Abstract
Objective
To determine the learning curve for double-port video-assisted thoracoscopic (VATS) lung segmentectomy performed by the same surgical team in our center.
Methods
We retrospectively collected clinical data from 193 patients who underwent double-port video-assisted thoracoscopic lung segmentectomy from March 2017 to March 2023. The operative time (OT) was analyzed using the cumulative sum (CUSUM) method, and two stages of the learning curve were obtained. Propensity score matching (PSM) was performed for age, sex, underlying disease, and single-segment resection via radius matching. The OT, estimated amount of intraoperative blood loss, and other complications were analyzed.
Results
We generated a graph of the CUSUM of the OT and found that the learning curve could be differentiated into two stages: the learning stage (1st to 95th surgery) and the proficiency stage (96th to 193rd surgery). Before PSM, there were significant differences in the OT, extent of lymph node station dissection, amount of drainage on the day of surgery, amount of drainage on the first postoperative day, estimated amount of intraoperative blood loss, and length of hospital stay after surgery. There were no significant differences in the average amount of drainage 3 days after surgery, postoperative tube time, or number of intraoperative revolutions. However, after PSM, there were significant differences in the OT, number of lymph node stations removed, amount of drainage on the day of surgery, and amount of drainage on the first postoperative day. There were no significant differences in the estimated amount of intraoperative blood loss, length of hospital stay after surgery, average amount of drainage for 3 days after surgery, postoperative tube time, or number of intraoperative revolutions.
Conclusion
In our center, the learning curve for double-port video-assisted thoracoscopic lung segmentectomy transitions from the learning stage to the proficiency stage when the number of surgical cases reaches 95. There were significant differences in the OT, number of lymph node stations removed, amount of drainage on the day of surgery, and amount of drainage on the first postoperative day.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-024-03180-1.
Keywords: Learning curve, Video-assisted thoracoscopic surgery, Segmentectomy, Cumulative sum, Propensity score matching
Introduction
Background
Lung cancer is one of the most common malignant tumors worldwide, including in China [1, 2]. It is a serious threat to human health. At present, surgery is an important treatment for lung cancer [3]. The surgical treatments available include lobectomy, segmentectomy, wedge resection, and sleeve resection. With the popularization of lung CT screening, early lung cancer can be effectively detected and diagnosed. During the progression of lung cancer from the central type to the peripheral type, segmentectomy has become a widely accepted early lung cancer resection technique because of its minimal amount of trauma and relatively good preservation of lung function. Its overall survival rate is better than that of lobectomy [4]. For small peripheral non-small cell lung cancer, lung segmentectomy is the standard surgical treatment [5, 6]. However, there are few studies on the learning curve for double-port video-assisted thoracoscopic lung segmentectomy [7].
Objectives
To enable surgeons to better learn how to perform double-port video-assisted thoracoscopic lung segmentectomy, we retrospectively analyzed the clinical data of 193 patients who underwent the procedure conducted by the same surgical team in our center. Draw up the learning curve on the basis of this data and evaluated the correlation between the stages of the learning curve [8].
Ethical statement
Data collection and analyses were in accordance with the ethical standards of the ethics committee (institutional review board no. IRB-AF/SC-04/02.0) and with the Declaration of Helsinki of 1975 revised in 2000.
Methods
Study design
The learning curve for this procedure was determined using the cumulative sum (CUSUM) method. Propensity score matching (PSM) was conducted to exclude the influence of covariates on the findings and evaluate the correlation between the stages of the learning curve [8].
Setting
We retrospectively collected clinical data from 193 patients who underwent double-port video-assisted thoracoscopic lung segmentectomy from March 2017 to March 2023. All surgeries were performed by the same surgical team at the First Affiliated Hospital of Harbin Medical University. The surgical team consisted of one surgeon, three assistants, two scrub nurses and one anesthesiologist. The leading surgeon has performed hundreds of thoracoscopic surgeries before this study. All surgeries were performed by the leading surgeon. The first double-port video-assisted thoracoscopic lung segmentectomy was performed in March 2017.
Participants and data sources
Lung cancer patients who underwent thoracoscopic segmental lung resection in our department between March 2017 and March 2023 were included in this study. The inclusion criteria were as follows: (1) tumor size (maximum diameter assessed by computed tomography [CT]) ≤ 2 cm; (2) ground-glass opacity (GGO) ≥ 50%; (3) anatomical lung segmental resection (inclusion of patients with multiple foci and simultaneous surgical resection was allowed, but the resected segmental lesion was required to be the main lesion); (4) Lung function or blood gas analysis indexes that met one of the following conditions: (a) forced expiratory volume in one second (FEV1) measured value > 1.5 and measured/predicted value > 70%; (b) oxygen partial pressure > 80 mmHg. The exclusion criteria included: (1) previous surgical history; (2) history of therapy (chemotherapy, radiotherapy, or others); and (3) lung function or blood gas analysis indices that did not meet the specified requirements.
Variables
We recorded several patient variables, including sex, age, basic diseases (e.g., diabetes mellitus and hypertension), the types of surgery, operative time (OT), estimated amount of intraoperative blood loss, amount of lymph node station dissection, amount of drainage on the day of surgery, amount of drainage on the first day after surgery, average amount of drainage for 3 days after surgery, postoperative tube time, length of hospital stay after surgery, and number of intraoperative revolutions were recorded. The covariates included sex, age, basic diseases and the types of surgery.
