Abstract
Prostate cancer is a leading cause of cancer-related death in men worldwide. Luteinizing hormone-releasing hormone receptor (LHRH-R) agonists and antagonists are known to achieve castration-level testosterone suppression; however, long-term data comparing the survival benefits of these therapies are insufficient to inform treatment decisions. Furthermore, the advent of next-generation hormonal agents (NHAs), such as abiraterone and enzalutamide, have shifted the paradigm of managing prostate cancer. Although LHRH-R agonists and antagonists remain the cornerstone treatment across various stages of prostate cancer, they are increasingly administered with NHAs, because the combination treatment confers a survival advantage. Nevertheless, the differences in efficacy and safety profiles among various combinations of LHRH-R agonists and antagonists and NHAs remain unclear. Hence, this narrative review is aimed at providing a comprehensive overview of the long-term outcomes of various LHRH-R agonists and antagonists. Key data from major clinical studies are summarized, categorized by disease stage. LHRH-R agonists and antagonists, particularly goserelin, have demonstrated long-term survival benefits in patients with localized and locally advanced prostate cancer. The clinical outcomes of different LHRH-R agonists and antagonists in combination with NHAs have also been evaluated. Among the various combinations, goserelin plus abiraterone appears to have a manageable safety profile with relatively low rates of hot flushes and fatigue. Overall, long-term survival data and safety profiles should be considered in selecting optimal combination therapies for prostate cancer treatment.
Keywords: Luteinizing hormone-releasing hormone receptor agonists, luteinizing hormone-releasing hormone receptor antagonists, prostate cancer, long-term survival, next-generation hormonal agents
Introduction
Prostate cancer (PC) is the second most common cancer and the fifth leading cause of cancer-related death in men worldwide1. In 2022, an estimated 1,466,718 new cases and 396,773 deaths were attributed to this disease2. The understanding of PC as androgen-dependent tumors has provided the rationale for achieving and maintaining serum testosterone concentrations at castration levels in patients with PC3.
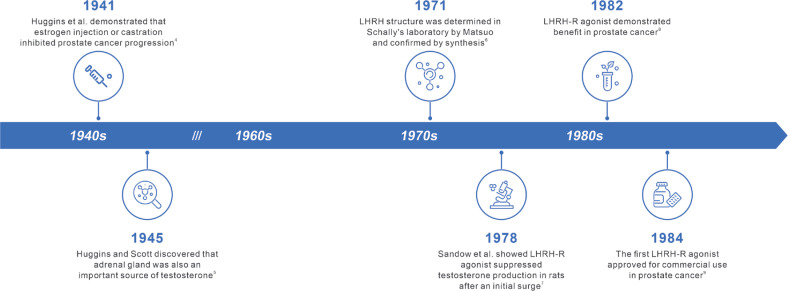
The first report of medical castration dates to 1941, when Huggins et al.4 demonstrated that estrogen injection or castration inhibits PC progression. Subsequently, in 1945, Huggins and Scott5 discovered that the adrenal gland is also an important source of testosterone, thus stimulating interest in medical castration of adrenal androgens. Another milestone occurred when Schally et al.6 elucidated the structure and physiological functions of luteinizing hormone-releasing hormone (LHRH) and described a method to synthesize it. This seminal study sparked great interest in LHRH analogues, and subsequently led to preclinical demonstration of testosterone suppression after an initial surge and demonstration of benefits in PC7,8. Subsequently, several LHRH analogues, known as luteinizing hormone-releasing hormone-receptor (LHRH-R) agonists, were developed and approved for clinical use9. The timeline of events leading to the development of LHRH-R agonists is presented in Figure 1. LHRH-R antagonists, which were initially developed for contraceptive purposes, have also been investigated as treatments for PC and demonstrated to be as effective as LHRH-R agonists10.
Figure 1.
Timeline of events in the development of LHRH-R agonists. LHRH-R, luteinizing hormone-releasing hormone receptor.
To date, androgen deprivation therapy (ADT) modalities include orchiectomy, LHRH-R agonists, LHRH-R antagonists, estrogens, and anti-androgens, which suppress the secretion of testicular androgens through different routes. LHRH-R agonists and antagonists, the primary pharmacological castration options in ADT, effectively lower androgen levels and thereby inhibit PC cell growth11. Mounting evidence indicates their efficacy and safety in clinical trials; however, given the complexity of the data, determining the optimal treatments in different treatment settings is difficult. Whereas previous meta-analyses and comparative reviews suggest that different LHRH-R agonists and antagonists yield comparable castration effects3,12, limited reports have compared their effects on long-term survival, the primary efficacy endpoint.
Although most patients respond to initial treatment with ADT, castration-resistant PC (CRPC) eventually develops. In CRPC, intracellular androgen levels are elevated, and the androgen receptor (AR) is overexpressed. To address these issues, novel compounds such as next-generation hormonal agents (NHAs) have been developed to block AR signaling13. Combining LHRH-R agents with NHAs improves overall survival benefits beyond those of LHRH-R agents alone in patients with metastatic, hormone-sensitive PC and castration-resistant PC14–17. Nevertheless, limited evidence is available comparing the efficacy and safety profiles of various LHRH-R agonists and antagonists combined with NHAs.
This narrative review is aimed at exploring and comparing the long-term treatment outcomes of various LHRH-R agonists and antagonists, and reviewing the efficacy of those agents in combination with NHAs; the safety profiles of various treatments are also discussed. We hope to provide valuable insights to inform the use of LHRH-R agonists/antagonists in various clinical settings.
Mechanisms of action of LHRH-R agonists, antagonists, and NHAs
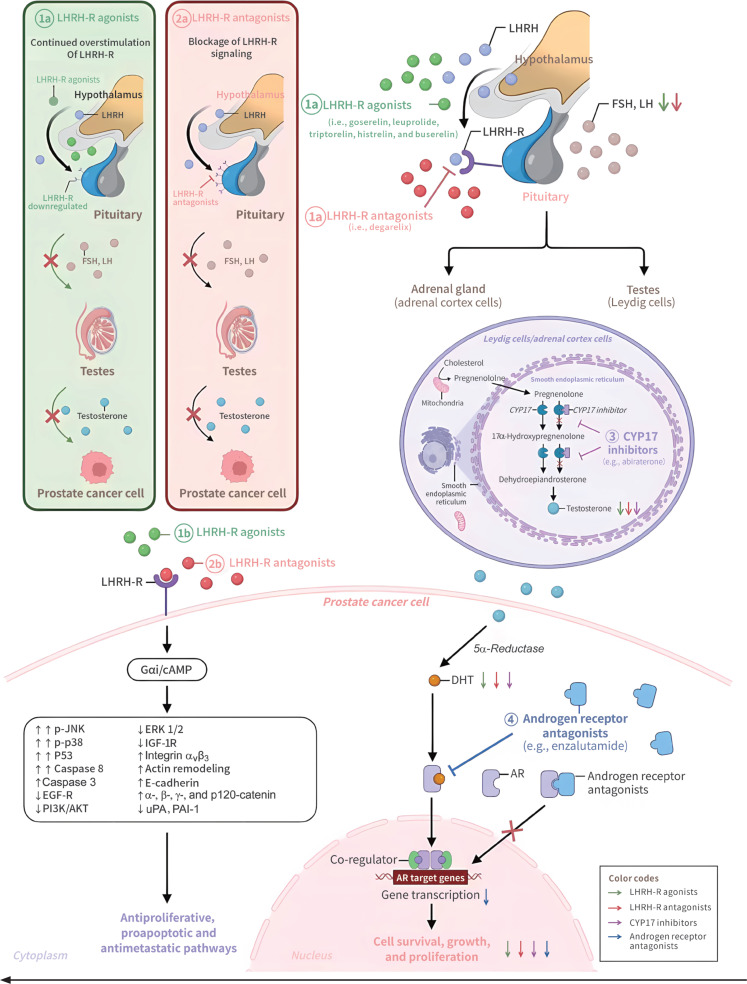
The mechanisms of action of LHRH-R agonists and antagonists are depicted in Figure 2. LHRH produced within the hypothalamus binds the LHRH-Rs and stimulates the anterior pituitary gland to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH)10,11. LH in turn binds the LH receptors in the testes, and induces the production and secretion of testosterone. Testosterone, together with other androgen precursors produced in the adrenal gland, is converted by 5α-reductase to 5α-dihydrotestosterone (DHT) in prostate cells; subsequently, DHT binds the AR, thus resulting in downstream gene amplification and expression. Constitutive activation of the AR signaling plays key roles in the survival, growth, and proliferation of PC cells11.
Figure 2.
Schematic diagram of mechanisms of action for LHRH-R agonists, LHRH-R antagonists, CYP17 inhibitors, and androgen receptor antagonists: (1a) LHRH-R agonists bind the LHRH-R and elicit an initial gonadotropin surge. Prolonged stimulation of LHRH-R along the hypothalamic-pituitary-gonadal axis eventually suppresses LH and testosterone. (2a) LHRH-R antagonists block interaction of LHRH with LHRH-R by directly occupying the binding sites on LHRH-R. (1b and 2b) In prostate cancer cells, LHRH-R is activated not only by LHRH-R agonists but also by LHRH-R antagonists, thereby leading to Gαi/cAMP activation. The Gαi/cAMP pathway triggers inactivation of tyrosine kinase receptors (e.g., EGF-R and IGF-1R) and interferes with downstream molecular pathways (e.g., MAPK and PI3K/AKT cascades), thus resulting in antiproliferative, apoptotic, antimetastatic, and antiangiogenic effects in prostate cancer cells. (3) CYP17 inhibitors suppress the synthesis of testosterone targeting the CYP17A. (4) AR antagonists inhibit DHT binding to the androgen receptor, thereby preventing androgen receptor nuclear translocation and subsequently blocking AR-mediated transcription. AKT, protein kinase B; AR, androgen receptor; cAMP, cyclic adenosine monophosphate; DHT, dihydrotestosterone; ERK, extracellular signal-regulated kinase; FSH, follicle-stimulating hormone; LH, luteinizing hormone; EGF-R, epidermal growth factor receptor; IGF-1R, insulin-like growth factor-1 receptor; LHRH, luteinizing hormone-releasing hormone; LHRH-R, LHRH receptor; MAPK, mitogen-activated protein kinase; p-JNK, phospho-c-Jun N-terminal kinase; PAI-1, plasminogen activator inhibitor-1; PI3K, phosphoinositide 3-kinase; uPA, urokinase plasminogen activator.
