Abstract
Myocardial injury (MI) is a common occurrence in clinical practice caused by various factors such as ischemia, hypoxia, infection, metabolic abnormalities, and inflammation. Such damages are characterized by a reduction in myocardial function and cardiomyocyte death that can result in dangerous outcomes such as cardiac failure and arrhythmias. An endoplasmic reticulum stress (ERS)-induced unfolded protein response (UPR) is triggered by several stressors, and its intricate signaling networks are instrumental in both cell survival and death. Cardiac damage frequently triggers ERS in response to different types of injuries and stress. High levels of ERS can exacerbate myocardial damage by inducing necrosis and apoptosis. To target ERS in MI prevention and treatment, current medical research is focused on identifying effective therapy approaches. Traditional Chinese medicine (TCM) is frequently used because of its vast range of applications and low risk of adverse effects. Various studies have demonstrated that active components of Chinese medicines, including polyphenols, saponins, and alkaloids, can reduce myocardial cell death, inflammation, and modify the ERS pathway, thus preventing and mitigating cardiac injury. Thus, this paper aims to provide a new direction and scientific basis for targeting ERS in MI prevention and treatment. We specifically summarize recent research progress on the regulation mechanism of ERS in MI by active ingredients of TCM.
Keywords: Natural products, Endoplasmic reticulum stress, Myocardial injury, Unfolded protein response, Cardiovascular disease
Graphical abstract
Highlights
-
•
Advances in Natural Products Targeting Endoplasmic Reticulum Stress for Myocardial Injuries.
-
•
Mechanisms of natural products ameliorating myocardial injury by regulating endoplasmic reticulum stress.
-
•
Natural products are promising in the prevention and treatment of various myocardial injury diseases.
1. Introduction
The endoplasmic reticulum (ER) is a multifunctional organelle that plays a critical role in protein and lipid production, processing, and transport [1]. Apart from synthesizing cellular lipids like glycerophospholipids, ceramides, and cholesterol, it also coordinates protein folding and translocation, transmembrane, and secretion. Additionally, ER controls cellular signaling, storage, and uptake of Ca2+ [2]. Endoplasmic reticulum stress (ERS) is the competition of ER homeostasis, causing the accumulation of misfolded and unfolded proteins, brought on by cellular events that are pathologic [3].
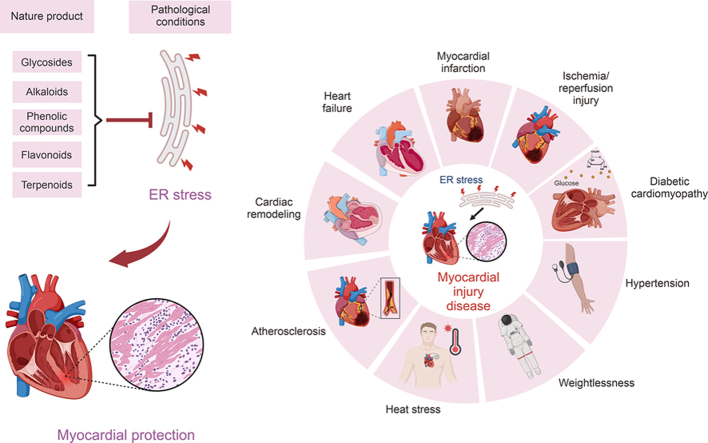
Myocardial damage is a crucial component of cardiovascular illnesses diseases (CVDs), the leading cause of death and morbidity globally [4]. Myocardial injury (MI) can develop due to diverse pathological conditions such as hypertension, ischemic heart disease, heart failure, myocardial infarction, metabolic cardiomyopathy, space weightlessness, and heat stress, among others [[5], [6], [7]].
Pathophysiologic factors that contribute to developing cardiovascular diseases, such as inflammation, hypoxia, and metabolic disorder, place substantial demands on the ER protein folding machinery, leading to ERS [[8], [9], [10]]. The non-adaptive unfolded protein response (UPR) triggers apoptosis, leading to ERS and damages cardiomyocytes, while oxidative stress and inflammation become imminent due to ERS, ultimately impacting CVDs [9,11]. Targeting ERS, particularly non-adaptive UPR, has become a practical therapeutic option for managing diseases due to the significant role played by ERS in CVDs' pathophysiology.
Although many drugs can treat CVDs, using existing medications in the long-term results in adverse effects such as rhabdomyolysis, renal failure, and hemorrhage [12]. The invention of novel medications has benefited significantly from natural products derived from plants, animals, and microorganisms, fostering human healthcare [13]. Numerous researches have shown that several natural substances can protect damaged myocardium by targeting ERS pathways. Therefore, natural compounds targeting the ERS pathway are significant as an effective treatment approach to minimize myocardial damage.
2. Methodology
A comprehensive search of electronic databases such as Scopus, PubMed, Web of Science, and Google Scholar was carried out using specific grid terms, including “ERS”, “ERS and myocardial protection”, “ERS and MI”, and “natural products of myocardial protection”. Moreover, bibliographies of all retrieved publications up to January 2024 were also reviewed for information on in vivo, in vitro, and human clinical investigations where natural products were employed to target at least one mediator of ERS involved in cardiovascular diseases.
3. ERS
The accumulation of unfolded proteins in the lumen of the ER, caused by nutritional shortages, hypoxia, genetic mutations, infections, disruptions in Ca2+ homeostasis, redox imbalance, altered protein glycosylation, and overexpression of folding-deficient proteins, results in ERS [14,15]. ERS is characterized by the buildup of unfolded proteins, and in response, the adaptive mechanism called the UPR is activated to reestablish ER homeostasis [16]. The UPR stimulates two distinct pathways, adaptive and pro-apoptotic. To restore ER homeostasis, four essential pathways are maintained: a) reducing protein production to prevent the further accumulation of unfolded and misfolded proteins, b) transcriptionally activating ER chaperone genes such as glucose regulated protein78 (GRP78)/binding-immunoglobulin protein (BiP) or glucose regulatory protein94 (GRP94) to promote protein folding, c) transcriptionally upregulating ER-associated degradation (ERAD) component genes to increase degradation of unfolded proteins via the ubiquitin-proteasome pathway, and d) initiating apoptosis to eliminate ERS-damaged cells, thereby ensuring survival of the organism [15]. However, in chronic ERS, if the adaptive UPR pathway fails to restore homeostasis, apoptotic pathways such as protein kinase RNA (PKR)-like ER kinase (PERK), calcium signaling, inositol-requiring enzyme 1 (IRE1)-c-Jun N-terminal kinase (JNK)-mediated apoptosis, and C/EBP homologous protein(CHOP)-mediated apoptosis can result in cell death.
3.1. Adaptive pathways of the UPR
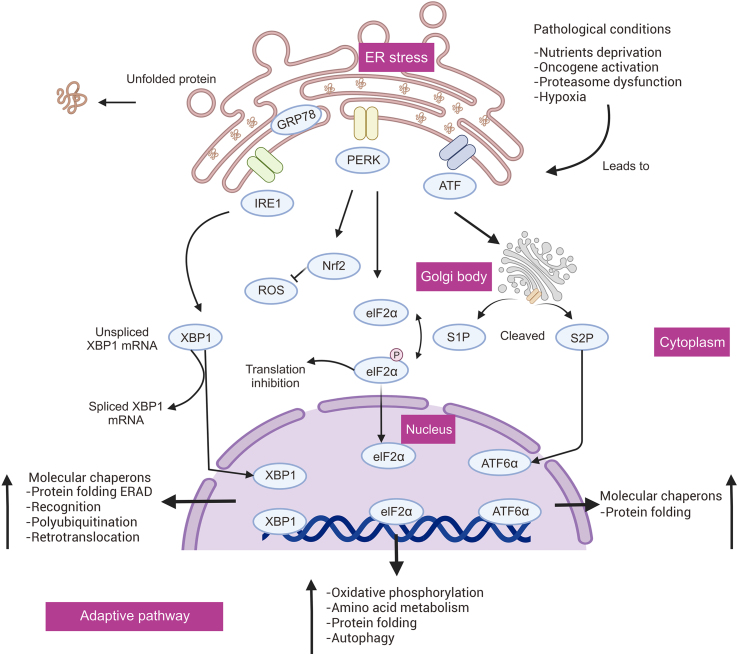
Adaptive mechanisms, such as the UPR and ERAD, are enacted to combat high ERS. The UPR is an adaptive response to reduce ERS conditions, which is triggered by protein buildup in the ER lumen during protein misfolding. In contrast, ERAD regulates the ER lumen's capacity to eliminate misfolded proteins [17]. Three separate branches of the UPR, controlled by IRE1, PERK, and activating transcription factor 6 (ATF6), respectively, initiate when ER transmembrane sensors detect the accumulation of unfolded proteins in the ER [18]. The UPR attempts to restore protein deposition, but sustained activation of the UPR results in a maladaptive response [17]. Normally, the ER molecular chaperone GRP78/BiP binds to the luminal structural domain to keep the transmembrane sensors of the UPR's adaptive route inactivated: IRE1, PERK, and ATF6 [19]. However, the unfolded protein binds to GRP78 and dissociates it from the transmembrane sensors to activate them and induce the UPR [20]. When the UPR is active, three signaling pathways (IRE1, PERK, and ATF6) are triggered to promote protein folding and restore ER homeostasis (Fig. 1).
Fig. 1.
Endoplasmic reticulum stress (ERS) activates adaptive signaling pathways. ER: endoplasmic reticulum; GRP78: glucose regulated protein78; IRE1: inositol-requiring enzyme 1; PERK: protein kinase RNA (PKR)-like ER kinase; ATF: activating transcription factor; XBP1: X-box-binding protein 1; ROS: reactive oxygen species; Nrf2: nuclear factor erythroid-2-related factor 2; eIF2α: eukaryotic translation initiation factor 2α; S1P: site 1 protease; S2P: site 2 protease; ATF6: activating transcription factor 6; ERAD: ER-associated degradation.
3.1.1. IRE1 pathway
IRE1, an ER transmembrane protein of the UPR, is expressed in intestinal epithelial cells and produces two isoforms: IRE1α and IRE1β, which are identified globally in human beings. Upon dissociation of GRP78, IRE1-activated ribonucleic acid endonuclease splices X-box-binding protein 1 (XBP1) mRNA, which encodes a basic leucine-zipper (b-ZIP) transcription factor. This factor upregulates genes that promote folded proteins, such as protein disulfide isomerase (PDI), and genes necessary for ERAD, such as ER degradation-enhanced-alpha-mannose-like protein (EDEM) [20,21]. ERAD, in association with the cytoplasmic ubiquitin-proteasome system (UPS), decreases the accumulation of unfolded proteins, thus restoring ER homeostasis [22]. Molecular chaperones and folding enzymes form a well-established recognition system of ERAD, which selectively transports unfolded proteins for destruction and guides the precise folding of released proteins [23]. Molecular chaperones facilitate proper protein folding, prevent protein aggregation, and regulate mitochondrial and protein breakdown [24].
3.1.2. ATF6 pathway
ATF6 is a transmembrane protein located on the ER that has two isoforms, namely ATF6α and ATF6β. During chronic ERS, GRP78 dissociates from ATF6 and leads to its activation. S1 and S2 proteases then cleave ATF6, resulting in cytoplasmic fragments of ATF6-α that are subsequently transported to the nucleus. These fragments act as transcription factors that regulate the production of XBP1. Specifically, ATF6α heterodimerizes with XBP1 to promote ERAD of the gene [25,26].
3.1.3. PERK pathway
PERK is another UPR-type ER transmembrane protein [27]. In times of ERS, the binding of GRP78 to the luminal structural domain is disrupted, leading to activation of PERK. As a result, the alpha-subunit of eukaryotic translation initiation factor 2 (eIF2) oligomerizes, causing the phosphorylation of Ser51 [28], which in turn halts eIF2 phosphorylation and decreases protein synthesis, influx into the ER lumen, and ER protein loading. Moreover, to attenuate oxidative stress, active PERK phosphorylates nuclear factor erythroid-2-related factor 2 (NrF2), which regulates the expression of genes that respond to antioxidants, such as heme oxygenase 1 (HO-1) and glutathione S-transferase [29].
3.2. Pro-apoptotic pathway
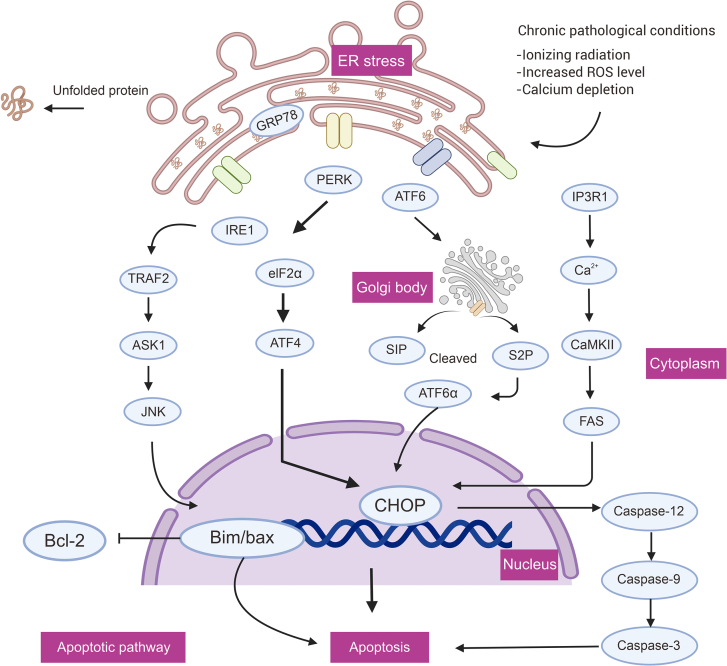
During chronic ERS, if the adaptive mechanisms of the UPR fail to restore in vivo equilibrium, several pro-apoptotic signaling pathways will trigger, leading to cell death due to the malfunction of ER [30]. The UPR apoptotic pathway comprises IRE1-JNK, PERK-CHOP, ATF6-CHOP, and the IP3R1-Ca2+ channel, which regulates the inositol 1,4,5-trisphosphate receptor type 1 (IP3R1) and Ca2+ levels. Apoptosis ensues if the UPR does not stabilize and control ER, as mediated by CHOP, IRE1/tumor necrosis factor receptor-associated factor 2 (TRAF2), and Ca2+-related signaling pathways [30,31](Fig. 2).
Fig. 2.
Endoplasmic reticulum stress (ERS) activates pro-apoptotic signaling pathways. ER: endoplasmic reticulum; GRP78: glucose regulated protein78; IRE1: inositol-requiring enzyme 1; PERK: protein kinase RNA (PKR)-like ER kinase; ATF: activating transcription factor; eIF2α: eukaryotic translation initiation factor 2α; ATF4: activating transcription factor 4; ATF6: activating transcription factor 6; TRAF2: tumor necrosis factor receptor-associated factor 2; ASK1: apoptotic signaling kinase-1; JNK: c-Jun N-terminal kinase; S1P: site 1 protease; S2P: site 2 protease; IP3R1: inositol 1,4,5-trisphosphate receptor type 1; CaMKII: Ca2+/calmodulin-dependent protein kinase II; FAS: Fas cell surface death receptor; CHOP: C/EBP homologous protein; Bcl-2: BCL2 apoptosis regulator.
3.2.1. IRE1α and CHOP related apoptosis
Chronic ERS triggers the activation of IRE1α, which then phosphorylates and binds to TRAF2 in cytoplasmic structural regions. This interaction activates apoptotic signaling kinase-1 (ASK1) [32,33]. ASK1, by inactivating the anti-apoptotic BCL2 apoptosis regulator (Bcl-2) protein, activates kinases such as JNK, which stimulate BAK and BAX. These, in turn, release cytochrome C and cause apoptosis via the intrinsic apoptotic pathway [34]. Furthermore, CHOP is an apoptotic marker in ERS, and is often referred to as the growth arrest and DNA damage-inducible gene (GADD153). In severe and irreversible ERS, the PERK-eIF2-activating transcription factor 4 (ATF4)-CHOP pathway is preferred, while all three UPR apoptotic pathways support programmed death in CHOP [35].
