Abstract
Objectives
Survivors of intracerebral haemorrhage (ICH) are at high risk of incident depression, which is modified by social determinants of health (SDOH) and associated with worse functional outcomes. We sought to determine the role of prestroke SDOH in depression incidence after ICH to better characterise post-ICH outcomes.
Study design
We analysed data from a cohort study of ICH survivors without prestroke depression, presenting at Massachusetts General Hospital between 2006 and 2017. We collected information from electronic health records (EHR), follow-up interviews and CT/MRI. The relationship between social vulnerability, air quality and post-ICH depression incidence within 12 months of acute haemorrhage was investigated using logistic regression models that also included EHR and CT/MRI information as predictors.
Results
Participants were 576 survivors, median age of 72 (IQR=61–81), 317 (55%) self-reported as male and 482 (84%) as white. 204 (35%) were diagnosed with depression within 12 months of ICH. Hospital admission longer than 1 week (OR 1.80, 95% CI 1.08 to 3.00), cerebral amyloid angiopathy (CAA) burden (OR 1.45, 95% CI 1.25 to 1.68) and social vulnerability (OR 3.03, 95% CI 1.49 to 6.19) were associated with depression incidence post-ICH.
Conclusions
In addition to CAA burden and patient location 1-week post-ICH, social vulnerability was independently associated with depression among ICH survivors. Our findings suggest that social vulnerability influences ICH outcomes. Future studies should investigate how poststroke clinical care interventions can address SDOH effects to reduce incident depression and improve outcomes among ICH survivors.
Keywords: CEREBROVASCULAR DISEASE, DEPRESSION, STROKE
WHAT IS ALREADY KNOWN ON THIS TOPIC
Examinations of individual social determinants of health have identified associations with disparate poststroke outcomes; however, little is known about the compounding effects of broad social vulnerability and biological determinants on the risk of poor poststroke outcomes.
WHAT THIS STUDY ADDS
The Social Vulnerability Index (SVI), a comprehensive measure representing multiple upstream determinants, has a strong predictive relationship with depression incidence post intracerebral haemorrhage and increases the risk of poor outcomes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings highlight the strength and importance of the SVI and consider multiple compounding social determinants as a research tool to better understand disparate poststroke outcomes.
Background and objectives
Intracerebral haemorrhage (ICH), an acute manifestation of underlying cerebral small vessel disease (CSVD), is the most severe form of stroke and accounts for nearly half of all stroke-related morbidity and mortality.1 2 Depression is common after stroke, affecting about one-third of survivors 12 months post-ICH,3 and is associated with poor functional outcomes and increased mortality.4 Therefore, effective identification and treatment of those at risk for depression following an acute ICH are critical aspects of long-term care for stroke survivors.
CSVD is a common cause of primary ICH.5 Cerebral amyloid angiopathy (CAA) and hypertensive arteriopathy underlie most ICH cases.6 CSVD neuroimaging markers are associated with depression incidence.7 8 Both CSVD9 and depression10 are associated with social determinants of health (SDOH).11 12 However, our understanding of the structural factors contributing to depression among ICH survivors remains limited.
The SDOH paradigm considers health as an outcome of socially constructed mechanisms that perpetuate health inequities.13 Low social support,10 poverty10 and chronic exposure to neighbourhood air pollutants14 are associated with an increased risk for depression. Specific to ICH survivors, higher median neighbourhood income predicts greater improvement in hypertension severity after ICH15 and lower educational attainment among ICH survivors living with depression increases the risk of recurrent ICH.16
As these determinants compound to increase the risk for poorer poststroke outcomes, we sought to clarify the roles of social and biological determinants in depression incidence among ICH survivors through the development of a diagnostic post-ICH depression prediction model. We used longitudinal data from an ongoing, single-centre prospective study of haemorrhagic stroke at Massachusetts General Hospital (MGH) to investigate the relationship between SDOH and depression incidence.
Methods
Source of data
Retrospective analyses were performed on data from a cohort study of haemorrhagic stroke patients presenting to MGH, a single-site tertiary care referral centre in Boston, Massachusetts, USA. Participants were patients admitted between January 2006 and July 2017 with an acute primary ICH. Participants were 18 or older. Diagnoses were determined by the attending stroke neurologist and confirmed by CT scan obtained within 24 hours of symptoms’ onset.
Inclusion and exclusion criteria
Patients with intracranial haemorrhage due to trauma, conversion of an ischaemic infarct, rupture of a vascular malformation or aneurysm, or brain tumour were ineligible. Patients who (1) died within 90 days of the haemorrhage; (2) did not consent or were unable to complete cognitive screening, (3) did not undergo MRI within 90 days or (4) were diagnosed with depression before ICH8 were excluded.
