Abstract
Nucleophilic aromatic substitutions (SNAr) of alkoxides on pentafluoroaryl ethers are explored as a first step in a synthesis sequence to generate all-cis 2,3,5,6-tetrafluorocyclohexyl-1,4-dialkyl ethers 1. The SNAr reaction was explored both experimentally and theoretically to rationalize ortho/para/meta selectivities. tert-Butyl deprotection of products followed by phenol alkylations introduces versatility to the synthesis. The final Rh(CAAC) 3 catalyzed aryl hydrogenation step of intermediate tetrafluoroaryl-1,4-diethers generated cyclohexane products 1. This chemistry introduces a new class of Janus fluorocyclohexane derivatives with ether substituents placed 1,4- to each other.
Introduction
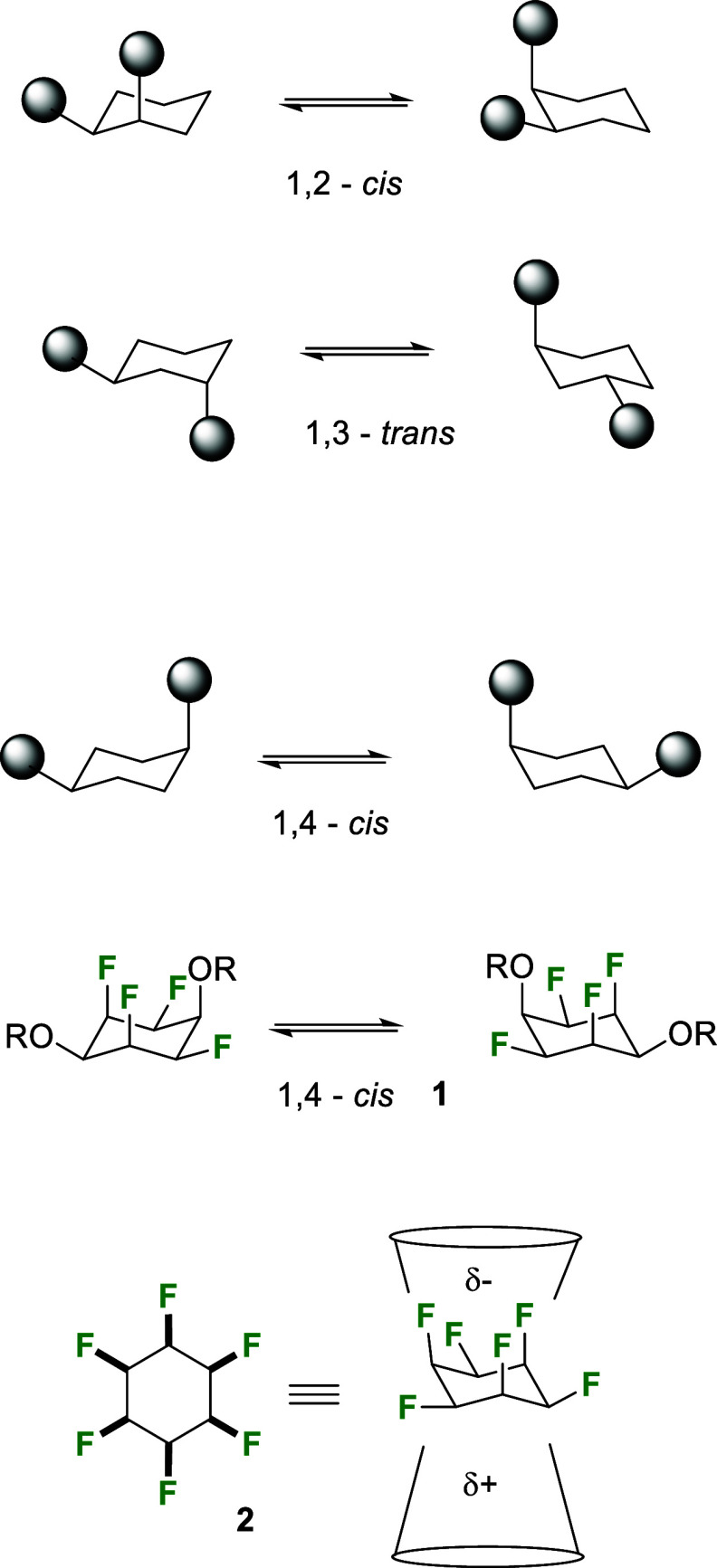
There has been a recent discussion regarding the synthesis and properties of “thermodynamically disfavored” cyclohexanes,1 where there is an ax/eq or eq/ax conformational ambiguity, such as is found in cis-1,2, trans-1,3, and cis-1,4 cyclohexanes, as illustrated in Figure 1. Chemistry to access such substitutions will generally tend toward thermodynamically preferred diequatorial isomers, and thus, specific methods have to be devised to achieve these thermodynamically disfavored cis-configurations. The relatively low representation of such compounds across the large organic molecule demographic runs counter to a wider consensus that molecules with an increased dimensionality offer new prospects for uncovering innovative properties from materials2 to bioactives.3
Figure 1.
“Thermodynamically disfavored” cyclohexanes and all-cis-1,2,3,4,5,6-hexafluorocyclohexane 2.
