Abstract
Abstract
Introduction
Ischaemic heart disease (IHD) is a pathological process characterised by a blockage or non-obstructive accumulation of atherosclerotic plaques in the epicardial arteries. Percutaneous coronary intervention (PCI) is widely used in clinical practice to treat IHD. However, angina post PCI (APPCI) impairs quality of life and portends a worse prognosis. Hence, an effective and safe treatment option remains an urgent need for patients with APPCI. In recent years, there has been an increasing body of clinical trials regarding the use of acupuncture for the prevention and treatment of APPCI, and the results have indicated it might be a promising therapeutic intervention. However, to our knowledge, the potential benefits of acupuncture for the prevention and treatment of APPCI to justify either their recommendation or their clinical role have not been evaluated.
Methods and analysis
PubMed, Embase, Cochrane Library, Web of Science Core Collection, Chinese Biomedical Database, Chinese National Knowledge Infrastructure, Wanfang Database and VIP Database will be searched from inception to 8 June 2024 without language restrictions. Two investigators will independently screen studies, extract data, assess the risk of bias from randomised controlled trials (RCTs) and assess the risk of bias. The third reviewer will arbitrate any disagreements. Data synthesis and analysis will be conducted using the RevMan V.5.4 software. Subgroup analyses, sensitivity analysis, meta-regression and bias reporting assessments will be performed if necessary and appropriate. Finally, the quality of evidence from RCTs will be assessed using the Grading of Recommendations Assessment, Development, and Evaluation System tool.
Ethics and dissemination
Ethical approval is unnecessary since no private or confidential patient data will be included. The systematic review will be published in peer-reviewed journals.
PROSPERO registration number
CRD42024562116.
Keywords: Acupuncture, Randomized Controlled Trial, Angina Pectoris
STRENGTHS AND LIMITATIONS OF THIS STUDY.
To the best of our knowledge, this study is the first systematic review and meta-analysis to evaluate the efficacy and safety of acupuncture for the prevention and treatment of angina post percutaneous coronary intervention, and this study will strictly follow the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines.
This study will systematically review quantitative data from multiple databases.
Various acupuncture styles, the choice of acupoints, the duration, severity of the disease and control treatments may cause considerable heterogeneity.
This study will include trials without language restrictions, ensuring a more comprehensive analysis and reducing the likelihood of omitting significant studies.
Introduction
Ischaemic heart disease (IHD) is also known as coronary artery disease (CAD), coronary heart disease (CHD), and atherosclerotic cardiovascular disease.1,3 IHD is a pathological process characterised by a blockage or non-obstructive accumulation of atherosclerotic plaques in the epicardial arteries. It is classified as acute coronary syndrome (ACS) or chronic coronary syndrome.1 4 According to the results of the Global Burden of Disease study, IHD is a leading cause of death worldwide, both men and women and poses a considerable burden on human health.35,8 With the intensification of population ageing and the continuous influence of metabolic risk factors, the incidence and mortality of CHD are on the rise, and its high mortality and disability rates seriously threaten the health of Chinese residents and significantly increase the burden of prevention and social burden.9
Percutaneous coronary intervention (PCI) is widely used to treat CHD in clinical practice. It is often used to improve the symptoms of patients with chronic coronary syndromes who do not respond to drug therapy and to improve the prognosis of ACSs.10 Although vascular stenosis has resolved in patients with CAD who have undergone PCI, many patients develop recurrent or persistent angina pectoris post PCI (APPCI) with optimal antianginal medications, and the incidence of APPCI can be as high as 20–40% at short-term to medium-term follow-up.11 12 With more advanced CHD, multiple comorbidities and the ageing of the social population, the incidence of APPCI is still growing. A study of the clinical and economic burden associated with APPCI found that the total medical costs of patients with APPCI were 1.8 times higher within 1 year of index PCI than those without angina.11 APPCI impairs quality of life and portends a worse prognosis.13 14 Hence, an effective and safe treatment option remains an urgent need for patients with APPCI.
Acupuncture has been widely used among angina in China and has gradually been accepted worldwide because of its efficacy. In recent years, there has been an increasing body of clinical trials regarding the use of acupuncture for the prevention and treatment of APPCI, and the results have indicated it might be a promising therapeutic intervention.15,19 However, to our knowledge, the potential benefits of acupuncture for the prevention and treatment of APPCI to justify either their recommendation or their clinical role have not been evaluated.
