Abstract
This article proposes a novel approach for improving the efficiency of fragrance designing and the accuracy of automatic fragrance formula creation based on empirical fragrance formulas and graph traversal algorithms. By effectively extracting the composition information and further analyzing the combination of fragrance materials in 210 fragrance formulas, a relational network model was constructed in the form of a graph to illustrate the relationship between the ingredients used in the formulas. Additionally, a fragrance ingredients information database of 344 common ingredients was constructed and used as a reference for perfumers when setting algorithmic constraints based on their experience. Finally, an automatic fragrance formula creation algorithm was established by constructing a relational network subgraph and finding fragrance formula solutions with the help of depth-first search algorithm that satisfies the constraint conditions and combining with appropriate statistical strategy that could determine the usage of each component in the new fragrance formula. By testing the algorithm with the goal of creating a floral fragrance, the resulting formula well fulfilled our expectations and had practical application value.
Keywords: Fragrance formula, Automatic formula creation, Traversal algorithm, Olfactory profiles
1. Introduction
Fragrances are integral to the marketability of various consumer products ranging from perfumes to detergents and personal care products [1]. Their significance stems from the fact that fragrances serve as a distinguishing factor for these products, influencing consumers' purchasing and repurchasing decisions [2,3]. However, fragrance design has traditionally been a time-consuming and labor-intensive process that relies on a perfumer's experience and creativity [4]. And fragrance formulas have long been considered the cornerstone of the fragrance industry.
With the rapid development of scientific technology, fragrance research has benefited greatly from the widespread application of chemical component separation, analytical techniques, chemometrics multivariate analysis techniques, and artificial intelligence and computer technology. These advancements have provided a systematic way to optimize the fragrance design process [[5], [6], [7], [8]]. At the same time, the flavor and fragrance industry has made significant progress, with many mature aroma components that have undergone long-term experimentation and optimization, making them valuable data resources [7,9,10]. In this context, the utilization of product engineering in fragrance design provides a valuable means to incorporate scientific knowledge into an area that has traditionally relied solely on empiricism and experimentation [[11], [12], [13]].
As our understanding of fragrance design has advanced, it has become increasingly clear that empirical fragrance formulas developed by fragrance specialists can be represented as a data structure resembling a knowledge graph, where nodes represent fragrance raw materials and edges reflect their relationships. This graph-like structure offers a useful means to analyze and optimize fragrance formulas through the application of computational methods. Graph traversal algorithms can facilitate the identification of intrinsic connections among various materials in empirical fragrance formulas, enabling systematic and insightful analysis of the empirical formula data [[14], [15], [16]]. This, in turn, provides a foundation for the automatic generation of novel fragrance formulas through the application of computational tools. Among various graph algorithms, the Depth First Search (DFS) algorithm is considered a fundamental graph traversal algorithm, renowned for its efficacy in solving graph traversal problems [17,18]. Furthermore, DFS serves as a building block for other graph theory algorithms, including graph connectivity problems, topological sorting, and bipartite graph matching, among others [[19], [20], [21]]. Its pivotal role in addressing graph-related problems and broader implications in the field ensure its ongoing importance.
In order to improve the efficiency of fragrance design, this paper aims to comprehensively investigate the characteristics of flavor materials and the corresponding empirical fragrance formulas. With the aid of computers, empirical fragrance formulas can be represented as a relationship graph model, and the DFS algorithm leveraged to transform the fragrance goal into a graph traversal constraint. This approach enables the construction of a relationship subgraph that satisfies the imposed constraint, thereby facilitating the generation of new fragrance formulas. Additionally, suitable statistical methods can be employed to determine the optimal dosage of each fragrance component in the newly generated formula, resulting in an intelligent and automatic fragrance design process.
2. Materials and methods
2.1. Description of the study area and study period
The experiment was conducted in Changsha, Hunan Province, China. Geographically, it lies in the central-south part of China at approximately 28°12′ N latitude and 112°59′ E longitude. The region experiences an average annual rainfall ranging from 1300 to 1500 mm, with a primary rainy season from April to June and a secondary rainy period from August to September. Changsha's elevation is about 38 m above sea level, and it has an average annual temperature of approximately 18 °C. This study, conducted from January 2022 to July 2023, encompassed multiple seasons and regional climate variations. This timeframe allowed for a thorough observation of seasonal environmental fluctuations and their impact on the research objectives.
2.2. Materials
All fragrance ingredients and solvents used in the fragrances design process were food grade and supplied by Aladdin Reagent (Shanghai) Co., Ltd., China. The equipment used included an electronic analytical balance (sensitivity 0.0001g, Mettler Toledo X3603SX, Switzerland).
2.3. Olfactory evaluations
In the sensory evaluation, 18 expert panelists (12 women and 6 men, all non-smokers), aged 20–55 years, were employed. These evaluations took place across two consecutive days in a controlled environment with stable temperature and consistent lighting conditions. The evaluation sessions were scheduled at the same time each day for all samples and stimuli, ensuring minimal variation in conditions. Each of the 18 sensory attributes, which represent distinct olfactory families, was assessed for every sample. These attributes included hay, resinous, fresh, fruity, woody, roasted, spicy, green, herbal, floral, chocolatey, beany, milky, sweet, balsamic, smoky, winy, and sour. Panelists rated the intensity of each attribute on a 0–9 scale, with 1-point increments. The specific criteria for these ratings were detailed in Table 1 of the study. To maintain objectivity, a blinded assessment approach was adopted, where each sample was identified only by a unique 3-digit code. The sequence of sample evaluation was randomized. For statistical robustness, outlier screening was conducted using JMP software. The ratings for each attribute were then averaged using arithmetic mean to derive the final scores for each sample [1].
Table 1.
Scoring criteria of olfactory evaluation.
| Sensory intensity | Score |
|---|---|
| None | 0 |
| Weak | 1∼2 |
| Slightly weak | 3∼4 |
| Moderate | 5 |
| Slightly strong | 6∼7 |
| Strong | 8∼9 |
2.4. Depth-first search algorithm
The depth-first search (DFS) algorithm is a recursive algorithm that follows a path as far as possible, and backtracks when a dead end is reached, i.e., when all the neighbors of a given node have already been visited. By doing so, DFS visits all the nodes in the graph in a depth-first order, which can be useful for many different types of problems [17,18]. The DFS algorithm has been extensively studied and applied in various domains, such as computer science, mathematics, physics, biology, and social sciences [22]. Many researchers have explored different aspects of the DFS algorithm, such as its time and space complexity, correctness and completeness properties, practical implementations, and extensions and variations [18]. The DFS algorithm can be implemented using either a recursive approach or an iterative approach with an explicit stack [23]. In both cases, the algorithm consists of four main steps.
