Abstract
The aim of the present study was to explore the role of ovarian cancer G protein-coupled receptor 1 (OGR1) in osteoclast differentiation and activity induced by extracellular acid. The impact of extracellular acidification on osteoclasts was investigated. Briefly, osteoclasts were generated from RAW 264.7 cells using 100 ng/ml receptor activator of nuclear factor-κB ligand in cell culture media at pH 6.8 or 7.4. Tartrate-resistant acid phosphatase (TRAP) staining and the bone resorption pit assay were used to detect the effects of extracellular acid on the number and absorptive capacity of osteoclasts. Intracellular Ca2+ levels were analyzed using laser scanning confocal microscopy. Reverse transcription-quantitative PCR was used to detect the expression levels of genes associated with osteoclast formation and bone erosion. The role of OGR1 in the acid-stimulated formation and bone resorption of osteoclasts was also investigated. The results showed that in the pH 6.8 medium group the number of osteoclasts was 511.2±54.72 and the area of bone absorption was 4,184.88±277.14 µm2; both were significantly higher than those in the pH 7.4 medium group (all P<0.01). Inhibition of OGR1 using copper ion (Cu2+) reduced the number of osteoclasts and the area of bone resorption in the pH 6.8 medium group (all P<0.05). Furthermore, extracellular acid (pH 6.8) was able to induce a transient increase of Ca2+ levels in osteoclasts; however, inhibition of OGR1 using Cu2+ effectively attenuated the acid-induced increase of Ca2+ in osteoclasts. In addition, the elevation in Ca2+ levels was inhibited when BAPTA, a cytoplasmic Ca2+ chelator with cellular permeability, was added to the cells; however, the extracellular Ca2+-chelating agent ethylene glycol tetraacetic acid did not inhibit the acid-stimulated increase in Ca2+. Treatment with the phospholipase C inhibitor U73122 also inhibited the acid-stimulated increase of Ca2+ in osteoclasts. Furthermore, the mRNA expression levels of TRAP, matrix metalloproteinase-9, osteoclast-related receptor, nuclear factor-activated T cell 1 (NFATc1), cathepsin K and integrin β3 were elevated in the pH 6.8 medium group compared with those in the pH 7.4 medium group (all P<0.05). By contrast, the inhibition of OGR1 using Cu2+ partially reduced the effects of the acidic environment on osteoclast differentiation and activity-related gene expression (all P<0.05). In addition, the mRNA and protein expression levels of calcineurin were increased in osteoclasts in the pH 6.8 group compared with those in the pH 7.4 group (P<0.05), whereas blocking OGR1 suppressed the expression of acid-induced calcineurin. The mRNA expression levels of NFATc1 in osteoclasts were also increased in the pH 6.8 medium group compared with those in the pH 7.4 medium group (P<0.05). By contrast, the specific calcineurin inhibitor cyclosporine A significantly inhibited the acid-induced expression of NFATc1 in osteoclasts. In conclusion, the present study revealed that extracellular acidification may increase osteoclast differentiation and bone resorption activity. Furthermore, OGR1-mediated Ca2+ elevation could have a crucial role in osteoclasts by regulating the Ca2+-calcineurin-NFATc1 signaling pathway and downstream signaling.
Keywords: acidosis, osteoclast, ovarian cancer G protein-coupled receptor 1, proton sensing receptor
Introduction
A suitable pH environment in the body is vital for cellular functions, because the protein conformation and activity of various cytokines are regulated by the hydrogen ion (H+) concentration in the local microenvironment; therefore, changes in the pH value of the local microenvironment can widely affect the function of proteases and cytokines in bone cells, and thus alter bone metabolism (1). Metabolic disorders can be associated with numerous diseases, such as chronic obstructive pulmonary disease or chronic kidney disease (2,3). Metabolic acidosis is the most frequent type of acid-base imbalance reported in the clinic. Clinical data have shown that lung and kidney disease may lead to abnormal changes in bone metabolism and a marked increase in bone absorption, resulting in decreased bone mineral density and a tendency toward osteoporosis (4). Osteoporosis is the most common type of bone disease, which is a systemic disease characterized by low bone mass, microstructural damage of bone tissue and easily fractured bones (5). Bone metabolism is regulated by the exact coactivity of osteoblasts and osteoclasts. Osteoclasts are an initiating factor in bone modeling and remodeling (6). The chronic metabolic acidosis observed in the clinic can lead to a decrease in bone mass, which may be caused by enhanced osteoclast activity (7).
Osteoclasts are terminally differentiated, multinucleated cells that mainly perform the function of bone resorption as components of the bone tissue. Receptor activator of nuclear factor (NF)-κB ligand (RANKL) is crucial for osteoclast formation, activity and survival (8). The osteoclast differentiation and formation pathway is activated by the binding of RANKL to RANK protein on the anterior cell membrane of osteoclasts; this results in the fusion of mononuclear cells into mature osteoclasts (9). Nuclear factor-activated T cell 1 (NFATc1) is a key factor in osteoclast differentiation; it is induced by the upstream RANKL signaling pathway, Ca2+-related co-stimulatory signaling pathways and Ca2+-independent signaling pathways (10). Activated calcineurin can bind to NFATc1 in the cytoplasm and dephosphorylate it, induced by elevation of Ca2+; the translocation of the activated NFATc1 into the nucleus then affects various downstream targets and finally leads to the fusion of monocytes into mature osteoclasts (11). Hence, the Ca2+-calcineurin-NFATc1 signaling pathway serves a critical role in osteoclast maturation.
At present, it is generally accepted that a number of elements can affect osteoclast biology, including mechanical stimulation, inflammation, estrogen deficiency and aging (12-15). Research has shown that acidosis stimulates osteoclast activity. Notably, osteoclasts have been reported to possess almost no activity in pH 7.4 medium; however, when the pH reaches a plateau of 6.8, the number of pits increases (7). Goldhaber and Rabadjija (16) and Meghji et al (17) showed that acidosis can activate mature osteoclasts in the skull to stimulate bone resorption and release calcium. By contrast, the addition of protons in the form of hydrochloric acid to neonatal mouse live calvaria maintained in a chemically defined medium in tissue culture for 1 week increased calcium release. The same amounts of protons added to the media of devitalized calvaria caused no increase in calcium release into the medium. When calcitonin, a well-recognized inhibitor of osteoclastic function, was added it was able to inhibit calcium release, suggesting that acid-induced calcium release may be related to osteoclasts. In addition, extracellular acidosis has been shown to increase osteoclast absorption activity by prolonging the lifespan of osteoclasts, and enhancing attachment to bone and migration (18).
