Abstract
Introduction
The balance of trace elements plays an important role in diabetic kidney disease (DKD) patients. However, studies on the differences in urinary trace elements across different DKD stages are scarce. This study aimed to explore the associations between nine essential trace elements and DKD.
Research design and methods
This cross-sectional analysis included 830 diabetic patients. Participants were classified into non-DKD (NDKD) and DKD, the latter was further grouped into mid and end DKD based on estimated glomerular filtration rate (eGFR), and the case and control were matched based on age and sex. The concentration of urinary trace elements was measured with inductively coupled plasma mass spectrometry.
Results
Urinary concentrations of copper (Cu) and manganese (Mn) in DKD patients were significantly higher than that of NDKD patients, whereas that of iron (Fe), cobalt, selenium, and nickel (Ni) of DKD were lower. Positive correlations between urinary Mn/Cu and the risk of mid-stage and end-stage DKD were revealed by conditional logistic regression, while Fe and Ni were negatively associated with the risk of DKD. In mixed effect analyses, no significant trend was found for joint trace element exposure and risk of mid DKD, while negative associations between combined effects of trace elements and the risk of end DKD were observed.
Conclusions
This study revealed different associations between trace elements and the risk of mid and end DKD using both single and mixture effect modeling. The results suggested that the urinary trace element profile might be associated with the progression of DKD, which provides important insights for understanding the pathogenesis of DKD and developing individualized nutritive management strategies.
Keywords: Diabetes Complications, Kidney Diseases, Case-Control Studies
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have established associations between the level of trace elements and the risk of diabetic kidney disease (DKD), but their role in the progression of DKD remains to be investigated.
WHAT THIS STUDY ADDS
In patients with mid to end DKD, iron and nickel were related to reduced risk, while copper and manganese were associated with increased risk of DKD. Urinary zinc exhibited a protective effect in mid DKD but showed a weaker effect in end DKD. An overall increase in the concentration of trace elements in urine was associated with a reduced risk of DKD.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study offers valuable information for understanding the role of trace elements in the progression of DKD and for developing personalized nutrition management strategies.
Introduction
Diabetic kidney disease (DKD), also known as diabetic nephropathy, is a complication of diabetes that coexists with chronic kidney disease (CKD), manifested by elevated urinary albumin excretion and/or decreased glomerular filtration rate.1 There are currently about 500 million diabetic patients in the world.2 Approximately 40% of type 2 diabetes mellitus patients and 30% of type 1 diabetes mellitus patients develop DKD, a leading cause of CKD and end-stage renal disease (ESRD).3 4 DKD affects renal function, altering the normal process of waste and excess fluid clearance from the body.5 Additionally, DKD is associated with a high mortality rate. Kidney disease increases the risk of death in diabetic patients by 31.1%, escalating with the severity of the disease.3 Mitochondrial dysfunction with increased reactive oxygen species (ROS) and superoxide production has been postulated as the primary initiating event in the development of diabetic complications.6 7 Iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), and other elements are constituents of superoxide. Loss of homeostasis of these trace elements in ESRD patients significantly increases morbidity and mortality. Therefore, the balance of trace elements should be considered at every stage of CKD.8
Numerous studies have linked diabetes to alterations in trace element levels, often associated with oxidative stress and the advancement of DKD.9,11 For example, Fe, Cu, Mn, and Zn can influence oxidative stress and inflammatory responses in the body through their involvement in redox reactions. Essential trace elements, such as Fe, are critical for cellular metabolism and homeostasis, and their imbalance, particularly elevated Fe levels in gestational diabetes with DKD, can contribute to oxidative stress and tissue damage.12 13 Excess accumulation of Fe and Cu promotes the generation of ROS, increasing oxidative stress and resulting in kidney tissue damage. Mn is an essential element for antioxidant enzymes, such as Mn superoxide dismutase (SOD); both excess and deficiency of Mn can disrupt the balance of these enzymes, weakening antioxidant defense mechanisms and increasing the risk of kidney damage. Similarly, insufficient Zn intake has been associated with kidney disease, and low serum Zn levels may raise the risk of DKD.14,16 Other elements like calcium and Cu are also connected to kidney failure, with the Cu/Zn ratio proposed as a marker for renal failure.17 18 In DKD patients, disrupted regulation of these elements due to factors such as diet, uremia, inflammation, or dialysis can lead to deficiencies or toxicities.19 This imbalance may result in the accumulation of trace elements in the kidneys, causing chronic damage, hypertension, proteinuria, and disease progression.20 Consequently, comprehending the homeostasis of trace elements is crucial for improving DKD prognosis and retarding disease progression.
Current research on the associations between DKD and bodily trace element concentrations has predominantly focused on trace elements exposure in the early stages of DKD. However, few studies have analyzed or compared the benefits and risks of various trace elements across different stages of DKD. Moreover, most research has been limited to the analysis of single trace elements and has not examined the associations of mixed exposures to multiple trace elements with DKD progression. This paper addresses these gaps by leveraging data from a population of diabetic patients to establish the associations between multiple trace elements and mid-to-end DKD.
Materials and methods
Study population
This study, conducted at Henan Province People’s Hospital, Henan Province, China. Diabetic patients were considered for enrollment. All patients (n=834) were 20–90 years old and had visited the nephrology department at least twice between March 2021 and June 2021. Trained investigators administered epidemiological questionnaires through face-to-face interviews, while first morning urine samples were collected from participants. According to previous studies, morning urine samples were representative and stable for detecting urine biomonitoring indicators.21 Those who did not provide sufficient urine samples or questionnaire data (n=4) were excluded from the current study, leaving 830 diabetes patients for further analysis (online supplemental figure S1). Written informed consent was obtained from all participants.
Type 2 diabetic kidney disease definition
Definition of diabetes
Diabetes was defined as a fasting glucose level≥7.0 mmol/L, self-reported physician-diagnosed diabetes or use of antidiabetic medications (insulin or oral hypoglycemic agents) according to the National Guidelines for Prevention and Treatment of Diabetes Mellitus (2022).22
Diabetic kidney disease definition
The diagnosis of DKD was based on the Chinese Guidelines for the Prevention and Treatment of Diabetic Kidney Disease (2021 edition). DKD was either defined by a urine albumin-to-creatinine ratio≥30 mg/g or an estimated glomerular filtration rate (eGFR)<60 mL/min/1.73 m2, in the absence of other primary causes of kidney damage. Included participants were divided into non-DKD (NDKD), mid and end DKD. We defined patients with diabetes and an eGFR decline within the range of 15–59 mL/min/1.73 m2 as mid stage, and patients with diabetes with an eGFR less than 15 mL/min/1.73 m2 accompanied by albuminuria as end stage.