-
1.
Initialization: The algorithm begins by selecting a starting node and initializing a stack or a recursive call with this node. In addition, a Boolean array or set is used to keep track of the visited nodes.
-
2.
Recursive Procedure: In the recursive approach, the algorithm recursively visits each neighbor of the current node until there are no unvisited nodes left. The recursive procedure involves marking a node as visited and then recursively visiting all its unvisited neighbors. In the iterative approach, the algorithm uses an explicit stack to maintain a list of the nodes to be visited. As each node is visited, its unvisited neighbors are added to the stack.
-
3.
Marking of Visited Nodes: After visiting a node, the algorithm marks it as visited, usually using a Boolean array or set.
-
4.
Backtracking: If the current node has no unvisited neighbors, the algorithm backtracks to the previous node and continues the search process from there until all nodes have been visited.
2.5. Construction of fragrance ingredients information database (FIID)
The core of this article focuses on the fragrance formulation algorithm, which incorporates both qualitative and quantitative data of fragrance ingredients, as well as empirical fragrance formulas. The qualitative and quantitative data of fragrance ingredients used in intelligent fragrance creation include their names, material types (synthetic or natural), solubility, vapor pressure at 25 °C, olfactory profiles, odor threshold, and type of notes (top, middle, or base) [10,[24], [25], [26]]. By collecting and compiling these descriptive indicators for 344 fragrance ingredients, including 158 natural and 186 synthetic ingredients commonly used within the food industry and our own company, a Fragrance Ingredients Information Database (FIID) was ultimately established for calling upon the digital fragrance formulation algorithm. FIID serves mainly as a point of reference for perfumers to establish constraints for subsequent digital fragrance formulation algorithms, as well as to provide targeted directions for perfumers based on the evaluation results of digital fragrance formulas. As an example, Table 2 illustrates the basic information of linalool.
Table 2.
Descriptive indicators of linalool.
| Number | Item | Details |
|---|---|---|
| 1 | Material name | Linalool |
| 2 | Material type | Synthesized ingredient |
| 3 | Solubility | Soluble in ethanol and 1,2-propanediol |
| 4 | Vapor pressure | 0.17 mmHg (25 °C) |
| 5 | Olfactory profiles | 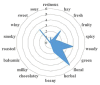 |
| 6 | Odor threshold | 6 ppba |
| 7 | Type of note | Middle note |
Parts(mL) of compound per billion(108) parts(mL) of water [27].
2.6. Construction of empirical formulas database
We collected 210 empirical fragrance formulas based on our company's existing products. These were adjusted and verified by perfumers and stored as empirical formulas in a fragrance database, with the main odors and their intensity scores, ranked in the top three, as the labels. This database was designed to establish a relationship diagram of ingredients (Table 3). An example of the formula composition for empirical formula 1# is shown in Table 4.
Table 3.
Empirical fragrance formulas used in fragrance database.
| Formula number | Main odors | Formula number | Main odors | Formula number | Main odors |
|---|---|---|---|---|---|
| 1# | Sweet, fruity, milky | 71# | Hay, sweet, fresh | 141# | Fruity, sweet, sour |
| 2# | Spicy, herbal, green | 72# | Floral, herbal, green | 142# | Fruity, sweet, sour |
| 3# | Fruity, sweet, winy | 73# | Spicy, sweet, floral | 143# | Fruity, sweet, green |
| 4# | Fruity, winy, sweet | 74# | Spicy, herbal, green | 144# | Floral, green, fresh |
| 5# | Herbal, green, sweet | 75# | Woody, sweet, herbal | 145# | Hay, sweet, fresh |
| 6# | Roasted, sweet, smoky | 76# | Herbal, woody, sweet | 146# | Floral, sweet, fruity |
| 7# | Spicy, herbal, sweet | 77# | Floral, sweet, resinous | 147# | Fruity, winy, sweet |
| 8# | Sour, fruity, milky | 78# | Spicy, sweet, green | 148# | Green, fruity, sweet |
| 9# | Sreen, hay, fresh | 79# | Fruity, winy, green | 149# | Floral, sweet, fruity |
| 10# | Sour, milky, fruity | 80# | Hay, sweet, roasted | 150# | Sweet, fruity, floral |
| 11# | Green, sweet, herbal | 81# | Sweet, floral, fruity | 151# | Milky, sweet, resinous |
| 12# | Winy, fruity, sour | 82# | Beany, milky, hay | 152# | Roasted, milky, sweet |
| 13# | Woody, sweet, resinous | 83# | Floral, green, sweet | 153# | Woody, smoky, herbal |
| 14# | Floral, sweet, spicy | 84# | Green, sweet, floral | 154# | Resinous, sour, fruity |
| 15# | Sweet, balsamic, spicy | 85# | Milky, sweet, beany | 155# | Floral, sweet, sour |
| 16# | Milky, sweet, fruity | 86# | Herbal, spicy, sweet | 156# | Sweet, woody, hay |
| 17# | Floral, woody, resinous | 87# | Balsamic, sweet, resinous | 157# | Hay, green, sweet |
