ABSTRACT
Background
Traditional transradial access (TRA) is widely used for coronary and non‐coronary interventions with significant improvements in procedural outcomes; however, it is associated with RAO that precludes repeat use of the same artery for possible future TRI and other purposes. Distal radial access (DRA) has been proposed as an effective alternative to decrease RAO rates. Published literature describing the RAO rate after DRA versus TRA from various RCT and clinical registries has shown conflicting results.
Objectives
This study compared the forearm radial artery occlusion (RAO) rate assessed by Doppler ultrasound between distal and conventional radial access at 1‐year follow‐up after the initial procedure.
Methods
TENDERA was a multicenter, randomized controlled study comparing DRA versus TRA for coronary diagnostic and interventional procedures using 5 or 6F hydrophilic‐coated sheaths. The primary endpoint was forearm RAO at 12 months after radial access. The secondary endpoints included puncture time, sheath insertion and total procedure time, radiation dose, and vascular access site‐related complications.
Results
Eight hundred and fifty patients were randomized to either TRA (n = 418) and DRA (n = 432) groups. In the intention‐to‐treat analysis, the rate of forearm RAO at 12 months was observed in 39 patients (4.6%) and was significantly reduced in the DRA group compared with the TRA group (2.5% vs. 6.7%, RR 2.59 [95% CI 1.29–5.59], p = 0.010). Analysis in per protocol population has shown consistent results with forearm RAO rate 2.8% in the DRA group versus 6.5% in the TRA group (p = 0.008). The crossover rate was higher (4.6% vs. 1%, p = 0.013) and median hemostasis time was shorter (156.5 min vs. 180 min, p < 0.001) with DRA. Overall bleeding (BARC 1–2) and postprocedure hematoma > 5 cm occurred less frequently in the DRA group compared with the TRA group (3.2% vs. 20.5%, p < 0.001% and 9.0% vs. 27.0%, p < 0.001, respectively). No significant differences were observed in total procedure time and radiation dose between groups.
Conclusions
DRA for coronary diagnostic and interventional procedures is associated with reduced forearm RAO rate and shorter hemostasis time, but a longer sheath insertion time and higher crossover rate compared with TRA.
Trial Registration
1. Introduction
Over the three decades since its implementation into clinical practice, TRA has been established as the safest access‐site choice for coronary diagnostic and revascularization procedures regardless of clinical presentation. Previous data have shown many advantages of TRA over the transfemoral approach including the reduction of access‐site‐related complications, such as bleeding and other major vascular complications [1, 2]. Moreover, TRA is associated with improved quality of life and lower mortality rate, especially in high‐risk patients [3, 4, 5]. Based on these data, TRA is supported as a default approach for coronary procedures by both the ESC/EACTS and ACC/AHA/SCAI clinical practice guidelines on myocardial revascularization [6, 7].
Because of the above‐mentioned features of TRA, this arterial access is also getting widely accepted for non‐coronary diagnostic and interventional procedures. However, despite obvious advantages, several problems related to TRA remain unresolved. In addition to commonly observed spasm, radial artery catheterization often results in arterial diameter reduction, negative remodeling, or thrombotic occlusion up to 9% [8, 9, 10, 11]. Radial artery occlusion (RAO) after TRA procedures is the most frequent complication and may limit the clinical benefit of TRA. The potential ischemic effect of RAO is eliminated by an extensive local arterial network, and multiple anastomoses in the wrist area, although an occluded artery precludes repeat access through the same radial artery or possible surgical use. Real‐world data show that RAO rates remain high even in experienced radial centers [9, 11].
Distal radial access (DRA) in the anatomical snuffbox or in the dorsum of the hand has evolved during the past decade firstly as access for recanalization of the occluded radial artery after previous TRA [12, 13] and subsequently as an access site for transradial procedures [14].
Previous RCTs have shown conflicting results regarding the reduction of the RAO rate after DRA versus conventional TRA. The aim of this study was to evaluate the differences in RAO rates between DRA and TRA.
2. Methods
2.1. Study Design and Oversight
TENDERA (Traditional ENtry point versus Distal puncturE of Radial Artery) trial was a prospective, multicenter, open‐label randomized controlled trial designed to evaluate the clinical benefit of DRA compared with conventional TRA comparing the incidence of forearm RAO up to 1 year after the procedure. Since DRA is a relatively new procedure operators involved in this study were required to have successfully completed at least 100 DRA procedures, including > 30 coronary interventions.
The protocol was approved by the ethics committee at each participating clinical center, and all patients provided written informed consent.
2.2. Study Population and Randomization
Patients were enrolled at seven clinical centers from different regions of the Russian Federation (Supporting Information S2: Table 1). Consecutive all‐comer patients > 18 years of age with chronic or acute coronary syndrome, excluding STEMI, who had an indication for coronary angiography and/or PCI were prospectively enrolled in this study after signing written informed consent. Patients with a history of previous percutaneous interventions through the same radial artery, coagulopathy, life expectancy < 1 year, and with radial artery diameter < 1.5 mm were excluded. Enrolled patients underwent diagnostic coronary arteriography or PCI using 5–6F hydrophilic coated introducer sheaths (Terumo Interventional Systems, Japan, Merit Medical, USA or Lepu Medical, China) no longer than 16 cm, as the standard access device. Patients were followed up to 1 year after hospital discharge.
Eligible patients were randomly assigned to DRA versus TRA. Concealed allocation of study access method was performed using a web‐based interactive randomization system, and randomization was achieved with a computer‐generated random sequence.
2.3. Study Procedures
Before randomization the operators performed ultrasound examination of the radial artery in its forearm and the snuffbox, to evaluate eligibility (artery diameter at intended puncture site > 1.5 mm). The choice of left or right radial artery was left to the operator's discretion, and intravenous access for the medication was recommended in the contralateral arm. The wrist circumference was measured in all enrolled patients before the procedure.