3.2.2. Calcium signaling apoptotic pathway
Calcium signaling pathways have been linked to CHOP-mediated apoptosis. Activation of CHOP-dependent ER oxidase 1 alpha promotes calcium release via IP3R1 [36], which in turn activates the calcium-sensing enzyme Ca2+/calmodulin-dependent protein kinase II (CaMKII). This leads to the activation of various downstream apoptotic pathways such as Fas cell surface death receptor (FAS), JNK activation, and mitochondrial apoptogen release [37]. Furthermore, matrix-interacting molecule 1 (Stim1) has a crucial role in ERS through its regulation of the calcium pathway. Stim1 senses Ca2⁺ levels in the ER through its EF-hand structural domain [38] and translocates to the plasma membrane when Ca2⁺ is depleted in the ER. In this state, Stim1 interacts with and activates ORAI1, a subunit of the Ca2⁺ release-activated Ca2⁺ channel (CRAC), which facilitates calcium entry into the cell [39]. Dysfunctional Stim1 directly affects Ca2⁺ levels in the ER, leading to the initiation or exacerbation of ERS. If Stim1 is impaired, it may result in an imbalance of Ca2⁺ homeostasis in mitochondria, thus affecting mitochondrial structure and function [40]. This imbalance leads to cytochrome C release from mitochondria and reduces mitochondrial membrane potential, which ultimately induces mitochondria-driven apoptosis [41].
4. An overview of advances in ERS in MI disease
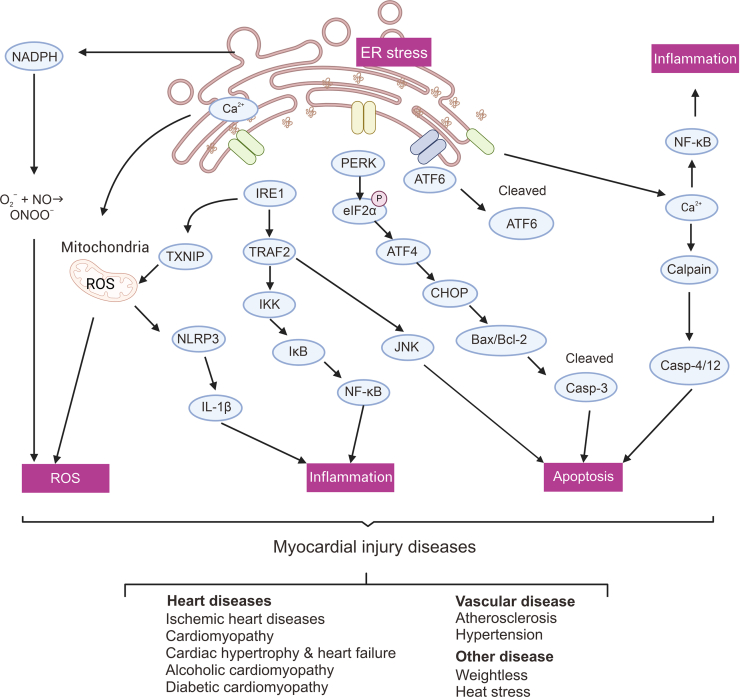
MI and vascular endothelial damage are typical consequences of a range of prevalent cardiovascular conditions, including hypertension, coronary heart disease, and heart failure, which are all triggered by varied stress stimuli. Preserving homeostasis in the ER is intricately linked to the proper functioning of the cardiovascular system. ERS serves as a contributor to, and a result of, various cardiovascular diseases such as stroke, hypertension, ischemic heart disease, heart failure and different cardiomyopathies (Fig. 3). This cycle of disease has the potential to trap the body [9,11].
Fig. 3.
Endoplasmic reticulum stress (ERS)-induced unfolded protein responses (UPRs) are associated with various signaling pathways in myocardial injury (MI). NADPH: nicotinamide adenine dinucleotide phosphate oxidase; ER: endoplasmic reticulum; ROS: reactive oxygen species; TXNIP: thioredoxin interacting protein; NLRP3: NLR family fyrin domain containing 3; IL-1β: interleukin 1beta; IRE1: inositol-requiring enzyme 1; TRAF2: tumor necrosis factor receptor-associated factor 2; IKK: inhibitor of kappa B kinase; NF-κB: nuclear factor kappa-B; IκB: inhibitor of NF-κB; PERK: protein kinase RNA (PKR)-like ER kinase; eIF2α: eukaryotic translation initiation factor 2α; ATF4: activating transcription factor 4; CHOP: CCAAT/enhancer-binding protein homologous protein; Bcl-2: BCL2 apoptosis regulator; ATF6: activating transcription factor 6.
4.1. ERS and ischemic heart disease
After ischemia/reperfusion (I/R), myocardial infarction is a common cause of MI, characterized by the production of pro-inflammatory cytokines, oxygen and nutrient depletion, and sudden load of oxygen free radicals into the heart, leading to inflammation and myocardial death [42]. The activation of pro-apoptotic pathways and the UPR can respond to these stimuli [43,44]. While pathogenic ERS induces apoptosis, the adaptive UPR can be helpful in myocardial I/R injury. Supporting this, genetically modified mice and pharmacological methods have further shown the advantageous adaptive role of ATF6 in myocardial I/R injury by promoting ERAD and misfolded protein degradation in the ER [45]. In mice with I/R injury, XBP1s is cardioprotective, and XBP1 mRNA splicing takes place and positively regulates ER activity in cardiac I/R injury. Utilizing strategies to activate the UPR may function as endogenous adaptive stress responses to counteract pathologic ERS caused by I/R injury. However, downstream PERK targets, such as CHOP, p53 upregulated apoptosis regulator (PUMA), and tribbles homolog 3 (TRIB3) may induce ERS-induced cell death following myocardial I/R injury. In mice with myocardial I/R injury, PAK2 downregulation precedes ERS, caspase-12 activation, ROS generation, apoptosis, and Ca2+ overload. Understanding the distinct functions of UPR elements and ERS in myocardial I/R injury could pave the way for the development of therapeutic intervention techniques.
4.2. ERS and heart failure
An increasing body of literature has indicated that ERS significantly influences heart failure and cardiac remodeling. Various factors, such as hypoxia and acute/chronic inflammatory reactions, can cause ERS in cardiomyocytes [46]. Structural alterations in ER and UPR components in cardiomyocytes have been found in patients with heart failure or cardiac hypertrophy [47,48]. The proliferation of ER tubules, observed during electron microscope examination, is a common characteristic of degenerating cardiomyocytes, indicating an overload of the ER in this state. Nonetheless, the adaptive UPR maintains cardiac homeostasis, whereas excessive ERS and stress-induced apoptosis are primarily due to cardiac pressure overload [49]. Elevated levels of CHOP and caspase-12 in failing human hearts signify an association between UPR activation and the pathogenesis of HF in humans. Likewise, in a model of azithromycin-induced heart failure, increased levels of GRP78 and CHOP were observed [50]. In this regard, apoptosis generated by ERS could be considered as the shared pathophysiological mechanism of heart failure caused by a variety of reasons. Overall, the findings suggest that molecules linked to ERS could be utilized as molecular targets in the treatment of heart failure and hypertrophy.
4.3. ERS and cardiomyopathy
ERS is a variable associated with the pathophysiology of cardiomyopathy [51]. Risk factors such as alcohol consumption, free fatty acid levels, and hyperglycemia can cause ERS, which, in turn, exacerbates the disease [52,53]. Increased levels of BiP, CaMKII, CHOP, mitogen-activated protein kinase (MAPK), and cleaved ATF6 are linked to autoimmune cardiomyopathies resulting in apoptosis, ventricular dilatation, and cardiac failure [54,55]. Furthermore, ERS and non-adaptive UPR contribute to diabetic cardiomyopathy (DCM) by inducing apoptosis via the PERK and ATF6 pathways [56]. Therefore, a supplement of taurine ursodeoxycholic acid (TUDCA) that reduces oxidative stress and encourages cell survival signals has been proposed to improve myocardial metabolism and alleviate cardiac injury [57]. On the other hand, ERS signaling pathways (PERK-eukaryotic translation initiation factor 2α (eIF2α)-ATF4, ATF6, and IRE1-XBP1) are activated in ethanol-induced dilated cardiomyopathy leading to apoptosis and altering cardiac mentation and remodeling. The significance of cholesterol metabolism in the development of metabolic cardiomyopathy has been acknowledged in recent years. Sterol regulatory element-binding proteins (SREBPs) have specifically been noted to affect the processing and synthesis of ATF6 [58,59], hence contributing to ERS [60]. Regulating the activity of SREBPs may improve ERS, myocardial metabolism, and blood supply, and attenuate the symptoms of metabolic cardiomyopathy [61].
4.4. ERS and atherosclerosis
Atherosclerosis is a primary pathological basis for numerous CVDs, and a prevalent risk factor for myocardial damage, causing significant impairment in people's quality of life. Multiple atherosclerosis risk factors lead to ERS, including reactive nitrogen generation, oxidative stress, hyperhomocysteinemia, and the accumulation of free cholesterol in macrophages [46,62].
Molecular pathways linked to dysfunctional UPR and ERS have been found to play a role in the development of atherosclerosis. Judging from the elevated levels of the ERS markers BiP and CHOP in human coronary artery lesions, we can conclude that ERS and the ERS response are involved in the development of atherosclerosis and the induction of smooth muscle cell apoptosis [63]. Endothelial cells that suffer from shear stresses promoting atherosclerosis exhibit a marked increase in ERS [64]. Additionally, according to transcriptional profiling, genes linked to ERS were consistently found to be upregulated in the endothelium of all atherosclerosis-prone areas [65].
Dong et al. [66] suggested that by preserving intracellular Ca2+ homeostasis and sarcoplasmic reticulum calcium transporting ATPase (SERCA) activity, 5′ adenosine monophosphate-activated protein kinase (AMPK) acts as a physiological inhibitor of ERS, as they found the suppression of endothelial cells' SERCA activity, and a dramatic increase in ERS when AMPK deficiency was present. Atherosclerosis poses a significant threat to public health, causing a high number of morbidity and deaths from CVDs. Therefore, tackling ERS and aberrant UPR might be a promising direction for treating and preventing atherosclerosis.
4.5. ERS and other MI diseases
Various factors that lead to myocardial damage have come to light in addition to prevalent cardiovascular diseases. Heat stress influences heart failure, myocardial damage and cardiac insufficiency [67]. Heat exposure leads to increased ROS levels causing oxidative damage to the heart tissue, while heat shock-driven ERS causes apoptosis. Heat stress-induced cardiac damage triggered significantly increased levels of cardiomyocyte angiotensin II (Ang II) and heart troponin I (cTn-I), which was linked to higher levels of ERS-related proteins GRP78 and CHOP. As space exploration programs continue, novel health issues have emerged. It has been found that the heart undergoes cardiac remodeling and MI when it's weightless. Ca2+ overload and ERS, elevated levels of cardiac trophoblast protein T (cTnT), creatine kinase-MB (CK-MB) in blood, and cardiomyocyte death are some of the consequences of activation of the Erk1/2 and CaMKII/histone deacetylase 4 (HDAC4) signaling pathways in weightless environments. Panax quinquefolium triggers the AMPK pathway, thereby repairing the damaged myocardium and reducing this injury. Furthermore, ERS is linked to numerous cardiovascular disorders and causes significant physiological dysfunction in cardiomyocytes. ERS-related substances may offer novel therapeutic approaches for targeted therapy aimed at alleviating cardiac damage.
5. Natural products targeting ERS for the treatment of MI
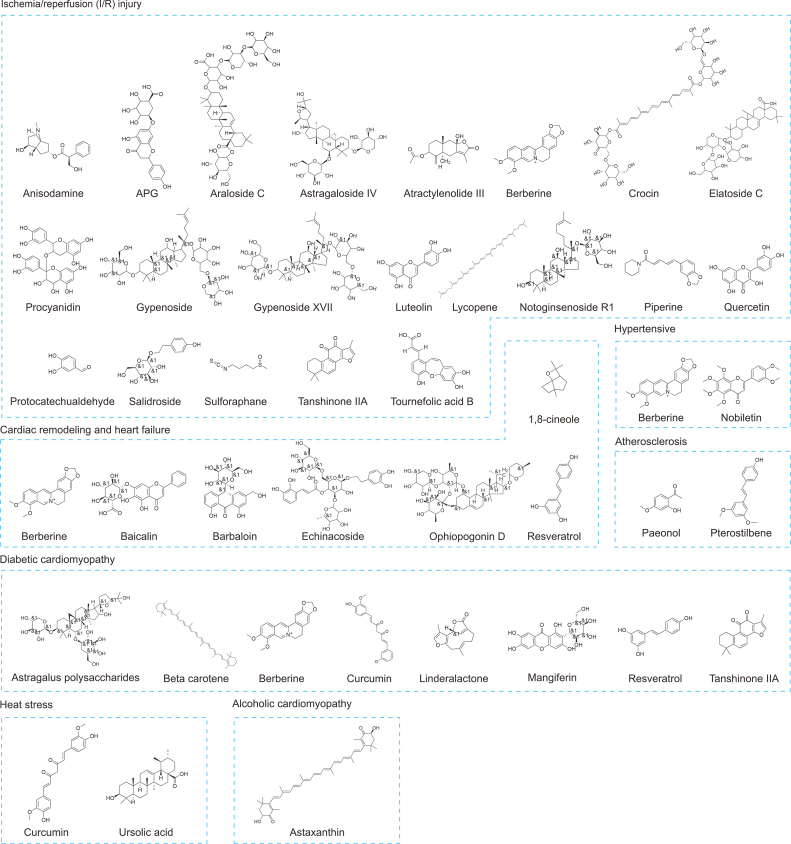
Cardiovascular disorders related to myocardial damage have been extensively studied in connection with natural compounds obtained from plants, bacteria, algae, and fungi that target ERS [68]. For this purpose, we summarized the mechanism of action of several active ingredients of Chinese herbal medicines used for the treatment of MI in Table 1 [[69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117]], and presented in Fig. 4.
Table 1.
Mechanisms of Chinese herbal medicine active ingredients regulating endoplasmic reticulum stress (ERS).