Natural LHRH is a decapeptide (pGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH2). LHRH-R agonists (for example, goserelin, triptorelin, leuprolide, histrelin, and buserelin) are synthetic peptides that mimic endogenous LHRH but bear modifications at the sixth and/or tenth amino acid that improve their half-life and potency18. LHRH-R agonists bind the LHRH-R and elicit an initial gonadotropin surge, which is described as a “flare-up” phenomenon that may last approximately 1 week. Concomitant therapy with an anti-androgen decreases the incidence of clinical flares, and sustained overstimulation of LHRH-R by LHRH-R agonists causes downregulation and desensitization of the receptors, thus decreasing gonadotropin production by the pituitary gland and subsequently decreasing testosterone levels19. Decreasing testosterone to castration levels can usually be achieved within 2–4 weeks of treatment20. In addition, LHRH-R agonists inhibit the mitogenic activity of growth factors such as insulin-like growth factor 1 and epidermal growth factor and their downstream signaling pathways in PC cells, in another mechanism explaining their anti-proliferative and pro-apoptosis effects21,22. In contrast, LHRH-R antagonists block interaction of LHRH with LHRH-R by directly occupying the binding sites on LHRH-R, thus decreasing LH and FSH production, and consequently testosterone levels. In this category, degarelix is a decapeptide with high affinity and selectivity toward human LHRH-R23. The presence of substitutions at multiple sites in LHRH and accompanying structural changes are believed to be associated with the release of histamine and the occurrence of severe hypersensitivity reactions24. LHRH-R antagonists induce sex hormone depletion rapidly and do not cause an initial serum testosterone flare10. Additionally, LHRH-R antagonists bind the LHRH-R in PC cells and interfere with growth factor receptor signaling pathways, thereby suppressing tumor growth and promoting apoptosis22. LHRH-Rs are also expressed in CRPC cells, where the binding to both agonists and antagonists results in anti-tumor effects mediated via the Gαi-cAMP signaling pathway22.
Although the mechanisms of action of ADT plus radiation or surgery have not been established, the antitumor efficacy of radiotherapy improves in combination treatment with ADT, which enhances the radiation effects and decreases micro-metastases; similarly, the addition of ADT to prostatectomy can also prevent micro-metastases25–29.
LHRH-R agents effectively inhibit tumor growth in castration-sensitive PC; however, tumors inevitably become castration resistant over time. The mechanisms responsible for castration-resistant status are mediated primarily by AR signaling, although AR-independent mechanisms have also been reported30. Hence, novel drugs have been developed to target the AR signaling axis in CRPC. Abiraterone acetate is a sterol ester that selectively inhibits CYP17 (a combination of 17α-hydrolase and 17,20-lyase inhibition)31. By blocking CYP17, a key enzyme in the androgen synthesis pathway, abiraterone acetate significantly decreases intracellular testosterone levels by suppressing its synthesis in the adrenal glands and testes, and within cancer cells32. The metabolite abiraterone also has inhibitory activity against CYP17 and enzymes involved in DHT synthesis, and additionally has competitive AR antagonism functions33. Novel non-steroidal anti-androgens, such as the benzamide enzalutamide, exhibit greater affinity toward the AR than first-generation anti-androgens34,35, which act as partial agonists but still allow AR transfer to the nucleus. The novel non-steroidal anti-androgens block AR transfer and therefore suppress any possible agonist-like activity. Inhibition of AR signaling results in blockade of downstream gene expression, and inhibition of PC cell survival and growth. The combination of LHRH-R agents and anti-androgens36,37, also known as maximal androgen blockade, not only inhibits the action of circulating androgens but also decreases intracellular testosterone levels by blocking androgen synthesis and nuclear transfer of ARs. Combining LHRH-R agents with anti-androgens improves anticancer activity toward castration-resistant PC and is currently an important treatment strategy for the disease. The properties of the various drug classes discussed herein are presented in Table S1.
LHRH-R agonists and antagonists provide long-term benefits across various stages of PC
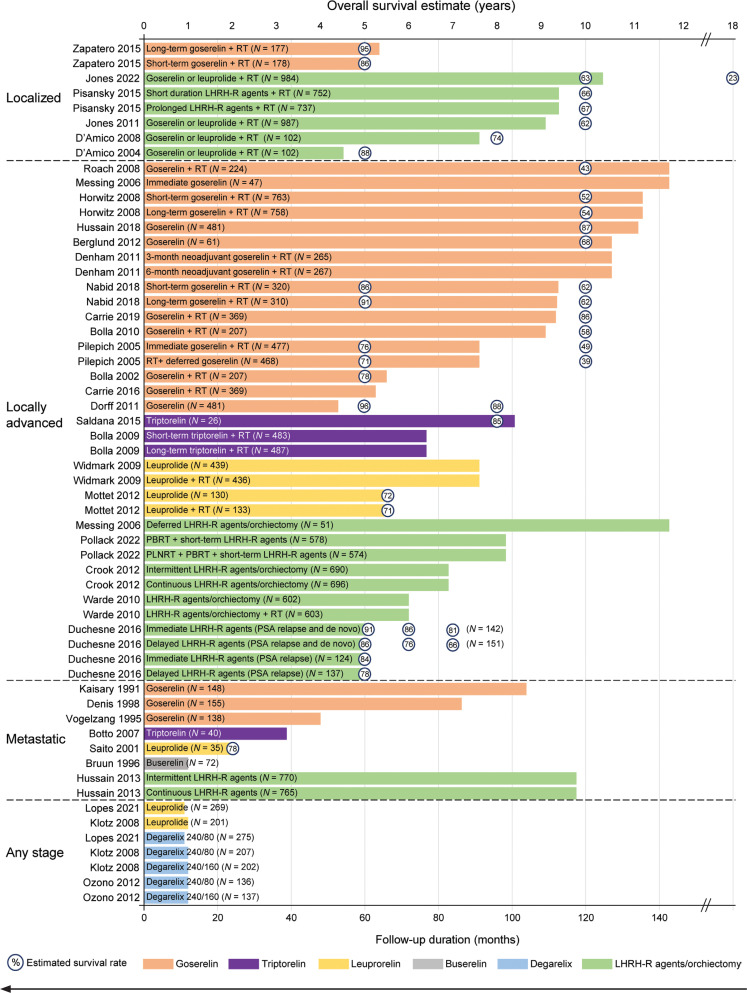
The long-term survival outcomes of patients with PC receiving treatment with various LHRH-R agonists and antagonists in clinical trials are summarized in Figure 3 and Table S2.
Figure 3.
Follow-up durations and overall survival estimates for LHRH-R agonist and antagonist treatment of prostate cancer. Lengths of bars represent the study follow-up duration for the treatment groups, and numbers in circles represent the estimated survival rate. For example, Zapatero et al.38 reported a 5-year survival estimate of 95% with 64 months of study follow-up in the long-term goserelin + radiotherapy group. Some studies did not report survival estimates; therefore, some bars in the figure do not have survival rate percentages. Note: 13 of 46 patients in Messing et al.39 underwent bilateral orchiectomy, and the others received goserelin. LHRH-R, luteinizing hormone-releasing hormone receptor; PSA, prostate-specific antigen; RT, radiotherapy.
The durations of follow-up varied widely across studies, from 12 months20,40–42 to 143 months39,43. Generally, studies involving goserelin38,39,43–61 had longer follow-up durations, and were more likely to report long-term survival estimates than studies involving leuprolide, triptorelin, degarelix, or unspecified LHRH-R agonists and antagonists, particularly in the settings of locally advanced and metastatic PC (Figure 3)38,39,62–67. Studies of goserelin had a median follow-up duration as high as 143 months39,43, whereas the corresponding durations were limited to 101 months for triptorelin and 91 months for leuprolide68,69. In contrast, most studies with short-term follow-up (1–3 years)20,40–42,70,71 did not provide long-term survival estimates. Several studies with intermediate-term follow-up (4–5 years)47,58,61,66 reported long-term survival estimates beyond the study follow-up duration. Given the challenges associated with estimating survival from extrapolation of survival data72, particularly given that > 70% of death events were not observed at the end of a study73, reporting of long-term follow-up data is expected to be particularly informative for patients with PC, in whom intermediate-term survival rates typically exceed 70%. In this regard, studies with long-term follow-up durations (beyond 5 years) should provide valuable insights into the effects of LHRH-R agents on patient survival, to inform treatment decisions regarding various agents.
LHRH-R agents are used in various settings for PC treatment; therefore, studies on LHRH-R agonists/antagonists are discussed herein in the context of localized38,44–47,62, locally advanced39,43,48–58,63–66,68,69,74,75, or metastatic40,59–61,67,70,71,76–79 disease stages.
LHRH-R agents in localized PC
For localized PC, the combination of LHRH-R agonists and non-steroidal anti-androgens has been extensively studied in patients undergoing radiotherapy38,44–47,62. As shown in Figure 3, the DART01/05 GICOR study enrolled patients with localized PC using a specific LHRH-R agonist (goserelin) and reported 5-year survival estimates. Other studies of LHRH-R agonists in this setting either did not specify the type of LHRH-R used or reported pooled data from several LHRH-R agonists. Typically, LHRH-R agonists were administered as short-term (4 or 6 months) ADT or prolonged (36 weeks or 28 months) ADT before radiotherapy. For patients treated with radiotherapy plus short-term ADT, the estimated 5-, 8-, 10-, and 18-year survival rates were 86%–88%, 74%, 62%–67%, and 23%, respectively38,44–47,62. In patients treated with radiotherapy and prolonged ADT, the estimated 5-year and 10-year survival rates were 95% and 67%, respectively.
