Abstract
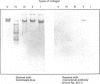
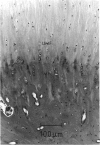
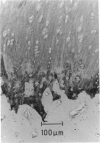
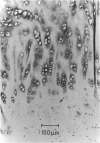
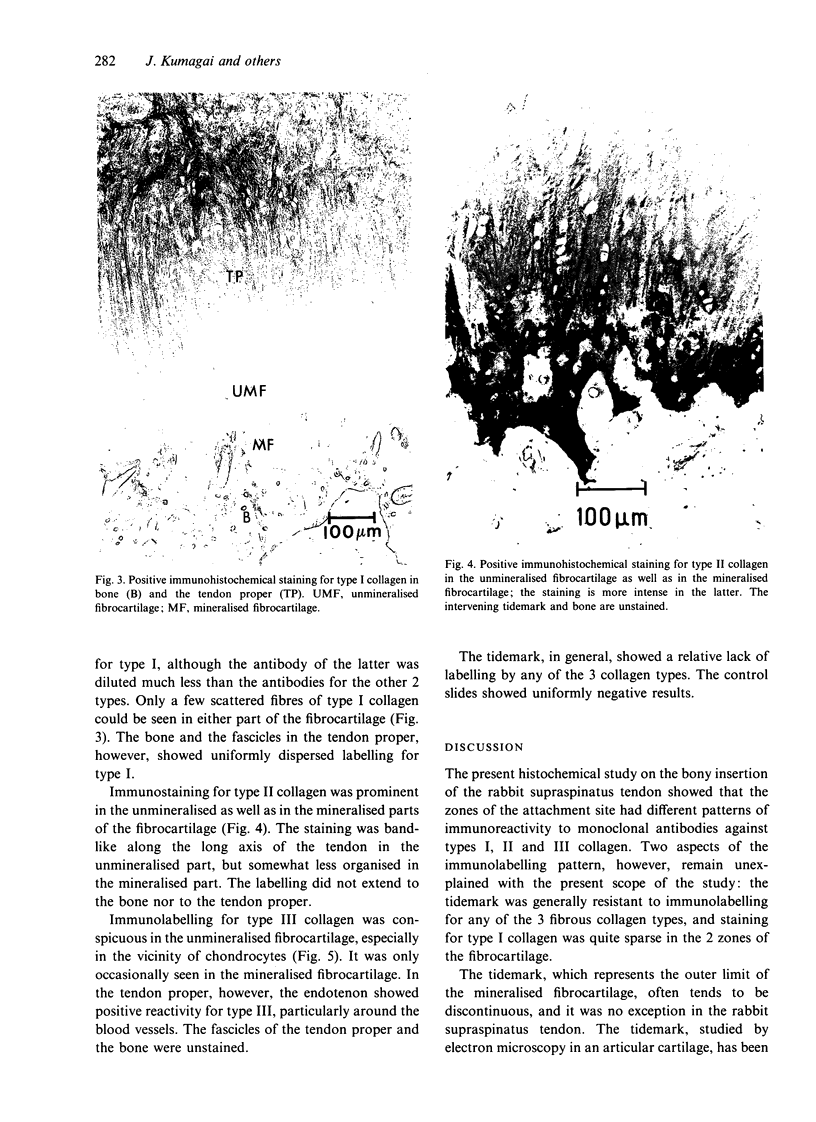
The collagen fibres in the area of attachment of the supraspinatus tendon to bone were studied immunohistochemically in 12 mature female New Zealand white rabbits. The labelling of type I collagen was uniformly prominent in the bone as well as in the fascicles of the tendon proper but inconspicuously scattered in the unmineralised and mineralised zones of the fibrocartilage. Type II collagen, not detected in the tendon proper, was widespread in both zones of the fibrocartilage. Type III collagen, on the other hand, appeared to be confined mainly to the zone of unmineralised fibrocartilage, in addition to its presence in the endotenon of the tendon proper. The region of the tidemark failed to show immunostaining for any of the collagen fibre types. In conclusion, this study demonstrates that, although all the principal fibrous collagen types are constituents of the supraspinatus tendon at its attachment site, the distribution pattern of immunolabelling varies from zone to zone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin M., Evans E. J., Copp L. The histology of tendon attachments to bone in man. J Anat. 1986 Dec;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Evans E. J. Fibrocartilage. J Anat. 1990 Aug;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M., Tyers R. N., Ralphs J. R. Age-related changes in tendon fibrocartilage. J Anat. 1991 Dec;179:127–136. [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Nimni M. E. Collagen types. Molecular structure and tissue distribution. Clin Orthop Relat Res. 1992 Sep;(282):250–272. [PubMed] [Google Scholar]
- Cetta G., Tenni R., Zanaboni G., De Luca G., Ippolito E., De Martino C., Castellani A. A. Biochemical and morphological modifications in rabbit Achilles tendon during maturation and ageing. Biochem J. 1982 Apr 15;204(1):61–67. doi: 10.1042/bj2040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. R., Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am. 1970 Jan;52(1):1–20. [PubMed] [Google Scholar]
- Evans E. J., Benjamin M., Pemberton D. J. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. J Anat. 1990 Aug;171:155–162. [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Yankowski R., Bashey R. I. Identification of two new collagen alpha-chains in extracts of lathyritic chick embryo tendons. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1298–1306. doi: 10.1016/0006-291x(78)91277-9. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ralphs J. R., Tyers R. N., Benjamin M. Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anat Embryol (Berl) 1992;185(2):181–187. doi: 10.1007/BF00185920. [DOI] [PubMed] [Google Scholar]
- Redler I., Mow V. C., Zimny M. L., Mansell J. The ultrastructure and biomechanical significance of the tidemark of articular cartilage. Clin Orthop Relat Res. 1975 Oct;(112):357–362. [PubMed] [Google Scholar]
- Rentrop M., Knapp B., Winter H., Schweizer J. Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986 May;18(5):271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- Rhodes R. K., Miller E. J. Physicochemical characterization and molecular organization of the collagen A and B chains. Biochemistry. 1978 Aug 22;17(17):3442–3448. doi: 10.1021/bi00610a003. [DOI] [PubMed] [Google Scholar]
- Rufai A., Benjamin M., Ralphs J. R. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl) 1992 Dec;186(6):611–618. doi: 10.1007/BF00186984. [DOI] [PubMed] [Google Scholar]
- Sakakibara K., Ooshima A., Igarashi S., Sakakibara J. Immunolocalization of type III collagen and procollagen in cirrhotic human liver using monoclonal antibodies. Virchows Arch A Pathol Anat Histopathol. 1986;409(1):37–46. doi: 10.1007/BF00705405. [DOI] [PubMed] [Google Scholar]
- Yulis C. R., Lederis K. Co-localization of the immunoreactivities of corticotropin-releasing factor and arginine vasotocin in the brain and pituitary system of the teleost Catostomus commersoni. Cell Tissue Res. 1987 Feb;247(2):267–273. doi: 10.1007/BF00218308. [DOI] [PubMed] [Google Scholar]