Abstract
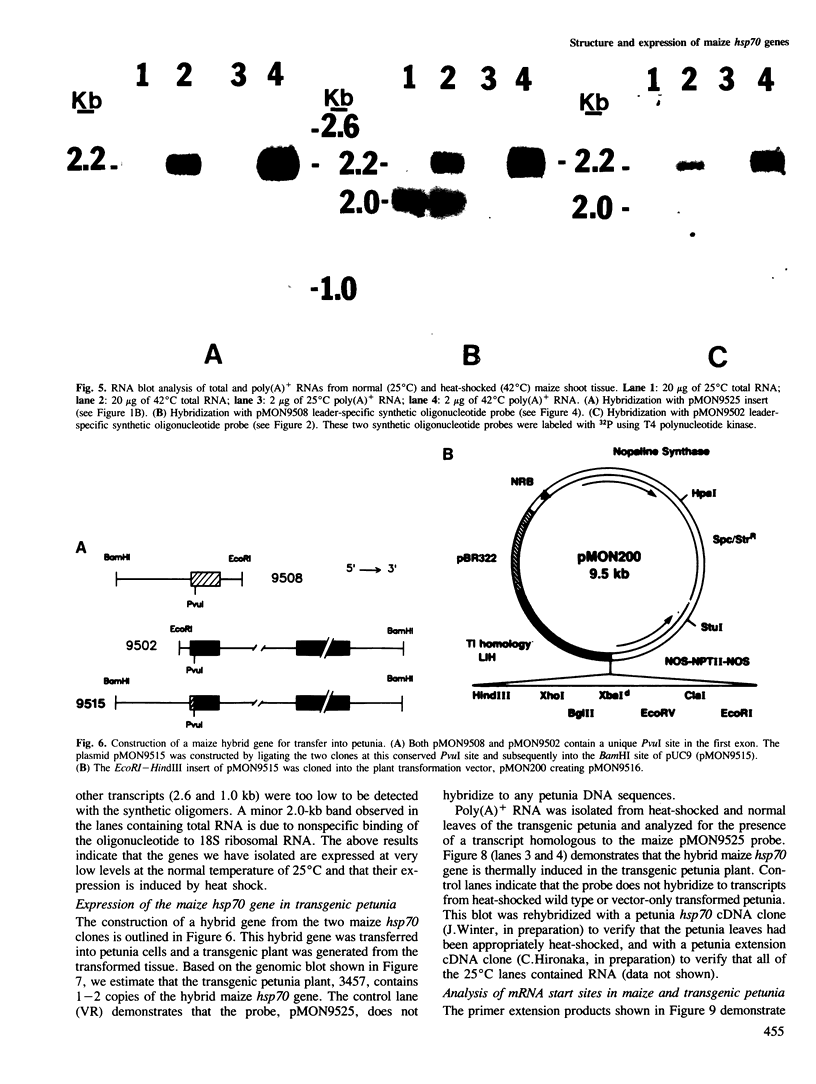
We have isolated and sequenced two maize genomic clones that are homologous to the Drosophila hsp70 gene. One of the maize hsp70 clones contains the entire hsp70 coding region and 81 nucleotides of the 5' nontranslated sequence. The predicted amino acid sequence for this maize protein is 68% homologous to the hsp70 of Drosophila. The second maize hsp70 clone contains only part of the coding sequence and 1.1 kb of the 5' flanking sequence. This 5' flanking sequence contains two sequences homologous to the consensus heat-shock-element sequence. Both maize genes are thermally inducible and each contains an intron in the same position as that of the heat-shock-cognate gene, hsc1, of Drosophila. The presence of an intron in the maize genes is a distinguishing feature in that no other thermally inducible hsp70 genes described to date contain an intron. We have constructed a hybrid hsp70 gene containing the entire hsp70 coding sequence with an intron, and 1.1 kb of the 5' flanking sequence. We demonstrate that this hybrid gene is thermally inducible in a transgenic petunia plant and that the gene is expressed from its own promoter.