Depending on whether a single segment was resected, the types of surgery were divided into single-lung segmentectomy and combined-lung segmentectomy. Single-lung segmentectomy was described as surgery targeting only a single lung segment, while combined-lung segmentectomy included segment–segment, segment–wedge, and other lung segment–lung tissue resections.
Surgical methods
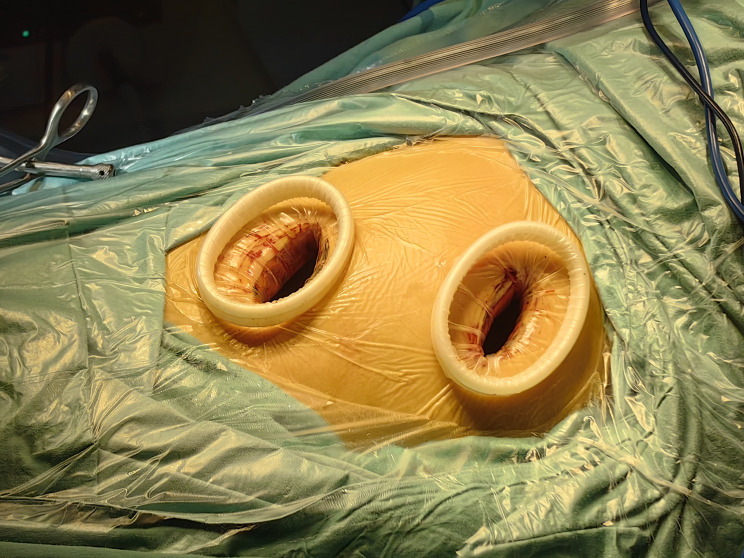
All patients underwent preoperative three-dimensional lung reconstruction using Mimics Medical 21.0.0.406 (Materialise N.V., Leuven, Belgium) and/or CT-guided puncture for lesion localization. The patients received general anesthesia with intravenous compound anesthesia and mechanical ventilation through a double-lumen endotracheal tube. Segmentectomy was performed using double-port thoracoscopy, with patients positioned in the lateral decubitus position. An observation port, approximately 5 centimeters in length, was created in the seventh or eighth intercostal space (ICS) along the midaxillary line on the affected side. An operating port, approximately 3 centimeters in length, was placed in the third or fourth ICS along the anterior axillary line on the affected side (see Figs. 1 and 2). The corresponding veins, arteries, and bronchi of the lung segment where the goal lesion was located were freed in three dimensions. A Johnson Endo-GIA cut stapler was utilized for cutting and anastomosis. The specimen was removed using a pickup bag, and a frozen section was performed to confirm the presence of cancer. Lymph node dissection followed. After surgery, the ribcage was rinsed, and hemostasis was achieved. The bronchial and lung stumps were inspected for air leaks. Following the placement of a drainage tube, the incision was sutured in layers (see Figs. 3 and 4).
Fig. 1.
Positions of the surgical ports for two-port video-assisted thoracoscopic surgery segmentectomy
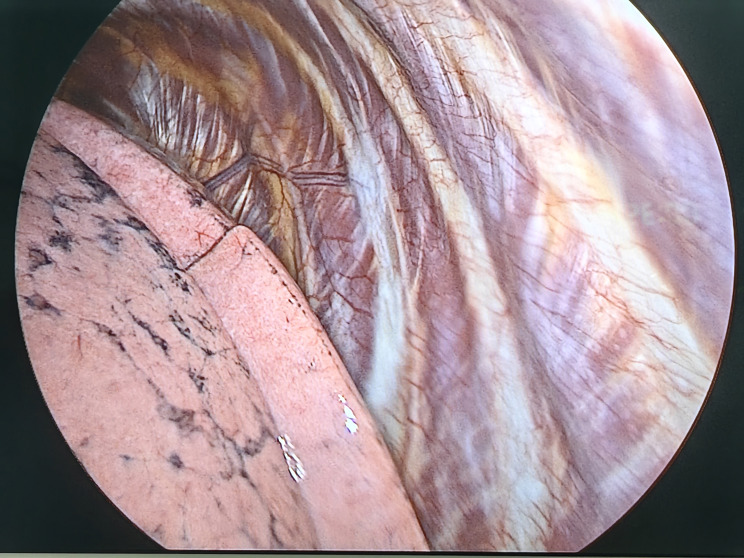
Fig. 2.
