Abstract
Cavitary sarcoidosis is a rare form and represents non-caseating granulomatous diseases of the lungs exhibiting a narrow range of differential diagnoses. The peculiarity of this case lies in the difficulty of distinguishing atypical manifestations of pulmonary sarcoidosis, such as cystic lesions, from cavernous tuberculosis. Both possess similar clinical and radiological features. We report a case of a 36-year-old male, who has been under observation since 2019 without active complaints. Chest computed tomography revealed bilateral reticulo-nodular changes with the formation of extensive consolidation areas and cavities, аs well as mediastinal lymphadenopathy. This article represents clinical case of patient with recurrent cavitary sarcoidosis and underscores the necessity for monitoring and adjusting of the treatment of this complex condition because their course may be punctuated by several complications including pleural effusion, hemoptysis, aspergilloma, and pneumothorax.
Keywords: Asymptomatic cavitary sarcoidosis, Recurrent episodes, Granulomas, Morphology
1. Introduction
Sarcoidosis is a systemic inflammatory disease with an unknown etiology, characterized by the activation of T cells, the production of numerous chemokines and cytokines at the site of inflammation, and the subsequent damage to various organs [1]. Sarcoidosis frequently affects the lung, associated with morbidity and mortality. According to up-to-date research, the incidence of sarcoidosis is 141 per 100,000 among African Americans and 49.8 per 100,000 among Caucasians, showing considerable variations [2]. According to the prevalence of sarcoidosis including the statistical database of Asia may contains ranging from 0.56 to 4.00 per 100,000 [3]. Korean a nationwide population-based study reported incidence of sarcoidosis as 0.81 per 100,000 [3]. It is important to mention that the highest prevalence occurs in men between the ages of 30 and 50, and in women between the ages of 50 and 60 [2]. Previous research has shown that 50 % of individuals may have asymptomatic illness, which can be detected by a chest X-ray (CXR) conducted for unrelated purposes. The occurrence of fortuitous finding on CXR is currently infrequent, with a reported prevalence of 8.4 % among patients [4].
The radiologic characteristics of sarcoidosis are peribronchovascular nodules, non-resolving consolidation, and fibrocystic lesions [5]. While 80 %–90 % of individuals have lymph nodes implicated in their sarcoidosis at some point during the disease, some forms of adenopathy are highly infrequent. The expansion of the bronchopulmonary nodes, the right paratracheal nodes, and the nodes in the aorticopulmonary window on both sides is the most common intrathoracic combination of adenopathy in sarcoidosis. Traction bronchiectasis, cavities, cysts, and bullous lesions are common manifestations of chronic pulmonary sarcoidosis. On the other hand, the occurrence of cavitation as the initial manifestation, known as a cavitary sarcoidosis, is extremely rare [6]. Cavities can be identified either at the initial presentation or may emerge over weeks or even years. Cavities, whether solitary or multiple, typically exhibit a round shape with smooth margins [7]. These cavities may undergo spontaneous resolution, and if not, they almost invariably respond to corticosteroid therapy [7]. While spontaneous remission of the disease is common, it is noteworthy that over 50 % of patients will necessitate treatment. Additionally, up to 30 % of patients may experience a chronic progressive non-remitting disease, characterized by pronounced pulmonary fibrosis, leading to substantial morbidity and, in some cases, mortality [8]. We present this case report to highlight the necessity for proper diagnostic measures, including high resolution CT and additional histopathological investigation, and appropriate pharmacological treatment in rare cases of non-typical asymptomatic cavitary sarcoidosis.
2. Case presentation
Patient T., a 36-year-old male, during the time of a regular health check-up in 2019, had shadows in the upper lobes of both lungs detected in a computed tomography (CT) of the chest. The patient denied a history of tuberculosis or contact with tuberculosis patients. On auscultation, vesicular breath sounds were similar on both sides. Examination of other bodily systems, including eyes and skin, did not reveal any abnormalities. The patient's medical history contained no systemic diseases or allergies. There was no exposing to professional influences. The patient denied smoking. The patient did not report any persistent complaints. His vital signs were normal including the blood oxygen saturation level (SpO2), except the cavitary lesions of the lungs on high resolution CT (HRCT) of the chest (Fig. 1A).