This study will include and systematically synthesise the eligible randomised controlled trials (RCTs) without language restrictions. To the best of our knowledge, this meta-analysis is the first attempt to assess the available evidence of acupuncture for treating APPCI. Hopefully, this study may yield helpful information for the people concerned. Therefore, this protocol intends to adopt the method of system valuation and meta-analysis of acupuncture for angina post-PCI to evaluate its efficacy and safety.
Methods and analysis
Design and registration of the review
This study has been registered on PROSPERO, and the protocol will be based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols statement (PRISMA-P).20 The PRISMA-P checklist is shown in online supplemental appendix S1.
Eligibility criteria
Types of studies
All clinical RCTs of acupuncture treating APPCI will be included without restrictions on the country, and there will be no language limitation.
Reviews, case reports, theory discussions, animal experiments, other diseases, conference summaries and other non-randomised clinical studies will be excluded.
Types of participants
This study will enrol patients undergoing PCI in IHD, regardless of gender, age, occupation, education, severity, etc.
Types of interventions
All types of acupuncture therapies should be treated in the experimental group. Regardless of stimulation approaches, acupoint selection and additional therapies will be included.
The control group will include sham acupuncture, acupuncture on corresponding non-acupoints, drugs and a waiting list. We will also exclude studies comparing acupuncture and other complementary and alternative therapies. If there are uncertain interventions, this study will be excluded.
Types of outcome measures
Primary outcomes
The main primary outcome is the severity of angina-related clinical evaluation symptoms assessed by validated and reliable scales such as the Seattle angina questionnaire (SAQ) score, the Canadian Cardiovascular Society (CCS) angina score, the Visual Analogue Scale and the dose of nitroglycerin. One of the standardised scales (eg, CCS, SAQ) is more helpful in ensuring consistency of results.
Secondary outcomes
Initial time and incident rate of major adverse cardiovascular events.
Serum inflammatory factors, such as high-sensitivity C-reactive protein, interleukin-6.
Nature and adverse effect rate (relevant symptoms caused by acupuncture).
Data sources and search strategy
Potentially eligible studies published since inception to 8 June 2024 will be retrieved from the following eight electronic databases: PubMed, Embase, Cochrane Library, Web of Science Core Collection, Chinese Biomedical Database, Chinese National Knowledge Infrastructure, Wanfang Database, VIP Database.21
Considering the particularity of the databases, we will develop special search strategies for each. The search strategy for PubMed is shown in table 1, and more is shown in online supplemental appendix S2.
Table 1. Search strategy for PubMed.
| Order | Search items |
| #1 | Percutaneous Coronary Intervention(MeSH Terms) |
| #2 | ((((((((((Coronary Intervention, Percutaneous(Title/Abstract)) OR (Coronary Interventions, Percutaneous(Title/Abstract))) OR (Intervention, Percutaneous Coronary(Title/Abstract))) OR (Interventions, Percutaneous Coronary(Title/Abstract))) OR (Percutaneous Coronary Interventions(Title/Abstract))) OR (Percutaneous Coronary Revascularization(Title/Abstract))) OR (Coronary Revascularization, Percutaneous(Title/Abstract))) OR (Coronary Revascularizations, Percutaneous(Title/Abstract))) OR (Percutaneous Coronary Revascularizations(Title/Abstract))) OR (Revascularization, Percutaneous Coronary(Title/Abstract))) OR (Revascularizations, Percutaneous Coronary(Title/Abstract)) |