Inconsistent overall survival results were reported in studies comparing long-term vs. short-term ADT (with LHRH-R agonists/antagonists) in patients with PC undergoing radiotherapy. The results should be interpreted in view of differences in study designs and patient populations; in particular, radiation dosage, ADT agent, and category of disease risk might have influenced the overall survival outcomes with long-term ADT38. The DART01/05 GICOR study demonstrated that radiotherapy plus prolonged (28 months) goserelin treatment yielded a higher 5-year survival rate than radiotherapy plus short-term (4 months) goserelin treatment (95% vs. 86%, P = 0.009)38. In contrast, the RTOG 9910 study indicated that prolonged-duration (36 weeks) LHRH-R agonist treatment did not lead to higher 10-year survival rates (67% vs. 66%) or 10-year disease-specific survival (96% vs. 95%) than short duration (16 weeks) LHRH-R agonist treatment, after 113 months of follow-up62. Of note, apart from the various ADT agents used, the radiation dosage in the DART01/05 GICOR study (range, 76–82 Gy) was higher than that in the RTOG 9910 study (70.2 Gy). Moreover, more patients in the DART01/05 GICOR study than the RTOG 9910 study had high-risk disease (90% vs. 15%); therefore, the benefits of long-term ADT plus radiotherapy in overall survival might be more evident in patients with high-risk PC than in those with low-risk disease38. Existing overall survival evidence favors the use of goserelin as either short-term or long-term ADT, although evidence for other particular ADT agents is limited.
LHRH-R agents in locally advanced PC
Combining ADT with radiotherapy is currently the standard of care for patients with intermediate- and high-risk localized PC80. Here, we summarize the survival outcomes of ADT in this patient population across various treatment settings, including ADT concomitant with radiotherapy, ADT before or after radical prostatectomy, and ADT at biochemical recurrence after radical treatment.
ADT concomitant with radiotherapy
In studies reporting concomitant use of ADT (goserelin43,48,51,52,54–56, leuprolide69,74, triptorelin75, or unspecified63,65) with radiotherapy, the initiation of ADT ranged from 5 months before radiotherapy to the first day of irradiation, with durations ranging from 3 months to disease progression. The TROG 96.01 trial, exploring neoadjuvant ADT (goserelin) given concurrently with radiotherapy, showed that 6-month neoadjuvant treatment resulted in a significantly lower 10-year all-cause mortality rate (29.2% vs. 42.5%) and PC-specific mortality rate (11.4% vs. 22.0%) than radiotherapy alone; however, 3-month neoadjuvant ADT had no effects on the all-cause mortality rate and PC-specific mortality rate51. Another study, RTOG 8610, revealed that the addition of 4 months of goserelin (2 months before and concurrent with radiotherapy) appeared to confer overall survival benefits beyond those of radiotherapy alone (10-year overall survival: 42.6% vs. 33.8%)43.
The EORTC 22863 study showed that initiation of goserelin on the first day of irradiation and continuing for 3 years increased 10-year overall survival and disease-free survival beyond that with radiotherapy alone54. Therefore, ADT concomitant with radiotherapy is a preferred treatment option for locally advanced PC. Regarding the optimal duration of ADT, a phase 3 study by Bolla et al.75 showed that long-term (36 months) ADT (triptorelin) led to a higher 5-year overall survival rate, with a follow-up of 6.4 years, than short-term (6 months) ADT (triptorelin). Meanwhile, the DART01/05 GICO study also reported that 2 years of ADT (goserelin) combined with radiotherapy increased 5-year overall survival and biochemical control beyond those with short-term ADT combined with radiotherapy38. The RTOG 92-02 study showed that long-term goserelin treatment (24 months) plus radiotherapy achieved more favorable disease-free survival, disease-specific survival, and biochemical recurrence but did not result in superior survival to short-term ADT plus radiotherapy48. Although a study by Nabid et al.52 in 2018 indicated no difference in survival when patients received 36 vs. 18 months of ADT (goserelin) combined with radiotherapy, the study enrolled few patients with disease stage of T3 or above. Current treatment guidelines recommend radiotherapy combined with short-term (4–6 months) ADT for unfavorable intermediate-risk PC, and endorse radiotherapy combined with long-term (2–3 years) over short-term ADT in patients with high- or very-high-risk disease80.
The survival benefits associated with a combination of goserelin and radiotherapy are supported by substantial research with long-term survival follow-up data demonstrating a decade-long overall survival rate ranging from 43% to 62%43,52. Additionally, studies indicated that leuprorelin plus radiotherapy resulted in an overall survival rate of 72% after 67 months of follow-up74, whereas triptorelin combined with radiotherapy led to a 5-year overall mortality of 19.0%, with 77 months follow-up75.
ADT before or after radical prostatectomy
Studies investigating the effects of neoadjuvant ADT before radical prostatectomy have associated combination therapy with downstaging, a decrease in positive margins, and a diminished incidence of positive lymph node involvement. The small prospective, phase 2, single-arm SWOG 9109 study50 showed that neoadjuvant ADT (4-month goserelin) followed by radical prostatectomy resulted in a 10-year survival rate of 68%. However, because of a lack of long-term follow-up data demonstrating survival benefits beyond those achieved with alternative treatments, neoadjuvant ADT before radical prostatectomy remains controversial.
In the SWOG S9921 study, high survival rates were observed for patients undergoing radical prostatectomy, with a 5-year survival rate of 96% after 53 months of follow-up58 and a 10-year survival rate of 87% after 134 months of follow-up in patients treated with 2 years of ADT (goserelin)49. All patients enrolled in the SWOG S9921 study had high risk features, such as high pathologic Gleason score, high preoperative prostate-specific antigen (PSA) level, seminal vesicle invasion, lymph node involvement, and positive margins. In the EST 3886 study, patients with nodal metastases who underwent radical prostatectomy and subsequently received immediate goserelin or bilateral orchiectomy had a median survival of 13.9 years39. These results suggest that ADT after radical prostatectomy can provide long-term survival benefits in patients with high-risk disease or lymph node involvement.
ADT at biochemical recurrence after radical treatment
Several clinical trials have investigated the efficacy of combining salvage radiotherapy with ADT after radical prostatectomy. The GETUG-AFU 16 study indicated that 6 months of goserelin treatment plus salvage radiotherapy significantly increased 10-year biochemical progression, biochemical progress-free survival, and metastasis-free survival, although addition of ADT to salvage radiotherapy did not increase the 10-year overall survival rate53. The NRG Oncology/RTOG 0534 SPPORT study compared the treatment of prostate bed radiotherapy (PBRT), 6 months of ADT plus PBRT, and 6 months of ADT plus PBRT and pelvic lymph node radiotherapy (PLNRT). With a median follow-up of 8.2 years, the 5-year distant metastasis incidence was lowest in the ADT plus PBRT and PLNRT group, whereas the ADT plus PBRT group had a lower distant metastasis incidence than the PBRT group63. Nevertheless, no significant difference in overall survival was observed. Furthermore, the data from the RTOG 9601 study suggested that the addition of 2-year anti-androgen therapy (bicalutamide) to salvage radiotherapy in patients experiencing biochemical recurrence after RP increased both disease-specific survival and overall survival81. These randomized controlled trials support adding ADT to salvage radiotherapy81. However, because of methodological discrepancies and differences in patient populations in clinical trials, which type and duration of ADT should be added to salvage radiotherapy is not yet clear.
In summary, long-term survival benefits have been observed with ADT across various treatment settings for locally advanced PC. Existing evidence of overall survival benefits was most robust for goserelin across various settings, given the available estimated 10-year survival rates43,48,52,54,55. Because overall survival is the “gold standard” endpoint for assessing efficacy in cancer treatment trials, therapeutic agents with long-term survival data available, such as goserelin, may warrant prioritized consideration in treatment decisions.
LHRH-R agents in metastatic PC
An ADT-containing regimen is the recommended first-line therapy for advanced/metastatic PC. Notable findings have been reported regarding the efficacy of LHRH-R agonists in metastatic PC, including goserelin59–61, leuprolide70, triptorelin71, histrelin76–79, and buserelin40, although long-term follow-up is lacking. In EORTC 30853, goserelin plus flutamide resulted in a median survival of 34 months in patients with bone metastases60. After 24 months of treatment with leuprorelin, the overall survival rate was 78% in patients with stage D1 and D2 PC70. For triptorelin, the median survival was 37.5 months in patients with previously untreated locally advanced or metastatic PC, after a follow-up duration of 38.8 months71.
With the advancement of NHAs, LHRH-R agents combined with NHAs have emerged as viable treatment options for metastatic PC. Unfortunately, no studies have shown the comparative efficacy and safety among various LHRH-R agents in the combination treatment.
LHRH-R agents have comparable safety profiles
Limited data are available on the adverse events associated with treatment with single-agent LHRH-R agonists or antagonists in patients with localized PC, because available studies have focused primarily on safety profiles during radiotherapy plus ADT treatment38,44,47,62. The most common adverse events across LHRH-R agonists include hot flushes, skeletal pain, headache, constipation, fatigue, sexual dysfunction, general pain, testicular atrophy, joint disorder, osteoporosis, and metabolic alterations3,12. Many of these common events may be attributable to decreased testosterone levels. The decline in luteinizing hormone and follicle-stimulating hormone caused by LHRH-R agonists during ADT treatment can lead to release of norepinephrine, thus contributing to dysregulation of peripheral vasodilatation and the occurrence of hot flushes82. Hot flushes and rash have been reported in 55% and 3% of patients, respectively, during 8 weeks of ADT before radiotherapy in patients with localized PC45. ADT treatment has been associated with an elevated rate of non-metastatic bone fractures, probably because of osteoporosis12. Increased risk of cardiovascular events has been reported after treatment with LHRH-R agonists and possibly antagonists; however, the evidence is mixed12,83. Psychological complaints and anemia are unlikely to be the reasons for increased fatigue levels with ADT84. Sleep disturbances associated with ADT-associated hormonal alterations can also contribute to fatigue85. Furthermore, mitochondrial dysfunction in the central nervous system may cause fatigue during ADT86. The safety profiles of goserelin, leuprorelin, and triptorelin are generally comparable12. Goserelin and degarelix also exhibit similar safety profiles, although injection site reactions are more common with degarelix83.