2.3.1. Transradial Procedure
For conventional TRA, radial artery puncture was performed as described previously [15]. The patient's hand with supinated palm placed in the extended position a100a tourniquet or dedicated hand fixation device. It was recommended to puncture the artery 2 cm proximal to the styloid process of the radius bone.
For distal access the hand was positioned with the thumb upward, the palm extended and fixed using the same tools as in the case of conventional TRA. After finding the pulse in the snuffbox or the dorsum of the hand manually, the artery was punctured using a micropuncture needle with a 30–45° entry angle to the skin. After successful puncture and wire advancement, sheath placement and the rest part of the coronary intervention were similar to that for conventional TRA.
Following successful hydrophilic sheath (5 or 6F) placement, a bolus of 200 μg of nitroglycerin and 5000 IU of unfractionated heparin were administered. For PCI, an additional 2000 IU or 70–100 IU/kg dose of Heparin was given to maintain activated clotting time (ACT) at 250–300 s. A coronary diagnostic procedure or PCI was performed using 5 or 6F catheters. Exchange of 5F to a larger (6F) catheter or to a guiding catheter with different shapes during PCI was allowed according to the indication and guide catheter selection was as per the operator's decision.
The operators were encouraged to use ultrasound to guide arterial puncture and perform single wall puncture using an open needle. In two cases, glycoprotein IIb/IIIa receptor blockers were used according to the indications. Standard 0.035″ J‐tipped guide wire was used for delivering diagnostic or guiding catheters into the aorta.
2.3.2. Hemostasis Protocol
2.3.2.1. Traditional Radial Access Hemostasis
After completion of the coronary diagnostic or interventional procedure, radial hemostasis was achieved with a dedicated closure device, as per the operator's discretion. A patent hemostasis protocol was recommended according to the previously published study [16]. Radial artery patency during hemostasis was assessed using the reverse Barbeau test using a plethysmographic sensor device.
2.3.2.2. Distal Radial Access Hemostasis
A compression bandage was applied to the distal radial puncture site to obtain dry hemostasis. Post‐hemostasis management was carried out using a dedicated protocol (Supporting Information S2: Table 2).
2.4. Study Endpoints and Definitions
The primary endpoint of this study was forearm RAO detected at 12 months after the TRA or DRA procedure, assessed by an independent physician not involved in this study. Duplex ultrasound examination was used for RAO detection. Forearm RAO was considered if no flow on color Doppler or low‐velocity signal and/or monophasic blood flow was detected distal to the puncture point, suggesting the presence of collateral flow. Radial artery patency was also evaluated at 24 h, 1 week, 30 days, 6 and 12 months after the procedure.
The secondary endpoints included puncture time, sheath insertion time, total procedure time, air Kerma, major bleeding defined according to the BARC criteria [17], vascular access site complications recorded as a hematoma (≥ 5 cm) using EASY classification [18], compartment syndrome, arterio‐venous fistula and formation of pseudoaneurysm, access‐related pain rated by self‐reported visual analog scale (Supporting Information S2: Table 3).
Puncture time was defined as the time needed from the first contact of the needle with skin to obtain reliable bleed back in the hub of the needle. Sheath insertion time was defined as the time from the first contact of the needle with the skin to the uncomplicated sheath placement into the lumen of the target artery. This also defined as a successful attempt at vessel cannulation at the randomized site.
If the initial attempt to obtain vascular access failed because of the inability to insert guidewire due to deflection of the wire in a distal direction or inability to successfully advance the sheath into the DRA, vessel damage or severe pain at the puncture site, further attempts were continued at another access site, with the choice of the alternate access artery based on the operator's discretion. All these cases were considered as a crossover event. All endpoint descriptions and inclusion/exclusion criteria are shown in detail in Supporting Information S2: Table 4.
2.4.1. Hand Function Evaluation
Dynamometric tests were performed in 456 patients in the per‐protocol population (228 patients in either TRA and DRA groups) before, at discharge, 1 week, 1, 6 and 12 months in the follow‐up period using Hydraulic Hand Evaluation Kit (Jamar® Hydraulic Hand Evaluation Kit, China). Wrist and thumb forces were recorded in kilograms.
2.5. Sample Size and Statistical Analysis
The sample size was calculated assuming the primary endpoint of forearm RAO for conventional TRA as 5.0% [8, 19, 20, 21]. For the DRA group 1.0% of forearm RAO rate was assumed based on previous studies [22, 23, 24]. Overall, a sample size of 420 patients per group was deemed adequate to achieve a statistical power of 80% and two‐sided α error of 0.05, allowing for a drop‐out rate of 20%. Continuous variables are presented as mean ± SD or median and interquartile ranges (Q1–Q3). Categorical data are reported as absolute values and proportions. Differences between variables were examined using Fisher's exact test or the Wilcoxon rank sum test, as appropriate. A binary logistic regression model with all clinically relevant variables was used to estimate odds ratio (OR) with 95% confidence interval bounds. Statistical significance was assumed at a p < 0.05. All statistical tests were performed using software R version 4.2.3.
3. Results
In this study, we enrolled 850 patients. Four hundred and thirty‐two patients were randomized to DRA and 418 patients were randomized to TRA. Details of the study flowchart are shown in Figure 1. Fifty‐five patients were excluded, and the final population consisted 795 patients for final analysis (Figure 1).
Figure 1.