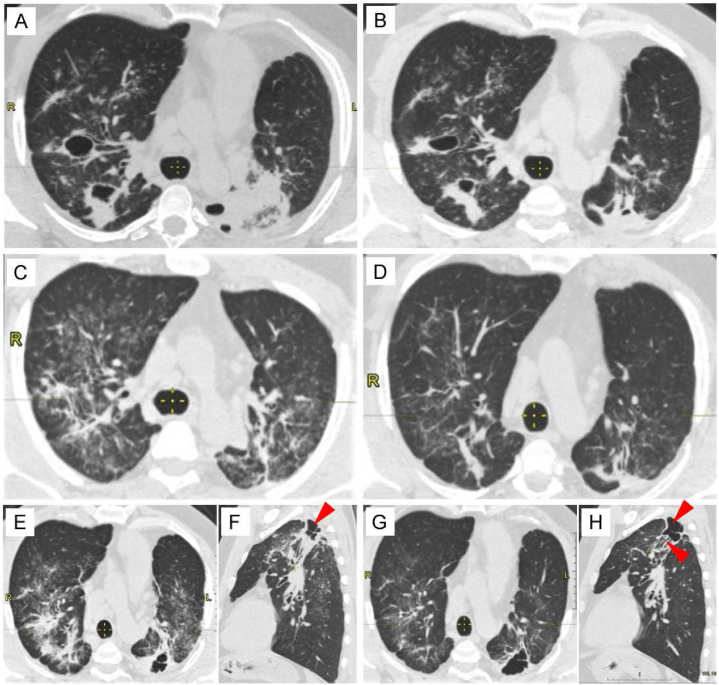
Fig. 1.
The image illustrates the disease progression timeline, CT findings and treatment strategies. A – E, G - High-resolution computed tomography in axial projection, (F, H- sagittal planes) curvilinear reconstruction of the chest. The red arrows in (F) and (H) indicate a multichambered cavity with irregular inner walls, located on the left in S2.
Complete blood count and biochemistry parameters were within normal range. The purified protein derivative (PPD) test conducted on May 2019 was negative, and sputum acid resistant bacteria (ARB) samples were consistently negative in three consecutive evaluations. The diagnosis of tuberculosis was expelled. The patient demonstrated normal parameters by spirometry (Table 1). HRCT of the chest revealed bilaterally enlarged mediastinal lymph nodes (Fig. 1), in the upper lobes on both sides, areas of infiltration with cavities of decay were noted. The level of angiotensin-converting enzyme (ACE) was 15 U/L (normal range within 12–68 U/L). C-reactive protein, rheumatoid factor, Mantoux test, antinuclear antibody (ANA) and antineutrophil autoantibodies (ANCA) were negative. Similarly, other investigations such as complete blood count, liver, renal function tests and calcium level were within normal limits.
Table 1.
Pulmonary function test readings demonstrated no disturbances in spirography.
| August 2019 |
January 2024 |
|||||
|---|---|---|---|---|---|---|
| Actual | Predicted | Predicted % | Actual | Predicted | Predicted % | |
| FVC (L) | 4.17 | 4.62 | 90.44 | 3.75 | 4.57 | 82.00 |
| FEV1 (L) | 3.33 | 3.85 | 86.55 | 3.35 | 3.79 | 88.37 |
| FEV1/FVC (%) | 79.85 | 81.45 | 98.04 | 89.20 | 80.73 | 110.49 |
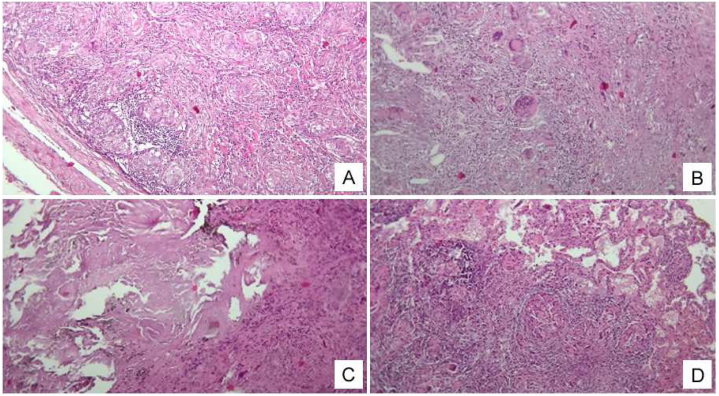
Next, three months later, i.e. in August 2019, the patient admitted to the thoracic surgery department of the National Scientific Medical Center, where a video-assisted thoracoscopic biopsy of the lung and mediastinal lymph node was performed for verifying the diagnosis of pulmonary sarcoidosis. Results of video-assisted thoracoscopic surgery (VATS) lung biopsy showed compact non-caseating epithelioid granulomas (Fig. 2). The samples of lung tissue are shown in Fig. 2C, D where multiple epithelioid cell granulomas with multiple multinucleated macrophages of the Pirogov-Langhans type and the type of foreign bodies are detected. Between these granulomas of varying densities, lymphocytic infiltration is observed. Some granulomas are separated from adjacent tissues by a thin layer of