Predictor: medical history and demographic information collected at baseline and follow-up
Participants were interviewed in person by study staff at baseline.8 17 Medical history, social and demographic information, including self-reported race and ethnicity, were recorded. Study staff blinded to baseline and neuroimaging information conducted follow-up phone interviews 3, 6 and, 12 months after the index ICH to capture follow-up data, which was also supplemented by a semiautomated review of electronic health records (EHR).
Predictor: CT and MRI data capture
Admission CT scans were analysed for ICH location, haematoma volume and intraventricular blood volume according to previously validated methods.15 MRIs were obtained using a 1.5 or 3.0 Tesla scanner.15 Neuroimaging markers of CSVD severity were rated according to the Standards for Reporting Vascular Changes on Neuroimaging consensus criteria.18 ICH cases were classified by aetiology based on the location of ICH and cerebral microbleeds (CMB), and the presence of superficial siderosis (cSS) as CAA-related and as previously described.8 19 Then, a CAA burden score was created on an ordinal scale from 0 to 6. This score was based on the presence and count of lobar CMB, centrum semiovale, enlarged perivascular spaces, deep white matter hyperintensities and focal or disseminated cSS, without lacunes.20 21
Predictor: SDOH data
Information on participant sociodemographic characteristics (veteran status, marital status, educational attainment and preferred language) was gathered from EHR using a previously validated algorithm.15 16 22 Then, EHR were manually reviewed for other available data points to minimise the amount of missing data.
In accordance with recommendations by the National Institutes of Health, using the PhenX toolkit, two structural SDOH domains were identified for inclusion in this study, social vulnerability and air quality.
Social vulnerability was measured using the Social Vulnerability Index (SVI). The SVI uses data on social factors collected by The Geospatial Research, Analysis and Services Programme within the Agency for Toxic Substances and Disease Registry at the Centers for Disease Control, to score and rank census tracts within each state from high to low social vulnerability. The SVI categorises 14 social factors into 4 themes: (1) socioeconomic status, (2) household composition, (3) minority status and language and (4) housing and transportation. Census tracts are then ranked on these social factors and themes. Rank values range from 0 to 1 with higher values indicating greater vulnerability.
Air quality was measured using the US Environmental Protection Agency’s Air Quality Index (AQI) data. The AQI ranges from 0 to 500 with higher values indicating greater levels of air pollution and health concern. Values between 0 and 50 are considered satisfactory with air pollution posing little or no risk, 51–100 are considered acceptable although there may be a risk for some people sensitive to air pollution and 100–150 are considered unhealthy for certain sensitive groups of people.
Outcome: depression following ICH
Depression incidence was defined as participants meeting the following criteria within 12 months: (1) documented evidence in medical records of a depression diagnosis (by Diagnostic and Statistical Manual of Mental Disorders 5; DSM-5 criteria), as confirmed by a board-certified neuropsychiatrist blinded to SDOH data; (2) billing information consistent with a new depression diagnosis and (3) four-item version of the Geriatric Depression Scale (GDS) score ≥2.16 18 23
Statistical methods
Wilcoxon rank sum tests, χ2 tests and Fisher’s exact tests (all two tailed) were performed to investigate associations between sociodemographic information and post-ICH depression. Logistic regression models were created to test the association of structural determinants, demographic and clinical factors with depression incidence post-ICH. All models included patient age, sex, race, education, ICH location and volume, and patient location 1-week post-ICH as predictors. Patient age was included as a continuous numeric variable and sex was dichotomised as female or male. Education was represented by three levels: completed less than high school, completed high school and completed more than high school. ICH location as lobar, deep or cerebellar and ICH volume as a continuous numeric variable. Last, patient location 1-week post-ICH was represented by four levels: home, acute rehab, nursing home or still in hospital.
Model 1: The literature informed model also included five structural determinants associated with depression and worse recovery outcomes post-ICH. Specifically, community-level poverty, unemployment, per capita income (PCI), low community-level educational attainment (defined as less than a high school diploma) and median AQI score were included. Model 2: The SVI-informed model included the overall SVI rank score as a predictor. Model 3: The comprehensive model included the overall SVI rank score and the CAA burden score. AQI was handled as a continuous numeric variable, while structural predictors and the CAA burden score were handled as ordinal rank variables. For all models, adjusted R2 were examined, and likelihood ratio tests (LRT) were compared using the BIC method to determine the variability explained by the models and to assess model fit.
Data were examined for distribution, normality and missing values. Single random imputation or single imputation using the appropriate marker of central tendency was used to address missing values. The missingness rate for each predictor was less than 20%. Findings at p<0.05 were considered statistically significant. All analyses were performed by using R software (The R Foundation for Statistical Computing), V.4.2.2.