| Active ingredients | Chemical formula | Mechanism and effect | The disease of myocardial injury | Experimental model | Dose | Refs. |
|---|---|---|---|---|---|---|
| Anisodamine | C17H23NO4 | ↓GRP78, CHOP, Cleaved Caspase-3 | Myocardial injury after cardiac arrest and resuscitation in rats | Cardiac and I/R injury and arrest Wistar rats (in vivo) | 10 mg/kg | [69] |
| Apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside | C30H26O12 | ↓PERK, p-PERK, eIF2α, p-eIF2α, CHOP, Bax, Cleaved Caspase-3, ↑Bcl-2, p-AMPK, AMPK, | Myocardial hypoxia-reperfusion injury | Myocardial ischemia/reperfusion injury isolated heart of adult SD rats (in vivo) Hypoxia/Reoxygenation injury in primary neonatal rat cardiomyocytes (in vitro) |
4 μM | [70] |
| Araloside C | C53H84O23 | ↓PERK, eIF2α, ATF6, CHOP, Caspase-12, ↑HSP90 | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes | 12.5 μM | [71] |
| Astragaloside IV | C41H68O14 | ↓p-eIF2α, CHOP, Caspase-3, Caspase-9, ROS, ↑Bcl-2 | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 50, 10, 5, 1 and 0.5 μM |
[72] |
| Atractylenolide Ⅲ | C15H20O3 | ↓GRP78, Caspase-12, Caspase-3, Ca2+ | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 15, 30 and 60 μM | [73] |
| Berberine | C20H18NO4]+ | ↓ PERK, eIF2α, ATF4, CHOP | Myocardial hypoxia-reperfusion injury | Myocardial I/R injured in SD rats (in vivo) Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) |
200 mg/kg/d for 2 week; 50 μM | [74] |
| Crocin | C44H64O24 | ↓GRP78, Caspase 3, Bax, miR-34a, ↑SIRT1, Nrf2, HO-1 | Myocardial hypoxia-reperfusion injury | I/R-induced left ventricular dysfunction and infarct in C57BL/6 mice (in vivo) Hypoxia/Reoxygenation injury in primary neonatal mouse cardiomyocytes (in vitro) |
50 mg/kg/d for 1 week; 10 μM | [75] |
| Elatoside C | C46H74O16 | ↓GRP78, CHOP, Caspase-12, JNK | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 25 μM | [76] |
| Grape seed proanthocyanidins | C30H26O13 | ↓GRP78, CHOP, eIF2α, p-PERK | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 50, 100 and 200 μM | [77] |
| Gypenoside | C47H80O17 | ↓CHOP, GRP78, CHOP, Caspase-12, PERK, eIF2α, ATF4 ↑PI3K, Akt, p-GSK3β | Myocardial hypoxia-reperfusion injury; coronary heart diseases | Myocardial I/R injured in Wistar rats (in vivo) Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) |
200 mg/kg for 1 week | [78] |
| Gypenoside XVII | C48H82O18 | ↓GRP78, PERK, IRE1, P-JNK, CHOP, Bad, BAX, Caspase-12, p38-MAPK pathway ↑Bcl-2, PI3K/Akt pathway | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 5, 10 and 20 μM | [79] |
| Luteolin | C15H10O6 | ↓GRP78, p-eIF2α, IRE1α, XBP-1, ATF6, CHOP, Bax, Caspase-3, ↑Bcl-2 | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 10 μM | [80] |
| Lycopene | C40H56 | ↓CHOP, GRP78, ATF6, eIF2α, sXBP-1, Bax/Bcl-2, | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in primary neonatal rat cardiomyocytes (in vitro) | 5 μM | [81] |
| Notoginsenoside R1 | C47H80O18 | ↓Calnexin, BiP, CHOP, Caspase-3, ↑RyR2 | Myocardial hypoxia-reperfusion injury | Hypoxia/Reoxygenation injury in H9c2 cardiomyocytes, HL-1 cells, and primary cultured neonatal cardiomyocytes from Sprague–Dawley rats (in vitro) | 20, 40, 80 and 100μg/mL | [82] |
| Panax quinquefolium saponin | − | ↓GRP78, Calreticulin, CHOP, Bax, ↑Bcl-2 | Myocardial infarction | Acute myocardial infarction in SD rats (in vivo) | 50 and 100 mg/kg for 4 week | [83] |
| Piperine | C17H19NO3 | ↓GRP78, CHOP, Caspase-12, BiP, ↑p-PI3K, p-AKT | Myocardial ischemia/reperfusion injury | Hypoxia/Reoxygenation injury in primary neonatal rat cardiomyocytes from Sprague-Dawley (in vitro) | 20 μM | [84] |
| Protocatechualdehyde | C7H6O3 | ↓eIF2α, p-eIF2α, Ero1-Lα, PERK, CHOP, BiP, IRE1α, ATF6, Caspase-3, BAX, HIF-1α, ↑Bcl-2 | Myocardial ischemia/reperfusion injury | Oxygen-glucose deprivation/reoxygenation in H9c2 cardiomyocytes, and primary neonatal rat cardiomyocytes from Sprague Dawley (in vitro) | 5 μM | [85] |
| Quercetin | C15H10O7 | ↓PERK, CHOP, Caspase-12, ↑SIRT1, TMBIM6 | Myocardial ischemia/reperfusion injury | Hypoxia/reoxygenation in H9c2 cardiomyocytes (in vitro) | 150 mg/L | [86] |
| Salidroside | C14H20O7 | ↓p-PERK, p-eIF2α, CHOP, p-PERK, ↑AMPK | Myocardial ischemia/reperfusion injury | Myocardial ischemia in SD rats (in vivo) I/R injury in H9c2 cardiomyocytes (in vitro) |
40 mg/kg; 10 μM | [87] |
| Sulforaphane | C6H11NOS2 | ↓GRP78, CHOP, caspase-12, SIRT1, PERK, eIF2α, ↑Bcl-2 | Myocardial ischemia/reperfusion injury | Ischemia/reperfusion injury in H9c2 cardiomyocytes (in vitro) | 0.1–5 μM | [89] |
| Tanshinone IIA | C19H18O3 | ↓GRP78, CHOP, Caspase-12, ↑miR-133 | Myocardial injury | Tunicamycinc induced injury in primary neonatal rat cardiomyocytes (in vitro) | 10 μM | [90] |
| Tournefolic acid B | C17H12O6 | ↓CHOP, Caspase-12, JNK↑SOD、CAT, GSH-Px, p-PI3K, p-AKT, Bcl-2/Bax ratio | Myocardial ischemia/reperfusion injury | Hypoxia/reoxygenation injury in H9c2 cardiomyocytes (in vitro) | 0.5, 1 and 2 μM | [91] |
| Baicalin | C21H18O11 | ↓CHOP, ↑eNOS, NO | Clotrimazole-induced apoptosis in cardiomyocytes | Hypoxia/Reoxygenation injury in primary neonatal rat cardiomyocytes from SD rats (in vitro) | 12.5, 25 and 50 μM | [92] |
| Barbaloin | C21H22O9 | ↓GRP78, CHOP, PERK, p-PERK, ATF4, Caspase-12, Caspase-3, CNPY2 | Myocardial I/R injured in SD rats (in vivo) | 20 mg/kg/d for 1 week | [93] | |
| Berberine | C20H18NO4 | ↓CHOP, Caspase-12, Caspase-3 ↑Bcl-2/Bax ratio | Cardiac remodeling of heart failure after myocardial infarction | Myocardial infarction in Wistar rats (in vivo) | 20 mg/kg for 4 week | [94] |
| Echinacoside | C35H46O20 | ↓GRP78, P–I/REα, p-PERK, ATF6, CHOP, NADPH, ROS | Heart failure | Isoprenaline (ISO)-induced HF rats (in vivo) Isoprenaline (ISO)-induced primary cardiomyocytes of neonatal from Sprague–Dawley rats (in vitro) |
10 mg/kg/d for 2 week | [95] |
| Ophiopogonin D | C44H70O16 | ↓Bip, Bax, Perk、ATF4, Caspase-12, CHOP | Heart failure | Isoprenaline (ISO)-induced cardiac hypertrophy injury in H9c2 cardiomyocytes (in vitro) | 10 μM | [96] |
| Resveratrol | C14H12O3 | ↓GRP78, GRP94, CHOP, ↑Bcl-2/Bax ratio | Cardiac hypertrophy | Isoprenaline (ISO)-induced cardiac hypertrophy injury in H9c2 cardiomyocytes (in vitro) | 50 μM | [97] |
| 1, 8-cineole | C10H18O | ↓GRP78, PERK, ATF4, CHOP, ROS, Bcl-2/Bax ratio, p-Caspase 3 | Heart failure | Isoprenaline (ISO)-induced cardiac hypertrophy injury in H9c2 cardiomyocytes (in vitro) | 10, 15, 20, 25, 30, 35 and 40 μM; 120, 60 and 30 mg/kg/d for 4 week | [98] |
| Astragalus polysaccharides | C10H7ClN2O2S | ↓ATF6, PERK, CHOP | Diabetic cardiomyopathy | Streptozotocin-induced type 1 diabetic ratsl (in vivo) High Glucose (HG)-Induced H9c2 cardiomyocytes (in vitro) |
1 g/kg/d for 16 week; 0.8 mg/mL | [99] |
| Beta carotene | C40H56 | ↓ROS, Bax, ATF4, GRP78, CHOP, Beclin 1, p62, LC3II/LC3I ratio, ↑Bcl-2, p-PI3K, p-AKT, p-mTOR, SOD, GSH-Px | Diabetic cardiomyopathy | Advanced glycation end products-induced H9c2 cardiomyocytes (in vitro) | 40 μM | [100] |
| Berberine | C20H18NO4]+ | ↓XBP1, ↑AMPK, p-eNOS | Diabetes-Associated Endothelial Dysfunction | High-fat diet and streptozotocin induced diabetes in C57BL/6 J mice (in vivo) High sugar or clindamycin-treated HUVECs cell (in vitro) |
11.3, 14.5, 12.2 and 1 μM | [101] |
| Curcumin | C21H20O6 | ↓GRP78, CHOP, Caspase 3, BAX, | Diabetic cardiomyopathy | Palmitate treated H9c2 cardiomyocytes (in vitro) | 10 μM | [102]. |
| Curcumin | C21H20O6 | ↓p-PERK, p-eIF2α, and ATF4, ↑LC3-II | Lipotoxic cardiomyopathy | Palmitate treated H9c2 cardiomyocytes (in vitro) | 100 μM | [103] |
| Linderalactone | C15H16O3 | ↓ATF6, p-ERK, p-JNK, p38, CHOP, MAPKs | Diabetic cardiomyopathy | Streptozotocin-induced type 1 diabetes in C57BL/6 J mice (in vivo) | 2.5 or 5 mg/kg for 5 week | [104] |
| Mangiferin | C19H18O11 | ↓GRP78, CHOP, TXNIP, NLRP3, Caspase-3,↑AMPK, NO | Endothelial dysfunction induced by diabetes | High sugar treated EA.hy926 cells (in vitro) | 10 μM | [105] |
| Resveratrol | C14H12O3 | ↓PERK, eIF2α, ATF6, CHOP, IRE1α, JNK, ↑SIRT1 | Diabetic cardiomyopathy | Streptozotocin-induced diabetes in Sprague–Dawley rats (in vivo) High sugar treated H9c2 cardiomyocytes (in vitro) |
10 μM | [106] |
| Tanshinone IIA | C19H18O3 | ↓GRP78, ATF4, ATF6, XBP-1s, P-eIF2α, CHOP, ↑SIRT1, | Diabetic cardiomyopathy | High-sugar, high-fat diet induces diabetes in C57BL/6 J mice (in vivo) High sugar treated primary neonatal rat cardiomyocytes from C57BL/6 J mouse (in vitro) |
10, 50 and 250 mg/kg/day for 14 week; 1.5 and 6.25 μM | [107] |
| Black tea | − | ↓ATF3, ATF6, p-eIF2α, ROS | Hypertension-associated endothelial dysfunction | Angiotensin II (Ang II)-induced hypertension in Sprague–Dawley rats (in vivo) | 15 mg/kg/day for 2 week | [108] |
| Berberine | C20H18NO4]+ | ↓AFT3, ATF4, XBP1, COX2, ROS, ↑AMPK | Spontaneously hypertensive | carotid artery tissue of Spontaneously hypertensive rats (in vivo) | 1 μM | [109] |
| Nobiletin | C21H22O8 | ↓GRP78, GRP94, CHOP, Caspase-3 | Renal vascular hypertension and left ventricular remodeling | Renal vascular hypertensive Wistar rats (in vivo) | 100 mg/kg/d for 4 week | [110] |
| Paeonol | C9H10O3 | ↓GRP78, ATF6, eIF2α, ROS, ↑AMPK, PPARδ, NO | Atherosclerosis | Tunicamycin induced C57BJ/6 J and PPARδ wild type (WT) mouse aortas (in vivo) Tunicamycin-induced human umbilical vein endothelial cells, H5V cells (in vitro) |
0.1 μM | [111] |
| Pterostilbene | C16H16O3 | ↓PERK, eIF2α, ATF4, CHOP, ↑SIRT1 | Atherosclerosis | TNF-α- induced H9c2 cells (in vitro) | 0.1, 0.5, or 1 μM | [112] |
| Curcumin | C21H20O6 | ↓GRP78, CHOP, cTn-I, Ang II, ↑Bcl-2 | Heat-stress-induced cardiac injury | Heat stress exposure C57BL/6 J mice (in vivo) | 50, 100 and 200 mg/kg/day for 4 week | [113] |
| Rosa canina L, methanolic extract | − | ↓p-PERK, p-eIF2α, CHOP, Caspase 8 | Heat-stress-induced cardiac injury | Heat stress exposure Wistar rats (in vivo) | 500/1000 mg/mL | [114] |
| Ursolic acid | C30H48O3 | ↓p-PERK, p-eIF2α, CHOP, ↑Mcl-1 | Heat stress | Heat stress exposure ICR rats (in vivo) | 20 or 40 mg/kg | [115] |
| Panax quinquefolium saponin | − | ↓Erk1/2, CaMKII, HDAC4, CK-MB, cTnT, IMA, ↑AMPK | Cardiac remodeling induced by simulated microgravity | Unloading induced cardiac remodeling SD rats (in vivo) | 200 mg/kg/d for 8 week | [116] |
| Astaxanthin | C40H52O4 | ↓GRP78, p-PERK, p-eIF2α, ATF4, ATF6, p-IRE1, XBP-1, CHOP, Caspase-12 | Alcoholic cardiomyopathy | Ethanol induced Alcoholic cardiomyopathy in C57BL/6 J mice (in vivo) Ethanol treated H9c2 cardiomyocytes and primary cardiomyocytes (in vitro) |
20 mg/kg/d for 8 week; 10 μM | [117] |
GRP78: glucose regulated protein78; CHOP: C/EBP homologous protein; I/R: ischemia/reperfusion; PERK: protein kinase RNA (PKR)-like endoplasmic reticulum (ER) kinase; eIF2α: eukaryotic translation initiation factor 2α; Bcl-2: BCL2 apoptosis regulator; AMPK: 5′ adenosine monophosphate-activated protein kinase; SD rats: Sprague-Dawley rats; ATF6: activating transcription factor 6; HSP90: heat shock protein 90 alpha family class A member 1; ROS: reactive oxygen species; ATF: activating transcription factor; SIRT1: silencing information regulator 1; Nrf2: nuclear factor erythroid-2-related factor 2; HO-1: heme oxygenase 1; JNK: c-Jun N-terminal kinase; RyR2: type 2 ranibodin receptor; Bip: binding-immunoglobulin protein; HIF-1α: hypoxia inducible factor 1 subunit alpha; TMBIM6: transmembrane BAX inhibitor-1 motif-containing 6; CNPY2: cardiomyocyte-specific protein canopy homolog 2; NADPH: nicotinamide adenine dinucleotide phosphate oxidase; LC3II: microtubule associated protein 1 light chain 3 beta; XBP1: X-box-binding protein 1; MAPK: mitogen-activated protein kinase; TXNIP: thioredoxin interacting protein; NLRP3: NLR family fyrin domain containing 3; eNOS: endothelial-type nitric oxide synthase; HUVECs: human umbilical vein endothelial cells; NO: nitric oxide; ATF3: activating transcription factor 3; COX2: cyclooxygenase-2; PPARδ: peroxisome proliferator activated receptor gamma; cTn-I: cardiac troponin I; TNF-α: tumor necrosis factor-α; Ang II: angiotensin II; Mcl-1: MCL1 apoptosis regulator, BCL2 family member; GRP94: glucose regulatory protein94; HDAC4: histone deacetylase 4; CK-MB: creatine kinase-MB; cTnT: cardiac trophoblast protein T; CaMKII: Ca2+/calmodulin-dependent protein kinase II; IMA: ischemia-modified albumin; −: no data.
Fig. 4.
Molecular structure of natural products targeting endoplasmic reticulum stress (ERS) for treatment of myocardial injury (MI). APG: apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside.
5.1. Natural products target ERS mediated MI in I/R injury
5.1.1. Anisodamine
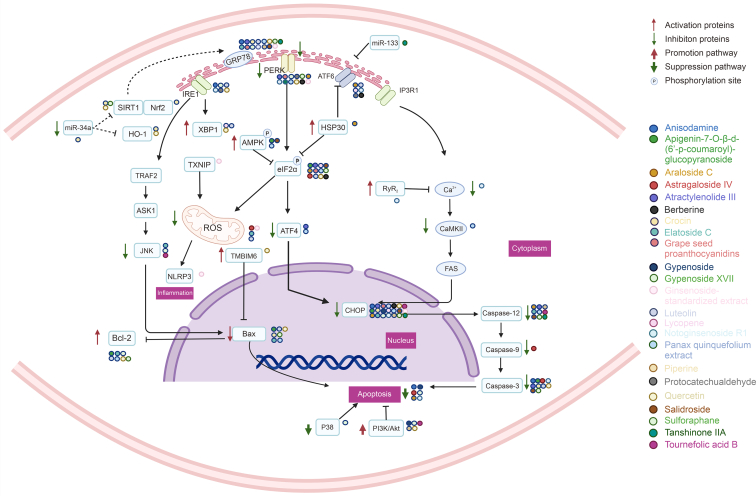
Anisodamine (Fig. 4) is a versatile tropane alkaloid derived from the Chinese herb Hyoscyamus niger L. Its unique vascular properties make it an essential compound in pharmacological research. Specifically, Anisodamine positively impacts stress markers in the ER including IRE-1α, CHOP, ATF4, and thioredoxin interacting protein (TXNIP) [118]. By reducing free radical-induced cellular damage, its administration negates the harmful consequences of stress on the body. Tropine alkaloids have also been noted to improve cardiovascular health by preventing cardiac arrest (CA) and resuscitation-induced I/R injury [119]. Similarly, Anisodamine safeguards the rat heart from calcium-mediated damage, as well as resuscitation-induced injury by inhibiting ERS proteins, such as GRP78, CHOP, and cleaved caspase-3 (Fig. 5 and Table 1). This leads to a significant decrease in cardiomyocyte death [69].
Fig. 5.
Natural products target endoplasmic reticulum stress (ERS) mediated myocardial injury (MI) in ischemia/reperfusion (I/R) injury. SIRT1: silencing information regulator 1; Nrf2: nuclear factor erythroid-2-related factor 2; HO-1: heme oxygenase 1; TRAF2: tumor necrosis factor receptor-associated factor 2; ASK1: apoptotic signaling kinase-1; JNK: c-Jun N-terminal kinase; Bcl-2: BCL2 apoptosis regulator; IRE1: inositol-requiring enzyme 1; XBP1: X-box-binding protein 1; TXNIP: thioredoxin interacting protein; ROS: reactive oxygen species; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; NLRP3: NLR family fyrin domain containing 3; TMBIM6: transmembrane BAX inhibitor-1 motif-containing 6; PERK: protein kinase RNA (PKR)-like endoplasmic reticulum (ER) kinase; AMPK: 5′ adenosine monophosphate-activated protein kinase; eIF2α: eukaryotic translation initiation factor 2α; ATF: activating transcription factor; HSP30: heat shock proteins30; RyR2: ryanodine receptor 2; CaMKII: Ca2+/calmodulin-dependent protein kinase II; CHOP: CCAAT/enhancer-binding protein homologous protein; p38: p38 mitogen-activated protein (MAP) kinase.