We adopted the following methods to ensure the accurate handling of borderline cases in diabetes and DKD pathology. First, standardized measurement and laboratory testing methods were used for diagnosis and inclusion. Only long-term diabetes patients (over 12 months) were included, and cases with other primary kidney diseases were excluded. For cases near threshold values, repeated testing was conducted to confirm the diagnosis.
Covariate data
Detailed physical examinations were conducted, including measurements of height, weight, and blood pressure, following recommended standard procedures. Questionnaires and medical records were used to collect characteristics of participants, including smoking, education, occupation, and related disease history. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.23 Covariates of current study included sex, age (<40, 40–60, >60 years), BMI (<24, 24–28, >28 kg/m2), smoking status (never, ever), education (high school graduate or less, college graduate or higher), occupation (heavy physical work, light physical work), diabetes-specific complications (no, yes), cardiovascular diseases (no, yes), and other systemic diseases (no, yes). Definition of occupation, diabetic-specific complications, cardiovascular diseases and other systemic diseases can be found in Covariates definition of online supplemental information.
Trace metal elements measurement
Morning urine samples from participants were collected and frozen within 2 hours, and stored at −80°C for subsequent analysis. The urinary concentrations of nine trace elements (Fe, Cu, chromium (Cr), cobalt (Co), Zn, selenium (Se), vanadium (V), nickel (Ni), and Mn) were determined using inductively coupled plasma mass spectrometry (NexION 300D ICP-MS; PerkinElmer, USA). The methodology was based on previously published literature with slight modifications.24 In brief, the sample was first nitrate-treated overnight at 4°C. Subsequently, it was diluted with ultrapure water, thoroughly shaken, and filtered through a 0.22 µm water-compatible filter head before analysis. The specific pretreatment methods were described in online supplemental information. For internal quality assurance and control, test tubes were prewashed by soaking overnight in nitric acid. The limit of detection (LOD) for urinary trace elements is between 0.004 and 4.657 µg/L (online supplemental table S1). A standard quanlity control (QC) sample was measured after every 20 samples. Therefore, a pooled urine sample was used to ensure the precise and accurate detection of these elements. The within-day and between-day coefficients of variation for urinary elements were less than 15% (online supplemental table S2). The recovery rates for all elements ranged from 72.6% to 121.8% (online supplemental table S2). The detected data were adjusted for specific gravity (SG), and it has been reported that urine SG and creatinine corrections are valid and interchangeable correction standards.25 Trace elements concentrations were adjusted according to the following equation: Pc=P × ((SGm-1)/(SGi-1)),26 where P represents the measured concentration of trace elements as in ng/mL, SGm is the median of SG of all samples (1.020), and SGi the SG of individual samples. Trace elements levels below LOD were replaced by LOD divided by the square root of two.
Statistical analysis
Baseline characteristics according to the development of DKD in diabetes were assessed using t-tests or Mann-Whitney U tests (continuous variables), and χ2 tests (categorical variables). Continuous variables were presented as median (IQR) and categorical variables in percentages. Urine trace elements concentrations with skewed distributions were log2-transformed. Spearman’s rank correlation analysis was used to examine correlations between urine trace elements.
In this study, we used the propensity score matching (PSM) method to match age and sex by using a multivariate logistic regression model with 1:1 optimal matching. After matching, we assessed the quality of the matches using standardized mean differences (SMD). We ensured that the SMD values for all age and sex variables were below 0.1, thereby confirming the balance in baseline characteristics between the two groups post-matching (online supplemental table S3).
In the single analyte models, conditional logistic regression models were used to estimate ORs and 95% CIs for the associations between urinary elements and DKD in patients with diabetes. Urinary elements concentrations were categorized into tertiles according to their distributions. To better understand the potential non-linear relationships and interactions between the urine trace elements and the risk of developing DKD, we employed restricted cubic spline (RCS) in logistic regression models with four knots.
To deal with potential non-linear and interactive relationships among multiple trace elements, we used the Weighted Quantile Sum (WQS) regression, Quantile G-Computation (QGcomp) and Bayesian Kernel Machine Regression (BKMR) model to estimate the joint and single effects of multiple elements exposures on DKD patients. WQS regression was employed to quantify the cumulative effect of elements on DKD risk. This model calculates a weighted index reflecting the combined effect of all trace elements. To evaluate the robustness of our estimates, we split the data into 40% for training and 60% for testing, and fitting models 1000 times to discern both positive and negative relationships between elements and DKD.
QGcomp was adopted to assess the aggregate influence of elements on DKD risk, calculating risk shifts corresponding to an equal quantile rise in nine urinary elements. Differing from WQS, QGcomp does not necessitate a uniform direction of elements associations with DKD risk, with the combined weight totals being equal to 1 in both positive and negative weights.
BKMR model was used to examine the potential non-linear dose response between each trace element and DKD, the combined effects of trace element mixture on DKD, and the possible interaction between trace elements. Confounding factors included in the BKMR model were the same as those in the adjusted single models. To fit the BKMR model, a Markov Chain Monte Carlo sampler was run for 10,000 iterations. In order to ensure the robustness of the BKMR model results, we also conducted a sensitivity analysis using the BKMR model without elements extreme value treatment.
All statistical analyses were conducted in R (V.4.3.2) with the following packages, ‘survival’, ‘rms’, ‘gWQS’, ‘qgcomp’, and ‘bkmr’, respectively. The threshold for statistical significance was set at p<0.05 (two-tailed).