| #3 | #1 OR #2 |
| #4 | ((((((((((Acupuncture (MeSH Terms)) OR Acupuncture Therapy(MeSH Terms)) OR Acupressure(MeSH Terms)) OR Meridians(MeSH Terms)) OR moxibustion(MeSH Terms)) OR Acupuncture Points(MeSH Terms)) OR Electroacupuncture(MeSH Terms)) OR Cupping Therapy(MeSH Terms)) OR Auriculotherapy(MeSH Terms)) OR Acupuncture, Ear(MeSH Terms)) OR Bloodletting Therapy(MeSH Terms) |
| #5 | ((((((((((((((((((((((((((Acupunctur*(Title/Abstract)) OR (Pharmaco*punctur*(Title/Abstract))) OR (“Zhen Jiu”(Title/Abstract))) OR (ZhenJiu(Title/Abstract))) OR (acupressur*(Title/Abstract))) OR (Shiatsu(Title/Abstract))) OR (“Zhi Ya”(Title/Abstract))) OR (“Tui Na”(Title/Abstract))) OR (acupotom*(Title/Abstract))) OR (“manual acupuncture”(Title/Abstract))) OR (“warm acupuncture”(Title/Abstract))) OR (meridian*(Title/Abstract))) OR (“jing luo”(Title/Abstract))) OR (jingluo(Title/Abstract))) OR (“ching lo”(Title/Abstract))) OR (chinglo(Title/Abstract))) OR (moxibustion*(Title/Abstract))) OR (moxabustion*(Title/Abstract))) OR (acupoint*(Title/Abstract))) OR (needl*(Title/Abstract))) OR (electroacupunctur*(Title/Abstract))) OR (electro-acupunctur*(Title/Abstract))) OR (“Cupping Therap*“(Title/Abstract))) OR (“Auricular Acupunctur*“(Title/Abstract))) OR (“Ear Acupunctur*“(Title/Abstract))) OR (“Bloodletting Therap*"(Title/Abstract))) |
| #6 | #4 OR #5 |
| #7 | randomized controlled trial(MeSH Terms) |
| #8 | (randomized controlled trial(Title/Abstract)) OR (random*(Title/Abstract)OR placebo(Title/Abstract)OR RCT(Title/Abstract)) |
| #9 | #7 OR #8 |
| #10 | #3 AND #6 AND #9 |
RCTs on acupuncture treatment in patients undergoing PCI will be searched for independently by two reviewers in those sources.
Data collection and analysis
Study selection and data extraction
Selection of studies: EndNote V.21 will be used to manage the search results from the databases mentioned above.
Two researchers (XZ and YY) will be trained to extract data independently.
In the first step, two reviewers (XZ and YY) will have professional training about the review’s background, purpose and process.
In the second step, the two reviewers will independently select and record by their titles, abstracts and keywords according to inclusion criteria, and ineligible studies will be removed from the trash in EndNote V.21.
In the third step, the two reviewers will independently read the full-text version of the remaining studies to select those that meet the inclusion criteria. The two reviewers will cross-check the election results. Any divergences in the data obtained will be arbitrated by the third reviewer (YR). Each unique study ID will be allocated to each eligible study, comprising the first author’s last name and the year of publication (eg, Zhou 2023).
In the fourth step, the two reviewers will independently extract the data into a self-designed data extraction form. The data extraction form will include basic information (article title, authors, publication date, country), study design (study type, sample size, characteristics of participants, intervention details, duration, outcome measures) and conclusions. Details of acupuncture therapy will be covered according to Revised Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the Consolidated Standards of Reporting Trials statement (STRICTA).22
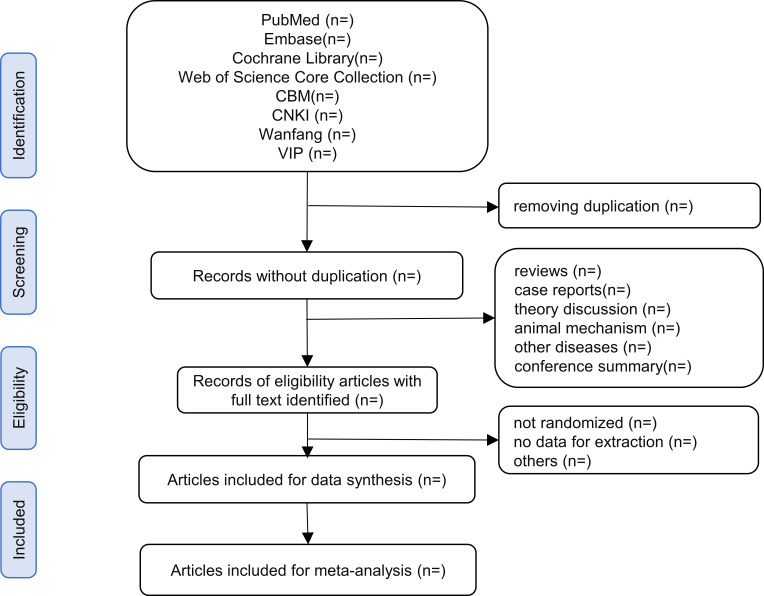
In the fifth step, the two reviewers will cross-check the completed data extraction forms. The third reviewer will resolve any diversities. The entire study selection process for this review will be shown in a PRISMA-P flow chart (figure 1).