5.1.2. Apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside (APG)
APG (Fig. 4) is reported as the primary active compound found in Clematis tangutica [120]. Research studies have shown that the ERS-mediated apoptotic pathway plays a crucial role in the pathology of cardiac I/R injury [121]. Pre-treatment of primary cardiomyocytes with APG resulted in the activation of the AMPK signaling pathway, significantly inhibiting myocardial I/R-induced ERS, increasing cell viability and reducing apoptosis which ultimately attenuates myocardial injuries and improves cardiac function (Fig. 5 and Table 1). Therefore, APG could be considered as a natural substance with pharmacological pre-treatment activity, which indicates promising new therapeutic approaches for myocardial I/R injury [70].
5.1.3. Araloside C
Araloside C (Fig. 4) is a cardioprotective triterpenoid that is primarily isolated from Aralia elata [122]. Through the inhibition of the ERS pathway (PERK/eIF2α/ATF6) via heat shock protein 90 alpha family class A member 1 (HSP90), araloside C has exhibited the ability to enhance cell survival. It achieves this by reducing the expression of ERS pro-apoptotic proteins CHOP and caspase-12, while also hindering lactate dehydrogenase (LDH) leakage (Fig. 5 and Table 1). Overall, araloside C serves to prevent cardiomyocyte apoptosis and alleviate I/R injury in cardiomyocytes [71].
5.1.4. Astragaloside IV
Astragaloside IV (Fig. 4), a saponin obtained from Radix Astragali, has been identified for having cardioprotective properties. Specifically, it has been found to effectively reduce the production of ROS by inhibiting the phosphorylation of eIF2α and the expression of pro-apoptotic proteins, such as caspase-3, caspase-9, and CHOP. Consequently, it has demonstrated the ability to prevent apoptosis caused by oxidative stress and ERS. In turn, it has improved the survival rates of H9c2 cells following I/R treatment [72]. The anti-inflammatory and anti-endothelial cell loss effects of Astragaloside IV have also been observed, which are facilitated by the inhibition of TXNIP/NLR family fyrin domain containing 3 (NLRP3) [123] (Fig. 5 and Table 1).
5.1.5. Atractylenolide III
Atractylenolide III (Fig. 4), the principal active component found in Atractylodes macrocephala Koidz, has demonstrated beneficial effects in various pharmacological studies, including antioxidant and antiallergic properties [124]. Atractylenolide III is known to exhibit strong binding activity with ERS essential protein GRP78. Consequently, it is able to alleviate ERS, reduce cardiomyocyte apoptosis and protect cardiomyocytes by down-regulating the levels of GRP78, caspase-12 and caspase-3 proteins, and GRP78 and caspase-12 mRNA in hydrogen peroxide-induced H9c2 cardiomyocytes [73] (Fig. 5 and Table 1).
5.1.6. Berberine
Berberine (Fig. 4), an isoquinoline-derived alkaloid, is present in Rhizoma coptidis, which has been a crucial component of traditional Chinese medicine (TCM) for over 2,500 years. Its primary use has been to treat dysentery and infectious diarrhea [125]. Berberine effectively suppresses ERS caused by myocardial I/R injury through the down-regulation of the protein kinase RNA-like ER kinase (PERK) and eIF2α phosphorylation, and expression of ATF4 and CHOP in heart tissues. Moreover, Berberine exhibited a reduction in the myocardial infarct size induced by I/R injury, a promotion of cardiac function and a prevention of myocardial apoptosis and oxidative damage during in vivo tests performed on rats that underwent I/R surgery [74] (Fig. 5 and Table 1).
5.1.7. Crocin
Crocin, a bioactive compound found in Crocus sativus L, has demonstrated numerous pharmacological actions. Studies on crocin have shown that it exerts antioxidant, anti-inflammatory, anticancer, anti-atherosclerotic, and antidepressant effects [126]. Crocin has also been found to reduce cardiomyocyte death rate in animal models and primary cardiomyocytes induced by I/R. By preventing I/R damage, crocin decreases the expression of proteins such as Bax, caspase-3, GRP78, and CHOP while concomitantly enhancing the expression of Bcl-2. These protective effects on cardiomyocytes may be attributed to the regulation of ERS mediated through the miR-34a/silencing information regulator 1(SIRT1)/Nrf2 pathway [75] (Fig. 5 and Table 1).
5.1.8. Elatoside C
Communia araliae is a plant that is distributed extensively in northeast China, eastern Russia, Japan, and Korea [127]. Its roots and bark have been used in TCM as tonic, antiarrhythmic, anti-arthritic, antihypertensive, and antidiabetic agents [128]. Elatoside C (Fig. 4), a major triterpenoid compound that is isolated from aralia, has been demonstrated to inhibit ERS, by inhibiting GRP78, CHOP, JNK, and caspase-12, in I/R-induced cardiomyocyte apoptosis. Consequently, this improves mitochondrial membrane potential, cell viability, increases the Bcl-2/Bax ratio and reduces mitochondrial ROS. Therefore, elatoside C might represent an effective therapeutic strategy for mitigating myocardial I/R injury [76] (Fig. 5 and Table 1).
5.1.9. Grape seed proanthocyanidins (GSPs)
Proanthocyanidins are a family of polyphenolic flavonoid compounds found in vegetables and fruits. These compounds contain dimers, trimers, and other oligomers of catechins, epicatechins, and manatee esters. GSPs (Fig. 4) are highly effective antioxidants that can scavenge free radicals as well as vitamin C, E, or beta-carotene [129]. The therapeutic effects of GSPs vary depending on the dosage, with 100 μM demonstrating a cardioprotective effect in H9c2 cardiomyocytes subjected to I/R injury. The use of this dosage reduced the expression of apoptosis-related proteins such as GRP78, CHOP, eIF2α, and p-PERK (Fig. 5 and Table 1), thereby minimizing cardiomyocyte apoptosis and protecting H9c2 cardiomyocytes from I/R injury [77].
5.1.10. Gypenoside
Gynostemma pentaphyllum is an herb commonly used in folk medicine and consumed as herbal tea in various Asian countries. Among its bioactive compounds, gypenoside (Fig. 4) has been identified as the most potent in terms of its anti-inflammatory and antioxidative properties. Studies have shown that gypenoside plays a significant role in protecting against I/R injury in coronary heart disease by reducing oxidative stress. Specifically, gypenoside has been found to inhibit expression of ERS-related proteins and reduce cardiomyocyte apoptosis through blocking the CHOP pro-apoptotic pathway and activating the PI3K/Akt pathway (Fig. 5 and Table 1), thereby ameliorating structural cardiac damage caused by I/R injury [78].
5.1.11. Gypenoside XVII
Gypenoside XVII (Fig. 4), a compound derived from Panax notoginseng [130], was found to inhibit myocardial apoptosis and reduce cardiac insufficiency in H9c2 cardiomyocytes. This effect was attributed to the activation of the PI3K/Atk signaling pathway by gypenoside XVII, which delayed the onset of ERS, and to the inhibition of the p38 signaling pathway. Gypenoside XVII significantly suppressed the up-regulation of pro-apoptotic and ERS-associated proteins, namely GRP78, PERK, IRE1, p-JNK, and CHOP (Fig. 5 and Table 1). Moreover, it upregulated the expression of Bcl-2, an anti-apoptotic protein, which contributed to delaying the onset of apoptosis and protecting injured cardiomyocytes [79].
5.1.12. Luteolin
Luteolin (Fig. 4) is a flavonoid found in plants like honeysuckle and foods like carrots, peppers, and celery. Apart from possessing anti-inflammatory properties, it also has endothelial-improving qualities. When tested on the myocardial I/R cell model, several flavonoid interventions, including luteolin, quercetin, rutin, soybean sapogenins, and lignans, significantly improved the survival of cardiomyocytes. This improvement is attributed to the modulation of ERS signature proteins, as well as the expression of various pro- and anti-apoptotic proteins like CHOP, Bcl-2, Bax, and caspase-3 (Fig. 5 and Table 1). Overall, such interventions can attenuate the rate of apoptosis and ameliorate MI [80].
5.1.13. Lycopene
Lycopene (Fig. 4), a member of the carotenoid family, is primarily present in red fruits like tomatoes. Research in epidemiology indicates an inverse association between lycopene levels in serum and the risk of sudden cardiac death or acute myocardial infarction [131]. In primary mouse cardiomyocytes treated with hypoxia/reoxygenation, lycopene demonstrated a reduction in apoptotic cardiomyocytes and a decrease in the expression of several pro-apoptotic proteins such as CHOP, GRP78, ATF6, eIF2α, and sXBP-1 (Fig. 5 and Table 1). This suggests lycopene could impede cardiomyocyte apoptosis induced by I/R by suppressing ERS and decreasing reactive oxygen species (ROS) [81].
5.1.14. Notoginsenoside R1
Notoginsenoside R1 (Fig. 4), which is a saponin derived from Panax notoginseng, has been shown to mitigate ERS by inhibiting oxidative stress pathway and altering the course of I/R damage. Specifically, intervention with notoginsenoside R1 can delay the development of ERS in H9c2 cells by reducing the expression of ERS response proteins including GRP78, p-PERK, ATF6, and IRE1 [43]. Moreover, this compound has been found to scavenge free radicals and elevate the activity of antioxidant enzymes, thus promoting cardioprotective activity and preventing cell death in response to I/R-induced cellular injury [132]. Furthermore, notoginsenoside R1 exerts its effect by downregulating the expression of calcium-linked proteins, BiP, CHOP, and caspase-3 by modulating ryanodine receptor 2 (RyR2)-mediated ER calcium release (Fig. 5 and Table 1), thereby reducing ERS-induced cardiomyocyte injury and apoptosis [82].
5.1.15. Panax quinquefolium extract
Panax Quinquefolium is a species of plant belonging to the family Pentaphyllaceae. American ginseng stem and leaf extracts have been studied for their chemical composition and health benefits for many years. The extracts contain various constituents such as saponins, amino acids, carbohydrates, volatile oils, inorganic elements, and fatty acids which contribute to its benefits to the metabolism [133]. Panax Quinquefolium has been found to have potential positive effects on the cardiovascular system. Treatment with Panax Quinquefolium can prevent excessive ERS-associated apoptosis which has a cardioprotective effect in ventricular remodeling following acute myocardial infarction. The treatment can also enhance the expression of Bcl-2 protein while considerably lowering the expression of GRP78, CHOP, and Bax proteins. In rats, treatment with Panax Quinquefolium also resulted in decreased ERS, reduced left ventricular dilation, reduced infarct size, as well as enhanced left ventricular ejection percentage and shortening fraction [83]. Panax Quinquefolium can further block endoplasmic stress-mediated cardiomyocyte apoptosis through the PERK-eIF2α-ATF4-CHOP pathway during I/R and myocardial infarction-induced cardiomyocyte apoptosis [134]. Additionally, Panax Quinquefolium can prevent cell death caused by ERS in cardiomyocytes by decreasing caspase-12 activation levels and the expression of GRP78 and CHOP proteins [84] (Fig. 5 and Table 1).
5.1.16. Piperine
Piperine (Fig. 4) possesses various pharmacological effects, and one that has attracted attention is its capacity to inhibit ERS-induced apoptosis. Myocardial I/R damaged cells displayed elevated apoptosis, along with an increase in the expression of pro-apoptotic proteins such as BiP, CHOP, GRP78, and caspase-12. However, the pretreatment of cardiomyocytes with piperine reversed this trend. This suggests that piperine may have the ability to impede apoptosis onset by adjusting ERS. Furthermore, piperine caused a shift in the expression of PI3K and Akt, which were suppressed before. Through the PI3K/Akt signaling pathway (Fig. 5 and Table 1), piperine manifests its cardioprotective effects, primarily by diminishing ERS-induced apoptosis [84].
5.1.17. Protocatechualdehyde
Protocatechualdehyde (Fig. 4), a natural phenolic compound found in Salvia miltiorrhiza Bunge, has been shown to provide protective effects to cardiomyocytes [135]. In particular, protocatechualdehyde demonstrates anti-apoptotic properties in H9c2 cells exposed to oxygen-glucose deprivation and reoxygenation (OGD/R). Using bioinformatics and experimental studies, it has been revealed that protocatechualdehyde primarily acts by inhibiting the PERK/IRE1α/ATF6α signaling pathway (Fig. 5 and Table 1), leading to the prevention of cardiomyocyte apoptosis mediated by ERS [85].
5.1.18. Quercetin
Quercetin (Fig. 4), a flavonoid naturally found in high concentrations in fruits and vegetables, is known for its antioxidant properties. Quercetin has been shown to restore ER homeostasis in a model of ERS-induced toxicity in human umbilical vein endothelial cells (HUVECs). Specifically, quercetin reduces the levels of GRP78, CHOP, and caspase-3 [136] to effectively regulate ERS. Additionally, quercetin can enhance mitochondrial autophagy by modulating the expression of SIRT1 and transmembrane BAX inhibitor-1 motif-containing 6 (TMBIM6) to suppress hypoxia/reoxygenation-mediated ROS generation. This eventually inhibits oxidative stress damage [86]. The stress-responsive kinase Pak2 gene-deficient mice models showed that quercetin can alleviate ER dysfunction through the IRE1/XBP1s signaling pathway [137]. Moreover, Shu et al. [138] demonstrated that quercetin can significantly scavenge free radicals, reduce lipid peroxidation, and improve peroxidase activity. Quercetin reduces GRP78, p-PERK, and p-eIF2α levels, increases HO-1 expression, and thus mitigates ERS via the PI3K/Akt signaling pathway (Fig. 5 and Table 1), improving cardiomyocyte survivability and reducing cardiac injury incidence.
5.1.19. Salidroside
Salidroside (Fig. 4), a phenylpropanoid glycoside obtained from Rhodiola rosea Linn [139], is reputed for its various pharmacological effects, and it is considered defensive against ischemic heart disease [140]. ERS and stress-induced apoptosis were curtailed in cardiomyocytes [88]. Pre-administration of Salidroside in a myocardial I/R rat model conspicuously reduced the extent of the infarction and decreased serum creatine kinase and lactate dehydrogenase levels. Salidroside seems to protect the injured myocardium by activating the AMPK signaling pathway, attenuating ERS, and ameliorating apoptosis [87] (Fig. 5 and Table 1).
5.1.20. Sulforaphane
Sulforaphane (Fig. 4) is a dietary isothiocyanate that occurs naturally in cruciferous vegetables such as broccoli, cabbage, and cauliflower [141]. It has been found to suppress proteins involved in ERS-dependent apoptosis, namely, GRP78, CHOP, and caspase-12, using a model of I/R-induced injury in newborn rat cardiomyocytes [142]. Sulforaphane activates the SIRT1 pathway, which reduces I/R injury. By inhibiting the UPR and decreasing activation of the PERK/eIF2α pathway, SIRT1 protects cardiomyocytes from ERS-induced death [89] (Fig. 5 and Table 1). Moreover, sulforaphane's role in reducing ERS helps to improve the viability of cardiomyocytes by reducing damage to the mitochondrial membrane potential, lowering the Bcl-2/Bax ratio, and preventing the activity of caspase-3.
5.1.21. Tanshinone IIA
Tanshinone IIA (Fig. 4) can indirectly regulate ERS by regulating microRNAs, and directly regulate ERS-related proteins [90]. MicroRNAs are small noncoding RNA molecules that are involved in various physiological and pathophysiological processes, and are often used to control signaling via the control components of the signaling pathways, providing delicate and comprehensive regulation of cellular pathways. The expression of MiR-133 was significantly reduced in apoptosis of clostridium-treated cardiomyocytes, and upregulation of MiR-133 protected cardiomyocytes from apoptosis [143] (Fig. 5 and Table 1).
5.1.22. Tournefolic acid B
Tournefolic acid B (Fig. 4) is a novel compound derived from Clinopodium chinense (Benth.) Kuntze, a traditional Chinese medicinal herb widely recognized for its potent anti-inflammatory, hypoglycemic, anti-tumor and anti-radiation properties. Recent studies have found that tournefolic acid B exhibits potent antioxidant activity in hypoxic/reoxygenated H9c2 cells, regulating the levels of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px). Furthermore, tournefolic acid B has been shown to activate PI3K and Akt phosphorylation, inhibiting the production of CHOP and caspase-12, thus reducing apoptosis generated by ERS (Fig. 5 and Table 1). Moreover, it increases the Bcl-2/Bax ratio, reduces JNK phosphorylation and has a protective effect on damaged myocardium [91].