Results
Demographic characteristics
This study analyzed 830 participants at different stages of DKD, comprising 539 men and 291 women (table 1). The proportion of DKD in diabetic patients in this study was 48%. Significant differences in age distribution between mid DKD and end DKD were observed (p=0.003). Most of the participants had a high school education or below. There were more light physical workers in mid than end DKD patients (p<0.001). The proportion of diabetes-specific complications and cardiovascular diseases was significantly higher in the mid DKD than NDKD (p<0.001), and the prevalence of other systemic diseases was less in end DKD compared with mid.
Table 1. Basic characteristics of the study participants by DKD stages.
| Characteristics | NDKD | Mid DKD | End DKD | P value* |
| n (%) | 432 (52.0) | 258 (31.1) | 140 (16.9) | |
| Sex (%) | 0.430 | |||
| Male | 280 (64.8) | 162 (62.8) | 97 (69.3) | |
| Female | 152 (35.2) | 96 (37.2) | 43 (30.7) | |
| Age, years, n (%) | ||||
| <40 | 55 (12.7) | 24 (9.3) | 6 (4.3) | 0.003 |
| 40–60 | 213 (49.3) | 106 (41.1) | 66 (47.1) | |
| >60 | 164 (38.0) | 128 (49.6) | 68 (48.6) | |
| BMI, m/kg2 n (%) | ||||
| <24 | 156 (36.1) | 74 (28.7) | 38 (27.1) | 0.104 |
| 24–28 | 188 (43.5) | 116 (45.0) | 70 (50.0) | |
| >28 | 88 (20.4) | 68 (26.4) | 32 (22.9) | |
| Smoke, n (%) | 0.111 | |||
| Never | 286 (66.2) | 163 (63.2) | 79 (56.4) | |
| Ever | 146 (33.8) | 95 (36.8) | 61 (43.6) | |
| Education, n (%) | <0.001 | |||
| High school graduate or less | 198 (45.8) | 102 (72.9) | 172 (66.7) | |
| College graduate or higher | 234 (54.2) | 38 (27.1) | 86 (33.3) | |
| Occupation, n (%) | <0.001 | |||
| Heavy physical work | 80 (18.5) | 76 (29.5) | 72 (51.4) | |
| Light physical work | 352 (81.5) | 182 (70.5) | 68 (48.6) | |
| Anemia | <0.001 | |||
| No | 361 (85.5) | 181 (72.1) | 22 (15.8) | |
| Yes | 61 (14.5) | 70 (27.9) | 117 (84,2) | |
| Diabetes-specific complications, n (%)† | 0.088 | |||
| No | 38 (8.8) | 15 (5.8) | 17 (12.1) | |
| Yes | 394 (91.2) | 243 (94.2) | 123 (87.9) | |
| Cardiovascular diseases, n (%)‡ | <0.001 | |||
| No | 197 (45.6) | 71 (27.5) | 10 (7.1) | |
| Yes | 235 (54.4) | 187 (72.5) | 130 (92.9) | |
| Other systemic diseases, n (%)§ | <0.001 | |||
| No | 76 (17.6) | 61 (23.6) | 49 (35.0) | |
| Yes | 356 (82.4) | 197 (76.4) | 91 (65.0) | |
VP values were calculated using the Wilcoxon rank-sum test for continuous variables and the chi-squareχ2 test for categorical variables. P values in bold represent values less than 0.05
Diabetic S-specific Ccomplications in this paper mainly included diabetic foot, diabetic ketosis, fundus disease, diabetic retinopathy, diabetic nephropathy, diabetic peripheral vascular disease, diabetic peripheral neuropathy, and diabetic macrovascular disease.
The Ccardiovascular Ddiseases in this paper mainly include peripheral atherosclerosis, coronary heart disease, cardiovascular disease, cerebrovascular disease, and hypertension.
Other Ssystemic Ddiseases in this paper mainly include respiratory system diseases, digestive system diseases, fatty liver, and other related diseases of the endocrine system.
BMI, body mass index; DKD, diabetic kidney diseaseNDKD, non-DKD
Trace element analysis revealed significant differences across DKD stages (table 2). Urinary concentrations of Fe, Co, Se and Ni in DKD were significantly lower than those of NDKD, and end-stage concentrations of these elements were lower than mid stage (p<0.001). The concentration of urinary Cu and Mn was higher in DKD than in NDKD, and higher in end DKD (p<0.001). Zn was different, being highest in mid DKD patients and lowest in end DKD patients.
Table 2. Concentrations of trace elements in urine among DKD stages.
| Elements | NDKD (ng/mL) | Mid DKD (ng/mL) | End DKD (ng/mL) | P value* |
| Iron | 785.0 (506.1–1154.0) | 645.5 (421.2–1024.3) | 328.9 (191.5–566.3) | <0.001 |
| Copper | 209.0 (110.6–373.3) | 223.6 (132.2–405.2) | 305.8 (183.9–435.2) | <0.001 |
| Chromium | 72.7 (59.6–89.2) | 76.2 (59.3–94.0) | 74.7 (53.3–93.6) | 0.266 |
| Cobalt | 0.55 (0.42–0.79) | 0.54 (0.36–0.78) | 0.34 (0.22–0.53) | <0.001 |
| Zinc | 558.5 (323.4–833.1) | 609.5 (397.5–904.0) | 473.1 (249.8–786.5) | 0.002 |
| Selenium | 20.3 (13.6–28.6) | 18.5 (11.0–26.0) | 17.6 (10.0–21.5) | <0.001 |
| Vanadium | 44.8 (31.4–65.9) | 46.3 (30.2–69.4) | 46.7 (32.2–67.7) | 0.767 |
| Nickel | 11.4 (8.10–15.8) | 9.03 (5.92–13.42) | 5.15 (2.69–7.66) | <0.001 |
| Manganese | 1.55 (0.95–2.46) | 1.86 (1.04–3.36) | 2.79 (1.67–4.33) | <0.001 |
Note: Trace elements are described using median (IQR). The concentrations of trace elements in urine were corrected for specific gravity ().
VP values were calculated using the Wilcoxon rank-sum test for continuous variables.
DKD, diabetic kidney diseaseNDKD, non-DKD
In the correlation analysis of trace elements, the study observed that most of the elements had a low correlation (online supplemental figure S1), except Fe, Co and Ni. Additionally, mild correlations existed between Cu and V, Mn (0.42 to 0.53), while Se was negatively correlated with Cu, and V with Cr (−0.24 to –0.15).