In this paper, we explore the preparation of cis-1,4 cyclohexyl ethers 1, where the cyclohexane ring also has four fluorine atoms arranged around the ring and with an all-cis configuration. This is an extension of our current interest in facially polarized Janus cyclohexanes.4 All-cis-1,2,3,4,5,6-hexafluorocyclohexane 2 has attracted some interest due to its high polarity, particularly as it is a cyclohexane, which as a class is generally found to be hydrophobic.5 In the interconverting chair conformations of this cyclohexane, there are always triaxial C–F bonds pointing in the same direction, which impart a strong molecular dipole. It is the coalignment of the axial C–F bonds, which contribute most significantly to its the molecular polarity.6
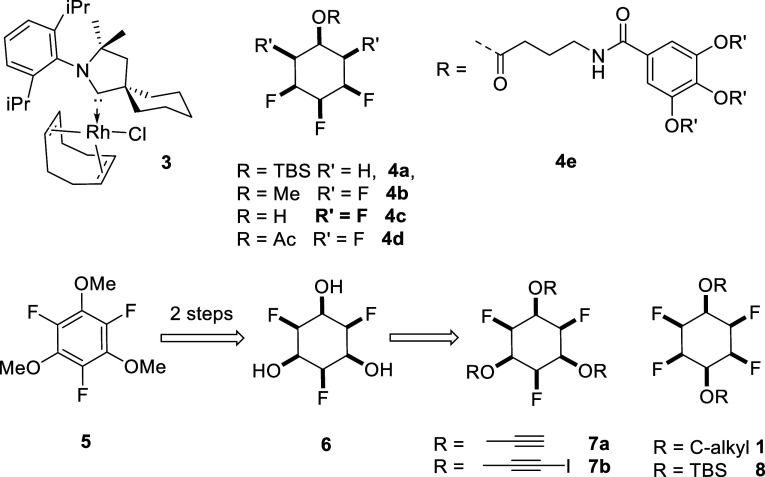
These systems have been termed Janus cyclohexanes7 as they have two faces (electropositive-H and electronegative-F face). The first preparation of 2 in our laboratory5 from inositol was significantly improved by the Glorius laboratory,8 who developed a direct aryl hydrogenation of hexafluorobenzene. The method involves high pressure hydrogenations (∼50 bar H2) using a Rh catalyst, which was applied to aryl hydrogenations by Zeng et al.9 The catalyst 3 shown in Figure 2 contains the strongly electron-donating cyclic alkyl amino carbene ligand (CAAC) and reactions are carried out in low polarity solvents. This combination suppressed competing dehydrofluorination reactions, generating a series of Janus cyclohexanes with good efficiency. For the preparation of ethers, Glorius et al.8c exemplified the aryl hydrogenation of fluorophenyl silyl (TBS) ethers to the corresponding all-cis pentafluorocyclohexyl ethers, such as 4a. Subsequently, von Delius’s laboratory10 demonstrated aryl hydrogenation to methyl ether 4b from the pentafluoroanisole precursor. This methyl ether could be converted to alcohol 4c and derivatized to acetate 4c and more elaborate esters, such as 4e, which acted as monomers displaying interesting dynamic behavior due to self-assembly equilibria between the Janus rings. Most recently,11 von Delius’s laboratory has extended to examples of all-cis 1,3,5-triethers 7a and 7b (via triol 6), which have been prepared by aryl hydrogenations of trifluorotrimethoxybenzene 5 followed by demethylation. The three ether substituents in 7a and 7b adopt a thermodynamically favorable triequatorial conformation, and as a consequence, the three C–F bonds are triaxial. This arrangement is attractive as it offers trisubstituted cyclohexanes with a large dipole due to the oriented C–F bonds.
Figure 2.
CAAC catalyst 3 and Janus ether substituted cyclohexanes.8−10
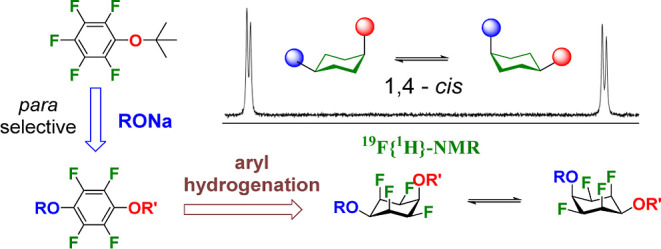
We address here the synthesis of all-cis tetrafluoro disubstituted cyclohexanes 1, where alkyl ether substituents are cis-1,4 to each other. They are a class of Janus cyclohexanes where oxygens replace the 1,4-fluorines; however, there will always be an axial and an equatorial C-OR bond in interconverting chair conformations rendering these “thermodynamically disfavored”. The only example of a 1,4-ether that we are aware of is bis silyl ether 8, which was reported in the original Glorius paper.8 The new class of Janus 1,4-dialkyl ethers may offer some advantages in that they will have more complex 3D arrangements and will be more dynamic in supramolecular assemblies. The route to these 1,4-cyclohexyl diethers exploits a para selective SNAr reaction on tButO-pentafluorophenol ether 10 and then aryl hydrogenations offer access to molecules of class 1.