| 18# | Herbal, green, sweet | 88# | Sweet, balsamic, fruity | 158# | Woody, floral, sweet |
| 19# | Roasted, chocolatey, milky | 89# | Green, sweet, fresh | 159# | Sweet, hay, balsamic |
| 20# | Hay, herbal, sweet | 90# | Spicy, sweet, herbal | 160# | Spicy, herbal, sweet |
| 21# | Herbal, green, sweet | 91# | Herbal, floral, green | 161# | Herbal, green, spicy |
| 22# | Winy, floral, fruity | 92# | Hay, woody, sweet | 162# | Spicy, sweet, herbal |
| 23# | Sweet, hay, herbal | 93# | Hay, roasted, sour | 163# | Herbal, spicy, floral |
| 24# | Hay, sweet, balsamic | 94# | Floral, spicy, sweet | 164# | Sweet, floral, woody |
| 25# | Floral, herbal, green | 95# | Spicy, herbal, green | 165# | Spicy, sweet, woody |
| 26# | Herbal, floral, hay | 96# | Spicy, sweet, herbal | 166# | Roasted, smoky, sweet |
| 27# | Fruity, floral, sweet | 97# | Floral, fruity, sweet | 167# | Spicy, sweet, sour |
| 28# | Fruity, sweet, smoky | 98# | Sweet, balsamic, resinous | 168# | Woody, sweet, floral |
| 29# | Winy, fruity, sweet | 99# | Balsamic, resinous, sweet | 169# | Fruity, sweet, green |
| 30# | Fruity, winy, sweet | 100# | Woody, herbal, green | 170# | Floral, woody, fruity |
| 31# | Smoky, herbal, roasted | 101# | Balsamic, sweet, smoky | 171# | Sweet, spicy, balsamic |
| 32# | Milky, roasted, sour | 102# | Hay, sweet, roasted | 172# | Winy, fruity, sweet |
| 33# | Beany, sweet, milky | 103# | Chocolatey, sweet, roasted | 173# | Hay, sweet, roasted |
| 34# | Spicy, sweet, floral | 104# | Fruity, sweet, sour | 174# | Smoky, sweet, roasted |
| 35# | Sour, fruity, milky | 105# | Spicy, herbal, sweet | 175# | Fruity, floral, sweet |
| 36# | Floral, green, fresh | 106# | Green, sweet, fruity | 176# | Floral, sweet, fresh |
| 37# | Herbal, sweet, sour | 107# | Herbal, sweet, woody | 177# | Hay, roasted, sweet |
| 38# | Herbal, sweet, hay | 108# | Fruity, sweet, winy | 178# | Spicy, sweet, herbal |
| 39# | Fruity, sweet, sour | 109# | Sweet, fruity, spicy | 179# | Sweet, woody, hay |
| 40# | Fruity, fresh, sweet | 110# | Sweet, floral, fruity | 180# | Winy, milky, sweet |
| 41# | Spicy, sweet, herbal | 111# | Green, fresh, sweet | 181# | Floral, sweet, fresh |
| 42# | Herbal, sweet, hay | 112# | Fruity, winy, sweet | 182# | Fruity, winy, sweet |
| 43# | Fruity, sweet, floral | 113# | Spicy, sweet, herbal | 183# | Roasted, smoky, sweet |
| 44# | Spicy, sweet, woody | 114# | Sweet, fruity, milky | 184# | Green, resinous, herbal |
| 45# | Hay, roasted, sweet | 115# | Fruity, sweet, floral | 185# | Floral, sweet, green |
| 46# | Hay, sour, sweet | 116# | Spicy, sweet, woody | 186# | Chocolatey, beany, roasted |
| 47# | Woody, herbal, floral | 117# | Woody, smoky, spicy | 187# | Fruity, sweet, floral |
| 48# | Sweet, floral, fresh | 118# | Spicy, herbal, sweet | 188# | Herbal, sweet, hay |
| 49# | Sweet, fruity, roasted | 119# | Smoky, chocolatey, roasted | 189# | Herbal, sweet, green |
| 50# | Milky, sweet, beany | 120# | Floral, sweet, green | 190# | Sweet, resinous, beany |
| 51# | Herbal, smoky, sweet | 121# | Balsamic, sweet, resinous | 191# | Smoky, herbal, woody |
| 52# | Floral, sweet, fruity | 122# | Roasted, chocolatey, milky | 192# | Fresh, herbal, sweet |
| 53# | Sweet, chocolatey, winy | 123# | Hay, balsamic, sweet | 193# | Spicy, sweet, green |
| 54# | Herbal, woody, sweet | 124# | Green, fruity, floral | 194# | Floral, woody, sweet |
| 55# | Floral, fruity, sweet | 125# | Milky, sweet, chocolatey | 195# | Spicy, sweet, herbal |
| 56# | Spicy, sweet, woody | 126# | Fruity, sweet, beany | 196# | Hay, sweet, herbal |
| 57# | Chocolatey, woody, sweet | 127# | Spicy, herbal, sweet | 197# | Herbal, woody, floral |
| 58# | Chocolatey, smoky, balsamic | 128# | Fruity, sweet, fresh | 198# | Hay, sweet, roasted |
| 59# | Milky, sweet, fruity | 129# | Fruity, sweet, green | 199# | Fruity, sweet, herbal |
| 60# | Fruity, sweet, sour | 130# | Spicy, sweet, floral | 200# | Herbal, woody, green |
| 61# | Milky, fruity, sweet | 131# | Floral, sweet, fresh | 201# | Floral, fruity, sweet |
| 62# | Roasted, sweet, chocolatey | 132# | Green, sweet, floral | 202# | Green, herbal, resinous |
| 63# | Floral, green, fresh | 133# | Herbal, woody, spicy | 203# | Herbal, green, sweet |
| 64# | Fruity, sweet, fresh | 134# | Resinous, green, herbal | 204# | Floral, sweet, fresh |
| 65# | Balsamic, sweet, resinous | 135# | Fruity, sweet, green | 205# | Roasted, smoky, sweet |
| 66# | Herbal, green, sweet | 136# | Beany, milky, sweet | 206# | Herbal, woody, green |
| 67# | Herbal, sweet, woody | 137# | Herbal, woody, green | 207# | Sweet, balsamic, woody |
| 68# | Milky, sweet, beany | 138# | Sweet, fruity, balsamic | 208# | Green, floral, woody |
| 69# | Floral, sweet, fresh | 139# | Fruity, sweet, herbal | 209# | Woody, resinous, sweet |
| 70# | Green, floral, herbal | 140# | Fruity, sweet, resinous | 210# | Milky, sweet, beany |
Table 4.