5.2. Natural products target ERS mediated MI in cardiac remodeling and heart failure
5.2.1. Baicalin
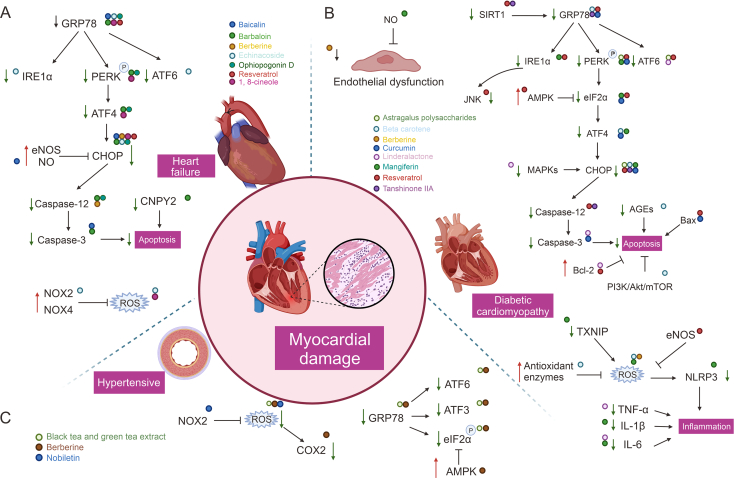
For centuries, Scutellaria baicalensis Georgi roots have been extensively used in traditional medicine to promote overall wellness, reduce inflammation, and fight infections [144]. The compound baicalin, which originates from the roots of Scutellaria baicalensis Georgi, has demonstrated several beneficial effects in neonatal rat cardiomyocytes, such as restoring the activity of endothelial-type nitric oxide synthase (eNOS) and enhancing nitric oxide (NO) bioavailability, as well as mitigating clathrin-induced ERS-induced death by downregulating the pro-apoptotic protein CHOP [92]. Moreover, baicalin (Fig. 4) exhibited the potential to alleviate cardiomyocyte apoptosis, counteract MI, and improve left ventricular remodeling through a reduction in the expression levels of GRP78, GRP94, and caspase-3, as demonstrated in research [145] (Fig. 6A and Table 1).
Fig. 6.
Natural products target endoplasmic reticulum stress (ERS) mediated myocardial injury (MI) in heart failure, diabetic cardiomyopathy (DCM) and hypertensive. GRP78: glucose rregulated protein78; IRE1: inositol-requiring enzyme 1; PERK: protein kinase RNA (PKR)-like endoplasmic reticulum (ER) kinase; ATF6: activating transcription factor 6; ATF4: activating transcription factor 4; CHOP: CCAAT/enhancer-binding protein homologous protein; eNOS: endothelial-type nitricoxide synthase; NO: nitric oxide; CNPY2: cardiomyocyte-specific protein canopy homolog 2; NOX2: (nicotinamide adenine dinucleotide phosphate oxidase) NADPH oxidase 2; NOX4: NADPH oxidase 4; ROS: reactive oxygen species; COX2: cyclooxygenase-2; SIRT1: silencing information regulator 1; GRP78: glucose regulated protein78; JNK: c-Jun N-terminal kinase; AMPK: 5′ adenosine monophosphate-activated protein kinase; eIF2α: eukaryotic translation initiation factor 2α; MAPKs: mitogen-activated protein kinases; Bcl-2: BCL2 apoptosis regulator; AGEs: advanced glycosylation end products; TXNIP: thioredoxin interacting protein; NLRP3: NLR family fyrin domain containing 3; TNF-α: tumor necrosis factor-α; IL-1β: interleukin 1beta; IL-6: interleukin-6; ATF3: activating transcription factor 3.
5.2.2. Barbaloin
Aloe, a succulent plant belonging to the lily family and the genus Aloe, is known to possess anti-inflammatory, antioxidant, and immune-boosting properties. While historically used as TCM for treating digestive disorders, recent research highlights its potential use in treating cardiovascular disease through the compound barbaloin [146]. Barbaloin (Fig. 4) has been shown to modulate the expression of ERS and pro-apoptosis-related proteins, including GRP78, PERK, p-PERK, ATF4, CHOP, caspase-3, and caspase-12, which help reduce cardiomyocyte injury. Notably, barbaloin may exert cardioprotective effects by reducing cardiomyocyte apoptosis through the cardiomyocyte-specific protein canopy homolog 2 (CNPY2) [93] (Fig. 6A and Table 1).
5.2.3. Berberine
In a rat model of myocardial infarction, berberine inhibited cardiac remodeling in post-infarction heart failure by attenuating MI and inhibiting cardiomyocyte apoptosis through the inhibition of ERS and modulation of CHOP and caspase-12 apoptotic pathways [94] (Fig. 6A and Table 1).
5.2.4. Echinacoside
Echinacoside (Fig. 4), the natural phenylethane glycoside found in the TCM Cistanche deserticola, has been shown to possess potent antioxidant and anti-inflammatory effects [147]. In an isoprenaline-induced heart failure model, echinacoside was observed to inhibit the expression of ERS pro-apoptotic proteins, including GRP78, p-IRE1α, p-PERK, ATF6, and CHOP, which in turn suppressed cardiomyocyte apoptosis. Additionally, echinacoside down-regulated the expression of nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidase 2 (NOX2) and NADPH oxidase 4 (NOX4), reduced ROS levels (Fig. 6A and Table 1), and improved cardiac function by protecting the damaged myocardium from further injury [95].
5.2.5. Ophiopogonin D
Ophiopogon japonicus is a renowned traditional Chinese medicinal herb that is known to nourish yin, moisten the lungs, and alleviate cough. Of the many active ingredients present in the herb, ophiopogonin D (Fig. 4) has exhibited significant biological activity that enhances cardiovascular function while possessing anti-inflammatory, antioxidant, and anti-aging properties. When isoproterenol was used to induce ERS in rat cardiomyocytes, the expression of BiP, PERK, and ATF4 were notably upregulated. Furthermore, the expression of pro-apoptotic molecules, such as CHOP, Bax, and caspase-12, were also elevated. However, pretreatment of the H2c9 cell line with maitake saponin effectively suppressed the upregulation of these molecules (Fig. 6A and Table 1). As a result, the cardiomyocytes maintained their ER Ca2+ homeostasis, experienced reduced apoptosis, and alleviated MI [96].
5.2.6. Resveratrol
Resveratrol (Fig. 4) is a polyphenol antioxidant present in red wine, grape seeds, and grape skins. It has been found to possess cardioprotective properties. In particular, resveratrol enhances the Bcl-2/Bax ratio and suppresses ERS proteins including CHOP, GRP78, and GRP94, thereby offering protection against isoproterenol-triggered cardiomyocyte hypertrophy and death [97]. The anthracycline antibiotic adriamycin is known for its efficacy against multiple types of cancers, but it causes heart failure and cardiac issues. H9c2 cardiomyocytes undergo apoptosis due to ERS, which is the source of anthracycline antibiotics like adriamycin. Nonetheless, resveratrol blocks the expression of ERS markers proteins (GRP78 and CHOP) and activates the SIRT1 pathway (Fig. 6A and Table 1), thereby restoring H9c2 cell viability against adriamycin induced cardiotoxicity [97].
5.2.7. 1, 8-cineole
1,8-cineole (Fig. 4) is the primary component of Sugemule-3, a monoterpene [148]. Studies have revealed the ability of 1,8-cineole to decrease isoproterenol (ISO)-induced apoptosis and enhance H9c2 cardiomyocyte survival after ISO injury. To protect injured myocardium and lower cardiomyocyte apoptosis, 1,8-cineole primarily controls ERS and reduces the expression of pro-apoptotic proteins (GRP78, PERK, ATF4 and CHOP) associated with ROS generation (Fig. 6A and Table 1). Additionally, 1,8-cineole has been shown to alleviate ISO-induced cardiac injury and reduce cardiac hypertrophy, cytoplasmic vesicle formation, myofiber loss, and fibrosis. The findings of this study lay the foundation for potential treatments of cardiac hypertrophy-associated diseases, such as heart failure [98].
5.3. Natural products target ERS mediated MI in DCM
5.3.1. Astragalus polysaccharides
Astragalus polysaccharides (Fig. 4) is the primary bioactive constituent extracted from Radix Astragali roots and is widely used in medicinal applications [149]. Research over the past two decades has shown astragalus polysaccharides to possess antioxidant, antidiabetic, and immunomodulatory activities. It has also exhibited cardioprotective effects against DCM [150]. In a rat model of DCM, astragalus polysaccharides was found to improve cardiac function and attenuate myocardial apoptosis primarily through modulation of the ERS pathway. Astragalus polysaccharides was able to down-regulate the expression of pro-apoptotic molecules such as ATF6 and PERK in the ER, while lentiviral overexpression of CHOP protein revealed that the protective effect of astragalus polysaccharides was blocked (Fig. 6B and Table 1). It is therefore hypothesized that astragalus polysaccharides may reduce cardiomyocyte apoptosis and protect damaged myocardium by decreasing the expression of pro-ERS proteins [99].
5.3.2. Beta carotene
Beta-carotene (Fig. 4) is a well-known precursor to vitamin A, present in a variety of fruits and vegetables and has been suggested to decrease the incidence of cancer and cardiovascular disease [151]. DCM is characterized by cardiac dysfunction due to the formation of advanced glycosylation end products (AGEs), resulting from protein glycosylation [152]. H9c2 cell assays showed that beta-carotene significantly inhibits AGEs-induced cell death and apoptosis, reducing intracellular ROS production and decreasing the rate at which antioxidant enzymes decompose. Furthermore, beta-carotene reduces the expression of pro-apoptotic and ERS proteins such as ATF4, GRP78, and CHOP via the PI3K/Akt/mTOR pathway (Fig. 6B and Table 1). It also reduces the incidence of ERS and autophagy and mitigates the damage to cardiomyocytes that is caused by oxidative stress [100].
5.3.3. Berberine
Berberine mitigated ROS production and downregulated the expression of proteins related to the ERS by suppressing oxidative stress and ERS, ultimately ameliorating the endothelial cell aberrations resulting from Kawasaki disease and diabetes [101,153] (Fig. 6B and Table 1).
5.3.4. Curcumin
Curcumin (Fig. 4) is a rhizome extract belonging to the class of Curcumae Longae Rhizoma. It is an agent of interest owing to its favorable pharmacological properties such as anticancer, anti-inflammatory, antidiabetic, and antioxidant benefits [154]. Curcumin has been found to be effective in treating different chronic conditions, including cardiovascular illnesses, autoimmune disorders, and different malignancies. In a palmitic acid (PA)-induced H9c2 cell model, curcumin was able to reverse the upregulation of ERS marker proteins and pro-apoptotic proteins (CHOP, GRP78, Caspase-3, Bax) [102] (Fig. 6B and Table 1).
Despite its many benefits, curcumin's hydrophobicity and volatility limit its use. However, recent research has revealed that the effects of curcumin can be significantly maximized by binding it to nanoparticles (curcumin-NPs). Curcumin-NPs reduced apoptosis in lipotoxic PA-treated cardiomyocytes and strongly upregulated the expression of LC3-II while suppressing the expression of p-PERK, p-eIF2α, and ATF4. This suggests that curcumin-NPs can prevent cardiomyocytes from undergoing apoptosis by blocking the ERS apoptotic pathway and stimulating the autophagy system [103].
5.3.5. Linderalactone
Linderalactone (Fig. 4), a sesquiterpene lactone extracted from the root of Aconitum carmichaelii Debeaux, shows great potential as a treatment for dilated cardiomyopathy [155]. Studies have revealed that linderalactone decreases ribosomal reduction, balloon-like degeneration, and ER dilatation - all processes related to ERS. In addition, it inhibits ERS mediated by the diabetes-activated MAPKs/ATF6 pathway (Fig. 6B and Table 1). Furthermore, linderalactone suppresses myocardial tissue hypertrophy as well as inflammatory responses in cardiomyocytes. As a result, it can be an effective therapy option to mitigate MI in DCM [104].
5.3.6. Mangiferin
Mangiferin (Fig. 4), a flavonoid found in mango, long-clawed I/Ris, or robinia has been extensively used in the treatment of diabetes [156]. Recent studies have shown that mangiferin can effectively reduce oxidative stress caused by ERS in EA.hy926 cells (human umbilical vein cell line) that were exposed to high glucose levels. This result is achieved by reducing ROS generation and attenuating IRE1α phosphorylation. Moreover, it also lowers the TXNIP levels and NLRP3 inflammatory vesicle activation, thereby decreasing the production of interleukin 1beta (IL-1β) and interleukin-6 (IL-6) and preventing inflammation. Increasing the generation of NO which helps in regulating endothelial homeostasis, mangiferin could potentially play a role in treating diabetic cardiovascular problems. Besides, mangiferin also protects the myocardium from ERS-induced injury by decreasing the activation of the PERK/eIF2α/ATF4/CHOP pathway while increasing SIRT1-mediated eIF2α deacetylation [105] (Fig. 6B and Table 1).
5.3.7. Resveratrol
It has been demonstrated that methoxylated resveratrol derivatives can improve endothelial dysfunction and have vasoprotective effects. These derivatives achieve this effect by reducing oxidative stress and ERS through the AMPK/SIRT1/eNOS pathway [157]. Resveratrol also improves cardiac function, reduces cardiomyocyte apoptosis, and increases SIRT1 in diabetic rats. Moreover, it improves ERS through the PERK/eIF2α, ATF6/CHOP, and IRE1α/JNK pathways. Therefore, resveratrol is a potential treatment for DCM [106]. Remarkably, metal ions are also crucial to the cardioprotective action of resveratrol. By releasing Zn2+ and blocking the opening of the mitochondrial permeability transition pore, resveratrol suppresses the production of ER chaperone proteins such as GRP78/94, as well as ER apoptotic proteins like CHOP, caspase-12, and JNK [158] (Fig. 6B and Table 1).
5.3.8. Tanshinone IIA
Tanshinone IIA, the primary active component of Salvia miltiorrhiza, has been found to exhibit preventive properties against cardiovascular disease and diabetes [159]. One common complication in diabetic patients is DCM, which can lead to heart failure through abnormal glucolipid metabolism and induction of ERS [160]. Tanshinone IIA was observed to inhibit the activity of GRP78 through SIRT1, inhibit the expression of ERS-related proteins and mRNAs (Fig. 6B and Table 1), improve the pathological morphology of cardiomyocytes, reduce cellular mortality, and consequently slow down MI [107].
5.4. Natural products target ERS mediated MI in hypertensive
5.4.1. Black tea and green tea extract
In most Western countries, tea products made from the leaves of the tea tree (Camellia sinensis) are the primary source of total polyphenol intake [161]. Regular tea drinking has been linked to the prevention of cardiovascular disease and reversal of endothelial dysfunction [162]. During the fermentation process used to produce black tea, catechins are converted to theaflavins and thearubigin polymers [162]. Elevated homocysteine levels in hypertension patients cause endothelial cells to experience ERS [163]. Black tea extract (BT) and theaflavin-3,3′-gallate (TF3) have been shown to reduce endothelial damage linked to hypertension by reducing ERS. Treatment with BT extract and TF3 decreased levels of homocysteine metabolizing enzymes and ROS levels while also reversing the rise in aortic ERS indicators (activating transcription factor 3 (ATF3), ATF6, and eIF2α) elevation in rats given Ang II. Furthermore, this medication restored normal blood pressure in hypertensive rats and enhanced endothelium-dependent diastole in the aorta, carotid, and renal arteries. Therefore, black tea shows potential as a supplement for hypertensive patients as it has a distinct cardioprotective action and reduces vascular dysfunction [108]. On the other hand, green tea has an abundance of flavonoids that include catechins such as epicatechin, epicatechin gallate, epigallocatechin, and epigallocatechin gallate [164]. Nicotine, the primary alkaloid in tobacco, causes cardiotoxic injury and toxicity in cardiomyocyte cultures. Rats treated with green tea extract and epigallocatechin gallate showed significant normalization in toxicity by decreasing the expression level of the chaperone stress marker GRP78, increasing catalase activity and ultimately reducing oxidative stress and apoptosis [165] (Fig. 6C and Table 1).
5.4.2. Berberine
Berberine was found to prevent ERS caused by myocardial ischemia-reperfusion in the heart and demonstrated antihypertensive benefits in rats with spontaneous hypertension [166]. In a rat model of renal vascular hypertension, berberine was shown to activate AMPK, leading to the inhibition of eIF2α phosphorylation, ATF3, ATF6, and XBP1 levels, as well as ROS-mediated cyclooxygenase-2 (COX2)-dependent contraction [109] (Fig. 6C and Table 1). These investigations provide further evidence of berberine's advantageous vascular effects in managing hypertension, which is critical for the prompt treatment of myocardial damage.