Operative field of the observation hole
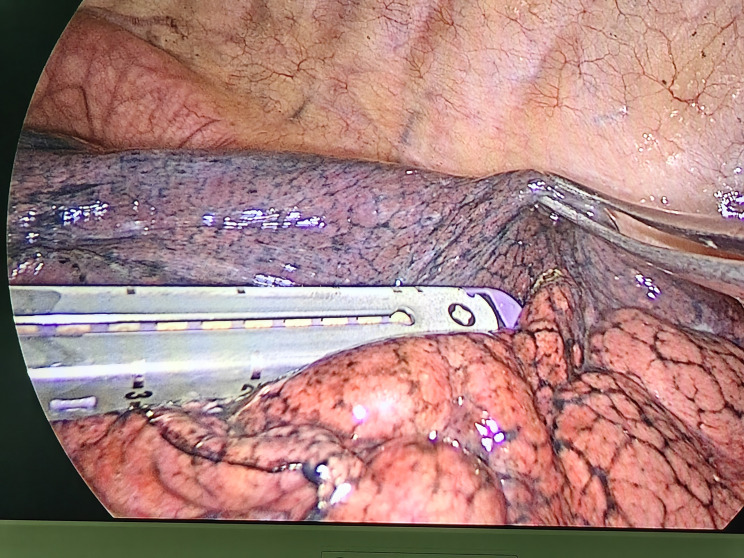
Fig. 3.
The use of endostaplers
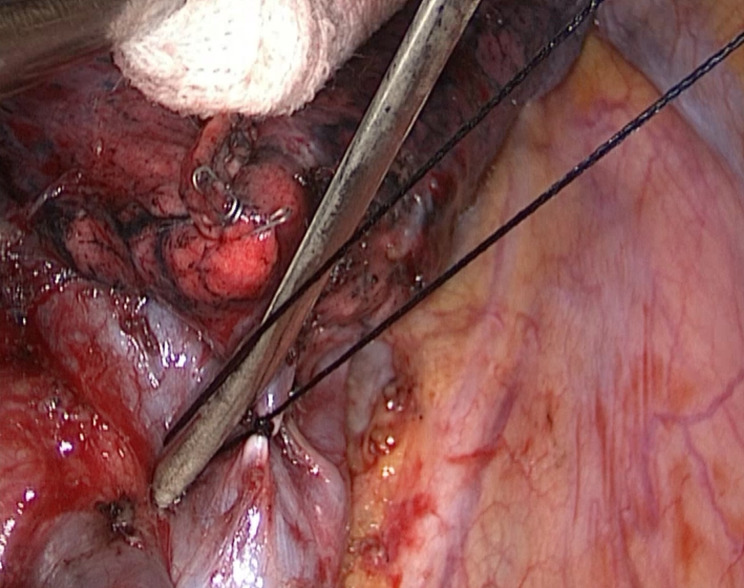
Fig. 4.
Silk ligatures on vessels
Statistical methods
All the data were processed using IBM Statistical Package for Social Sciences (SPSS) v27.0 (IBM Co., Armonk, NY, USA) and STATA 17.0 MP–Parallel Edition(Stata Corp LLC, College Station, TX, USA). The CUSUM values of the OT for each patient were obtained using the CUSUM method. The first CUSUM value was calculated as follows: CUSUM (OT1) = OT1 − mean OT of all patients, while the remaining CUSUM values were calculated as follows: CUSUM (OTn) = CUSUMn−1 + OTn − mean OT of all patients [9]. IBM SPSS Statistics 27.0 was used to generate scatter plots, with the number of surgical cases plotted on the horizontal axis and the CUSUM value of the OT on the vertical axis. Linear relationships were excluded, and curve estimation was performed. R² was used to determine the goodness of fit of the curve, and the maximum value of R² was considered the best curve model [10]. The point where the slope changed positively, which corresponded to the vertex of the curve, served as the minimum number of surgical cases required to cross the learning curve. This curve was divided into the learning stage and the proficiency stage.
Count data are expressed as percentages. Normally distributed data were tested for normality, with normally distributed data presented as the means ± standard deviations (SDs) and nonnormally distributed data presented as medians (interquartile ranges). Student’s t test, the Wilcoxon rank-sum test, the chi-square test, and a nonparametric test were used to determine the mean differences between the curve stages and between the surgical groups. A probability value of < 0.05 was considered to indicate statistical significance.
The PSmatch2 package in STATA 17.0 MP–Parallel Edition was used for PSM analysis to reduce the effect of covariates in both stages, and logit regression models were utilized to generate propensity scores. Age, sex, underlying medical conditions, and single-segment resection were included in the PSM analysis. The patients were matched via radius matching, and the logit of the tendency value was matched using a caliper with a width equal to 0.2 of the SD of the tendency value [11] (0.026).
Results
Participants
Among the 193 cases of double-port video-assisted thoracoscopic lung segmentectomy performed by the same surgical team at our center between March 2017 and March 2023, six cases were converted to three-port thoracoscopic surgery, and one case was converted to open chest surgery. These converted cases were not excluded from the study, as they contributed to the accumulation of operator experience. Excluding these cases would result in an underestimation of the number of surgeries required for the operator to achieve proficiency. Notably, none of the patients experienced life-threatening complications, hospital readmissions, or deaths.