Results
Participants
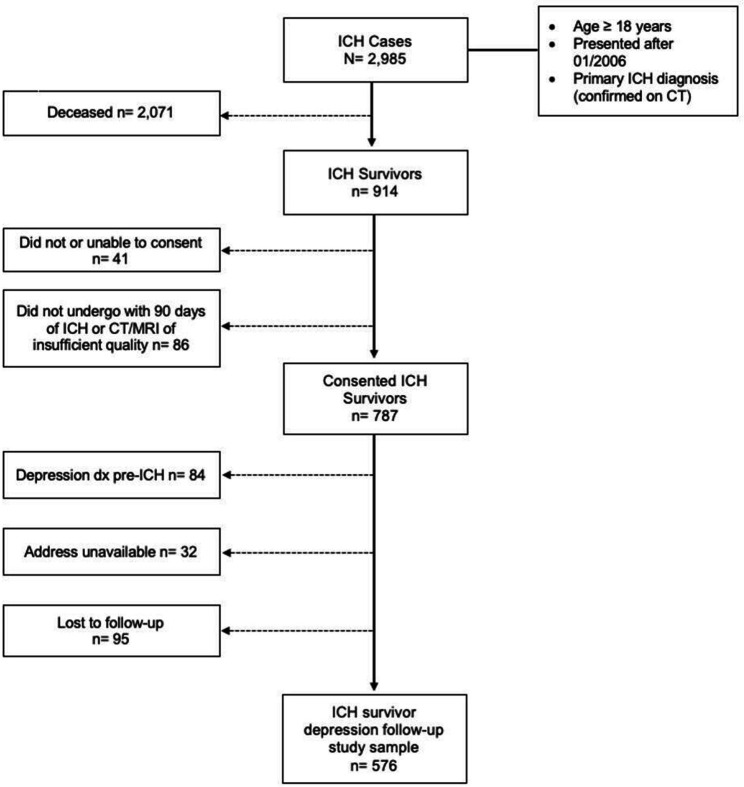
After screening, 914 ICH cases were enrolled (figure 1). We identified 576 eligible participants for study inclusion. The majority (2071/2985, 69.4%) of cases were excluded due to death within 90 days of the index ICH. Additionally, 84/914 (9.2%) were excluded due to a confirmed history of depression prior to the ICH event. Among study participants, 95/787 (12.1%) were lost to follow-up while 204/576 (35.4%) were diagnosed with depression within 12 months of ICH. We present patient, clinical and SDOH measures for all ICH survivors included in this study and survivors who experienced depression post-ICH in table 1. Participants self-reported largely as male (n=317, 55.0%), white (n=482, 83.7%) and were 72 years of age (median, IQR=61–81). Most participants reported a history of hypertension (n=428, 74.3%), while many reported a history of diabetes (n=116, 20.3%), prior ICH (n=7, 1.2%), atrial fibrillation (n=93, 16.4%), coronary artery disease (CAD, n=97, 8.1%) and pre-ICH dementia diagnosis (n=46, 8.1%). 55% (n=317) of participants survived a lobar hematoma with a median volume of 10.3 (IQR=3.4–24.8) and an intraventricular haemorrhage median volume of 0, (IQR=0–0.91). Further, survivors had a median Glasgow Coma Score (GCS) of 15 (IQR=13–15), an ICH score of 1 (IQR=0–2) and a discharge modified Rankin Score of 4 (IQR=3–4). Most participants remained in the hospital 1-week post-ICH (n=271, 47.0%), while about a quarter (n=151, 26.2%) were discharged to an acute rehabilitation setting, 24.0% (n=138) were discharged home and few (n=16, 2.8%) were discharged to a nursing home. Participants lived in areas characterised by satisfactory air quality (median=38, IQR=32–39) and relatively low to moderate social vulnerability (median=0.34, IQR=0.18–061). All study participants had outcome data available (evidence of depression within 12 months of the index ICH, n=576), and therefore, were included in all regression models. The missing data rate for the predictors included was marginal, 0.7% age (n=4), 1.4% sex (n=8), 5.0% race (n=29), 1.4% education (n=8), 1.4% ICH location (n=8), 4.0% ICH volume (n=23), 1.4% 1-week location (n=8), 0.5% CAA score (n=3), 6.8% AQI (n=39) and 2.3% SVI rankings (n=13).
Figure 1. Study enrolment flow chart. ICH, intracerebral haemorrhage.