collagenized stroma. The pathological examination of the lung biopsy did not reveal evidence of angiitis. The necrotic areas associated with granulomatous inflammation are not linked to blood vessels, and there is no indication of destructive vasculitis. Necrotizing sarcoidosis has been excluded; the observed necrosis is homogenizing and eosinophilic, rather than caseous. The giant cells exhibit polymorphism with nuclei primarily located centrally, indicative of foreign body-type giant cells, though some have peripheral nuclei characteristic of Langhans-type giant cells. Mycobacterium tuberculosis (MTB) was not found using Ziehl-Neelsen staining, ruling out an infectious cause. This finding excludes the presence of granulomatous angiitis and necrotizing sarcoidosis.
Fig. 2.
Sarcoidosis of a lymph node and lung tissue. A - Multiple clearly demarcated ("stamped") sarcoid granulomas in the lymph node; B - Non-caseating granulomas (caseous necrosis is absent) in the lymph node, composed of epithelioid and multinucleated giant cells of the Langhans type. C - homogenized fibrinoid mass in the decay phase (forming cavities) in the lung tissue. D - epithelioid-cellular nodule in the interalveolar septum in the lung tissue. The main mass of the inflammatory infiltrate with multiple stamped granulomas. Lymphocytic infiltration is giant-cell. Staining: HxE. Magnification:10/х0,25.
The samples of the lymph node tissue with a fibrotic capsule (Fig. 2A and B), multiple giant multinucleated cells with non-caseous granulomas (caseous necrosis is absent) are represented. Granulomatous structures are delimited by layers of coarse-fibrous connective tissue, where there is a significant number of multinucleated cells of the Pirogov-Langhans type in the fibrotic capsule of the lymph node, and an accumulation of lymphoid cells with clear "stamped" boundaries.
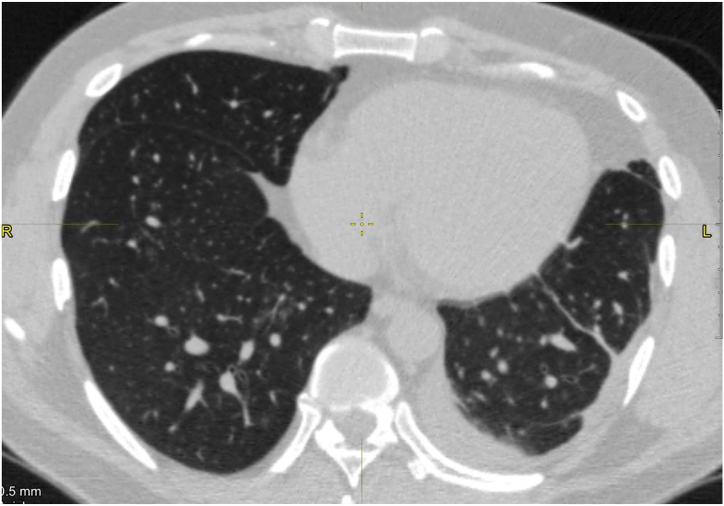
A CT chest scan demonstrates the presence of enlarged mediastinal bilateral lymph nodes with a diameter of up to 1.8 cm, and unilateral pleural effusion (Fig. 3) which indicates an increased risk of cavity sarcoidosis. Sarcoidosis diagnosis was confirmed on the basis of observing non-caseating granulomas on histology. Cavitations developed in the upper lobes of both lungs where consolidation was present. Subsequently, bullae appeared in the left upper lobe, indicating the ongoing presence of chronic sarcoidosis.
Fig. 3.
A high-resolution computed tomography axial projection, of the chest demonstrates pleural effusion on the left side.