Results and Discussion
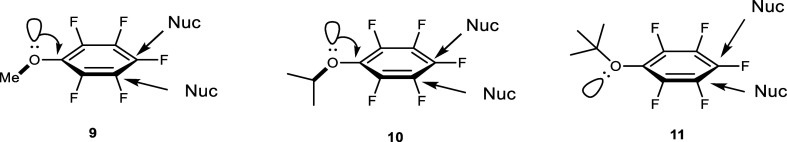
A general route to 1,4-disubstituted tetrafluorocyclohexyl ether 1 is outlined in Scheme 1. For SNAr reactions on pentafluoroaryl rings, a regiochemical preference for para over meta attack by nucleophiles is generally observed; although the bias is dependent on the indigenous substituent.
Scheme 1. Synthesis Routes to All-Cis Tetrafluorocyclohexyl Diethers 1a–1e from tButO Ether 11.
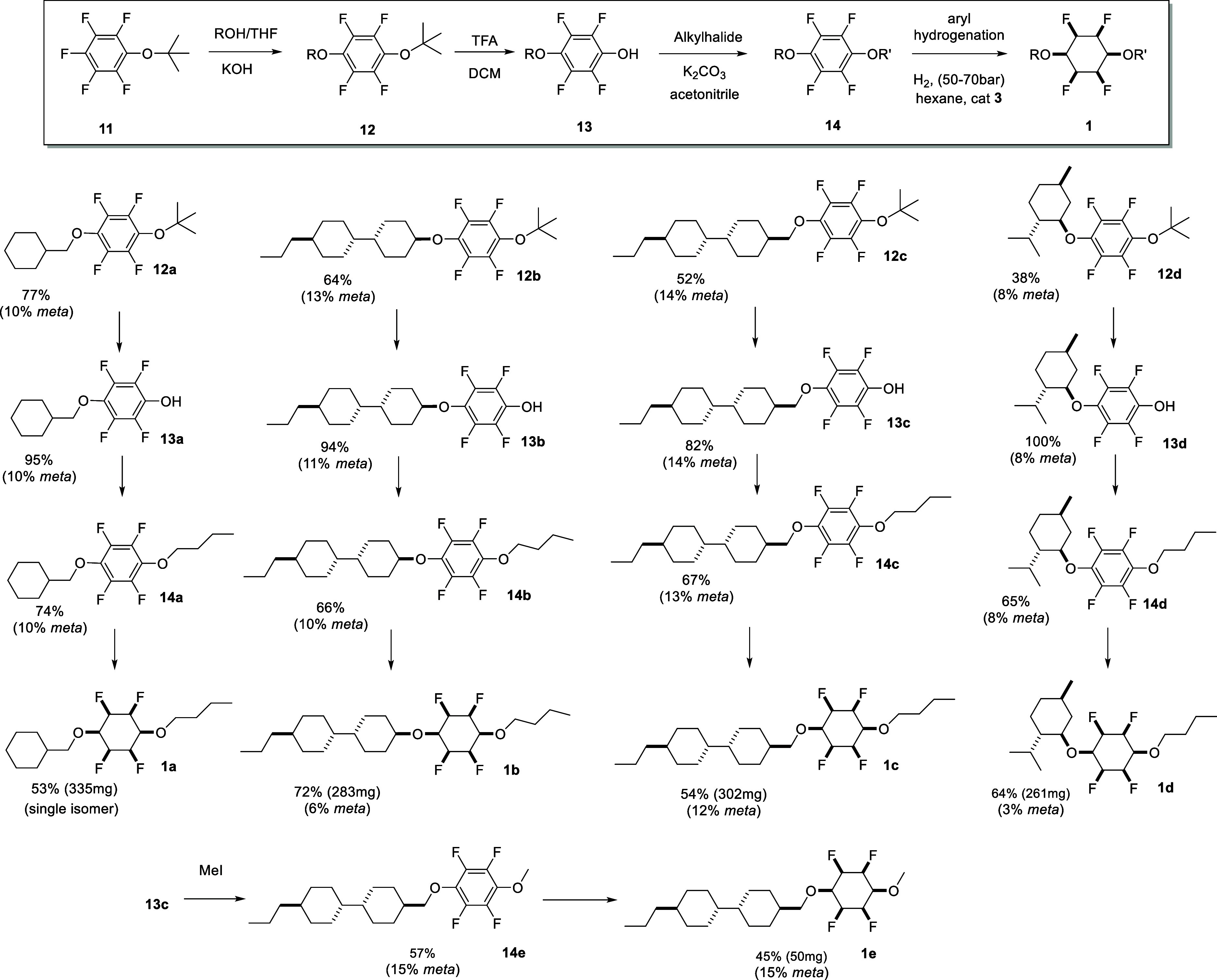
For example, SNAr reactions on C6F5X systems with electron-withdrawing groups (–NO2, –CN) are very highly selective for para products12 as is a –Ph substituent13 or even an unsubstituted –H substituent.14 For pentafluoroaryl ethers such as X = OMe, the para over the meta directing effect is much more modest relative to –H14 and –Ph,13 due to the mesomeric donor ability of the methoxyl group. In order to maximize the regiochemical preference in favor of para addition for an alkoxide nucleophile to a pentafluoroaryl ether, an exploration of progressively larger indigenous ether substituents (–OMe 9, –OiPr 10, and –OtBut 11) was conducted and with progressively larger alkoxide nucleophiles to enhance both steric and stereoelectronic factors. Reactions were conducted in THF with 9, 10, and 11 reacting with sodium methoxide (OMe–), iso-propoxide (iPrO–) and tert-butoxide (tButO–) as nucleophiles. The ortho/para/meta product ratios are represented in Table 1. It emerged that the meta/para ratio is the lowest in the reactions conducted on the tButO-aryl ether 11 and with tButO– as a nucleophile.