Table 1. Sample characteristics and demographics.
| ICH survivors | Study sample | Depression post-ICH | |
| N=1007 | n=576, 57% | n=204, 35% | |
| Demographics | |||
| Age at ICH event median, IQR | 70, 57–76 | 72, 61–81 | 71, 61–80 |
| Sex (male, %) | 567, 56.3 | 317, 55.0 | 106, 52.0 |
| Race and ethnicity (%) | |||
| White | 796, 79.0 | 482, 83.7 | 175, 85.8 |
| Black | 74, 7.3 | 33, 5.7 | 10, 4.9 |
| Asian | 75, 7.4 | 40, 6.9 | 13, 6.4 |
| Multiple, other and unknown | 62, 6.2 | 21, 3.6 | 6, 2.9 |
| Hispanic | 85, 8.4 | 34, 5.9 | 13, 6.4 |
| Education | |||
| Less than high school | 85, 8.4 | 40, 6.9 | 13, 6.4 |
| Completed high school | 658, 65.3 | 383, 66.5 | 127, 62.3 |
| Completed college or more | 264, 26.2 | 153, 26.6 | 64, 31.4 |
| Medical history | |||
| Hypertension | 792, 79.4 | 428, 74.7 | 156, 76.8 |
| Diabetes | 201, 20.2 | 116, 20.3 | 39, 19.3 |
| Prior ICH | 43, 4.3 | 7, 1.2 | 2, 1.0 |
| Atrial fibrillation | 153, 15.4 | 93, 16.4 | 34, 16.8 |
| Coronary artery disease | 149, 15.0 | 97, 8.1 | 41, 20.3 |
| Pre-ICH dementia | 43, 4.3 | 46, 8.1 | 17, 8.5 |
| Neuroimaging characteristics (median, IQR) | |||
| Lobar haematoma location % | 378, 37.5 | 317, 55.0 | 129, 63.2 |
| Haematoma volume | 9.2, 3.1–21.7 | 10.3, 3.4–24.8 | 12.9, 3.8–30.8 |
| Intraventricular volume | 0, 0–1.2 | 0, 0–0.91 | 0, 0–0.5 |
| Acute ICH characteristics (median, IQR) | |||
| Admission GCS score | 15, 13–15 | 15, 13–15 | 15, 13–15 |
| ICH score | 1, 0–2 | 1, 0–2 | 1, 0–2 |
| Discharge mRS | 4, 3–4 | 4, 3–4 | 4, 3–4 |
| Patient location one week post-ICH | |||
| Home | 266, 26.7 | 138, 24.0 | 42, 20.6 |
| Acute rehab | 269, 27.0 | 151, 26.2 | 52, 25.5 |
| Nursing home | 14, 1.4 | 16, 2.8 | 4, 2.0 |
| Still in hospital | 433, 43.5 | 271, 47.0 | 106, 52.0 |
| Structural determinants (median, IQR) | |||
| AQI: Median score | 38, 32–39 | 38, 32–39 | 38, 32–39 |
| SVI: overall percentile rank | 0.23, 0.23–0.46 | 0.34, 0.18–0.61 | 0.37, 0.21–0.65 |
.AQI, Air Quality Index; CSVD, cerebral small vessel disease; GCS, Glasgow Coma Scale; ICH, intracerebral haemorrhage; mRS, modified Rankin ScaleSVISocial Vulnerability Index
Given the relationships between SDOH and access to healthcare and research participation, SDOH were examined in all survivors, including those ruled ineligible, to inform results interpretation and examine a source of potential bias (online supplemental table 1). Those ineligible for participation were younger (median=67, IQR=55–77) and lived in areas with a lower SVI rank (median=23, IQR=023–0.46), indicating less social vulnerability. A larger proportion of those ruled ineligible self-reported as black (n=17, 8.1%) and Hispanic (n=24, 11.4%) compared with those ruled eligible. Further, ineligible patients were more likely to report a medical history of hypertension (n=171, 82.2%) and to have been discharged to an acute rehabilitation setting (n=66, 31.3%). Last, characteristics and SVI rank were examined among those who did not survive 90 days post-ICH. Overall, those who did not survive were older (median=77, IQR=69–84), reported a medical history of CAD (n=515, 26.4) and atrial fibrillation (n=492, 25.2%), to have experienced a lobar (n=884, 44.7%) and larger haemorrhage (median=23.0, IQR=6.6–58.8). Deceased patients lived in areas characterised similarly to those ruled ineligible (median SVI rank=0.23, IQR=0.23–0.46).
Unadjusted associations
Wilcoxon rank sum tests indicated that ICH volume (W=33 881, p=0.033) but not patient age (W=38 716, p=0.686) was significantly associated with depression incidence post-ICH. Further, a significant association was identified with the overall SVI rank (W=33 042, p=0.010) but not the socioeconomic component of the SVI (W=35 024, p=0.126). Kruskal-Wallis tests showed that median AQI scores (χ2(1)=7.17, p=0.007) and CAA burden scores (χ2(1)=18.71, p<0.001) were both associated with depression. While Pearson’s χ2 indicated a significant association between depression and lobar ICH (χ2(1)=8.08, p=0.004), no significant association was identified with patient sex (χ2(1)=1.02, p=0.321), educational attainment (χ2(2)=3.75, p=0.153) or race (Fisher’s exact test p=0.923). Additionally, no significant association was found for patient location 1-week post-ICH (χ2(3)=3.94, p=0.268).