A variety of factors may cause changes in local pH value in the body affecting osteoclasts and leading to a low bone mass, such as local ischemia and hypoxia, elevated lactic acid levels, resulting in bone lesion (7). Therefore, studies on the effect of an acidic microenvironment on osteoclasts may provide novel theories regarding the mechanism of bone metabolic disease in osteoporosis. Although numerous studies have indicated that various H+-sensing ion channels are expressed by osteoclasts (19,20), the mechanism of extracellular pH sensing is poorly understood. Ovarian cancer G protein-coupled receptor 1 (OGR1) belongs to the proton sensing G protein-coupled receptor family and exists in a variety of cells in the body, including bone cells. OGR1 can sense changes in H+ around a cell, and when the local pH value changes OGR1 can stimulate downstream signaling pathways, resulting in a series of modifications (21). OGR1 is involved in the physiological and pathological changes of multiple systems in the body, including cancer, asthma, inflammation and islet function (22-24). Previously, Ding et al (25) reported that the OGR1 receptor is expressed on endothelial progenitor cells (EPCs), and the proliferation, migration and angiogenesis of EPCs was shown to be delayed in acidic medium. By contrast, when OGR1 was suppressed, the opposite results were obtained. Yuan et al (26) revealed that OGR1 is expressed on chondrocytes in the intervertebral disc of rats, and the expression of OGR1 was increased when stimulated by acid. In addition, an acidic environment could promote the apoptosis of intervertebral disc chondrocytes through the OGR1-mediated downstream Ca2+ signaling pathway. Similarly, OGR1 is expressed in osteoblasts, osteoclasts and osteoclast precursor cells (21). It has been reported that OGR1 is inhibited by Cu2+ (27). Although it has been confirmed that extracellular acidification promotes the proliferation of osteoclasts, the specific mechanism underlying the effect of extracellular acidification on osteoclasts remains unclear.
The aim of the present study was to investigate how extracellular acidification stimulates OGR1, which affects osteoclast formation and bone erosion, to understand the mechanism of bone remodeling and osteoporosis.
Materials and methods
Medium preparation
The pH value of the cell culture medium was set using HCl and NaHCO3 solutions. RAW 264.7 cells (murine spleen) were obtained from the Peking Union Medical College (Beijing, China). RAW 264.7 cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) of different pH values (pH 6.8 or 7.4) supplemented with 10% heat-inactivated fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin at 37˚C, in a 95% humidity and 5% CO2 atmosphere. The culture medium was replaced every 24 h.
Tartrate-resistant acid phosphatase (TRAP) staining
Cu2+ containing medium was prepared by adding a solution of CuCl2 (Sigma-Aldrich; Merck KGaA). The RAW 264.7 cells were cultured in different pH cell media supplemented with 100 ng/ml RANKL (PeproTech, Inc.) at a density of 20,000 cells/cm2 for 3 days. (The OGR1 inhibitor group contained 4 µM Cu2+). Subsequently, the cells were fixed with 4% paraformaldehyde for 15 min at 22˚C. The TRAP staining kit (Sigma-Aldrich; Merck KGaA; cat. no. GMS11016) was used according to the manufacturer's protocol. The criteria for osteoclasts included: i) ≥3 nuclei; and ii) positive TRAP staining. The number of osteoclasts was calculated in 10 random non-overlapping fields under a CX-21 microscope (Olympus Corporation; magnification, x100) and the average was calculated. This part of the experiment was repeated 6 times (n=6 per group).
Resorption pit assay
Bovine femur bones were purchased from the Tianjin Institute of Orthopedics (Tianjin, China). The cortex of the bovine femoral shaft was cut longitudinally into 100-µm slices using tungsten carbide blades (SP1600; Leica Microsystems GmbH) and was then washed three times in distilled water (10 min/wash). After 24 h of UV irradiation, the sections were soaked in 75% ethanol for 24 h at 22˚C, washed three times with sterile distilled water and stored in DMEM at 4˚C. Subsequently, RAW 264.7 cells were cultured on bovine cortical bone slices at a density of 20,000 cells/cm2. The cells were induced by 100 ng/ml RANKL in different pH cell media for 3 days. The osteoclasts were then removed using sonication with 30 KHz for 10 min at 22˚C, fixed with 2.5% glutaraldehyde for 30 min at 4˚C, dehydrated in an ethanol gradient (30-50-70-85-95-100% ethanol), each concentration of ethanol for 20 min, dried with vacuum extraction, sprayed with gold for sputter coating and detected by scanning electron microscopy (LEO; Zeiss AG). For each bone slice, 10 regions were randomly selected with no overlap, and Image Pro Plus (version 6.0; Media Cybernetics, Inc.) was used to calculate the bone pit area. This part of the experiment was repeated 6 times (n=6 per group).
Measurement of Ca2+ levels
Ca2+ changes were detected as previously described (26). The RAW 264.7 cells were cultured in pH 7.4 medium supplemented with 100 ng/ml RANKL (PeproTech, Inc.) at a density of 20,000 cells/cm2 for 3 days. Prior to adding acid, cells were treated with 4 µM OGR1-inhibitor Cu2+ (Sigma-Aldrich; Merck KGaA) for 3 days at 37˚C, the extracellular Ca2+ chelator 0.5 mM EGTA (MilliporeSigma) for 30 min at 37˚C, the cell-permeable cytosolic Ca2+ chelator 50 µM BAPTA (MilliporeSigma) for 30 min at 37˚C and the phospholipase C inhibitor 10 µM U73122 (MilliporeSigma) for 30 min at 37˚C. Cells on cover glass were washed three times with PBS and incubated with 4 mM Fluo-3-AM (Biotium, Inc.) and 0.02% Pluronic F-127 (Biotium, Inc.) for 45 min at 37˚C in the dark. The cells were detected using laser scanning confocal microscopy (Leica Microsystems, Inc.). The fluorescence of intracellular Fluo-3-based measurement of Ca2+ levels was detected at excitation and emission wavelengths of 488 and 525 nm, respectively. Gray scale images were acquired at different points before and <10 min after fluorescence microscopy according to previous research (26), and then further analysis was carried out. After recording the baseline fluorescence intensity for ~100 sec, a pipette was immediately used to add an extracellular solution of pH 6.8 (0.1 ml) to osteoclasts. Subsequently, the fluorescence intensity was recorded for 500 sec without interruption using a laser scanning confocal microscopy (Leica Microsystems, Inc.). Fluorescence intensity was observed using LSM 5 Image software (version 5; Zeiss AG). This part of the experiment was repeated 6 times (n=6 per group).