LHRH-R agonists and antagonists in combination with NHAs
PC treated with ADT eventually becomes castration resistant87. Consequently, several new compounds targeting the androgen axis have been developed. The use of LHRH-R agonists/antagonists with NHAs has led to improvements in clinical outcomes in patients with PC. Among the various NHAs available, abiraterone and enzalutamide have been extensively studied. However, adverse events appear to be more frequent with the addition of NHAs to LHRH-R agonists or antagonists than LHRH-R agonists or antagonists alone17,88,89. A recent meta-analysis has shown that NHAs are associated with elevated risk of cognitive toxic effects, fatigue, and falls90.
This section focuses on studies involving single-agent LHRH-R agonists or antagonists combined with abiraterone or enzalutamide91–101. Studies that did not specify the LHRH-R agonists or antagonists used in combination with NHA are not included. Because only several included studies reported overall survival outcomes, the effects on PSA, testosterone, and disease progression, as well as safety results, are reviewed.
LHRH-R agents can be combined with NHAs in castration-resistant and hormone-sensitive PC
Studies examining combinations of LHRH-R agonists and antagonists with abiraterone or enzalutamide are summarized in Table 191–101, including neoadjuvant91–97, salvage98,99, and advanced100,101 treatment stages.
Table 1.
Efficacy of LHRH agonists and antagonists in combination with next-generation hormonal agents in prostate cancer*
| Author year; study phase (type of study) | Patient population | Race | Treatment duration | Regimen | N | Follow-up duration | Testosterone | PSA | Progression outcome |
|---|---|---|---|---|---|---|---|---|---|
| Neoadjuvant | |||||||||
| Cho 201591; phase 2 (single arm) | Intermediate-risk disease (GS of 7, or PSA 10–20) or high-risk disease (T3/4, GS 8–10 or PSA > 20) | NR | 24 weeks (12 weeks of neoadjuvant followed by concurrent phase with radiation therapy) | Goserelin or leuprolide + abiraterone + RT | 22 | 21 months | Testosterone decline: 99.9% | Median pre-radiation PSA at 12 weeks: 0.05 ng/mL. All 21 men who complied with at least 3 months of abiraterone had a pre-radiation PSA nadir: ≤ 0.3 ng/mL. Post radiation PSA undetectable in 19/22 patients and: all patients who completed the neoadjuvant and concurrent portions of the study (n = 16). |
21/22 patients had not experienced biochemical relapse |
| Shee 202292; phase 2 (single arm) | High-risk localized or regional PC, having ≥ 2 of the following criteria: cT3a/b, PSA ≥ 20 ng/mL, GS 8–10, ≥ 33% core involvement on prostate biopsy; or ≥ 1 cm pelvic lymph node(s) | White: 54.5%; Black: 18.2%; Asian: 9.1%; unknown: 18.2% | 24 months before RT | Leuprolide + enzalutamide | 11 | 35.5 months | Median time to testosterone < 50 ng/dL from initiation of ADT: 1.73 months; median nadir testosterone: 18 ng/dL | Achieved PSA-CR (PSA nadir ≤ 0.3 ng/mL) at 120 days of ADT: 90.9%. Median time to PSA-CR was: 4.20 months, and median nadir PSA was: 0.015 ng/mL. |
Biochemical recurrence: 0% at 24 months and 11% at 36 months |
| Bastos 202293; phase 2 (randomized trial) | High-risk localized PC, GS ≥ 8 and/or cT3N0-1 and/or PSA ≥ 20 ng/mL | NR | 3 months neoadjuvant before RP | Goserelin + abiraterone + RP | 31 | 2.6 years | Testosterone recovery: 84% | NR | Biochemical relapse: 32% pCR or MRD: 7%; complete PSMA response: 23%. |
| Karzai 201994; phase 2 (single arm) | Newly diagnosed, high-risk PC | NR | 6 months neoadjuvant before RP | Goserelin + enzalutamide + RP | 33 | NR | NR | Median on-study PSA was: 9.58 ng/dL. Median PSA after 6 months of goserelin + enzalutamide was: < 0.02 (0.02–0.35 ng/mL). |
NR |
| Efstathiou 201995; phase 2 (randomized trial) | PC without metastasis, ≥ T1c with GS 8–10 or ≥ T2b with GS of 7 and PSA ng/mL | NR | 3 months before RP | Leuprolide + abiraterone + RP | 44 | ≥ 4 years | NR | PSA ≤ 0.1 ng/mL: 84% | 3-year relapse-free survival: 75%; biochemical recurrence: 44% |
| Leuprolide + RP | 21 | ≥ 4 years | NR | PSA ≤ 0.1 ng/mL: 5% | 3-year relapse-free survival: 71%; biochemical recurrence: 59% | ||||
| McKay 201996; phase 2 (randomized trial) | GS ≥ 7 or ≥ 1 cm tumor on MRI, PSA > 20 ng/mL, or T3 | White: 90%; Black or African American: 6%; other: 4% | 24 weeks neoadjuvant before RP | Leuprolide + enzalutamide + abiraterone + RP | 50 | NR | NR | PSA ≤ 0.2 ng/mL (% patients): 96% (before RP) | pCR or MRD at RT: 30% |
| White: 80%; Black or African American: 12%; Asian: 8% | Leuprolide + enzalutamide + RP | 25 | NR | NR | PSA ≤ 0.2 ng/mL (% patients): 100% (before RP) | pCR or MRD at RT: 16% | |||
| Taplin 201497; phase 2 (randomized trial) | Localized PC and 1 of the following: PSA > 10 ng/mL, PSA velocity > 2 ng/mL per year (in preceding 12 months), and GS ≥ 7 | NR | 24 weeks neoadjuvant before RP | Leuprolide (24 w) + RP | 28 | 24 weeks | Testosterone (mean): baseline 429.4 → 12 w: 17.3 → 24 w: 5.3 ng/dL | 24 w PSA (median): 0.06 ng/dL 24 w PSA ≤ 0.2 ng/mL: 82% |
pCR or MRD: 48% |
| NR | Leuprolide (24 w) + abiraterone (24 w) + RP | 30 | 24 weeks | Testosterone (mean): baseline 425.1 → 12 w: 6.7 → 24 w: 15.5 ng/dL | 24 w PSA (median): 0.04 ng/dL 24 w PSA ≤ 0.2 ng/mL: 93% |
pCR or MRD: 62% | |||
| Salvage | |||||||||
| Freedland 202398; phase 3 (randomized trial) | High-risk (PSA doubling time of ≤ 9 months and a PSA level of ≥ 2 ng/mL above nadir after radiation therapy or ≥ 1 ng/mL after radical prostatectomy with or without postoperative radiation therapy, testosterone ≥ 150 ng/dL, and an ECOG PS 0 or 1 | White: 82.5%; Asian: 7.3%; Black: 4.5; other: 2.8%; not reported: 2.8% | 36 weeks if the PSA at week 36 was < 0.2 ng/mL and restarted with increased PSA | Leuprolide + enzalutamide | 355 | 60.7 months | NR | Free from PSA progression at 5 years: 97.4%; 90.9% of patients had treatment suspended for a median of 20.2 months. | 5-year metastasis-free survival: 87.3% |
| White: 83.1%; Asian: 7.3%; Black: 4.2; other: 1.4%; not reported: 3.9% | Enzalutamide | 355 | 60.7 months | NR | Free from PSA progression at 5 years: 88.9%; 85.9% of patients had treatment suspended for a median of 11.1 months | 5-year metastasis-free survival: 80.0% | |||
| White: 84.1%; Asian: 7.3%; Black: 4.5; other: 2.5%; not reported: 1.4% | Leuprolide | 358 | 60.7 months | NR | Free from PSA progression at 5 years:70.0%; 67.8% of patients had treatment suspended for a median of 16.8 months. | 5-year metastasis-free survival: 71.4% | |||
| Autio 202199; phase 2 (randomized trial) | Had undergone an RP for localized PC; experienced biochemical recurrence; PSA doubling time at the time of trial entry: ≤ 9 months; testosterone level ≤ 150 ng/dL | White: 80%; Black or African American: 15%; unknown: 5% | 8 months | Degarelix + abiraterone | 41 | 18 months | Median time to testosterone recovery (from treatment start): 56 weeks; undetectable PSA with testosterone recovery at 18 months: 17.1% | Undetectable PSA level at 8 months: 87.8% | PSA progression: 64.4 weeks |
| White: 93%; Black or African American: 7% | Degarelix | 42 | Median time to testosterone recovery: 52.9 weeks; undetectable PSA with testosterone recovery at 18 months: 11.9% | Undetectable PSA level at 8 months: 66.7% | PSA progression: 54.9 weeks | ||||
| White: 90%; Black or African American: 10% | Abiraterone | 39 | Median time to testosterone recovery: 36 weeks; undetectable PSA with testosterone recovery at 18 months: 5.1% | Undetectable PSA level at 8 months: 83.8% | PSA progression: 37.5 weeks | ||||
| Advanced | |||||||||
| Maluf 2021100; phase 2 (randomized trial) | (1) Locally advanced PC with positive lymph nodes, not candidates for radical surgery or RT and PSA ≥ 2 ng/mL; (2) high-risk biochemical recurrence: PSA ≥ 4 ng/mL and PSA doubling time < 10 months, or PSA ≥ 20 ng/mL; or (3) metastatic CSPC and PSA ≥ 2 ng/mL | NR | 25 weeks (study treatment continued only at investigator discretion) | Goserelin + abiraterone | 42 | 14 months | Testosterone level < 50 ng/dL (castration level) at week 25: 97.5% | PSA ≤ 0.2 ng/mL (% patients): 75.6% (at week 25); PSA decline ≥ 50%: 100%; PSA decline ≥ 80%: 100% | Radiographic progression (at week 25): 3.1% |
| George 2023101; phase 3 (randomized trial) | Advanced PC eligible for at least 1 year of continuous ADT | NR | 48 weeks | Leuprolide + enzalutamide | 9 | NR | Sustained testosterone suppression below castration levels (< 50 ng/dL) from day 29 through 48 weeks: 90.9% (concomitant enzalutamide or docetaxel) | NR | NR |
| NR | Relugolix + enzalutamide | 20 | NR | Sustained testosterone suppression below castration levels (< 50 ng/dL) from day 29 through 48 weeks: 95.8% (concomitant enzalutamide or docetaxel); testosterone (median): 12.71 ng/dL | NR | NR | |||
ADT, androgen deprivation therapy; CSPC, castration-sensitive prostate cancer; GS, Gleason score; MRD, minimal residual disease; MRI, magnetic resonance imaging; NR, not reported; PC, prostate cancer; pCR, pathologic complete response; PSA, prostate-specific antigen; PSA-CR, PSA complete response; PSMA, prostate specific membrane antigen; RP, radical prostatectomy; RT, radiotherapy; w, weeks.
