Abstract
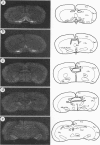
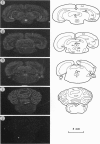
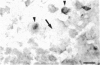
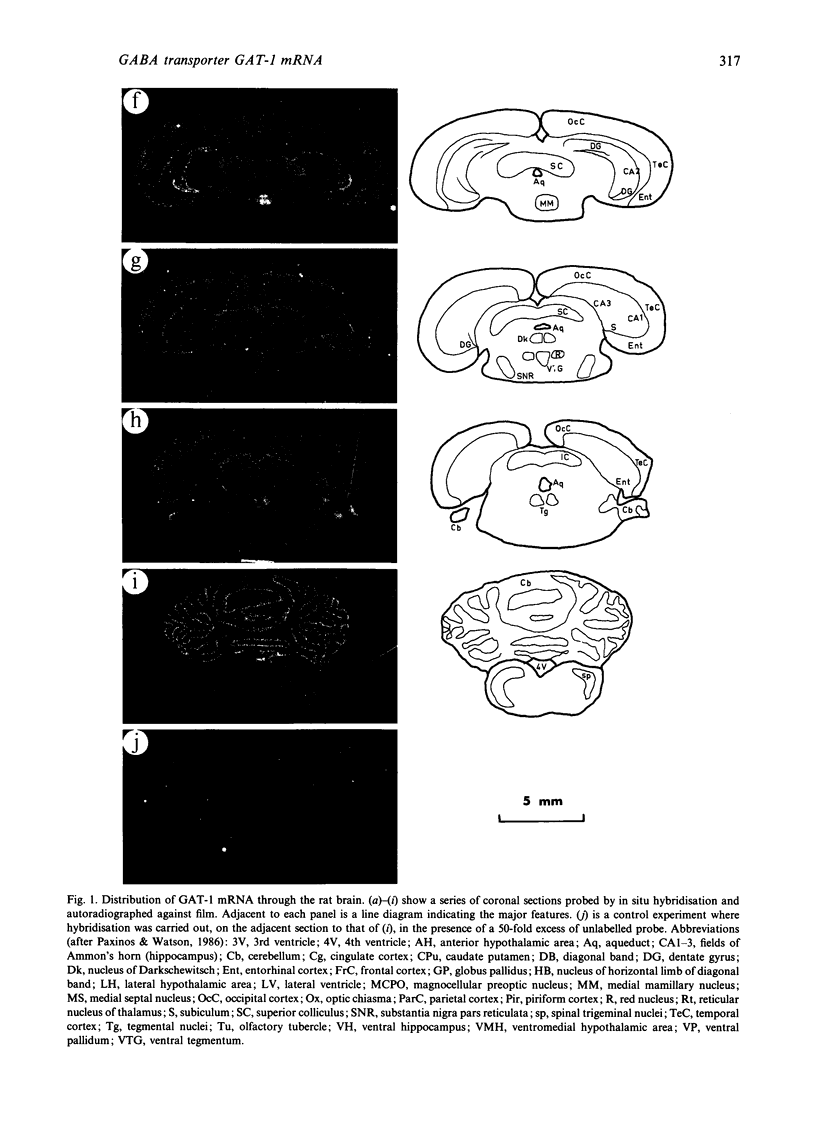
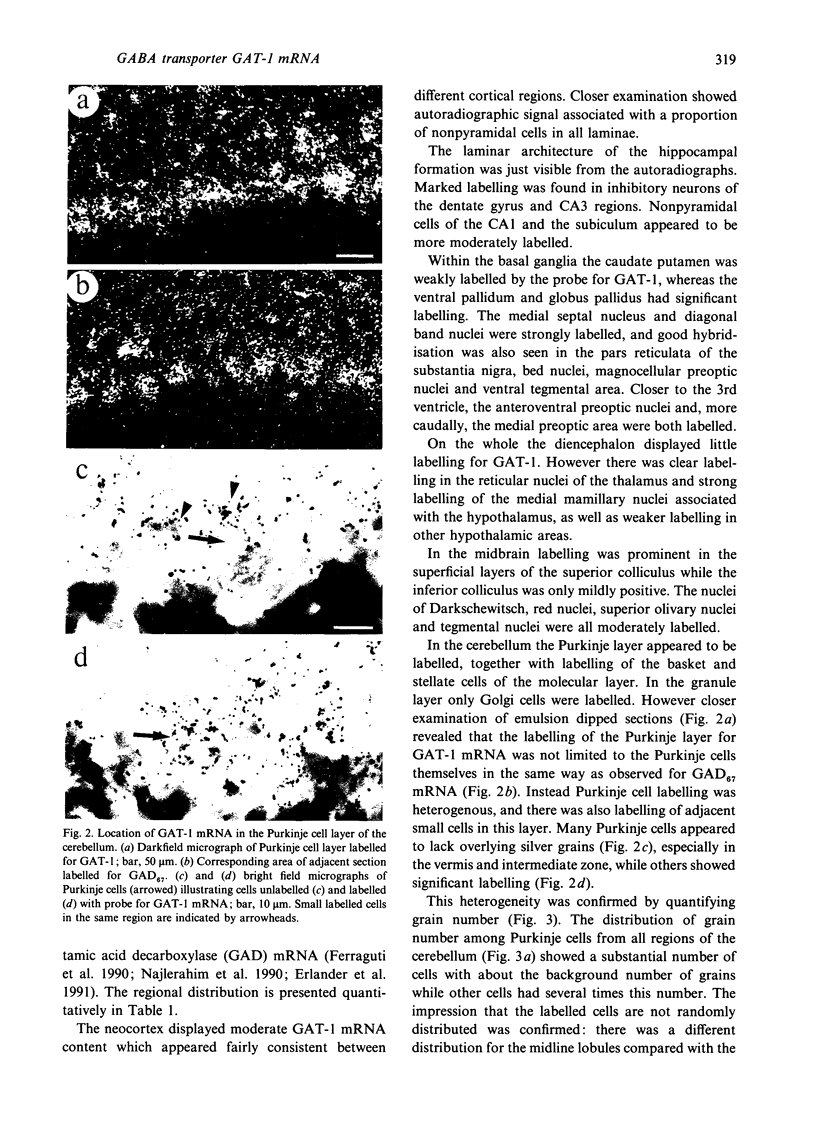
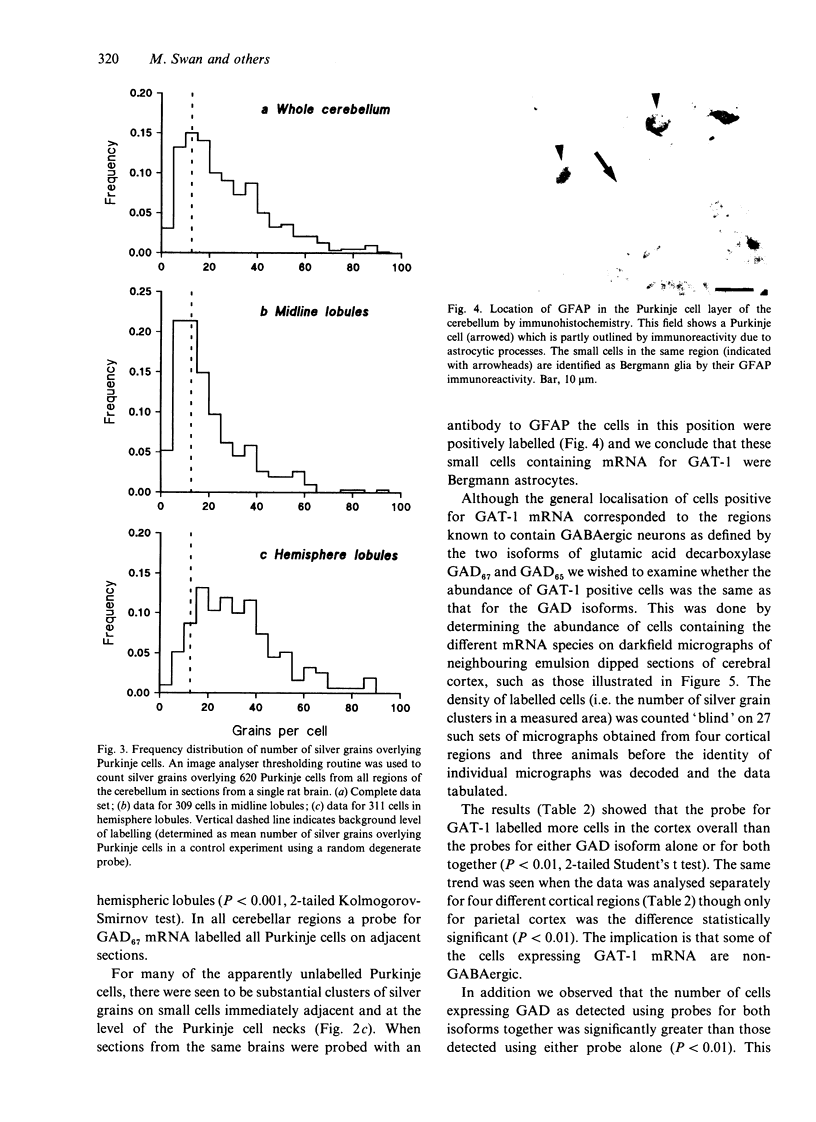
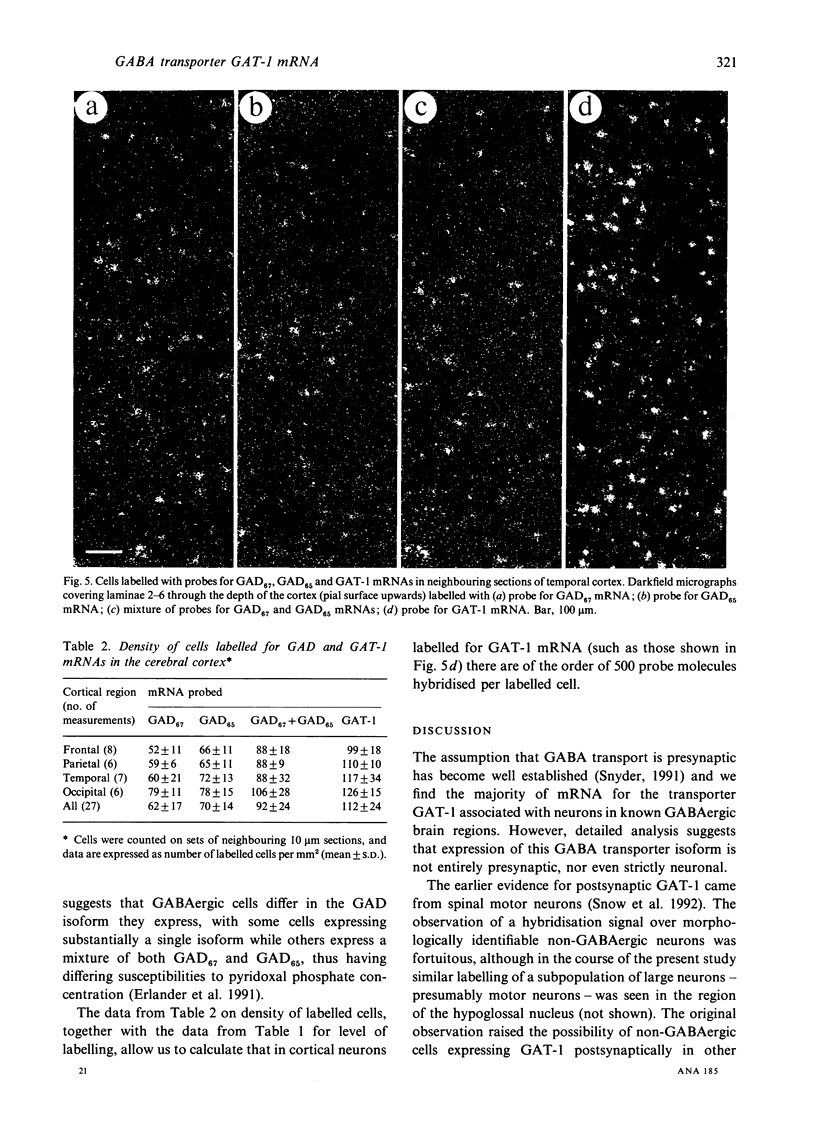
Cells containing mRNA for the gamma-aminobutyric acid (GABA) transporter GAT-1 were identified in rat brain by in situ hybridisation. They were found in most of the known locations of GABAergic neurons, as defined by the distribution of mRNA for glutamic acid decarboxylase, the synthetic enzyme for GABA. Within the cerebellum there was substantial labelling of basket and stellate cells in the molecular layer, and of Golgi cells but no others in the granule cell layer. Many Purkinje cells were unlabelled while others, particularly in the hemispheres, were moderately labelled. Many of the Purkinje cells negative for GAT-1 mRNA had adjacent intensely labelled small cells whose size and position corresponded to Bergmann glia. Numerical comparison of cells labelling for GAT-1 mRNA and the mRNAs for the two known isoforms of glutamic acid decarboxylase were made on serial sections of cerebral cortex. Cells positive for GAT-1 mRNA were more numerous, indicating that expression of the transporter is not just limited to GABAergic cells and we suggest that it may also be expressed postsynaptically by some non-GABAergic neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahima R. S., Tagoe C. N., Harlan R. E. Type II corticosteroid receptor-like immunoreactivity in the rat cerebellar cortex: differential regulation by corticosterone. Neuroendocrinology. 1992 Jun;55(6):683–694. doi: 10.1159/000126188. [DOI] [PubMed] [Google Scholar]