Learning curve analysis
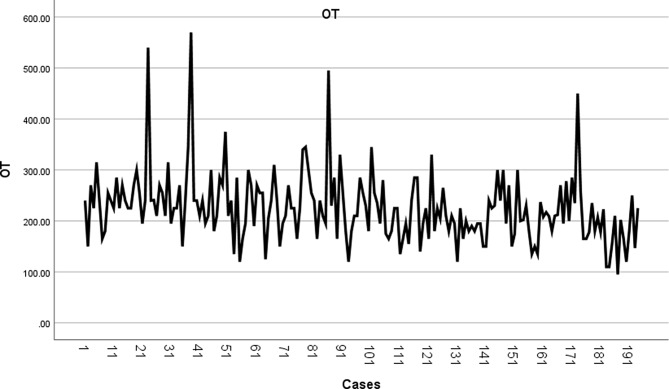
The OT for lung segmentectomy was drawn in the order in which surgery was performed (Fig. 5). The OT gradually decreased, but the change trend was unclear. The CUSUM value of the OT for each patient was obtained using the CUSUM method.
Fig. 5.
The OT for lung segmentectomy
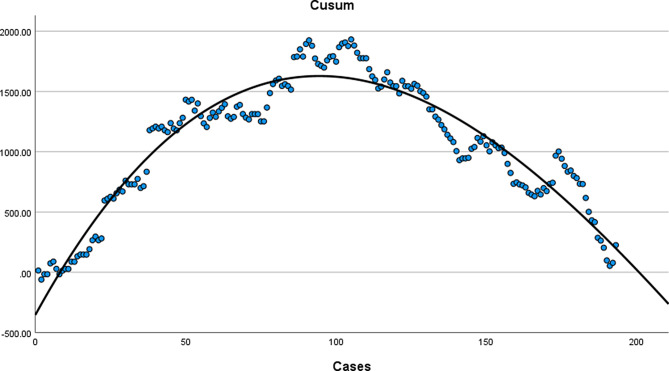
The cubic curve was judged to be the best-fitting model based on a maximum R² value of 0.911, and the corresponding curve equation was y = 0.0004 x³ − 0.2960 x² + 45.4710 x − 353.8535 (Fig. 6). The slope of the curve showed positive and negative shifts at the 95th surgery; the 95th case was the inflection point of the CUSUM curve. The ascending slope of the surgical composition curve from the 1st to the 95th cases was the first stage of the learning curve—the learning stage. Conversely, the descending slope of the surgical composition curve from the 96th to the 193rd cases was the second stage of the learning curve—the proficiency stage. The cumulative summation scores and slope scores are provided in Supplementary Table 1.
Fig. 6.
CUSUM values and the corresponding curve equations
Propensity score matching analyses
A comparison of the various parameters of the two stages identified based on CUSUMOT is shown in Table 1. There were no significant differences in sex, age, or type of surgery between the two groups. There were significantly more patients with underlying disease (e.g., diabetes mellitus and hypertension) in the proficiency stage than in the learning stage (P = 0.003). Therefore, there was selectivity bias in this study. The presence of bias and confounding factors could affect the accuracy of the findings. Owing to the retrospective nature of the study, the selected samples were tested for the influence of confounding factors. To reduce the effect of bias and confounding factors and increase comparisons between the groups, we used PSM to control for the effects of confounders.
Table 1.
The various parameters of the two stages identified based on CUSUMOT
| Learning stage (95 cases) | Proficiency stage (98 cases) | P-value | |
|---|---|---|---|
| Sex | 0.881 | ||
| Male | 33 (34.7%) | 36 (36.7%) | |
| Female | 62 (62.3%) | 62 (63.3%) | |
| Age (year) | 53.2 ± 9.4 | 55.4 ± 10.6 | 0.103 |
| Basic diseases | 9 | 26 | 0.003 |
| Hypertension | 8 | 22 | |
| Diabetes mellitus | 4 | 10 | |
| Type of surgery | 0.372 | ||
| Single-lung segmentectomy | 62 (62.3%) | 58 (59.2%) | |
| Combined-lung segmentectomy | 33 (34.7%) | 40 (40.8%) |
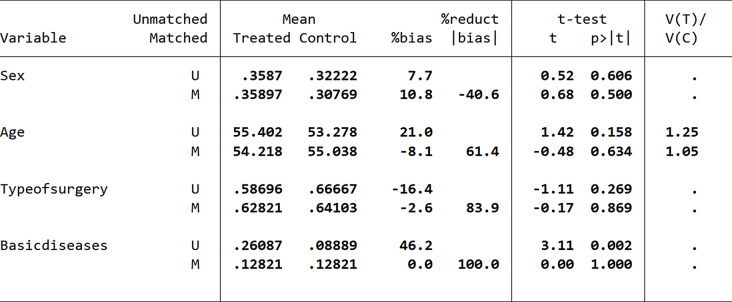
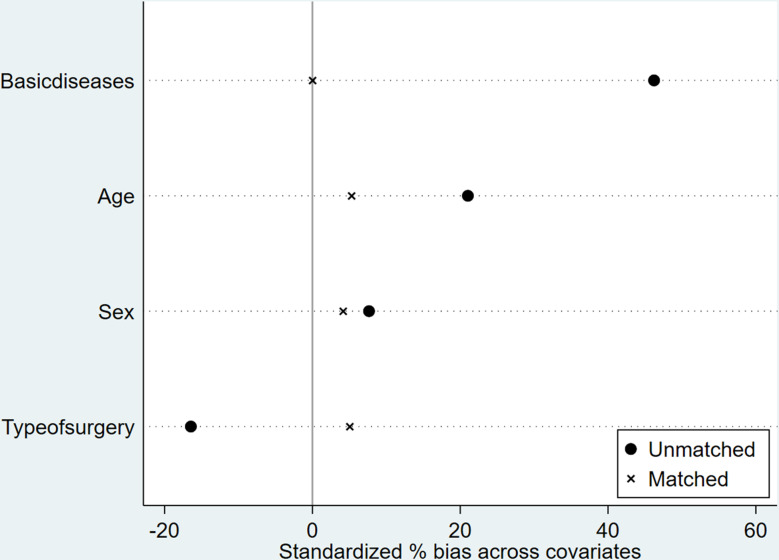
Initially, we utilized proximity matching for the propensity score matching analysis. During the balance test, we discovered that the bias value for the sex variable after matching was 10.8, exceeding the acceptable threshold of 10 (Fig. 7). This finding indicated that significant differences persisted among the influencing factors, and the balance test was unsuccessful, demonstrating that the proximity matching did not effectively eliminate the influence of covariates. Consequently, we decided to implement radius matching as an alternative approach to enhance the matching analysis.