Exposure to single element and incidence of DKD
The conditional logistic regression results showed the association between trace elements and different stages of DKD (table 3). Urinary Fe and Ni showed a significant negative association with DKD both mid (OR (95% CI): Fe, 0.77 (0.62, 0.95); Ni, 0.50 (0.38, 0.66)) and end (OR (95% CI): Fe, 0.35 (0.21, 0.60); Ni, 0.30 (0.17, 0.53)) after adjusted. Mn and Cu showed a positive correlation with mid DKD after adjusted (OR (95% CI): Mn, 1.27 (1.10, 1.47); Cu, 1.21 (1.02, 1.44)), with risk increases of 74% and 47% in end DKD patients compared with mid DKD (OR (95% CI): Mn, 1.74 (1.20, 2.53); Cu, 1.79 (1.14, 2.83)). Co showed an insignificant association in mid DKD, and showed a negative correlation in end after adjusted (OR (95% CI): Co, 0.70 (0.58, 0.86)). Most of the elements showed a negative correlation between the two stages of DKD, the positive correlation between Cu and Mn and DKD shown in table 3.
Table 3. Single model between trace elements and DKD stages after PSM.
| Elements | Mid DKD | End DKD | ||||||
| Model 1 (unadjusted)OR (95% CI)* | P value | Model 2 (adjusted)OR (95% CI)† | P value | Model 1 (unadjusted)OR (95% CI)* | P value | Model 2 (adjusted)OR (95% CI)† | P value | |
| Iron | 0.81(0.67, 0.97) | 0.025 | 0.77(0.62, 0.95) | 0.014 | 0.32(0.22, 0.46) | <0.001 | 0.35(0.21, 0.60) | <0.001 |
| Copper | 1.23(1.06, 1.43) | 0.007 | 1.21(1.02, 1.44) | 0.027 | 2.03(1.51, 2.73) | <0.001 | 1.79(1.14, 2.83) | 0.012 |
| Chromium | 1.67(1.18, 2.35) | 0.003 | 1.71(1.17, 2.50) | 0.006 | 1.26 (0.84, 1.90) | 0.257 | 1.24 (0.65, 2.35) | 0.518 |
| Cobalt | 1.02 (0.87, 1.20) | 0.813 | 0.99 (0.83, 1.19) | 0.932 | 0.70(0.58, 0.86) | 0.001 | 0.70(0.51, 0.94) | 0.020 |
| Zinc | 1.15 (0.98, 1.34) | 0.090 | 1.12 (0.95, 1.32) | 0.182 | 0.78(0.63, 0.98) | 0.034 | 0.81 (0.55, 1.21) | 0.302 |
| Selenium | 0.92(0.86, 1.00) | 0.047 | 0.94 (0.86, 1.02) | 0.123 | 0.86(0.76, 0.97) | 0.015 | 0.97 (0.82, 1.16) | 0.778 |
| Vanadium | 0.93 (0.75, 1.16) | 0.533 | 0.83 (0.64, 1.06) | 0.139 | 0.93 (0.72, 1.21) | 0.602 | 0.60(0.37, 0.98) | 0.040 |
| Nickel | 0.55(0.43, 0.69) | <0.001 | 0.50(0.38, 0.66) | <0.001 | 0.29(0.20, 0.43) | <0.001 | 0.30(0.17, 0.53) | <0.001 |
| Manganese | 1.25(1.10, 1.42) | 0.001 | 1.27(1.10, 1.47) | 0.001 | 1.93(1.48, 2.51) | <0.001 | 1.74(1.20, 2.53) | 0.004 |
Note: Association between 9nine trace elements and mid DKD and end DKD using conditional logistic regression.
Bolded values denote statistically significant odds ratios (OR), defined as P < 0.05 and 95% confidence intervals (CI) excluding the null value of 1.
Model 1 is an unadjusted model.
Model 2 was adjusted by sex, BMI, age, smokinge status, education, occupation, diabetes -specific complications, cardiovascular diseases, and other systemic diseases.
BMIbody mass indexDKD, diabetic kidney diseasePSMpropensity score matching
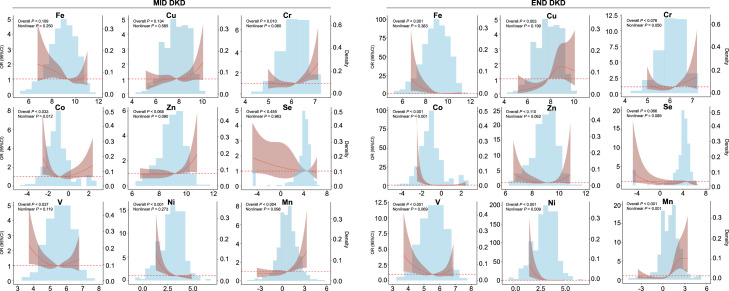
This study used RCS with four knots to model the association of nine trace elements with two stages of DKD (figure 1). Overall, the elements showed similar risk trends in mid DKD and end DKD. In mid DKD, urinary Fe, Co, V, and Ni only had certain risks below 77.5 µg/L, 2.32 µg/L, 18.3 µg/L, 7.19 µg/L, respectively, with Co showing a significant non-linear association (p for non-linear both <0.05). A similar risk trend of these elements was observed in end DKD (Fe: 88.5 µg/L, Co: 0.84 µg/L, V: 22.2 µg/L, Ni: 11.3 µg/L). Cr, Zn, and Mn exhibited increased DKD risk with concentrations exceeding corresponding thresholds across all stages of DKD. A strong U-shaped relationship was noted between predicted Mn and end DKD, with the risk of end DKD decreasing when Mn concentration was below 1.46 µg/L and reaching the highest risk when above 5.15 µg/L (p for non-linear both <0.05). Additionally, the trend of Cu in mid DKD was not significant, with Cu concentration in end DKD showing the lowest OR at 42.7 µg/L and highest risk at 72.4 µg/L.