The purpose of the following models is to identify biological and social drivers of health associated with depression incidence among ICH survivors. Commonly collected clinical information is directly incorporated into all models and used to find SVI overall rank scores to characterise the social vulnerability per patient area and median AQI (based on census tracts found using patient addresses). Specifically, patient age, sex, race, educational attainment, ICH location and volume and location 1-week post-ICH are included in all the following models. Additionally, CAA burden scores were included in the last model which were created from visual reads of patient CT/MRIs. Model outputs indicate the probability of incident depression post-ICH considering these biological and social drivers such that OR greater than 1.0 indicate an increased probability of depression. As the following models largely include commonly collected clinical data, they may be appropriate for incorporation into clinical use to identify patients at risk for depression post-ICH. The following models provide support for the consideration of social vulnerability and CAA burden in risk prediction.
SDOH and depression risk after ICH
Model 1 (table 2) shows the results of the logistic regression with depression post-ICH as an outcome and predictor variables informed by the literature (BIC=831.5, highest). The predictors in the literature supported regression model collectively provided a better fit to the data, in comparison to a null model, as indicated by an LRT (χ2(19)=37.35, p=0.005). The literature informed model indicated that experiencing a lobar ICH (OR 1.69, 95% CI 1.12 to 2.56, p=0.012) and remaining in the hospital 1-week post-ICH (OR 1.69, 95% CI 1.12 to 2.56, p=0.039), as opposed to a cerebellar or deep ICH and being discharged home, were associated with increased odds of depression.
Table 2. Multivariable analyses model 1: literature informed model.
| Determinate category | Coef. | SE | Z value | OR | 95% CI | P value |
| Patient | ||||||
| Age | −0.004 | 0.008 | −0.525 | 1.00 | 0.98 to 1.01 | 0.599 |
| Sex: male | −0.302 | 0.187 | −1.615 | 0.74 | 0.51 to 1.07 | 0.106 |
| Race | ||||||
| White | REF | REF | REF | REF | REF | REF |
| Black | −0.504 | 0.414 | −1.216 | 0.60 | 0.27 to 1.36 | 0.224 |
| Asian | −0.058 | 0.369 | −0.158 | 0.94 | 0.46 to 1.94 | 0.874 |
| Multiple/unknown | −0.292 | 0.527 | −0.554 | 0.75 | 0.27 to 2.10 | 0.579 |
| Education | ||||||
| Less than high school | REF | REF | REF | REF | REF | REF |
| High school | 0.687 | 0.418 | 1.643 | 1.15 | 0.54 to 2.44 | 0.100 |
| College or more | 0.137 | 0.384 | 0.358 | 1.99 | 0.88 to 4.51 | 0.720 |
| Clinical | ||||||
| ICH location: lobar | 0.528 | 0.211 | 2.499 | 1.69 | 1.12 to 2.56 | 0.012 |
| ICH volume | 0.006 | 0.005 | 1.207 | 1.01 | 1.00 to 1.02 | 0.227 |
| One week location | ||||||
| Home | REF | REF | REF | REF | REF | REF |
| Acute rehab | 0.220 | 0.273 | 0.807 | 1.25 | 0.73 to 2.13 | 0.420 |
| Nursing home | −0.249 | 0.634 | −0.393 | 0.78 | 0.23 to 2.70 | 0.695 |
| Still in hospital | 0.524 | 0.254 | 2.059 | 1.69 | 1.12 to 2.56 | 0.039 |
| Structural | ||||||
| SVI: socioeconomic rank | ||||||
| Poverty | 0.297 | 0.604 | 0.492 | 1.35 | 0.41 to 4.39 | 0.622 |
| Unemployed | 0.273 | 0.521 | 0.524 | 1.31 | 0.47 to 3.65 | 0.600 |
| PCI | −0.928 | 0.863 | −1.074 | 0.40 | 0.07 to 2.15 | 0.283 |
| No high school diploma | 0.805 | 0.662 | 1.217 | 2.24 | 0.61 to 8.19 | 0.224 |
| AQI: Median score | 0.055 | 0.024 | 2.348 | 1.06 | 1.01 to 1.11 | 0.019 |
AQIAir Quality IndexICHintracerebral haemorrhagePCIper capita incomeSVISocial Vulnerability Index
Model 2 (table 3) includes predictors informed by the SVI paradigm (BIC=813.5). The SVI-informed model provided a better fit to the data, compared with a null model, as indicated by an LRT, χ2(15)=30.66, p=0.006. This model indicated that associations between experiencing a lobar ICH (OR=1.76, 95% CI 1.17 to 2.67, p=0.007), hospital stay (OR 1.62, 95% CI 0.99 to 2.67, p=0.055) and depression incidence remained significant. Notably, greater social vulnerability was associated with increased odds of depression (OR 3.02, 95% CI 1.51 to 6.07, p=0.002).