A month later, starting from September 2019, the patient was treated with oral corticosteroids approximately for 5 years. First, it should be noted that the presence of diffuse reticulo-nodular pattern, with areas of lung consolidation bilaterally, has been observed at this point of treatment (Fig. 1A). Against the background of areas of consolidation, two large cavities are visible on the right in S2 (sized 34.7 mm × 22.3 mm) and S3 (30.8 mm × 15.2 mm). On the left, within the area of consolidation, several cavities ranging in size from 0.4 mm to 13.8 mm are visible. The patient was initiated on tablet prednisolone 30 mg with a tapering dosage over 6 months, which led to a reduction in the size of cavities and consolidation areas as seen in HRCT findings after 3 months (Fig. 1B). Treatment was given for a total of 6 months from the onset of detection of sarcoidosis. After 1 year, a follow-up chest HRCT showed resolving of pulmonary shadows (Fig. 1C). Positive changes were noted on the chest CT after 1.5 years, indicating a reduction in consolidation areas (Fig. 1D), and leading to the discontinuation of prednisolone. However, pulmonary cavitation appeared on chest HRCT 6 months later (Fig. 1E and F). Due to radiologically negative dynamics and the activation of the disease, steroid therapy was resumed at a dose of 20 mg per day for 6 months. A follow-up chest CT 3 months after resuming of prednisolone revealed linear fibrosis on the right lung in S2 and S3, absence of consolidation zone, and one multichamber cavity with irregular inner walls on the left in S2 (Fig. 1G and H). Upon further observation, despite of the changes in CT, no complaints have been reported by the patient. His vital signs were normal including the blood oxygen saturation level (SpO2) and pulmonary function test before the commencement of steroid therapy once the diagnosis of sarcoidosis has been established and 5 years and half later in January 2024 (Table 1).
DISCUSSION: This case report highlighted that sarcoidosis can manifest without symptoms or clinical signs but with a variety of radiological abnormalities that require rapid recognition and thorough assessment by dynamic monitoring and CT scans. Pulmonary function tests provide insight into the effects of sarcoidosis on the lungs, but they may not always align with radiographic staging results, as seen in our case.
Diagnosis of sarcoidosis at the beginning stage may be complicated and require strong differential diagnosis. Systematic review with 556 asymptomatic patients with suspected radiographic stage 1 sarcoidosis demonstrated that the diagnosis was confirmed in 85 % of the patients, while 11 % was undifferentiated and 1.9 % patients had an alternative diagnosis such as tuberculosis and lymphoma [9].
Spontaneous remission occurs frequently, so treatment may not always be necessary unless the disease presents symptoms or leads to progressive organ damage or dysfunction. For instance, only one-third of patients with stage II and III disease experience spontaneous regression of radiographic abnormalities [10]. Previous research indicated that wall thickening consistently correlated with subsequent infectious complications. Most frequent complications from cavitary lesions were as follows: hemoptysis (25 %), aspergilloma (15 %), pneumothorax, and other infections [11]. Pleuritis, as complication in our case, can also present but very rarely in about 1 % of individuals diagnosed with sarcoidosis [3]. The pleural effusion in our case was diagnosed due to sarcoidosis, and was additionally supported by the presence of other typical features of this disease, such as granulomatous changes in the lungs and mediastinal lymph nodes. Sarcoidosis can cause pleuritis and, as a result, pleural effusion, although such cases are relatively rare, as mentioned above.
Treating the disease is crucial in cases where there is a risk of organ failure. Despite an increase in studies dedicated to the pulmonary sarcoidosis in recent years, the available information remains limited, and there is no consensus on how to monitor the disease's activity. A retrospective study of cavitary sarcoidosis showed that the disease often remained chronic despite of ongoing treatment, with only 15 % of patients recovering [12]. Furthermore, those with stage III and IV disease have a five-fold higher risk of developing chronic respiratory impairment compared to patients with stage I disease [10].
In another research, patients without symptoms and any improvement in their radiographic results were randomly assigned to receive either glucocorticoids for a minimum of 18 months or glucocorticoids only if their condition worsened clinically. The group that received treatment demonstrated superior functional outcomes at 5 years [1]. The documented incidence of illness recurrence following the cessation of glucocorticoid treatment after a period of 2 years varies between 20 % and 80 % [13].