Table 1. Ortho/Para/Meta Ratios of Products from Reactions of 9–11 with Alkoxide Nucleophilesa.
| nucleophile |
9 (MeO) |
10 (iPrO) |
11 (tButO) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ortho | para | meta | ortho | para | meta | ortho | para | meta | |
| MeONa | 0.55 | 1.27 | 1.0 | 0.0 | 1.8 | 1.0 | 0.0 | 3.7 | 1.0 |
| iPrONa | 0.49 | 1.34 | 1.0 | 0.0 | 1.7 | 1.0 | 0.0 | 4.3 | 1.0 |
| tButONa | 0.28 | 1.81 | 1.0 | 0.0 | 2.4 | 1.0 | 0.0 | 7.1 | 1.0 |
Conditions: 9, 10 or 11 (1.0 equiv), alcohol (0.5 equiv), NaH (0.75 equiv), THF (3 mL), 2.5 h, 50 °C. (Ratios are an averages of triplicates).
It is not clear where the origin of this selectivity arises. In aryl ethers (anisoles), the MeO– substituent tends to adopt a planar orientation with respect to the aromatic ring, maximizing oxygen lone pair conjugation into the ring whereas the tButO– group adopts a perpendicular conformation, which will reduce the donor potential of the oxygen lone pairs.15 However, the ortho fluorines in 9 also direct the –OMe to a more favored perpendicular conformation,16 so stereoelectronically the MeO- and tButO-ethers are more equivalent in this perfluorinated series. In order to explore these effects further, reaction profiles with an alkoxide nucleophile were conducted computationally for MeO- ether 9 and tButO-ether 11. In each case, reaction profiles were modeled for para and meta trajectories and with MeO– and tButO– as nucleophiles.
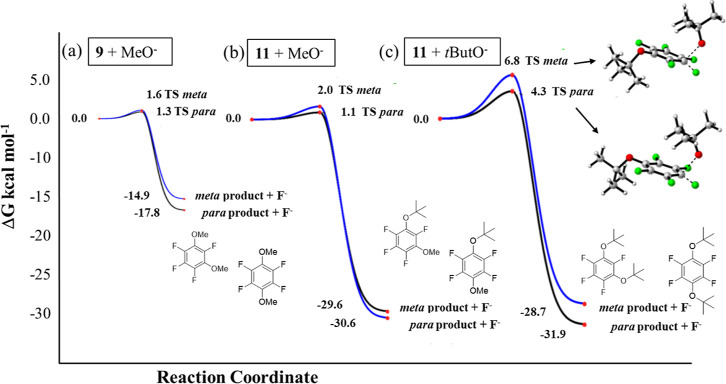
It proved challenging to locate transition states (TSs), but they could be found employing a developing version of Autobench17 for energy reaction barriers. Considering the methoxyl ether 9 as the substrate and with MeO– as the nucleophile, the energies of the TSs are very similar and only a little (1.3 and 1.6 kcal mol–1) above the ground state energy of 9, as illustrated in Figure 3a. In each case, the reaction is highly exergonic (ΔG0 = −14.9 and −17.8 kcal mol–1) with a high barrier to reversibility and thus the meta/para ratio appears to be determined kinetically with the meta TS a little higher (ΔΔG‡ = 0.8 kcal mol–1) than that of the para TS (Figure 4). When 11 is the substrate and with MeO– again as the nucleophile, the reaction follows a trend similar to that of 9, with a ΔΔG‡ = 0.9 kcal mol–1, but the reaction is very much more exergonic for the meta/para products (ΔG0 = −29.6 and −30.6 kcal mol–1), as illustrated in Figure 3b. On the other hand, when tButO– is the nucleophile combining with tButO ether 11, there is an increase in the reaction energy barriers to ΔΔG‡ = 2.5 kcal mol–1 favoring the para TS, and the reaction is also high exergonic, as illustrated in Figure 3c. In all of these reactions, intermediate Meisenheimer complexes could not be located computationally, and these reactions appear to progress through a concerted SNAr substitution process. Concerted processes have already been proposed for SNAr of perfluoroaromatics,18,19 and this general mechanism for SNAr reactions was evaluated by Jacobsen20 and has been discussed more widely.21
Figure 3.
Reaction profiles for SNAr reactions of (a) 9 with OMe–; (b) 11 with OMe–; and (c) 11 with tButO–; each attacking either at the meta or para positions calculated at the M06-2X/6-311++G** level. The para reaction course is kinetically favored in each case.
Figure 4.
