Lancet. 2023;402:1012–6. doi: 10.1016/S0140-6736(23)01358-2. [DOI] [PubMed] [Google Scholar]
- 7.European Respiratory Society European respiratory society position statement on asthma and the environment. 2021
- 8.Barratt AL, Bell KJ, Charlesworth K, et al. High value health care is low carbon health care. Med J Aust. 2022;216:67–8. doi: 10.5694/mja2.51331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60:2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 11.Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200:e70–88. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 13.Newman SP. Principles of metered-dose inhaler design. Respir Care. 2005;50:1177–90. [PubMed] [Google Scholar]
- 14.The Thoracic Society of Australia and New Zealand (TSANZ) List of accredited respiratory labs. [1-Sep-2023]. https://thoracic.org.au/lab-accreditation-program/list-of-accredited-respiratory-labs/ Available. Accessed.
- 15.Janson C, Henderson R, Löfdahl M, et al. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax. 2020;75:82–4. doi: 10.1136/thoraxjnl-2019-213744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Paolo ER, Spaggiari S, Pannatier A, et al. Stop using the flotation technique and start weighing salbutamol pressurised metered-dose inhalers without dose counters. Swiss Med Wkly. 2015;145:w14162. doi: 10.4414/smw.2015.14162. [DOI] [PubMed] [Google Scholar]
- 17.Rizan C, Bhutta MF, Reed M, et al. The carbon footprint of waste streams in a UK hospital. J Clean Prod. 2021;286:125446. doi: 10.1016/j.jclepro.2020.125446. [DOI] [Google Scholar]
- 18.Australian Government Department of Climate Change Energy the Environment and Water Australian national greenhouse accounts factors workbook. 2023
- 19.National Transport Commission . Melbourne: National Transport Commission; 2022. Carbon dioxide emissions intensity for new Australian light vehicles 2021. [Google Scholar]
- 20.Grissinger M. Shared metered dose inhalers among multiple patients: can cross-contamination be avoided? P T. 2013;38:434–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Wojciechowski WV. The common canister protocol using the Monaghan AeroChamber reveals no cross-contamination and potential cost savings. Respir Care. 2000;45 [Google Scholar]
- 22.Wojciechowski WV, Moseley AL. Analysis of cross-contamination of metered dose inhalers when using the respironics optichamber under the common canister protocol. American Association for Respiratory Care International Congress; New Orleans. 2004. [Google Scholar]
- 23.Dunlevy CL, Roman SB. Surveillance of reservoir cross-contamination with multiple patient MDI use. American Society of Health-System Pharmacists Mid-year Meeting; Atlanta, Georgia. 1997. [Google Scholar]
- 24.Hinson D. Incidence of contamination of metered dose inhaler canisters when used with multiple patients using spacer devices. American Society of Health-System Pharmacists Mid-year Meeting; Atlanta, Georgia. 1997. [Google Scholar]
- 25.Kamburoff PL, Rime FJ. Oral and inhaled salbutamol as a bronchodilator. Br J Dis Chest. 1970;64:46–54. doi: 10.1016/s0007-0971(70)80048-1. [DOI] [PubMed] [Google Scholar]
- 26.Barnes PJ, Pride NB. Dose-response curves to inhaled beta-adrenoceptor agonists in normal and asthmatic subjects. Br J Clin Pharmacol. 1983;15:677–82. doi: 10.1111/j.1365-2125.1983.tb01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borg BM, Reid DW, Walters EH, et al. Bronchodilator reversibility testing: laboratory practices in Australia and New Zealand. Med J Aust. 2004;180:610–3. doi: 10.5694/j.1326-5377.2004.tb06121.x. [DOI] [PubMed] [Google Scholar]
- 28.Chebbout C. Inhaler disposal: where do our patients put them when finished?. East Midlands Thoracic Society Conference; 2021. [Google Scholar]
- 29.Sivarajasingam V. BJGP Life; 2021. [12-Feb-2024]. Understanding patients’ knowledge of inhaler recycling.https://bjgplife.com/understanding-patients-knowledge-of-inhaler-recycling/ Available. Accessed. [Google Scholar]
- 30.Murphy A, Howlett D, Gowson A, et al. Understanding the feasibility and environmental effectiveness of a pilot postal inhaler recovery and recycling scheme. NPJ Prim Care Respir Med. 2023;33:5. doi: 10.1038/s41533-023-00327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munro S. Life Sciences; 2021. [30-Oct-2024]. Trends and future challenges in inhaled drug delivery.https://247biopharma.com/article/trends-and-future-challenges-in-inhaled-drug-delivery/ Available. Accessed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.