The updated European Respiratory Society guidelines (2021) recommend initiation steroid treatment for untreated patients with significant pulmonary sarcoidosis who are at high risk of future mortality or permanent disability, to improve and/or preserve forced vital capacity (FVC) and quality of life. Treatment decisions should be based on assessing the risk of mortality, organ dysfunction, and the impact on quality of life [13].
The described clinical case presents a rare scenario of asymptomatic, recurrent cavitary sarcoidosis. It is essential for these patients to receive an early diagnosis of the disease in order to prevent critical morbidity and mortality. An early diagnosis can be achieved through instrumental methods such as high-resolution CT scans (during a routine check-up), combined with histopathological analysis and additional tests such as bacterial cultures if there are suspicious lesions in the lung tissue.
3. Limitations
The presented clinical case has some inherent limitations. This was a rare case of asymptomatic cavitary sarcoidosis, the specific traits and features of the clinical and histological data in this patient make it difficult to generalize the conclusions. Therefore, we did not conduct additional tests for bacterial and fungal cultures on the lung tissue samples although these infections can cause cavitary lesions and need to be differentiated from sarcoidosis [14]. During the observation period, we repeated the Diaskintest, which came back negative. In the future, given the importance of additional serological tests for a more comprehensive evaluation, these should be included in our clinical guidelines in order to improve diagnostic accuracy.
4. Conclusion
In summary, cavitary lesions in pulmonary sarcoidosis typically occur in active and more severe stages of the disease. Their progression and development are difficult to predict, and they can lead to serious complications. There is currently uncertainty regarding how to manage asymptomatic patients with recurring cavitary features, even if their pulmonary function and clinical symptoms remain stable. More research is needed to accurately determine the incidence and best management strategies for cavitary lesions in sarcoidosis.
CRediT authorship contribution statement
Alexey Pak: Writing – review & editing, Validation, Supervision, Project administration, Formal analysis, Data curation, Conceptualization. Abai Baigenzhin: Writing – review & editing, Supervision, Project administration, Methodology, Data curation, Conceptualization. Zhanar Zarkumova: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology. Elmira Chuvakova: Writing – original draft, Resources, Methodology, Funding acquisition, Conceptualization. Manana Peradze: Writing – review & editing, Visualization, Methodology, Formal analysis. Lina Zaripova: Writing – review & editing, Writing – original draft, Formal analysis, Data curation, Conceptualization.
5. Patient perspective
Upon discovery of specific details on the CT images during a regular check-up, the patient was informed about the possible diagnosis, the progression of the condition, and the possible outcomes if left untreated. The patient fully agreed with the proposed additional diagnostic tests and treatments, was satisfied with all procedures and manipulations, and had no treatment-related complications. The patient did not express any complaints regarding the financial costs associated with the treatment.
Ethics statement
Written informed consent was acquired from the patient and the patient consented to the publishing of all images, clinical data, and other data included in the manuscript.
Data availability statement
The research data were not published in publicly available repositories because all relevant data are included to the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
Abbreviations
- ACE –
Angiotensin-converting enzyme
- ANCA –
Antinuclear antibody
- ANCA –
Antineutrophil autoantibodies
- ARB –
Acid Resistant Bacteria
- CT –
Computed Tomography
- CXR –
Chest X-Ray
- FVC –
Forced vital capacity
- HRCT –
High-Resolution Computed Tomography
- PPD –
Purified Protein Derivative
- SpO2 –
Saturation of peripheral oxygen
References