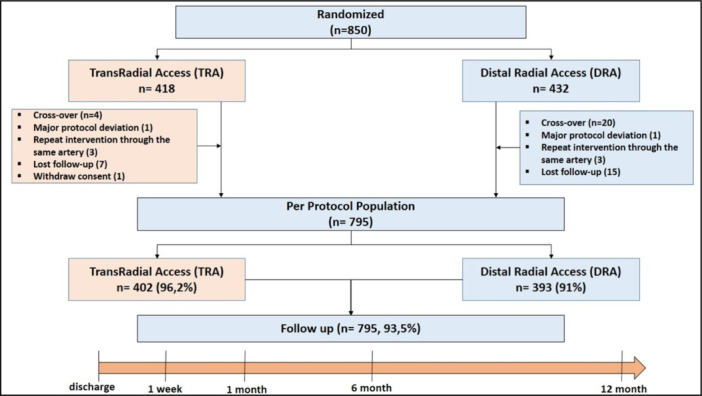
Study flowchart. [Color figure can be viewed at wileyonlinelibrary.com]
3.1. Primary Endpoint
According to intention‐to‐treat analysis primary endpoint occurred in 39 (4.6%) patients at 12 months after the TRI procedure. Forearm RAO was observed in 28 patients in the conventional TRA group compared with 11 patients in the DRA group (6.7% vs. 2.5%, RR 2.59 [95% CI 1.29–5.59], p = 0.010). Analysis in the per protocol group has shown consistent results with forearm RAO of 6.5% in the conventional TRA group versus 2.8% in the DRA group (p = 0.008) (Table 4).
Table 4.
Primary and secondary outcomes.
| ITT group | PP group | ||||||
|---|---|---|---|---|---|---|---|
| TRA (n = 418) | DRA (n = 432) | p value | TRA (n = 402) | DRA (n = 393) | p value | ||
| Forearm RAO | |||||||
| At discharge (24 h) | 15 (3.6%) | 5 (1.2%) | 0.039 | 14 (3.5%) | 4 (1.0%) | 0.029 | |
| 1 week | 17 (4.1%) | 8 (1.9%) | 0.071 | 17 (4.2%) | 7 (1.8%) | 0.060 | |
| 1 month | 27 (6.5%) | 11 (2.5%) | 0.012 | 26 (6.5%) | 11 (2.8%) | 0.012 | |
| 6 months | 28 (6.7%) | 11 (2.5%) | 0.008 | 26 (6.5%) | 11 (2.8%) | 0.008 | |
| 12 months | 28 (6.7%) | 11 (2.5%) | 0.008 | 26 (6.5%) | 11 (2.8%) | 0.008 | |
| Puncture time, s | 13.0 [5.0–30.0] | 18.0 [8.0–48.0] | < 0.001 | 12.0 [5.0–28.0] | 18.0 [8.0–48.0] | < 0.001 | |
| Sheath insertion time, s | 35.0 [23.0–56.0] | 44.0 [28.0–78.5] | < 0.001 | 35.0 [23.0–55.0] | 43.0 [28.0–80.0] | < 0.001 | |
| Total procedure time, min | 20.0 [8.0–35.0] | 20.0 [10.0–35.0] | 0.315 | 20.0 [9.0–35.0] | 20.0 [9.8–35.0] | 0.501 | |
| Radiation dose, mGy | 996.9 [554.1–1839.1] | 924.5 [493.1–1709.5.0] | 0.238 | 1001.9 [14.1–8.087.0] | 927.4 [21.2–0.591.0] | 0.244 | |
| Time to hemostasis, min | 180.0 [120.0–460.0] | 156.5 [125.0–195.0] | < 0.001 | 180.0 [120.0–420.0] | 156.5 [125.0–197.2] | 0.005 | |
| Postprocedure bleeding (BARC 1–2) (n/N), % | 20.5 (86/418) | 3.2 (14/432) | < 0.001 | 20.9 (84/402) | 3.6 (14/393) | < 0.001 | |
| Hematoma ≥ 5 cm at discharge (24 h) (n/N), % | 27.0 (113/418) | 9.0 (39/432) | < 0.001 | 27.3 (110/402) | 9.7 (38/393) | < 0.001 | |
| Pain scale score | 0.0 [0.0; 2.0] | 1.0 [0.0; 2.0] | 0.992 | 0.0 [0.0; 2.0] | 1.0 [0.0; 2.0] | 0.992 | |
| Vascular complications | |||||||
| Compartment syndrome | 0 | 0 | — | 0 | 0 | — | |
| Arterio‐venous fistula | 0 | 0 | — | 0 | 0 | — | |
| False aneurysm | 0.2% (1/418) | 0 (0/393) | 0.495 | 0.3% (1/402) | 0 (0/393) | 0.496 | |
| Infection | 0 | 0 | — | 0 | 0 | — | |
| Radial artery spasm | 23.9 (100/418) | 23.6 (102/432) | 0.872 | 100 (24.9%) | 110 (28%) | 0.870 | |
Notes: Values are in % (n/N), median [IQR].
Baseline characteristics of the two groups of patients are shown in Table 1.
Table 1.
Baseline clinical and demographic characteristics.
| TRA (n = 418) | DRA (n = 432) | p value | |
|---|---|---|---|
| Age, years | 63.0 ± 9.9 | 62.7 ± 10.0 | 0.707 |
| Male | 277 (66%) | 279 (65%) | 0.614 |
| Body mass index, kg/m2 | 29.3 ± 4.6 | 29.2 ± 4.8 | 0.714 |
| Wrist circumference, cm | 19.1 ± 2.0 | 19.1 ± 2.1 | 0.710 |
| Diabetes | 109 (26%) | 116 (27%) | 0.816 |
| Tobacco smoker | 129 (31%) | 130 (30%) | 0.823 |
| Hypertension | 365 (87%) | 378 (88%) | > 0.999 |
| Hyperlipidemia | 172 (42%) | 183 (43%) | 0.727 |
| ACS (NSTEMI) | 60 (14%) | 61 (14%) | 0.922 |
| Chronic Kidney Disease (CKD) | 82 (20%) | 95 (22%) | 0.398 |
| Antiplatelet medication | |||
| Aspirin | 308 (73.7%) | 344 (79.6%) | 0.043 |
| Clopidogrel | 165 (39.5%) | 193 (44.7%) | 0.127 |
| Ticagrelor | 66 (15.8%) | 86 (19.9%) | 0.128 |
| Oral anticoagulants | 36 (8.6%) | 29 (6.7%) | 0.305 |
| DAPT | 197 (47.1%) | 206 (47.7%) | 0.891 |
| SAPT | 122 (29.2%) | 132 (30.6%) | 0.708 |
| Triple therapy (DAPT+oral anticoagulant) | 12 (2.9%) | 13 (3%) | > 0.999 |
| Lipid‐lowering therapy | 328 (78.5%) | 367 (85%) | 0.016 |
Notes: Values are mean ± SD or %.