Figure 1. Restricted cubic spline analysis of the risk association between DKD at different stages and urinary element concentrations. Restricted cubic spline plots from logistic regression models showing adjusted HRs and 95% CIs in elements concentration for DKD stages. Straight bars are used to show the distribution of each element in different DKD stages. The outliers of the mean ±3 SD are removed when the histogram is drawn. All models were adjusted by sex, BMI, age, smoking status, education, occupation, diabetes-specific complications, cardiovascular diseases, and other systemic diseases. BMI, body mass index; Cu, copper; Co, cobalt; Cr, chromium; DKD, diabetic kidney disease; Fe, iron; Mn, manganese; Ni, nickel; Se, selenium; V, vanadium; Zn, zinc.
Analysis between trace element mixture and DKD
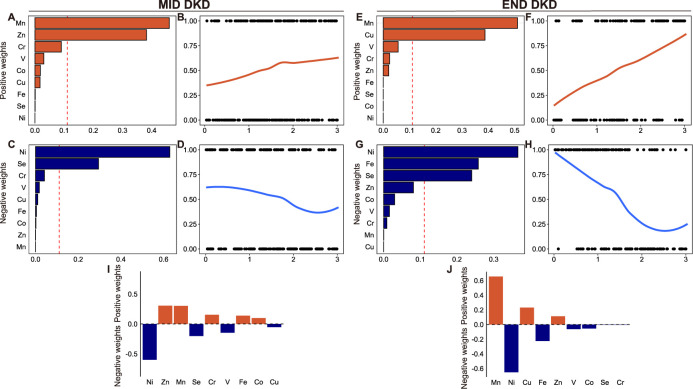
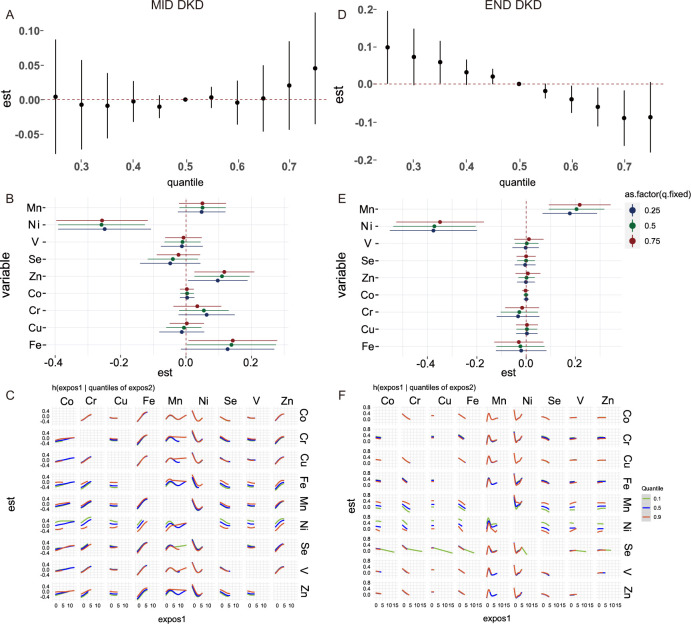
The results of the WQS index of the positive and negative weights with mid and end DKD are shown in figure 2. Mn (0.46) and Zn (0.38) are the two highest-weight contributors associated with mid DKD, whereas in end DKD, the main contributors are Mn (0.51) and Cs (0.39). Ni has the highest negative weight in both mid stage (0.63) and end stage (0.37). As demonstrated in (figure 2E), which revealed the weights of each element for QGcomp model indexes, Ni has the largest negative weight, followed by positive weights for Zn and Mn in mid DKD. Mn has the largest positive weight, followed by Ni with a negative weight in end DKD. In the mixture model of BKMR (figure 3), there is no significant correlation and trend between the mixture and the mid DKD. In end DKD, this study found that increasing the overall concentration of elements can reduce the risk of DKD. In the BKMR of the single element model, when other elements are fixed at the 25th, 50th, and 75th percentiles, Ni consistently showed a significant negative correlation in both DKD stages. Additionally, in end DKD, Mn also exhibited a significant positive correlation, and the negative correlation of Ni was not affected. Here, interactions between every two elements. When the concentration of Mn is fixed at the 90th percentile, the influence exposure trends of other elements become stable in mid DKD. However, in end DKD, this phenomenon was not observed; an interaction between Ni and Mn at the 90th percentile was identified instead.
Figure 2. Associations between the mixture and different stages of DKD by WQS and QGcomp. (A–D, F–I) The plot shows the distribution of estimated weights for each trace element and their association with mid and end stages of DKD with covariate adjusted. The red reference lines indicate the cut-off to distinguish elements with significant weights greater than zero. By default, the cut-off is set to the inverse of the number of elements in the mixture. (E, J) Baseline QGcomp model without bootstrapping with covariate adjusted. Red represents the positive weight; blue represents the negative weight. Covariates adjusted for sex, BMI, age, smoking status, education, occupation, diabetes-specific complications, cardiovascular diseases, and other systemic diseases. BMI, body mass index; Cu, copper; Co, cobalt; Cr, chromium; DKD, diabetic kidney disease; Fe, iron; Mn, manganese; Ni, nickel; QGcomp, Quantile G-Computation; Se, selenium; V, vanadium; WQS, Weighted Quantile Sum; Zn, zinc.
Figure 3. The BKMR analysis for associations between multiple trace elements and different DKD stages. (A, D) Overall effect of the element mixtures (Y-axis: the estimated change in risk of DKD and 95 % credible interval; X-axis: quantile of cumulative mixture). (B, E) Single element effect (Y-axis: different kinds of element; X-axis: the estimated change in risk of DKD and 95 % credible intervals). This plot compares associations of each element with DKD when a single element was at the 75th versus 25th percentile, when all the other elements were fixed at either the 25th (red line), 50th (green line), or 75th percentile (blue line). (C, F) Bivariate exposure-response functions for each of the elements presented on the right longitudinal axis when the other element presented on the upper coordinate axis holding at different quantiles (25th (red line), 50th (green line) and 75th (blue line)) and other three elements were held at the median (Y-axis: the estimated change in risk of DKD Covariates adjusted for sex, BMI, age, smoking status, education, occupation, diabetes-specific complications, cardiovascular diseases, and other systemic disease. BKMR, Bayesian Kernel Machine Regression; BMI, body mass index; Cu, copper; Co, cobalt; Cr, chromium; DKD, diabetic kidney disease; Fe, iron; Mn, manganese; Ni, nickel; Se, selenium; V, vanadium; Zn, zinc.