Reverse transcription-quantitative PCR (RT-qPCR)
The RAW 264.7 cells were cultured with 100 ng/ml RANKL in different pH cell media for 3 days and the cells were then collected. TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract total RNA from the cells according to the manufacturer's instructions. cDNA was synthesized from total RNA using the All-in-One™ First-Strand cDNA Synthesis Kit according to manufacturer's protocol (GeneCopoeia, Inc.) and oligo (dT) primers. For the analysis of osteoclast-related genes the following primers were used: Calcineurin forward, 5'-AGTGTTCTCAGTTCTCAG-3' and reverse, 5'-TTCATCAGCCTCAATAGC-3'; integrin β3 forward, 5'-TGACTCGGACTGGACTGGCTA-3' and reverse, 5'-CACTCAGGCTCTTCCACCACA-3'; cathepsin K forward, 5'-CCTCTCTTGGTGTCCATACA-3' and reverse, 5'-ATCTCTCTGTACCCTCTGCA-3'; matrix metalloproteinase (MMP)-9 forward, 5'-GCTGACTACGATAAGGACGGCA-3' and reverse, 5'-GCGGCCCTCAAAGATGAACGG-3'; NFATc1 forward, 5'-TCCAAAGTCATTTTCGTGGA-3' and reverse, 5'-CTTTGCTTCCATCTCCCAGA-3'; TRAP forward, 5'-AAATCACTCTTTAAGACCAG-3' and reverse, 5'-TTATTGAATAGCAGTGACAG-3'; osteoclast-related receptor (OSCAR) forward, 5'-TGATTGGCACAGCAGGAG-3' and reverse, 5'-AAGGCACAGGAAGGAAATAGAG-3'; and β-actin forward, 5'-GGGAAATCGTGCGTGACATT-3' and reverse, 5'-GGAACCGCTCATTGCCAAT-3'. The reaction conditions were set according to the Reverse transcription kit instructions (GeneCopoeia, Inc.). qPCR was performed according to the following steps: Pre-denaturation at 95˚C for 1 min, followed by 40 cycles of denaturation at 95˚C for 10 sec, annealing at 60˚C for 20 sec and extension at 72˚C for 15 sec. β-actin was used as the internal reference gene, and the relative expression levels of key genes were calculated using the 2-ΔΔCq method (28). This part of the experiment was repeated 5 times (n=5 per group).
Western blotting
The protein levels of calcineurin in RAW 264.7 cells cultured in different pH cell culture media containing 100 ng/ml RANKL for 3 days were measured by western blotting. Cells were placed in lysis buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris, 20 mM glucose, pH 7.4), containing a protease inhibitor mixture. Protein concentration was measured using the BCA-200 Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). The lysates (20 µg) were resolved via denaturing SDS-PAGE on 12% gels and were transferred to Immobilon polyvinylidene difluoride membranes, 5% BSA Blocking Buffer for 30 min at room temperature. The following primary antibodies were used for immunoblot: calcineurin (cat. no: ab8553; Abcam; 1:100), and β-actin (cat. no: ab8227; Abcam; 1:500). The membranes were incubated with primary antibodies against calcineurin for 12 h at 37˚C after which they were washed in Tris-buffered saline with 0.05% Tween 20 and were incubated with HRP-conjugated secondary antibody (1:500; cat. no. Sc-3916; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. Protein semi-quantification was carried out using ECL kit (Wuhan Boster Biological Technology, Ltd.) and protein band intensities were analyzed using Image J software (version 1.52; National Institutes of Health). This part of the experiment was repeated 5 times (n=5 per group).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using SPSS (version 16.0; SPSS, Inc.). Prior to presenting the data, the normality of the distributions were tested. Statistical analysis was performed with one-way analysis of variance (ANOVA) followed by Tukey's post hoc test and the unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of acid on the number of TRAP-positive osteoclasts
The number of osteoclasts formed in different pH cell culture media is shown in Fig. 1. The number of osteoclasts in the pH 6.8 and 7.4 medium groups was 511.2±54.72 and 163±56.76, respectively, and there was a statistically significant difference between the pH 6.8 and 7.4 medium groups (P<0.01). However, the number of osteoclasts in the pH 6.8 and 7.4 medium groups was reduced to 311.8±72.84 and 110±59.44, respectively, when the OGR1 inhibitor Cu2+ was added to the medium. Notably, there was a significant difference between the pH 6.8 medium group and the pH 6.8 medium + OGR1 inhibitor group (P<0.05).
Figure 1.
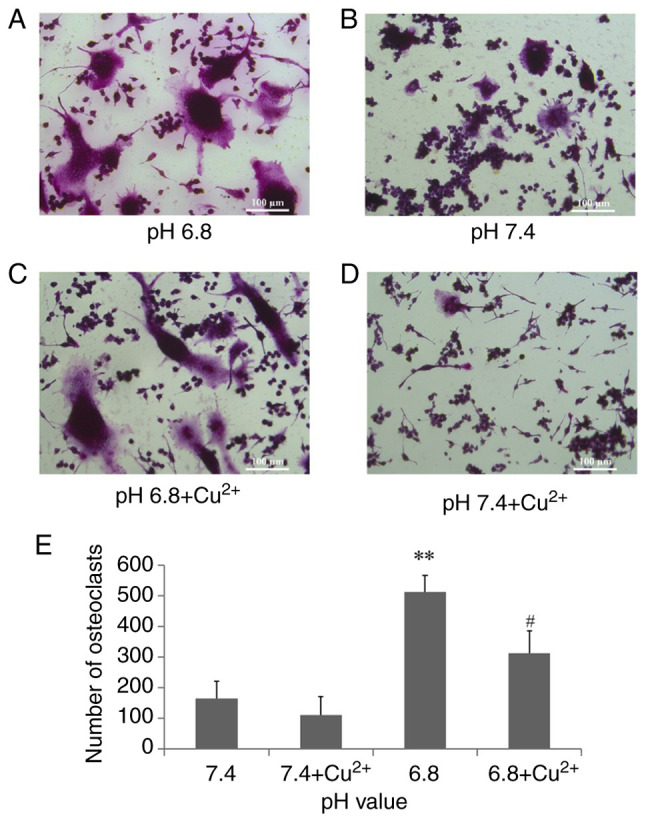
Number of osteoclasts cultured in different pH cell media for 3 days (sample size, n=6). (A) More TRAP-positive multinucleated osteoclasts were formed in an acidic environment, at pH 6.8. (B) In the neutral medium, there were fewer TRAP-positive multinucleated osteoclasts. (C) Inhibition of OGR1 with Cu2+ (CuCl2, 100 µM) reversed the acid-induced increase in osteoclasts. (D) Inhibition of OGR1 with Cu2+ in neutral medium. (E) Histogram of the number of osteoclasts. **P<0.01 vs. pH 7.4; #P<0.05 vs. pH 6.8. Cu2+, copper ion; OGR1, ovarian cancer G protein-coupled receptor 1; TRAP, tartrate-resistant acid phosphatase.