5.4.3. Nobiletin
Increased oxidative stress is a typical pathophysiologic response in pressure overload-induced cardiac hypertrophic diseases. Nobiletin (Fig. 4) which is a compound extracted from citrus fruit peels, possesses strong antioxidant properties [167]. In vivo experiments, Nobiletin significantly ameliorated pressure overload-induced cardiac hypertrophy by inhibiting GRP78, CHOP, and caspase-12 expression, and thus improving ERS in cardiac hypertrophy (Fig. 6C and Table 1). Moreover, Nobiletin's ability to reduce cardiomyocyte apoptosis may be attributed to its inhibition of NADPH oxidase. It has also been found that Nobiletin has a positive effect on cardiac hypertrophy as it alleviates ERS by inhibiting NOX2 expression, consequently reducing apoptosis. Thus, Nobiletin can attenuate the hypertrophic response of cardiomyocytes [110].
5.5. Natural products target ERS mediated MI in atherosclerosis
5.5.1. Paeonol
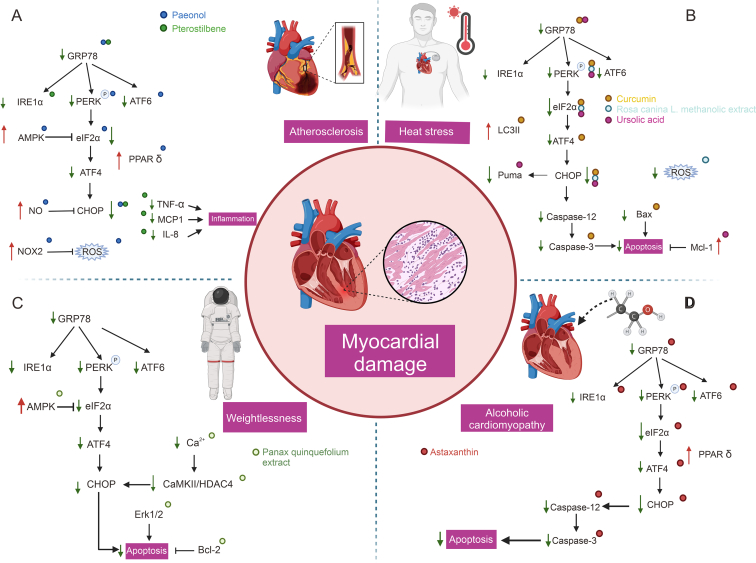
Paeonol (Fig. 4), a major phenolic component derived from peony bark, is widely used in TCM [168]. It has been found to enhance AMPK expression and PPAR δ activity in isolated mouse aortic cells and HUVECs, thereby alleviating ERS. Peoniflorin, on the other hand, inhibits the expression of ERS-related proteins (GRP78, ATF6, eIF2α, PERK). As a result, it reduces the overproduction of ROS, improves NO bioavailability, and alleviates vascular endothelial dysfunction [111,169] (Fig. 7A and Table 1).Additionally, paeoniflorin has been found to reduce NADPH subunits, further strengthening its therapeutic effect [170]. Furthermore, paeonol has been known to protect endothelial function in mice induced by clathrin in vivo by inhibiting ROS related to ERS via NOX2 [171].
Fig. 7.
Natural products target endoplasmic reticulum stress (ERS) mediated myocardial injury (MI) in atherosclerosis, heat stress, alcoholic cardiomyopathy and weightlessness. GRP78: glucose regulated protein78; IRE1: inositol-requiring enzyme 1; PERK:protein kinase RNA (PKR)-like ER kinase; ATF6: activating transcription factor 6; AMPK: 5′ adenosine monophosphate-activated protein kinase; eIF2α: eukaryotic translation initiation factor 2α; ATF4: activating transcription factor 4; PPAR δ: peroxisome proliferator activated receptor gamma; NO: nitric oxide; CHOP: CCAAT/enhancer-binding protein homologous protein; NOX2: nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidase 2; ROS: reactive oxygen species; TNF-α, tumor necrosis factor-α; MCP1: monocyte chemoattractant protein-1; IL-8: interleukin 8; LC3II: microtubule associated protein 1 light chain 3 beta; Mcl-1: MCL1 apoptosis regulator, BCL2 family member; CaMKII: Ca2+/calmodulin-dependent protein kinase II; HDAC4: histone deacetylase 4; Bcl-2: BCL2 apoptosis regulator.
5.5.2. Pterostilbene
Pterostilbene (Fig. 4) is a natural plant antitoxin found in blueberries displaying strong anti-inflammatory properties. In HUVECs, pterostilbene therapy demonstrated a reduction in ER-associated markers like GRP78, p-IRE1, and p-eIF2α, which are associated with inflammation induced by TNF-α. The reduced signaling of ERS diminished the expression of various inflammatory cytokines, including intercellular adhesion molecule-1 (ICAM1), interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1), soluble E-selectin, and matrix metalloproteinase 9 (MMP9) (Fig. 7A and Table 1). Ultimately, these changes exhibited by pterostilbene provide protection against inflammation in vascular endothelial cells [112].
5.6. Natural products target ERS mediated MI in other MI diseases
5.6.1. Heat stress
5.6.1.1. Curcumin
Curcumin mitigated HS (HS)-induced physiological disturbances in cardiac injury by reversing ER-mediated cardiomyocyte apoptosis, decreasing cardiac troponin I (cTn-I), and increasing cardiomyocyte Ang II levels, while reducing the expression of GRP78 and CHOP [113] (Fig. 7B and Table 1).
5.6.1.2. Rosa canina L. Methanolic extract
The Rosa canina extract possesses a wide range of medicinal benefits, such as its antioxidant, antimicrobial, anticancer, antidiabetic, anti-inflammatory, and immunomodulatory effects [172]. In vivo experiments revealed that treatment with Rosa canina extract effectively reduced the overproduction of ROS induced by HS in cardiomyocytes. Specifically, heat-stressed cardiomyocytes demonstrated increased expression of pro-apoptotic proteins, including CHOP, caspase 8, p-PERK, and p-eIF2α, while Rosa canina extract was able to effectively inhibit the expression of these pro-apoptotic proteins (Fig. 7B and Table 1), thereby providing a protective effect upon injured myocardium that has been exposed to HS [114].
5.6.1.3. Ursolic acid
HS has been found to be associated with multiple cardiovascular disorders and it severely hampers the functioning of cardiomyocytes [173]. The principal mechanism behind apoptosis induced by HS is the activation of the PERK-eIF2α-CHOP UPR, which results in increased production of the Puma protein. Ursolic acid (Fig. 4) safeguards the cells by restoring the intracellular redox state, triggering production of the anti-apoptotic protein MCL1 apoptosis regulator, BCL2 family member (Mcl-1), and blocking CHOP-induced overexpression of Puma. Ursolic acid supplementation is believed to be an effective measure in reducing the harmful impacts of HS on cardiomyocytes, given its antioxidant and anti-apoptotic properties against cardiac injury caused by ERS [115] (Fig. 7B and Table 1).
5.6.2. Weightlessness
5.6.2.1. Panax quinquefolium extract
Upon exposure to microgravity, studies have demonstrated the occurrence of cardiac shrinkage and MI. However, the beneficial effects of panax quinquefolium on heart remodeling induced by weightlessness have also been demonstrated. Specifically, panax quinquefolium has been found to notably reduce serum CK-MB, cTnT, and ischemia-modified albumin (IMA) levels. Moreover, it exerts a positive effect by activating AMPK, while simultaneously inhibiting the Erk1/2 and CaMKII/HDAC4 signaling pathway stress and Ca2+ overload (Fig. 7C and Table 1). As a result, panax quinquefolium leads to structural improvement of the impaired cardiac structure, while also decreasing weightlessness-induced cardiomyocyte apoptosis [117].
5.6.3. Alcoholic cardiomyopathy (ACM)
5.6.3.1. Astaxanthin
Astaxanthin (Fig. 4) belongs to the family of lutein, an oxygenated derivative of carotenoids. Studies have indicated the protective effects of astaxanthin on myocardial damage and anti-apoptosis [174]. Recently, astaxanthin has been found to reduce the accumulation of fat and glutamate-induced apoptosis by reducing ERS [175]. Chronic use of ethanol results in ACM, in which cardiac damage plays a crucial role in its progression. The development of ethanol-induced cardiomyocyte damage is associated with ERS. In vivo and in vitro investigations in ACM, reveal that astaxanthin may inhibit the activation of the PERK-eIF2α-ATF4, ATF6, and IRE1-XBP1 ERS signaling pathway induced by ethanol, leading to reduced expression of pro-apoptotic proteins, e.g., GRP78, CHOP, and caspase-12 (Fig. 7D and Table 1). As a result, it may reduce myocardial morphological changes and cardiac damage in dilated cardiac remodeling [116].
6. Discussion
In recent years, there has been a rapid increase in studies targeting natural agents to ameliorate MI by targeting ERS. Particularly in hypoxia/reperfusion injury, numerous natural products have shown potential therapeutic effects, providing new ideas for drug development for MI. Additionally, the innovation of new drug delivery modes has attracted extensive attention from researchers, with nanoparticle drug delivery emerging as a promising field for the treatment of MI. Innovative drug delivery routes can improve the accuracy and efficacy of drug-targeted therapy, and curcumin nanoparticles have been demonstrated to be highly effective in treating MI-related diseases. Studies have confirmed the ability of curcumin-loaded nanoparticles to ameliorate cardiomyocyte injury and reduce apoptosis via AMPK [176], modulation of autophagy, and slowing down of ERS-related pathways [[177], [178]]. Natural products such as resveratrol [179], icariin [180], and notoginsenoside R1 [181] have also been applied in new drug delivery pathways and shown to have potential in targeting damaged myocardium and providing myocardial protection and repair.
However, research on natural drugs targeting ERS to ameliorate MI still has many deficiencies. While many studies have shown that drugs can affect the expression of ERS and apoptosis-related proteins, they have not elucidated the specific mechanisms through which natural small molecules affect protein expression. Further studies should focus on the direct mechanism of the action of natural drugs, including their effects on protein modification, gene transcription, and protein binding. This will lead to a more accurate understanding of the mechanism of action of natural drugs, promoting clinical trials and applications while minimizing safety risks and maximizing the therapeutic effects of natural drugs on MI.
Furthermore, our literature review has revealed that studies on MI by natural compounds require more standardization, with modeling methods, dosage, start time, and duration of drug administration all needing to be standardized. As such, future research must adopt more rigorous and standardized experimental designs, including the use of multiple cellular models to validate findings. In vivo and in vitro experiments are also essential for comprehensive results, while the control of dosage and duration of drug administration is crucial in ensuring accurate and reliable findings.
Encouragingly, other factors contributing to MI, such as metabolic abnormalities, heat stress, and weightlessness, are gaining more attention from researchers. Such a shift in focus is vital in improving MI of different etiology and providing new ideas and reference for the pharmacological treatment of MI.
7. Conclusions and perspectives
ERS is a biological response to changes in internal and external environments that occur when these environments change beyond a specific threshold. ER plays a crucial role in multiple regulatory mechanisms that impact the cardiovascular system, serving as the first site of cellular stress response. Unresolved ERS can cause cardiomyocytes and vascular cells to malfunction and undergo apoptosis, leading to reduced systolic and diastolic functions of the heart that can potentially cause heart failure. Given the role of ERS and its convergence in multiple pathogenic pathways, natural products with antioxidant, anti-inflammatory, and anti-apoptotic properties offer protection to cardiomyocytes from damage and death. Natural solid substances, such as polyphenols, alkaloids, saponins, and other phytochemicals, can reduce ERS by acting on various signaling pathways linked to the generation of ROS, inflammation, and apoptosis.
Despite the tremendous potential of natural compounds for treating MI, misconceptions about their use still abound. Firstly, their lack of standardization in dosage, potency, and purity makes using natural compounds challenging. This lack of standardization can lead to variability in therapeutic effects as well as potential safety issues. Secondly, natural compounds may interact with conventional medications, leading to adverse reactions or reduced efficacy. Hence, clinicians should consider potential drug interactions when combining herbal monomers with other drugs. Thirdly, while natural compounds are generally considered safe, high doses or prolonged use may still pose risks. Some herbal monomers have been reported to cause adverse reactions such as allergic reactions, gastrointestinal disturbances, and hepatotoxicity. Besides, since MI is a serious disease that requires comprehensive treatment and management, natural medicines are not complete substitutes for conventional medical treatments, and it is dangerous to forgo or delay receiving formal treatment. Finally, despite growing evidence supporting the therapeutic potential of herbal monomers, there are still research gaps in their mechanism of action, long-term safety, and optimal dosing regimen. Therefore, it is necessary for researchers to address these misconceptions in future studies to avoid safety risks and realize the full potential of natural medicines in MI.
The study of natural products that target ERS is still in its early stages despite the promising use of these agents in the prevention and treatment of cardiovascular disorders. Further research is required to fully understand the mechanism and impact of natural products that targetERS and to develop fresh concepts and approaches for managing cardiovascular disorders. Clinical trials are also required to confirm the efficacy and safety of natural products in managing cardiovascular disorders.