Discussion
In this study, we simultaneously measured nine trace elements from patients with different stages of DKD and evaluated the association between them, both in single and mixtures. Mn and Cu are positively correlated in all DKD stages, and Mn is still the most positively correlated element with the largest contribution and weight in the mixture model. Ni and Fe are the two elements negatively correlated in both mid and end DKD, Ni ranks first in the weight of the mixture, while Fe ranks second and has a significant correlation only in the end DKD. At the end DKD, there was a negative correlation trend between nine element mixtures and DKD. When the total mixture was lower than 50%, trace elements had a certain risk, which was mainly driven by Mn and Cu.
Recent epidemiological studies on kidney function suggest that besides genetic and lifestyle factors, sex and age show significant differences in CKD.27 The prevalence of DKD in this study was 48%, with no significant difference between men and women. This study used a case–control approach and employed the PSM method to individually match age and sex in mid and end DKD. Urine is a non-invasive and usually preferred biological monitoring medium, especially for some water-soluble trace elements.28 In the process of producing urine, the kidney is constantly exposed to and filters many toxins and pollutants, which makes it vulnerable to adverse effects. The levels of these trace elements in urine indirectly reflect the decline in kidney function, influencing the filtration and reabsorption of these elements.29 Fe deficiency anemia is a common comorbidity in CKD, found in 44% of our study. Clinically, Fe is often used to treat anemia associated with CKD,28 but excessive Fe storage can lead to infections and subsequently impair kidney function, causing abnormal Fe excretion.30 Experimental studies have observed that high exposure to Fe leads to increased urinary Fe excretion and kidney damage.31 In this study, Fe levels were found to be significantly lower in subjects with DKD, yet Fe served as a stronger protective factor against ESRD than for mid DKD. This might be due to kidney disease reducing the body’s overall Fe absorption.32 However, it is important to note that Fe²+, the active form involved in ferroptosis, can promote oxidative damage in DKD by triggering renal fibrosis and cell death. In contrast, Fe³+, stored in ferritin, is less harmful but may contribute to imbalances when improperly regulated. Ferroptosis has been proven to be a potential mechanism in the progression of DKD. Fe overload was found in DKD mice, leading to ferroptosis, which in turn triggers renal fibrosis and mediates renal cell death.33
Previous epidemiological and mechanistic studies have indicated that high exposure to Cu in both serum and urine can increase the risk of CKD and proteinuria, leading to poor kidney function.34 35 Previous studies have shown that excessive urinary Cu can also affect the development of DKD.36 Cu’s toxicity is largely due to its oxidative properties, especially in its Cu²+ form, which can catalyze the production of ROS, leading to oxidative stress and further kidney damage. The increase in urinary Cu excretion may be due to the dissociation of Cu from its carrier protein due to glomerular damage caused by acidification and kidney damage. An imbalance in Cu homeostasis may lead to impaired antioxidant capacity and the progression of DKD. Fe and Cu are both important biological oxidizing agents. They function as essential elements of SOD, which helps cells resist oxidative damage.37 However, when in overload, they can act as ROS and cause damage to renal tubular epithelial cells.34 However, the accumulation or reduction of element ions does not directly reflect ferroptosis or Cu death. The oxidation valence state of elements, such as Fe2+, promotes ferroptosis, while Fe3+ is usually an inert storage in ferritin. The toxicity of Cu also has different forms of toxicity compared with Cu2+. Therefore, future research needs to pay more attention to the determination of element valence states and the assessment of toxicity risks.
Cu is a cofactor of Cu, Zn-SOD, where Cu²+ directly participates in the catalytic cycle and reduces oxidative damage to cells, while Zn²+ plays a structural role and enhances stability. Zn plays a key role in regulating numerous cellular and subcellular processes in humans, such as DNA replication, energy metabolism, protein structure maintenance, and growth.38 In mid DKD, Zn levels are elevated, potentially as a protective response to renal stress, leveraging Zn’s antioxidant properties. Conversely, in end DKD, Zn levels decrease, signaling severe kidney dysfunction and impaired reabsorption of essential minerals.39 In advanced kidney disease, reduced renal function hampers Zn reabsorption, leading to lower urine Zn levels. Urinary Zn concentrations mirror kidney damage and dysfunction in DKD, with high levels possibly signifying compensatory responses in early stages and low levels indicating severe impairment in advanced stages.
A study has shown that supplementation with Se positively affects some inflammatory and oxidative stress markers in patients with DKD.40 41 Se is an essential trace element or metalloid that helps produce a variety of enzymes with antioxidant properties like glutathione peroxidase.42 A double-blind randomized controlled trial for Swedish elderly put forward a possible mechanism for the association between Se exposure and CKD that kidney function was dramatically improved with the supplemented effect of Se and coenzyme Q10.43
Mn is essential for enzymes like Mn SOD, protecting against oxidative stress in CKD and DKD, but its levels rise due to impaired kidney function, especially during dialysis, becoming a consistent risk factor for DKD.44 Conversely, Ni, despite its toxic potential in excess, plays a protective role at appropriate levels by modulating pathways related to renal disease development.45 and enhancing cellular defenses against oxidative stress and inflammation via the Nrf2/NLRP3 pathway.46 The pathway supports cellular resilience against environmental and metabolic stresses, which is critical in chronic conditions like DKD where oxidative stress and inflammation are prevalent. In an animal experiment, kidney damage indicators such as serum urea and creatinine were significantly increased in mice exposed to Ni alone, indicating that the kidneys were unable to eliminate these waste products, resulting in a decrease in the concentration of Ni in the urine.47 Research indicates that urinary metal levels, including Ni, are influenced by kidney function. In the middle and late stages of DKD, reduced glomerular filtration and tubular reabsorption may lead to lower urinary excretion of elements like Ni, which might not directly reflect a systemic reduction in body burden but rather impaired kidney function.48 Furthermore, Ni has been shown to activate antioxidant defenses, which can reduce oxidative stress, a key factor in the progression of DKD. This is consistent with studies suggesting that oxidative stress and inflammation are central to kidney damage in DKD, and protective effects from elements like Ni could vary across disease stages depending on the body’s oxidative stress response. Additionally, animal models and in vitro studies have shown that other elements and antioxidants play protective roles in reducing oxidative stress in CKD and diabetes-related conditions.49 50
Our study benefits from a large sample size and the use of PSM matching to assess the impact of nine urinary trace elements across various DKD stages. For the first time, we examine stage-specific variations in these elements and their disease progression risks. However, the cross-sectional design precludes establishing causality, and we did not differentiate between element valence states or metabolites. Although the recovery rates of trace elements Zn, Ni, and Se in urine were slightly below or above the ideal range, this study employed rigorous quality control measures, including within-day and between-day precision evaluations (with coefficients of variation, all within acceptable ranges) to ensure the robustness of the data.51 Moreover, urinary elements may not be ideal biomarkers for all substances, and our single measurement may not capture fluctuating levels. The hospital-based recruitment could introduce selection and confounding biases. Future research should use prospective cohorts and control for occupational exposures.