Formula composition of empirical formula 1# (with the main odors of sweet, fruity and milky).
| Number | Fragrance ingredient | Amount (wt. %) | Material type | Type of note |
|---|---|---|---|---|
| 1 | Acetic acid | 0.05 | Synthesized | Top |
| 2 | Ethyl acetate | 0.02 | Synthesized | Top |
| 3 | Butyric acid | 0.20 | Synthesized | Top |
| 4 | Ethyl butyrate | 0.60 | Synthesized | Top |
| 5 | Ethyl 2-methylbutyrate | 0.07 | Synthesized | Top |
| 6 | Cis-3-hexen-1-ol | 0.04 | Synthesized | Top |
| 7 | Cis-3-hexen-1-yl acetate | 0.02 | Synthesized | Top |
| 8 | Ethyl maltol | 0.30 | Synthesized | Middle |
| 9 | Geraniol | 0.01 | Synthesized | Middle |
| 10 | β-damascone | 0.08 | Synthesized | Middle |
| 11 | α-ionone | 0.05 | Synthesized | Middle |
| 12 | β-ionone | 0.01 | Synthesized | Middle |
| 13 | 4-(4-hydroxyphenyl)-2-butanone | 1.50 | Synthesized | Middle |
| 14 | Date tincture | 0.30 | Natural | Middle |
| 15 | Vanillin | 2.00 | Synthesized | Base |
| 16 | Ethyl vanillin | 1.50 | Synthesized | Base |
| 17 | 1,2-propanediol | 93.25 | Solvent | – |
| Total | 100.00 | – | – | |
2.7. Relationship diagram of ingredients used in empirical formulas
Denoted PF = {X1, X2, Xi …, Xn} be the set of empirical fragrance formulas, where Xi represents the i-th empirical formula and n is the total number of empirical formulas. Each empirical formula in PF belongs to a certain type of fragrance, such as empirical formula 3# with the main odors of floral and herbal. Each empirical formula Xi is composed of multiple fragrance ingredients and their corresponding amount information, i.e., Xi = {(C1i, P1i), (C2i, P2i), …, (Cti, Pti)}, where Cti is the fragrance ingredient and Pti is the corresponding amount (Table 5).
Table 5.
Information of Xi in empirical fragrance formulas.a.
| Number | Fragrance ingredient | Amount (wt. %) |
|---|---|---|
| 1 | C1i | P1i |
| 2 | C2i | P2i |
| … | … | … |
| t | Cti | Pti |
Exclude the solvents and their corresponding amounts from the formulas artificially.
Obtain the set of fragrance ingredients by taking the union of all ingredients included in the empirical formula sheets in PF. Define C as the resulting set in Equation (1):
| (1) |
Where m is the total number of fragrance ingredients. For each ingredient in C, count its occurrence in all empirical formulas to obtain . Sort the fragrance ingredients in C in descending order based on , and let the sorted set be = . Therefore, Xi can be represented by as shown in Equation (2):
| (2) |
If Xi does not include a certain ingredient, its amount is 0.
After this process, all empirical formulas (PF) can be represented as an m-by-n matrix, as shown in Equation (3):
| (3) |
Where the j-th row and i-th column of the matrix represent the amount of the j-th ingredient in the i-th formula.
Finally, construct a square adjacency matrix Q to represent the relationships between fragrance ingredients. For all n formulas, if the i-th fragrance ingredient appears in the same formula as the j-th fragrance ingredient k times, then , where k represents the weight of the edge connecting the two fragrance ingredients. Q is symmetric, so its diagonal elements that represent each ingredient itself can be set to 0.
2.8. Construction of an automatic fragrance formula creation algorithm (AFFC)
New fragrance formulas are generated by traversing the relationship diagram generated in section 2.7 using a depth-first search (DFS) algorithm. Prior to traversal, common constraint information must be established to guide the traversal process. This constraint information includes.
-
i.
Fragrance goal: defining the main odors desired for the new fragrance formula in order to provide the algorithm with an empirical formula containing the corresponding main fragrance style.
-
ii.
Mandatory ingredients for the new formula: primarily based on user preferences or selecting one or more ingredients that match the fragrance goal, set them as the starting or passing point for the graph traversal.
-
iii.
Rejected ingredients for the new formula: typically based on user preferences or usage experience, define ingredients that cannot be used in the new formula. To ensure graph connectivity, results containing the rejected ingredient(s) should be removed from the result set of the traversal. If only one result contains the rejected ingredient(s), the ingredient(s) should be removed directly from the formulation.
-
iv.
Quantity of ingredients in the new formula: setting the number of fragrance ingredients included in the generated formula.
-
v.
Other constraint information: such as the proportion of top note, middle note, and base note, the proportion of synthesized and natural ingredients, and other relevant ingredient information can be established as additional constraints during the traversal process to adjust the traversal path.
Using the DFS algorithm to implement the traversal process, the specific method is as follows.
-
i.
A query is made to the empirical formulas database to retrieve all empirical formulations that contain the desired main odors for the new fragrance.
-
ii.
Starting from the mandatory ingredient A, select the edge with the highest weight among the edges connected to A as the forward direction;
-
iii.
If B is the edge connected to A with the highest weight, select the edge with the highest weight among the edges connected to B, except for A, as the forward direction.
-
iv.
Set B as the new starting point and proceed according to the above steps;
-
v.
If there is only one edge connected to B, which is from A, backtrack to A and select the edge connected to A with the second highest weight as the forward direction while retaining B in the path and repeat the process.
-
vi.
Once all mandatory ingredients have been covered by the path, let L be the number of points traversed minus the number of rejected ingredients. Suppose the number of components in the preset formula is R. If L = R, stop the traversal. If L < R, continue the traversal until L = R. If L > R, select the point with the smallest degree (number of connected edges) in the traversed path for deletion and repeat until L = R.
-
vii.
Remove the rejected ingredients from the traversed path and output the remaining portion as the new formula ingredients.
Based on prior research and the structure of empirical formulas, we have developed a practical method associated with the dosage amount of raw materials in empirical formulas for predicting the usage amount of raw materials in fragrance formulas.
We obtain the corresponding formula ingredients information, denoted as T, based on the new formula composition generated above. We then calculate the ratio of the intersection size (i.e., the number of common ingredients between T and each empirical formula ) to the union size (i.e., number of ingredients in both T and ), denoted as Wi in Equation (4):
| (4) |
The usage amount p of ingredient c in T is then given by Equation (5):
| (5) |
Here, n is the number of empirical formulas, is the usage amount of ingredient c in the corresponding empirical formula, and is the sign function. When , is 0, otherwise, it is 1.