- Amara S. G. Neurotransmitter transporters. A tale of two families. Nature. 1992 Dec 3;360(6403):420–421. doi: 10.1038/360420d0. [DOI] [PubMed] [Google Scholar]
- Bennett J. P., Lowrie M. B. Are neurotransmitter carriers cell-specific markers? Trends Neurosci. 1992 Dec;15(12):483–484. doi: 10.1016/0166-2236(92)90096-q. [DOI] [PubMed] [Google Scholar]
- Blakely R. D., Berson H. E., Fremeau R. T., Jr, Caron M. G., Peek M. M., Prince H. K., Bradley C. C. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991 Nov 7;354(6348):66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Wu J. Y. Sagittal cerebellar microbands of taurine neurons: immunocytochemical demonstration by using antibodies against the taurine-synthesizing enzyme cysteine sulfinic acid decarboxylase. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4221–4225. doi: 10.1073/pnas.79.13.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuénod M., Do K. Q., Grandes P., Morino P., Streit P. Localization and release of homocysteic acid, an excitatory sulfur-containing amino acid. J Histochem Cytochem. 1990 Dec;38(12):1713–1715. doi: 10.1177/38.12.2254641. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tillakaratne N. J., Feldblum S., Patel N., Tobin A. J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991 Jul;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Ferraguti F., Zoli M., Aronsson M., Agnati L. F., Goldstein M., Filer D., Fuxe K. Distribution of glutamic acid decarboxylase messenger RNA-containing nerve cell populations of the male rat brain. J Chem Neuroanat. 