Abbreviations: ACS, acute coronary syndrome; DAPT, dual antiplatelet therapy; DRA, distal radial access; NSTEMI, non‐ST‐elevation myocardial infarction; SAPT, single antiplatelet therapy; TRA, conventional radial access.
As shown in Table 1, clinical and demographic characteristics were well balanced between the groups. The mean age of the patients was 62.9 ± 9.9, with 65.4% men, 26.5% with diabetes, and 41.8% with hyperlipidemia. The vast majority of patients presented with chronic coronary syndrome (85.8%) and 14.2% admitted with non‐STEMI. Patients received dual (47.4%) or single (30%) antiplatelet therapy, 7.6% and 2.9% were taking oral anticoagulants only or triple (DAPT+anticoagulant) therapy, respectively.
Procedural characteristics are shown in Table 2. As shown in Table 2, radial artery diameter was significantly larger at the forearm radial artery puncture site compared to the distal radial artery (2.60 ± 0.45 vs. 2.27 ± 0.38 mm, respectively, p < 0.001). Therefore, RA/sheath diameters ratio was significantly different between the groups (1.34 ± 0.25 vs. 1.18 ± 0.22, in TRA and DRA, respectively, p < 0.001).
Table 2.
Procedural characteristics.
| TRA (n = 418) | DRA (n = 432) | p value | |
|---|---|---|---|
| Side | |||
| Right | 249 (59.6%) | 285 (66%) | 0.056 |
| Ultrasound data | |||
| RA size, mm | 2.60 ± 0.45 | 2.27 ± 0.38 | < 0.001 |
| RA size males, mm | 2.69 ± 0.45 | 2.34 ± 0.37 | < 0.001 |
| RA size females, mm | 2.42 ± 0.39 | 2.13 ± 0.38 | < 0.001 |
| RA/sheath diameters ratio | 1.34 ± 0.25 | 1.18 ± 0.22 | < 0.001 |
| Vascular anomalies | |||
| RA ostium high take off | 24 (5.7%) | 15 (3.5%) | 0.14 |
| RA loop/marked tortuosity | 25 (6%) | 28 (6.5%) | 0.779 |
| Procedure details | |||
| No. of attempts | 1.78 ± 1.71 | 2.26 ± 2.39 | < 0.001 |
| Diagnostic angiography | 208 (49.8%) | 236 (54.6%) | 0.17 |
| PCI | 210 (50.2%) | 196 (45.4%) | 0.725 |
| Multivessel PCI | 131 (31.3%) | 126 (29.2%) | 0.502 |
| Left main PCI | 14 (3.3%) | 24 (5.6%) | 0.136 |
| CTO PCI | 41 (9.8%) | 52 (12%) | 0.324 |
| Procedure time, min | 20.0 [8.0; 35.0] | 20.0 [10.0; 35.0] | 0.315 |
| Puncture failed/crossover ratea | 4 (1.0%) | 20 (4.6%) | 0.001 |
| Time from sheath insertion to coronary ostia cannulation, s | 185.0 [135.0; 297.8] | 190.0 [135.0; 296.0] | 0.744 |
| Radial artery spasm | 100 (23.9%) | 102 (23.6%) | 0.872 |
| Crossover rate, % (n/N) | 1 (4/418) | 4,6 (20/432) | 0.013 |
| To the ipsilateral radial | 0 | 3.7 (16/432) | |
| To the contralateral distal | 1 (4/418) | 0.2 (1/432) | |
| To the ipsilateral ulnar | 0 | 0.5 (2/432) | |
| To femoral | 0 | 0.2 (1/432) | |
| Sheath used | |||
| 5F | 74 (17.7%) | 83 (19.2%) | 0.697 |
| 6F | 343 (82.1%) | 347 (80.3%) | |
| 7F | 1 (0.2%) | 2 (0.5%) | 0.924 |
| Any catheter change | 71 (17%) | 66 (15.3%) | 0.515 |
| Hemostasis | |||
| Dedicated compression device | 409 (97.8%) | 10 (2.3%) | < 0.001 |
| Bandage | 9 (2.2%) | 422 (97.7%) | < 0.001 |
| Total compression time, min | 180.0 [120.0; 460.0] | 156.5 [125.0; 195.0] | < 0.001 |
| TRA (n = 418) | DRA (n = 432) | p value | |
Notes: Values are in %, n/N, median (IQR) or mean ± SD.
Abbreviations: CTO, chronic total occlusion; PCI, percutaneous coronary intervention; RA, radial artery.
Intention‐to‐treat population.
Major vascular anomalies were observed in 92 out of 850 patients (10.8%) (detailed information about RA anomalies is shown in Supporting Information S1: Figure 3). Among these patients 39 had radial artery origin from brachial (36 [4.2%]) or axillary (3 [0.3%]) artery and in 53 out of 850 patients (6.2%) a radial artery loop or marked tortuosity of the artery course was observed. The incidence of radial artery spasm after successful puncture and sheath placement was similar in both groups (100 [23.9%] and 102 [23.6%] cases in TRA and DRA, respectively, p = 0.872). Overall, moderate/severe spasm observed in 73 cases (8.6%), of which severe spasm was detected in seven patients (0.8%). Severe spasm was the cause of the crossover in four out of seven cases. Puncture failure/crossover rate (intention‐to‐treat population) was significantly higher in the DRA group (4.6% vs. 1%, DRA vs. TRA, p = 0.013). The reasons for crossover were inability to advance the guidewire after successful puncture (16 [66.7%]), wire advancement to the distal direction instead of wiring forearm part of the radial artery (5 [20.8%]), and inability to successfully advance the introducer sheath (3 [12.5%]). All crossover cases in the TRA group were observed to the opposite distal access, while the majority of crossover cases in the DRA group occurred to the same side of conventional radial access (Table 2).