*A literature search was performed in core databases and supplemented by searching for studies published before February 2024.
In the neoadjuvant setting, LHRH-R agonists and antagonists plus NHAs are typically used before radiotherapy91,92 or radical prostatectomy93–97. In 2 clinical studies, neoadjuvant ADT plus NHA in combination with radiotherapy decreased testosterone to castration levels, PSA levels were undetectable, and biochemical recurrence rates of 5%–11% were observed during 21–35.5 months of follow-up; the regimens used in the studies were 12 weeks of goserelin or leuprolide plus abiraterone, followed by another 12 weeks of combination therapy concurrent with radiotherapy in patients with intermediate-risk or high-risk disease, as well as 24 months of leuprolide plus enzalutamide in patients with high-risk disease91,92.
Among patients with PC who received 3-month ADT plus NHA as neoadjuvant treatment before radical prostatectomy, the biochemical relapse rate was 32% with goserelin plus abiraterone after a follow-up of 2.6 years and 44% with leuprolide plus enzalutamide after a follow-up of ≥ 4 years93,95. Additionally, neoadjuvant therapy of 6 months ADT plus NHA before radical prostatectomy resulted in a median PSA level < 0.02 (range 0.02–0.35) ng/mL after treatment with goserelin plus enzalutamide94; 100% of patients had PSA ≤ 0.2 ng/mL with the combination of leuprolide and enzalutamide96; and 93% had PSA ≤ 0.2 ng/mL with leuprolide plus abiraterone97.
Among studies involving patients with biochemical recurrence after radical prostatectomy98,99, combination treatment with LHRH-R agonists/antagonists plus NHAs conferred notable clinical benefits in terms of decreased testosterone and PSA levels. The EMBARK study, a phase 3 study with specified LHRH-R agents, showed that combining leuprolide plus enzalutamide decreased the risk of PSA progression [hazard ratio (HR), 0.07] and improved overall survival [HR, 0.59 (immature overall survival data)] beyond that with leuprolide alone over 61 months of follow-up98, whereas the 5-year metastasis-free survival rate was 87.3% with leuprolide plus enzalutamide. In patients with biochemical recurrence with a PSA doubling time ≤ 9 months, 88% of patients had undetectable PSA after 8 months of treatment with degarelix plus abiraterone99.
In advanced PC, 25 weeks of goserelin plus abiraterone led to a ≥ 80% decline in PSA levels in all treated patients; 76% of patients had a PSA level ≤ 0.2 ng/mL and 98% of patients achieved testosterone levels of ≤ 50 ng/dL. Moreover, only 3.1% of patients showed radiographic progression at week 25100. Other combinations of LHRH-R agonists/antagonists plus NHAs yielded similar findings, with 96% and 91% of patients with advanced PC achieving testosterone levels ≤ 50 ng/dL with relugolix plus enzalutamide or docetaxel, and leuprolide plus enzalutamide or docetaxel, respectively101.
Reports on specific LHRH-R agonists or antagonists plus NHAs have shown consistent efficacy in terms of decreasing PSA and testosterone levels, and preventing disease progression. However, the above studies included patients with slightly varying baseline conditions and consisted predominantly of phase 2 trials with limited sample sizes. Therefore, concluding which combination therapy with LHRH-R agonists/antagonists plus NHAs is optimal is difficult without a head-to-head comparison or studies reporting identical treatment outcomes in the same treatment setting. The clinical benefits of adding an NHA to LHRH-R agonists or antagonists are not debated. Selection among various combinations should be informed by comparison of their safety profiles, which are discussed below.
Safety profiles of LHRH-R agents plus NHAs differ among combinations but remain manageable
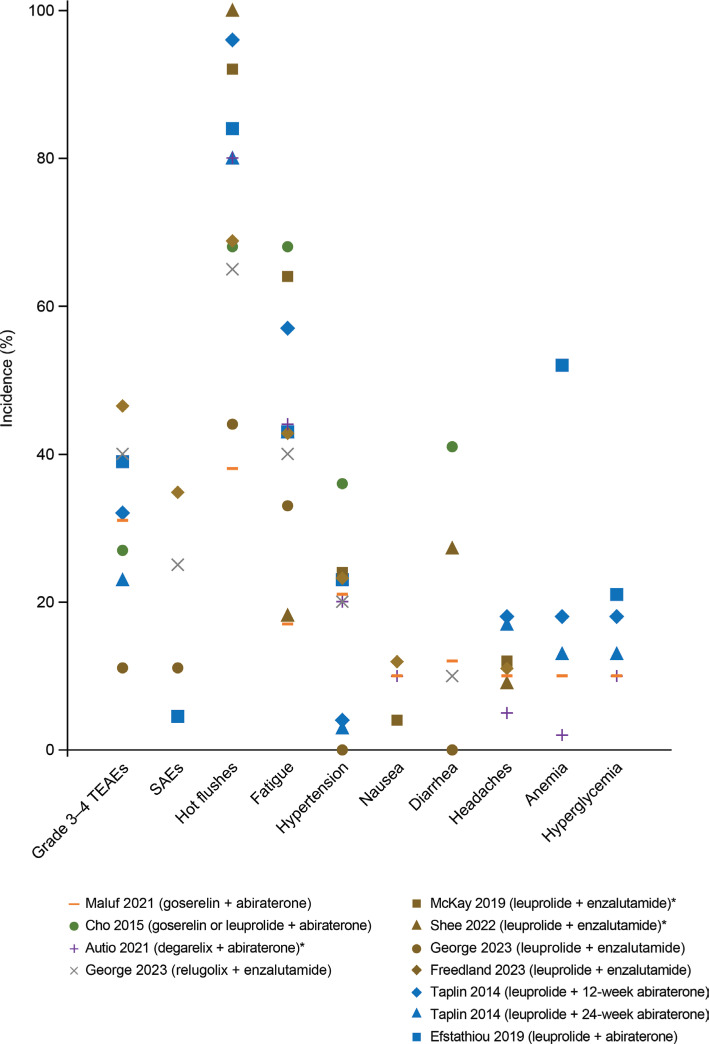
Whereas the safety profile of LHRH-R agonists or antagonists in combination with NHAs has been reviewed102,103, the focus has been primarily on NHAs rather than on the specific LHRH-R agonists or antagonists used. The incidence of common adverse events observed after treatment with single-agent LHRH-R agonists or antagonists in combination with abiraterone or enzalutamide is presented in Figure 491,92,95–101. The comparison of safety profiles of these combination therapies should be interpreted with caution, because of the inclusion of various LHRH-R agonists and antagonists, various NHAs, and the limited reporting of treatment-related adverse events in some studies.
Figure 4.
Incidence of adverse events in combination therapy with LHRH-R agonists and antagonists with NHAs. Each dot represents the percentage of adverse events reported in a study; a dot at 0% indicates that the incidence was reported as 0% in the article. AST, ALT, and hypertension were defined as an adverse event of special interest in Taplin et al.97. *Study reported only the incidence of treatment-related adverse events. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LHRH-R, luteinizing hormone-releasing hormone receptor; NHA, next-generation hormonal agent (i.e., abiraterone or enzalutamide). SAE, serious adverse events; TEAEs, treatment-emergent adverse events.
Most patients treated with combination therapy comprising LHRH-R agonists or antagonists plus abiraterone or enzalutamide experienced at least 1 treatment-related adverse event (93%–100%)92,95,97,98,100,101. The common adverse events (hot flushes, fatigue, headaches, hypertension, alanine transaminase increase, and aspartate aminotransferase increase) reported with the combination therapies were similar to the known adverse reactions associated with abiraterone or enzalutamide104,105. The incidence of nausea ranged from 4% to 12% across various treatment combinations96,98–100. In contrast, the incidence of hypertension (0%–36%)91,95,97,98,100,101, diarrhea (0%–41%)91,92,100,101, headaches (0%–18%)92,97,100, and anemia (0%–52%)95,97,100 varied widely among studies. Hyperglycemia, a known laboratory abnormality associated with abiraterone105, was noted in patients treated with goserelin (10%)100, leuprolide (13%–21%)95,97, or degarelix (10%)99 in combination with abiraterone.