1990 Sep-Oct;3(5):377–396. [PubMed] [Google Scholar]
- Gallo V., Upson L. M., Hayes W. P., Vyklicky L., Jr, Winters C. A., Buonanno A. Molecular cloning and development analysis of a new glutamate receptor subunit isoform in cerebellum. J Neurosci. 1992 Mar;12(3):1010–1023. doi: 10.1523/JNEUROSCI.12-03-01010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Hawkes R. Antigenic markers of cerebellar modules in the adult mouse. Biochem Soc Trans. 1992 May;20(2):391–395. doi: 10.1042/bst0200391. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kaufman D. L., Tobin A. J. Glutamic acid decarboxylase cDNA: nucleotide sequence encoding an enzymatically active fusion protein. J Neurosci. 1987 Sep;7(9):2768–2772. doi: 10.1523/JNEUROSCI.07-09-02768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T., Tauchi M., Dahl J. L. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1988;70(3):605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Larsson O. M., Griffiths R., Allen I. C., Schousboe A. Mutual inhibition kinetic analysis of gamma-aminobutyric acid, taurine, and beta-alanine high-affinity transport into neurons and astrocytes: evidence for similarity between the taurine and beta-alanine carriers in both cell types. J Neurochem. 1986 Aug;47(2):426–432. doi: 10.1111/j.1471-4159.1986.