Effects of acid on bone resorption
The bone resorption area in the pH 6.8 and 7.4 medium groups was 4,184.88±277.14 and 1.006±214.49 µm2, respectively, and there was a statistically significant difference between the pH 6.8 and 7.4 medium groups (P<0.01; Fig. 2). Furthermore, the bone resorption area in the pH 6.8 and 7.4 medium groups was 1.493.02±544.93 and 356±121.26 µm2, respectively, when the OGR1 inhibitor was added to the medium. Notably, there was a significant difference between the pH 6.8 medium group and the pH 6.8 medium + OGR1 inhibitor group (P<0.05).
Figure 2.
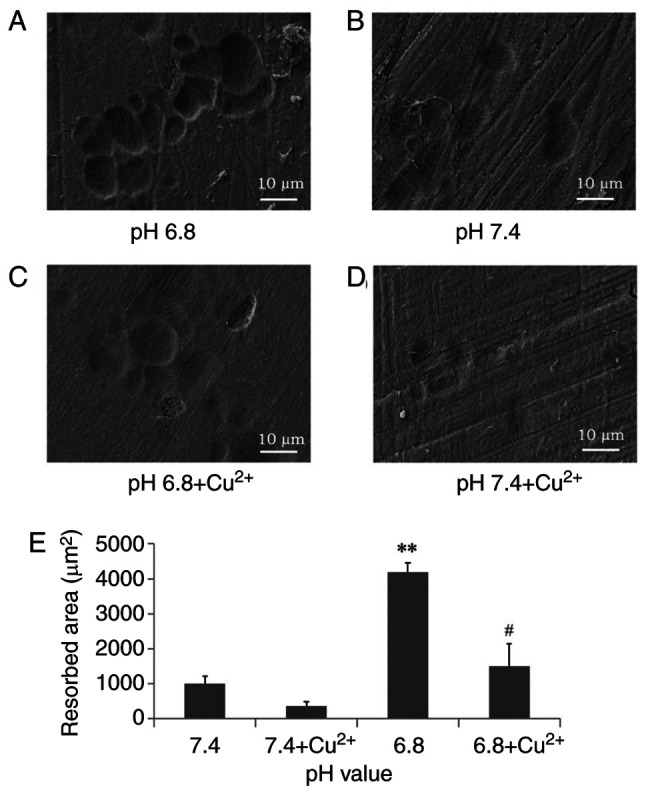
Resorption pit assay (n=6). (A) Osteoclasts form more bone pits in acidic medium at pH 6.8. (B) In the neutral medium, there were fewer resorption pits. (C) Treatment with Cu2+ inhibited acid-stimulated osteoclast resorption. (D) Inhibition of OGR1 with Cu2+ in neutral medium. (E) Analysis of bone resorption area. **P<0.01 vs. pH 7.4; #P<0.05 vs. pH 6.8. Cu2+, copper ion; OGR1, ovarian cancer G protein-coupled receptor 1.
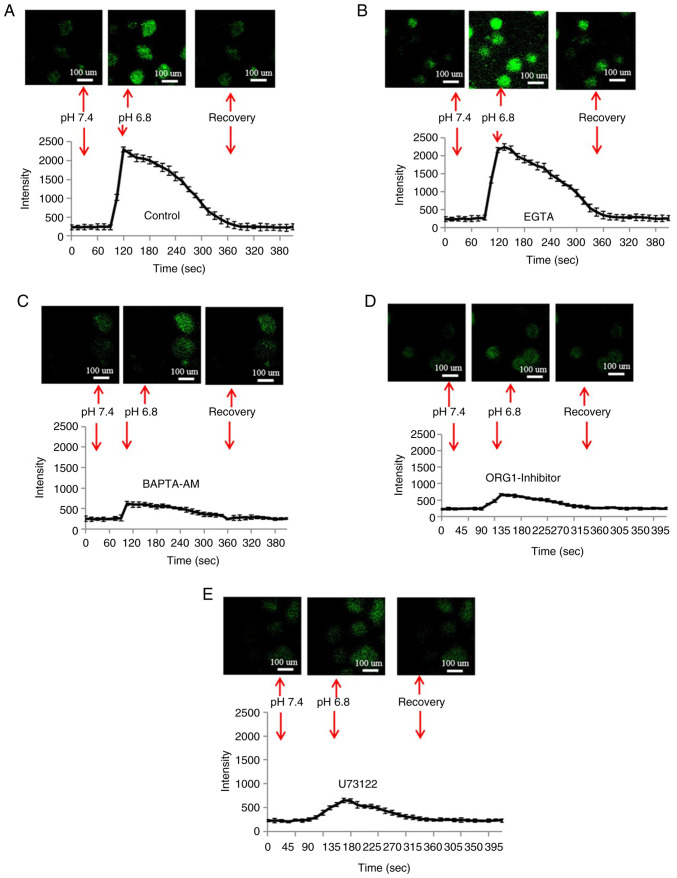
Acid-induced Ca2+ release
It is well known that upregulation of Ca2+ levels serves a key role in regulating osteoclasts (29). In the present study, extracellular acid (pH 6.8) was used to stimulate osteoclasts. The results of confocal microscopy are shown in Fig. 3A; the introduction of acid induced a transient increase in the fluorescence intensity of Ca2+ in the osteoclasts, then the fluorescence intensity of Ca2+ was gradually decreased.
Figure 3.
OGR1 is involved in the acid-induced increase of Ca2+ levels in osteoclasts (n=6). (A) Representative fluorescence change showing the elevation of Ca2+ levels in acidic medium at pH 6.8. (B) Chelation of extracellular calcium with EGTA (0.5 mM) did not inhibit acid-induced Ca2+ increase. (C) Restrain acid-induced elevation of Ca2+ levels with BAPTA-AM (50 mM). (D) Acid-induced elevation of Ca2+ levels in osteoclasts was reduced by the OGR1 inhibitor. (E) Inhibition of phospholipase C with U73122 (10 mM) suppressed the acid-induced rise of Ca2+.
The acid-induced temporary increase in osteoclast Ca2+ levels was not detected when the cell-permeable cytosolic Ca2+ chelator BAPTA was added to the cells (Fig. 3C). However, the extracellular Ca2+-chelating agent ethylene glycol tetraacetic acid did not inhibit acid-induced Ca2+ increase (Fig. 3B). Furthermore, the transient elevation in Ca2+ levels was attenuated in response to the OGR1-inhibitor Cu2+ (Fig. 3D) and treatment with the phospholipase C (PLC) inhibitor U73122 (Fig. 3E). These results suggested that the acid-stimulated increase in Ca2+ levels may be attributed Ca2+ release from intracellular stores via a PLC-dependent pathway.