Using multivariate logistic regression analysis the independent predictors of forearm RAO were traditional radial access (OR = 2.59 [95% CI 1.29–5.59], p = 0.01), RA/sheath diameter ratio < 1.1:1 (OR = 0.21 [95% CI 0.04–0.92], p = 0.048) and female gender (OR = 3.94 [95% CI 1.82–8.86], p < 0.001) (Table 3).
Table 3.
Multivariable regression analysis using clinical and anatomical variables.
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Traditional access site | 2.59 | 1.29–5.59 | 0.010 |
| Age, years | 0.99 | 0.95–1.03 | 0.619 |
| BMI, kg/m2 | 0.95 | 0.87–1.02 | 0.174 |
| Female | 3.94 | 1.82–8.86 | < 0.001 |
| Arterial hypertension | 1.27 | 0.45–4.58 | 0.677 |
| Diabetes mellitus | 1.36 | 0.63–2.82 | 0.417 |
| CKD | 0.51 | 0.16–1.29 | 0.187 |
| Smoking | 2.18 | 0.97–4.88 | 0.056 |
| DAPT | 0.77 | 0.36–1.66 | 0.501 |
| Oral anticoagulation therapy | 0.61 | 0.09–2.30 | 0.526 |
| Triple therapy (DAPT+oral anticoagulants) | 0.80 | 0.04–4.62 | 0.841 |
| Number of cannulation attempt | 1.01 | 0.83–1.17 | 0.907 |
| RA/sheath diameter ratio < 1.1:1 | 0.21 | 0.04–0.92 | 0.048 |
Abbreviations: CKD, chronic kidney dysfunction; DAPT, dual antiplatelet therapy; RA, radial artery.
3.1.1. Secondary Endpoints
Puncture time was significantly longer in the ITT group with DRA compared with TRA regardless of access side (left or right arm) (18.0 s [IQR: 8.0–48.0] and 13.0 s [IQR: 5.0–30.0] respectively, p < 0.001). Accordingly, in the ITT population sheath insertion time was significantly prolonged in the DRA group compared with the TRA group (44.0 s [IQR: 28.0–78.5] vs. 35.0 s [IQR: 23.0–56.0], respectively, p < 0.001) (see Table 4).
In the ITT group there were no differences between TRA and DRA in total procedure time (20 min [IQR: 8.0–35.0] vs. 20 min [IQR: 10.0–35.0], p = 0.315) and radiation dose (996.9 mGy [IQR: 554.1–1839.1] vs. 924.5 mGy [IQR: 493.1–1709.5], p = 0.238). Median time to hemostasis was 180.0 min [IQR: 120.0–460.0] versus 156.5 min [IQR: 125.0–195.0] for TRA and DRA groups, respectively (p < 0.001). Post procedure bleeding BARC 1–2 and hematoma ≥ 5 cm at discharge were observed more frequently in TRA versus DRA—20.5% vs. 3.2%, p < 0.001% and 27.0% versus 9.0%, p < 0.001, respectively. One patient developed pseudoaneurysm in the TRA group (0.2%). Analysis in per protocol population show similar results (see Table 4).
Hand and finger strength dynamometry data are shown in Figure 2.
Figure 2.
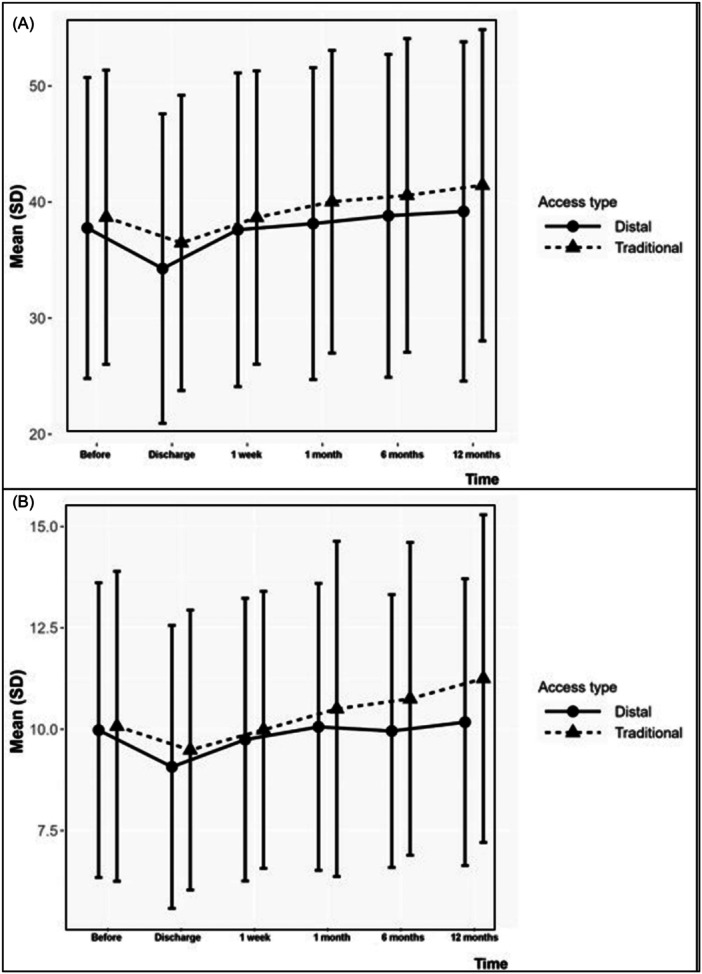
Wrist (A) and thumb (B) strength changes over time measured by hand dynamometry in both traditional and distal radial access groups.
