Abstract
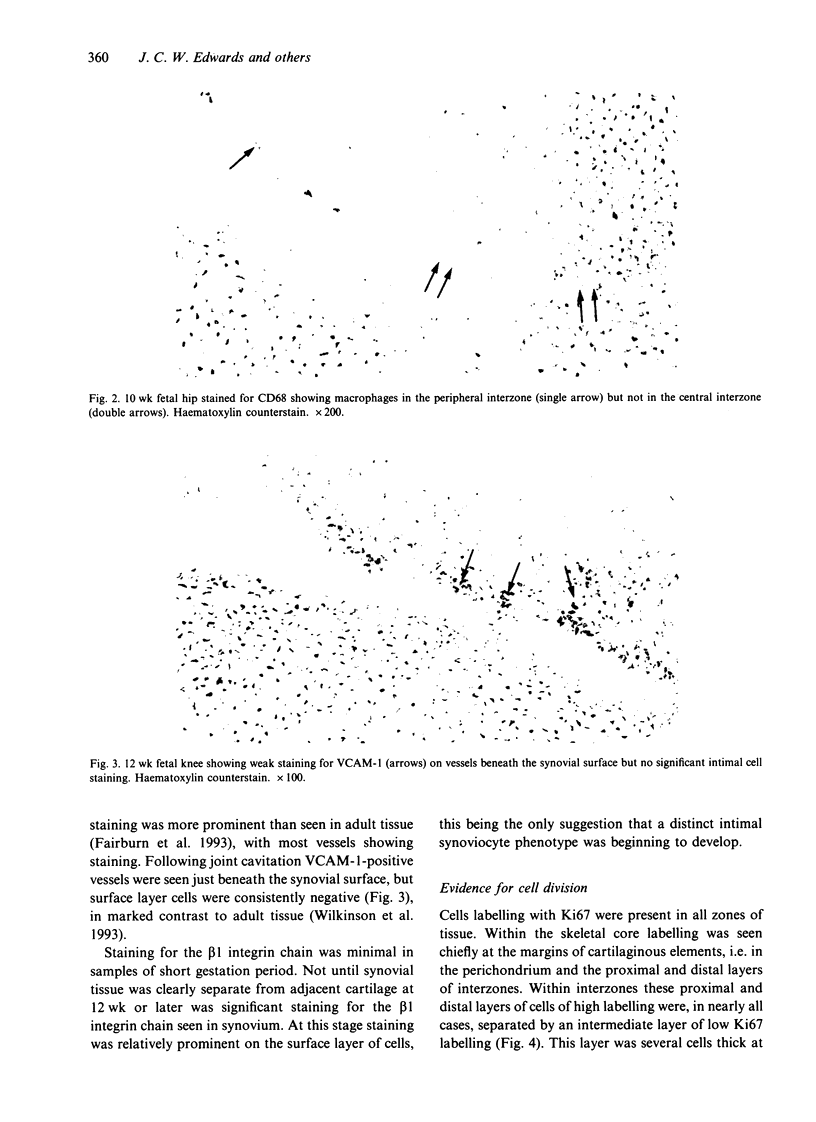
During fetal development, cavitation occurs within the primitive skeleton along planes destined to become the articular surfaces of synovial joints. A histochemical study of human fetal limbs was undertaken to identify the cell types involved in this cavitation and the possible role of interactions between cells and extracellular matrix. Cryostat sections were stained with antibodies to CD68, factor VIII related antigen, prolyl hydroxylase, beta 1 integrin, VCAM-1, proliferating cell nuclear antigen, chondroitin-4 sulphate, chondroitin-6-sulphate, hyaluronan synthase and CD44. Similar sections were reacted for uridine diphosphoglucose dehydrogenase (UDPGD) and acid phosphatase activity. Hyaluronan was demonstrated using an aggrecan core protein hyaluronan binding region probe. Macrophages were present prior to cavitation in the periphery of joint interzones but not at the presumptive joint line in the central interzone. Fibroblastic cells were present throughout. Absence of local VCAM-1 expression indicated that cavitation was temporally distinct from full fibroblast-like synoviocyte differentiation. CD44 was expressed by interzone cells at all stages. Staining for hyaluronan and hyaluronan synthase, but not chondroitin sulphates was present in the interzone before and at the time of cavitation. UDPGD activity was increased in a narrow band of cells at the presumptive joint line prior to cavitation. These findings suggest that joint cavitation is dependent on the behaviour of fibroblastic cells and/or adjacent chondrocytes, rather than macrophages. Since UDPGD activity is involved in hyaluronan synthesis, it is proposed that joint cavitation is facilitated by a rise in local hyaluronan concentration in an area of tissue where cohesion is dependent on the interaction between cellular CD44 and extracellular hyaluronan. As proposed by Toole et al. (1984) such a local rise in hyaluronan concentration may lead to a switch from intercellular cohesion to dissociation, leading to tissue cavitation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN H. DEVELOPMENT, MORPHOLOGY AND HISTOCHEMISTRY OF THE EARLY SYNOVIAL TISSUE IN HUMAN FOETUSES. Acta Anat (Basel) 1964;58:90–115. doi: 10.1159/000142577. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- BARKA T. A simple azo-dye method for histochemical demonstration of acid phosphatase. Nature. 1960 Jul 16;187:248–249. doi: 10.1038/187248a0. [DOI] [PubMed] [Google Scholar]
- Craig F. M., Bayliss M. T., Bentley G., Archer C. W. A role for hyaluronan in joint development. J Anat. 1990 Aug;171:17–23. [PMC free article] [PubMed] [Google Scholar]