3. Results and discussion
3.1. Intelligent creation of fragrance formula
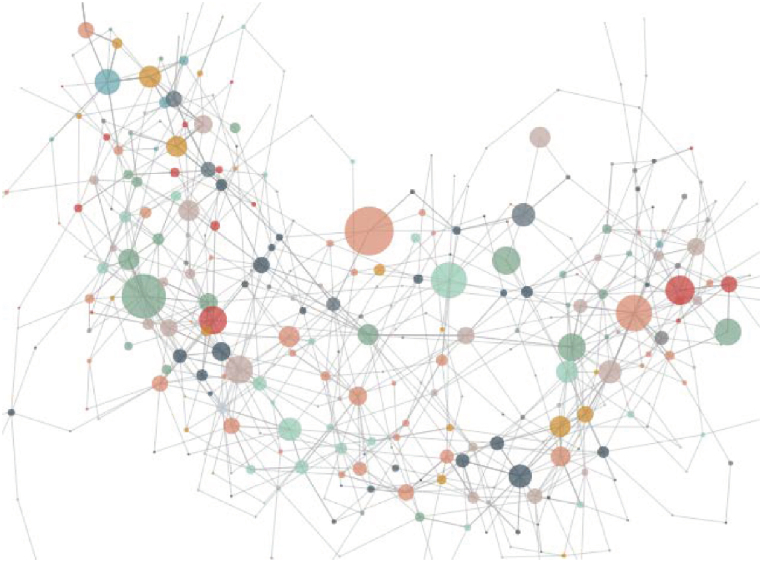
In this study, the goal of fragrance creation was focused on floral fragrances. A total of 52 empirical formulas with floral odor as main odors were selected from the empirical formulas database, involving 285 types of fragrance raw materials. These empirical formulas were organized into a 285 × 52 matrix, and a diagram illustrating the relationships of ingredients used in these empirical formulas was constructed using the methodology described in section 2.7 (Fig. 1) Each point on Fig. 1 represents a fragrance ingredient, with the size of the point indicating the number of uses of this ingredient in the empirical formula. In other words, the more frequently a certain fragrance ingredient appears in the formula, the larger the point is. The lines between the points denote that these two fragrance ingredients have been used together in at least one empirical formula.
Fig. 1.
Relationship diagram of empirical fragrance formulas.
To create a fragrance formula utilizing computer intelligence, the following constraint conditions were implemented based on traditional flower fragrance formulas and product requirements.
-
1)
The fragrance goal was set to “floral fragrance.”
-
2)
The formula was limited to a maximum of “20” fragrance ingredients.
-
3)
The mandatory ingredients “geraniol” and “benzyl acetate” were included to enhance floral odors.
-
4)
No rejected materials or other constraint conditions were specified.
Using the AFFC algorithm described in section 2.8 with the above constraint conditions, a semi-finished fragrance formula T0# containing only the selected ingredients was generated by the computer (Table 6).
Table 6.
Fragrance components of semi-finished formula T0# generated by AFFC algorithm.
| Number | Fragrance ingredient | Material type | Type of note |
|---|---|---|---|
| 1 | Benzyl acetate | Synthesized | Top |
| 2 | Phenethyl acetate | Synthesized | Top |
| 3 | Phenethyl alcohol | Synthesized | Top |
| 4 | Linalool | Synthesized | Top |
| 5 | Orange flower absolute | Natural | Top |
| 6 | D,l-menthol | Synthesized | Top |
| 7 | Nerolidol | Synthesized | Middle |
| 8 | Anisyl alcohol | Synthesized | Middle |
| 9 | Geraniol | Synthesized | Middle |
| 10 | Menthone | Synthesized | Middle |
| 11 | β-damascone | Synthesized | Middle |
| 12 | Ethyl maltol | Synthesized | Middle |
| 13 | 2,3,5-trimethyl pyrazine | Synthesized | Middle |
| 14 | β-ionone | Synthesized | Middle |
| 15 | Citronellal | Synthesized | Middle |
| 16 | Cassia bark oil | Natural | Middle |
| 17 | Lavender oil | Natural | Middle |
| 18 | Jasmine absolute | Natural | Base |
| 19 | Treemoss absolute | Natural | Base |
| 20 | Balsam peru | Natural | Base |
Based on the semi-finished fragrance formula T0#, the Wi values between formula T0# and the 52 empirical formulas used were automatically calculated by the computer according to Equation (4) in section 2.8, and the results are presented in Table 7.
Table 7.
Values of Wi between formula T0# and the 52 empirical formulas used.
| Number of i | W value | Number of i | W value |
|---|---|---|---|
| 1 | 0.1316 | 27 | 0.1862 |
| 2 | 0.1250 | 28 | 0.0426 |
| 3 | 0.2895 | 29 | 0.1500 |
| 4 | 0.0789 | 30 | 0.0189 |
| 5 | 0.1351 | 31 | 0.3912 |
| 6 | 0.2564 | 32 | 0.0910 |
| 7 | 0.2093 | 33 | 0.0278 |
| 8 | 0.2791 | 34 | 0.2162 |
| 9 | 0.2432 | 35 | 0.2195 |
| 10 | 0.1081 | 36 | 0.1842 |
| 11 | 0.2250 | 37 | 0.2368 |
| 12 | 0.0620 | 38 | 0.1676 |
| 13 | 0.0732 | 39 | 0.0271 |
| 14 | 0.0544 | 40 | 0.1514 |
| 15 | 0.0563 | 41 | 0.0859 |
| 16 | 0.0688 | 42 | 0.0786 |
| 17 | 0.0865 | 43 | 0.2452 |
| 18 | 0.3926 | 44 | 0.0812 |
| 19 | 0.0722 | 45 | 0.0468 |
| 20 | 0.0714 | 46 | 0.0725 |
| 21 | 0.3514 | 47 | 0.2764 |
| 22 | 0.0956 | 48 | 0.1096 |
| 23 | 0.0978 | 49 | 0.1358 |
| 24 | 0.2522 | 50 | 0.3580 |
| 25 | 0.1081 | 51 | 0.2021 |
| 26 | 0.0244 | 52 | 0.0232 |
According to Equation (5) in section 2.8, each component in formula T0# was automatically calculated in sequence to determine the required percentage usage amount. Any remaining percentage less than 100 % was compensated with a solvent. The resulting primary fragrance formula X1# was then obtained from the computer-generated formula that met our pre-defined fragrance targeting goals (Table 8).