Fig. 7.
Balance test results based on proximity matching
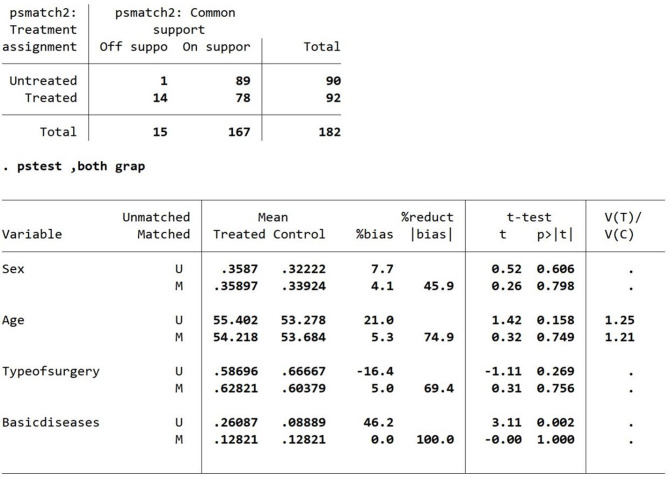
Radius matching was performed using a caliper width of 0.026. After the missing values were removed, eleven patients with missing clinical data were excluded. A total of 182 patients were included in the data matching, of whom 167 were conducted following analyses after successful case matching. The percent bias of the overall equilibrium test for PSM was less than 10, and the t value was less than 1.96 [12, 13] (Figs. 8 and 9). The P values were greater than 0.05, indicating that the match was excellent.
Fig. 8.
Balance test results based on radius matching
Fig. 9.
Balance test results based on radius matching
The OT, number of lymph node stations removed, amount of drainage on the day of surgery, and amount of drainage on the first day after surgery in the postlearning stage and the proficiency stage were 241.22 (40.04) and 196.56 (11.44), 4.72 (1.19) and 5.80 (0.53), 192.87 ± 25.73 and 238.57 (34.58), and 187.97 (32.07) and 223.42 (25.97), respectively. The P values were all less than 0.05, indicating significant differences. Conversely, there was no significant difference in the estimated amount of intraoperative blood loss, length of hospital stay after surgery, average amount of drainage for 3 days after surgery, postoperative tube time, or number of intraoperative revolutions (Table 2).
Table 2.
The parameters of the two stages identified before and after PSM
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Learning stage (95 cases) | Proficiency stage (98 cases) | P value | Learning stage (89 cases) | Proficiency stage (94 cases) | P value | |
| OT (min) | 240 (75) | 200 (60) | < 0.001 | 241.22 (40.04) | 196.56 (11.44) | < 0.001 |
| Estimated amount of intraoperative blood loss (mL) | 20 (30) | 20 (0) | < 0.001 | 39.38 (29.96) | 23.16 (10.50) | 0.850 |
| Lymph node station dissection | 5 (2) | 6 (2) | < 0.001 | 4.72 (1.19) | 5.80 (0.53) | 0.032 |
| Amount of drainage on the day of surgery (mL) | 180 (125) | 232.61 ± 109.53 | 0.019 | 192.87 ± 25.73 | 238.57 (34.58) | 0.010 |
| Amount of drainage on the first postoperative day (mL) | 200 (125) | 205 (81) | 0.002 | 187.97 (32.07) | 223.42 (25.97) | < 0.001 |
| Average amount of drainage for 3 days after surgery (mL) | 169.68 ± 56.91 | 176 (72) | 0.355 | 168.50 (12.25) | 173.78 (30.56) | 0.091 |
| Postoperative tube time (day) | 4 (3) | 4 (2) | 0.052 | 4.74 (0.85) |
4.08 (0.40) |
0.133 |
| Length of hospital stay after surgery (day) | 7 (3) | 6 (2) | 0.008 | 7.31 (0.85) |
6.50 (0.60) |
0.085 |
| Number of intraoperative revolutions | 4 (4.2%) | 3 (3.1%) | 0.718 | 0 (0) | 0 (0.06) | 0.333 |
| Death rate (%) | 0 | 0 | NA | 0 | 0 | NA |
Discussion
Key results
In this study, the learning curve for double-port video-assisted thoracoscopic lung segmentectomy in our center was determined by analyzing the OTs of 193 patients using the CUSUM method. With the 95th surgery as the inflection point, the learning curve was divided into two stages: the learning stage and the proficiency stage. The surgical data for the two stages were matched with propensity scores to compare the stages. The analysis showed that the OTs in the proficiency stage were significantly lower than the OTs in the learning stage. Lymph node station dissection, the amount of drainage on the day of surgery, and the amount of drainage on the first day after surgery increased significantly. There were no significant differences in the estimated amount of intraoperative blood loss, length of hospital stay after surgery, average amount of drainage for 3 days after surgery, postoperative tube time, or number of intraoperative revolutions.