Keywords: heat shock genes, maize, plant transformation
Full text
PDF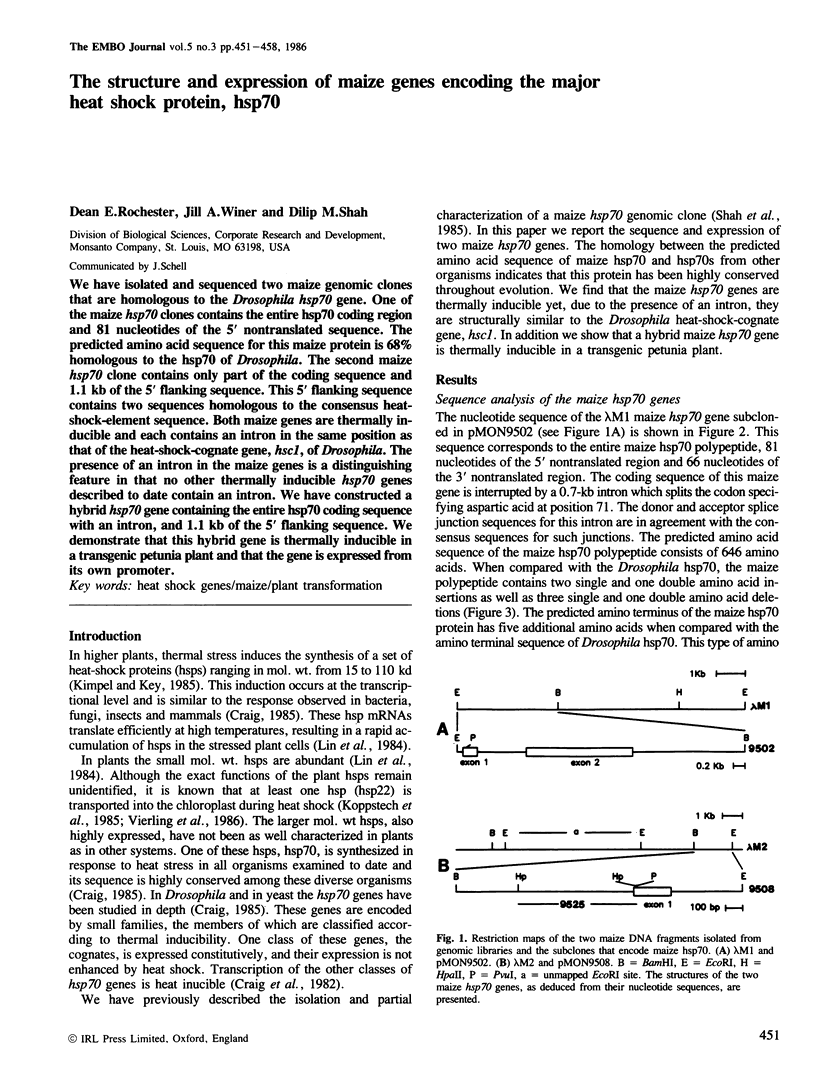






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Gurley W. B., Czarnecka E., Nagao R. T., Key J. L. Upstream sequences required for efficient expression of a soybean heat shock gene. Mol Cell Biol. 1986 Feb;6(2):559–565. doi: 10.1128/mcb.6.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Drosophila gene related to the major heat shock-induced gene is transcribed at normal temperatures and not induced by heat shock. Proc Natl Acad Sci U S A. 1982 Jan;79(2):525–529. doi: 10.1073/pnas.79.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Ingolia T. D., Slater M. R., Craig E. A. Saccharomyces cerevisiae contains a complex multigene family related to the major heat shock-inducible gene of Drosophila. Mol Cell Biol. 1982 Nov;2(11):1388–1398. doi: 10.1128/mcb.2.11.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppstech K., Meyer G., Schuster G., Ohad I. Synthesis, transport and localization of a nuclear coded 22-kd heat-shock protein in the chloroplast membranes of peas and Chlamydomonas reinhardi. EMBO J. 1985 Aug;4(8):1901–1909. doi: 10.1002/j.1460-2075.1985.tb03869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Freund R., Schweber M., Wensink P. C., Meselson M. Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5613–5617. doi: 10.1073/pnas.75.11.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia P. N., Rubenstein I., Phillips R. L., Wang A. S., Xiang L. Z. Localization of the 5S rRNA genes and evidence for diversity in the 5S rDNA region of maize. Gene. 1981 Oct;15(1):7–20. doi: 10.1016/0378-1119(81)90099-8. [DOI] [PubMed] [Google Scholar]
- Nagao R. T., Shah D. M., Eckenrode V. K., Meagher R. B. Multigene family of actin-related sequences isolated from a soybean genomic library. DNA. 1981;1(1):1–9. doi: 10.1089/dna.1.1981.1.1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller R. H., Jackson J. F., McAllister L. B., Schwartz J. H., Kandel E. R., Axel R. A family of genes that codes for ELH, a neuropeptide eliciting a stereotyped pattern of behavior in Aplysia. Cell. 1982 Apr;28(4):707–719. doi: 10.1016/0092-8674(82)90050-2. [DOI] [PubMed] [Google Scholar]
- Schöffl F., Baumann G. Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J. 1985 May;4(5):1119–1124. doi: 10.1002/j.1460-2075.1985.tb03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Raschke E., Nagao R. T. The DNA sequence analysis of soybean heat-shock genes and identification of possible regulatory promoter elements. EMBO J. 1984 Nov;3(11):2491–2497. doi: 10.1002/j.1460-2075.1984.tb02161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shure M., Wessler S., Fedoroff N. Molecular identification and isolation of the Waxy locus in maize. Cell. 1983 Nov;35(1):225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Spena A., Hain R., Ziervogel U., Saedler H., Schell J. Construction of a heat-inducible gene for plants. Demonstration of heat-inducible activity of the Drosophila hsp70 promoter in plants. EMBO J. 1985 Nov;4(11):2739–2743. doi: 10.1002/j.1460-2075.1985.tb03997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling E., Mishkind M. L., Schmidt G. W., Key J. L. Specific heat shock proteins are transported into chloroplasts. Proc Natl Acad Sci U S A. 1986 Jan;83(2):361–365. doi: 10.1073/pnas.83.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]