Table 8.
Complete intelligent fragrance formula X1# generated by AFFC algorithm.
| Number | Fragrance ingredient | Amount(wt. %) | Material type | Type of note |
|---|---|---|---|---|
| 1 | Benzyl acetate | 0.39 | Synthesized | Top |
| 2 | Phenethyl acetate | 0.88 | Synthesized | Top |
| 3 | Phenethyl alcohol | 2.12 | Synthesized | Top |
| 4 | Linalool | 0.15 | Synthesized | Top |
| 5 | Orange flower absolute | 0.26 | Natural | Top |
| 6 | D,l-menthol | 0.47 | Synthesized | Top |
| 7 | Nerolidol | 0.45 | Synthesized | Middle |
| 8 | Anisyl alcohol | 1.76 | Synthesized | Middle |
| 9 | Geraniol | 1.11 | Synthesized | Middle |
| 10 | Menthone | 0.47 | Synthesized | Middle |
| 11 | β-damascone | 0.52 | Synthesized | Middle |
| 12 | Ethyl maltol | 0.51 | Synthesized | Middle |
| 13 | 2,3,5-trimethylpyrazine | 0.19 | Synthesized | Middle |
| 14 | β-ionone | 0.49 | Synthesized | Middle |
| 15 | Citronellal | 0.04 | Synthesized | Middle |
| 16 | Cassia bark oil | 0.72 | Natural | Middle |
| 17 | Lavender oil | 0.05 | Natural | Middle |
| 18 | Jasmine absolute | 0.13 | Natural | Base |
| 19 | Treemoss absolute | 0.97 | Natural | Base |
| 20 | Balsam peru | 0.18 | Natural | Base |
| 21 | Solvent | 88.14 | Solvent | – |
| Total | 100.00 | – | – | |
3.2. Optimization of the primary fragrance formula generated by AFFC algorithm
A small sample of the formula X1# was prepared according to the specifications in Table 8 and allowed to mix and settle appropriately. Subsequently, the olfactory profile of the sample was evaluated using the method outlined in Section 2.3.
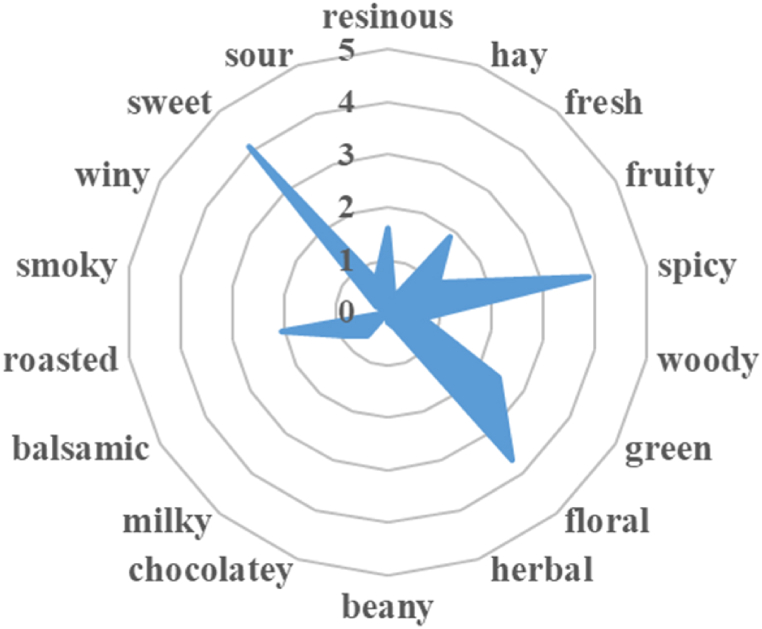
The results of the evaluation (Fig. 2) indicated that the formula X1# had predominant notes of sweet (4.10 points), spicy (3.88 points), and floral (3.66 points), accompanied by green (2.45 points), roasted (2.05 points), and fresh (1.86 points) aromas. Heavy use of ingredients with spicy and roasted notes resulted in poor overall harmony and comfort, which deviated from the intended goal of creating a floral fragrance. Therefore, it is necessary to adjust the algorithm constraints to optimize the formula based on the characteristics of the raw materials used.
Fig. 2.
The olfactory profiles of fragrance formula X1#.
Based on the olfactory profiles of fragrance formula X1# and the characteristics of its materials as references according to FIID, the spicy cinnamon oil and roasted notes of 2,3,5-trimethylpyrazine were manually removed as constraint conditions for the AFFC algorithm. The new traversal constraint conditions for the algorithm were adjusted as follows.
-
1)
The fragrance goal was set to “floral fragrance.”
-
2)
The formula was limited to a maximum of “20” fragrance ingredients.
-
3)
Mandatory ingredients set for new formula were “geraniol” and “benzyl acetate”.
-
4)
Reject the use of “cinnamon oil” and “2,3,5-trimethylpyrazine” in the new formula.
After running the aforementioned AFFC algorithm, the optimized complete fragrance formula X2# was obtained (Table 9).
Table 9.
Optimized intelligent fragrance formula X2# generated by AFFC algorithm.
| Number | Fragrance ingredient | Amount (wt. %) | Material type | Type of note |
|---|---|---|---|---|
| 1 | Benzyl acetate | 0.26 | Synthesized | Top |
| 2 | Phenethyl acetate | 1.21 | Synthesized | Top |
| 3 | Phenethyl alcohol | 1.56 | Synthesized | Top |
| 4 | Linalool | 0.13 | Synthesized | Top |
| 5 | Linalyl acetate | 0.12 | Synthesized | Top |
| 6 | D,l-menthol | 0.63 | Synthesized | Top |
| 7 | β-damascenone | 0.25 | Synthesized | Middle |
| 8 | Nerolidol | 0.23 | Synthesized | Middle |
| 9 | Anisyl alcohol | 1.19 | Synthesized | Middle |
| 10 | Geraniol | 0.12 | Synthesized | Middle |
| 11 | γ-valerolactone | 0.48 | Synthesized | Middle |
| 12 | Maltol | 0.63 | Synthesized | Middle |
| 13 | Styralyl acetate | 0.05 | Synthesized | Middle |
| 14 | β-ionone | 0.26 | Synthesized | Middle |
| 15 | Geranium oil | 0.30 | Natural | Middle |
| 16 | Spearmint oil | 0.14 | Natural | Middle |
| 17 | Aglaia odorata flower oil | 0.06 | Natural | Middle |
| 18 | Jasmine absolute | 0.17 | Natural | Base |
| 19 | Treemoss absolute | 0.58 | Natural | Base |
| 20 | Balsam peru | 0.36 | Natural | Base |
| Solvent | 91.27 | Solvent | ||
| Total | 100.00 | – | ||
Likewise, a small sample of the formula X2# was prepared according to Table 9 and made it mix and settle appropriately, and the olfactory profiles of this sample was evaluated according to the method mentioned in section 2.3.