Effect of acid on gene expression
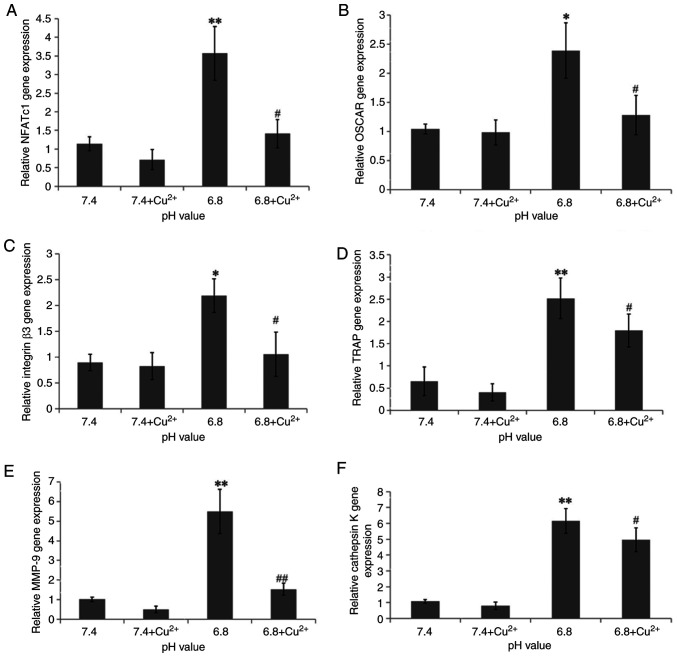
To investigate the influence of acidification on osteoclast differentiation and absorption, the mRNA expression levels of NFATc1, OSCAR, integrin β3, TRAP, MMP-9 and cathepsin K were assessed by RT-qPCR (Fig. 4). Following acidification, the expression levels of TRAP, MMP-9, OSCAR, NFATc1, cathepsin K and integrin β3 were increased compared with those in the pH 7.4 medium group; the relative expression levels were 2.52, 5.498, 2.39, 3.572, 6.158 and 2.19, respectively. By contrast, in response to treatment with the OGR1 inhibitor copper ion (Cu2+), the expression levels of TRAP, MMP-9, OSCAR, NFATc1, cathepsin K and integrin β3 were significantly decreased; the relative expression levels were 1.91, 1.526, 1.28, 1.412, 4.966 and 1.056, respectively. The present study showed that an upregulation of OGR1 may result in an increase in the expression levels of TRAP, MMP-9, OSCAR, NFATc1, cathepsin K and integrin β3, whereas the results were reversed when OCR1 was inhibited. These findings indicated that osteoclast differentiation and bone resorption may be affected by OGR1 in extracellular acidification.
Figure 4.
Relative mRNA expression levels of genes in osteoclasts cultured in different pH cell culture media (n=5). mRNA expression levels of (A) NFATc1, (B) OSCAR, (C) integrin β3, (D) TRAP, (E) MMP-9 and (F) cathepsin K in osteoclasts after 3 days of treatment with different pH cell culture media. *P<0.05, **P<0.01 vs. pH 7.4; #P<0.05, ##P<0.01 vs. pH 6.8. Cu2+, copper ion; MMP-9, matrix metalloproteinase-9; NFATc1, nuclear factor-activated T cell 1; OSCAR, osteoclast-related receptor; TRAP, tartrate-resistant acid phosphatase.
Effects of extracellular acid on the calcineurin-NFATc1 signaling pathway
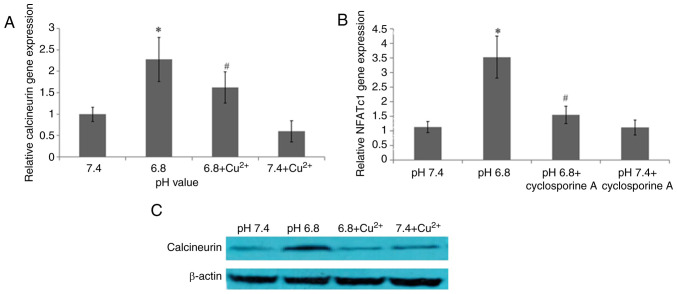
The effects of OGR1 on the calcineurin-NFATc1 signaling pathway were investigated. The mRNA expression levels of calcineurin and NFATc1 were detected by RT-qPCR (Fig. 5A and B). The expression levels of calcineurin and NFATc1 in the pH 6.8 medium group were 2.276 and 3.528, respectively, which was significantly increased compared with that in the pH 7.4 medium group. The expression of calcineurin in the pH 6.8 medium group was decreased to 1.624 upon treatment with the OGR1-inhibitor Cu2+. Upon treatment with 1 mM cyclosporine A (Novartis International AG) for 30 min at 37˚C, a calcineurin inhibitor, the expression levels of NFATc1 were decreased. To further verify the aforementioned observations, the expression level of calcineurin was detected by western blotting (Fig. 5C). Compared with in the pH 7.4 medium group, the expression levels of calcineurin were increased in the pH 6.8 medium group but were decreased after addition of the OGR1 inhibitor. These findings suggested that OGR1 may mediate osteoclast differentiation and absorption induced by extracellular acidification through the calcineurin-NFATc1 signaling pathway.
Figure 5.
Effects of extracellular acidification on the calcineurin-NFATc1 signaling pathway of osteoclast differentiation (n=5). (A) mRNA expression levels of calcineurin following treatment with an OGR1 inhibitor. (B) Effects of an OGR1 inhibitor on the protein expression levels of calcineurin in osteoclasts. (C) mRNA expression levels of NFATc1 in osteoclasts treated with the inhibitor of calcineurin cyclosporine A. *P<0.05 vs. pH 7.4; #P<0.05 vs. pH 6.8. NFATc1, nuclear factor-activated T cell 1; OGR1, ovarian cancer G protein-coupled receptor 1.
Discussion
In the present study, the results showed that acid promoted RAW 264.7 monocyte cell fusion into mature osteoclasts and enhanced bone resorption activity. The proton-sensing receptor OGR1 was revealed to serve an important role in acid-stimulated osteoclast differentiation and absorption through Ca2+ release from intracellular stores and downstream Ca2+-sensitive signaling pathways. Ca2+-sensitive signaling acts on the activation of the calcineurin-NFAT axis, which is indispensable for osteoclast differentiation and bone resorption. It is well known that the bone absorption efficiency of osteoclasts depends on their number and absorptive activity (30). Osteoclasts are sensitive to acid stimulation and acid can enhance osteoclast bone erosion. Notably, it has been reported that a decrease of <0.1 units in pH value can result in a doubling of bone resorption activity (31). Apart from human osteoclasts, bird osteoclasts have also been shown to be activated in response to acid stimulation (32,33). A previous study also demonstrated that when osteoclasts were cultured for 4 h, the number of osteoclasts at pH 6.74 medium was greater than that at pH 7.4(33). There are two main categories of membrane pH sensors: i) Acid-sensitive ion channels and ii) proton-sensitive G protein-coupled receptors (25,34). Several studies have reported that the expression of OGR1 is upregulated during osteoclastogenesis, and that it is closely related to the osteoclast response to acid (19,35). The present findings indicated that more TRAP-positive and bone pit-resorptive mature osteoclasts were detected in an extracellular acidified environment; however the number of osteoclasts and resorption capacity decreased when an OGR1 blocker was used (Cu2+).