CRediT authorship contribution statement
Kai Yang: Writing – original draft. Ping Zhang: Writing – original draft. Jixin Li: Writing – review & editing. Genming Zhang: Writing – original draft. Xing Chang: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
References
- 1.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Hetz C., Zhang K., Kaufman R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebeaupin C., Vallée D., Hazari Y., et al. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018;69:927–947. doi: 10.1016/j.jhep.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z., Zhang S. Endoplasmic reticulum stress: A key regulator of cardiovascular disease. DNA Cell Biol. 2023;42:322–335. doi: 10.1089/dna.2022.0532. [DOI] [PubMed] [Google Scholar]
- 6.Tuday E.C., Berkowitz D.E. Microgravity and cardiac atrophy: No sex discrimination. J. Appl. Physiol. (1985) 2007;103:1–2. doi: 10.1152/japplphysiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 7.Qian L., Song X., Ren H., et al. Mitochondrial mechanism of heat stress-induced injury in rat cardiomyocyte. Cell Stress Chaperones. 2004;9:281–293. doi: 10.1379/CSC-20R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Díaz-Bulnes P., Saiz M.L., López-Larrea C., et al. Crosstalk between hypoxia and ER stress response: A key regulator of macrophage polarization. Front. Immunol. 2020;10 doi: 10.3389/fimmu.2019.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S., Binder P., Fang Q., et al. Endoplasmic reticulum stress in the heart: Insights into mechanisms and drug targets. Br. J. Pharmacol. 2018;175:1293–1304. doi: 10.1111/bph.13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Xu L., Gillette T.G., et al. The unfolded protein response in ischemic heart disease. J. Mol. Cell. Cardiol. 2018;117:19–25. doi: 10.1016/j.yjmcc.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groenendyk J., Agellon L.B., Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu. Rev. Physiol. 2013;75:49–67. doi: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- 12.Dieterle T. Side effects and interactions of frequently used cardiovascular drugs. Ther. Umsch. 2015;72:701–710. doi: 10.1024/0040-5930/a000740. [DOI] [PubMed] [Google Scholar]
- 13.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 14.Senft D., Ronai Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40:141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki H., Nishitoh H. Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes. 2013;4:306–333. doi: 10.3390/genes4030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang J., Qi L. Quality control in the endoplasmic reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018;43:593–605. doi: 10.1016/j.tibs.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuck S., Prinz W.A., Thorn K.S., et al. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 2009;187:525–536. doi: 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oslowski C.M., Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acosta-Alvear D., Zhou Y., Blais A., et al. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olzmann J.A., Kopito R.R., Christianson J.C. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherepanova N., Shrimal S., Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr. Opin. Cell Biol. 2016;41:57–65. doi: 10.1016/j.ceb.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 25.Bommiasamy H., Back S.H., Fagone P., et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato Y., Nadanaka S., Okada T., et al. Luminal domain of ATF6 alone is sufficient for sensing endoplasmic reticulum stress and subsequent transport to the Golgi apparatus. Cell Struct. Funct. 2011;36:35–47. doi: 10.1247/csf.10010. [DOI] [PubMed] [Google Scholar]
- 27.Verfaillie T., Rubio N., Garg A.D., et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C.Y., Schröder M., Kaufman R.J. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 29.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 30.Bhandary B., Marahatta A., Kim H.R., et al. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder M., Kaufman R.J. ER stress and the unfolded protein response. Mutat. Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 32.Nishitoh H., Matsuzawa A., Tobiume K., et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urano F., Wang X., Bertolotti A., et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 34.Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battson M.L., Lee D.M., Gentile C.L. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H355–H367. doi: 10.1152/ajpheart.00437.2016. [DOI] [PubMed] [Google Scholar]
- 36.Li G., Mongillo M., Chin K.T., et al. Role of ERO1-alpha-mediated stimulation of inositol 1, 4, 5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmins J.M., Ozcan L., Seimon T.A., et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gudlur A., Zeraik A.E., Hirve N., et al. Calcium sensing by the STIM1 ER-luminal domain. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-06816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen N.T., Han W., Cao W., et al. Store-operated calcium entry mediated by ORAI and STIM. Compr. Physiol. 2018;8:981–1002. doi: 10.1002/cphy.c170031. [DOI] [PubMed] [Google Scholar]
- 40.Nan J., Li J., Lin Y., et al. The interplay between mitochondria and store-operated Ca2+ entry: Emerging insights into cardiac diseases. J. Cell. Mol. Med. 2021;25:9496–9512. doi: 10.1111/jcmm.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scorrano L., Oakes S.A., Opferman J.T., et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 42.Hausenloy D.J., Yellon D.M. Preconditioning and postconditioning: United at reperfusion. Pharmacol. Ther. 2007;116:173–191. doi: 10.1016/j.pharmthera.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Thuerauf D.J., Marcinko M., Gude N., et al. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ. Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 44.Severino A., Campioni M., Straino S., et al. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2007;50:1029–1037. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 45.Martindale J.J., Fernandez R., Thuerauf D., et al. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ. Res. 2006;98:1186–1193. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 46.Dickhout J.G., Carlisle R.E., Austin R.C. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: Endoplasmic reticulum stress as a mediator of pathogenesis. Circ. Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 47.Ni L., Zhou C., Duan Q., et al. β-AR blockers suppresses ER stress in cardiac hypertrophy and heart failure. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortega A., Roselló-Lletí E., Tarazón E., et al. Endoplasmic reticulum stress induces different molecular structural alterations in human dilated and ischemic cardiomyopathy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nie J., Duan Q., He M., et al. Ranolazine prevents pressure overload-induced cardiac hypertrophy and heart failure by restoring aberrant Na+ and Ca2+ handling. J. Cell. Physiol. 2019;234:11587–11601. doi: 10.1002/jcp.27791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lou Y., Wang Z., Xu Y., et al. Resveratrol prevents doxorubicin-induced cardiotoxicity in H9c2 cells through the inhibition of endoplasmic reticulum stress and the activation of the Sirt1 pathway. Int. J. Mol. Med. 2015;36:873–880. doi: 10.3892/ijmm.2015.2291. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y., Sowers J.R., Ren J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018;14:356–376. doi: 10.1038/s41574-018-0009-1. [DOI] [PubMed] [Google Scholar]
- 52.Yang L., Zhao D., Ren J., et al. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim. Biophys. Acta. 2015;1852:209–218. doi: 10.1016/j.bbadis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Pei Z., Deng Q., Babcock S.A., et al. Inhibition of advanced glycation endproduct (AGE) rescues against streptozotocin-induced diabetic cardiomyopathy: Role of autophagy and ER stress. Toxicol. Lett. 2018;284:10–20. doi: 10.1016/j.toxlet.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 54.Eden E.R. The formation and function of ER-endosome membrane contact sites. Biochim. Biophys. Acta. 2016;1861:874–879. doi: 10.1016/j.bbalip.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kilpatrick B.S., Eden E.R., Hockey L.N., et al. An endosomal NAADP-sensitive two-pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henne W.M., Zhu L., Balogi Z., et al. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J. Cell Biol. 2015;210:541–551. doi: 10.1083/jcb.201503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radwan E., Bakr M.H., Taha S., et al. Inhibition of endoplasmic reticulum stress ameliorates cardiovascular injury in a rat model of metabolic syndrome. J. Mol. Cell. Cardiol. 2020;143:15–25. doi: 10.1016/j.yjmcc.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 58.Brown M.S., Radhakrishnan A., Goldstein J.L. Retrospective on cholesterol homeostasis: The central role of scap. Annu. Rev. Biochem. 2018;87:783–807. doi: 10.1146/annurev-biochem-062917-011852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen S., Shen M., Kuang L., et al. SIRT1/SREBPs-mediated regulation of lipid metabolism. Pharmacol. Res. 2024;199 doi: 10.1016/j.phrs.2023.107037. [DOI] [PubMed] [Google Scholar]
- 60.Ye J., Rawson R.B., Komuro R., et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 61.Ayyappan J.P., Lizardo K., Wang S., et al. Inhibition of SREBP improves cardiac lipidopathy, improves endoplasmic reticulum stress, and modulates chronic chagas cardiomyopathy. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M., Wang Y., Han B., et al. Activation of aldehyde dehydrogenase 2 slows down the progression of atherosclerosis via attenuation of ER stress and apoptosis in smooth muscle cells. Acta Pharmacol. Sin. 2018;39:48–58. doi: 10.1038/aps.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myoishi M., Hao H., Minamino T., et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 64.Feaver R.E., Hastings N.E., Pryor A., et al. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells, Arterioscler. Thromb. Vasc. Biol. 2008;28:1534–1541. doi: 10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Civelek M., Manduchi E., Riley R.J., et al. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ. Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dong Y., Zhang M., Liang B., et al. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim Y.H., Kim H., Hong Y.C. Variation in mortality of ischemic and hemorrhagic strokes in relation to high temperature. Int. J. Biometeorol. 2013;57:145–153. doi: 10.1007/s00484-012-0542-x. [DOI] [PubMed] [Google Scholar]
- 68.Choy K.W., Murugan D., Mustafa M.R. Natural products targeting ER stress pathway for the treatment of cardiovascular diseases. Pharmacol. Res. 2018;132:119–129. doi: 10.1016/j.phrs.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Yin X.-L., Shen H., Zhang W., et al. Inhibition of endoplasm reticulum stress by anisodamine protects against myocardial injury after cardiac arrest and resuscitation in rats. Am. J. Chin. Med. 2011;39:853–866. doi: 10.1142/S0192415X11009251. [DOI] [PubMed] [Google Scholar]
- 70.Feng Y., Lu Y., Liu D., et al. Apigenin-7-O-β-d-(-6″-p-coumaroyl)-glucopyranoside pretreatment attenuates myocardial ischemia/reperfusion injury via activating AMPK signaling. Life Sci. 2018;203:246–254. doi: 10.1016/j.lfs.2018.04.048. [DOI] [PubMed] [Google Scholar]
- 71.Du Y., Wang M., Liu X., et al. Araloside C prevents hypoxia/reoxygenation-induced endoplasmic reticulum stress via increasing heat shock protein 90 in H9c2 cardiomyocytes. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Y.-X., Jin Y.-C., Jin H.-Q., et al. Astragaloside IV protects cardiomyocytes from hypoxic injury by regulating endoplasmic reticulum stress via eIF2α/CHOP signaling pathway. Pharmazie. 2023;78:196–200. doi: 10.1691/ph.2023.1946. [DOI] [PubMed] [Google Scholar]
- 73.Zuo M.-Y., Tang T.-J., Zhou P., et al. [Mechanism of atractylenolide III in alleviating H9c2 cell apoptosis through ROS/GRP78/caspase-12 signaling pathway based on molecular docking] Zhongguo Zhong Yao Za Zhi. 2022;47:4436–4445. doi: 10.19540/j.cnki.cjcmm.20220304.401. [DOI] [PubMed] [Google Scholar]
- 74.Liao Y., Chen K., Dong X., et al. Berberine inhibits cardiac remodeling of heart failure after myocardial infarction by reducing myocardial cell apoptosis in rats. Exp. Ther. Med. 2018;16:2499–2505. doi: 10.3892/etm.2018.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Yuan B., Cheng B., et al. Crocin alleviates myocardial ischemia/reperfusion-induced endoplasmic reticulum stress via regulation of miR-34a/Sirt1/Nrf2 pathway. Shock. 2019;51:123–130. doi: 10.1097/SHK.0000000000001116. [DOI] [PubMed] [Google Scholar]
- 76.Wang M., Meng X., Yu Y., et al. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis. 2014;19:1727–1735. doi: 10.1007/s10495-014-1039-3. [DOI] [PubMed] [Google Scholar]
- 77.Wang X., Jia D., Zhang J., et al. Grape seed proanthocyanidins protect cardiomyocytes against hypoxia/reoxygenation injury by attenuating endoplasmic reticulum stress through PERK/eIF2α pathway. Mol. Med. Rep. 2017;16:9189–9196. doi: 10.3892/mmr.2017.7756. [DOI] [PubMed] [Google Scholar]
- 78.Yu H., Zhang H., Zhao W., et al. Gypenoside protects against myocardial ischemia-reperfusion injury by inhibiting cardiomyocytes apoptosis via inhibition of CHOP pathway and activation of PI3K/Akt pathway in vivo and in vitro. Cell. Physiol. Biochem. 2016;39:123–136. doi: 10.1159/000445611. [DOI] [PubMed] [Google Scholar]
- 79.Yu Y., Wang M., Chen R., et al. Gypenoside XVII protects against myocardial ischemia and reperfusion injury by inhibiting ER stress-induced mitochondrial injury. J. Ginseng Res. 2021;45:642–653. doi: 10.1016/j.jgr.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim D.S., Kwon D.Y., Kim M.S., et al. The involvement of endoplasmic reticulum stress in flavonoid-induced protection on cardiac cell death caused by ischaemia/reperfusion. J. Pharm. Pharmacol. 2010;62:197–204. doi: 10.1211/jpp.62.02.0007. [DOI] [PubMed] [Google Scholar]
- 81.Xu J.Q., Chen B., Hu H.X., et al. Lycopene protects against hypoxia/reoxygenation injury in mouse cardiomyocytes by inhibiting endoplasmic reticulum stress induced apoptosis. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44:518–523. doi: 10.3760/cma.j.issn.0253-3758.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Chen J., Xue R., Li L., et al. Panax notoginseng saponins protect cardiac myocytes against endoplasmic reticulum stress and associated apoptosis through mediation of intracellular calcium homeostasis. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M., Wang X., Wang C., et al. Panax quinquefolium saponin attenuates ventricular remodeling after acute myocardial infarction by inhibiting chop-mediated apoptosis. Shock. 2013;40:339–344. doi: 10.1097/SHK.0b013e3182a3f9e5. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Chen Z., Cai Y. Piperine protects against myocardial ischemia/reperfusion injury by activating the PI3K/AKT signaling pathway. Exp. Ther. Med. 2021;21 doi: 10.3892/etm.2021.9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wan Y., Wang Y., Guo Q., et al. Protocatechualdehyde protects oxygen-glucose deprivation/reoxygenation-induced myocardial injury via inhibiting PERK/ATF6α/IRE1α pathway. Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173723. [DOI] [PubMed] [Google Scholar]
- 86.Chang X., Zhang T., Meng Q., et al. Quercetin improves cardiomyocyte vulnerability to hypoxia by regulating SIRT1/TMBIM6-related mitophagy and endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/5529913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian X., Huang Y., Zhang X., et al. Salidroside attenuates myocardial ischemia/reperfusion injury via AMPK-induced suppression of endoplasmic reticulum stress and mitochondrial fission. Toxicol. Appl. Pharmacol. 2022;448 doi: 10.1016/j.taap.2022.116093. [DOI] [PubMed] [Google Scholar]
- 88.Wang F., Wang J., Liang X., et al. Ghrelin inhibits myocardial pyroptosis in diabetic cardiomyopathy by regulating ERS and NLRP3 inflammasome crosstalk through the PI3K/AKT pathway. J. Drug Target. 2024;32:148–158. doi: 10.1080/1061186X.2023.2295268. [DOI] [PubMed] [Google Scholar]
- 89.Prola A., Pires Da Silva J., Guilbert A., et al. SIRT1 protects the heart from ER stress-induced cell death through eIF2α deacetylation. Cell Death Differ. 2017;24:343–356. doi: 10.1038/cdd.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feng J., Li S., Chen H. Tanshinone IIA ameliorates apoptosis of cardiomyocytes induced by endoplasmic reticulum stress. Exp. Biol. Med. 2016;241:2042–2048. doi: 10.1177/1535370216660634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu Y., Xing N., Xu X., et al. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine. 2019;52:178–186. doi: 10.1016/j.phymed.2018.09.168. [DOI] [PubMed] [Google Scholar]
- 92.Shen M., Wang L., Yang G., et al. Baicalin protects the cardiomyocytes from ER stress-induced apoptosis: Inhibition of CHOP through induction of endothelial nitric oxide synthase. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui Y., Wang Y., Liu G. Protective effect of Barbaloin in a rat model of myocardial ischemia reperfusion injury through the regulation of the CNPY2-PERK pathway. Int. J. Mol. Med. 2019;43:2015–2023. doi: 10.3892/ijmm.2019.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao G., Yu L., Gao W., et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016;37:354–367. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ni Y., Zhang J., Zhu W., et al. Echinacoside inhibited cardiomyocyte pyroptosis and improved heart function of HF rats induced by isoproterenol via suppressing NADPH/ROS/ER stress. J. Cell. Mol. Med. 2022;26:5414–5425. doi: 10.1111/jcmm.17564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang G., Wang Y., Ruan P., et al. Protective effect of ophiopogonin D against isoproterenol-induced cardiomyocyte injury and targets. Zhongguo Zhong Yao Za Zhi. 2022;47:2721–2728. doi: 10.19540/j.cnki.cjcmm.20211216.702. [DOI] [PubMed] [Google Scholar]
- 97.Lin Y., Zhu J., Zhang X., et al. Inhibition of cardiomyocytes hypertrophy by resveratrol is associated with amelioration of endoplasmic reticulum stress. Cell. Physiol. Biochem. 2016;39:780–789. doi: 10.1159/000447788. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y., Zhang X., Fu Y., et al. 1, 8-cineole protects against ISO-induced heart failure by inhibiting oxidative stress and ER stress in vitro and in vivo. Eur. J. Pharmacol. 2021;910 doi: 10.1016/j.ejphar.2021.174472. [DOI] [PubMed] [Google Scholar]
- 99.Sun S., Yang S., An N., et al. Astragalus polysaccharides inhibits cardiomyocyte apoptosis during diabetic cardiomyopathy via the endoplasmic reticulum stress pathway. J. Ethnopharmacol. 2019;238 doi: 10.1016/j.jep.2019.111857. [DOI] [PubMed] [Google Scholar]