In TRA group hand pinch strength changed from 40.0 kg [IQR: 30.0–49.0] at baseline to 42.0 kg [IQR: 30.0–51.0] at the 12 months (p = 0.264), whereas in the DRA group hand strength was 38.0 kg [IQR: 28.0–48.0] and 40.0 kg [IQR: 28.0–50.0] at the same points, respectively (p = 0.458). Also, in the TRA group thumb pinch strength test increased from 10.0 kg [IQR: 7.0–12.0] before the coronary intervention to 11.0 kg [IQR: 8.5.0–14.0] at 12 months in follow‐up (p = 0.216) and in the DRA group thumb strength changed from 9.5 kg [IQR: 7.0–12.5] to 10.0 kg [IQR: 30.0–49.0] at baseline and 12 months after the interventions, respectively (p = 0.784).
Interestingly, hand pinch strength test mildly worsened in both groups immediately after the procedure comparing to baseline value: −5% and −10.5% for TRA and DRA groups with no significant difference between them (p = 0.443 and p = 0.069, respectively). The same trend occurred for thumb strength test before and immediately after the procedure: −10% and −5.3% for TRA and DRA groups (p = 0.775 and p = 0.291, respectively). Nevertheless, hand/thumb pinch strength test improved over time for both TRA and DRA groups.
4. Discussion
The TENDERA was multicenter RCT initiated by physicians who pioneered distal radial access for percutaneous coronary and non‐coronary interventions. Since acute or late RAO is the “Achilles’ heel” of TRI, many radialists investigated different ways to solve this problem, including recommendations how to implement best clinical practice for prevention of RAO [25]. The rationale of DRA use is to lessen forearm RAO for possible repeat transradial interventions or arterio‐venous fistula formation for dialysis. The TENDERA multicenter investigator initiated RCT showed that the forearm RAO rate was significantly lower in the DRA group compared to the TRA group.
Our findings are comparable to the overall incidence of RAO reported in previously published studies, ranging from 2.5% to 8.4% after conventional TRA [8, 9, 10, 11, 26], and from 0% to 5% following DRA [19, 22, 23, 24, 27]. With adherence to best‐practice recommendations for prevention of RAO several studies have reported RAO < 3%, but these trials were performed in radial centers with highly experienced interventionalists in conventional TRA with specific hemostasis protocols and without direct comparison to DRA. Extremely low RAO rates were reported in the recently published DISCO‐RADIAL trial in both TRA and DRA populations with no significant difference in forearm RAO rates between groups [28]. This may have likely been caused by a smaller female gender population, a risk factor for RAO and the use of thin‐walled introducers by the investigators in DISCO‐RADIAL trial with likely less vessel wall trauma and favorable artery/sheath diameters ratio [29]. As suggested, RAO has two different mechanisms: thrombus formation in the early (~7 days) period after the initial TRI and inflammation and negative remodeling of the vessel lumen in follow‐up (up to 3 months). In our study, we observed both mechanisms of RAO—in 25 patients (2.9%) in early period (during first 7 days) and in 14 (1.7%) patients up to 12 months after TRI. Interestingly, RAO cases nearly doubled in follow‐up (19 cases) comparing to the number of RAO cases at discharge (20 cases). Early thrombotic occlusion and late inflammation/negative remodeling of RA are associated with patient and procedure‐related factors. In depth analysis we found out, that following factors were associated with late RAO: female gender, decreased kidney function, puncture time, repeat bleeding, and repeat hemostasis (see Supporting Information S2: Table 5).
RAO rate reported in “real world” practice widely varies (1%–33%) [11, 19], and several strategies have been proposed to mitigate this complication [16, 21, 25, 26]. Adopting DRA as a default strategy may complement several other strategies (patent hemostasis [16], ipsilateral ulnar artery compression [19, 30], shorter hemostasis time [21]) for reduction of RAO. The higher crossover rate (7%–28%) in previously published studies [22, 24] may be attributable partly to lack of operator experience. We recommend ultrasound guidance for distal radial puncture for beginners and less experienced operators for better understanding of radial anatomy, RA course in snuffbox area for correct puncture and catheterization. In experienced hands with sufficient learning curve ultrasound guidance is not needed anymore. As reported by our study investigators, ultrasound guidance was performed in few cases (in 11 out of 850 patients, 1.3%) and only for the distal access. Moreover, as shown in another randomized study, completion of the learning curve was associated with a reduction of crossover rate in the DRA group [28]. Other factors contributing to the higher crossover rate with DRA could be the smaller diameter of the radial artery in the snuffbox, tortuosity course in the snuffbox area, as well as deflection of the wire in the distal direction. We reported significantly lower crossover rate (4.6%) in the DRA group, presumably due to the implementation of “Distal Radial First” concept in our routine practice and hence the extensive experience of researchers participating in our study. The crossover rate mentioned in our study is comparable with earlier data reported from another experienced radial center [31]. Nevertheless, the number of puncture attempts and time required for successful sheath placement were significantly higher in the DRA group even in our cohort.
Our data showed no difference in total procedure time and radiation dose between the two groups. This is in contrast to our data in previous RCT where higher radiation dose was reported in the DRA group compared with the conventional TRA group [24]. This may be a result of the increasing experience of our study operators.
The time needed for hemostasis was significantly shorter and post procedure bleeding was less frequent in DRA groups compared with the TRA group. Also, DRA was associated with significantly less frequent large hematomas (EASY Grade I or more [18]) at discharge. One of the possible explanations for the easy and secure hemostasis after DRA could be the smaller diameter of the radial artery in its distal part coupled with its location over the bony floor, formed by carpal bones, which provides effective and reliable hemostatic compression. Reduction of hemostasis time in combination with persistent blood flow in the forearm part of the radial artery during compression hemostasis (similar to patent hemostasis principle, described earlier [16]) could be two main factors contributing to lesser forearm RAO after DRA.