Conclusion
Trace elements play an important role in the different courses of DKD patients. This study explored nine trace elements in the urine of patients with mid-to-late DKD based on single and mixed analysis. In this study, it was found that Fe and Ni consistently exhibit a negative correlation in mid-to-late DKD patients, while Cu and Mn are positive correlation factors in the mid and end stages. In the mixture, the protective factor of Ni is stronger than Mn in the mid DKD. In the end DKD, the risk of Mn is greater. In addition, Mn also interacts with Ni, Se, and Cu in the mid DKD. Overall, elevated concentrations of multiple trace elements in urine can reduce the risk of DKD. Our findings may have important implications for disease prognosis and health management of DKD patients. Further research is needed in the future to verify our results and elucidate the potential mechanisms of the effects of multiple elements on the condition of DKD.
supplementary material
Footnotes
Funding: The authors would like to thank all the participants who consent to be enrolled in the study. This work was supported by the Funding of Zhongyuan Scholar Workstation (234400510024), Science and Technology Project of Science and Technology Department of Henan Province (242102311062), Medical Science and Technology Research Program of Henan Province (SBGJ202302002), and Science and Technology Project of Science and Technology Department of Henan Province (242102310299).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Ethics Committee of Henan Province People’s Hospital (Approval: 2021 Lunshen NO.78).
Contributor Information
Tianrui Gao, Email: gaotianruiii@163.com.
Jia Lv, Email: lvjia150313@163.com.
Lizhen Lu, Email: lulizhen1112@163.com.
Lijuan Guo, Email: 18855189118@163.com.
Weitian Tang, Email: tangwt0707@sina.com.
Fengmin Shao, Email: fengminshao@126.com.
Shiwei Zhu, Email: 13625557845@163.com.
Yuchen Zhang, Email: 1579141283@qq.com.
Ruiqi Jia, Email: 17856589075@163.com.
Jing Zhou, Email: jzhou128@126.com.
Chang Gao, Email: changgao1993@outlook.com.
Yue Gu, Email: guyuesunny@zzu.edu.cn.
Data availability statement
The data cannot be made publicly available due to privacy and ethical issue, and are available on request from the corresponding author.
References
- 1.Tervaert TWC, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–63. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Idowu AA, Ajose AO, Adedeji AT, et al. Microalbuminuria, Other Markers of Nephropathy and Biochemical Derangementsin Type 2 Diabetes Mellitus: Relationships and Determinants. Ghana Med J. 2017;51:56–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 7.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature New Biol. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Liu F, Zhang X, et al. Benefits and risks of essential trace elements in chronic kidney disease: a narrative review. Ann Transl Med. 2022;10:1400. doi: 10.21037/atm-22-5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CC, Huang YL. Chromium, zinc and magnesium status in type 1 diabetes. Curr Opin Clin Nutr Metab Care. 2015;18:588–92. doi: 10.1097/MCO.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 10.Tapiero H, Tew KD. Trace elements in human physiology and pathology: zinc and metallothioneins. Biomedicine & Pharmacotherapy. 2003;57:399–411. doi: 10.1016/S0753-3322(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 11.Prasad AS. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu L, Yan J, Zhang Q, et al. Association between Serum Ferritin and Blood Lipids: Influence of Diabetes and hs-CRP Levels. J Diabetes Res. 2020;2020:4138696. doi: 10.1155/2020/4138696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo EF, Li HX, Qin YH, et al. Role of ferroptosis in the process of diabetes-induced endothelial dysfunction. World J Diabetes. 2021;12:124–37. doi: 10.4239/wjd.v12.i2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Timimi DJ, Sulieman DM, Hussen KR. Zinc status in type 2 diabetic patients: relation to the progression of diabetic nephropathy. J Clin Diagn Res. 2014;8:CC04–8. doi: 10.7860/JCDR/2014/10090.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C-C, Shih C-T, Lee C-H, et al. Changes in Trace Elements During Early Stages of Chronic Kidney Disease in Type 2 Diabetic Patients. Biol Trace Elem Res. 2018;186:330–6. doi: 10.1007/s12011-018-1314-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Lee J, Kim K-N, et al. Association between Dietary Mineral Intake and Chronic Kidney Disease: The Health Examinees (HEXA) Study. IJERPH. 2018;15:1070. doi: 10.3390/ijerph15061070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Stojsavljević A, Ristić-Medić D, Krstić Đ, et al. Circulatory Imbalance of Essential and Toxic Trace Elements in Pre-dialysis and Hemodialysis Patients. Biol Trace Elem Res. 2022;200:3117–25. doi: 10.1007/s12011-021-02940-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonelli M, Wiebe N, Hemmelgarn B, et al. Trace elements in hemodialysis patients: a systematic review and meta-analysis. BMC Med. 2009;7:25. doi: 10.1186/1741-7015-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasama RK. Trace minerals in patients with end-stage renal disease. Semin Dial. 2010;23:561–70. doi: 10.1111/j.1525-139X.2010.00793.x. [DOI] [PubMed] [Google Scholar]
- 21.Deng M, Gao T, Tao L, et al. Are human exposure assessment the same for non-persistent organic chemicals? -from the lens of urinary variability and predictability. Sci Total Environ. 2023;868:161542. doi: 10.1016/j.scitotenv.2023.161542. [DOI] [PubMed] [Google Scholar]
- 22.Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442–58. doi: 10.1002/dmrr.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, Shen Z, Gu W, et al. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes Metab. 2023;25:3390–9. doi: 10.1111/dom.15238. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Li X, Zhang W, et al. Aluminum Exposure and Gestational Diabetes Mellitus: Associations and Potential Mediation by n-6 Polyunsaturated Fatty Acids. Environ Sci Technol. 2020;54:5031–40. doi: 10.1021/acs.est.9b07180. [DOI] [PubMed] [Google Scholar]