- 100.Zhao G., Zhang X., Wang H., et al. Beta carotene protects H9c2 cardiomyocytes from advanced glycation end product-induced endoplasmic reticulum stress, apoptosis, and autophagy via the PI3K/Akt/mTOR signaling pathway. Ann. Transl. Med. 2020;8 doi: 10.21037/atm-20-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Zhou C., Zhang X., et al. Coptisine attenuates diabetes-associated endothelial dysfunction through inhibition of endoplasmic reticulum stress and oxidative stress. Molecules. 2021;26 doi: 10.3390/molecules26144210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guan G., Lei L., Lv Q., et al. Curcumin attenuates palmitic acid-induced cell apoptosis by inhibiting endoplasmic reticulum stress in H9C2 cardiomyocytes. Hum. Exp. Toxicol. 2019;38:655–664. doi: 10.1177/0960327119836222. [DOI] [PubMed] [Google Scholar]
- 103.Gu Y., Xia H., Chen X., et al. Curcumin nanoparticles attenuate lipotoxic injury in cardiomyocytes through autophagy and endoplasmic reticulum stress signaling pathways. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.571482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han X., Zhou W., Zhang J., et al. Linderalactone mitigates diabetic cardiomyopathy in mice via suppressing the MAPK/ATF6 pathway. Int. Immunopharmacol. 2023;124 doi: 10.1016/j.intimp.2023.110984. [DOI] [PubMed] [Google Scholar]
- 105.Song J., Li J., Hou F., et al. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism. 2015;64:428–437. doi: 10.1016/j.metabol.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 106.Guo R., Liu W., Liu B., et al. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int. J. Cardiol. 2015;191:36–45. doi: 10.1016/j.ijcard.2015.04.245. [DOI] [PubMed] [Google Scholar]
- 107.Wu S., Lu D., Gajendran B., et al. Tanshinone IIA ameliorates experimental diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress in cardiomyocytes via SIRT1. Phytother. Res. 2023;37:3543–3558. doi: 10.1002/ptr.7831. [DOI] [PubMed] [Google Scholar]
- 108.San Cheang W., Yuen Ngai C., Yen Tam Y., et al. Black tea protects against hypertension-associated endothelial dysfunction through alleviation of endoplasmic reticulum stress. Sci. Rep. 2015;5 doi: 10.1038/srep10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian X.Y., Wong W.T., Leung F.P., et al. Oxidative stress-dependent cyclooxygenase-2-derived prostaglandin f(2α) impairs endothelial function in renovascular hypertensive rats. Antioxid. Redox Signal. 2012;16:363–373. doi: 10.1089/ars.2010.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang N., Wei W., Yang Z., et al. Nobiletin, a polymethoxy flavonoid, protects against cardiac hypertrophy induced by pressure-overload via inhibition of NAPDH oxidases and endoplasmic reticulum stress. Cell. Physiol. Biochem. 2017;42:1313–1325. doi: 10.1159/000478960. [DOI] [PubMed] [Google Scholar]
- 111.Choy K.-W., Mustafa M.R., Lau Y.S., et al. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 2016;116:51–62. doi: 10.1016/j.bcp.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 112.Liu J., Fan C., Yu L., et al. Pterostilbene exerts an anti-inflammatory effect via regulating endoplasmic reticulum stress in endothelial cells. Cytokine. 2016;77:88–97. doi: 10.1016/j.cyto.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y., Jiang W., Liu X., et al. Curcumin supplementation improves heat-stress-induced cardiac injury of mice: Physiological and molecular mechanisms. J. Nutr. Biochem. 2020;78 doi: 10.1016/j.jnutbio.2019.108331. [DOI] [PubMed] [Google Scholar]
- 114.Nasrolahi A., Hosseini L., Farokhi-Sisakht F., et al. Cardioprotective effect of Rosa canina L. methanolic extract on heat shock induced cardiomyocyte injury: An experimental study. J. Cardiovasc. Thorac. Res. 2020;12:286–293. doi: 10.34172/jcvtr.2020.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang Y., Li C., Xiang X., et al. Ursolic acid prevents endoplasmic reticulum stress-mediated apoptosis induced by heat stress in mouse cardiac myocytes. J. Mol. Cell. Cardiol. 2014;67:103–111. doi: 10.1016/j.yjmcc.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 116.Sun H., Ling S., Zhao D., et al. Panax quinquefolium saponin attenuates cardiac remodeling induced by simulated microgravity. Phytomedicine. 2019;56:83–93. doi: 10.1016/j.phymed.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 117.Wang W., Liu T., Liu Y., et al. Astaxanthin attenuates alcoholic cardiomyopathy via inhibition of endoplasmic reticulum stress-mediated cardiac apoptosis. Toxicol. Appl. Pharmacol. 2021;412 doi: 10.1016/j.taap.2020.115378. [DOI] [PubMed] [Google Scholar]
- 118.Yuan X., Zheng Y., Chen C., et al. Anisodamine inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in rhabdomyolysis-induced acute kidney injury. Apoptosis. 2017;22:1524–1531. doi: 10.1007/s10495-017-1414-y. [DOI] [PubMed] [Google Scholar]
- 119.Jia L., Chen W., Shen H., et al. Effects of anisodamine on microcirculation of the asystole rats during the cardiopulmonary resuscitation. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:737–739. [PubMed] [Google Scholar]
- 120.Cai M., Ma Y., Zhang W., et al. Apigenin-7-O-β-D-(-6’’-p-coumaroyl)-glucopyranoside treatment elicits neuroprotective effect against experimental ischemic stroke. Int. J. Biol. Sci. 2016;12:42–52. doi: 10.7150/ijbs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Minamino T., Kitakaze M. ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 2010;48:1105–1110. doi: 10.1016/j.yjmcc.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 122.Wang M., Tian Y., Du Y., et al. Protective effects of araloside C against myocardial ischaemia/reperfusion injury: Potential involvement of heat shock protein 90. J. Cell. Mol. Med. 2017;21:1870–1880. doi: 10.1111/jcmm.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y., Li Q., Zhao W., et al. Astragaloside IV and cycloastragenol are equally effective in inhibition of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in the endothelium. J. Ethnopharmacol. 2015;169:210–218. doi: 10.1016/j.jep.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 124.Li H., Yu W., Yang Y., et al. Combination of Atractylenolide I, Atractylenolide III, and Paeoniflorin promotes angiogenesis and improves neurological recovery in a mouse model of ischemic Stroke. Chin. Med. 2024;19 doi: 10.1186/s13020-023-00872-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J., Ljn J. Advance on study in anti-tumor mechamism of bererine (Ber) Zhongguo Zhong Yao Za Zhi. 2007;32:881–883. , 934. [PubMed] [Google Scholar]
- 126.Hire R.R., Srivastava S., Davis M.B., et al. Antiproliferative activity of crocin involves targeting of microtubules in breast cancer cells. Sci. Rep. 2017;7 doi: 10.1038/srep44984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nhiem N.X., Lim H.Y., Kiem P.V., et al. Oleanane-type triterpene saponins from the bark of Aralia elata and their NF-κB inhibition and PPAR activation signal pathway. Bioorg. Med. Chem. Lett. 2011;21:6143–6147. doi: 10.1016/j.bmcl.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 128.Wang M., Xu X., Xu H., et al. Effect of the total saponins of Aralia elata (Miq) Seem on cardiac contractile function and intracellular calcium cycling regulation. J. Ethnopharmacol. 2014;155:240–247. doi: 10.1016/j.jep.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 129.Bagchi D., Bagchi M., Stohs S.J., et al. Cellular protection with proanthocyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002;957:260–270. doi: 10.1111/j.1749-6632.2002.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 130.Yu Y., Sun G., Luo Y., et al. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci. Rep. 2016;6 doi: 10.1038/srep21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Karppi J., Laukkanen J.A., Mäkikallio T.H., et al. Low serum lycopene and β-carotene increase risk of acute myocardial infarction in men. Eur. J. Public Health. 2012;22:835–840. doi: 10.1093/eurpub/ckr174. [DOI] [PubMed] [Google Scholar]
- 132.Wang L., Zhang W., Liu Q., et al. A standardized notoginseng extract exerts cardioprotection by attenuating apoptosis under endoplasmic reticulum stress conditions. J. Funct. Foods. 2015;16:20–27. [Google Scholar]
- 133.Kitts D.D., Wijewickreme A.N., Hu C. Antioxidant properties of a North American ginseng extract. Mol. Cell. Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 134.Liu M., Xue M., Wang X., et al. Panax quinquefolium saponin attenuates cardiomyocyte apoptosis induced by thapsigargin through inhibition of endoplasmic reticulum stress. J. Geriatr. Cardiol. 2015;12:540–546. doi: 10.11909/j.issn.1671-5411.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gu M., Wang X., Su Z., et al. One-step separation and purification of 3, 4-dihydroxyphenyllactic acid, salvianolic acid B and protocatechualdehyde from Salvia miltiorrhiza Bunge by high-speed counter-current chromatography. J. Chromatogr. A. 2007;1140:107–111. doi: 10.1016/j.chroma.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 136.Suganya N., Bhakkiyalakshmi E., Suriyanarayanan S., et al. Quercetin ameliorates tunicamycin-induced endoplasmic reticulum stress in endothelial cells. Cell Prolif. 2014;47:231–240. doi: 10.1111/cpr.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Binder P., Wang S., Radu M., et al. Pak2 as a novel therapeutic target for cardioprotective endoplasmic reticulum stress response. Circ. Res. 2019;124:696–711. doi: 10.1161/CIRCRESAHA.118.312829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shu Z., Yang Y., Yang L., et al. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019;10:203–215. doi: 10.1039/c8fo01256c. [DOI] [PubMed] [Google Scholar]
- 139.Lan X., Chang K., Zeng L., et al. Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu L., Wei T., Chang X., et al. Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-κB/AP1 pathway. Inflammation. 2015;38:1589–1598. doi: 10.1007/s10753-015-0134-0. [DOI] [PubMed] [Google Scholar]
- 141.Danilov C.A., Chandrasekaran K., Racz J., et al. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Y., Wang S., Liu B., et al. Sulforaphane prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating SIRT1 and subsequently inhibiting ER stress. Acta Pharmacol. Sin. 2016;37:344–353. doi: 10.1038/aps.2015.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu C., Hu Y., Hou L., et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J. Mol. Cell. Cardiol. 2014;75:111–121. doi: 10.1016/j.yjmcc.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 144.Chen H., Hsu J.H., Liou S., et al. Baicalein, an active component of Scutellaria baicalensis Georgi, prevents lysophosphatidylcholine-induced cardiac injury by reducing reactive oxygen species production, calcium overload and apoptosis via MAPK pathways. BMC Complement. Altern. Med. 2014;14 doi: 10.1186/1472-6882-14-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dai H., Zhang X., Yang Z., et al. Effects of baicalin on blood pressure and left ventricular remodeling in rats with renovascular hypertension. Med. Sci. Monit. 2017;23:2939–2948. doi: 10.12659/MSM.902536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cao Z., Tian Y., Hao J., et al. Barbaloin inhibits ventricular arrhythmias in rabbits by modulating voltage-gated ion channels. Acta Pharmacol. Sin. 2018;39:357–370. doi: 10.1038/aps.2017.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liang Y., Chen C., Xia B., et al. Neuroprotective effect of echinacoside in subacute mouse model of Parkinson’s disease. Biomed Res. Int. 2019;2019 doi: 10.1155/2019/4379639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang Y., Zhen D., Fu D., et al. 1, 8-cineole attenuates cardiac hypertrophy in heart failure by inhibiting the miR-206-3p/SERP1 pathway. Phytomedicine. 2021;91 doi: 10.1016/j.phymed.2021.153672. [DOI] [PubMed] [Google Scholar]
- 149.Zhang K., Pugliese M., Pugliese A., et al. Biological active ingredients of traditional Chinese herb Astragalus membranaceus on treatment of diabetes: A systematic review. Mini Rev. Med. Chem. 2015;15:315–329. doi: 10.2174/1389557515666150227113431. [DOI] [PubMed] [Google Scholar]
- 150.Liu M., Qin J., Hao Y., et al. Astragalus polysaccharide suppresses skeletal muscle myostatin expression in diabetes: Involvement of ROS-ERK and NF-κB pathways. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/782497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Paz E., Martín Á., Estrella A., et al. Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocoll. 2012;26:17–27. [Google Scholar]
- 152.Yamagishi S.I., Nakamura N., Matsui T. Glycation and cardiovascular disease in diabetes: A perspective on the concept of metabolic memory. J. Diabetes. 2017;9:141–148. doi: 10.1111/1753-0407.12475. [DOI] [PubMed] [Google Scholar]
- 153.Xu M., Qi Q., Men L., et al. Berberine protects Kawasaki disease-induced human coronary artery endothelial cells dysfunction by inhibiting of oxidative and endoplasmic reticulum stress. Vascul. Pharmacol. 2020;127 doi: 10.1016/j.vph.2020.106660. [DOI] [PubMed] [Google Scholar]
- 154.Pawar R.S., Toppo F.A., Mandloi A.S., et al. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian J. Pharmacol. 2015;47:160–166. doi: 10.4103/0253-7613.153422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jani N.A., Sirat H.M., Ahmad F., et al. New sesquiterpene dilactone and β-carboline alkaloid and the α-glucosidase inhibitory activity of selected phytochemicals from Neolitsea cassia (L.) Kosterm. Nat. Prod. Res. 2022;36:4061–4069. doi: 10.1080/14786419.2021.1961134. [DOI] [PubMed] [Google Scholar]
- 156.Márquez L., García-Bueno B., Madrigal J.L.M., et al. Mangiferin decreases inflammation and oxidative damage in rat brain after stress. Eur. J. Nutr. 2012;51:729–739. doi: 10.1007/s00394-011-0252-x. [DOI] [PubMed] [Google Scholar]
- 157.Zhou C., Tan Y., Xu B., et al. 3,4’,5-trimethoxy-trans-stilbene alleviates endothelial dysfunction in diabetic and obese mice via activation of the AMPK/SIRT1/eNOS pathway. Antioxidants (Basel) 2022;11 doi: 10.3390/antiox11071286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.He Y., Fu Y., Xi M., et al. Zn2+ and mPTP mediate resveratrol-induced myocardial protection from endoplasmic reticulum stress. Metallomics. 2020;12:290–300. doi: 10.1039/c9mt00264b. [DOI] [PubMed] [Google Scholar]
- 159.Li Z., Xu S., Liu P. Salvia miltiorrhizaBurge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018;39:802–824. doi: 10.1038/aps.2017.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang F., Yao S., Xia H. SIRT1 is a key regulatory target for the treatment of the endoplasmic reticulum stress-related organ damage. Biomed. Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110601. [DOI] [PubMed] [Google Scholar]
- 161.Peters U., Poole C., Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am. J. Epidemiol. 2001;154:495–503. doi: 10.1093/aje/154.6.495. [DOI] [PubMed] [Google Scholar]
- 162.Hodgson J.M. Tea flavonoids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008;17:288–290. [PubMed] [Google Scholar]
- 163.Sutton-Tyrrell K., Bostom A., Selhub J., et al. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997;96:1745–1749. doi: 10.1161/01.cir.96.6.1745. [DOI] [PubMed] [Google Scholar]
- 164.Kris-Etherton P.M., Keen C.L. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr. Opin. Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 165.Nacerai H., Gregory T., Sihem B., et al. Green tea beverage and epigallocatecihin gallate attenuate nicotine cardiocytotoxicity in rat, Acta Pol. Pharm. 2017;74:277–287. [PubMed] [Google Scholar]
- 166.Liu J.C., Chan P., Chen Y.J., et al. The antihypertensive effect of the berberine derivative 6-protoberberine in spontaneously hypertensive rats. Pharmacology. 1999;59:283–289. doi: 10.1159/000028331. [DOI] [PubMed] [Google Scholar]
- 167.Murakami A., Nakamura Y., Ohto Y., et al. Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid. BioFactors. 2000;12:187–192. doi: 10.1002/biof.5520120130. [DOI] [PubMed] [Google Scholar]
- 168.Ha D.T., Trung T.N., Hien T.T., et al. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human HepG2 cells. J. Ethnopharmacol. 2010;131:417–424. doi: 10.1016/j.jep.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 169.Cheang W.S., Tian X.Y., Wong W.T., et al. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5’ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor δ pathway. Arterioscler. Thromb. Vasc. Biol. 2014;34:830–836. doi: 10.1161/ATVBAHA.113.301938. [DOI] [PubMed] [Google Scholar]
- 170.Choy K.W., Lau Y.S., Murugan D., et al. Chronic treatment with paeonol improves endothelial function in mice through inhibition of endoplasmic reticulum stress-mediated oxidative stress. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Montezano A.C., Touyz R.M. Oxidative stress, Noxs, and hypertension: Experimental evidence and clinical controversies. Ann. Med. 2012;44:S2–S16. doi: 10.3109/07853890.2011.653393. [DOI] [PubMed] [Google Scholar]
- 172.Deliorman Orhan D., Hartevioğlu A., Küpeli E., et al. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007;112:394–400. doi: 10.1016/j.jep.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 173.Stafoggia M., Forastiere F., Agostini D., et al. Factors affecting in-hospital heat-related mortality: A multi-city case-crossover analysis. J. Epidemiol. Community Health. 2008;62:209–215. doi: 10.1136/jech.2007.060715. [DOI] [PubMed] [Google Scholar]
- 174.Fan C., Sun J., Fu X., et al. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang J.P., Shin J.H., Seo S.H., et al. Effects of antioxidants in reducing accumulation of fat in hepatocyte. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19092563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang J., Wang Y., Bao C., et al. Curcumin-loaded PEG-PDLLA nanoparticles for attenuating palmitate-induced oxidative stress and cardiomyocyte apoptosis through AMPK pathway. Int. J. Mol. Med. 2019;44:672–682. doi: 10.3892/ijmm.2019.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sharifiaghdam Z., Dalouchi F., Sharifiaghdam M., et al. Curcumin-coated gold nanoparticles attenuate doxorubicin-induced cardiotoxicity via regulating apoptosis in a mouse model. Clin. Exp. Pharmacol. Physiol. 2022;49:70–83. doi: 10.1111/1440-1681.13579. [DOI] [PubMed] [Google Scholar]
- 178.Boarescu P.M., Chirilă I., Bulboacă A.E., et al. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7847142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang L., Zhu K., Zeng H., et al. Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity. Int. J. Nanomedicine. 2019;14:6061–6071. doi: 10.2147/IJN.S211130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zheng Y., Lu L., Yan Z., et al. mPEG-icariin nanoparticles for treating myocardial ischaemia. Artif. Cells Nanomed. Biotechnol. 2019;47:801–811. doi: 10.1080/21691401.2018.1554579. [DOI] [PubMed] [Google Scholar]
- 181.Li H., Zhu J., Xu Y., et al. Notoginsenoside R1-loaded mesoporous silica nanoparticles targeting the site of injury through inflammatory cells improves heart repair after myocardial infarction. Redox Biol. 2022;54 doi: 10.1016/j.redox.2022.102384. [DOI] [PMC free article] [PubMed] [Google Scholar]