Limitations
The limitations of this study include its single-center retrospective nature and relatively small sample size. Although PSM analysis was performed on sex, age, underlying medical conditions, and type of surgery, the influence of confounding factors on the findings of the study was controlled to some extent. However, uncontrollable and unobservable influencing factors may still be present. In this study, the OT was used as the only parameter for judging the learning curve. The estimated amount of intraoperative blood loss, postoperative tube time, length of hospital stay after surgery, and long-term efficacy could also be included as comprehensive factors to assess the learning curve.
Interpretation
In 2022, Dimitrovska et al. [7] conducted a retrospective study of the learning curve for double-port thoracoscopic segmentectomy derived from 86 patients over 5 months. In their study, the inflection point of the learning curve was the 47th surgery. In 2021, Kuang et al. [14] performed a retrospective study of the learning curve for single-port thoracoscopic segmentectomy derived from 148 patients over 2 years, and the inflection point of the learning curve was the 36th surgery. In 2021, Gao et al. [15] retrospectively studied the learning curve for single-port thoracoscopic segmentectomy derived from 148 cases over 5 years, and the inflection point of the learning curve was the 58th surgery. In 2018, Hamada et al. [16] conducted an 11-year prospective study of the learning curve for thoracoscopic anatomical segmentectomy derived from 252 patients, and the inflection point of the learning curve was the 84th surgery. The existing findings demonstrate that thoracic surgeons with skilled video-assisted thoracoscopic surgical techniques need only a relatively short time to learn about two-port video-assisted thoracoscopic segmentectomy and reach proficiency, which is consistent with the present findings [7, 14–20]. Our study was a retrospective analysis of 193 cases of surgery performed within 6 years. In contrast, the time span is longer than the above research, the frequency of practice is lower than the above research, and the number of surgical cases required to reach the proficiency stage is more than the above research. According to the above comparison, we believe that with the extension of the study time span and the decrease of the practice frequency, the inflection point of the learning curve becomes delayed. The occurrence of inflection points in the learning curve for lung segmentectomy can be influenced by the time span during the learning stage. In the process of learning lung segmentectomy, continuous repeated practice in a short period can advance the inflection point, helping to reach the proficiency stage faster.
In 2016, Guo et al. [21] reported that the number of lymph node dissections affects the recurrence and metastasis rates. In 2022, Riquet et al. [22] reported that the prognosis of patients with lung cancer is positively correlated with the number of lymph nodes dissected during radical lung cancer resection. With the gradual deepening of the understanding of lymph node dissection, people pay more and more attention to lymph node dissection [23, 24]. And we incorporated the lymph node dissection time into the overall OT. We adjusted the number of groups for lymph node clearance from 4.72 (1.19) in the learning phase to 5.80 (0.53) in the proficient phase. Of the existing research, Wu et al. [20] showed no statistical difference in the number of lymph node dissection at the three different stages. Increasing the number of lymph node dissection during the process of proficiency may also be one of the reasons for the late appearance of the learning curve inflection point in our study [25]. As the number of lymph nodes dissected groups increases, there is a corresponding increase in the amount of postoperative drainage, which is consistent with the results of our study. Therefore, we recommend that surgeons aim to remove an appropriate number of lymph nodes in the early stages of segmental lung resection, as this may expedite the achievement of proficiency.
In our study, there were significantly more patients with basic disease in the proficiency stage than in the learning stage. This may be due to the cautious selection of patients in the initial phase of the study. There was no statistical difference in the basic condition section of patients in the existing studies by Wu et al., Dimitrovska et al., and Hamada et al. Therefore, This can also be the cause of the inflection point of the learning curve shifting backward or the amount of drainage increasing.
We did not perform bronchoscopy, transthoracic biopsy, or PET-CT prior to surgery. Pulmonary nodules to be segmentally resected are generally small, which leads to difficult maneuvers and high false-negative rates. So we do not perform transthoracic biopsy or bronchoscopy before surgery. PET-CT has no significant advantage in the differential diagnosis of pGGN and solid component ≤ 8 mm lung nodules [26]. The use of PET can be avoided for nonsolid nodules (ground glass opacity or mixed nodules) and replaced by thin-section CT of the lungs which performs well in these circumstances [27]. Plus, PET-CT is more expensive, so we don’t routinely perform PET-CT before the surgery, but use thin-section CT to replace it.