In contrast to previous observational studies reporting a higher incidence of hand and finger neuropathy (0.9%–1.4%) after DRA [26, 30], our data showed no difference in self‐reported pain scores or findings of wrist and thumb dynamometry tests.
Based on our study findings, DRA offers an elegant strategy to significantly lower the risk of forearm RAO, decrease hemostasis time, and no significant intraprocedural or hand function penalty.
5. Study Limitations
Some limitations of this study need to be acknowledged. First, the COVID‐19 pandemic was responsible for slow enrollment, high dropout rate, and temporary interruption of our study. Indeed, disruption of regular workflow in catheterization laboratories of participating clinics may have had a negative impact on learning curve of operators and consequently could have led to increased technical failure and crossover rate in DRA patients. Second, despite the study protocol mandating the use of hydrophilic introducer sheaths, heterogeneity among patients occurred because of different brands of sheaths being used at different participating sites, which may have affected outcomes. The dropout rate was higher in the DRA group that may have also affected the outcomes.
6. Conclusions
In the TENDERA randomized multicenter trial DRA for coronary diagnostic and percutaneous interventions was associated with a statistically significant decrease in forearm RAO rate, shorter time needed for hemostasis, less frequent postprocedural bleeding, and significant hematoma formation. However, DRA was associated with a significantly higher crossover rate, number of puncture attempts, and longer sheath insertion time.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting information.
Supporting information.
Acknowledgments
The authors acknowledge the technologists and nurses of catheterization laboratories of all participating centers for their support. The authors are also deeply grateful to Anastasya Vasilyeva for her valuable assistance throughout the study. All authors have reported that they have no relationship relevant to the contents of this paper to disclose. There was no extramural funding associated with this trial.
References
- 1. Jolly S. S., Yusuf S., Cairns J., et al., “Radial Versus Femoral Access for Coronary Angiography and Intervention in Patients with Acute Coronary Syndromes (RIVAL): A Randomised, Parallel Group, Multicentre Trial,” Lancet 377, no. 9775 (April 2011): 1409–1420. [DOI] [PubMed] [Google Scholar]
- 2. Louvard Y., Benamer H., Garot P., et al., “Comparison of Transradial and Transfemoral Approaches for Coronary Angiography and Angioplasty in Octogenarians (The OCTOPLUS Study),” American Journal of Cardiology 94, no. 9 (November 2004): 1177–1180. [DOI] [PubMed] [Google Scholar]
- 3. Choussat R., “Vascular Complications and Clinical Outcome after Coronary Angioplasty with Platelet IIb/IIIa Receptor Blockade. Comparison of Transradial vs Transfemoral Arterial Access,” European Heart Journal 21, no. 8 (April 2000): 662–667. [DOI] [PubMed] [Google Scholar]
- 4. Valgimigli M., Frigoli E., Leonardi S., et al., “Radial Versus Femoral Access and Bivalirudin Versus Unfractionated Heparin in Invasively Managed Patients with Acute Coronary Syndrome (MATRIX): Final 1‐year Results of a Multicentre, Randomised Controlled Trial,” Lancet 392, no. 10150 (September 2018): 835–848. [DOI] [PubMed] [Google Scholar]
- 5. Gargiulo G., Giacoppo D., Jolly S. S., et al., “Effects on Mortality and Major Bleeding of Radial Versus Femoral Artery Access for Coronary Angiography or Percutaneous Coronary Intervention: Meta‐Analysis of Individual Patient Data From 7 Multicenter Randomized Clinical Trials,” Circulation 146, no. 18 (November 2022): 1329–1343. [DOI] [PubMed] [Google Scholar]
- 6. Neumann F. J., Sousa‐Uva M., Ahlsson A., et al., “2018 ESC/EACTS Guidelines on Myocardial Revascularization,” European Heart Journal 40, no. 2 (January 2019): 87–165. [DOI] [PubMed] [Google Scholar]
- 7. Lawton J. S., Tamis‐Holland J. E., Bangalore S., et al., “2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines,” Circulation. 145, no. 3 (January 2022): e4–e17. [DOI] [PubMed] [Google Scholar]
- 8. Avdikos G., Karatasakis A., Tsoumeleas A., Lazaris E., Ziakas A., and Koutouzis M., “Radial Artery Occlusion After Transradial Coronary Catheterization,” Cardiovascular Diagnosis and Therapy 7, no. 3 (June 2017): 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Uhlemann M., Möbius‐Winkler S., Mende M., et al., “The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications,” JACC: Cardiovascular Interventions 5 1 (2012): 36–43. [DOI] [PubMed] [Google Scholar]
- 10. Chen Y., Ke Z., Xiao J., et al., “Subcutaneous Injection of Nitroglycerin at the Radial Artery Puncture Site Reduces the Risk of Early Radial Artery Occlusion After Transradial Coronary Catheterization: A Randomized, Placebo‐Controlled Clinical Trial,” Circulation: Cardiovascular Interventions 11, no. 7 (July 2018): e006571. [DOI] [PubMed] [Google Scholar]
- 11. Kotowycz M. A. and Džavík V., “Radial Artery Patency after Transradial Catheterization,” Circulation: Cardiovascular Interventions 5, no. 1 (February 2012): 127–133. [DOI] [PubMed] [Google Scholar]
- 12. Babunashvili A. and Dundua D., “Recanalization and Reuse of Early Occluded Radial Artery Within 6 Days after Previous Transradial Diagnostic Procedure,” Catheterization and Cardiovascular Interventions 77, no. 4 (March 2011): 530–536. [DOI] [PubMed] [Google Scholar]
- 13. Corcos T., “Distal Radial Access for Coronary Angiography and Percutaneous Coronary Intervention: A State‐Of‐The‐Art Review,” Catheterization and Cardiovascular Interventions 93, no. 4 (March 2019): 639–644. [DOI] [PubMed] [Google Scholar]
- 14. Kaledin A.. "New Access To Facilitate EnDovascular Operations: First‐In‐Man Study." Abstracts of EuroPCR 2014. Eurointervention May 2014: 53.