Ca2+ is a versatile second messenger that participates in the regulation of a large number of physiological activities from the beginning to the end of life (36). The essential roles of Ca2+ influx regulate the differentiation, proliferation, activation and apoptosis of bone cells (37). Nevertheless, the way OGR1 modulates the proliferation and activation of osteoclasts through Ca2+ signaling remains unclear. Osteoclasts originate from the blood mononuclear macrophage system and are terminal differentiated cells, which can be fused from mononuclear progenitor cells to form giant multinucleated cells in various ways, such as the NF-κB pathway and Wnt pathway. Notably, osteoclast differentiation is a complex process that requires multiple signaling factors. NF-κB is activated by RANKL in the cytoplasm, activated NF-κB moves into the nucleus (nuclear translocation) and acts together with NFATc2 to induce initial NFAT1 gene expression in the process of osteoclast differentiation (38). N-terminal domains bind to calcineurin phosphorylation.
NFATc1 serves an important role in regulating osteoclast maturation; its N-terminal domain binds to calcineurin causing dephosphorylation to mediate the nuclear translocation of NFAT, and the C-terminal domain specifically binds to the DNA sequence and acts together with activator protein 1 (38,39). The GenBank accession numbers for these sequences are U02079 (murine NFAT1a), U43341 (NFAT1b) and U43342 (NFAT1c). The sustained transcription of NFATc1 is mainly maintained by Ca2+ and calcineurin pathways. When free Ca2+ in the cell increases, dephosphorylation of NFATc1 by calcineurin leads to Ca2+ translocating to the nucleus to serve a transcriptional role; notably, it activates osteoclast differentiation gene transcription (40). NFATc1 deficiency in mice has been reported to be embryonically fatal (41). Notably, mice with NFATc1 knockout fail to develop mature osteoclasts and undergo osteolysis (42). However, the ectopic expression of NFATc1 effectively enables osteoclast precursor cells to differentiate into osteoclasts in the absence of RANKL stimulation (43). The current study indicated that OGR1 may induce Ca2+ release from intracellular stores in an acidic environment. OGR1 inhibitors, such as Cu2+, and the Ca2+ chelator BAPTA-AM, may markedly inhibit the acid-induced elevation of Ca2+ in osteoclasts by preventing NFATc1 nuclear translocation. It was also shown that the increase in Ca2+ levels was suppressed in osteoclasts treated with the selective PLC blocker U73122. These findings indicated that the phosphatidylinositol signaling pathway may participate in Ca2+ elevation. It may be hypothesized that the extracellular hydrogen ion (H+) binds to cell OGR1 to induce PLC, after which, induced phosphatidylinositol 4,5-bisphosphate may produce diacylglycerol and inositol-1,4,5-triphosphate (IP3). IP3 can combine with the endoplasmic reticulum ligand gated channel to activate the Ca2+ channel, thereby increasing Ca2+. These results suggested that the Ca2+-calcineurin-NFATc1 pathway may have an important role in acid-induced osteoclast formation and activation.
Increasing evidence has revealed that the expression of multiple genes related to osteoclast differentiation and activity are closely controlled by NFATc1; these include TRAP, OSCAR, cathepsin K and integrin β3 (37,40,44). Osteoclast bone erosion is a complex process. The role of integrin promotes osteoclast migration and attachment to the bone and integrins αv and β3 are cell adhesion receptors mediate the interaction between the extracellular matrix (the organic and inorganic components of bone) and cells during bone resorption (45). In bone remodeling, type II carbonic anhydrase is one of the characteristic enzymes of mature osteoclasts, which can inversely catalyze the production of H+ and provide a source of acid for osteoclast demineralization (46). The high concentration of H+ in the environment cannot break down minerals, but creates an acidic microenvironment for hydrolases, such as MMP-9, TRAP and cathepsin K, and is thus involved in bone resorption (47,48). MMP-9 and cathepsin K not only degrade organic components of bone, but also help osteoclast cells move (48). The current study showed that acid could promote the gene expression of MMP-9, TRAP and cathepsin K, suggesting that extracellular acidification may enhance the bone resorption capacity of osteoclasts. OSCAR serves an important role in osteoclast formation and bone metabolism, and NFATc1 regulates the expression of OSCAR in osteoclasts. In addition, OSCAR can further activate NFATc1, forming a positive feedback loop and promoting osteoclast differentiation (49). The present study showed that extracellular acidification increased OSCAR mRNA expression, suggesting that the acidic medium promoted RANKL to induce RAW 264.7 cells to differentiate into mature osteoclasts.
There were certain limitations in the present study. Firstly, the main purpose of the study was to explore and confirm the relationship between extracellular acidification and the Ca2+-calcineurin-NFATc1 signaling pathway. However, new targets of this signaling pathway were not further investigated. Our research team aims to identify new targets in this signaling pathway to correct bone metabolism disorders. For example, future studies could focus on ORG1 inhibitors in specific parts of the bone, such as the femoral head for topical treatment of femoral head necrosis. The Ca2+-calcineurin-NFATc1 signaling pathway also can be used as a new therapeutic target to reduce osteoclast activity to treat osteoporosis. Moderate increases in skeletal extracellular fluid pH may also be a future research direction. Furthermore, the lack of in vivo experiments is another limitation of the present study. We aim to identify the mechanism underlying the relationship between bone and the local acidic microenvironment through animal modeling. Finally, an OGR1 inhibitor was used in the present study, which verified the experimental results and excluded potentially confounding factors, even though it is not considered a specific inhibitor for osteoclasts. Specific inhibitors need to be further developed. However, the present study identified some notable findings; the relationship between osteoclasts and an acidic microenvironment was revealed, and the Ca2+-calcineurin-NFATc1 signaling pathway was explored.
In conclusion, the present study revealed that osteoclast formation and absorption were markedly enhanced in response to extracellular acid. By contrast, blocking OGR1 suppressed the differentiation and activation of osteoclasts in an acidic environment. Notably, OGR1 may increase Ca2+ levels via activation of the Ca2+-calcineurin-NFATc1 signaling pathway, and could thus promote osteoclast differentiation and activity. Numerous studies have been performed on the effects of intervention factors on osteoclasts. For examples, naringin promotes the apoptosis of osteoclasts by regulating the activity of the mitochondrial apoptosis pathway (50). However, the pH value of osteoclast culture medium was not considered in the experimental method; however, most researchers have not paid attention to the pH value of the osteoclast culture medium during experimentation, which can lead to deviations in the results because osteoclasts are sensitive to acidic environments. Notably, the Ca2+-calcineurin-NFATc1 signaling pathway can be used as a new therapeutic target for bone metabolic disease. For example, osteoporosis can be treated by blocking the Ca2+-calcineurin-NFATc1 signaling pathway to inhibit the bone resorption activity of osteoclasts. In addition, methods can be developed to improve the local pH of the femoral head, thereby reducing the activity of osteoclasts, to treat femoral head necrosis. Notably, the relationship between bone and the local acidic microenvironment requires additional investigation in the future.