There are several access options available for thoracoscopic segmental lung resection, and available studies show that single- and multi-port thoracoscopic techniques are similar in terms of safety and efficacy in patients with lung cancer [28, 29]. In contrast, the single-port thoracoscopic technique has the advantage of less injury, less postoperative pain, and less damage to the chest wall and intercostal nerves [29]. Compared with the single-port thoracoscopic technique, the double-port thoracoscopic technique is more effective in exposing the surgical field by pulling, it is more convenient to use the cutting anastomosis from different holes, the instruments collide with each other to a lesser extent, and the drainage effect is more precise. And compared to the multi-port thoracoscopic technique, there is less damage to the patient and shorter postoperative drainage time [30]. In summary, the surgical procedure of choice in our centre is the double-port thoracoscopic technique for lung segmental resection.
Regarding the selection of the length of the operating hole and the observation hole, the small operating hole brings less pain and faster recovery for patients after surgery, but it will increase the difficulty of surgical operation. The operation hole in the higher position is often only used by the leading surgeon himself, so only 3 cm holes can be opened to reduce the pain of patients and accelerate the recovery of patients. The observation hole at the lower position often requires two surgeons to use together, and the instruments may collide with each other, so we recommend opening a 5 cm long observation hole to reduce the difficulty of operation.
Conclusion
In summary, a CUSUM analysis using surgical times identified two stages of the learning curve. The data suggest that at our institution, the inflection point of the learning curve occurs after completion of 95 procedures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- VATS
Video-assisted thoracoscopic surgery
- OT
The operative time
- CUSUM
Cumulative sum
- PSM
Propensity score matching
- GGO
Ground-glass opacity
- FEV1
Forced expiratory volume in one second
- ICS
Intercostal space
Author contributions
B. Y.: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Writing – original draft. X. Y.: Data curation. Z. Z.: Conception; Data curation. T. S.: conceptualization; formal analysis; methodology. Y. Z.: Data curation. W. Z.: Data curation; Software. D. W.: Data curation. H. C.: Conceptualization; methodology; software; writing – review & editing.All authors reviewed the manuscript.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen P, Liu Y, Wen Y, Zhou C. Non-small Cell Lung Cancer in China. Cancer Commun (Lond). 2022;42(10):937–70. 10.1002/cac2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics. 2022. CA Cancer J Clin 2022, 72 (1), 7–33. 10.3322/caac.21708 [DOI] [PubMed]
- 3.Klapper J, D’Amico TA. VATS Versus Open surgery for Lung Cancer Resection: moving toward a minimally invasive Approach. J Natl Compr Canc Netw. 2015;13(2):162–4. 10.6004/jnccn.2015.0023. [DOI] [PubMed] [Google Scholar]
- 4.Bongiolatti S, Salvicchi A, Mugnaini G, Vokrri E, Viggiano D, Gonfiotti A, Lavorini F, Voltolini L. Does thoracoscopic basal pyramid segmentectomy really offer functional advantages in comparison with thoracoscopic Lower Lobectomy? Interdisciplinary Cardiovasc Thorac Surg. 2023;36(2):ivad018. 10.1093/icvts/ivad018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomori H, Yamazaki I, Machida Y, Otsuki A, Cong Y, Sugimura H, Oyama Y. Lobectomy versus Segmentectomy: a propensity score-matched comparison of postoperative complications, pulmonary function and prognosis. Interact Cardiovasc Thorac Surg. 2022;34(1):57–65. 10.1093/icvts/ivab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe S-I, Asamura H. West Japan oncology group and Japan clinical oncology group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607–17. 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrovska NT, Bao F, Yuan P, Hu S, Chu X, Li W. Learning curve for two-Port Video-assisted thoracoscopic surgery lung segmentectomy. Interact Cardiovasc Thorac Surg. 2022;34(3):402–7. 10.1093/icvts/ivab236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Zhang Y, Guo Y, Li W, Huang Y, Wu T, Jiang G, Dai J. Prognosis of Lung Cancer Associated with cystic airspaces: a propensity score matching analysis. Lung Cancer. 2021;159:111–6. 10.1016/j.lungcan.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Xu S, Liu B, et al. Learning curve study of the Da Vinci robotic sharpectomy. Chin Clin J Thorac Cardiovasc Surg. 2024;31(5):689–94. [Google Scholar]
- 10.Ou J. Comparison of Fitting effects of SPSS and Excel for pharmacokinetic parameters. of Extravenous Administration; 2007.