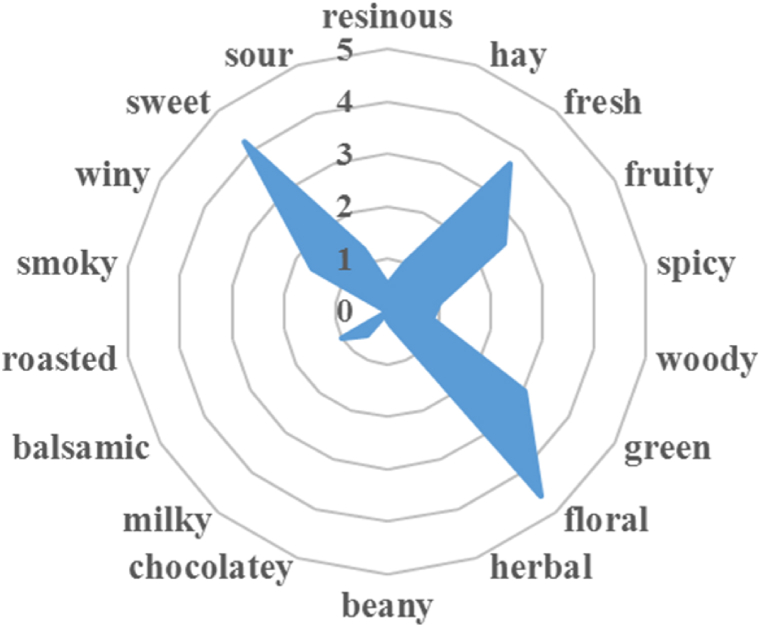
As depicted in Fig. 3, the olfactory sensory evaluation revealed that formula X2# was primarily composed of floral (4.58 points), sweet (4.22 points), and fresh (3.66 points) notes, complemented by green (3.02 points), fruity (2.58 points), and wine (1.65 points) notes. The overall olfactory performance of this formula exhibited excellent consistency and stability, and it also had a comfortable and harmonious scent with long-lasting effects. The achieved results well aligned with the objective of creating a floral fragrance with practical value.
Fig. 3.
The olfactory profiles of fragrance formula X2#.
In summary, we introduced an advanced algorithm designed to analyze the interrelationships among ingredients in empirical fragrance formulas in this study. Leveraging statistical analysis, this algorithm strategically selected key components for devising innovative formulas. The algorithm's efficacy was significantly influenced by the quality of the empirical fragrance formulas it processed. For instance, an ingredient like 'A', if ubiquitous in empirical formulas, is consistently included in new formulations. Conversely, an ingredient such as 'B', absent from the empirical datasets, is similarly excluded from the new formulations. This presents a notable challenge in the algorithm's functionality. Currently, addressing these imbalances necessitates manual intervention, involving strategic inclusion or exclusion of certain ingredients in the new formulas, to align them with predefined research objectives.
4. Conclusion
This article presents a novel approach to improving the efficiency and accuracy of fragrance design using empirical fragrance formulas and graph traversal algorithms. We constructed a database of 344 common fragrance ingredients that allowed perfumers to set algorithmic constraints based on their experience and the characteristics of these materials. By analyzing the composition of 210 empirical fragrance formulas and constructing a relational network model in graph form, we illustrated the relationships between the ingredients used in these formulas. Finally, we developed an automatic fragrance formula creation (AFFC) algorithm that constructs a subgraph of the relational network and finds fragrance formula solutions based on the depth-first search algorithm, satisfying the constraint conditions, and combining the appropriate statistical strategy to determine the use of each component in the new fragrance formula. The algorithm introduced in our study innovatively utilized empirical fragrance formulas to determine various components and their respective quantities. Through empirical validation, it demonstrated satisfactory performance. However, this algorithm exhibited a significant dependency on empirical formula data. Any deviations in the empirical formulas or the inclusion of personal preferences in raw material selection markedly affected the algorithm's outcomes, leading to a decrease in its innovative capacity. Most notably, the algorithm was unable to accommodate ingredients not previously encountered in the empirical formula data. The results of testing the AFFC algorithm for creating a floral fragrance produced a formula with primary fragrance notes of floral, sweet, and fresh, exhibiting good consistency and stability which meets perfumers' requirements. Overall, this study provides a novel and convenient fragrance development approach for fragrance developers, which addresses the diversity deficiency problem caused by individual preferences and improves the efficiency of traditional fragrance development.
CRediT authorship contribution statement
Jiaxiao Cai: Writing – original draft, Methodology, Data curation. Suxing Tuo: Validation, Methodology, Formal analysis, Data curation. Yanchun Li: Formal analysis, Data curation. Hongbing Lu: Validation, Formal analysis, Data curation. Yizi Wu: Validation, Supervision, Software, Methodology. You Zou: Visualization, Validation, Supervision, Software. Zhen Ma: Resources, Investigation. Yuqi Cui: Methodology, Investigation, Data curation. Bo Kong: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Kejun Zhong: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Data availability statement
Data will be made available on request.
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study because the study used anonymized, published data.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the State Tobacco Monopoly Administration of China for funding this research work through the project no. 110202202002 and no. 110202001001(YX-01).
Contributor Information
Bo Kong, Email: kongb0706@hngytobacco.com.
Kejun Zhong, Email: zhongkejun@yeah.net.