Acknowledgements
Not applicable.
Funding Statement
Funding: This project was supported by the National Natural Science Foundation of China (grant no. 82000763) and the Tianjin Municipal Health Commission (grant no. KJ20009).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
All authors contributed to study conception and design. Material preparation, data collection and analysis were performed by FL and XM. SB, XS and JM interpreted the data. The first draft of the manuscript was written by FL, and all authors commented on previous versions of the manuscript. FL and XM confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hanon C, Savarino J, Thomas C. Blood lactate and acid-base balance of world-class amateur boxers after three 3-min rounds in international competition. J Strength Cond Res. 2015;29:942–946. doi: 10.1519/JSC.0000000000000736. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Gao H, Zhao L, Wang J. Osteoporosis in COPD patients: Risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16:487–496. doi: 10.1111/crj.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.William Whitehouse R, Ahmad G, Kirwadi A, Matthew Howard J. Imaging of chronic kidney disease-mineral and bone disorder. Radiol Clin North Am. 2022;60:547–559. doi: 10.1016/j.rcl.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Yormaz B, Cebeci H, Yılmaz F, Süerdem M. Bone mineral density in emphysema and chronic bronchitis phenotypes in hospitalized male chronic obstructive pulmonary disease patients. Clin Respir J. 2020;14:47–53. doi: 10.1111/crj.13099. [DOI] [PubMed] [Google Scholar]
- 5.Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104:873–884. doi: 10.1016/j.mcna.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. 2020;9(2073) doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503:103–109. doi: 10.1016/j.abb.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Amin N, Boccardi V, Taghizadeh M, Jafarnejad S. Probiotics and bone disorders: The role of RANKL/RANK/OPG pathway. Aging Clin Exp Res. 2020;32:363–371. doi: 10.1007/s40520-019-01223-5. [DOI] [PubMed] [Google Scholar]
- 9.Chun KH, Jin HC, Kang KS, Chang TS, Hwang GS. Poncirin inhibits osteoclast differentiation and bone loss through down-regulation of NFATc1 in vitro and in vivo. Biomol Ther (Seoul) 2020;28:337–343. doi: 10.4062/biomolther.2018.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohatgi N, Zou W, Collins PL, Brestoff JR, Chen TH, Abu-Amer Y, Teitelbaum SL. ASXL1 impairs osteoclast formation by epigenetic regulation of NFATc1. Blood Adv. 2018;2:2467–2477. doi: 10.1182/bloodadvances.2018018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B, Dai X, Wang W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+ -calcineurin-NFATc1 pathway. J Cell Physiol. 2019;234:6831–6841. doi: 10.1002/jcp.27432. [DOI] [PubMed] [Google Scholar]
- 12.Kadow-Romacker A, Hoffmann JE, Duda G, Wildemann B, Schmidmaier G. Effect of mechanical stimulation on osteoblast- and osteoclast-like cells in vitro. Cells Tissues Organs. 2009;190:61–68. doi: 10.1159/000178022. [DOI] [PubMed] [Google Scholar]
- 13.Tong X, Ganta RR, Liu Z. AMP-activated protein kinase (AMPK) regulates autophagy, inflammation and immunity and contributes to osteoclast differentiation and functionabs. Biol Cell. 2020;112:251–264. doi: 10.1111/boc.202000008. [DOI] [PubMed] [Google Scholar]
- 14.Gan Z, Huang J, Xu M, Yuan X, Shang X, Chen X, Chen K. Micheliolide prevents estrogen deficiency-induced bone loss via inhibiting osteoclast bone resorption. Aging (Albany NY) 2023;15:10732–10745. doi: 10.18632/aging.205111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Sun Y. Aging of the bone. Adv Exp Med Biol. 2018;1086:189–197. doi: 10.1007/978-981-13-1117-8_12. [DOI] [PubMed] [Google Scholar]
- 16.Goldhaber P, Rabadjija L. H+ stimulation of cell-mediated bone resorption in tissue culture. Am J Physiol. 1987;253:E90–E98. doi: 10.1152/ajpendo.1987.253.1.E90. [DOI] [PubMed] [Google Scholar]
- 17.Meghji S, Morrison MS, Henderson B, Arnett TR. pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. Am J Physiol Endocrinol Metab. 2001;280:E112–E119. doi: 10.1152/ajpendo.2001.280.1.E112. [DOI] [PubMed] [Google Scholar]
- 18.Ahn H, Kim JM, Lee K, Kim H, Jeong D. Extracellular acidosis accelerates bone resorption by enhancing osteoclast survival, adhesion, and migration. Biochem Biophys Res Commun. 2012;418:144–148. doi: 10.1016/j.bbrc.2011.12.149. [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Yoon HJ, Kang WY, Cho S, Seong SJ, Lee HW, Yoon YR, Kim HJ. G protein-coupled receptor 84 controls osteoclastogenesis through inhibition of NF-κB and MAPK signaling pathways. J Cell Physiol. 2018;233:1481–1489. doi: 10.1002/jcp.26035. [DOI] [PubMed] [Google Scholar]
- 20.Kanaya K, Iba K, Abe Y, Dohke T, Okazaki S, Matsumura T, Yamashita T. Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a high bone turnover state in ovariectomized mice. J Orthop Res. 2016;34:566–573. doi: 10.1002/jor.23047. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 22.Wiley SZ, Sriram K, Salmerón C, Insel PA. GPR68: An emerging drug target in cancer. Int J Mol Sci. 2019;20(559) doi: 10.3390/ijms20030559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutter S, van Haaften WT, Hünerwadel A, Baebler K, Herfarth N, Raselli T, Mamie C, Misselwitz B, Rogler G, Weder B, et al. Intestinal activation of pH-sensing receptor OGR1 [GPR68] contributes to fibrogenesis. J Crohns Colitis. 2018;12:1348–1358. doi: 10.1093/ecco-jcc/jjy118. [DOI] [PubMed] [Google Scholar]
- 24.Mogi C, Nakakura T, Okajima F. Role of extracellular proton-sensing OGR1 in regulation of insulin secretion and pancreatic β-cell functions. Endocr J. 2014;61:101–110. doi: 10.1507/endocrj.ej13-0380. [DOI] [PubMed] [Google Scholar]