- 15. Ludwig J., Achenbach S., and Flachskampf F., “Transradial Approach: A Modified Puncture Technique for Arterial Access,” EuroIntervention 6, no. 2 (June 2010): 280–282. [DOI] [PubMed] [Google Scholar]
- 16. Pancholy S., Coppola J., Patel T., and Roke‐Thomas M., “Prevention of Radial Artery Occlusion‐Patent Hemostasis Evaluation Trial (PROPHET Study): A Randomized Comparison of Traditional Versus Patency Documented Hemostasis after Transradial Catheterization,” Catheterization and Cardiovascular Interventions 72, no. 3 (September 2008): 335–340. [DOI] [PubMed] [Google Scholar]
- 17. Mehran R., Rao S. V., Bhatt D. L., et al., “Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report from the Bleeding Academic Research Consortium,” Circulation 123, no. 23 (June 2011): 2736–2747. [DOI] [PubMed] [Google Scholar]
- 18. Shroff A., Siddiqui S., Burg A., and Singla I., “Identification and Management of Complications of Transradial Procedures,” Current Cardiology Reports 15, no. 4 (April 2013): 350. [DOI] [PubMed] [Google Scholar]
- 19. Koutouzis M. J., Maniotis C. D., Avdikos G., Tsoumeleas A., Andreou C., and Kyriakides Z. S., “Ulnar Artery Transient Compression Facilitating Radial Artery Patent Hemostasis (ULTRA): A Novel Technique to Reduce Radial Artery Occlusion after Transradial Coronary Catheterization,” Journal of Invasive Cardiology 28, no. 11 (2016): 451–454. [PubMed] [Google Scholar]
- 20. Rashid M., Kwok C. S., Pancholy S., et al., “Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta‐Analysis,” Journal of the American Heart Association 5, no. 1 (January 2016): e002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashmi K. A., Iqbal Z., Hashmi A. A., et al., “The Frequency of Radial Artery Occlusion Following Cardiac Catheterization with the Use of Transradial Pneumatic Compression Band,” BMC Research Notes 13, no. 1 (October 2020): 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chugh Y., Kanaparthy N. S., Piplani S., et al., “Comparison of Distal Radial Access Versus Standard Transradial Access in Patients with Smaller Diameter Radial Arteries (The Distal Radial Versus Transradial Access in Small Transradial Arteriesstudy: D.A.T.A ‐ S.T.A.R Study),” Indian Heart Journal 73, no. 1 (January‐February 2021): 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eid‐Lidt G., Rivera Rodríguez A., Jimenez Castellanos J., Farjat Pasos J. I., Estrada López K. E., and Gaspar J., “Distal Radial Artery Approach to Prevent Radial Artery Occlusion Trial,” JACC: Cardiovascular Interventions 14, no. 4 (February 2021): 378–385. [DOI] [PubMed] [Google Scholar]
- 24. Tsigkas G., Papageorgiou A., Moulias A., et al., ““Distal or Traditional Transradial Access Site for Coronary Procedures: A Single‐Center, Randomized Study,” JACC: Cardiovascular Interventions. 15 1 (2022): 22–32. [DOI] [PubMed] [Google Scholar]
- 25. Bernat I., Aminian A., Pancholy S., et al., “Best Practices for the Prevention of Radial Artery Occlusion After Transradial Diagnostic Angiography and Intervention,” JACC: Cardiovascular Interventions 12, no. 22 (November 2019): 2235–2246. [DOI] [PubMed] [Google Scholar]
- 26. Hahalis G., Aznaouridis K., Tsigkas G., et al., “Radial Artery and Ulnar Artery Occlusions Following Coronary Procedures and the Impact of Anticoagulation: ARTEMIS (Radial and Ulnar ARTEry Occlusion Meta‐AnalysIS) Systematic Review and Meta‐Analysis,” Journal of the American Heart Association 6, no. 8 (August 2017): e005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizuguchi Y., Izumikawa T., Hashimoto S., et al., “Efficacy and Safety of the Distal Transradial Approach in Coronary Angiography and Percutaneous Coronary Intervention: A Japanese Multicenter Experience,” Cardiovascular Intervention and Therapeutics 35, no. 2 (April 2020): 162–167. [DOI] [PubMed] [Google Scholar]
- 28. Aminian A., Sgueglia G. A., Wiemer M., et al., “Distal Versus Conventional Radial Access for Coronary Angiography and Intervention: The DISCO RADIAL Trial,” JACC: Cardiovascular Interventions 15 12 (June 2022): 1191–1201. [DOI] [PubMed] [Google Scholar]
- 29. Aminian A., Saito S., Takahashi A., et al., “Comparison of a New Slender 6 Fr Sheath with a Standard 5 Fr Sheath for Transradial Coronary Angiography and Intervention: RAP and BEAT (Radial Artery Patency and Bleeding, Efficacy, Adverse evenT), a Randomised Multicentre Trial,” EuroIntervention 13 5 (August 2017): e549–e556. [DOI] [PubMed] [Google Scholar]
- 30. Condello F., Cacia M., Sturla M., et al., “Simultaneous Radial and Ipsilateral Ulnar Artery Compression Versus Isolated Radial Artery Compression after Conventional Radial Access for Coronary Angiography And/Ora Intervention: A Systematic Review and Meta‐Analysis,” Journal of Clinical Medicine 11, no. 23 (November 2022): 7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee J. W., Park S. W., Son J. W., Ahn S. G., and Lee S. H., “Real‐World Experience of the Left Distal Transradial Approach for Coronary Angiography and Percutaneous Coronary Intervention: A Prospective Observational Study (LEDRA),” EuroIntervention 14, no. 9 (October 2018): e995–e1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