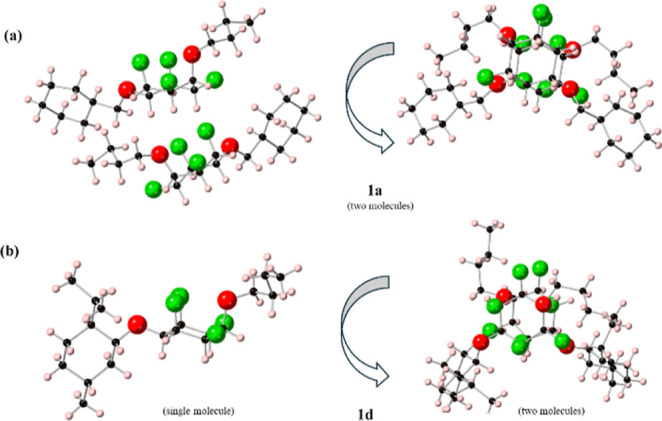
X-ray structures of 1,4-diethers (a) 1a and (b) 1d viewed from two trajectories.
With the knowledge that the para/meta ratio is most influenced by an incipient tButO ether, 11 emerged as an attractive starting material in order to most effectively influence the para/meta regiochemistry. SNAr reactions generated the tButO ether products 12, predominantly as the para products, with lesser levels of meta isomers (≤15%), as illustrated in Scheme 1. The ethers were then conveniently cleaved to aryl ether phenols 13 by treatment with trifluoroacetic acid. Subsequent alkylation of phenols 13, in this case with 1-bromobutane or methyl iodide-generated diaryl ethers 14.
The resultant ethers 14 were then subject to aryl hydrogenations (H2 50 bar, cat 3)8 to generate a range of all-cis tetrafluoro-substituted cyclohexyl diethers 1. The approach could reasonably be applied quite widely although the reactions explored here were conducted with well-known liquid crystal motifs as the targets.22
In the case of 1b–e, differential scanning calorimetry and polarized optical microscopy analysis showed significant levels of crystalline polymorphism in each case, although no LC phases were observed during both the heating and cooling processes (see Figures S1–S4). In all Janus cis-tetrafluorocyclohexane 1b–e, the alkoxy moieties introduced at the 1,4-positions occupy axial and equatorial positions, forming a bent structure, which appears to prevent LC phase formation.
1,4-Diether products 1a–e were solids and single crystals of 1a and 1d were obtained and submitted for X-ray structure analysis. The resultant structures are illustrated in Figure 4. In each case, the structures adopt the expected chair conformations in the solid state and, consequently, the 1,4-substitution dictates an axial and an equatorial ether bond in each cyclohexane. In both cases, it is the n-butyl ether that adopts the axial orientation. One feature of both structures is that the cyclohexane rings do not stack directly above each other; instead, they rotate such that the C–O bonds eclipse C–F bonds in the ring stacks. Essentially, there is less order in ring stacking relative to compounds where the substituents are all equatorial.23
In the solid state, only a single conformer is adopted; however, in solution, 19F-[1H}NMR analysis indicates that these compounds adopt ∼1:1 mixed ratios of presumably the chair conformers, when the ether substituents are nonequivalent and with these nonequivalent substituents switching between axial and equatorial orientations. This is obvious in the representative NMR spectra of 1a, as illustrated in Figure 5.
Figure 5.
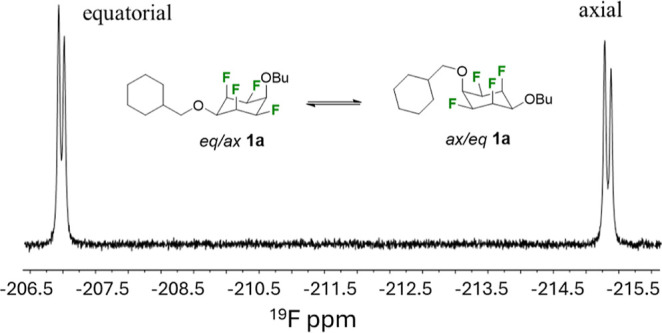
19F{1H}-NMR of 1a showing an equilibrium mix of eq/ax1a and ax/eq1a in solution.
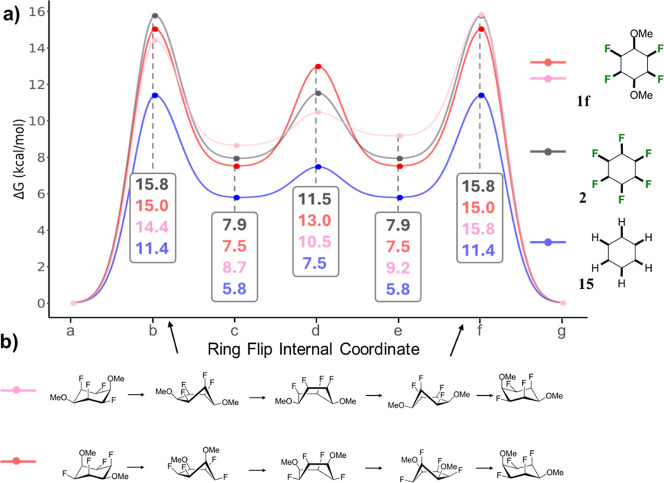
The pathway and energy barrier to this interconversion was explored theoretically for the symmetric tetrafluoro dimethyl ether 1f and barriers to interconversion were compared relative to all-cis-hexafluorofluorocyclohexane 2 and to cyclohexane 15 itself to act as points of reference. Calculations were carried out at the M06-2X/6-311++G** theory level.24,25 The resultant interconversion pathways and energy barriers are illustrated in Figure 6.