Figure 1. Flow chart of the study selection process.
Assessment of risk bias
Two review authors will independently measure the risk of bias in the included studies using the Cochrane risk of bias assessment tool. The assessment of the risk of bias will consist of the following seven items: sequence generation, allocation concealment, participant blinding, the blindness of result evaluators, incomplete outcome data, selective outcome reporting and other biases. The risk level will be categorised as low risk of bias, unclear risk of bias and high risk of bias. If necessary, the third reviewer (YR) will help us judge the consistency. Manager (RevMan) V.5.4 will be used to generate the risk of bias.
When the articles’ data are insufficient or ambiguous, one of the authors will contact the original author to request detailed information about the research by email or telephone or estimate the data. These studies will be excluded if missing data is unavailable or the author cannot be contacted. We will conduct a limited analysis based on available data and discuss the potential impact of missing data.
Measures of treatment effect and assessment of heterogeneity testing
Measures of treatment effect will be analysed by RevMan V.5.4.
Dichotomous data will be expressed as relative risk with 95% CIs, and continuous variables will be described as the mean difference (MD) or standardised MD with 95% CI.
The significance level α will be set at 0.05. I2 will be selected to identify statistical heterogeneity across studies in all analyses based on Cochrane Handbook V.5.4. If there is no significant heterogeneity among the studies (I²<50%, p>0.1), a fixed-effect model will be employed for the analysis. Conversely, if significant heterogeneity is present, subgroup analyses will be conducted to clarify the sources of heterogeneity further. If heterogeneity cannot be resolved, a random-effects model will be used for the analysis.
As potential heterogeneity is unavoidable, we will first consider the sources of clinical heterogeneity and identify covariates. These may include variations in acupuncture interventions (such as electroacupuncture, manual acupuncture and auricular acupuncture). Patients with ACSs and stable CAD are included in our study population. The study population may be a source of heterogeneity. In addition, other covariates that may affect outcome indicators, such as the age of the population, duration of the disease, duration of treatment, and treatment in the control group, are also collected for subgroup analyses.
Sensitivity analysis
We will conduct a sensitivity analysis to determine the robustness and quality of the results. The principal criteria include methodological quality, sample size and data analysis techniques. The meta-analysis will be operated repeatedly. If studies have significant differences in assessment results of risk of bias, we will exclude studies with a high risk of bias from analysis.
Grading the quality of evidence
We will evaluate the quality of evidence according to the Grades of Recommendations Assessment, Development and Evaluation (GRADE) tool.23 Two investigators (XZ and YY), who will have been trained on GRADE, will independently perform a quality assessment of the evidence. Based on GRADE, the quality level will be classified as high, moderate, low or critically low. Any controversy will be handled by the third reviewer (YR).
Assessment of reporting bias
Reporting bias includes publication bias, time lag bias, duplicate publication bias, outcome reporting bias, etc. To avoid reporting bias as much as possible, we will search the most important databases, international general healthcare databases, subject-specific electronic bibliographic databases, citation index databases, dissertations and theses databases, grey literature databases and clinical trials registry platforms. Moreover, more than 10 studies are included in the meta-analysis. In that case, we will evaluate the publication bias by the Egger’'s regression test, and the evaluation will be presented in the form of funnel plots.
Ethics and dissemination
Ethical approval is unnecessary since no private and confidential patient data will be included. The systematic review will be published in peer-reviewed journals.
supplementary material
Footnotes
Funding: This study was supported by the NSFC (National Natural Science Foundation of China, Grant number 81873239).
Prepub: Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-090964).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Xin Zhou, Email: zhouxin@stu.cdutcm.edu.cn.
Yanwei Li, Email: liyw0305@163.com.
Yongjun Yin, Email: cdxnyyj@cdutcm.edu.cn.
Yilan Wang, Email: wangyilan@stu.cdutcm.edu.cn.
Wen Xie, Email: 1131649799@qq.com.
Yulan Ren, Email: renxg2468@163.com.