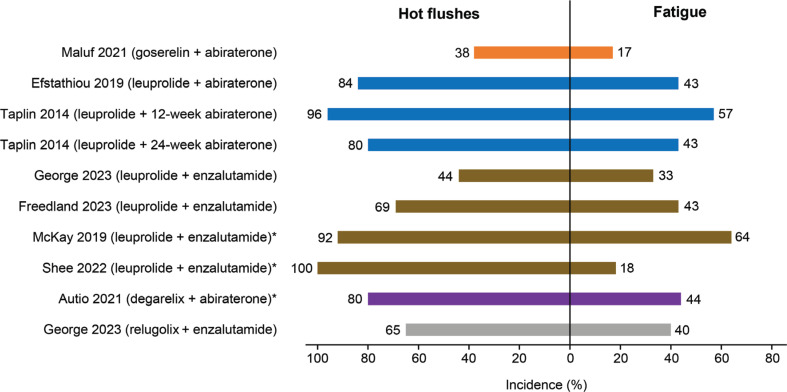
Among common adverse events observed after treatment with LHRH-R agonists or antagonists plus abiraterone or enzalutamide, considerable differences have been observed in the incidence of hot flushes and fatigue among the treatment combinations (Figure 5)92,95–101. Combination therapy containing goserelin has shown a manageable safety profile and appears to compare favorably with other LHRH-R agonists in terms of hot flushes and fatigue. Studies have reported that the incidence of hot flushes was lower with goserelin plus abiraterone (38%)100 than with leuprolide plus abiraterone (80%–96%)95,97 or leuprolide plus enzalutamide (44%–100%)96,98,101. The incidence of fatigue was also lower with goserelin plus abiraterone (17%)100 than with degarelix plus abiraterone (44%)99, relugolix plus enzalutamide (40%)101, leuprolide plus abiraterone (43%–57%)95,97, or leuprolide plus enzalutamide (18%–64%)92,96,98,101. These findings may be particularly important for treatment selection, because fatigue and hot flushes are considered the most bothersome adverse effects of ADT, and some patients may discontinue treatment because of effects on quality of life82,106,107.
Figure 5.
Incidence of hot flushes and fatigue associated with combination therapy comprising LHRH-R agonists and antagonists with NHAs. Incidence is rounded to whole numbers. *The study reported only the incidence of treatment-related adverse events. LHRH-R, luteinizing hormone-releasing hormone receptor; NHAs, next-generation hormonal agents (i.e., abiraterone or enzalutamide).
Although the mechanisms underlying fatigue and hot flushes associated with LHRH-R agonists or antagonists plus NHAs remain unclear, the inclusion of an AR antagonist in combination therapy has been suggested to increase the incidence of hot flushes and fatigue, which are typical effects of this drug class108. In studies involving patients with any stage or advanced stage PC, the rates of fatigue and hot flushes were similar among single-agent LHRH-R agonists and antagonists in phase 3 studies20,109,110. Previous phase 3 studies showed similar incidences of fatigue and hot flushes between ADT plus abiraterone and ADT plus placebo111. However, ADT plus enzalutamide led to higher incidences of fatigue and hot flushes than ADT alone88. Differences in the incidence of hot flushes among combination regimens might possibly be attributable to differences in the levels of testosterone fluctuations after treatment initiation. For example, patients who underwent orchiectomy (complete elimination of testosterone) had a lower incidence of hot flushes than those who were treated with LHRH-R agonists (rapid testosterone decrease)61,112, and the incidence of hot flushes decreased over time with ADT113. Although the data were not collected from a single study, differences in profiles of testosterone levels in the first few days after treatment initiation have been reported among LHRH-R agonists and antagonists112,114. The combination of goserelin and abiraterone achieves testosterone stabilization more rapidly than other combinations, thus potentially resulting in a lower incidence of hot flushes. Although the mechanism remains unclear, enzalutamide has been associated with greater risk of fatigue than abiraterone115,116. Therefore, a combination regimen with a manageable safety profile and lower incidence of fatigue and hot flushes, such as goserelin plus abiraterone, may be an option when a combination therapy is indicated.
Concluding remarks and perspectives
Overall survival is typically considered the gold standard for evaluating cancer treatment outcomes117. However, many studies investigating LHRH-R agonists and antagonists in PC have focused on short-term or intermediate-term follow-up, whereas long-term survival data for this class of agents are often lacking. More than half of the studies using a single LHRH-R agonist and antagonist agent with long-term survival follow-up were conducted in patients with locally advanced PC. Among the available LHRH-R agonists and antagonists, goserelin has been extensively studied and has long-term survival data available across various stages of PC. Given that long-term survival data provide valuable insights to inform treatment decisions, therapeutic agents with data available for long-term outcomes, such as goserelin, are an important tool in the armamentarium for PC management.
LHRH-R agonists or antagonists are also commonly used in combination with NHAs for treatment of metastatic PC. However, because of the heterogeneity in study designs and the lack of head-to-head comparisons, determining the optimal combination of LHRH-R agonists or antagonists with NHAs is challenging. Because the safety profile of a therapy is an important consideration in treatment decisions, combination regimens with tolerable safety profiles, in addition to acceptable efficacy, are preferred. Because the rate of adverse events varies widely across various treatment combinations, combination regimens containing agents with manageable safety profiles, such as goserelin and abiraterone, which have been associated with diminished rates of hot flushes and fatigue, may be useful options to be considered in combination therapy. Nonetheless, further studies are warranted to provide robust evidence supporting the selection of the optimal combination therapy in various treatment settings.
Supporting Information
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed the analysis: Hao Zeng.
Collected the data: Jinge Zhao, Junru Chen, and Guangxi Sun.
Contributed data or analysis tools: Jinge Zhao, Junru Chen, Guangxi Sun, Pengfei Shen, and Hao Zeng.
Performed the analysis: Jinge Zhao, Junru Chen, Guangxi Sun, Pengfei Shen, and Hao Zeng.
Wrote the paper: Jinge Zhao.
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO International Agency for Research on Cancer. Global Cancer Observatory 2022: Prostate. [Accessed February 5, 2024]. https://gco.iarc.who.int/media/globocan/factsheets/cancers/27-prostate-fact-sheet.pdf . [Google Scholar]
- 3.Raja T, Sud R, Addla S, Sarkar KK, Sridhar PS, Talreja V, et al. Gonadotropin-releasing hormone agonists in prostate cancer: a comparative review of efficacy and safety. Indian J Cancer. 2022;59:S142–S59. doi: 10.4103/ijc.IJC_65_21. [DOI] [PubMed] [Google Scholar]
- 4.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22:232–40. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 5.Huggins C, Scott WW. Bilateral adrenalectomy in prostatic cancer. Ann Surg. 1945;122:1031–41. [PubMed] [Google Scholar]
- 6.Schally AV, Kastin AJ, Arimura A. Hypothalamic follicle-stimulating hormone (FSH) and luteinizing hormone (LH)-regulating hormone: structure, physiology, and clinical studies. Fertil Steril. 1971;22:703–21. [PubMed] [Google Scholar]
- 7.Sandow J, Von Rechenberg W, Jerzabek G, Stoll W. Pituitary gonadotropin inhibition by a highly active analog of luteinizing hormone-releasing hormone. Fertil Steril. 1978;30:205–9. doi: 10.1016/s0015-0282(16)43461-8. [DOI] [PubMed] [Google Scholar]
- 8.Tolis G, Ackman D, Stellos A, Mehta A, Labrie F, Fazekas AT, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci U S A. 1982;79:1658–62. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtonen M, Kellokumpu-Lehtinen PL. The past and present of prostate cancer and its treatment and diagnostics: a historical review. SAGE Open Med. 2023;11:20503121231216837. doi: 10.1177/20503121231216837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford ED, Heidenreich A, Lawrentschuk N, Tombal B, Pompeo ACL, Mendoza-Valdes A, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24–38. doi: 10.1038/s41391-018-0079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton EM, Lynch T. Are all gonadotrophin-releasing hormone agonists equivalent for the treatment of prostate cancer? A systematic review. BJU Int. 2018;122:371–83. doi: 10.1111/bju.14168. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto N. Novel agents for castration-resistant prostate cancer: early experience and beyond. Int J Urol. 2016;23:114–21. doi: 10.1111/iju.12907. [DOI] [PubMed] [Google Scholar]
- 14.Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 17.James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338–51. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreau JP, Delavault P, Blumberg J. Luteinizing hormone-releasing hormone agonists in the treatment of prostate cancer: a review of their discovery, development, and place in therapy. Clin Ther. 2006;28:1485–508. doi: 10.1016/j.clinthera.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Tolkach Y, Joniau S, Van Poppel H. Luteinizing hormone-releasing hormone (LHRH) receptor agonists vs antagonists: a matter of the receptors? BJU Int. 2013;111:1021–30. doi: 10.1111/j.1464-410X.2013.11796.x. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102:1531–8. doi: 10.1111/j.1464-410X.2008.08183.x. [DOI] [PubMed] [Google Scholar]
- 21.Marelli MM, Moretti RM, Dondi D, Motta M, Limonta P. Luteinizing hormone-releasing hormone agonists interfere with the mitogenic activity of the insulin-like growth factor system in androgen-independent prostate cancer cells. Endocrinology. 1999;140:329–34. doi: 10.1210/endo.140.1.6402. [DOI] [PubMed] [Google Scholar]
- 22.Fontana F, Marzagalli M, Montagnani Marelli M, Raimondi M, Moretti RM, Limonta P. Gonadotropin-releasing hormone receptors in prostate cancer: molecular aspects and biological functions. Int J Mol Sci. 2020;21:9511. doi: 10.3390/ijms21249511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonesson A, Koechling W, Stalewski J, Tanko LB, Rasmussen BB. Metabolite profiles of degarelix, a new gonadotropin-releasing hormone receptor antagonist, in rat, dog, and monkey. Drug Metab Dispos. 2011;39:1895–903. doi: 10.1124/dmd.111.039883. [DOI] [PubMed] [Google Scholar]
- 24.Millar RP, Lu ZL, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR. Gonadotropin-releasing hormone receptors. Endocr Rev. 2004;25:235–75. doi: 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- 25.Zietman AL, Nakfoor BM, Prince EA, Gerweck LE. The effect of androgen deprivation and radiation therapy on an androgen-sensitive murine tumor: an in vitro and in vivo study. Cancer J Sci Am. 1997;3:31–6. [PubMed] [Google Scholar]