Figure 6.
(a) Comparative cyclohexyl interconversion energy profiles for dimethoxy ether 1f, all-cis1,2,3,4,5,6-exafluorocyclohexane 2 and cyclohexane 15 showing the relative energies of intermediates. Energy values in kcal mol–1. (b) Schematic of the two interconversion pathways for cyclohexane 1f.
The values computed here for the TSs and, also the twisted boat intermediates, are similar to those reported in previous studies for both all-cis1,2,3,4,5,6-hexafluorocyclohexane 2(26) and cyclohexane 15(27) and, therefore, we assume that the calculated conformational energy profile for 1f is relatively accurate. It is notable that the highest barrier to interconversion for 1f at the “half-chair” structure is close to that for 2 at its “half-chair” intermediate, suggesting a similar level of negative electrostatic interaction between the C–O and C–F bonds in 1f, and C–F and C–F bonds in 2 as the conformational profile progresses through these two intermediates. In fact, the “half-chair” TS in 1f is 0.8 kcal mol–1 lower in energy compared to 2 (15.0 against 15.8 kcal mol–1), which is attributed to the formation of OC–H···F stabilizing contacts formed between methoxy groups and fluorine atoms (Figure S8). These half–chair TSs are ∼4.0 kcal mol–1 above that for cyclohexane 15, where only C–H/C–H bond contacts are present. There are two conformational profiles for 1f, corresponding to two possible symmetrical boat intermediate structures where either two C–O or two C–F bonds occupy the apical positions of the TS structure (see Figure 6b). Ethoxylation at positions 1 and 4 on the fluorinated systems does not significantly affect the ring-flip energy barriers, which are around 15 kcal mol–1 and easily surmountable at room temperature. As a result, it is expected that asymmetric compounds 1a–e, despite showing a single conformer in the solid state, are in a rapid equilibrium between both chair conformers in solution.
In conclusion, we present a synthesis to all-cis 2,3,5,6-tetrafluorocyclohexanes 1 carrying 1,4-diether moieties, as a new class of Janus cyclohexanes. These were prepared by SNAr chemistry with various alkoxide nucleophiles on pentafluoroaryl ether 11. Cleavage of the tButO ethers 12 followed by alkylation of the resultant phenols 13, allowed a range of 1,4 diethers 14 to be prepared, and these were subjected to aryl hydrogenations to generate cyclohexanes of class 1. Low levels of the meta isomers were evident from the SNAr reaction, a reaction that was investigated computationally. This analysis indicated a maximum energy difference (∼2.5 kcal mol–1) between para and meta TSs, for tButO ether 11 and tBuO– as a nucleophile, which is consistent with the experimental outcomes. These “thermodynamically disfavored” compounds form less ordered arrangements in the solid state than previous prepared all-cis-fluorocyclohexanes.23,28
Acknowledgments
We thank EPSRC (EP/S030506) UK, and FAPESP Brazil are thanked for a Young Researcher Award to R.A.C. (#2018/03910-1) and Scholarships to B.A.P. (#2023/14064-2 and #2022/10156-7). CNPq is acknowledged for a fellowship to R.A.C. (#306912/2023-6) and FAEPEX for research funding. CENAPAD-SP and CESUP computational resources are also gratefully acknowledged.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.4c02345.
Experimental details and analyses of prepared compounds 1a–1e, copies of 1H, 13C{1H} and 19F{1H} NMR spectra, X-ray data 1a and 1d, details of computation reaction barriers for SNAr reactions on 9 and 11, and interconversion barriers for cyclohexanes 1f, 2, and 15 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Li Y.; Shi H.; Yin G. Synthetic techniques for thermodynamically disfavoured substituted six-membered rings. Nat. Rev. Chem 2024, 8, 535–550. 10.1038/s41570-024-00612-3. [DOI] [PubMed] [Google Scholar]
- a Chang J.; Chen F.; Li H.; Suo J.; Zheng H.; Zhang J.; Wang Z.; Valtchev V.; Qiu S.; Fang Q. Three-dimensional covalent organic frameworks with nia nets for efficient separation of benzene/cyclohexane mixtures. Nat. Commun. 2024, 15, 813. 10.1038/s41467-024-45005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ma X.; Scott T. F. Approaches and challenges in the synthesis of three-dimensional covalent-organic frameworks. Commun. Chem. 2018, 1, 98. 10.1038/s42004-018-0098-8. [DOI] [Google Scholar]