- Craig F. M., Bentley G., Archer C. W. The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development. 1987 Mar;99(3):383–391. doi: 10.1242/dev.99.3.383. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Wilkinson L. S., Pitsillides A. A. Palisading cells of rheumatoid nodules: comparison with synovial intimal cells. Ann Rheum Dis. 1993 Nov;52(11):801–805. doi: 10.1136/ard.52.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn K., Kunaver M., Wilkinson L. S., Cambridge G., Haskard D., Edwards J. C. Intercellular adhesion molecules in normal synovium. Br J Rheumatol. 1993 Apr;32(4):302–306. doi: 10.1093/rheumatology/32.4.302. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Mason D. Y., Pulford K., Falini B., Bliss E., Gatter K. C., Stein H., Clarke L. C., McGee J. O. Immunohistological analysis of human mononuclear phagocytes and dendritic cells by using monoclonal antibodies. Lab Invest. 1986 Mar;54(3):322–335. [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Klewes L., Turley E. A., Prehm P. The hyaluronate synthase from a eukaryotic cell line. Biochem J. 1993 Mar 15;290(Pt 3):791–795. doi: 10.1042/bj2900791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulyk W. M., Kosher R. A. Temporal and spatial analysis of hyaluronidase activity during development of the embryonic chick limb bud. Dev Biol. 1987 Apr;120(2):535–541. doi: 10.1016/0012-1606(87)90256-9. [DOI] [PubMed] [Google Scholar]
- MUNARON G. Osservazioni istofisiche ed istochimiche sul mesenchima intermedio delle articolazioni embrionali e sui suoi derivati. Boll Soc Ital Biol Sper. 1954 Jul;30(7):919–922. [PubMed] [Google Scholar]
- Mehdizadeh S., Bitensky L., Chayen J. The assay of uridine diphosphoglucose dehydrogenase activity: discrimination from xanthine dehydrogenase activity. Cell Biochem Funct. 1991 Apr;9(2):103–110. doi: 10.1002/cbf.290090207. [DOI] [PubMed] [Google Scholar]
- Mitrovic D. Development of the articular cavity in paralyzed chick embryos and in chick embryo limb buds cultured on chorioallantoic membranes. Acta Anat (Basel) 1982;113(4):313–324. doi: 10.1159/000145566. [DOI] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripellino J. A., Klinger M. M., Margolis R. U., Margolis R. K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985 Oct;33(10):1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- Sorrell J. M., Mahmoodian F., Schafer I. A., Davis B., Caterson B. Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem. 1990 Mar;38(3):393–402. doi: 10.1177/38.3.1689338. [DOI] [PubMed] [Google Scholar]
- Underhill C., Dorfman A. The role of hyaluronic acid in intercellular adhesion of cultured mouse cells. Exp Cell Res. 1978 Nov;117(1):155–164. doi: 10.1016/0014-4827(78)90438-x. [DOI] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]
- Wilkinson L. S., Edwards J. C., Poston R. N., Haskard D. O. Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993 Jan;68(1):82–88. [PubMed] [Google Scholar]
- Wilkinson L. S., Pitsillides A. A., Worrall J. G., Edwards J. C. Light microscopic characterization of the fibroblast-like synovial intimal cell (synoviocyte). Arthritis Rheum. 1992 Oct;35(10):1179–1184. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]