Table 3. Multivariable analyses model 2: social vulnerability model.
| Determinate category | Coef. | SE | Z value | OR | 95% CI | P value |
| Patient | ||||||
| Age | −0.003 | 0.008 | −0.415 | 1.00 | 0.98 to 1.01 | 0.678 |
| Sex: male | −0.258 | 0.186 | −1.387 | 0.77 | 0.54 to 1.11 | 0.166 |
| Race | ||||||
| White | REF | REF | REF | REF | REF | REF |
| Black | −0.584 | 0.410 | −1.424 | 0.56 | 0.25 to 1.25 | 0.154 |
| Asian | −0.046 | 0.366 | −0.126 | 0.96 | 0.47 to 1.96 | 0.900 |
| Multiple/unknown | −0.303 | 0.528 | −0.574 | 0.74 | 0.26 to 2.08 | 0.566 |
| Education | ||||||
| Less than high school | REF | REF | REF | REF | REF | REF |
| High school | 0.697 | 0.414 | 1.685 | 1.11 | 0.53 to 2.34 | 0.092 |
| College or more | 0.105 | 0.381 | 0.274 | 2.01 | 0.89 to 4.52 | 0.784 |
| Clinical | ||||||
| ICH location: lobar | 0.568 | 0.211 | 2.693 | 1.76 | 1.17 to 2.67 | 0.007 |
| ICH volume | 0.005 | 0.005 | 1.047 | 1.01 | 1.00 to 1.01 | 0.295 |
| One week location | ||||||
| Home | REF | REF | REF | REF | REF | REF |
| Acute rehab | 0.271 | 0.272 | 0.999 | 1.31 | 0.77 to 2.23 | 0.318 |
| Nursing home | −0.249 | 0.632 | −0.394 | 0.78 | 0.23 to 2.69 | 0.694 |
| Still in hospital | 0.485 | 0.253 | 1.921 | 1.62 | 0.99 to 2.67 | 0.055 |
| Structural | ||||||
| SVI: overall rank | 1.106 | 0.356 | 3.111 | 3.02 | 1.51 to 6.07 | 0.002 |
ICHintracerebral haemorrhageSVISocial Vulnerability Index
Model 3 (table 4) included the addition of the CAA burden score (BIC=809.5, lowest). This comprehensive model was also a better fit to the data than the null model χ2(15)=34.61, p=0.002. Model 3 also indicated that the associations between a lobar ICH (OR 1.55, 95% CI 1.01 to 2.37, p=0.043), hospital stay (OR 1.80, 95% CI 1.08 to 3.00, p=0.025) and depression remained significant. Again, the relationship between overall SVI rank was significantly associated with increased odds of depression (OR 3.03, 95% CI 1.49 to 6.19, p=0.002). Notably, evidence of CAA burden as measured by the CAA score was also significantly associated with increased odds of depression (OR 1.45, 95% CI 1.25 to 1.68, p<0.001).
Table 4. Multivariable analyses model 3: comprehensive model.
| Determinate category | Coef. | SE | Z value | OR | 95% CI | P value |
| Patient | ||||||
| Age | −0.012 | 0.008 | −1.470 | 0.99 | 0.97 to 1.00 | 0.142 |
| Sex: male | −0.331 | 0.191 | −1.734 | 0.72 | 0.49 to 1.04 | 0.083 |
| Race | ||||||
| White | REF | REF | REF | REF | REF | REF |
| Black | −0.555 | 0.416 | −1.333 | 0.57 | 0.25 to 1.30 | 0.183 |
| Asian | −0.002 | 0.376 | −0.006 | 1.00 | 0.48 to 2.08 | 0.995 |
| Multiple/unknown | −0.294 | 0.541 | −0.543 | 0.75 | 0.26 to 2.15 | 0.587 |
| Education | ||||||
| Less than high school | REF | REF | REF | REF | REF | REF |
| High school | −0.065 | 0.391 | −0.165 | 0.94 | 0.44 to 2.02 | 0.869 |
| College or more | 0.542 | 0.423 | 1.282 | 1.72 | 0.75 to 3.94 | 0.200 |
| Clinical | ||||||
| ICH location: lobar | 0.439 | 0.217 | 2.025 | 1.55 | 1.01 to 2.37 | 0.043 |
| ICH volume | 0.007 | 0.005 | 1.374 | 1.01 | 1.00 to 1.02 | 0.169 |
| 1-week location | ||||||
| Home | REF | REF | REF | REF | REF | REF |
| Acute rehab | 0.374 | 0.280 | 1.337 | 1.45 | 0.84 to 2.52 | 0.181 |
| Nursing home | −0.130 | 0.637 | −0.204 | 0.88 | 0.25 to 3.06 | 0.838 |
| Still in hospital | 0.586 | 0.261 | 2.243 | 1.80 | 1.08 to 3.00 | 0.025 |
| Structural | ||||||
| SVI: overall rank | 1.110 | 0.364 | 3.052 | 3.03 | 1.49 to 6.19 | 0.002 |
| CT/MRI | ||||||
| CAA burden | 0.371 | 0.076 | 4.854 | 1.45 | 1.25 to 1.68 | <0.001 |
CAAcerebral amyloid angiopathy.ICHintracerebral haemorrhageSVISocial Vulnerability Index
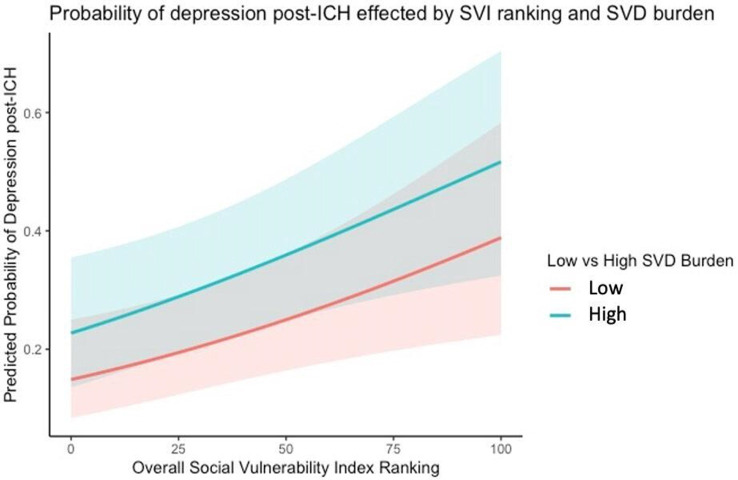