- a Wang Z.; Sharma P. P.; Rathi B.; Xie M.; De Clercq E.; Pannecouque C.; Kang D.; Zhan P.; Liu X. Escaping from Flatland: Multiparameter Optimization Leads to the Discovery of Novel Tetrahydropyrido[4,3-d]pyrimidine Derivatives as Human Immunodeficiency Virus-1 Non-nucleoside Reverse Transcriptase Inhibitors with Superior Antiviral Activities against Non-nucleoside Reverse Transcriptase Inhibitor-Resistant Variants and Favorable Drug-like Profiles. J. Med. Chem. 2023, 66, 8643–8665. 10.1021/acs.jmedchem.3c00275. [DOI] [PubMed] [Google Scholar]; b Lovering F. Escape from Flatland 2: Complexity and Promiscuity. MedChemComm 2013, 4, 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- O’Hagan D. The Emergence and Properties of Selectively Fluorinated ‘Janus’ Cyclohexanes. Chem. Rec. 2023, 23, e202300027 10.1002/tcr.202300027. [DOI] [PubMed] [Google Scholar]
- Keddie N. S.; Slawin A. M. Z.; Lebl T.; Philp D.; O’Hagan D. All-cis 1,2,3,4,5,6-Hexafluorocyclohexane is a Facially Polarized Cyclohexane. Nat. Chem. 2015, 7, 483–488. 10.1038/nchem.2232. [DOI] [PubMed] [Google Scholar]
- a Lecours M. J.; Marta R. A.; Steinmetz V.; Keddie N. S.; Fillion E.; O’Hagan D.; McMahon T. B.; Hopkins W. S. Interaction of B12F122- with All-cis 1,2,3,4,5,6 Hexafluorocyclohexane in the Gas Phase. J. Phys. Chem. Lett. 2017, 8, 109–113. 10.1021/acs.jpclett.6b02629. [DOI] [PubMed] [Google Scholar]; b Ziegler B. E.; Lecours M.; Marta R. A.; Featherstone J.; Fillion E.; Hopkins W. S.; Steinmetz V.; Keddie N. S.; O’Hagan D.; McMahon T. B. Janus Face Aspect of All-cis 1,2,3,4,5,6-Hexafluorocyclohexane Dictates Remarkable Anion and Cation Interactions In the Gas Phase. J. Am. Chem. Soc. 2016, 138, 7460–7463. 10.1021/jacs.6b02856. [DOI] [PubMed] [Google Scholar]
- Santschi N.; Gilmour R. A Janus Cyclohexane Ring. Nat. Chem. 2015, 7, 467–468. 10.1038/nchem.2240. [DOI] [PubMed] [Google Scholar]
- a Nairoukh Z.; Wollenburg M.; Schlepphorst C.; Bergander K.; Glorius F. The Formation of All-cis-(Multi)Fluorinated Piperidines by a Dearomatization-Hydrogenation Process. Nat. Chem. 2019, 11, 264–270. 10.1038/s41557-018-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wiesenfeldt M. P.; Knecht T.; Schlepphorst C.; Glorius F. Silylarene Hydrogenation: A Strategic Approach that Enables Direct Access to Versatile Silylated Saturated Carbo- and Heterocycles. Angew. Chem., Int. Ed. 2018, 57, 8297–8300. 10.1002/anie.201804124. [DOI] [PubMed] [Google Scholar]; c Wiesenfeldt M. P.; Nairoukh Z.; Li W.; Glorius F. Hydrogenation of Fluoroarenes. Direct Access to All-cis (Multi)Fluorinated Cycloalkanes. Science 2017, 357, 908–912. 10.1126/science.aao0270. [DOI] [PubMed] [Google Scholar]
- a Zhang X.; Ling L.; Luo M.; Zeng X. Accessing Difluoromethylated and Trifluoromethylated cis-Cycloalkanes and Saturated Heterocycles: Preferential Hydrogen Addition to the Substitution Sites for Dearomatization. Angew. Chem., Int. Ed. 2019, 58, 16785–16789. 10.1002/anie.201907457. [DOI] [PubMed] [Google Scholar]; b Wei Y.; Rao B.; Cong X.; Zeng X. Highly Selective Hydrogenation of Aromatic Ketones and Phenols Enabled by Cyclic (Amino)(alkyl)carbene Rhodium Complexes. J. Am. Chem. Soc. 2015, 137, 9250–9253. 10.1021/jacs.5b05868. [DOI] [PubMed] [Google Scholar]
- a Haridas S. V.; Shyshov O.; von Delius M. Supramolecular Polymerization of All-cis Fluorinated Cyclohexanes: Influence of Side Chains. Org. Mater. 2023, 5, 166–174. 10.1055/s-0043-1761314. [DOI] [Google Scholar]; b Shyshov O.; Haridas S. V.; Pesce L.; Qi H.; Gardin A.; Bochicchio D.; Kaiser U.; Pavan G. M.; von Delius M. Living Supramolecular Polymerization of Fluorinated Cyclohexanes. Nat. Commun. 2021, 12, 3134. 10.1038/s41467-021-23370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Shyshov O.; Siewerth K. A.; von Delius M. Evidence for Anion-Binding of All-cis Hexafluorocyclohexane in Solution and Solid State. Chem. Commun. 2018, 54, 4353–4355. 10.1039/C8CC01797B. [DOI] [PubMed] [Google Scholar]
- Haridas S. V.; von Delius M. Synthesis and Supramolecular Properties of All-cis-2,4,6-Trifluorocyclohexane-1,3,5-triol. Chem. Commun. 2024, 60, 606–609. 10.1039/D3CC05510H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon J.; Hollyhead W. B.; Patrick C. R.; Wilson K. V. 1184. Kinetics of nucleophilic substitution in polyfluoro-aromatic compounds. Part I. The reaction of sodium methoxide with some pentafluorophenyl-compounds. J. Chem. Soc. 1965, 6375–6379. 10.1039/jr9650006375. [DOI] [Google Scholar]