- 26.Zietman AL, Prince EA, Nakfoor BM, Shipley WU. Neoadjuvant androgen suppression with radiation in the management of locally advanced adenocarcinoma of the prostate: experimental and clinical results. Urology. 1997;49:74–83. doi: 10.1016/s0090-4295(97)00173-8. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski JM, Hanlon AL, Joon DL, Meistrich M, Hachem P, Pollack A. Effect of sequencing of androgen deprivation and radiotherapy on prostate cancer growth. Int J Radiat Oncol Biol Phys. 2003;57:24–8. doi: 10.1016/s0360-3016(03)00539-x. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 29.Stewart GD, Nanda J, Katz E, Bowman KJ, Christie JG, Brown DJ, et al. DNA strand breaks and hypoxia response inhibition mediate the radiosensitisation effect of nitric oxide donors on prostate cancer under varying oxygen conditions. Biochem Pharmacol. 2011;81:203–10. doi: 10.1016/j.bcp.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Congregado Ruiz B, Rivero Belenchon I, Lendinez Cano G, Medina Lopez RA. Strategies to re-sensitize castration-resistant prostate cancer to antiandrogen therapy. Biomedicines. 2023;11:1105. doi: 10.3390/biomedicines11041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin L, Hu Q. CYP17 inhibitors –abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol. 2014;11:32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Xu W, Xiao YT, Huang H, Gu D, Ren S. Targeting signaling pathways in prostate cancer: mechanisms and clinical trials. Signal Transduct Target Ther. 2022;7:198. doi: 10.1038/s41392-022-01042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–51. doi: 10.1038/nature14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schalken J, Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016;117:215–25. doi: 10.1111/bju.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prostate Cancer Trialists Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–8. [PubMed] [Google Scholar]
- 37.Samson DJ, Seidenfeld J, Schmitt B, Hasselblad V, Albertsen PC, Bennett CL, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–76. doi: 10.1002/cncr.10647. [DOI] [PubMed] [Google Scholar]
- 38.Zapatero A, Guerrero A, Maldonado X, Alvarez A, Gonzalez San Segundo C, Cabeza Rodríguez MA, et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:320–7. doi: 10.1016/S1470-2045(15)70045-8. [DOI] [PubMed] [Google Scholar]
- 39.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472–9. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 40.Bruun E, Frimodt-Møller C. The effect of Buserelin versus conventional antiandrogenic treatment in patients with T2-4NXM1 prostatic cancer. A prospective, randomized multicentre phase III trial. The “Danish Buserelin Study Group”. Scand J Urol Nephrol. 1996;30:291–7. doi: 10.3109/00365599609182309. [DOI] [PubMed] [Google Scholar]
- 41.Lopes RD, Higano CS, Slovin SF, Nelson AJ, Bigelow R, Sørensen PS, et al. Cardiovascular safety of degarelix versus leuprolide in patients with prostate cancer: the primary results of the PRONOUNCE randomized trial. Circulation. 2021;144:1295–307. doi: 10.1161/CIRCULATIONAHA.121.056810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozono S, Ueda T, Hoshi S, Yamaguchi A, Maeda H, Fukuyama Y, et al. The efficacy and safety of degarelix, a GnRH antagonist: a 12-month, multicentre, randomized, maintenance dose-finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol. 2012;42:477–84. doi: 10.1093/jjco/hys035. [DOI] [PubMed] [Google Scholar]
- 43.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 44.Jones CU, Pugh SL, Sandler HM, Chetner MP, Amin MB, Bruner DW, et al. Adding short-term androgen deprivation therapy to radiation therapy in men with localized prostate cancer: long-term update of the NRG/RTOG 9408 randomized clinical trial. Int J Radiat Oncol Biol Phys. 2022;112:294–303. doi: 10.1016/j.ijrobp.2021.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–18. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 46.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. J Am Med Assoc. 2008;299:289–95. doi: 10.1001/jama.299.3.289. [DOI] [PubMed] [Google Scholar]
- 47.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. J Am Med Assoc. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 49.Hussain M, Tangen CM, Thompson IM, Jr, Swanson GP, Wood DP, Sakr W, et al. Phase III intergroup trial of adjuvant androgen deprivation with or without mitoxantrone plus prednisone in patients with high-risk prostate cancer after radical prostatectomy: SWOG S9921. J Clin Oncol. 2018;36:1498–504. doi: 10.1200/JCO.2017.76.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berglund RK, Tangen CM, Powell IJ, Lowe BA, Haas GP, Carroll PR, et al. Ten-year follow-up of neoadjuvant therapy with goserelin acetate and flutamide before radical prostatectomy for clinical T3 and T4 prostate cancer: update on Southwest Oncology Group Study 9109. Urology. 2012;79:633–7. doi: 10.1016/j.urology.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–9. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 52.Nabid A, Carrier N, Martin AG, Bahary JP, Lemaire C, Vass S, et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur Urol. 2018;74:432–41. doi: 10.1016/j.eururo.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Carrie C, Magné N, Burban-Provost P, Sargos P, Latorzeff I, Lagrange JL, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20:1740–9. doi: 10.1016/S1470-2045(19)30486-3. [DOI] [PubMed] [Google Scholar]
- 54.Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 55.Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma – long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 56.Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 57.Carrie C, Hasbini A, de Laroche G, Richaud P, Guerif S, Latorzeff I, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–56. doi: 10.1016/S1470-2045(16)00111-X. [DOI] [PubMed] [Google Scholar]
- 58.Dorff TB, Flaig TW, Tangen CM, Hussain MH, Swanson GP, Wood DP, Jr, et al. Adjuvant androgen deprivation for high-risk prostate cancer after radical prostatectomy: SWOG S9921 study. J Clin Oncol. 2011;29:2040–5. doi: 10.1200/JCO.2010.32.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaisary AV, Tyrrell CJ, Peeling WB, Griffiths K. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol. 1991;67:502–8. doi: 10.1111/j.1464-410x.1991.tb15195.x. [DOI] [PubMed] [Google Scholar]
- 60.Denis LJ, Keuppens F, Smith PH, Whelan P, de Moura JL, Newling D, et al. Maximal androgen blockade: final analysis of EORTC phase III trial 30853. EORTC Genito-Urinary Tract Cancer Cooperative Group and the EORTC Data Center. Eur Urol. 1998;33:144–51. doi: 10.1159/000019546. [DOI] [PubMed] [Google Scholar]
- 61.Vogelzang NJ, Chodak GW, Soloway MS, Block NL, Schellhammer PF, Smith JA, Jr, et al. Goserelin versus orchiectomy in the treatment of advanced prostate cancer: final results of a randomized trial. Zoladex Prostate Study Group. Urology. 1995;46:220–6. doi: 10.1016/s0090-4295(99)80197-6. [DOI] [PubMed] [Google Scholar]
- 62.Pisansky TM, Hunt D, Gomella LG, Amin MB, Balogh AG, Chinn DM, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: radiation therapy oncology group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–9. doi: 10.1200/JCO.2014.58.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollack A, Karrison TG, Balogh AG, Gomella LG, Low DA, Bruner DW, et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): an international, multicentre, randomised phase 3 trial. Lancet. 2022;399:1886–901. doi: 10.1016/S0140-6736(21)01790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS, Horwitz EM, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warde PR, Mason MD, Sydes MR, Gospodarowicz MK, Swanson GP, Kirkbride P, et al. Intergroup randomized phase III study of androgen deprivation therapy (ADT) plus radiation therapy (RT) in locally advanced prostate cancer (CaP) (NCIC-CTG, SWOG, MRC-UK, INT: T94-0110; NCT00002633) J Clin Oncol. 2010;28 Abstract CRA4504. [Google Scholar]
- 66.Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D’Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17:727–37. doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 67.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–25. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saldana C, Salomon L, Rousseau B, Chaubet-Houdu M, Joly C, Allory GP, et al. Weekly paclitaxel versus ADT alone in localized high-risk prostate cancer: results of a single-institution phase II trial. J Clin Oncol. 2015;33 Abstract 37. [Google Scholar]
- 69.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–8. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 70.Saito S, Nakashima J, Nakajima Y, Ikeuchi K, Shibayama T, Nagakura K, et al. Clinical efficacy of leuprolide acetate and combined treatment with estramustine for advanced prostate cancer. Nihon Hinyokika Gakkai Zasshi. 2001;92:682–93. doi: 10.5980/jpnjurol1989.92.682. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 71.Botto H, Rouprêt M, Mathieu F, Richard F. Multicentre randomized trial comparing triptorelin medical castration versus surgical castration in the treatment of locally advanced or metastatic prostate cancer. Prog Urol. 2007;17:235–9. doi: 10.1016/s1166-7087(07)92270-8. [DOI] [PubMed] [Google Scholar]
- 72.Willigers BJA, Ouwens M, Briggs A, Heerspink HJL, Pollock C, Pecoits-Filho R, et al. The role of expert opinion in projecting long-term survival outcomes beyond the horizon of a clinical trial. Adv Ther. 2023;40:2741–51. doi: 10.1007/s12325-023-02503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vickers A. An evaluation of survival curve extrapolation techniques using long-term observational cancer data. Med Decis Making. 2019;39:926–38. doi: 10.1177/0272989X19875950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mottet N, Peneau M, Mazeron JJ, Molinie V, Richaud P. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol. 2012;62:213–9. doi: 10.1016/j.eururo.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 75.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 76.Shore N, Cookson MS, Gittelman MC. Long-term efficacy and tolerability of once-yearly histrelin acetate subcutaneous implant in patients with advanced prostate cancer. BJU Int. 2012;109:226–32. doi: 10.1111/j.1464-410X.2011.10370.x. [DOI] [PubMed] [Google Scholar]