- 25.Muscat JE, Liu A, Richie JP., Jr A comparison of creatinine vs. specific gravity to correct for urinary dilution of cotinine. Biomarkers. 2011;16:206–11. doi: 10.3109/1354750X.2010.538084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duty SM, Calafat AM, Silva MJ, et al. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20:604–10. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- 27.Bodhini D, Morton RW, Santhakumar V, et al. Impact of individual and environmental factors on dietary or lifestyle interventions to prevent type 2 diabetes development: a systematic review. Commun Med (Lond) 2023;3:133. doi: 10.1038/s43856-023-00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S. Occupational, environmental and lifestyle factors associated with spontaneous abortion. Reprod Sci. 2011;18:915–30. doi: 10.1177/1933719111413298. [DOI] [PubMed] [Google Scholar]
- 29.Kang E, Han M, Kim H, et al. Baseline General Characteristics of the Korean Chronic Kidney Disease: Report from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD) J Korean Med Sci. 2017;32:221–30. doi: 10.3346/jkms.2017.32.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sonnweber T, Theurl I, Seifert M, et al. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant. 2011;26:977–87. doi: 10.1093/ndt/gfq483. [DOI] [PubMed] [Google Scholar]
- 31.van Raaij SEG, Rennings AJ, Biemond BJ, et al. Iron handling by the human kidney: glomerular filtration and tubular reabsorption both contribute to urinary iron excretion. Am J Physiol Renal Physiol. 2019;316:F606–14. doi: 10.1152/ajprenal.00425.2018. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Zhuang J, Chen Q, et al. Chronic exposure to polyvinyl chloride microplastics induces liver injury and gut microbiota dysbiosis based on the integration of liver transcriptome profiles and full-length 16S rRNA sequencing data. Sci Total Environ. 2022;839:155984. doi: 10.1016/j.scitotenv.2022.155984. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wang J. Ferroptosis, a Rising Force against Renal Fibrosis. Oxid Med Cell Longev. 2022;2022:1–12. doi: 10.1155/2022/7686956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kung WJ, Shih CT, Lee CH, et al. The Divalent Elements Changes in Early Stages of Chronic Kidney Disease. Biol Trace Elem Res. 2018;185:30–5. doi: 10.1007/s12011-017-1228-3. [DOI] [PubMed] [Google Scholar]
- 35.Tsai CC, Wu CL, Kor CT, et al. Prospective associations between environmental heavy metal exposure and renal outcomes in adults with chronic kidney disease. Nephrology (Carlton) 2018;23:830–6. doi: 10.1111/nep.13089. [DOI] [PubMed] [Google Scholar]
- 36.Gembillo G, Labbozzetta V, Giuffrida AE, et al. Potential Role of Copper in Diabetes and Diabetic Kidney Disease. Metabolites. 2022;13 doi: 10.3390/metabo13010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng Y, Abreu IA, Cabelli DE, et al. Superoxide dismutases and superoxide reductases. Chem Rev. 2014;114:3854–918. doi: 10.1021/cr4005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Damianaki K, Lourenco JM, Braconnier P, et al. Renal handling of zinc in chronic kidney disease patients and the role of circulating zinc levels in renal function decline. Nephrol Dial Transplant. 2020;35:1163–70. doi: 10.1093/ndt/gfz065. [DOI] [PubMed] [Google Scholar]
- 40.Singh R, Barden A, Mori T, et al. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 41.Bahmani F, Kia M, Soleimani A, et al. The effects of selenium supplementation on biomarkers of inflammation and oxidative stress in patients with diabetic nephropathy: a randomised, double-blind, placebo-controlled trial - Expression of concern. Br J Nutr. 2022;127:155. doi: 10.1017/S000711452100204X. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias P, Selgas R, Romero S, et al. Selenium and kidney disease. J Nephrol. 2013;26:266–72. doi: 10.5301/jn.5000213. [DOI] [PubMed] [Google Scholar]
- 43.Alehagen U, Aaseth J, Alexander J, et al. Selenium and Coenzyme Q10 Supplementation Improves Renal Function in Elderly Deficient in Selenium: Observational Results and Results from a Subgroup Analysis of a Prospective Randomised Double-Blind Placebo-Controlled Trial. Nutrients. 2020;12:3780. doi: 10.3390/nu12123780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287:13541–8. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad MSA, Ashraf M. Essential roles and hazardous effects of nickel in plants. Rev Environ Contam Toxicol. 2011;214:125–67. doi: 10.1007/978-1-4614-0668-6_6. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Dai X, Hu S, et al. Nickel Induces Pyroptosis via the Nrf2/NLRP3 Pathway in Kidney of Mice. Biol Trace Elem Res. 2024;202:3248–57. doi: 10.1007/s12011-023-03922-7. [DOI] [PubMed] [Google Scholar]
- 47.Lin X-Y, Liang J-H, Jiao D-D, et al. Using Fe biofortification strategies to reduce both Ni concentration and oral bioavailability for rice with high Ni. J Hazard Mater. 2023;452:131367. doi: 10.1016/j.jhazmat.2023.131367. [DOI] [PubMed] [Google Scholar]
- 48.Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–36. doi: 10.1038/s41581-020-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koya D, Hayashi K, Kitada M, et al. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:S250–3. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Quan F, Cao Q, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2021;28:231–43. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linsinger TPJ. Use of recovery and bias information in analytical chemistry and estimation of its uncertainty contribution. TrAC. 2008;27:916–23. doi: 10.1016/j.trac.2008.08.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data cannot be made publicly available due to privacy and ethical issue, and are available on request from the corresponding author.