References
- 1.Khan MA, Hashim MJ, Mustafa H, et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus. 2020;12:e9349. doi: 10.7759/cureus.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howick J, Webster RK, Rees JL, et al. TIDieR-Placebo: A guide and checklist for reporting placebo and sham controls. PLoS Med. 2011;17:e1003294. doi: 10.1371/journal.pmed.1003294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nowbar AN, Gitto M, Howard JP, et al. Mortality From Ischemic Heart Disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005375. doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 5.Zhao D, Liu J, Wang M, et al. Epidemiology of cardiovascular disease in China: current features and implications. Nat Rev Cardiol. 2019;16:203–12. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 6.Naghavi M, Ong KL, Aali A, et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet. 2024;403:2100–32. doi: 10.1016/S0140-6736(24)00367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392:1859–922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack M, Gopal A. Epidemiology, traditional and novel risk factors in coronary artery disease. Cardiol Clin. 2014;32:323–32. doi: 10.1016/j.ccl.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Xiao S, Zhang L, Wu Q, et al. Development and Validation of a Risk Nomogram Model for Predicting Revascularization After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome. CIA . 2021;Volume 16:1541–53. doi: 10.2147/CIA.S325385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannini F, Candilio L, Mitomo S, et al. A Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2018;11:1797–810. doi: 10.1016/j.jcin.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 11.Crea F, Bairey Merz CN, Beltrame JF, et al. Mechanisms and diagnostic evaluation of persistent or recurrent angina following percutaneous coronary revascularization. Eur Heart J. 2019;40:2455–62. doi: 10.1093/eurheartj/ehy857. [DOI] [PubMed] [Google Scholar]
- 12.Niccoli G, Montone RA, Lanza GA, et al. Angina after percutaneous coronary intervention: The need for precision medicine. Int J Cardiol. 2017;248:14–9. doi: 10.1016/j.ijcard.2017.07.105. [DOI] [PubMed] [Google Scholar]
- 13.Collet C, Collison D, Mizukami T, et al. Differential Improvement in Angina and Health-Related Quality of Life After PCI in Focal and Diffuse Coronary Artery Disease. JACC Cardiovasc Interv. 2022;15:2506–18. doi: 10.1016/j.jcin.2022.09.048. [DOI] [PubMed] [Google Scholar]
- 14.Ajmal M, Chatterjee A, Acharya D. Persistent or Recurrent Angina Following Percutaneous Coronary Revascularization. Curr Cardiol Rep. 2022;24:1837–48. doi: 10.1007/s11886-022-01820-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Liang D, Wang F, et al. Efficacy of electroacupuncture pretreatment for myocardial injury in patients undergoing percutaneous coronary intervention: A randomized clinical trial with a 2-year follow-up. Int J Cardiol. 2015;194:28–35. doi: 10.1016/j.ijcard.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, Yang J, Lei D, et al. Effect of thumbtack needling therapy on clinical symptoms and inflammatory factors of unstable angina pectoris with syndrome of blood stasis due to qi deficiency in patients with coronary heart disease after percutaneous coronary intervention. China Med Herald. 2023;20:85–9. [Google Scholar]
- 17.Xianrun L, Zhaohui L, Zhaohui L, et al. Efficacy study of the combination therapy of red and green Low - Power Laser Irradiation and percutaneous coronary intervention for UA patients. LASER JOURNAL. 2013:68–9. doi: 10.3969/j.issn.0253-2743.2013.06.028. [DOI] [Google Scholar]
- 18.Chen J, Zhang S, Lin J, et al. Observation on Efficacy of Acupoint Application of Compound Danshen Dripping Pills in Treatment of Pectoralgia After Percutaneous Coronary Intervention. Eval and Anal of Drug-Use in Hosps of China. 2017;17:889–91. doi: 10.14009/j.issn.1672-2124.2017.07.008. [DOI] [Google Scholar]
- 19.Lifang P, Kena B, Fang Z, et al. Rehabilitation Effect of Acupoint Massage Combined with Exercise on Patients with Coronary Artery Stent Implantation. Health Educ Health Prom. 2019;14:274–6. doi: 10.16117/j.cnki.31-1974/r.201903025. [DOI] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng Y, Xia J, Chen Z, et al. Transcutaneous electrical acupoint stimulation (TEAS) for cancer-related fatigue: study protocol for a systematic review and meta-analysis. BMJ Open. 2021;11:e049318. doi: 10.1136/bmjopen-2021-049318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacPherson H, Altman DG, Hammerschlag R, et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement. J Evidence Based Medicine. 2010;3:140–55. doi: 10.1111/j.1756-5391.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Green S. The Cochrane Collaboration; 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]