- 77.Schlegel PN. Efficacy and safety of histrelin subdermal implant in patients with advanced prostate cancer. J Urol. 2006;175:1353–8. doi: 10.1016/S0022-5347(05)00649-X. [DOI] [PubMed] [Google Scholar]
- 78.Woolen S, Holzmeyer C, Nesbitt E, Siami PF. Long-term efficacy and tolerability of abdominal once-yearly histrelin acetate subcutaneous implants in patients with advanced prostate cancer. Prostate Cancer. 2014;2014:490315. doi: 10.1155/2014/490315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siami PF, Beasley K, Woolen SA, Zahn J. A retrospective study evaluating the efficacy and tolerability of intra-abdominal, once-yearly histrelin acetate subcutaneous implants in patients with advanced prostate cancer. UroToday Int J. 2012;5 doi: 10.1155/2014/490315. art 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 1.2023. J Natl Compr Canc Netw. 2022;20:1288–98. doi: 10.6004/jnccn.2022.0063. [DOI] [PubMed] [Google Scholar]
- 81.Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376:417–28. doi: 10.1056/NEJMoa1607529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan A, Lewis R, Hughes S. Managing hot flushes in men receiving androgen deprivation therapy for prostate cancer. Trends Urol Men’s Health. 2014;5:31–3. [Google Scholar]
- 83.Bahl A, Rajappa S, Rawal S, Bakshi G, Murthy V, Patil K. A review of clinical evidence to assess differences in efficacy and safety of luteinizing hormone-releasing hormone (LHRH) agonist (goserelin) and LHRH antagonist (degarelix) Indian J Cancer. 2022;59:S160–S74. doi: 10.4103/ijc.IJC_1415_20. [DOI] [PubMed] [Google Scholar]
- 84.Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–41. doi: 10.1016/s0959-8049(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 85.Feng LR, Barb JJ, Allen H, Regan J, Saligan L. Steroid hormone biosynthesis metabolism is associated with fatigue related to androgen deprivation therapy for prostate cancer. Front Cell Dev Biol. 2021;9:642307. doi: 10.3389/fcell.2021.642307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng LR, Wolff BS, Liwang J, Regan JM, Alshawi S, Raheem S, et al. Cancer-related fatigue during combined treatment of androgen deprivation therapy and radiotherapy is associated with mitochondrial dysfunction. Int J Mol Med. 2020;45:485–96. doi: 10.3892/ijmm.2019.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.European Association of Urology. EAU Guidelines on prostate cancer. [Accessed August 13, 2023]. https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2023_2023-06-13-141145_owmj.pdf . [Google Scholar]
- 88.Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–33. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 90.Nowakowska MK, Ortega RM, Wehner MR, Nead KT. Association of second-generation antiandrogens with cognitive and functional toxic effects in randomized clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2023;9:930–7. doi: 10.1001/jamaoncol.2023.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho E, Mostaghel EA, Russell KJ, Liao JJ, Konodi MA, Kurland BF, et al. External beam radiation therapy and abiraterone in men with localized prostate cancer: safety and effect on tissue androgens. Int J Radiat Oncol Biol Phys. 2015;92:236–43. doi: 10.1016/j.ijrobp.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shee K, de la Calle CM, Chang AJ, Wong AC, Feng FY, Gottschalk AR, et al. Addition of enzalutamide to leuprolide and definitive radiation therapy is tolerable and effective in high-risk localized or regional nonmetastatic prostate cancer: results from a phase 2 trial. Adv Radiat Oncol. 2022;7:100941. doi: 10.1016/j.adro.2022.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bastos DA, Coelho R, Cardili L, Galiza F, Ilario EN, Viana P, et al. Randomized phase II trial of neoadjuvant androgen deprivation therapy plus abiraterone and apalutamide for patients with high-risk localized prostate cancer: pathologic response and PSMA imaging correlates. J Clin Ocol. 2022;40 Abstract 5085. [Google Scholar]
- 94.Karzai F, Madan RA, VanderWeele DJ, Chun G, Bilusic M, Cordes LM, et al. A study of intense neoadjuvant testosterone lowering therapy with goserelin and enzalutamide (Enza) in high-risk prostate cancer (PC) with multiparametric MRI (mpMRI) J Clin Oncol. 2019;37 Abstract 63. [Google Scholar]
- 95.Efstathiou E, Davis JW, Pisters L, Li W, Wen S, McMullin RP, et al. Clinical and biological characterisation of localised high-risk prostate cancer: results of a randomised preoperative study of a luteinising hormone-releasing hormone agonist with or without abiraterone acetate plus prednisone. Eur Urol. 2019;76:418–24. doi: 10.1016/j.eururo.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKay RR, Ye H, Xie W, Lis R, Calagua C, Zhang Z, et al. Evaluation of intense androgen deprivation before prostatectomy: a randomized phase II trial of enzalutamide and leuprolide with or without abiraterone. J Clin Oncol. 2019;37:923–31. doi: 10.1200/JCO.18.01777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taplin ME, Montgomery B, Logothetis CJ, Bubley GJ, Richie JP, Dalkin BL, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705–15. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freedland SJ, de Almeida Luz M, De Giorgi U, Gleave M, Gotto GT, Pieczonka CM, et al. Improved outcomes with enzalutamide in biochemically recurrent prostate cancer. N Engl J Med. 2023;389:1453–65. doi: 10.1056/NEJMoa2303974. [DOI] [PubMed] [Google Scholar]
- 99.Autio KA, Antonarakis ES, Mayer TM, Shevrin DH, Stein MN, Vaishampayan UN, et al. Randomized phase 2 trial of abiraterone acetate plus prednisone, degarelix, or the combination in men with biochemically recurrent prostate cancer after radical prostatectomy. Eur Urol Open Sci. 2021;34:70–8. doi: 10.1016/j.euros.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maluf FC, Schutz FA, Cronemberger EH, Luz MA, Martins SPS, Muniz DQB, et al. A phase 2 randomized clinical trial of abiraterone plus ADT, apalutamide, or abiraterone and apalutamide in patients with advanced prostate cancer with non-castrate testosterone levels (LACOG 0415) Eur J Cancer. 2021;158:63–71. doi: 10.1016/j.ejca.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 101.George DJ, Saad F, Cookson MS, Saltzstein DR, Tutrone R, Bossi A, et al. Impact of concomitant prostate cancer medications on efficacy and safety of relugolix versus leuprolide in men with advanced prostate cancer. Clin Genitourin Cancer. 2023;21:383–92.e2. doi: 10.1016/j.clgc.2023.03.009. [DOI] [PubMed] [Google Scholar]
- 102.Saad F, Hamilou Z, Lattouf JB. A drug safety evaluation of enzalutamide to treat advanced prostate cancer. Expert Opin Drug Saf. 2021;20:741–9. doi: 10.1080/14740338.2021.1919620. [DOI] [PubMed] [Google Scholar]
- 103.Castellan P, Castellucci R, Marchioni M, De Nunzio C, Tema G, Primiceri G, et al. A drug safety evaluation of abiraterone acetate in the treatment of prostate cancer. Expert Opin Drug Saf. 2019;18:759–67. doi: 10.1080/14740338.2019.1648428. [DOI] [PubMed] [Google Scholar]
- 104.XTANDI (enzalutamide) [package insert] [Accessed September 7, 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203415s014lbl.pdf . [Google Scholar]
- 105.ZYTIGA (abiraterone acetate) [package insert] [Accessed September 7, 2023]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202379s031s033lbl.pdf . [Google Scholar]
- 106.De B, Lowenstein LM, Corrigan KL, Andring LM, Kuban DA, Cantor SB, et al. Patients’ preferences for androgen deprivation therapy in the treatment of intermediate-risk prostate cancer. MDM Policy Pract. 2022;7:23814683221137752. doi: 10.1177/23814683221137752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peeke P, Billes SK, Vetter A, Naghavi N, Le D, Smith M, et al. Feasibility of a novel wearable thermal device for management of bothersome hot flashes in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2023;27:736–42. doi: 10.1038/s41391-023-00771-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shpilsky J, Stevens J, Bubley G. An up-to-date evaluation of abiraterone for the treatment of prostate cancer. Expert Opin Pharmacother. 2021;22:1227–34. doi: 10.1080/14656566.2021.1915287. [DOI] [PubMed] [Google Scholar]
- 109.ClinicalTrials.gov. A degarelix trial in paitents with prostate cancer. [Accessed November 17, 2023]. NCT01242748. https://clinicaltrials.gov/study/NCT01242748. [Google Scholar]
- 110.Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–96. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 111.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–60. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 112.Peeling WB. Phase III studies to compare goserelin (Zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology. 1989;33:45–52. doi: 10.1016/0090-4295(89)90106-4. [DOI] [PubMed] [Google Scholar]
- 113.Dosani M, Morris WJ, Tyldesley S, Pickles T. The relationship between hot flashes and testosterone recovery after 12 months of androgen suppression for men with localised prostate cancer in the ASCENDE-RT trial. Clin Oncol (R Coll Radiol) 2017;29:696–701. doi: 10.1016/j.clon.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Nasser NJ. Androgen flare after LHRH initiation is the side effect that makes most of the beneficial effect when it coincides with radiation therapy for prostate cancer. Cancers (Basel) 2022;14:1959. doi: 10.3390/cancers14081959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreira RB, Debiasi M, Francini E, Nuzzo PV, Velasco G, Maluf FC, et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8:84572–8. doi: 10.18632/oncotarget.20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shah YB, Shaver AL, Beiriger J, Mehta S, Nikita N, Kelly WK, et al. Outcomes following abiraterone versus enzalutamide for prostate cancer: a scoping review. Cancers (Basel) 2022;14:3773. doi: 10.3390/cancers14153773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delgado A, Guddati AK. Clinical endpoints in oncology – a primer. Am J Cancer Res. 2021;11:1121–31. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.