- Kvíčala J.; Beneš M.; Paleta O.; Král V. Regiospecific Nucleophilic Substitution in 2,3,4,5,6-Pentafluorobiphenyl as Model Compound for Supramolecular Systems. Theoretical Study of Transition States and Energy Profiles, Evidence for Tetrahedral SN2 Mechanism. J. Fluorine Chem. 2010, 131, 1327–1337. 10.1016/j.jfluchem.2010.09.003. [DOI] [Google Scholar]
- Brooke G. M.; Burdon J.; Tatlow J. C. J. Aromatic Polyfluoro Compounds XII. Orientation Reactions of Pentafluorobenzene. Chem. Soc. 1962, 3253–3254. [Google Scholar]
- Tsuzuki S.; Houjou H.; Nagawa Y.; Hiratani K. High-Level Ab-initio Calculations of Torsional Potential of Phenol, Anisole, and o-Hydroxyanisole: Effects of Intramolecular Hydrogen Bond. J. Phys. Chem. A 2000, 104, 1332–1336. 10.1021/jp993607e. [DOI] [Google Scholar]
- Belyakov A. V.; Kieninger M.; Cachau R. E.; Ventura O. N.; Oberhammer H. Molecular Structure and Internal Rotation in 2,3,5,6-Tetrafluoroanisole as Studied by Gas-Phase Electron Diffraction and Quantum Chemical Calculations. J. Phys. Chem. A 2005, 109, 394–399. 10.1021/jp046975d. [DOI] [PubMed] [Google Scholar]
- Cormanich R. A.; da Silva G. D. Autobench V1.0: Benchmarking Automation for Electronic Structure Calculations. J. Chem. Inf. Model. 2024, 64, 3322–3331. 10.1021/acs.jcim.4c00250. [DOI] [PubMed] [Google Scholar]
- Sadowsky D.; McNeill K.; Cramer C. J. Dehalogenation of Aromatics by Nucleophilic Aromatic Substitution. Environ. Sci. Technol. 2014, 48, 10904–10911. 10.1021/es5028822. [DOI] [PubMed] [Google Scholar]
- Kikushima K.; Grellier M.; Ohashi M.; Ogoshi S. Transition-Metal-Free Catalytic Hydrodefluorination of Polyfluoroarenes by Concerted Nucleophilic Aromatic Substitution With a Hydrosilicate. Angew. Chem., Int. Ed. 2017, 56, 16191–16196. 10.1002/anie.201708003. [DOI] [PubMed] [Google Scholar]
- Kwan E. E.; Zeng Y.; Besser H. A.; Jacobsen E. N. Concerted Nucleophilic Aromatic Substitutions. Nat. Chem. 2018, 10, 917–923. 10.1038/s41557-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach S.; Smith A. J.; Pang J. H.; Poole D. L.; Tuttle T.; Chiba S.; Murphy J. A. Concerted Nucleophilic Aromatic Substitution Reactions. Angew. Chem., Int. Ed. 2019, 58, 16368–16388. 10.1002/anie.201902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P.; Bremer M. Nematic Liquid Crystals for Active Matrix Displays: Molecular Design and Synthesis. Angew. Chem., Int. Ed. 2000, 39, 4216–4235. . [DOI] [PubMed] [Google Scholar]
- Poskin T. J.; Piscelli B. A.; Yoshida K.; Cordes D. B.; Slawin A. M. Z.; Cormanich R. A.; Yamada S.; O’Hagan D. Janus Faced Fluorocyclohexanes for Supramolecular Assembly: Synthesis and Solid-State Structures of Equatorial Mono-Di- and Tri Alkylated Cyclohexanes and With Tri-Axial C-F Bonds to Impart Polarity. Chem. Commun. 2022, 58, 7968–7971. 10.1039/D2CC03010A. [DOI] [PubMed] [Google Scholar]
- Bannwarth C.; Ehlert S.; Grimme S. GFN2-xTB-An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. 10.1021/acs.jctc.8b01176. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Truhlar D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Pratik S. M.; Nijamudheen A.; Datta A. Janus All-cis-1,2,3,4,5,6-Hexafluorocyclohexane: A Molecular Motif for Aggregation-Induced Enhanced Polarization. ChemPhysChem 2016, 17, 2373–2381. 10.1002/cphc.201600262. [DOI] [PubMed] [Google Scholar]
- a Anet F. A. L.; Bourn A. J. R. Nuclear Magnetic Resonance Line-Shape and Double-Resonance Studies of Ring Inversion in Cyclohexane-d11. J. Am. Chem. Soc. 1967, 89, 760–768. 10.1021/ja00980a006. [DOI] [Google Scholar]; b Hendrickson J. B. Molecular geometry. VII. Modes of interconversion in the medium rings. J. Am. Chem. Soc. 1967, 89, 7047–7061. 10.1021/ja01002a038. [DOI] [Google Scholar]
- Clark J. L.; Taylor A.; Geddis A.; Neyyappadath R. M.; Piscelli B. A.; Yu C.; Cordes D. B.; Slawin A. M. Z.; Cormanich R. A.; Guldin S.; O’Hagan D. Supramolecular packing of alkyl substituted Janus face all-cis 2,3,4,5,6-pentafluorocyclohexyl motifs. Chem. Sci. 2021, 12, 9712–9719. 10.1039/D1SC02130C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.