Figure 2 shows the predicted probability of depression incidence post-ICH based on model 3. All predictors were held at their median or the condition which indicated lower odds of depression, except the overall SVI rank, which was allowed to randomly vary within the range 0–1. The probability of experiencing depression is greatest among those with a high burden of CAA and those living in areas characterised by greater social vulnerability.
Figure 2. Predicted probability of depression incidence post-ICH. ICH, intracerebral haemorrhage; SVD, small vessel disease; SVI, Social Vulnerability Index.
Discussion
We investigated the relationship between social vulnerability and air quality in depression incidence after primary ICH to better understand their role in poststroke outcomes. Our findings do not support a significant relationship between air quality and depression in this sample, understanding the nature of this relationship likely requires further investigation. Overall SVI rank was associated with depression after ICH. We demonstrated that the identified associations are independent of the relationships between established and commonly collected basic clinical and radiographic measures. Notably, we did not find associations between individual-level determinants such as age, marital or employment status, or race, suggesting that SVI may encompass upstream SDOH more accurately than the limited SDOH measures collected on hospital admission.
We found that no individual category of social vulnerability was strongly associated with depression post-ICH. However, when considering all categories together using the SVI, a strong predictive relationship emerged. This underscores the strength and importance of the SVI as a research tool. Unlike single SDOH factors previously reported, the SVI encompasses multiple determinants, capturing a broader context of social vulnerability. This comprehensive approach represents multiple upstream factors influencing post-ICH outcomes and interactions with the healthcare system, particularly in accessing poststroke care.
The association between lobar ICH and hospital admission longer than 1 week was consistently associated with depression. This finding likely reflects the severity of disability that is associated with larger and specifically lobar haemorrhages. Further, returning home post-ICH is likely most protective when the home environment can be characterised by low social vulnerability. Our findings indicate that clinical determinants capture important information about the characteristics of the index ICH that can inform risk assessment for depression post-ICH.
Limitations exist within our analysis. As this study sought to examine the role of SDOH in depression among ICH survivors, this criterion represents a survival bias. Additionally, a selection bias exists in that the goal of understanding post-ICH recovery selected for patients who were more likely to have lived in areas with lower social vulnerability and to have had increased access to follow-up care. Therefore, the strength of association between SVI and depression may be underestimated in this study. Further, we relied on reporting a depression diagnosis, billing codes and the GDS-4 to capture depression in our sample, likely leading to a missed diagnosis in many patients. Future studies will improve this approach by capturing key psychological components of depression.
As a study strength, we combined an in-depth survey of SDOH data with typically available clinical information. This consideration of clinical and social determinants is both novel and pivotal in addressing inequities in ICH outcomes.
We found social vulnerability is an independent factor in depression incidence after ICH. Our findings warrant further investigation of inclusion of structural SDOH in clinical stroke care and the design and conduct of future research studies.
supplementary material
The funding entities had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; and decision to submit the article for publication.