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H., Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992 Mar;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. R., López-Corcuera B., Mandiyan S., Nelson H., Nelson N. Molecular characterization of four pharmacologically distinct gamma-aminobutyric acid transporters in mouse brain [corrected]. J Biol Chem. 1993 Jan 25;268(3):2106–2112. [PubMed] [Google Scholar]
- Lolova I., Davidoff M. Immuno- and histochemical data on changed GABA transmission in aged rat cerebellum. J Hirnforsch. 1990;31(4):423–428. [PubMed] [Google Scholar]
- Lopez-Corcuera B., Liu Q. R., Mandiyan S., Nelson H., Nelson N. Expression of a mouse brain cDNA encoding novel gamma-aminobutyric acid transporter. J Biol Chem. 1992 Sep 5;267(25):17491–17493. [PubMed] [Google Scholar]
- Monyer H., Seeburg P. H., Wisden W. Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing. Neuron. 1991 May;6(5):799–810. doi: 10.1016/0896-6273(91)90176-z. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Harrison P. J., Barton A. J., Heffernan J., Pearson R. C. Distribution of messenger RNAs encoding the enzymes glutaminase, aspartate aminotransferase and glutamic acid decarboxylase in rat brain. Brain Res Mol Brain Res. 1990 May;7(4):317–333. doi: 10.1016/0169-328x(90)90082-o. [DOI] [PubMed] [Google Scholar]
- Ortega A., Eshhar N., Teichberg V. I. Properties of kainate receptor/channels on cultured Bergmann glia. Neuroscience. 1991;41(2-3):335–349. doi: 10.1016/0306-4522(91)90331-h. [DOI] [PubMed] [Google Scholar]