- 11.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in Observational studies. Pharm Stat. 2011;10(2):150–61. 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattanayak CW, Rubin DB, Zell ER. Propensity score methods for creating Covariate Balance in Observational studies. Revista Española De Cardiología. (English Edition). 2011;64(10):897–903. 10.1016/j.rec.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum PR, Rubin DB. The Central Role of the Propensity score in Observational studies for Causal effects. Biometrika. 1983;70(1):41–55. 10.1093/biomet/70.1.41. [Google Scholar]
- 14.Kuang Y, Zhang J, Niu L, et al. Clinical application and learning curve of the single-port thoracoscopic technique [J]. Chin Clin J Thorac Cardiovasc Surg. 2021;28(07):826–9. [Google Scholar]
- 15.Gao Y, Zhang Z, Fu W, et al. Learning curve of single-operated thoracoscopic lung resection for early non-small cell lung cancer [J]. Chin J Minim Invasive Surg. 2021;21(12):1072–6. [Google Scholar]
- 16.Hamada A, Oizumi H, Kato H, Suzuki J, Nakahashi K, Sho R, Sadahiro M. Learning curve for Port-Access Thoracoscopic anatomic lung segmentectomy. J Thorac Cardiovasc Surg. 2018;156(5):1995–2003. 10.1016/j.jtcvs.2018.06.082. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Lee JY, Choi JS, Sung SW. Comparison of Uniportal versus Multiportal Video-assisted thoracoscopic surgery Pulmonary Segmentectomy. Korean J Thorac Cardiovasc Surg. 2019;52(3):141–7. 10.5090/kjtcs.2019.52.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonfiotti A, Bongiolatti S, Borgianni S, Borrelli R, Jaus MO, Politi L, Tancredi G, Viggiano D, Voltolini L. Development of a video-assisted thoracoscopic lobectomy program in a single Institution: results before and after completion of the learning curve. J Cardiothorac Surg. 2016;11(1):130. 10.1186/s13019-016-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng K, Zheng B, Zhang S, Zheng W, Guo Z, Zhu Y, Chen C. Feasibility and learning curve of Uniportal Video-assisted thoracoscopic segmentectomy. J Thorac Disease. 2016;8(Suppl 3). 10.3978/j.issn.2072-1439.2016.02.14. [DOI] [PMC free article] [PubMed]
- 20.Wu W, Xu J, Wen W, Yu Y, Xu X, Zhu Q, Chen L. Learning curve of totally thoracoscopic pulmonary segmentectomy. Front Med. 2018;12(5):586–92. 10.1007/s11684-017-0566-z. [DOI] [PubMed] [Google Scholar]
- 21.Guo X, Tang S. Correlation between the number of surgical lymph nodes and postoperative thoracic drainage rate and prognosis of non-small cell lung cancer [J]. J Pract Cancer. 2016;31(01):76–8. [Google Scholar]
- 22.Riquet M, Arame A, Pricopi C. Subcarinal Lymph Node Importance Revisited. Ann Thorac Surg. 2018;105(2):666–7. 10.1016/j.athoracsur.2017.04.063. [DOI] [PubMed] [Google Scholar]
- 23.Verhagen AF, Schoenmakers MCJ, Barendregt W, Smit H, van Boven W-J, Looijen M, van der Heijden EHFM, van Swieten HA. Completeness of Lung Cancer surgery: is Mediastinal Dissection Common Practice? Eur J Cardiothorac Surg. 2012;41(4):834–8. 10.1093/ejcts/ezr059. [DOI] [PubMed] [Google Scholar]
- 24.Anami K, Yamashita S-I, Yamamoto S, Chujo M, Tokuishi K, Moroga T, Mori H, Kawahara K. Contralateral Mediastinal Lymph Node Micrometastases assessed by video-assisted thoracoscopic surgery in Stage I Non-small Cell Left Lung Cancer. Eur J Cardiothorac Surg. 2013;43(4):778–82. 10.1093/ejcts/ezs415. [DOI] [PubMed] [Google Scholar]
- 25.Chen WG, Zhang H, Wu WB, Early chest tube removal following single-direction versus conventional uniportal video-assisted thoracoscopic lobectomy: A retrospective cohort study. Chin J Clin Thorac Cardiovasc Surg. 2023,30(1):71–77
- 26.Chun EJ, Lee HJ, Kang WJ, Kim KG, Goo JM, Park CM, Lee CH et al. Differentiation between Malignancy and Inflammation in Pulmonary Ground-Glass Nodules: The Feasibility of Integrated 18F-FDG PET/CT. Lung Cancer. 2009;65 (2),180–186. 10.1016/j.lungcan.2008.11.015 [DOI] [PubMed]
- 27.Groheux D, Quere G, Blanc E, Lemarignier C, Vercellino L, de Margerie-Mellon C, Merlet P, Querellou S. FDG PET-CT for Solitary Pulmonary Nodule and Lung Cancer: Literature Review. Diagn Interv Imaging. 2016;97(10):1003–17. 10.1016/j.diii.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Song KS, Park CK, Kim JB. Efficacy of single-Port Video-assisted thoracoscopic surgery lobectomy compared with triple-Port VATS by Propensity score matching. Korean J Thorac Cardiovasc Surg. 2017;50(5):339–45. 10.5090/kjtcs.2017.50.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris CG, James RS, Tian DH, Yan TD, Doyle MP, Gonzalez-Rivas D, Cao C. Systematic review and Meta-analysis of Uniportal versus Multiportal Video-assisted thoracoscopic lobectomy for Lung Cancer. Ann Cardiothorac Surg. 2016;5(2):76–84. 10.21037/acs.2016.03.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y-F, Huang C-L, Hung W-H, Cheng C-Y, Wang B-Y. The Perioperative outcomes of Uniport versus two-Port and three-Port Video-assisted thoracoscopic surgery in Lung Cancer: a systematic review and Meta-analysis. J Cardiothorac Surg. 2022;17(1):284. 10.1186/s13019-022-02034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.