- 25.Ding S, Xu J, Zhang Q, Chen F, Zhang J, Gui K, Xiong M, Li B, Ruan Z, Zhao M. OGR1 mediates the inhibitory effects of acidic environment on proliferation and angiogenesis of endothelial progenitor cells. Cell Biol Int. 2019;43:1307–1316. doi: 10.1002/cbin.11179. [DOI] [PubMed] [Google Scholar]
- 26.Yuan FL, Wang HR, Zhao MD, Yuan W, Cao L, Duan PG, Jiang YQ, Li XL, Dong J. Ovarian cancer G protein-coupled receptor 1 is involved in acid-induced apoptosis of endplate chondrocytes in intervertebral discs. J Bone Miner Res. 2014;29:67–77. doi: 10.1002/jbmr.2030. [DOI] [PubMed] [Google Scholar]
- 27.Pereverzev A, Komarova SV, Korcok J, Armstrong S, Tremblay GB, Dixon SJ, Sims SM. Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone. 2008;42:150–161. doi: 10.1016/j.bone.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 30.Soysa NS, Alles N. Osteoclast function and bone-resorbing activity: An overview. Biochem Biophys Res Commun. 2016;476:115–120. doi: 10.1016/j.bbrc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Arnett TR, Spowage M. Modulation of the resorptive activity of rat osteoclasts by small changes in extracellular pH near the physiological range. Bone. 1996;18:277–279. doi: 10.1016/8756-3282(95)00486-6. [DOI] [PubMed] [Google Scholar]
- 32.Quélo I, Jurdic P. Differential regulation of the carbonic anhydrase II gene expression by hormonal nuclear receptors in monocytic cells: Identification of the retinoic acid response element. Biochem Biophys Res Commun. 2000;271:481–491. doi: 10.1006/bbrc.2000.2654. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto N, Daido S, Sun-Wada GH, Wada Y, Futai M, Nakanishi-Matsui M. Diversity of proton pumps in osteoclasts: V-ATPase with a3 and d2 isoforms is a major form in osteoclasts. Biochim Biophys Acta. 2014;1837:744–749. doi: 10.1016/j.bbabio.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Ren G, Zhou R, Ge J, Chen FH. The role of Ca2+ in acid-sensing ion channel 1a-mediated chondrocyte pyroptosis in rat adjuvant arthritis. Lab Invest. 2019;99:499–513. doi: 10.1038/s41374-018-0135-3. [DOI] [PubMed] [Google Scholar]
- 35.Abe-Ohya R, Ishikawa T, Shiozawa H, Suda K, Nara F. Identification of metals from osteoblastic ST-2 cell supernatants as novel OGR1 agonists. J Recept Signal Transduct Res. 2015;35:485–492. doi: 10.3109/10799893.2015.1015736. [DOI] [PubMed] [Google Scholar]
- 36.Wei X, Li H, Zhang Y, Li C, Li K, Ai K, Yang J : Ca2+-calcineurin axis-controlled NFAT nuclear translocation is crucial for optimal T cell immunity in an early vertebrate. J Immunol. 2020;204:569–585. doi: 10.4049/jimmunol.1901065. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Xu H, Han Z, Chen P, Yu Q, Lei Y, Li Z, Zhao M, Tian J. Pulsed electromagnetic field inhibits RANKL-dependent osteoclastic differentiation in RAW264.7 cells through the Ca2+-calcineurin-NFATc1 signaling pathway. Biochem Biophys Res Commun. 2017;482:289–295. doi: 10.1016/j.bbrc.2016.11.056. [DOI] [PubMed] [Google Scholar]
- 38.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: Regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue JI, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 40.Liang S, Zhang H, Du Y, Dou C, Liu S, Zhang L, Chen Y, Li R, Ma J, Li Z, et al. RANK deficiency ameliorates podocyte injury by suppressing calcium/calcineurin/NFATc1 signaling. Kidney Blood Press Res. 2018;43:1149–1159. doi: 10.1159/000492049. [DOI] [PubMed] [Google Scholar]
- 41.Kusumaningrum N, Lee DH, Yoon HS, Park CH, Chung JH. Ultraviolet light-induced gasdermin C expression is mediated via TRPV1/calcium/calcineurin/NFATc1 signaling. Int J Mol Med. 2018;42:2859–2866. doi: 10.3892/ijmm.2018.3839. [DOI] [PubMed] [Google Scholar]
- 42.Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, Takayanagi H. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med. 2005;202:1261–1269. doi: 10.1084/jem.20051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishikawa K, Iwamoto Y, Ishii M. Development of an in vitro culture method for stepwise differentiation of mouse embryonic stem cells and induced pluripotent stem cells into mature osteoclasts. J Bone Miner Metab. 2014;32:331–336. doi: 10.1007/s00774-013-0547-5. [DOI] [PubMed] [Google Scholar]
- 44.Zeng XZ, He LG, Wang S, Wang K, Zhang YY, Tao L, Li XJ, Liu SW. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol Sin. 2016;37:255–263. doi: 10.1038/aps.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang B, Liu Y, Zhao W, Zhao H, Yu H. Extracellular calcium regulates the adhesion and migration of osteoclasts via integrin αv β 3/Rho A/cytoskeleton signaling. Cell Biol Int. 2019;43:1125–1136. doi: 10.1002/cbin.11033. [DOI] [PubMed] [Google Scholar]
- 46.Barinda AJ, Ikeda K, Hirata KI, Emoto N. Macrophages highly express carbonic anhydrase 2 and play a significant role in demineralization of the ectopic calcification. Kobe J Med Sci. 2017;63:E45–E50. [PMC free article] [PubMed] [Google Scholar]
- 47.Castillo LM, Guerrero CA, Acosta O. Expression of typical osteoclast markers by PBMCs after PEG-induced fusion as a model for studying osteoclast differentiation. J Mol Histol. 2017;48:169–185. doi: 10.1007/s10735-017-9717-4. [DOI] [PubMed] [Google Scholar]
- 48.Kim JH, Kim M, Jung HS, Sohn Y. Leonurus sibiricus L. ethanol extract promotes osteoblast differentiation and inhibits osteoclast formation. Int J Mol Med. 2019;44:913–926. doi: 10.3892/ijmm.2019.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han SY, Kim YK. Berberine suppresses RANKL-induced osteoclast differentiation by inhibiting c-Fos and NFATc1 expression. Am J Chin Med. 2019;47:439–455. doi: 10.1142/S0192415X19500228. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Sun X, Ma J, Ma X, Zhao B, Zhang Y, Tian P, Li Y, Han Z. Naringin prevents ovariectomy-induced osteoporosis and promotes osteoclasts apoptosis through the mitochondria-mediated apoptosis pathway. Biochem Biophys Res Commun. 2014;452:629–635. doi: 10.1016/j.bbrc.2014.08.117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.