- Radian R., Ottersen O. P., Storm-Mathisen J., Castel M., Kanner B. I. Immunocytochemical localization of the GABA transporter in rat brain. J Neurosci. 1990 Apr;10(4):1319–1330. doi: 10.1523/JNEUROSCI.10-04-01319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray M., Priestley J. V. Differential expression of GABA transporter-1 messenger RNA in subpopulations of GABA neurones. Neurosci Lett. 1993 Jun 25;156(1-2):163–166. doi: 10.1016/0304-3940(93)90463-u. [DOI] [PubMed] [Google Scholar]
- Snow H., Lowrie M. B., Bennett J. P. A postsynaptic GABA transporter in rat spinal motor neurones. Neurosci Lett. 1992 Aug 31;143(1-2):119–122. doi: 10.1016/0304-3940(92)90246-4. [DOI] [PubMed] [Google Scholar]
- Snyder S. H. Neuroscience. Vehicles of inactivation. Nature. 1991 Nov 21;354(6350):187–187. doi: 10.1038/354187a0. [DOI] [PubMed] [Google Scholar]
- Uhl G. R. Neurotransmitter transporters (plus): a promising new gene family. Trends Neurosci. 1992 Jul;15(7):265–268. doi: 10.1016/0166-2236(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Wisden W., McNaughton L. A., Darlison M. G., Hunt S. P., Barnard E. A. Differential distribution of GABAA receptor mRNAs in bovine cerebellum--localization of alpha 2 mRNA in Bergmann glia layer. Neurosci Lett. 1989 Nov 20;106(1-2):7–12. doi: 10.1016/0304-3940(89)90193-6. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Davies M. Regulation of the gamma-aminobutyric acidA receptor by gamma-aminobutyric acid levels within the postsynaptic cell. J Neurochem. 1989 Nov;53(5):1648–1651. doi: 10.1111/j.1471-4159.1989.tb08565.x. [DOI] [PubMed] [Google Scholar]