Footnotes
Funding: The authors’ work on this study was supported by funding from the US National Institutes of Health (K23NS100816, R01NS093870, R01NS103924 and R01AG26484).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by the Massachusetts General Hospital Internal Review Board (2013P000494, 2006P000570). Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data used in this study will be made available to a qualified investigator on reasonable request.
Contributor Information
Dominique Lynn Popescu, Email: dpopescu@mgh.harvard.edu.
Jessica Abramson, Email: jrabramson@mgh.harvard.edu.
Sophia Keins, Email: skeins@mgh.harvard.edu.
Akashleena Mallick, Email: amallick1@mgh.harvard.edu.
Christina Kourkoulis, Email: ckourkoulis@mgb.org.
Christopher D Anderson, Email: cdanderson@mgb.org.
Jonathan Rosand, Email: jrosand@mgb.org.
Alessandro Biffi, Email: abiffi@mgh.havard.edu.
Nirupama Yechoor, Email: nyechoor@mgh.harvard.edu.
Data availability statement
Data are available on reasonable request.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation. 2019;139:10. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Poon MTC, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–7. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 3.Stern-Nezer S, Eyngorn I, Mlynash M, et al. Depression one year after hemorrhagic stroke is associated with late worsening of outcomes. NRE. 2017;41:179–87. doi: 10.3233/NRE-171470. [DOI] [PubMed] [Google Scholar]
- 4.Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2017;48:e30–43. doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 5.Cannistraro RJ, Badi M, Eidelman BH, et al. CNS small vessel disease: A clinical review. Neurol (ECronicon) 2019;92:1146–56.:24. doi: 10.1212/WNL.0000000000007654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol. 2011;7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rensma SP, van Sloten TT, Launer LJ, et al. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–73. doi: 10.1016/j.neubiorev.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castello JP, Pasi M, Kubiszewski P, et al. Cerebral Small Vessel Disease and Depression Among Intracerebral Hemorrhage Survivors. Stroke. 2022;53:523–31. doi: 10.1161/STROKEAHA.121.035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Brutto OH, Mera RM, Recalde BY, et al. On the Association Between Social Determinants of Health and Disability in Stroke-Free Older Adults Living in Rural Settings. The Three Villages Study. J Prim Care Community Health. 2020;11:2150132720961265. doi: 10.1177/2150132720961265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross-Denny B, Robinson MA. Using the Social Determinants of Health as a Framework to Examine and Address Predictors of Depression in Later Life. Ageing Int. 2017;42:393–412. doi: 10.1007/s12126-017-9278-6. [DOI] [Google Scholar]
- 11.Alegría M, NeMoyer A, Falgàs Bagué I, et al. Social Determinants of Mental Health: Where We Are and Where We Need to Go. Curr Psychiatry Rep. 2018;20:95. doi: 10.1007/s11920-018-0969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosendale N. Social Determinants of Health in Neurology. Neurol Clin. 2022;40:231–47. doi: 10.1016/j.ncl.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Ashcroft R. Health Inequities: Evaluation of Two Paradigms. Health Soc Work. 2010;35:249–56. doi: 10.1093/hsw/35.4.249. [DOI] [PubMed] [Google Scholar]
- 14.Borroni E, Pesatori AC, Bollati V, et al. Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ Pollut. 2022;292:118245. doi: 10.1016/j.envpol.2021.118245. [DOI] [PubMed] [Google Scholar]
- 15.Abramson JR, Castello JP, Keins S, et al. Biological and Social Determinants of Hypertension Severity Before vs After Intracerebral Hemorrhage. Neurol (ECronicon) 2022;98:e1349–60. doi: 10.1212/WNL.0000000000200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubiszewski P, Sugita L, Kourkoulis C, et al. Association of Selective Serotonin Reuptake Inhibitor Use After Intracerebral Hemorrhage With Hemorrhage Recurrence and Depression Severity. JAMA Neurol. 2020;78:1–8. doi: 10.1001/jamaneurol.2020.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keins S, Abramson JR, Castello JP, et al. Latent profile analysis of cognitive decline and depressive symptoms after intracerebral hemorrhage. BMC Neurol. 2021;21:481. doi: 10.1186/s12883-021-02508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds. Neurol (ECronicon) 2018;90:e119–26. doi: 10.1212/WNL.0000000000004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau KK, Li L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurol (ECronicon) 2017;88:2260–7. doi: 10.1212/WNL.0000000000004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Staals J, Makin SDJ, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurol (ECronicon) 2014;83:1228–34. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biffi A, Sonni A, Anderson CD, et al. Variants at APOE influence risk of deep and lobar intracerebral hemorrhage. Ann Neurol. 2010;68:934–43. doi: 10.1002/ana.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(SICI)1099-1166(199910)14:103.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.