Nomenclature
- PF
the set of empirical fragrance formulas
- Xi
empirical formula
- n
total number of empirical formulas
- Cti
fragrance ingredient
- Pti
amount of fragrance ingredient
- m
total number of fragrance ingredients
- nc
fragrance ingredients that occurrence in all empirical formulas
- C
fragrance ingredient set
sorted fragrance ingredient set
- Q
a square adjacency matrix
- k
weight of the edge connecting the two fragrance ingredients
- L
number of points traversed minus number of rejected ingredients
- R
number of components in preset formula
- T
fragrance ingredients used in auto-created formula
- Wi
ratio of the intersection size to the union size
- p
usage amount of fragrance ingredients in auto-created formula
References
- 1.Zhan W., Doro F., Teixeira M.A. A rapid approach to optimize the design of fragrances for fabric care products. Flavour Fragrance J. 2019;35(2):167–173. [Google Scholar]
- 2.Schifferstein H.N., Howell B.F. Using color-odor correspondences for fragrance packaging design. Food Qual. Prefer. 2015;46:17–25. [Google Scholar]
- 3.Teixeira M.A., Rodriguez O., Mata V.G., Rodrigues A.E. Perfumery quaternary diagrams for engineering perfumes. AIChE J. 2009;55(8):2171–2185. [Google Scholar]
- 4.Mata V.G., Gomes P.B., Rodrigues A.E. Engineering perfumes. AIChE J. 2005;51(10):2834–2852. [Google Scholar]
- 5.Figueiredo A.C., Barroso J.G., Pedro L.G., Scheffer J.J.C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragrance J. 2008;23(4):213–226. [Google Scholar]
- 6.Jennings W., Takayuki S. Qualitative analysis of flavor and fragrance volatiles by glass capillary gas chromatography. Journal of Chemical Education. 1981;58(12):A381. [Google Scholar]
- 7.Letizia C.S., Cocchiara J., Wellington G.A., Funk C., Api A.M. Monographs on fragrance raw materials. Food Chem. Toxicol. 2000;38 S1-+ [Google Scholar]
- 8.Teixeira M.A., Rodriguez O., Rodrigues A.E. Prediction model for the odor intensity of fragrance mixtures: a valuable tool for perfumed product design. Ind. Eng. Chem. Res. 2013;52(2):963–971. [Google Scholar]
- 9.Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 10.Wei J. Oxford University Press; 2007. Product Engineering: Molecular Structure and Properties. [Google Scholar]
- 11.Shang L., Liu C.J., Tomiura Y., Hayashi K. Machine-learning-based olfactometer: prediction of odor perception from physicochemical features of odorant molecules. Anal. Chem. 2017;89(22):11999–12005. doi: 10.1021/acs.analchem.7b02389. [DOI] [PubMed] [Google Scholar]
- 12.Teixeira M.A., Barrault L., Rodriguez O., Carvalho C.C., Rodrigues A.E. Perfumery radar 2.0: a step toward fragrance design and classification. Ind. Eng. Chem. Res. 2014;53(21):8890–8912. [Google Scholar]
- 13.Turin L. A method for the calculation of odor character from molecular structure. J. Theor. Biol. 2002;216(3):367–385. doi: 10.1006/jtbi.2001.2504. [DOI] [PubMed] [Google Scholar]
- 14.Meda H.S., Sen A.K., Bagchi A. 17th Annual Workshop on Information Technolgies & Systems (WITS) Paper. 2007. Detecting data flow errors in workflows: a systematic graph traversal approach. [Google Scholar]
- 15.Holdsworth J.J. Graph traversal and graph transformation. Theor. Comput. Sci. 2004;321(2–3):215–231. [Google Scholar]
- 16.Fleischer R., Trippen G. In: Experimental and Efficient Algorithms. Jansen K., Margraf M., Mastrolli M., Rolim J.D.P., editors. 2003. Experimental studies of graph traversal algorithms; pp. 120–133. Proceedings. [Google Scholar]
- 17.Cormen T.H., Leiserson C.E., Rivest R.L., Stein C. MIT press; 2022. Introduction to Algorithms. [Google Scholar]
- 18.Tarjan R. Depth-first search and linear graph algorithms. SIAM J. Comput. 1972;1(2):146–160. [Google Scholar]
- 19.Rahim R., Abdullah D., Simarmata J., Pranolo A., Ahmar A.S., Hidayat R., Napitupulu D., Nurdiyanto H., Febriadi B., Zamzami Z. Joint Workshop of KO2PI/2nd International Conference on Mathematics, Science, Technology, Education, and Their Applications (ICMSTEA) 2016. Iop, block architecture problem with depth first search solution and its application. Makassar, INDONESIA. [Google Scholar]
- 20.Xiong J., Li Z., Fan L., Liu D. 2010 IEEE Fifth International Conference on Bio-Inspired Computing: Theories and Applications (BIC-TA) IEEE; 2010. Improved depth first algorithm and its application in information retrieval; pp. 69–73. [Google Scholar]
- 21.Moseley H.N.B., Riaz N., Aramini J.M., Szyperski T., Montelione G.T. A generalized approach to automated NMR peak list editing: application to reduced dimensionality triple resonance spectra. J. Magn. Reson. 2004;170(2):263–277. doi: 10.1016/j.jmr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Hamel M. Angele. Combinatorial optimization: theory and algorithms (5th ed.) Comput. Rev. 2012;53(12):717–718. [Google Scholar]
- 23.Hopcroft J.E., Motwani R., Ullman J.D. Introduction to automata theory, languages, and computation. Acm Sigact News. 2001;32(1):60–65. [Google Scholar]
- 24.Teixeira M.A., Rodriguez O., Gomes P., Mata V., Rodrigues A. Butterworth-Heinemann; 2012. Perfume Engineering: Design, Performance and Classification. [Google Scholar]
- 25.Fisher C., Scott T.R. Royal Society of chemistry; 2007. Food Flavours: Biology and Chemistry. [Google Scholar]
- 26.Sell C.S. Royal Society of Chemistry; 2006. The Chemistry of Fragrances: from Perfumer to Consumer. [Google Scholar]
- 27.Van Gemert L. RID, CIVO-TNO; Zeist, The Netherlands: 1977. Compilation of Odor Threshold Values in Air and Water. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.