- 1.Crouser E.D., Maier L.A., Wilson K.C., Bonham C.A., Morgenthau A.S., Patterson K.C., Abston E., Bernstein R.C., Blankstein R., Chen E.S., Culver D.A., Drake W., Drent M., Gerke A.K., Ghobrial M., Govender P., Hamzeh N., James W.E., Judson M.A., Kellermeyer L., Knight S., Koth L.L., Poletti V., Raman S.V., Tukey M.H., Westney G.E., Baughman R.P. Diagnosis and detection of sarcoidosis. An official American thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020;201:e26–e51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y., Liang Z., Zheng Y., Qiao J., Wang P. Pulmonary sarcoidosis: from clinical features to pathology- narrative review. Ann. Palliat. Med. 2021;10:3438–3444. doi: 10.21037/apm-21-344. [DOI] [PubMed] [Google Scholar]
- 3.Jeon M.H., Kang T., Yoo S.H., Swan H.S., Kim H.J., Ahn H.S. The incidence, comorbidity and mortality of sarcoidosis in Korea, 2008-2015: a nationwide population-based study, Sarcoidosis, vasculitis, and diffuse lung diseases. official journal of WASOG. 2020;37:24–26. doi: 10.36141/svdld.v37i1.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mañá J., Rubio-Rivas M., Villalba N., Marcoval J., Iriarte A., Molina-Molina M., Llatjos R., García O., Martínez-Yélamos S., Vicens-Zygmunt V., Gámez C., Pujol R., Corbella X. Multidisciplinary approach and long-term follow-up in a series of 640 consecutive patients with sarcoidosis: cohort study of a 40-year clinical experience at a tertiary referral center in Barcelona, Spain. Medicine. 2017;96 doi: 10.1097/md.0000000000007595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunninghake G.W., Costabel U., Ando M., Baughman R., Cordier J.F., du Bois R., Eklund A., Kitaichi M., Lynch J., Rizzato G., Rose C., Selroos O., Semenzato G., Sharma O.P. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders, Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16:149–173. [PubMed] [Google Scholar]
- 6.Handa A., Dhooria S., Sehgal I.S., Agarwal R. Primary cavitary sarcoidosis: a case report, systematic review, and proposal of new diagnostic criteria, Lung India. official organ of Indian Chest Society. 2018;35:41–46. doi: 10.4103/lungindia.lungindia_225_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohatgi P.K., Schwab L.E. Primary acute pulmonary cavitation in sarcoidosis. AJR. American journal of roentgenology. 1980;134:1199–1203. doi: 10.2214/ajr.134.6.1199. [DOI] [PubMed] [Google Scholar]
- 8.Obi O.N., Saketkoo L.A., Russell A.M., Baughman R.P. Sarcoidosis: updates on therapeutic drug trials and novel treatment approaches. Front. Med. 2022;9 doi: 10.3389/fmed.2022.991783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sève P., Pacheco Y., Durupt F., Jamilloux Y., Gerfaud-Valentin M., Isaac S., Boussel L., Calender A., Androdias G., Valeyre D., El Jammal T. Sarcoidosis: a clinical overview from symptoms to diagnosis. Cells. 2021;10 doi: 10.3390/cells10040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungprasert P., Crowson C.S., Carmona E.M., Matteson E.L. Outcome of pulmonary sarcoidosis: a population-based study 1976-2013, Sarcoidosis, vasculitis, and diffuse lung diseases. official journal of WASOG. 2018;35:123–128. doi: 10.36141/svdld.v35i2.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzunhan Y., Nunes H., Jeny F., Lacroix M., Brun S., Brillet P.Y., Martinod E., Carette M.F., Bouvry D., Charlier C., Lanternier F., Planès C., Tazi A., Lortholary O., Baughman R.P., Valeyre D. Chronic pulmonary aspergillosis complicating sarcoidosis. Eur. Respir. J. 2017;49 doi: 10.1183/13993003.02396-2016. [DOI] [PubMed] [Google Scholar]
- 12.Hours S., Nunes H., Kambouchner M., Uzunhan Y., Brauner M.W., Valeyre D., Brillet P.Y. Pulmonary cavitary sarcoidosis: clinico-radiologic characteristics and natural history of a rare form of sarcoidosis. Medicine. 2008;87:142–151. doi: 10.1097/MD.0b013e3181775a73. [DOI] [PubMed] [Google Scholar]
- 13.Baughman R.P., Valeyre D., Korsten P., Mathioudakis A.G., Wuyts W.A., Wells A., Rottoli P., Nunes H., Lower E.E., Judson M.A., Israel-Biet D., Grutters J.C., Drent M., Culver D.A., Bonella F., Antoniou K., Martone F., Quadder B., Spitzer G., Nagavci B., Tonia T., Rigau D., Ouellette D.R. ERS clinical practice guidelines on treatment of sarcoidosis. Eur. Respir. J. 2021;58 doi: 10.1183/13993003.04079-2020. [DOI] [PubMed] [Google Scholar]
- 14.Valeyre D., Bernaudin J.F., Brauner M., Nunes H., Jeny F. Infectious complications of pulmonary sarcoidosis. J. Clin. Med. 2024;13 doi: 10.3390/jcm13020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The research data were not published in publicly available repositories because all relevant data are included to the manuscript.